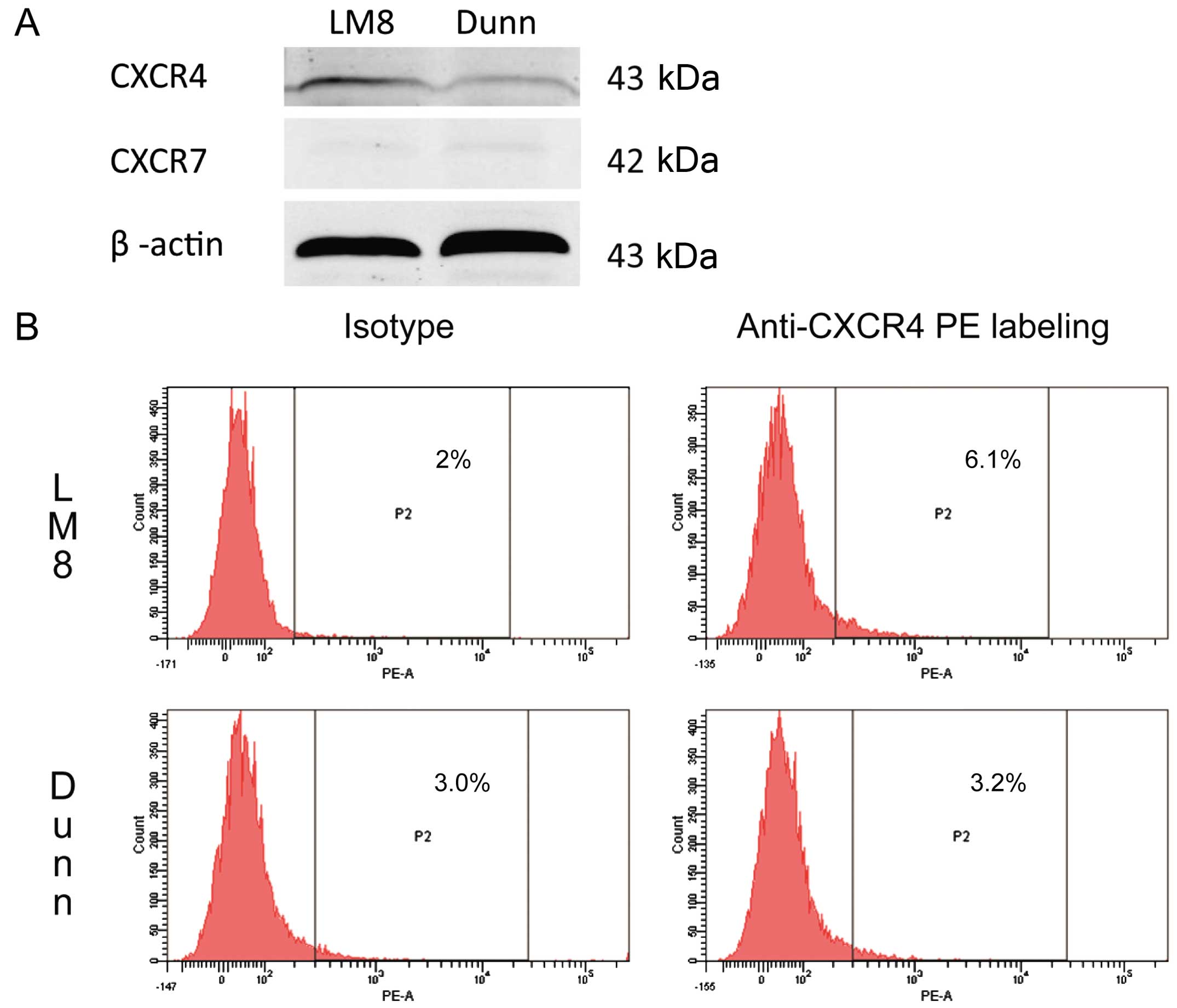
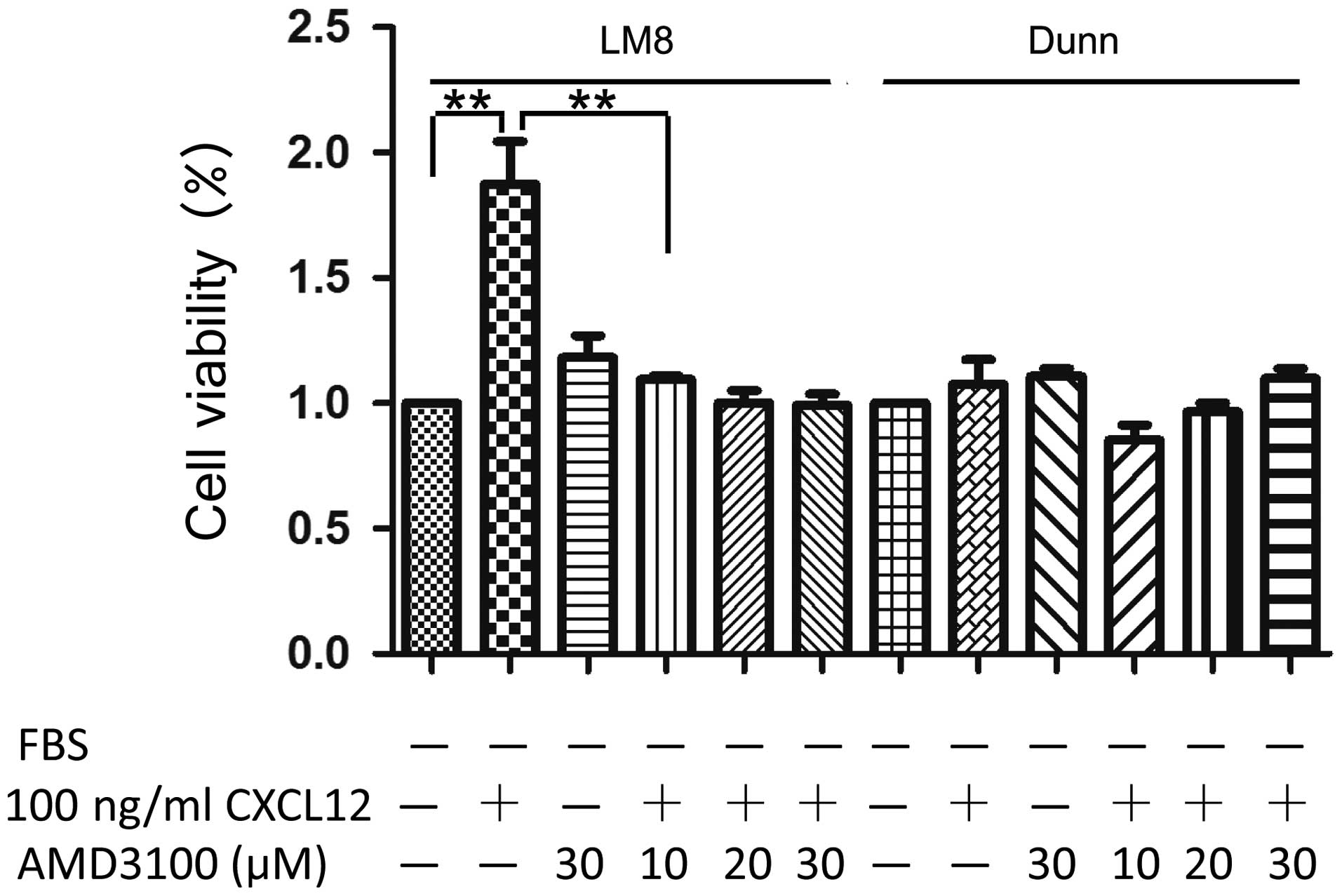
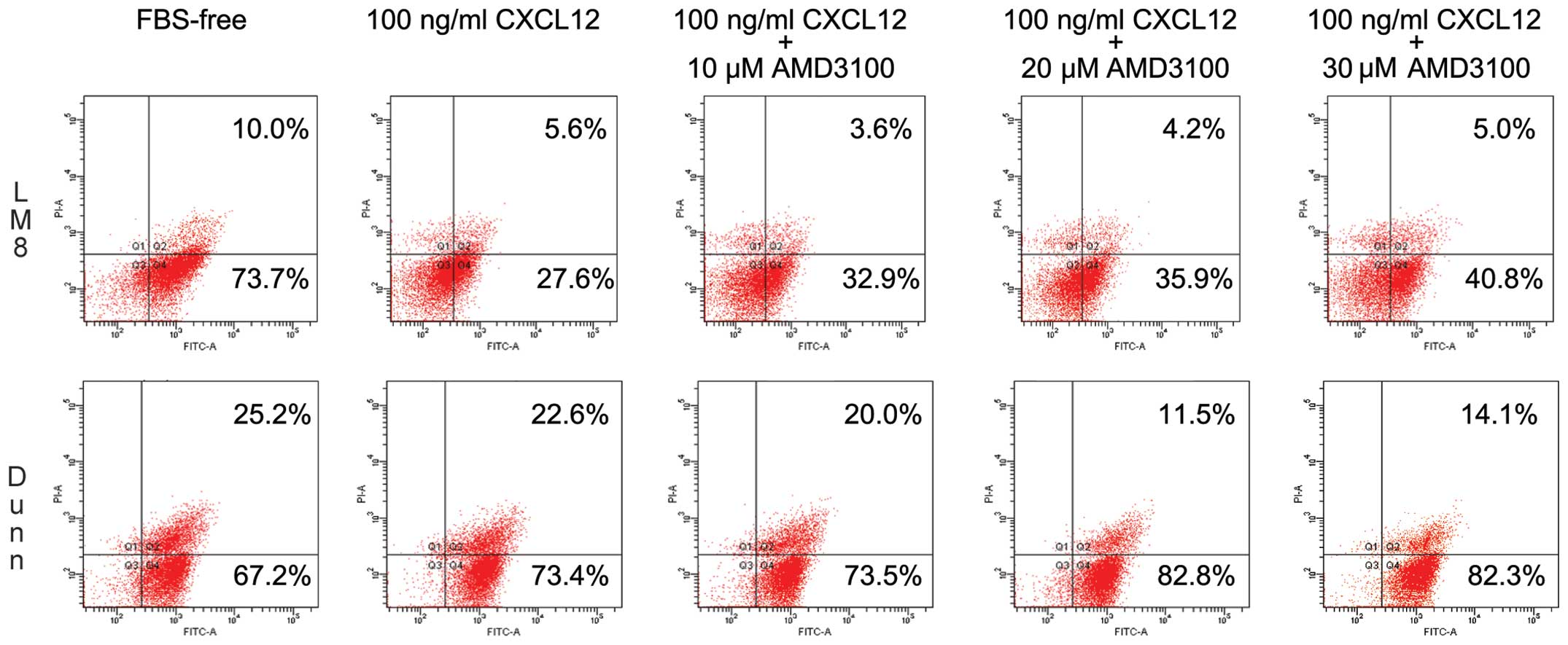
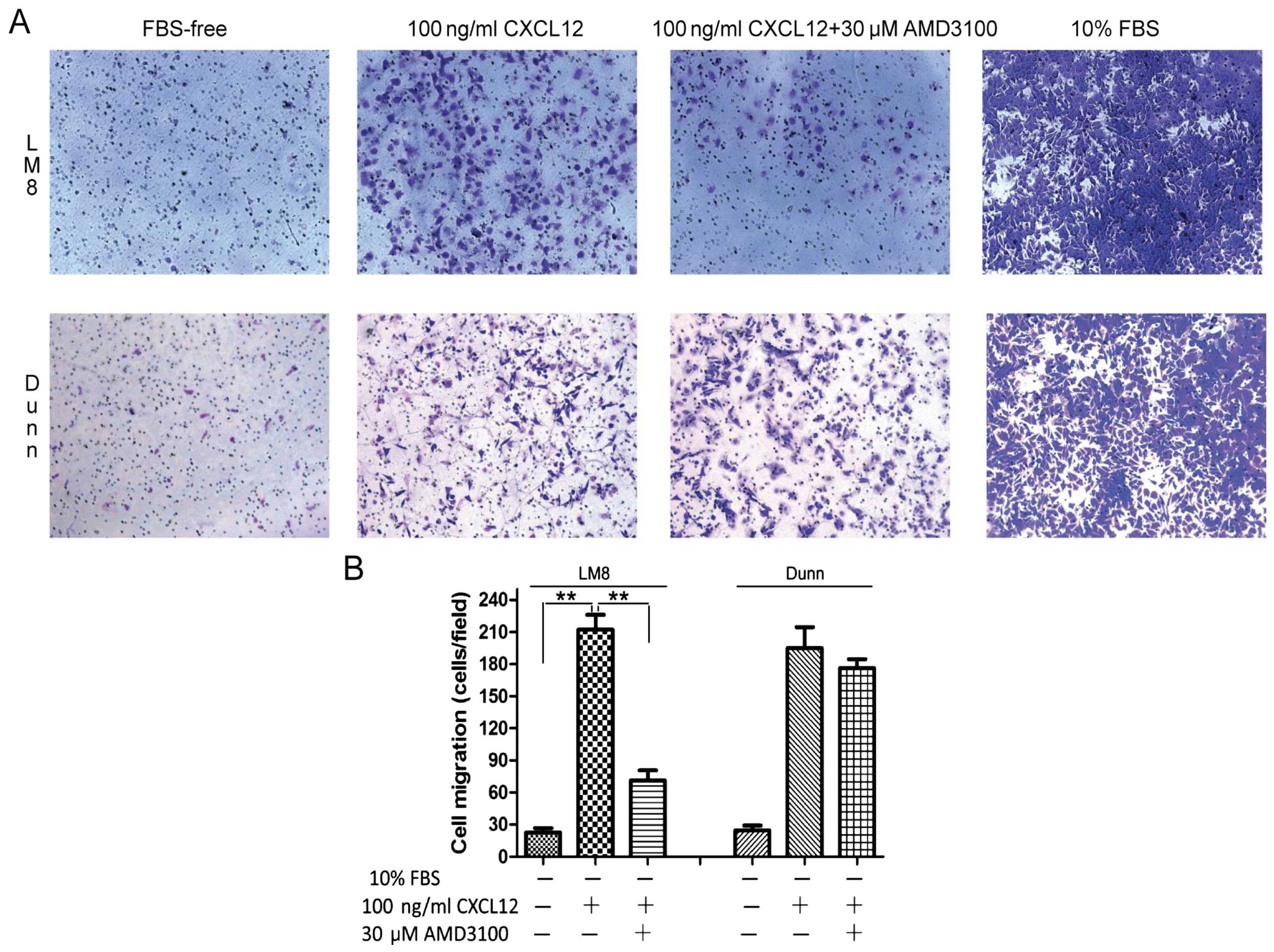
|
1
|
Mirabello L, Troisi RJ and Savage SA:
Osteosarcoma incidence and survival rates from 1973 to 2004: Data
from the Surveillance, Epidemiology, and End Results Program.
Cancer. 115:1531–1543. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mankin HJ, Hornicek FJ, Rosenberg AE,
Harmon DC and Gebhardt MC: Survival data for 648 patients with
osteosarcoma treated at one institution. Clin Orthop Relat Res.
429:286–291. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bentzen SM: Prognostic factor studies in
oncology: Osteosarcoma as a clinical example. Int J Radiat Oncol
