Introduction
Renal cell carcinoma (RCC) is the most common type
of kidney cancer, and the incidence has been rising steadily. Due
to the lack of symptoms at the early stages, approximately
one-third of patients present with advanced disease, either locally
advanced or metastatic (1,2). Early stage RCC is usually curable by
surgery. On the other hand, therapy for recurrent or metastatic
tumors still needs to be improved. RCC is generally refractory to
conventional anticancer treatments including chemotherapy and
radiotherapy. Although immunotherapy remains the effective therapy
when treating advanced RCC, long-term outcome has been
disappointing (1,3). Furthermore, targeted therapies have
been demonstrated to have a significant clinical response and
prolong patient survival (4–11).
However, these treatments are not effective enough to achieve a
complete response and also cause several adverse effects.
Therefore, novel approaches against RCC need to be developed to
improve patient prognosis.
Prostate cancer antigen-1 (PCA-1) has been
identified in human prostate cancer and its expression is
associated with disease progression and prognosis (12,13).
Furthermore, it was found to be identical to ALKBH3, a member of
the human AlkB homologs. In Escherichia coli (E.
coli), the alkB gene product was identified as a protein which
carries out DNA repair by oxidative demethylation and repairs both
DNA and RNA methylation (14–16).
Nine AlkB homologs have been identified in human tissues (17–20).
Among these human AlkB homologs, PCA-1/ALKBH3 has been reported to
have a protein structure and catalytic mechanisms of repairing DNA
and RNA quite similar to E. coli AlkB (17–24).
In addition to these physiological roles of PCA-1/ALKBH3 in humans,
recent studies have demonstrated that PCA-1/ALKBH3 is highly
expressed and associated with poor disease outcome in several human
types of cancers including prostate, non-small cell lung and
pancreatic cancer (12,13,25,26).
However, the precise functions and clinical significance of
PCA-1/ALKBH3 are still largely unknown.
The aim of the present study was to investigate the
clinical importance of PCA-1/ALKBH3 in human RCC. Furthermore, we
also aimed to clarify its therapeutic potential using siRNA
knockdown method.
Materials and methods
Patients
We analyzed 101 patients with RCC, who received
radical or partial nephrectomy from 2003 to 2008 at Nara Medical
University Hospital. The age of the patients ranged from 31 to 84
years (median 66 years), and the male to female ratio was 2.6:1.0.
None of these patients received preoperative anticancer treatments
including immunotherapy and arterial embolization therapy. They
were histopathologically composed of 81 cases (80%) of clear cell
type, 9 cases (9%) of granular cell type, 4 cases (4%) of papillary
cell type, 4 cases (4%) of spindle cell type, 2 cases (2%) of
cystic cell type and 1 case (1%) of mixed clear and spindle type.
The tumor stage was classified according to the UICC TNM
classification of renal tumors (27). Pathological grades were assigned
according to the criteria proposed by Fuhrman et al
(28). The present study was
approved by the Ethics Review Committee of Nara Medical University
Hospital.
Preparation of antisera
Anti-PCA-1/ALKBH3 antisera were prepared as
previously described against a synthetic PCA-1/ALKBH3 peptide
(amino acids 64–76) as the antigen (13). After a 0.5-mg aliquot of peptides
was emulsified and injected into rabbits, blood was collected at
2-week intervals. The relative activity of the antisera against the
synthetic peptide was tested by enzyme-linked immunosorbent
assay.
Immunohistochemistry
Immunohistochemical staining for PCA-1/ALKBH3 was
performed with a Dako Envision™ kit (Dako, Tokyo, Japan).
Formalin-fixed, paraffin-embedded tissues were cut into 5-µm
sections, deparaffinized and rehy-drated in a graded series of
ethanols. Antigen retrieval was carried out by heating the tissue
sections using a target retrieval solution, pH 9.0 (Dako). Then,
the samples were incubated for 5 min in peroxidase blocking
solution (Dako) to inhibit endogenous peroxidase. The sections were
then incubated overnight at 4°C with anti-human PCA-1/ALKBH3. A
subsequent reaction was carried out using secondary antibodies
(Dako) at 37°C for 30 min. Then, the sections were washed three
times with phosphate-buffered saline, and subsequently the color
was displayed with diaminobenzidine (DAB) (Dako) for ~5 min.
