Introduction
Hepatocellular carcinoma (HCC) is one of the most
prevalent types of cancer and the leading cause of human mortality
worldwide (1). Aberrant gene
expression and somatic mutations induced by genotoxic agents that
cause DNA damage remain one main process leading to the development
of HCC (2). Most patients are
diagnosed at a late stage and thus miss the most optimal period for
effective treatment. Systematic chemotherapy plays a crucial role
in HCC treatment especially for patients with terminally staged
tumors (3). Cisplatin is a widely
used chemotherapeutic agent for the treatment of HCC. However, a
high dose of cisplatin causes serious side effects and also kills
normal cells, and the long-term side effects of cisplatin, which
are carcinogenic can cause secondary cancers (4).
microRNAs (miRNAs) belong to a class of small,
endogenous, non-coding, single-stranded RNAs, which are ~22
nucleotides in length. Growing evidence indicates that miRNAs
functionally repress target proteins by pairing with the
3′-untranslated region (3′-UTR) of specific target mRNAs, inducing
mRNA degradation or translational repression (5–7).
Studies have demonstrated that miRNAs are involved in many
biological processes such as cell proliferation, differentiation,
development and apoptosis (8,9).
miRNAs also play important roles in the development of cancer, and
may act as either oncogenic molecules or tumor suppressors by
regulating respective target genes (10,11).
However, the role of miRNAs in cancer chemotherapy remains largely
unknown.
Herein, we provided evidence showing the absence of
miR-193b expression in HCC cells. Furthermore, we demonstrated that
miR-193b significantly enhanced the antitumor effect of cisplatin
in HCC therapy by targeting Mcl-1, which is an anti-apoptotic Bcl-2
family member (12). Furthermore,
the activation of caspase-3 was required for the sensitization of
HCC cells by miR-193b to cisplatin-mediated cell death.
Materials and methods
Reagents
Cisplatin, zVAD-fmk,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT),
dimethyl sulfoxide (DMSO) and the Annexin V-FITC Apoptosis
Detection kit were obtained from Sigma-Aldrich (St. Louis, MO,
USA). Antibodies for rabbit anti-human Mcl-1, rabbit anti-human
caspase-3, rabbit anti-human poly-ADP-ribose polymerase (PARP) and
rabbit anti-human β-actin were purchased from Cell Signaling
Technology (Danvers, MA, USA). miR-193b mimics, Mcl-1 siRNA and the
negative control oligonucleotide (NCO) were purchased from
GenePharma Company (China).
Clinical specimens
Forty pairs of HCC tumor and corresponding adjacent
non-tumor liver tissues were obtained from patients who underwent
hepatic tumor resection at the First Affiliated Hospital,
University of South China from May 2011 to March 2014. The use of
clinical tissues for this study was approved by the hospital’s
Protection of Human Subjects Committee.
Cell culture and transfection
Normal hepatic cell line L02 and HCC cell lines
including Huh7, HepG2 and PLC were obtained from the Institute of
Biochemistry and Cell Biology, Chinese Academy of Sciences
(Shanghai, China) and cultured in Dulbecco’s modified Eagle’s
medium (DMEM) basic medium with 10% fetal bovine serum (FBS) (both
from Gibco, Carlsbad, CA, USA) at 37°C in a humidified 5%
CO2 incubator. HepG2 cells were transfected with the
miR-193b mimic (AACUGGCCCUCAAAGUCCCGCU), NCO (GGC
CCUAAAGAACUUCCUCCCG), Mcl-1 siRNA (AAGUA UCACAGACGUUCUUU) and the
recombinant pMIR or recombinant pcDNA3.1 using Lipofectamine 2000
(Invitrogen, Carlsbad, CA, USA) according to the protocols of the
manufacturer.
Quantitative real-time polymerase chain
reaction (RT-qPCR)
Total RNA was extracted from the patient tissues or
the HepG2 cells using TRIzol (Invitrogen), and the cDNA was
synthesized using M-MLV reverse transcriptase (Invitrogen)
following the manufacturer’s instructions. The reverse
transcription of miR-193b was performed by stem-loop RT-qPCR method
(13) and the RT-primer sequences
are as follows: 5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTG
AGAGCGGGAC-3′. The expression of mature miR-193b was quantified by
real-time PCR using SYBR-Green (Takara, japan) according to the
manufacturer’s instructions. Quantification of U6 was used to
normalize the miRNA expression level using the 2−ΔΔCT
method (14). U6 forward primer was
5′-CTCGCTTCGGCAGCACA3′. The expression of Mcl-1 mRNA was also
determined by qPCR and the GAPDH mRNA was taken as the internal
control. The primer sequences are as follows: Mcl-1 forward,
5′-GGCTAAACAC TTGAAGACCA-3′ and reverse, 5′-TGGAAGAACTCCACA
AACC-3′; GAPDH forward, 5′-CACTCCTCCACCTTTGA-3′ and reverse,
5′-CCACCACCCTGTTGCTG-3′.
