Introduction
Epidermal growth factor receptor (EGFR) signaling is
known to be deregulated in many human tumors (1,2).
Causative are EGFR gene amplifications and mutations resulting in
receptor overexpression and constitutively active EGFR tyrosine
kinase activation. Due to its substantial role in progression and
pathogenesis of different carcinomas, huge efforts have been
undertaken to develop specific EGFR targeting approaches.
Monoclonal antibodies such as cetuximab or tyrosine kinase
inhibitors are clinically administered as monotherapy or in
multimodal concepts in combination with chemo- and/or radiotherapy
(3). Despite promising preclinical
data, clinical trials revealed EGFR targeting less effective in
prolonging overall survival as expected. Currently, cetuximab is
standard of care together with radiotherapy for head and neck
squamous cell carcinomas (HNSCC) (4,5). To
further optimize the efficacy of anti-EGFR treatment, it is
essential to fully understand EGFR-related intracellular
signaling.
Receptor tyrosine kinase signaling pathways are
structurally and functionally linked with integrin-associated
signaling to optimally regulate survival, proliferation,
differentiation, adhesion and migration (6–9).
Specific adapter molecules connect EGFR and integrins such as Nck2,
particularly interesting new cysteine-histidine rich 1 (PINCH1) and
integrin-linked kinase (ILK) (10–14).
Data suggest that PINCH1 binds to Nck2 via its LIM4 domain and with
its LIM1 domain to ILK (13,15).
The exact EGFR-integrin interaction and transactivation mechanisms
remain to be unraveled. However, ligand-dependent EGFR stimulation
and integrin-mediated cell-extracellular matrix (ECM) adhesion seem
inevitable for proper channeling of biochemical cues and control of
cellular sensitivity to cytotoxic agents (16–19).
Intriguingly, EGFR and integrin signaling have been shown to
critically contribute to the cellular radiation response and repair
processes involved in DNA double strand breaks (DSB), being the
most severe in mammalian cells (16,20–24).
Furthermore, both EGFR and integrin pathways participate in the
repair of radiation-induced DNA lesions involving the key DNA
damage recognition and repair proteins ATM and DNA-PK (23,25).
To address the role of the adapter proteins PINCH1
and Nck2 for EGFR signaling, cell survival and cellular
radiosen-sitivity, we investigated in human squamous cell carcinoma
(SCC) cells of the hypopharynx (FaDu) and the skin (A431) in a more
physiological 3D laminin-rich (lr) ECM-based cell culture model
(24,26). We found reduced clonogenic radiation
survival of 3D grown SCC cells to the same extent as for PINCH1 or
Nck2 knockdown, a finding correlative with impaired DSB repair.
Materials and methods
Antibodies and reagents
Antibodies against PINCH1 (BD Biosciences,
Heidelberg, Germany), EGFR, EGFR Y1068, EGFR Y1173, MAPK, MAPK
T202/Y204, Akt, Akt S473, Akt T308, FAK, FAK Y397, Src, Src Y416,
ATM, DNA-PK, Mre11, Rad50 and Nbs1 (Cell Signaling Technology,
Frankfurt, Germany), ATM S1981 (Rockland Immunochemicals Inc.,
Pottstown, PA, uSA), p53 binding protein 1 (53BP1; Novus
Biologicals, Cambridge, uK), phospho-Histone H2AX S139 (Millipore,
Darmstadt, Germany), β-actin (Sigma-Aldrich Chemie GmbH,
Taufkirchen, Germany) and horseradish peroxidase-conjugated donkey
anti-rabbit and sheep anti-mouse (Amersham, Freiburg, Germany)
antibodies were purchased as indicated. Coomassie was from Merck
(Darmstadt, Germany), complete protease inhibitor cocktail was from
Roche Diagnostics (Mannheim, Germany), BCA assay and SuperSignal
West Dura Extended Duration Substrate were from Thermo Fisher
Scientific (Karlsruhe, Germany), nitrocellulose membranes were from
Schleicher & Schuell, and oligofectamine from Invitrogen
(Karlsruhe, Germany).
3D cell culture
A431 and FaDu cells were purchased from the American
Type Culture Collection (ATCC; Manassas, VA, MA). Cells were
cultured in Dulbecco’s modified Eagle’s medium (DMEM; PAA
Laboratories, Cölbe, Germany) containing GlutaMAX-I supplemented
with 10% fetal calf serum and 1% non-essential amino acids (PAA
Laboratories) at 37°C in a humidified atmosphere containing 7%
CO2. For 3D cell culture, plates were coated with 1%
agarose (Sigma) to prevent cell attachment to the bottom of the
well. Laminin-rich extracellular matrix (lrECM; Cultrex 3D Culture
Matrix; Trevigen, Gaithersburg, MD, USA; BD Matrigel™ Basement
Membrane Matrix; BD Biosciences) was added to the cell culture
medium to obtain a final concentration of 0.5 mg/ml (26).
Radiation exposure
Irradiation was delivered at room temperature using
2 to 6 Gy single doses of 200 kV X-rays (Yxlon Y. Tu 320; Yxlon
International, Hamburg, Gemany; dose rate ~1.3 Gy/min at 20 mA)
filtered with 0.5 mm Cu. The absorbed dose was measured using a
Duplex dosimeter (PTW, Freiburg, Germany). The dose-rate was ~1.3
Gy/min at 20 mA, and applied doses ranged from 0 to 6 Gy.
Colony formation assay
Clonogenic survival under three-dimensional (3D)
growth conditions was determined in a 3D colony formation assay as
published (26). Briefly, single
cells were mixed with lrECM (Trevigen) to obtain a final
concentration of 0.5 mg/ml and placed in agarose-coated 96-well
plates. After 24 h, cetuximab was added to the medium to a final
concentration of 5 μg/ml. After 24 h cells received 0 to
6-Gy irradiation. Cetuximab remained in the cell culture medium for
the entire growth period. Cells were cultured for 9 days (A431) or
11 days (FaDu). Cell clusters with a minimum of 50 cells were
counted microscopically. Plating efficiencies: numbers of colonies
formed/numbers of cells plated and surviving fractions (SF):
numbers of colonies formed/numbers of cells plated (irradiated) x
plating efficiency (unirradiated)) were calculated. Each point on
survival curves represents the mean surviving fraction from at
least three independent experiments.
siRNA transfection
PINCH1 siRNA (sequence:
5′-GGACCUAUAUGAAUGGUUUTT-3′), Nck2 siRNA (sequence,
5′-GGGAAGAACAAACACUUCATT-3′) and a non-specific control (Co) siRNA
(sequence, 5′-GCAGCUAUAUGAAUGUUGUTT-3′) were obtained from Ambion
(Frankfurt, Germany). siRNA transfection was performed as
previously published (24).