Sections were counterstained with hematoxylin, dehydrated in
ethanol, cleared in xylene and coverslipped.
Evaluation of the immunostaining
Evaluation of the immunostaining was performed by a
consensus of two pathologists blinded to the clinicopathological
data. The staining intensity was graded on a scale of 0 to 2 (0, no
staining; 1, weak staining; and 2, strong staining). Specimens in
which one or more tumor areas with different staining intensities
were present were scored for the most prevalent intensity. The
PCA-1/ALKBH3 expression level was divided into two categories: low
(scale 0–1) and high (scale 2) expression.
Cell culture and reagents
The human RCC line, CAKI-1, was obtained from the
American Type Culture Collection (ATCC; Manassas, VA, USA). The
CAKI-1 cells were grown in RPMI-1640, supplemented with 10%
heat-inactivated fetal bovine serum and incubated at 37°C in a
humidified atmosphere of 5% CO2 in air.
Anti-poly(ADP-ribose) polymerase (PARP) and anti-caspase 3
antibodies were purchased from Cell Signaling Technology (Beverly,
MA, USA); anti-actin antibodies were purchased from Santa Cruz
Biotechnology (Santa Cruz, CA, USA).
Transfection of ALKBH3 siRNA
For our transfection analyses, CAKI-1 cells were
seeded in 6-well plates and transfected either with the control RNA
(Santa Cruz Biotechnology) or with 100 nmol/l of the siRNA of
PCA-1/ALKBH3. Transfections were carried out using the
Lipofectamine system (Invitrogen, Tokyo, Japan) in accordance with
the manufacturer’s protocol when cells achieved ~30% confluency.
The PCA-1/ALKBH3 siRNA duplexes, generated with 30-dTdT overhangs
and prepared by Qiagen (Tokyo, Japan), were chosen against the DNA
target sequences as follows: PCA-1/ALKBH3 (1) target sequence, 5′-CAGAGAGGATA
TAACTTATCA-3′ and (PCA-1/ALKBH3 (2)
target sequence, 5′-ATCGCTATCATCTTTAGGCAA-3′.
Reverse transcription-polymerase chain
reaction
Total RNA was extracted using TRIzol reagent and
subjected to reverse transcription (RT) and polymerase chain
reaction (PCR) using a One-Step RT-PCR kit (Qiagen) according to
the manufacturer’s protocol. The PCR conditions were 95°C for 30
sec, 55–60°C for 30 sec and 72°C for 1 min, for a total of 35
cycles. The PCR primers were: PCA-1/ALKBH3 forward,
5′-TACCACTGCTAAGAGCCATCTCC-3′; PCA-1 reverse,
5′-ACCTGCTGAGGTTCTTTGAACAC-3′; glyc-eraldehyde-3-phosphate
dehydrogenase (G3PDH) forward, 5′-ACCACAGTCCATGCCATCAC-3′ and G3PDH
reverse, 5′-TCCACCACCCTGTTGCTGTA-3′. The PCR products were analyzed
on a 1.5% agarose gel and visualized by ethidium bromide
staining.
Real-time reverse
transcription-polymerase chain reaction
Total RNA was isolated from the cells using RNAspin
Mini (GE Healthcare, Tokyo, Japan). cDNA was synthesized from 1
µg RNA using a High Capacity cDNA Reverse Transcription kit
(Applied Biosystems, Foster City, CA, USA) following a standard
protocol. Specific TaqMan primers and probes were obtained from
Applied Biosystems. cDNA was amplified in TaqMan® Fast
Universal PCR Master Mix with gene-specific primers and probe on
the StepOnePlus™ Real-Time PCR System. Expression of the gene was
normalized against mRNA expression of the housekeeping gene
β2-microglobulin. Real-time PCR experiments for each gene were
performed for three independent experiments.