Biol Phys. 49:513–518. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Namløs HM, Kresse SH, Müller CR, Henriksen
J, Holdhus R, Sæter G, Bruland OS, Bjerkehagen B, Steen VM and
Myklebost O: Global gene expression profiling of human
osteosarcomas reveals metastasis-associated chemokine pattern.
Sarcoma. 2012:6390382012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liberman J, Sartelet H, Flahaut M,
Mühlethaler-Mottet A, Coulon A, Nyalendo C, Vassal G, Joseph JM and
Gross N: Involvement of the CXCR7/CXCR4/CXCL12 axis in the
malignant progression of human neuroblastoma. PLoS One.
7:e436652012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Balkwill F: Cancer and the chemokine
network. Nat Rev Cancer. 4:540–550. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sun X, Charbonneau C, Wei L, Yang W, Chen
Q and Terek RM: CXCR4-targeted therapy inhibits VEGF expression and
chondrosarcoma angiogenesis and metastasis. Mol Cancer Ther.
12:1163–1170. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Teicher BA and Fricker SP: CXCL12
(SDF-1)/CXCR4 pathway in cancer. Clin Cancer Res. 16:2927–2931.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dewan MZ, Ahmed S, Iwasaki Y, Ohba K, Toi
M and Yamamoto N: Stromal cell-derived factor-1 and CXCR4 receptor
interaction in tumor growth and metastasis of breast cancer. Biomed
Pharmacother. 60:273–276. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liao YX, Zhou CH, Zeng H, Zuo DQ, Wang ZY,
Yin F, Hua YQ and Cai ZD: The role of the CXCL12-CXCR4/CXCR7 axis
in the progression and metastasis of bone sarcomas (Review). Int J
Mol Med. 32:1239–1246. 2013.PubMed/NCBI
|
|
11
|
Vandercappellen J, Van Damme J and Struyf
S: The role of CXC chemokines and their receptors in cancer. Cancer
Lett. 267:226–244. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Monnier J, Boissan M, L’Helgoualc’h A,
Lacombe ML, Turlin B, Zucman-Rossi J, Théret N, Piquet-Pellorce C
and Samson M: CXCR7 is up-regulated in human and murine
hepatocellular carcinoma and is specifically expressed by
endothelial cells. Eur J Cancer. 48:138–148. 2012. View Article : Google Scholar
|
|
13
|
Feng Y, Broder CC, Kennedy PE and Berger
EA: HIV-1 entry cofactor: Functional cDNA cloning of a
seven-transmembrane, G protein-coupled receptor. Science.