Twenty-four hours after delivery of 20 nM siRNA using
oligofectamine, cells were plated in 3D lrECM. Colony formation
assays and western blotting were carried out. Efficient PINCH1
knockdown was confirmed by western blotting, while Nck2 depletion
was analyzed on mRNA level by use of RT-PCR.
Total protein extracts and western
blotting
Cells cultured in 3D lrECM (Trevigen) were lysed
with modified RIPA buffer [50 mM Tris-HCl (pH 7.4), 1% Nonidet-P40,
0.25% sodium deoxycholate, 150 mM NaCl, 1 mM EDTA, complete
protease inhibitor cocktail, 1 mM NaVO4, 2 mM NaF].
Total protein extracts were separated by SDS-PAGE and transferred
onto nitrocellulose membranes. Probing and detection of specific
proteins with indicated antibodies were performed as previously
described (26).
Reverse transcription-PCR
For validating Nck2 knockdown total RNA was
extracted using the NucleoSpin RNA II kit (Macherey-Nagel, Düren,
Germany). cDNA was prepared with SuperScript™ III reverse
transcriptase kit according to the instructions of the manufacturer
(Invitrogen). RT-PCR was performed for Nck2 and G3PDH (Nck2-fw,
5′-TGCTGGACGACTCCAAGAC-3′ and Nck2-rev, 5′-AGCCCTTCTTCAGGCTGTTC-3′;
G3PDH-fw, 5′-ACCACAGTCCATGCCATCAC-3′ and G3PDH-rev,
5′-TCCACCACCCTGTTGCTGTA-3′; Eurofins MWG Operon, Ebersberg,
Germany) using 2 μl of cDNA and HotStar Taq polymerase
(Qiagen, Venlo, The Netherlands) according to standard PCR
protocols. Results of RT-PCR were analyzed using 1.5% agarose gels
(Sigma) with 0.1% ethidium bromide (Carl Roth GmbH & Co. KG,
Karlsruhe, Germany).
Immunofluorescence staining
For detection of residual DNA double-strand breaks
(rDSB), the phosphorylated histone H2AX S139 (γH2AX)/p53 binding
protein 1 (53BP1) foci assay was performed as published (26). Cells were grown in 0.5 mg/ml lrECM
(BD Matrigel™) under 3D conditions for 24 h, irradiated with 0 or 6
Gy and isolated 24 h post irradiation. γH2AX/p53BP1-positive
nuclear foci of 50 cells were counted microscopically with an
Axioscope 2 plus fluorescence microscope (Carl Zeiss AG, Jena,
Germany) and were defined as DSB.
Stimulation with EGF
Cells cultured in 3D lrECM (Trevigen) were serum
starved for 24 h followed by a 1 h-treatment with 5 μg/ml
cetuximab and 15-min stimulation with 10 nM EGF before cells were
harvested and whole cell lysates were used for western
blotting.
Data analysis
Data were expressed as means ± SD of at least three
independent experiments. To test statistical significance,
Student’s t-test was performed using Microsoft® Excel
2003. Results were considered statistically significant at P-values
of <0.05.
Results
Depletion of the adapter proteins PINCH1
and Nck2 enhances the radiosensitivity of 3D grown SCC cells
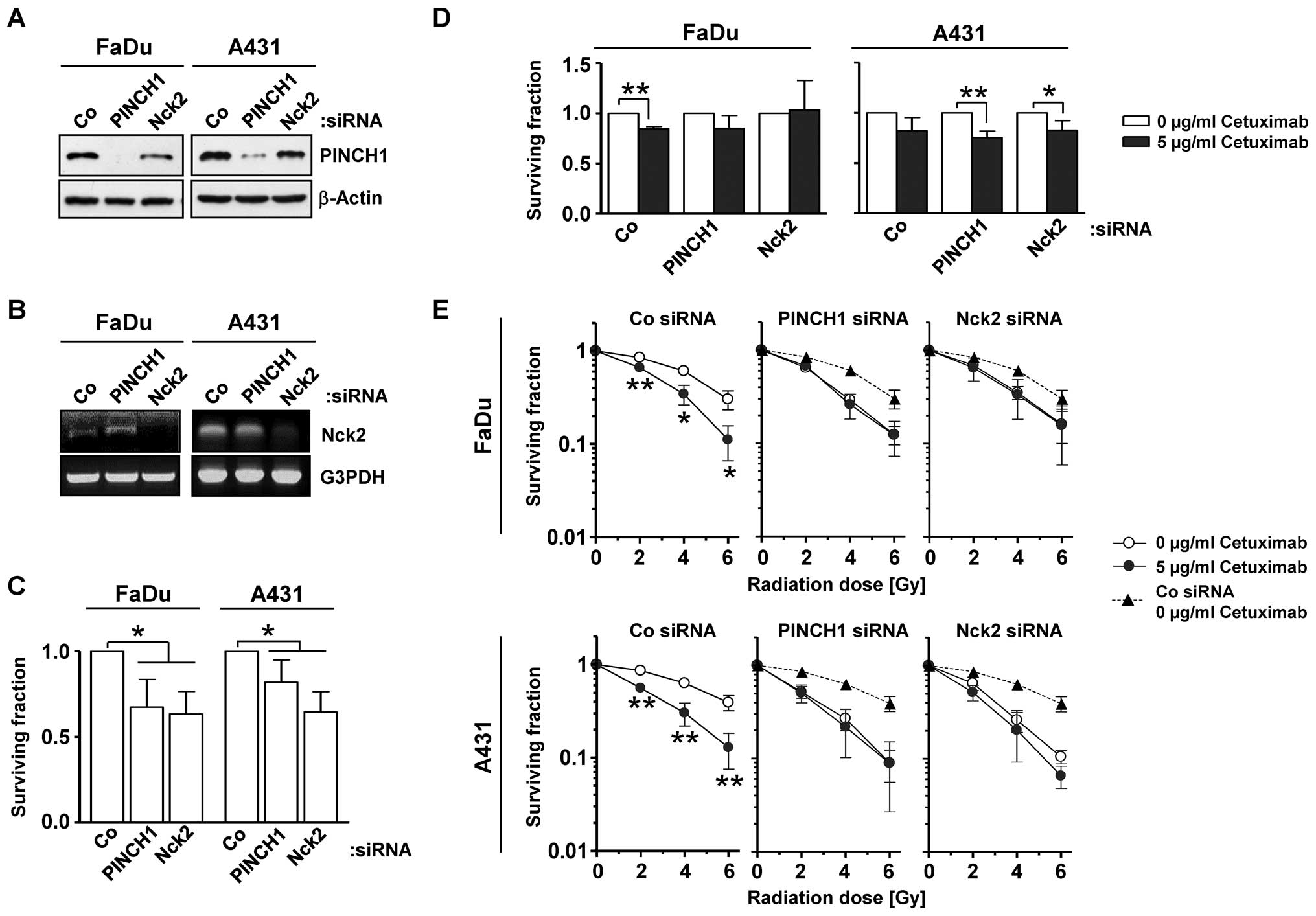
We commenced the present study by measuring the
effect of PINCH1 or Nck2 knockdown in SCC cells without and in
combination with the monoclonal anti-EGFR antibody cetuximab. While
the efficient PINCH1 (Fig. 1A) and
Nck2 (Fig. 1B) knockdown alone
caused significantly (P<0.05) reduced clonogenic survival in
both cell lines (Fig. 1C), its
combination with cetuximab resulted in only minor cell
line-specific alterations of clonogenicity relative to controls
(Fig. 1D). Intriguingly, depletion
of PINCH1 or Nck2 enhanced the radiosensitivity of FaDu and A431
cells compared to siRNA controls (Fig.