Western blot analysis
We resolved the cell lysates from the CAKI-1 cells
on SDS polyacrylamide gels and transferred them onto polyvinylidene
difluoride membranes (Millipore, Bedford, MA, USA), which were
blocked in 5% skimmed milk at room temperature for 1 h. The
membranes were then incubated with each of the antibodies described
in the previous section for 1 h, followed by incubation with
horseradish peroxidase-conjugated anti-mouse or anti-rabbit IgG
(Amersham Pharmacia Biotech, Piscataway, NJ, USA). We detected
peroxidase activity on X-ray film using an enhanced
chemiluminescence detection system.
Cell proliferation assay
The
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxypheny-l)-2-(4-sulfophenyl)-2H-tetrazo-lium,
inner salt (MTS) assay was used for evaluation of cell
proliferation. Aliquots of 2×103 cells/well were
cultured in 96-well plates. After 72 h, the MTS assay was performed
using CellTiter 96 Aqueous One Solution (Promega, Madison, WI, USA)
following the manufacturer’s instructions.
Statistical analysis
Comparisons among the clinicopatho-logical features
were evaluated using χ2 and Fisher’s exact tests as
appropriate. Statistical significance between two groups of
parametric data was evaluated using an unpaired Student’s t-test.
Survival curves were estimated using the Kaplan-Meier method, and
the significance of differences between survival curves was
determined using log-lank test. All tests were two-sided, and
P<0.05 was considered to indicate a statistically significant
result.
Results
PCA-1/ALKBH3 expression in RCC
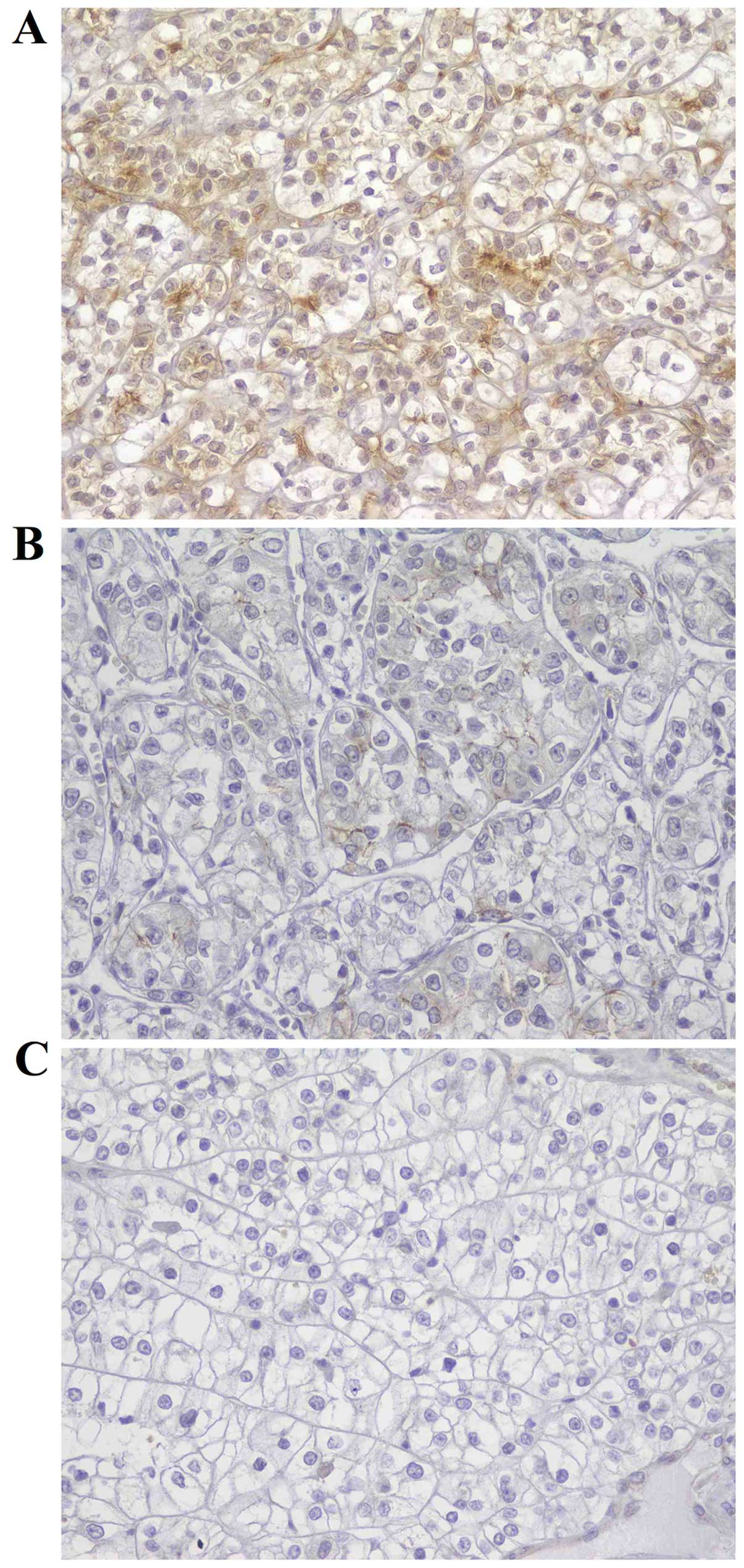
First, we evaluated the PCA-1/ALKBH3 expression in
actual human RCC tissues. Surgical specimens from 101 RCC patients
were examined by immunohistochemistry. The protein expression of