Plasmid construction
To construct the pMIR-Mcl-1 3′-UTR-WT plasmid, a
wild-type 3′-UTR segment of human Mcl-1 mRNA (927-2348 nt, GenBank
accession no. NM_001197320) containing the putative miR-193b
binding sequence was amplified by PCR using the following primers:
forward, 5′-AGGGCAAGAGGATTAT-3′ and reverse, 5′-CTGTAGAG
GGAGCAGAA-3′, and then cloned into the downstream of the firefly
luciferase gene in the pMIR-REPORT™ miRNA Expression Reporter
Vector (Life Technologies, Carlsbad, CA, USA). pMIR-Mcl-1
3′-UTR-MUT, which carried the mutated sequence in the complementary
site for the seed region of miR-193b (GGCCAGU to GGCGUGU) was
generated using the Site-Directed Mutagenesis kit (Takara) based on
the wild-type plasmid. The open reading frame of the Mcl-1 gene
without 3′-UTR was amplified by PCR with the cDNA as template and
cloned into the pcDNA3.1 vector (Invitrogen); the recombinant
plasmid was named pcDNA3.1-Mcl-1.
Luciferase reporter assay
HepG2 cells were incubated in 48-well plates. The
cells were co-transfected with 50 pmol/ml of either miR-193b mimics
or NCO plus 2 µg/ml of either pMIR-Mcl-1 3′-UTR-WT or
pMIR-Mcl-1 3′-UTR-MUT plasmid, and 100 ng/ml of the Renilla
luciferase pRL-TK vector (Promega, Madison, WI, USA). Cells were
collected 24 h after transfection and analyzed using the
Dual-Luciferase Reporter system (Promega) according to the
manufacturer’s instructions. Firefly luciferase activity was
normalized to the Renilla luciferase activity.
Western blot analysis
Protein extracts were separated by 12.5% SDS-PAGE
and transferred onto PVDF membranes. This was followed by probing
with rabbit primary antibodies against human Mcl-1 and β-actin. The
membranes were then incubated with a horseradish
peroxidase-conjugated secondary antibody or goat anti-rabbit IgG
(Cell Signaling Technology). After washing, the proteins were
visualized with an enhanced chemiluminescence detection kit
(Pierce, Rockford, IL, USA).
Measurement of cell viability by MMT
assay
HepG2 cells were seeded in triplicate in a 96-well