1E) and, notably, to the same extent as observed for the
combination of cetuximab plus X-ray irradiation (Fig. 1E). These data indicate PINCH1 and
Nck2 to play an important role in the cellular response to
radiation and to serve as critical determinants of EGFR associated
downstream signaling.
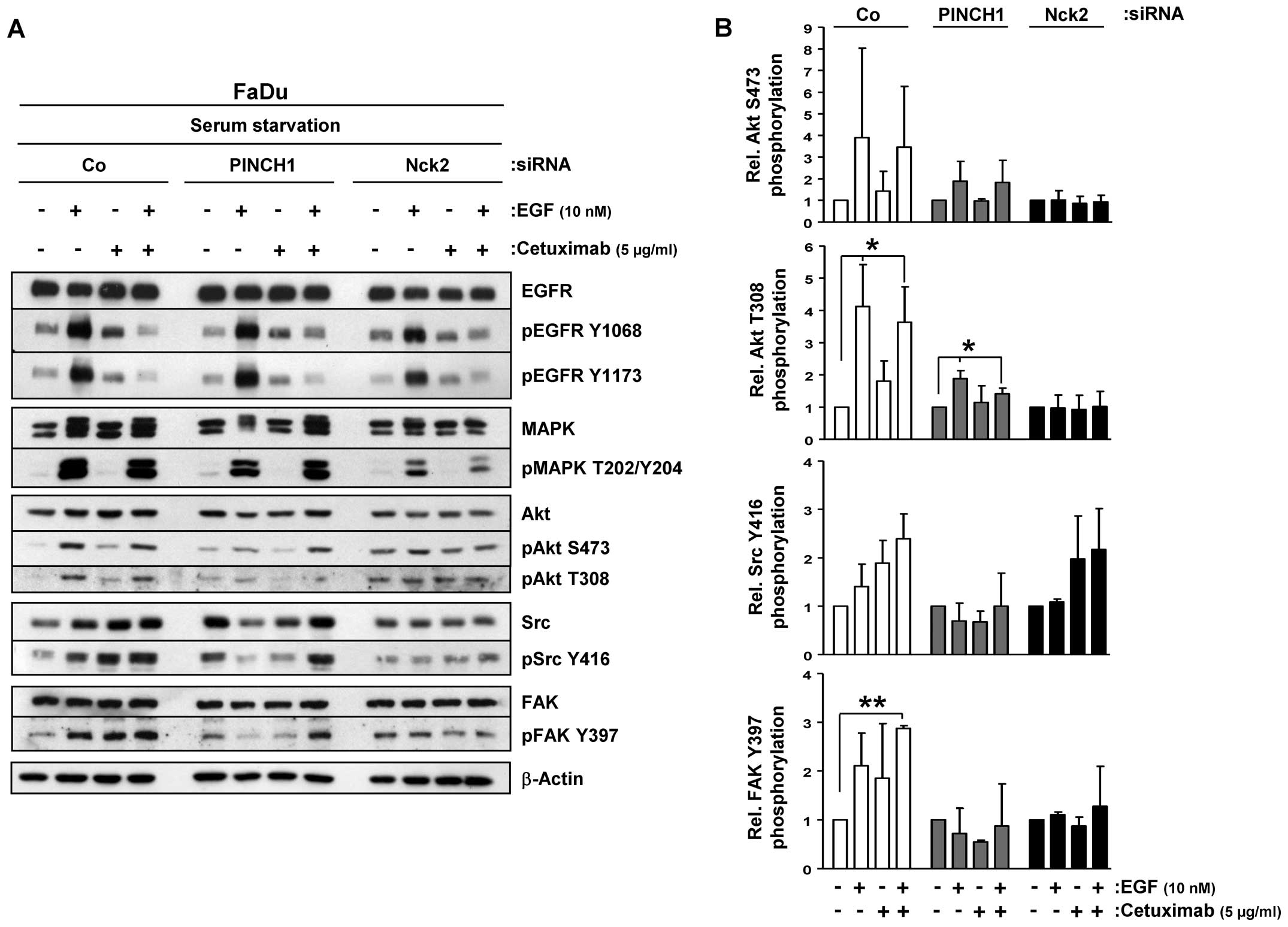
Cetuximab differentially impacts on EGFR
downstream signaling upon PINCH1 or Nck2 knockdown
To optimally assess the inhibitory efficacy of
cetuximab on EGFR and its downstream signaling, we serum-starved
our 3D cell cultures. upon EGF stimulation, EGFR tyrosine (Y)1068
and Y1173 phosphorylation were induced while cetuximab effectively
prevented this induction (Fig. 2A).