PCA-1/ALKBH3 was observed mainly in the cytoplasm of tumor cells
(Fig. 1). PCA-1/ALKBH3 expression
was detected in 55 of the 101 RCC specimens. Of these, 11 (10.9%)
were weakly positive and 44 (43.6%) were strongly positive.
Forty-six specimens (45.5%) exhibited negative staining.
Associations between PCA-1/ALKBH3 status
and the clinicopathological factors
According to the intensity of the
immunohistochemical staining for PCA-1/ALKBH3, 101 specimens were
divided into two categories: low (scale 0–1, n=57) and high (scale
2, n=44) expression. The associations between PCA-1/ALKBH3
expression and various clinico-pathological factors are shown in
Table I. The expression of
PCA-1/ALKBH3 was positively correlated with advanced pathological
T- and M-factors and TNM stage (P=0.025, 0.012 and 0.006,
respectively). Furthermore, the increased pre-operative serum
C-reactive protein (CRP) levels, which are known to be a negative
prognostic factor, were significantly correlated with the
PCA-1/ALKBH3 status (P<0.001). In contrast, there were no
significant correlations of PCA-1/ALKBH3 expression with age,
gender, Eastern Cooperative Oncology Group Performance Status
(ECOG-PS), N-factor, nuclear grade, histology, hemoglobin (Hb),
lactate dehydrogenese (LDH) or corrected Ca level.
 | Table IClinicopathological characteristics
of the RCC cases according to PCA-1 tumor expression. |
Table I
Clinicopathological characteristics
of the RCC cases according to PCA-1 tumor expression.
| PCA-1 low
(n=57) | PCA-1 high
(n=44) | P-value |
|---|
| Age at surgery
(years) | 63.7±11.6 | 63.2±11.4 | 0.84 |
| Gender | | | |
| Female | 15 | 13 | 0.82 |
| Male | 42 | 31 | |
| ECOG-PS | | | |
| 0 | 23 | 19 | 0.30 |
| 1 | 28 | 20 | |
| 2 | 6 | 5 | |
| T stage | | | |
| pT1 | 39 | 26 | 0.025 |
| pT2 | 2 | 8 | |