plate at a density of 3×103/well. After incubation for
12 h, the cells were transfected with miR-193b, Mcl-1 siRNA or NCO.
After incubation for 24 h, the HepG2 cells were treated with
cisplatin for another 48 h. MTT (20 ml, 5 mg/ml) was added to each
well and incubated for 4 h. Medium was aspirated and 100 µl
DMSO was added to each well. Absorbance was read at OD 570/655 nm
using the Bio-Rad Model 680 microplate reader (Bio-Rad, Hercules,
CA, USA). Results are represented as the ratio between the various
treatments and the negative control.
Apoptosis assay
HepG2 cells were transfected with miR-193b or NCO
for 48 h, and then cells were treated with cisplatin for another 48
h. After treatment, the cells were incubated with Annexin
V/propidium iodide (PI) for 15 min at room temperature according to
the manufacturer’s instructions and analyzed using flow cytometry
(Becton Dickinson, San Jose, CA, USA).
Statistical analysis
Data are expressed as mean ± SE. Student’s t-test
and ANOVA analysis were conducted with SPSS 14.0 software to assess
the statistical significance between treatments. A P<0.05 was
considered to indicate a statistically significant difference.
Results
Downregulation of miR-193b in HCC patient
tumor tissues and cell lines
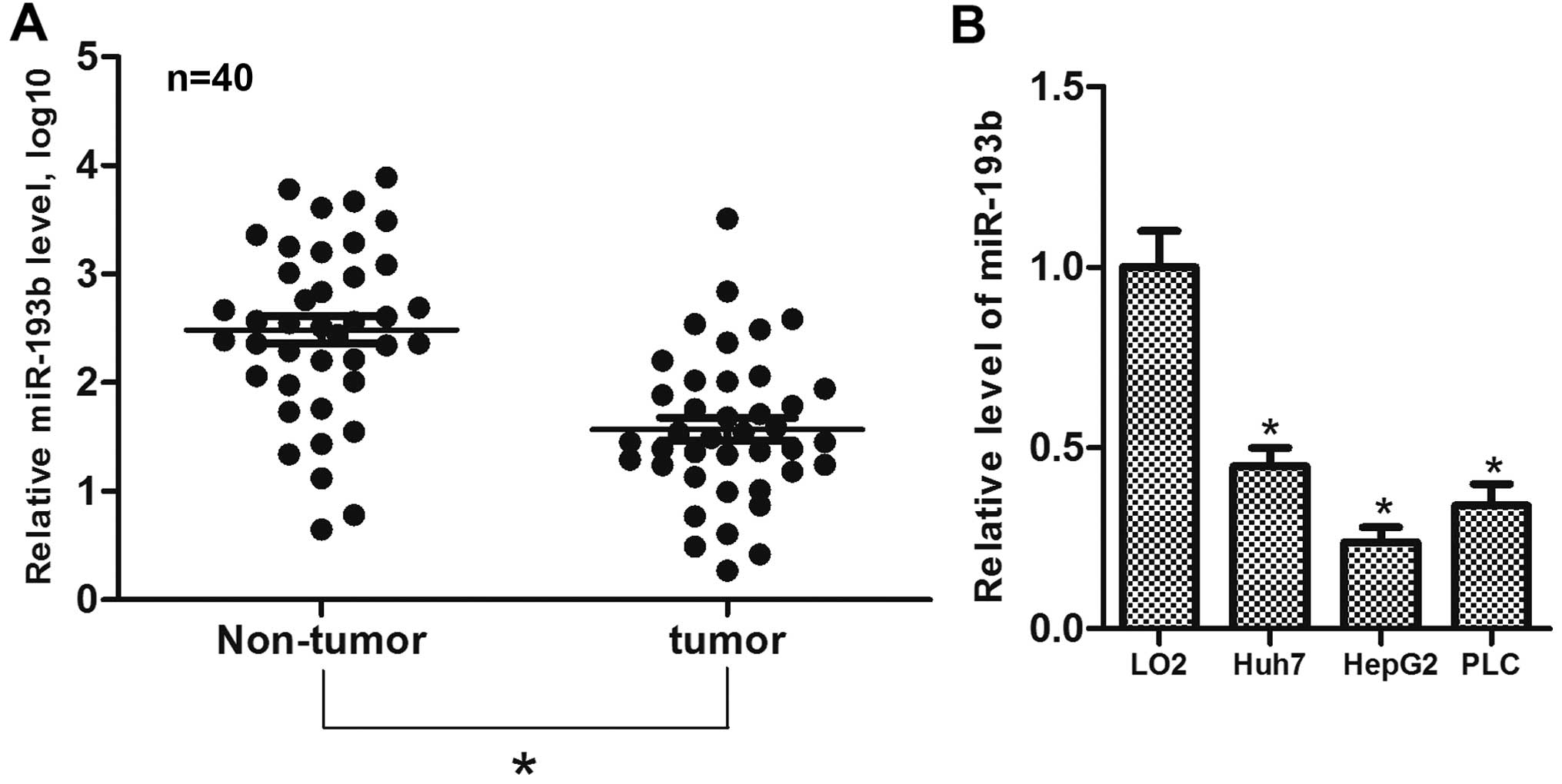
The expression of miR-193b in 40 paired samples of
clinical HCC tumor and adjacent normal liver tissues was determined
using RT-qPCR assays. We found that miR-193b expression was
significantly decreased in the HCC tumor tissues compared to this
level in the adjacent normal liver tissues (Fig. 1A). To validate this finding, the
expression of miR-193b was determined in liver cell lines. The
results showed that the expression of miR-193b was substantially
lower in the HCC cell lines than that in the L02 cells (Fig. 1B). These results suggest that
miR-193b may function as a tumor suppressor in HCC.
miR-193b sensitizes cisplatin-induced
apoptosis in HepG2 cells
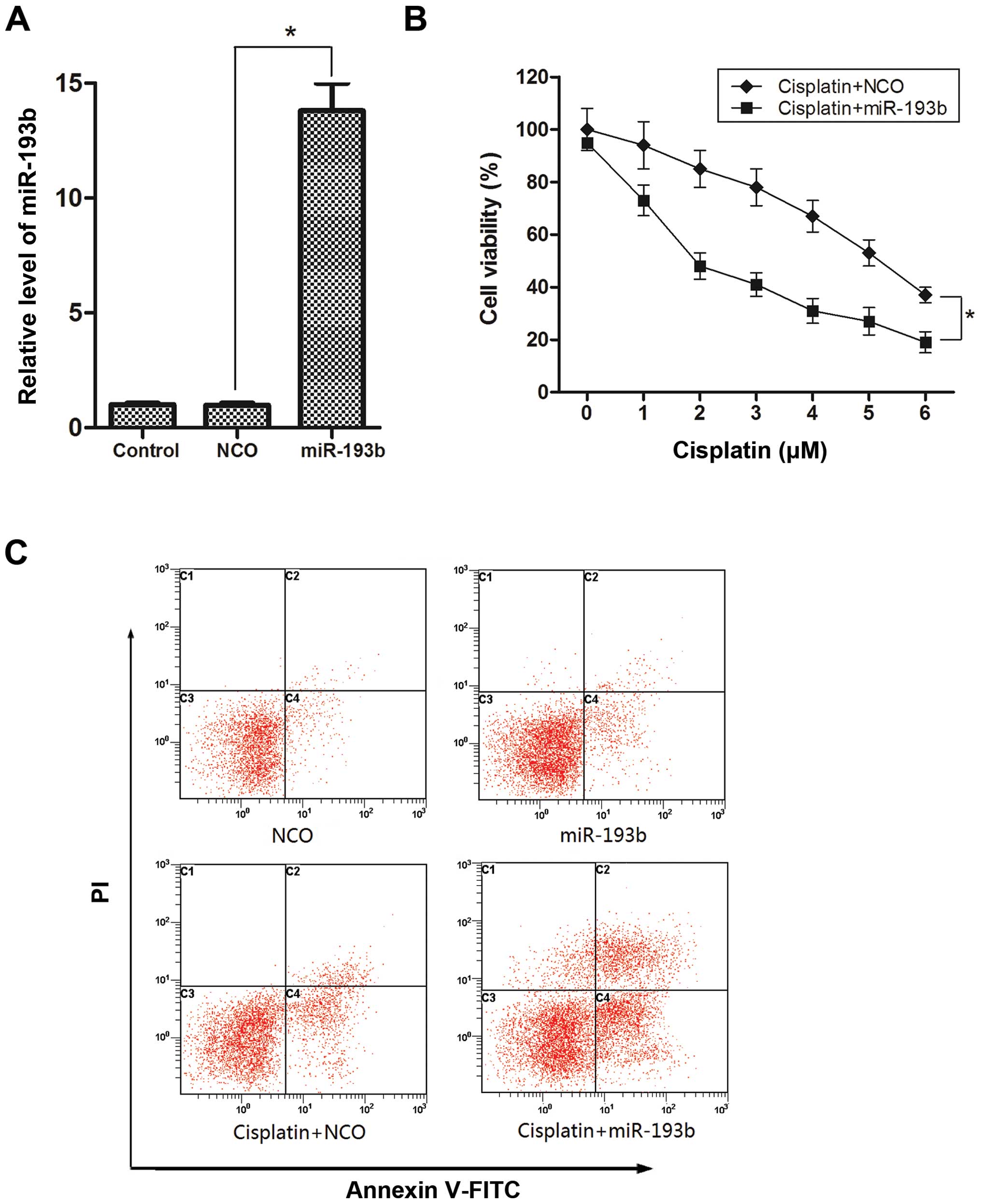
To explore the effect of miR-193b on cisplatin
treatment in HCC, we used miR-193b mimics to increase the