In spite of the effective EGFR blocking, increased MAPK threonine
(T)202/Y204 and Akt S473/T308 phosphorylation was detected upon EGF
application in cetuximab-treated FaDu cultures comparable to
untreated controls (Fig. 2A). In
contrast, FAK and Src, which have been shown to locate downstream
of EGFR, demonstrated enhanced phosphorylated upon EGF exposure,
which even increased when cetuximab was applied (Fig. 2).
When combined with PINCH1 or Nck2 depletion, the
phosphorylation pattern of EGFR remained similar to controls
(Fig. 2A). In contrast to PINCH1
depletion, MAPK showed attenuated phosphorylation upon EGF and
cetuximab exposure under Nck2 knockdown relative to siRNA controls
(Fig. 2). Independent from
cetuximab, phospho-Akt S473/T308 was slightly induced by EGF in
PINCH1 knockdown cultures and marginally induced under all tested
conditions in Nck2 depleted cells (Fig.
2). Similar patterns were observed for Src and FAK
phosphorylations. PINCH1 silencing prevented induction of Src and
FAK phosphorylation by EGF but enabled strong phosphorylation and
in cetuximab-treated, EGF-exposed cells (Fig. 2). Nck2 depletion facilitated
stimulation of Src Y416 phosphorylation upon EGF and EGF/cetuximab
without affecting FAK phosphorylation. These data suggest a
function of PINCH1 and Nck2 in EGFR signaling.
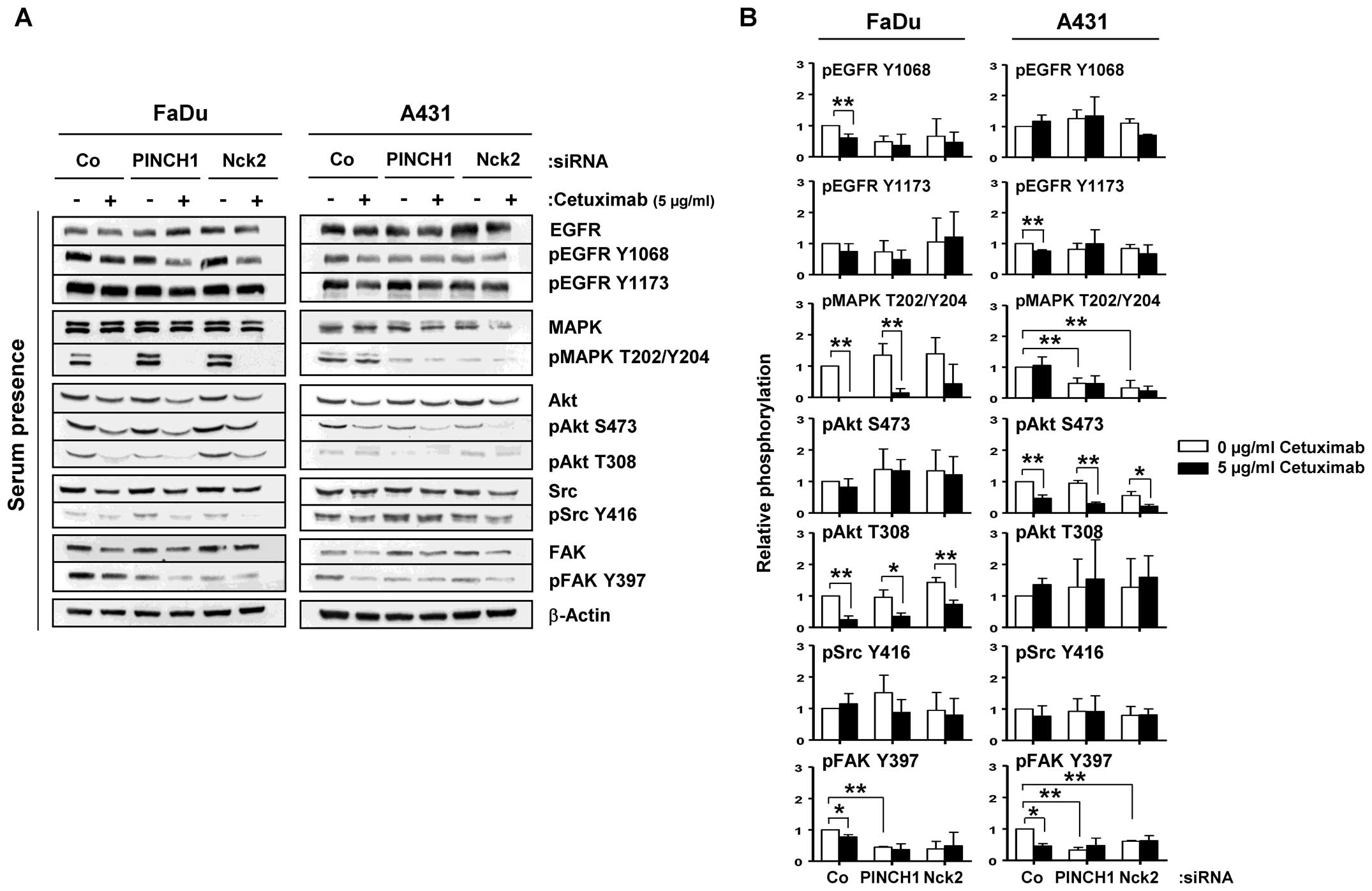
EGFR signaling is modulated in 3D PINCH1
and Nck2 knockdown cultures cell line-dependently
In the next step, we investigated EGFR signaling in
10% serum, 3D lrECM grown cell cultures to find signaling
modifications that contribute to the enhanced radiosensitivity seen
upon cetuximab treatment and PINCH1 or Nck2 knockdown. Cetuximab
and PINCH1 or Nck2 knockdown caused reduced Y1068 and unchanged
Y1173 phosphorylation of the EGFR in 3D lrECM FaDu cultures
(Fig. 3). While Akt serine (S)473
and Scr Y416 stayed stable, MAPK T202/Y204 and Akt T308
phosphorylation were significantly diminished by cetuximab but not
PINCH1 or Nck2 depletion in FaDu cells (Fig. 3). In A431 cells, EGFR, Akt T308 and
Src Y416 phosphorylation remained largely unmodified upon cetuximab
or knockdowns, while MAPK T202/Y204 and Akt S473 showed reduced
phosphorylation due to PINCH1 or Nck2 knockdown or cetuximab,
respectively (Fig. 3). The only
protein kinase showing similar modifications in both cell lines
upon knockdown and cetuximab was FAK at its Y397
autophosphorylation site (Fig. 3).