272:872–877. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wegner SA, Ehrenberg PK, Chang G, Dayhoff
DE, Sleeker AL and Michael NL: Genomic organization and functional
characterization of the chemokine receptor CXCR4, a major entry
co-receptor for human immunodeficiency virus type 1. J Biol Chem.
273:4754–4760. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang J, Loberg R and Taichman RS: The
pivotal role of CXCL12 (SDF-1)/CXCR4 axis in bone metastasis.
Cancer Metastasis Rev. 25:573–587. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun X, Cheng G, Hao M, Zheng J, Zhou X,
Zhang J, Taichman RS, Pienta KJ and Wang J: CXCL12/CXCR4/CXCR7
chemokine axis and cancer progression. Cancer Metastasis Rev.
29:709–722. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Balkwill F: The significance of cancer
cell expression of the chemokine receptor CXCR4. Semin Cancer Biol.
14:171–179. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ma M, Ye JY, Deng R, Dee CM and Chan GC:
Mesenchymal stromal cells may enhance metastasis of neuroblastoma
via SDF-1/CXCR4 and SDF-1/CXCR7 signaling. Cancer Lett. 312:1–10.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li X, Ma Q, Xu Q, Liu H, Lei J, Duan W,
Bhat K, Wang F, Wu E and Wang Z: SDF-1/CXCR4 signaling induces
pancreatic cancer cell invasion and epithelial-mesenchymal
transition in vitro through non-canonical activation of Hedgehog
pathway. Cancer Lett. 322:169–176. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim SY, Lee CH, Midura BV, Yeung C,
Mendoza A, Hong SH, Ren L, Wong D, Korz W, Merzouk A, et al:
Inhibition of the CXCR4/CXCL12 chemokine pathway reduces the
development of murine pulmonary metastases. Clin Exp Metastasis.
25:201–211. 2008. View Article : Google Scholar
|
|
21
|
de Nigris F, Rossiello R, Schiano C, Arra
C, Williams-Ignarro S, Barbieri A, Lanza A, Balestrieri A, Giuliano
MT, Ignarro LJ, et al: Deletion of Yin Yang 1 protein in
osteosarcoma cells on cell invasion and CXCR4/angiogenesis and
metastasis. Cancer Res. 68:1797–1808. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang CY, Lee CY, Chen MY, Yang WH, Chen
YH, Chang CH, Hsu HC, Fong YC and Tang CH: Stromal cell-derived
factor-1/CXCR4 enhanced motility of human osteosarcoma cells
involves MeK1/2, ERK and NF-kappaB-dependent pathways. J Cell
Physiol. 221:204–212. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guo M, Cai C, Zhao G, Qiu X, Zhao H, Ma Q,
Tian L, Li X, Hu Y, Liao B, et al: Hypoxia promotes migration and
induces CXCR4 expression via HiF-1α activation in human
osteosarcoma. PLoS One. 9:e905182014. View Article : Google Scholar
|
|
24
|
Walter I, Wolfesberger B, Miller I, Mair
G, Burger S, Gallè B and Steinborn R: Human osteosarcoma cells
respond to sorafenib chemotherapy by downregulation of the tumor
progression factors S100A4, CXCR4 and the oncogene FOS. Oncol Rep.