intracellular levels of miR-193b in the HepG2 cells, and the
overexpression of miR-193b was confirmed by qPCR (Fig. 2A). We subsequently used the MTT
assay to examine the effect of miR-193b on cisplatin treatment in
HepG2 cells. As shown in Fig. 2B,
the enforced expression of miR-193b plus cisplatin led to a
significant decrease in the viability of the HepG2 cells compared
to the viability in the cells treated with cisplatin combined with
NCO. Furthermore, we selected a moderate concentration of cisplatin
(2 µM) for the combination treatment with miR-193b to detect
the apoptosis induction. As shown in Fig. 2C, more apoptotic cells were observed
in the group treated with the combination of cisplatin and miR-193b
mimic than the single-treatment group. Together, these results
indicated that miR-193b efficiently sensitized the HepG2 cells to
cisplatin cytotoxicity in vitro.
Mcl-1 is the direct target of miR-193b in
HepG2 cells
In order to explore the molecular mechanisms
responsible for the sensitization by miR-193b to cisplatin
treatment, we used TargetScan database and found that Mcl-1 may be
a putative target of miR-193b (Fig.
3A). We then showed that the transfection of miR-193b
significantly suppressed the expression of Mcl-1 in the HepG2 cells
(Fig. 3B). To validate whether
Mcl-1 is an actual target of miR-193b, an Mcl-1 3′-UTR fragment
containing either a wild-type or mutant miR-193b binding sequence
(Fig. 3A) was cloned into the pMIR
reporter plasmid. After co-transfection with the pMIR reporter and
miR-193b, a reduction in luciferase activity was observed for the
wild-type construct-containing HepG2 cells. In contrast, the
luciferase activity of the mutant or empty reporter in the presence
of miR-193b was almost unaffected (Fig.
3C). More importantly, transfection of Mcl-1 siRNA exhibited a
function similar to miR-193b on sensitizing HepG2 cells to
cisplatin-inducing cytotoxicity (Fig.
3D). In addition, transfection with pcDNA3.1-Mcl-1 which
contained no 3′-UTR sequences totally overcame the suppression of