| pT3 | 16 | 8 | |
| pT4 | 0 | 2 | |
| Lymph node
involvement | | | |
| Absent | 53 | 39 | 0.50 |
| Present | 4 | 5 | |
| Distant
metastases | | | |
| Absent | 53 | 32 | 0.012 |
| Present | 4 | 12 | |
| TNM stage | | | |
| I | 40 | 26 | 0.006 |
| II | 0 | 2 | |
| III | 12 | 3 | |
| IV | 5 | 13 | |
| Nuclear grade | | | |
| 1 | 28 | 14 | 0.19 |
| 2 | 22 | 21 | |
| 3+4 | 7 | 9 | |
| Histology | | | |
| Clear cell | 49 | 32 | 0.13 |
| Non-clear
cell | 8 | 12 | |
| Hemoglobin
(mg/dl) | 13.8±1.6 | 13.3±2.2 | 0.26 |
| Lactate
dehydrogenese (IU/l) | 190±39 | 194±48 | 0.65 |
| Corrected Ca
(mg/dl) | 9.4±0.5 | 9.5±0.5 | 0.17 |
| C-reactive protein
(mg/dl) | 0.84±0.27 | 2.34±0.69 | 0.001 |
Patient prognosis
At the time of analysis, 13 out of 101 patients died
due to RCC. The median (range) time to death was 12.5 (2–23.4)
months. The estimated cancer-specific survival rate 5 years after
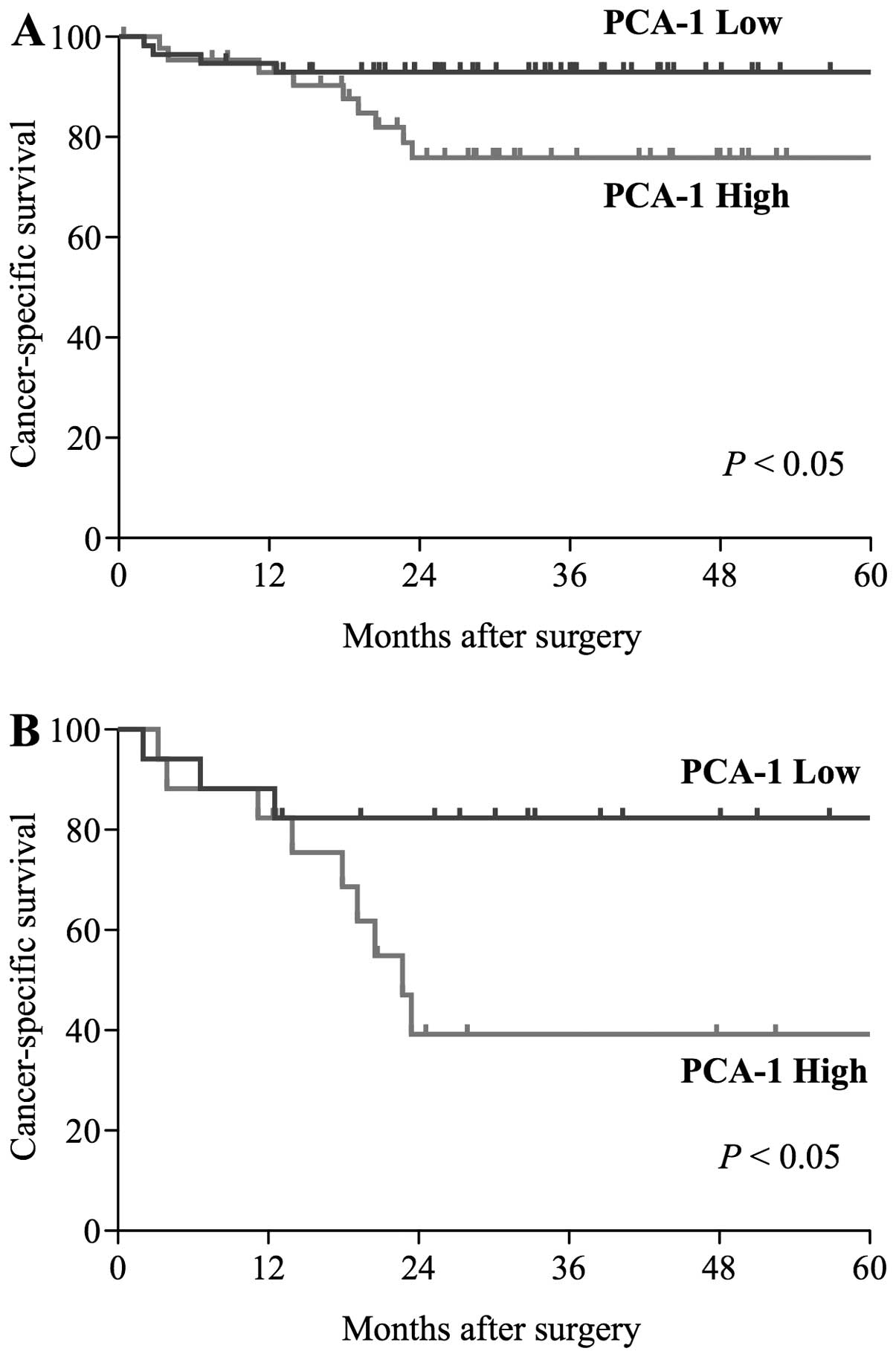
surgery was 85.9%. Patients with low PCA-1/ALKBH3 expression had a
significantly better prognosis than patients with high expression
(5-year survival rate, 92.9 vs. 75.9%; P<0.05; Fig. 2A). Moreover, 21 patients had a
metastatic lesion at the time of surgery and 13 patients developed
metastasis after surgery in the present study. In these metastatic
patients, 17 patients (50%) were classified in the PCA-1/ALKBH3
high group. According to Memorial Sloan-Kettering Cancer Center
(MSKCC) risk classification (29),
29 patients (85%) were defined in the intermediate risk group,
while 1 (3%) and 4 (12%) patients were defined in the favorable and
poor risk groups, respectively. The metastatic patients with low
PCA-1/ALKBH3 expression had a significantly better survival than
those with high expression (5-year survival rate, 82.4 vs. 39.2%;
P<0.05; Fig. 2B).