31:1147–1156. 2014.PubMed/NCBI
|
|
25
|
Brennecke P, Arlt MJ, Campanile C, Husmann
K, Gvozdenovic A, Apuzzo T, Thelen M, Born W and Fuchs B: CXCR4
antibody treatment suppresses metastatic spread to the lung of
intratibial human osteosarcoma xenografts in mice. Clin Exp
Metastasis. 31:339–349. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Baumhoer D, Smida J, Zillmer S, Rosemann
M, Atkinson MJ, Nelson PJ, Jundt G, von Luettichau I and Nathrath
M: Strong expression of CXCL12 is associated with a favorable
outcome in osteosarcoma. Mod Pathol. 25:522–528. 2012. View Article : Google Scholar
|
|
27
|
Scotton CJ, Wilson JL, Scott K, Stamp G,
Wilbanks GD, Fricker S, Bridger G and Balkwill FR: Multiple actions
of the chemokine CXCL12 on epithelial tumor cells in human ovarian
cancer. Cancer Res. 62:5930–5938. 2002.PubMed/NCBI
|
|
28
|
Sun YX, Wang J, Shelburne CE, Lopatin DE,
Chinnaiyan AM, Rubin MA, Pienta KJ and Taichman RS: Expression of
CXCR4 and CXCL12 (SDF-1) in human prostate cancers (PCa) in vivo. J
Cell Biochem. 89:462–473. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Domanska UM, Timmer-Bosscha H, Nagengast
WB, Oude Munnink TH, Kruizinga RC, Ananias HJ, Kliphuis NM, Huls G,
De Vries EG, de Jong IJ, et al: CXCR4 inhibition with AMD3100
sensitizes prostate cancer to docetaxel chemotherapy. Neoplasia.
14:709–718. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Smith MC, Luker KE, Garbow JR, Prior JL,
Jackson E, Piwnica-Worms D and Luker GD: CXCR4 regulates growth of
both primary and metastatic breast cancer. Cancer Res.
64:8604–8612. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Orimo A, Gupta PB, Sgroi DC,
Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL
and Weinberg RA: Stromal fibroblasts present in invasive human
breast carcinomas promote tumor growth and angiogenesis through
elevated SDF-1/CXCL12 secretion. Cell. 121:335–348. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhou Y, Larsen PH, Hao C and Yong VW:
CXCR4 is a major chemokine receptor on glioma cells and mediates
their survival. J Biol Chem. 277:49481–49487. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Barbero S, Bonavia R, Bajetto A, Porcile
C, Pirani P, Ravetti JL, Zona GL, Spaziante R, Florio T and
Schettini G: Stromal cell-derived factor 1alpha stimulates human
glioblastoma cell growth through the activation of both
extracellular signal-regulated kinases 1/2 and Akt. Cancer Res.
63:1969–1974. 2003.PubMed/NCBI
|
|
34
|
Ehtesham M, Mapara KY, Stevenson CB and
Thompson RC: CXCR4 mediates the proliferation of glioblastoma
progenitor cells. Cancer Lett. 274:305–312. 2009. View Article : Google Scholar :
|
|
35
|
Gros SJ, Kurschat N, Drenckhan A, Dohrmann
T, Forberich E, Effenberger K, Reichelt U, Hoffman RM, Pantel K,
Kaifi JT, et al: Involvement of CXCR4 chemokine receptor in
metastastic HER2-positive esophageal cancer. PLoS One.
7:e472872012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Berghuis D, Schilham MW, Santos SJ, Savola
S, Knowles HJ, Dirksen U, Schaefer KL, Vakkila J, Hogendoorn PC and
Lankester AC: The CXCR4-CXCL12 axis in Ewing sarcoma: Promotion of
tumor growth rather than metastatic disease. Clin Sarcoma Res.
2:242012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Miura K, Uniyal S, Leabu M, Oravecz T,
Chakrabarti S, Morris VL and Chan BM: Chemokine receptor
CXCR4-beta1 integrin axis mediates tumorigenesis of osteosarcoma
HOS cells. Biochem Cell Biol. 83:36–48. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hendrix CW, Collier AC, Lederman MM,
Schols D, Pollard RB, Brown S, Jackson JB, Coombs RW, Glesby MJ,
Flexner CW, et al AMD3100 HIV Study Group: Safety,
pharmacokinetics, and antiviral activity of AMD3100, a selective
CXCR4 receptor inhibitor, in HIV-1 infection. J Acquir immune Defic
Syndr. 37:1253–1262. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Devine SM, Flomenberg N, Vesole DH,
Liesveld J, Weisdorf D, Badel K, Calandra G and DiPersio JF: Rapid
mobilization of CD34+ cells following administration of
the CXCR4 antagonist AMD3100 to patients with multiple myeloma and
non-Hodgkin’s lymphoma. J Clin Oncol. 22:1095–1102. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cashen A, Lopez S, Gao F, Calandra G,
MacFarland R, Badel K and DiPersio J: A phase II study of
plerixafor (AMD3100) plus G-CSF for autologous hematopoietic
progenitor cell mobilization in patients with Hodgkin lymphoma.