These data demonstrate differential impact of cetuximab and PINCH1
or Nck2 depletion on EGFR signaling in 3D lrECM cell cultures grown
in 10% serum. Furthermore, the inconsistencies in signaling
modifications in the two tested SCC cell lines cannot explain the
similarity in radiosensitization as result from cetuximab treatment
or PINCH1 or Nck2 depletion.
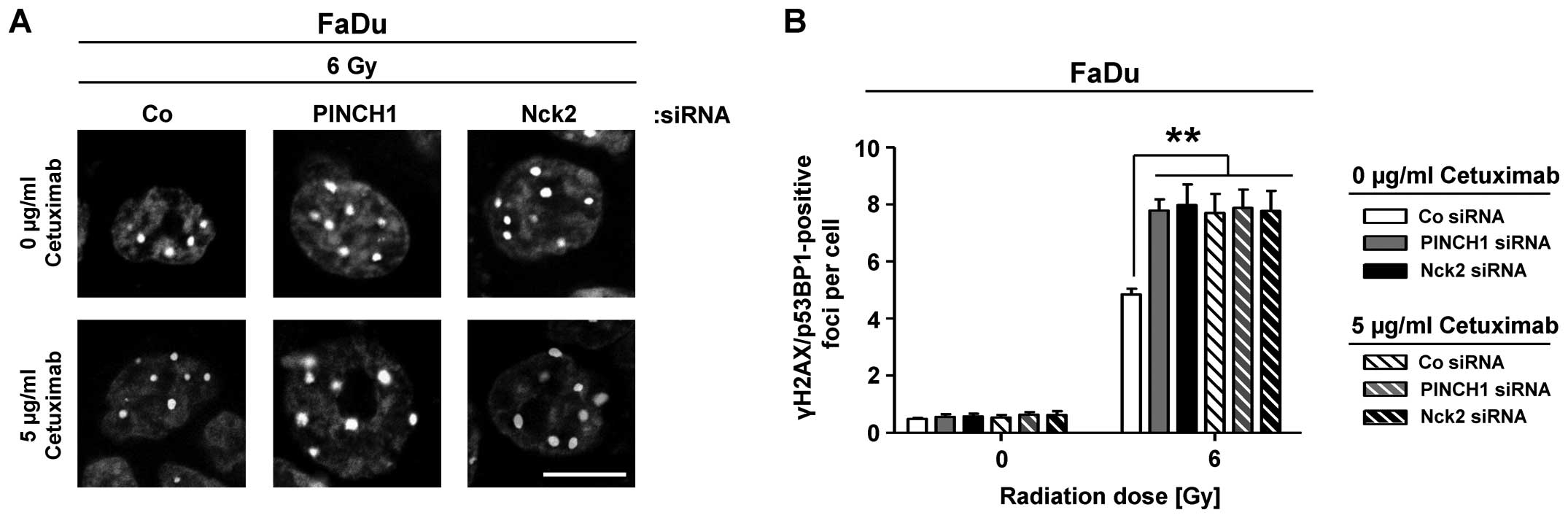
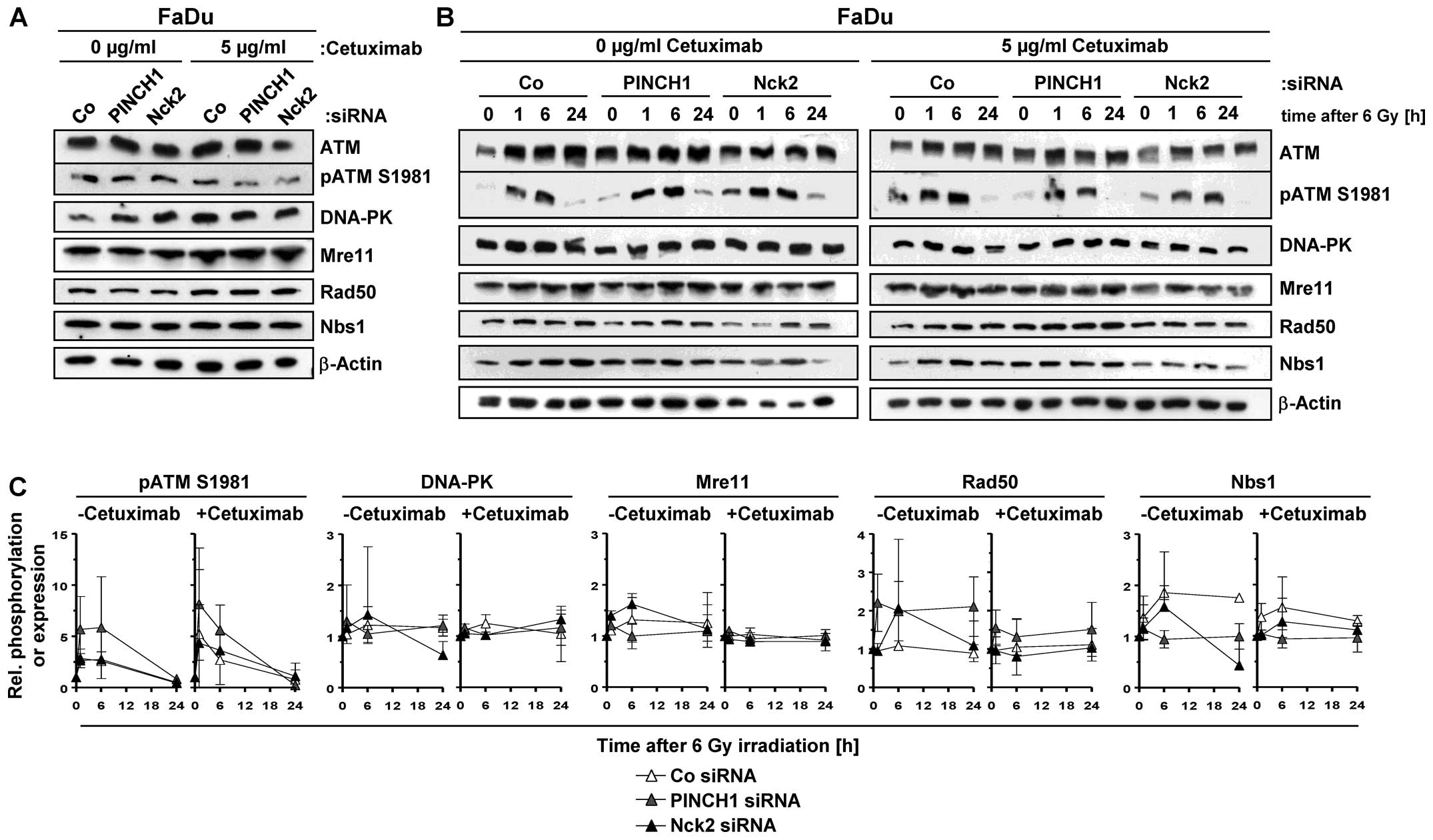
PINCH1 and Nck2 knockdown hampers DNA
double strand break repair
Based on these observations and in line with
radiosensitization, a highly significant and similar increase in
the number of γH2AX/53BP1-positive foci was observed in
6-Gy-irradiated cetuximab-treated or PINCH1 or Nck2 knockdown FaDu
cell cultures relative to corresponding controls (Fig. 4). Approximately 3 additional rDSB
were detectable under the different conditions.