Mcl-1 caused by miR-193b (Fig. 3E).
Taken together, these results suggest that the expression of Mcl-1
was negatively regulated by miR-193b in the HCC cells, which may
play an essential role in the synergism of miR-193b with cisplatin
treatment.
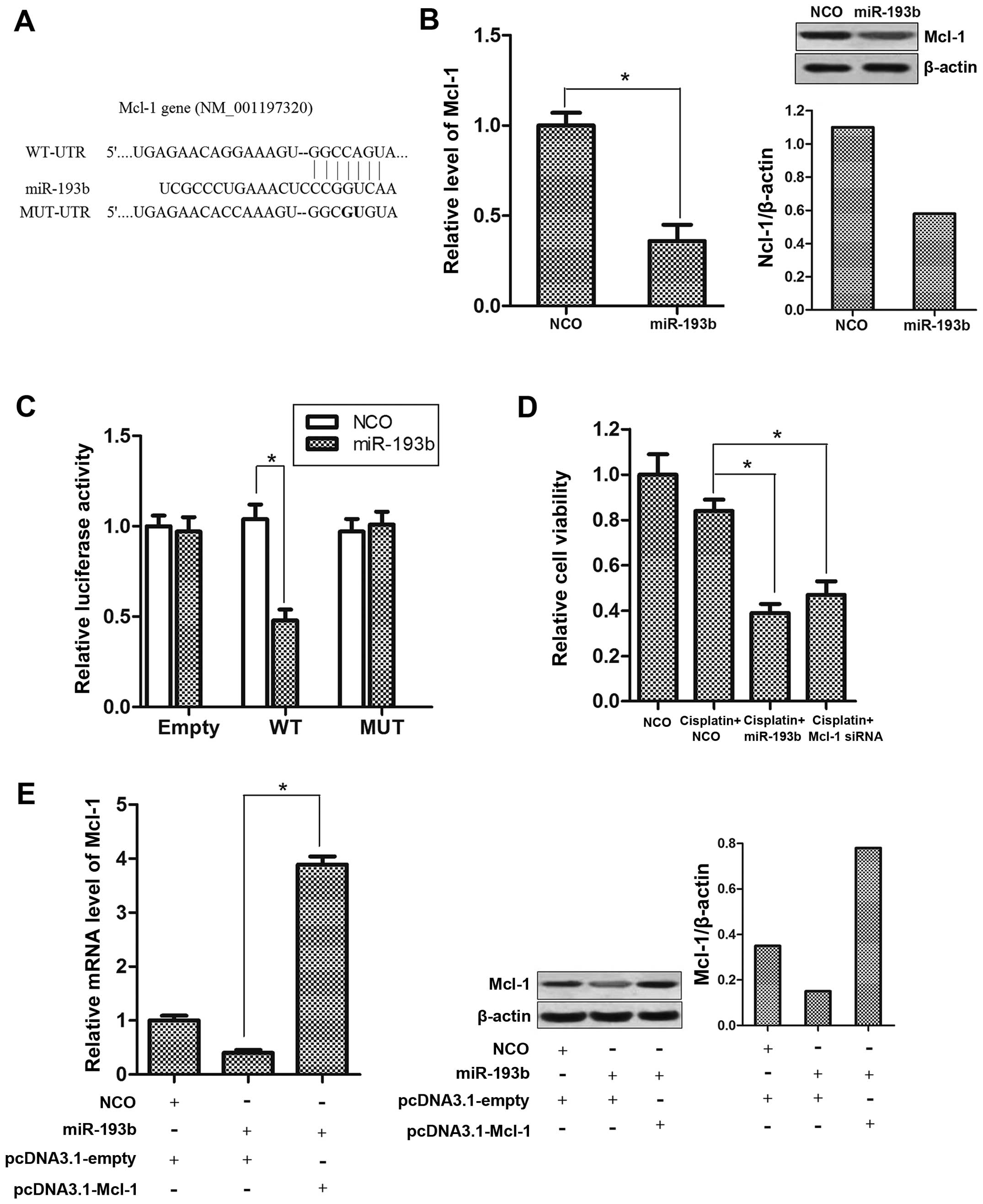 | Figure 3Mcl-1 is negatively regulated by
miR-193b in HCC cells. (A) Putative miR-193b binding sequence in
the 3′-UTR of Mcl-1 mRNA. Mutation was generated on the Mcl-1
3′-UTR sequence in the complementary site for the seed region of
miR-193b. (B) Mcl-1 expression level was determined after miR-193b
transfection by qPCR analysis and western blot analysis,
*P<0.05. (C) HepG2 cells were co-transfected with the
pMIR reporter and miR-193b, and incubation was carried out for 24
h. Firefly luciferase activity was measured and normalized to
Renilla luciferase, *P<0.05. (D) HepG2 cells
were treated with cisplatin (2 µM) plus NCO, miR-193b or
Mcl-1 siRNA (50 pmol/ml) for 48 h, and cell viability was
determined by MTT assay, *P<0.05. (E) pcDNA3.1-Mcl-1
(2 µg/ml) abolished the suppression of Mcl-1 caused by
miR-193b transfection. The expression of Mcl-1 was determined by
qPCR and western blot analysis, *P<0.05. HCC,
hepatocellular carcinoma; NCO, negative control oligonucleotide;
3′-UTR, 3′-untranslated region. |
miR-193b sensitizes cisplatin-induced
apoptosis by down-regulation of the expression of Mcl-1
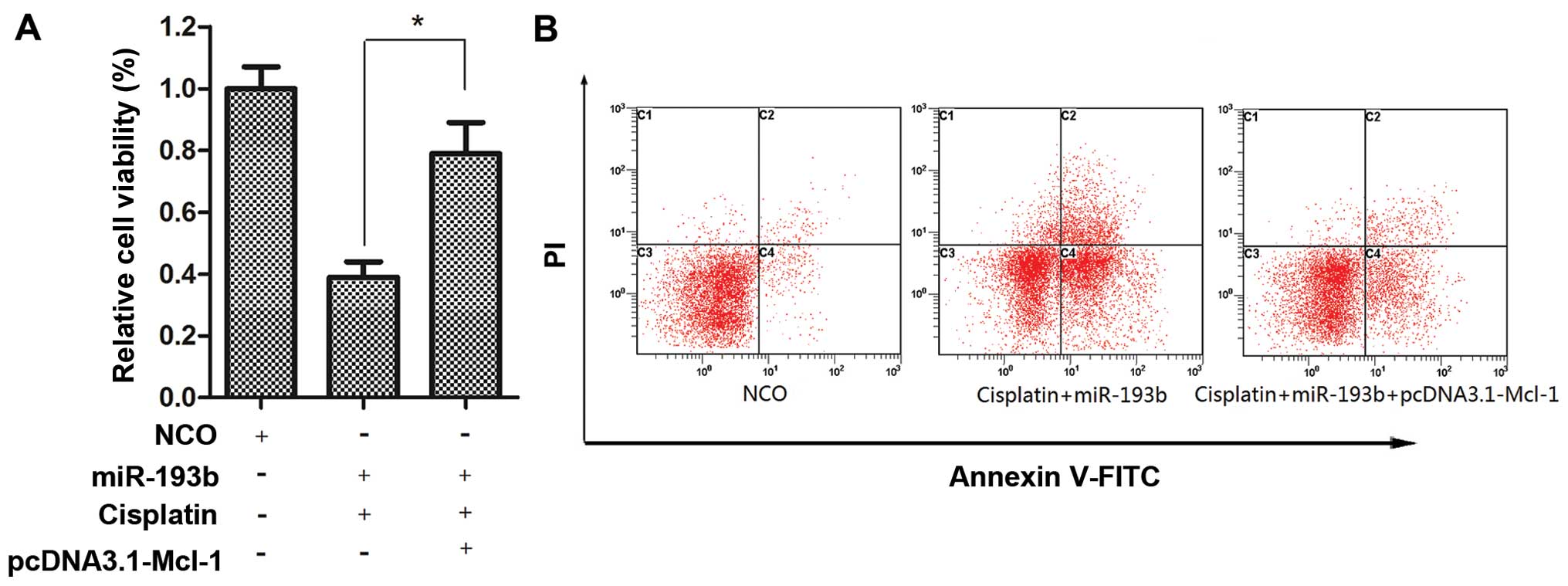
Our preceding results showed that miR-193b
sensitized cisplatin-induced apoptosis in HepG2 cells and Mcl-1 is
the direct target of miR-193b. To further confirm whether miR-193b
functions as a cisplatin sensitizer via the direct targeting of
Mcl-1, HepG2 cells were co-transfected with pcDNA3.1-Mcl-1 and
miR-193b for 24 h. Then, the culture media were replaced with fresh
medium and cisplatin was added, and incubation was carried out for
another 48 h. We found that the forced expression of Mcl-1
significantly decreased the growth inhibitory effect (Fig. 4A) and apoptotic rate (Fig. 4B) in the combined treatment of
cisplatin plus miR-193b.