Effect of small interfering RNA knockdown
of PCA-1/ALKBH3 in RCC cells
We investigated the therapeutic efficacy of
targeting PCA-1/ALKBH3 in a human RCC cell line, CAKI-1. To this
end, we employed conventional small interfering RNA (siRNA)
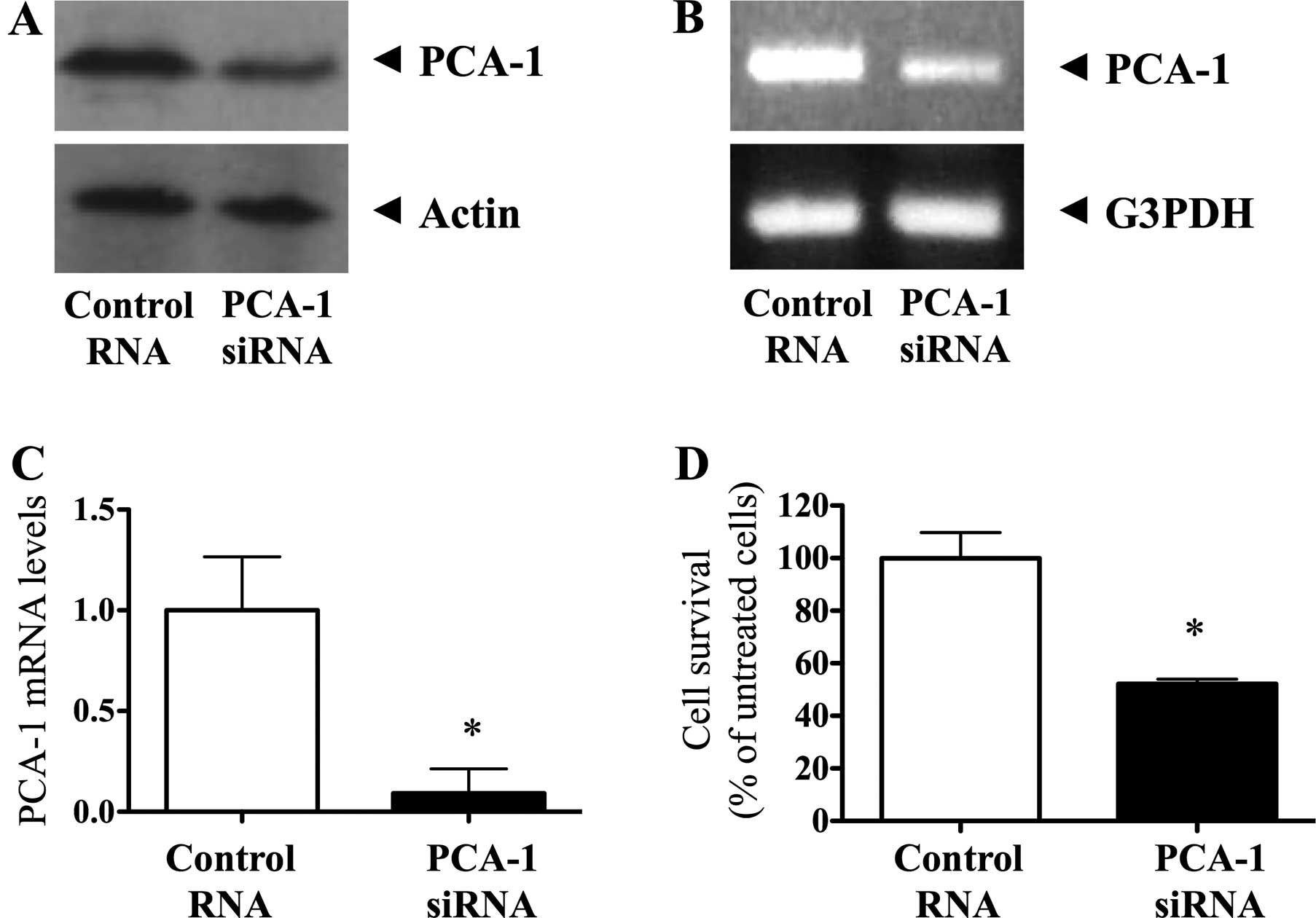
knockdown method. We first confirmed the PCA-1/ALKBH3 protein and
gene expression in the CAKI-1 cells using western blotting, RT-PCR
and real-time RT-PCR analysis. These data indicated that
PCA-1/ALKBH3 gene expression was successfully depleted by 100 nM
siRNA transfection and culture for 72 h (Fig. 3A-C). In the MTS assay, we found that
siRNA-mediated depletion of PCA-1/ALKBH3 significantly inhibited
the growth of CAKI-1 cells compared with that in the control
(Fig. 3D, P<0.001). There was
48% inhibition in CAKI-1 cell growth.
PCA-1/ALKBH3 knockdown induces apoptosis
in RCC cells
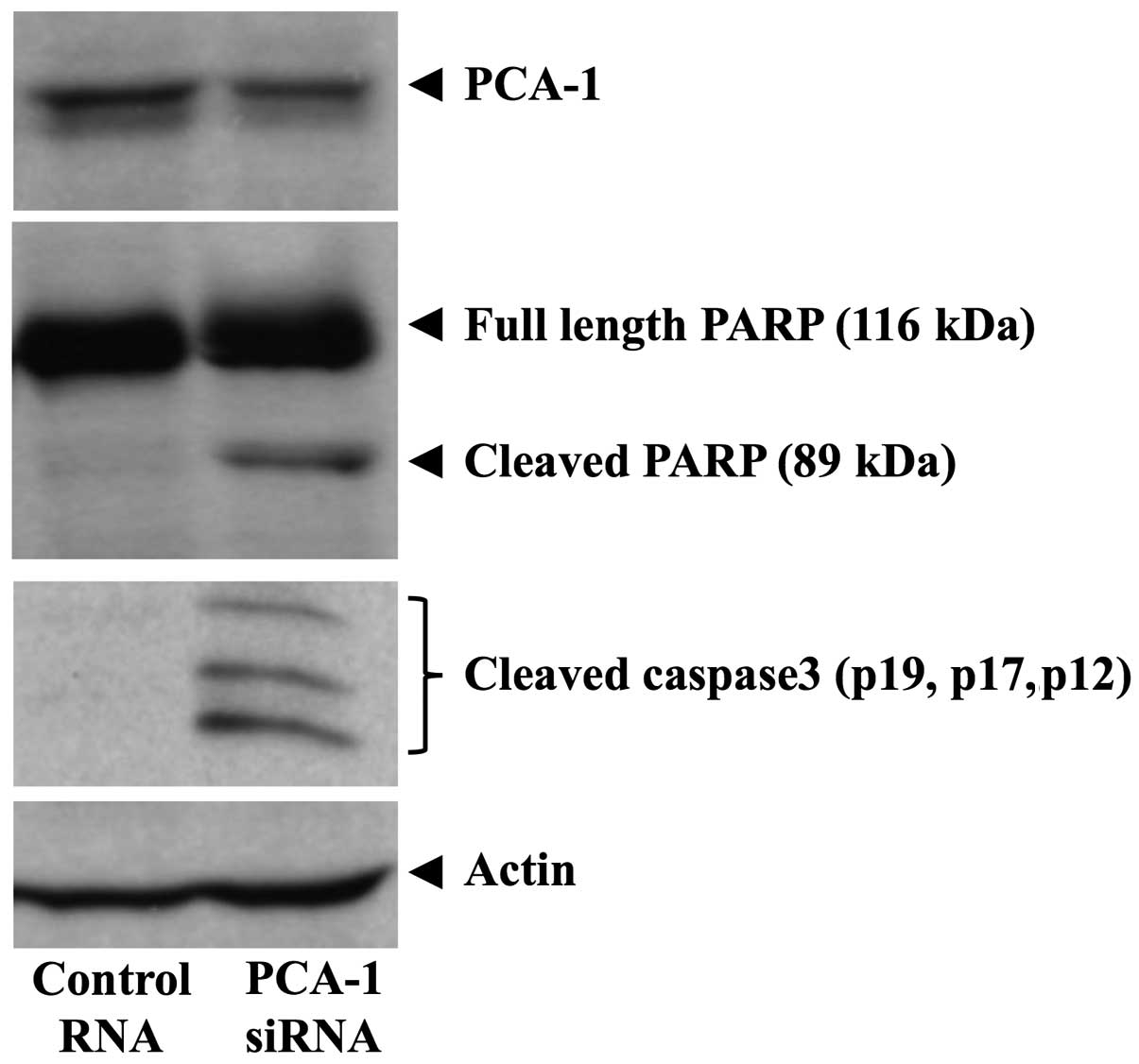
Furthermore, we evaluated apoptosis using
PARP-cleavage analysis. As a result, PCA-1/ALKBH3 knockdown induced
apoptosis in the CAKI-1 cells (Fig.
4). We also observed the cleavage of caspase-3 (Fig. 4). The results suggest that
PCA-1/ALKBH3 protects renal cancer cells from mitochondrial