To better understand the underlying mechanisms of
this elevated rDSB rate, we analyzed expression and phosphorylation
of a variety of key proteins of the DNA damage recognition and
repair machinery in cetuximab-treated and untreated cells as well
as upon PINCH1 or Nck2 silencing. Notably, a pattern of alterations
in the tested proteins that provides an explanatory basis for the
increased number of rDSB and radiosensitization upon PINCH1 or Nck2
knockdown or cetuximab administration was not observed (Fig. 5). Remarkable were the enhanced ATM
S1981 phosphorylation in PINCH1 knockdown cells and the differences
in Rad50 and Nbs1 expression under PINCH1 and Nck2 depletion
between cetuximab absence and presence (Fig. 5B and C). These observations suggest
that EGFR blocking using cetuximab or depletion of the adaptor
proteins PINCH1 or Nck2 promotes radiosensitization, which emanates
from hampered rDSB repair involving yet to be identified
mechanisms.
Discussion
EGFR downstream signaling is influenced by a variety
of extra- and intracellular factors. Among the intracellular
factors, there exist adapter proteins that structurally bridge EGFR
and integrins while their functional role in signal transduction
and cancer cell therapy resistance is less clear (6,9,14).
Focusing on the focal adhesion proteins PINCH1 and Nck2, we
investigated the function of these two proteins in EGFR signaling
and cellular radiation response of SCC cells grown under more
physiological 3D lrECM conditions. In the present study, we show
that: i) single knockdown of PINCH1 or Nck2 resulted in enhanced
radiosensitivity of SCC cells comparable with effects seen after
cetuximab treatment alone; ii) modifications in MAPK, Akt and FAK
phosphorylation occurred upon cetuximab treatment as well as PINCH1
or Nck2 depletion; and iii) tumor cell radiosensitization by
cetuximab and PINCH1 or Nck2 silencing is in accordance with
attenuated repair of DNA double strand breaks.
A connection between cell adhesion, cell-ECM
interactions and cancer therapy resistance has been demonstrated by
a large number of reports from our group and others (18,19,23,24,27–29).
Focal adhesion proteins seem to be part of fundamental signaling
hubs as their depletion or pharmacological inhibition cause reduced
cell viability and enhanced therapy resistance in different tumor
models (8,18,19,24,26,28–31).
Compared to PINCH1 not much is known about the function of the
adapter protein Nck2. PINCH1 is critically involved in regulating
cell shape, attachment, spreading and motility (15,32,33).
In line with previous in vitro and in vivo data
(30,34), knockdown of PINCH1 resulted in
enhanced radiosensitivity of the head and neck squamous cell
carcinoma cell line FaDu and the epidermoid cancer cell line A431.
In addition to Nck2 being involved in IGF-1 signaling (15), regulating cell migration (35,36)
and apoptosis (37), we show that
Nck2 is also key for EGFR downstream signaling and clonogenic
radiation survival.
Despite the cell line dependency of the shown
effects, both PINCH1 and Nck2 determine the involvement and the
phosphorylation of a spectrum of EGFR downstream located mediators
ranging from MAPK via Akt to FAK. In HT-1080 fibrosarcoma cells,
Chen et al (38) documented
a regulating role of PINCH1 in MAPK phosphorylation resulting in
increased levels of the proapoptotic protein Bim to trigger
activation of the intrinsic apoptosis pathway. We observed this
phenomenon of reduced MAPK phosphorylation in PINCH1−/−
embryonic mouse fibroblasts but not in human cancer cells, nor
under 3D lrECM cell culture conditions, which points at a great
dependence of MAPK phosphorylation on growth conditions and the
cancer cell model (30). For cell
survival, radiochemosensitivity, adhesion and spreading, PINCH1
serves as interacting platform for Akt and protein phosphatase 1α
as well as RSu-1 at its LIM5 domain (34,39,40).
In primitive endoderm cells, PINCH1 regulates JNK activation via
RSu-1 and Bax activity via integrin signaling (41). Nck2 also links to apoptosis
regulation in case of uV-radiation through enhanced caspase-3 and
PARP cleavage (37). While
modification of FAK upon EGFR inhibition is known (16,42),
alterations of FAK phosphorylation by PINCH1 are novel and
similarly observable for Nck2. These data strongly indicate that
EGFR signaling is more complex than first thought. PINCH1 and Nck2
mediate differential connections with downstream signaling proteins
on the basis of a unique, yet to be identified proteome expressed
in the tested cell lines.