miR-193b increases cisplatin-induced cell
death via the caspase-3-dependent pathway in HepG2 cells
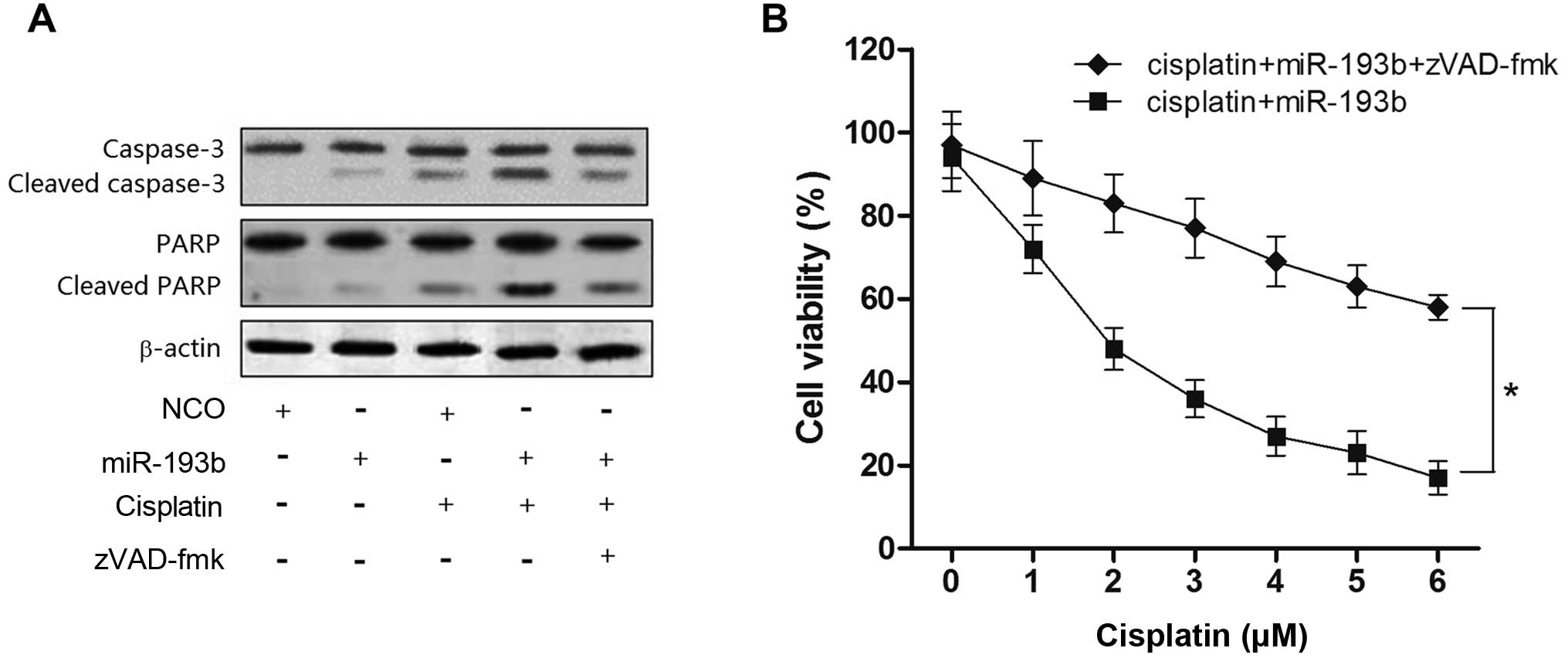
Since the preceding results proved that the
upregulation of miR-193b enhanced cell apoptosis caused by
cisplatin, we investigated whether the synergistic effect of
miR-193b on cisplatin-induced cell death is related with the
caspase pathway. As shown in Fig.