Biol Blood Marrow Transplant. 14:1253–1261. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang P, Dong L, Yan K, Long H, Yang TT,
Dong MQ, Zhou Y, Fan QY and Ma BA: CXCR4-mediated osteosarcoma
growth and pulmonary metastasis is promoted by mesenchymal stem
cells through VEGF. Oncol Rep. 30:1753–1761. 2013.PubMed/NCBI
|
|
42
|
Goguet-Surmenian E, Richard-Fiardo P,
Guillemot E, Benchetrit M, Gomez-Brouchet A, Buzzo P,
Karimdjee-Soilihi B, Alemanno P, Michiels JF, Schmid-Alliana A, et
al: CXCR7-mediated progression of osteosarcoma in the lungs. Br J
Cancer. 109:1579–1585. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Brennecke P, Arlt MJ, Muff R, Campanile C,
Gvozdenovic A, Husmann K, Holzwarth N, Cameroni E, Ehrensperger F,
Thelen M, et al: Expression of the chemokine receptor CXCR7 in
CXCR4-expressing human 143B osteosarcoma cells enhances lung
metastasis of intratibial xenografts in SCID mice. PLoS One.
8:e740452013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang Y, Yang CQ, Gao Y, Wang C, Zhang CL
and Zhou XH: Knockdown of CXCR7 inhibits proliferation and invasion
of osteosarcoma cells through inhibition of the PI3K/Akt and
β-arrestin pathways. Oncol Rep. 32:965–972. 2014.PubMed/NCBI
|
|
45
|
Zeng H, Sun M, Zhou C, Yin F, Wang Z, Hua
Y and Cai Z: Hematoporphyrin monomethyl ether-mediated photodynamic
therapy selectively kills sarcomas by inducing apoptosis. PLoS One.
8:e777272013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chen Z, Pan X, Georgakilas AG, Chen P, Hu
H, Yang Y, Tian S, Xia L, Zhang J, Cai X, et al:
Tetramethylpyrazine (TMP) protects cerebral neurocytes and inhibits
glioma by down regulating chemokine receptor CXCR4 expression.
Cancer Lett. 336:281–289. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ek ET, Dass CR and Choong PF: Commonly
used mouse models of osteosarcoma. Crit Rev Oncol Hematol. 60:1–8.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Balabanian K, Lagane B, Infantino S, Chow
KY, Harriague J, Moepps B, Arenzana-Seisdedos F, Thelen M and
Bachelerie F: The chemokine SDF-1/CXCL12 binds to and signals
through the orphan receptor RDC1 in T lymphocytes. J Biol Chem.
280:35760–35766. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kim HY, Hwang JY, Kim SW, Lee HJ, Yun HJ,
Kim S and Jo DY: The CXCR4 antagonist AMD3100 has dual effects on
survival and proliferation of myeloma cells in vitro. Cancer Res
Treat. 42:225–234. 2010. View Article : Google Scholar
|
|
50
|
Liu J, Wu Y, Wang B, Yuan X and Fang B:
High levels of glucose induced the caspase-3/PARP signaling
pathway, leading to apoptosis in human periodontal ligament
fibroblasts. Cell Biochem Biophys. 66:229–237. 2013. View Article : Google Scholar
|
|
51
|
Wang L, Pan J, Wang T, Song M and Chen W:
Pathological cyclic strain-induced apoptosis in human periodontal
ligament cells through the RhoGDIα/caspase-3/PARP pathway. PLoS
One. 8:e759732013. View Article : Google Scholar
|
|
52
|
Chinni SR, Sivalogan S, Dong Z, Filho JC,
Deng X, Bonfil RD and Cher ML: CXCL12/CXCR4 signaling activates
Akt-1 and MMP-9 expression in prostate cancer cells: The role of
bone microenvironment-associated CXCL12. Prostate. 66:32–48. 2006.
View Article : Google Scholar
|
|
53
|
Leelawat K, Leelawat S, Narong S and
Hongeng S: Roles of the MEK1/2 and AKT pathways in CXCL12/CXCR4
induced cholangiocarcinoma cell invasion. World J Gastroenterol.
13:1561–1568. 2007. View Article : Google Scholar : PubMed/NCBI
|