Notably, this concept is obviously not applicable
for the closely associated endpoints clonogenic radiation survival
and DNA double strand break repair. Both SCC cell lines are
similarly radiosensitized and display a similar number of
unrepaired DSB. These data are surprising and indicate that a
particular part of the intracellular signaling network is greatly
overlapping between PINCH1 and Nck2. Possibly, as both proteins
shuttle between focal adhesions and cell nucleus, the DNA damage
response is critically controlled by these proteins. While PINCH1
seems to interact with the nuclear transcription factor Wilms tumor
1 protein (43), Nck1 is supposed
to be carried into the nucleus by the suppressor of cytokine
signaling 7 as this protein possesses a nuclear import and export
sequence (44). upon DNA damage,
Nck1 expression impacts on cell cycle blockage and interferes with
ATM/ATR signaling. Nck2 also has a nuclear function in acting as
repressor of gene transcription of jun/fos promoter elements
induced by v-Abl (45).
In summary, our data suggest that the adapter
proteins PINCH1 and Nck2 critically participate in the regulation
of cellular radiosensitivity and EGFR function and downstream
signaling in 3D grown human SCC cells. Future work is warranted to
provide detailed information on the molecular circuitry how PINCH1
and Nck2 control EGFR signaling and cellular radiosensitivity.
Acknowledgments
The present research and authors were in part
supported by a grant from the Federal Ministry of Education and
Research, Germany (BMBF Contract 03ZIK041 to N.C.) and by the EFRE
Europäische Fonds für regionale Entwicklung, Europa fördert Sachsen
(100066308). The authors like to thank Claudia Förster and Inga
Lange for excellent technical assistance.
References
|
1
|
Mendelsohn J and Baselga J: Status of
epidermal growth factor receptor antagonists in the biology and
treatment of cancer. J Clin Oncol. 21:2787–2799. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chong CR and Jänne PA: The quest to
overcome resistance to EGFR-targeted therapies in cancer. Nat Med.
19:1389–1400. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nyati MK, Morgan MA, Feng FY and Lawrence
TS: Integration of EGFR inhibitors with radiochemotherapy. Nat Rev
Cancer. 6:876–885. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bonner JA, Harari PM, Giralt J, Cohen RB,
Jones CU, Sur RK, Raben D, Baselga J, Spencer SA, Zhu J, et al:
Radiotherapy plus cetuximab for locoregionally advanced head and
neck cancer: 5-year survival data from a phase 3 randomised trial,
and relation between cetuximab-induced rash and survival. Lancet
Oncol. 11:21–28. 2010. View Article : Google Scholar
|
|
5
|
Caudell JJ, Sawrie SM, Spencer SA, Desmond
RA, Carroll WR, Peters GE, Nabell LM, Meredith RF and Bonner JA:
Locoregionally advanced head and neck cancer treated with primary
radiotherapy: A comparison of the addition of cetuximab or
chemotherapy and the impact of protocol treatment. Int J Radiat
Oncol Biol Phys. 71:676–681. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yamada KM and Even-Ram S: Integrin
regulation of growth factor receptors. Nat Cell Biol. 4:E75–E76.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cabodi S, Moro L, Bergatto E, Boeri Erba
E, Di Stefano P, Turco E, Tarone G and Defilippi P: Integrin
regulation of epidermal growth factor (EGF) receptor and of
EGF-dependent responses. Biochem Soc Trans. 32:438–442. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Eke I, Storch K, Krause M and Cordes N:
Cetuximab attenuates its cytotoxic and radiosensitizing potential
by inducing fibronectin biosynthesis. Cancer Res. 73:5869–5879.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hehlgans S, Haase M and Cordes N:
Signalling via integrins: Implications for cell survival and
anticancer strategies. Biochim Biophys Acta. 1775:163–180.
2007.
|
|
10
|
Braverman LE and Quilliam LA:
Identification of Grb4/Nckbeta, a src homology 2 and 3
domain-containing adapter protein having similar binding and
biological properties to Nck. J Biol Chem. 274:5542–5549. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chiswell BP, Zhang R, Murphy JW, Boggon TJ
and Calderwood DA: The structural basis of integrin-linked
kinase-PINCH interactions. Proc Natl Acad Sci USA. 105:20677–20682.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tu Y, Li F and Wu C: Nck-2, a novel Src
homology2/3-containing adaptor protein that interacts with the
LIM-only protein PINCH and components of growth factor receptor
kinase-signaling pathways. Mol Biol Cell. 9:3367–3382. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vaynberg J, Fukuda T, Chen K, Vinogradova
O, Velyvis A, Tu Y, Ng L, Wu C and Qin J: Structure of an ultraweak
protein-protein complex and its crucial role in regulation of cell
morphology and motility. Mol Cell. 17:513–523. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Legate KR, Montañez E, Kudlacek O and
Fässler R: ILK, PINCH and parvin: The tIPP of integrin signalling.
Nat Rev Mol Cell Biol. 7:20–31. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu Z, Fukuda T, Li Y, Zha X, Qin J and Wu
C: Molecular dissection of PINCH-1 reveals a mechanism of coupling
and uncoupling of cell shape modulation and survival. J Biol Chem.
280:27631–27637. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Eke I and Cordes N: Dual targeting of EGFR
and focal adhesion kinase in 3D grown HNSCC cell cultures.
Radiother Oncol. 99:279–286. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Eke I, Sandfort V, Storch K, Baumann M,
Röper B and Cordes N: Pharmacological inhibition of EGFR tyrosine
kinase affects ILK-mediated cellular radiosensitization in vitro.
Int J Radiat Biol. 83:793–802. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Morello V, Cabodi S, Sigismund S,
Camacho-Leal MP, Repetto D, Volante M, Papotti M, Turco E and
Defilippi P: β1 integrin controls EGFR signaling and tumorigenic
properties of lung cancer cells. Oncogene. 30:4087–4096. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang F, Weaver VM, Petersen OW, Larabell
CA, Dedhar S, Briand P, Lupu R and Bissell MJ: Reciprocal
interactions between beta1-integrin and epidermal growth factor
receptor in three-dimensional basement membrane breast cultures: A
different perspective in epithelial biology. Proc Natl Acad Sci
USA. 95:14821–14826. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Eke I, Sandfort V, Mischkus A, Baumann M
and Cordes N: Antiproliferative effects of EGFR tyrosine kinase
inhibition and radiation-induced genotoxic injury are attenuated by
adhesion to fibronectin. Radiother Oncol. 80:178–184. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rodemann HP, Dittmann K and Toulany M:
Radiation-induced EGFR-signaling and control of DNA-damage repair.