5A, treatment with cisplatin plus miR-193b resulted in obvious
cleavage of caspase-3 and its substrate PARP, and the activation of
caspase-3 was impaired by the caspase inhibitor zVAD-fmk (15). In contrast, cisplatin treatment
alone showed a weak activation of caspase-3 in the HepG2 cells
(Fig. 5A). More importantly, cell
death induced by cisplatin combined with miR-193b treatment was
inhibited in the presence of zVAD-fmk, suggesting that the
sensitization by miR-193b to cisplatin-mediated cell death was
caspase-dependent (Fig. 5B).
Discussion
Studies have demonstrated that miR-193b acts as a
tumor suppressor in multiple cancer types. Mu et al
demonstrated that downregulation of miR-193b is significantly
correlated with differentiation, invasion and metastasis in gastric
cancer (16). Li et al
showed that the expression of miR-193b was markedly decreased in
pancreatic cancer and the transfection of miR-193b significantly
inhibited the proliferation, invasion and metastasis of Panc-1
cells (17). In the present study,
we showed that the expression of miR-193b was downregulated in
liver tumors and HCC cell lines, suggesting that miR-193b is a
tumor suppressor in HCC.
Previous research has confirmed that some specific
miRNAs enhance the antitumor effect of chemotherapeutic drugs in
multiple tumor types, including HCC (18–20).
However, the molecular mechanisms of sensitization to chemotherapy
caused by miRNAs remain unclear. Our findings demonstrated that
miR-193b sensitized HepG2 cells to cisplatin-dependent
cytotoxicity, inducing apoptosis.
Apoptosis is essential for normal development and
homeostasis in a healthy body (21,22).
Aberrant regulation of apoptosis plays an essential role in
multiple human diseases including cancer, inducing tumorigenesis
and acquired chemotherapy resistance (23,24).
The apoptosis pathway is largely mediated by the Bcl-2 family
proteins, which decide whether a cell continues to live or
undergoes death through the intrinsic or mitochondrial apoptotic
pathway (25,26). Among them, Mcl-1 is one of the
anti-apoptotic Bcl-2 family members, which plays a key role in
apoptosis-resistance and is often overexpressed in human cancers
(12). Recently, Mcl-1 has been
confirmed to be regulated by miRNAs and was found to be associated
with the curative effect of cisplatin in ovarian and breast cancer
(27,28). In the present study, we identified
miR-193b as a direct regulator of Mcl-1. Notably, knockdown of the
Mcl-1 gene by specific siRNA showed a function similar to miR-193b
on sensitizing HepG2 cells to cisplatin, inducing cell death. In
contrast, enforced expression of Mcl-1 by pcDNA3.1-Mcl-1 vector
̔rescued’ the HepG2 cells from apoptosis induced by cisplatin
combined with miR-193b. These data emphasize the key role of Mcl-1
in cisplatin-dependent apoptosis of HCC cells.
Since the activation of caspase is a key event in
the process of apoptosis, and the cleavage of caspase-3 is a common
step in both the extrinsic and apoptosis pathway (29), we further studied whether the
synergistic effect of miR-193b on cisplatin-inducing cell death is
caspase-3-dependent. According to our results, treatment with
cisplatin plus miR-193b led to obvious cleavage of caspase-3 and
its substrate PARP, and zVAD-fmk increased the resistance of HepG2
cells to cisplatin plus miR-193b-induced cell death. We, therefore,
conclude that miR-193b acts as a cisplatin sensitizer via the
caspase-3-dependent pathway in HCC chemotherapy.
In summary, the present study demonstrated that
miR-193b is downregulated in human liver cancer cells. Moreover,
miR-193b increased the sensitivity of HepG2 cells to the
chemotherapy agent cisplatin by promoting apoptosis. Importantly,
Mcl-1 was confirmed as a target of miR-193b in HCC, and the
overexpression of miR-193b enhanced the sensitivity of cancer cells
through the caspase-dependent apoptosis pathway. Our data
demonstrated an important role for miR-193b in cisplatin therapy,
which may provide a novel therapeutic strategy for the treatment of
HCC.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant no. 81172575), the Hunan
Provincial Natural Science Committee and Hengyang City Government
Unification Foundation of China (grant no. 12JJ9033) and the Hunan
Provincial Natural Science Foundation of China (grant no.
13JJ3079).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nault JC, Calderaro J, Di Tommaso L,
Balabaud C, Zafrani ES, Bioulac-Sage P, Roncalli M and Zucman-Rossi
J: Telomerase reverse transcriptase promoter mutation is an early
somatic genetic alteration in the transformation of premalignant
nodules in hepatocellular carcinoma on cirrhosis. Hepatology.
60:1983–1992. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kerr SH and Kerr DJ: Novel treatments for
hepatocellular cancer. Cancer Lett. 286:114–120. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Arslan C, Ozdemir E, Dogan E, Ozisik Y and
Altundag K: Secondary hematological malignancies after treatment of
non-metastatic breast cancer. J BUON. 16:744–750. 2011.
|
|
5
|
Roufayel R, Johnston DS and Mosser DD: The
elimination of miR-23a in heat-stressed cells promotes NOXA-induced
cell death and is prevented by HSP70. Cell Death Dis. 27:e15462014.
View Article : Google Scholar
|
|
6
|
Nair N and Gongora E: MicroRNAs as
therapeutic targets in cardiomyopathies: Myth or reality? Biomol
Concepts. 5:439–448. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang H, Cheng Y, Jia C, Yu S, Xiao Y and
Chen J: MicroRNA-29s could target AKT2 to inhibit gastric cancer
cells invasion ability. Med Oncol. 32:3422015. View Article : Google Scholar
|
|
8
|
Zhang L, Ge Y and Fuchs E: miR-125b can
enhance skin tumor initiation and promote malignant progression by
repressing differentiation and prolonging cell survival. Genes Dev.
28:2532–2546. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang S and Li K: MicroRNA-96 regulates
RGC-5 cell growth through caspase-dependent apoptosis. Int J Clin
Exp Med. 7:3694–3702. 2014.PubMed/NCBI
|
|
10
|
Wolfson B, Eades G and Zhou Q: Roles of
microRNA-140 in stem cell-associated early stage breast cancer.
World J Stem Cells. 6:591–597. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mo ZH, Wu XD, Li S, Fei BY and Zhang B:
Expression and clinical significance of microRNA-376a in colorectal
cancer. Asian Pac J Cancer Prev. 15:9523–9527. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Woo SM, Min KJ, Seo BR, Nam JO, Choi KS,
Yoo YH and Kwon TK: Cafestol overcomes ABT-737 resistance in
Mcl-1-overexpressed renal carcinoma Caki cells through
downregulation of Mcl-1 expression and upregulation of Bim
expression. Cell Death Dis. 5:e15142014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen C, Ridzon DA, Broomer AJ, Zhou Z, Lee
DH, Nguyen JT, Barbisin M, Xu NL, Mahuvakar VR, Andersen MR, et al:
Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic
Acids Res. 33:e1792005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
15
|
Amaral C, Borges M, Melo S, da Silva ET,
Correia-da-Silva G and Teixeira N: Apoptosis and autophagy in
breast cancer cells following exemestane treatment. PLoS One.
7:e423982012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mu YP, Tang S, Sun WJ, Gao WM, Wang M and
Su XL: Association of miR-193b down-regulation and miR-196a
up-regulation with clinicopathological features and prognosis in
gastric cancer. Asian Pac J Cancer Prev. 15:8893–8900. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li J, Kong F, Wu K, Song K, He J and Sun
W: miR-193b directly targets STMN1 and uPA genes and suppresses
tumor growth and metastasis in pancreatic cancer. Mol Med Rep.
10:2613–2620. 2014.PubMed/NCBI
|
|
18
|
Liu R, Liu X, Zheng Y, Gu J, Xiong S,
Jiang P, Jiang X, Huang E, Yang Y, Ge D, et al: MicroRNA-7
sensitizes non-small cell lung cancer cells to paclitaxel. Oncol
Lett. 8:2193–2200. 2014.PubMed/NCBI
|
|
19
|
Boyerinas B, Park SM, Murmann AE, Gwin K,
Montag AG, Zillhardt M, Hua YJ, Lengyel E and Peter ME: Let-7
modulates acquired resistance of ovarian cancer to Taxanes via
IMP-1-mediated stabilization of multidrug resistance 1. Int J
Cancer. 130:1787–1797. 2012. View Article : Google Scholar
|
|
20
|
Koga C, Kobayashi S, Nagano H, Tomimaru Y,
Hama N, Wada H, Kawamoto K, Eguchi H, Konno M, Ishii H, et al:
Reprogramming using microRNA-302 improves drug sensitivity in
hepatocellular carcinoma cells. Ann Surg Oncol. 21(Suppl 4):
S591–S600. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Creagh EM: Caspase crosstalk: integration
of apoptotic and innate immune signalling pathways. Trends Immunol.
35:631–640. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Childs BG, Baker DJ, Kirkland JL, Campisi
J and van Deursen JM: Senescence and apoptosis: Dueling or
complementary cell fates? EMBO Rep. 15:1139–1153. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Setia S, Nehru B and Sanyal SN: Celecoxib
prevents colitis associated colon carcinogenesis: An upregulation
of apoptosis. Pharmacol Rep. 66:1083–1091. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang P, Tuo L, Wu Q and Cao X:
Licochalcone-A sensitizes human esophageal carcinoma cells to
TRAIL-mediated apoptosis by proteasomal degradation of XIAP.
Hepatogastroenterology. 61:1229–1234. 2014.PubMed/NCBI
|
|
25
|
Lee HG, Lee JM, Shin SJ, Kwon SH, Lee GS,
Song CH, Choi ES, Cha SD and Cho CH: Salinomycin inhibited cell
proliferation and induced apoptosis in human uterine leiomyoma
cells. Obstet Gynecol Sci. 57:501–506. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao J, Li X, Zou M, He J, Han Y, Wu D,
Yang H and Wu J: miR-135a inhibition protects A549 cells from
LPS-induced apoptosis by targeting Bcl-2. Biochem Biophys Res
Commun. 452:951–957. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rao YM, Shi HR, Ji M and Chen CH: miR-106a
targets Mcl-1 to suppress cisplatin resistance of ovarian cancer
A2780 cells. J Huazhong Univ Sci Technolog Med Sci. 33:567–572.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang R, Li Y, Dong X, Peng L and Nie X:
miR-363 sensitizes cisplatin-induced apoptosis targeting in Mcl-1
in breast cancer. Med Oncol. 31:3472014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Geserick P, Wang J, Feoktistova M and
Leverkus M: The ratio of Mcl-1 and Noxa determines ABT737
resistance in squamous cell carcinoma of the skin. Cell Death Dis.
5:e14122014. View Article : Google Scholar : PubMed/NCBI
|