Int J Radiat Biol. 83:781–791. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang S, Peet CR, Saker J, Li C, Armstrong
EA, Kragh M, Pedersen MW and Harari PM: Sym004, a novel anti-EGFR
antibody mixture, augments radiation response in human lung and
head and neck cancers. Mol Cancer Ther. 12:2772–2781. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Eke I, Zscheppang K, Dickreuter E,
Hickmann L, Mazzeo E, Unger K, Krause M and Cordes N: Simultaneous
β1 integrin-EGFR targeting and radiosensitization of human head and
neck cancer. J Natl Cancer Inst. 107:1072015. View Article : Google Scholar
|
|
24
|
Eke I, Deuse Y, Hehlgans S, Gurtner K,
Krause M, Baumann M, Shevchenko A, Sandfort V and Cordes N:
β1Integrin/FAK/cortactin signaling is essential for
human head and neck cancer resistance to radiotherapy. J Clin
Invest. 122:1529–1540. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Storch K and Cordes N: Focal
adhesion-chromatin linkage controls tumor cell resistance to radio-
and chemotherapy. Chemother Res Pract. 2012:3192872012.PubMed/NCBI
|
|
26
|
Eke I, Schneider L, Förster C, Zips D,
Kunz-Schughart LA and Cordes N: EGFR/JIP-4/JNK2 signaling
attenuates cetuximab-mediated radiosensitization of squamous cell
carcinoma cells. Cancer Res. 73:297–306. 2013. View Article : Google Scholar
|
|
27
|
Cordes N and Meineke V: Cell
adhesion-mediated radioresistance (CAM-RR). Extracellular
matrix-dependent improvement of cell survival in human tumor and
normal cells in vitro. Strahlenther Onkol. 179:337–344. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cordes N, Seidler J, Durzok R, Geinitz H
and Brakebusch C: beta1-integrin-mediated signaling essentially
contributes to cell survival after radiation-induced genotoxic
injury. Oncogene. 25:1378–1390. 2006. View Article : Google Scholar
|
|
29
|
Storch K, Eke I, Borgmann K, Krause M,
Richter C, Becker K, Schröck E and Cordes N: Three-dimensional cell
growth confers radioresistance by chromatin density modification.
Cancer Res. 70:3925–3934. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sandfort V, Eke I and Cordes N: The role
of the focal adhesion protein PINCH1 for the radiosensitivity of
adhesion and suspension cell cultures. PLoS One. 5:e130562010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Estrugo D, Fischer A, Hess F, Scherthan H,
Belka C and Cordes N: Ligand bound beta1 integrins inhibit
procaspase-8 for mediating cell adhesion-mediated drug and
radiation resistance in human leukemia cells. PLoS One. 2:e2692007.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ito S, Takahara Y, Hyodo T, Hasegawa H,
Asano E, Hamaguchi M and Senga T: The roles of two distinct regions
of PINCH-1 in the regulation of cell attachment and spreading. Mol
Biol Cell. 21:4120–4129. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fukuda T, Chen K, Shi X and Wu C: PINCH-1
is an obligate partner of integrin-linked kinase (ILK) functioning
in cell shape modulation, motility, and survival. J Biol Chem.
278:51324–51333. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Eke I, Koch U, Hehlgans S, Sandfort V,
Stanchi F, Zips D, Baumann M, Shevchenko A, Pilarsky C, Haase M, et
al: PINCH1 regulates Akt1 activation and enhances radioresistance
by inhibiting PP1alpha. J Clin Invest. 120:2516–2527. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Funasaka K, Ito S, Hasegawa H, Goldberg
GS, Hirooka Y, Goto H, Hamaguchi M and Senga T: Cas utilizes Nck2
to activate Cdc42 and regulate cell polarization during cell
migration in response to wound healing. FEBS J. 277:3502–3513.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Labelle-Côté M, Dusseault J, Ismaïl S,
Picard-Cloutier A, Siegel PM and Larose L: Nck2 promotes human
melanoma cell proliferation, migration and invasion in vitro and
primary melanoma-derived tumor growth in vivo. BMC Cancer.
11:4432011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Errington TM and Macara IG: Depletion of
the adaptor protein NCK increases uV-induced p53 phosphorylation
and promotes apoptosis. PLoS One. 8:e762042013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen K, Tu Y, Zhang Y, Blair HC, Zhang L
and Wu C: PINCH-1 regulates the ERK-Bim pathway and contributes to
apoptosis resistance in cancer cells. J Biol Chem. 283:2508–2517.
2008. View Article : Google Scholar
|
|
39
|
Gonzalez-Nieves R, Desantis AI and Cutler
ML: Rsu1 contributes to regulation of cell adhesion and spreading
by PINCH1-dependent and -independent mechanisms. J Cell Commun
Signal. 7:279–293. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Stanchi F, Grashoff C, Nguemeni Yonga CF,
Grall D, Fässler R and Van Obberghen-Schilling E: Molecular
dissection of the ILK-PINCH-parvin triad reveals a fundamental role
for the ILK kinase domain in the late stages of focal-adhesion
maturation. J Cell Sci. 122:1800–1811. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Montanez E, Karaköse E, Tischner D,
Villunger A and Fässler R: PINCH-1 promotes Bcl-2-dependent
survival signalling and inhibits JNK-mediated apoptosis in the
primitive endoderm. J Cell Sci. 125:5233–5240. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Rea K, Sensi M, Anichini A, Canevari S and
Tomassetti A: EGFR/MEK/ERK/CDK5-dependent integrin-independent FAK
phosphorylated on serine 732 contributes to microtubule
depolymerization and mitosis in tumor cells. Cell Death Dis.
4:e8152013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang D, Li Y, Wu C and Liu Y: PINCH1 is
transcriptional regulator in podocytes that interacts with WT1 and
represses podocalyxin expression. PLoS One. 6:e170482011.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kremer BE, Adang LA and Macara IG: Septins
regulate actin organization and cell-cycle arrest through nuclear
accumulation of NCK mediated by SOCS7. Cell. 130:837–850. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Jahn T, Seipel P, Coutinho S, Miething C,
Peschel C and Duyster J: Grb4/Nckbeta acts as a nuclear repressor
of v-Abl-induced transcription from c-jun/c-fos promoter elements.
J Biol Chem. 276:43419–43427. 2001. View Article : Google Scholar : PubMed/NCBI
|