pathway-mediated apoptosis.
Discussion
Humans are continuously exposed to agents that
methylate DNA and RNA. Such agents initiate abnormal methylation on
genes. When DNA methylation is dysregulated, the harmful
methylation contributes to several disease conditions including
cancer. Therefore, the enzymatic functions of repairing these
abnormalities are critical for maintaining DNA and RNA integrity.
It has been reported that AlkB proteins play an important roles in
the demethylation of DNA (14–16).
At least 9 AlkB homologs (ALKBH1–8, FTO) have been identified in
human tissues (17–20). Among the diverse functions of the
AlkB family, there are only limited number of studies that have
described its role in cancer biology. For example, ALKBH8 was
reported to contribute to the progression of human bladder cancer
(30). Another study showed that
ALKBH2 knockdown enhanced the sensitivity of human lung cancer
cells to cisplatin-based chemotherapy (31). In contrast, the overexpression of
ALKBH2 was found to inhibit the cell growth in gastric cancer
(32). Taken together, each AlkB
family member has distinctive role and exerts various functions in
each type of cancer.
Among the human AlkB homologs, ALKBH3 is known as a
unique member which demethylates RNA besides repairing methylated
DNA. In tumors, PCA-1/ALKBH3 has originally been identified in
human prostate cancer (12,13). PCA-1/ALKBH3 has been shown to
significantly contribute to the tumor growth and clinical outcome
in several types of cancers including not only prostate, but also
lung and pancreatic cancer (25,26).
Furthermore, PCA-1/ALKBH3 was also reported to be one of the
candidate gene associated with the risk of papillary thyroid cancer
(33). In contrast, it has been
reported that PCA-1/ALKBH3 plays a protective role in chronic
inflammation-associated colon carcinogenesis (34). Taken together, the precise role of
PCA-1/ALKBH3 in human types of cancers remains to be fully
elucidated. Since there is no report to address the roles of the
AlkB family in RCC, we investigated the clinical significance of
PCA-1/ALKBH3 in RCC.
First, we found that PCA-1/ALKBH3 protein was
expressed in approximately half of the RCC tissues. Furthermore, we
also found that intense PCA-1/ALKBH3 expression was significantly
associated with advanced pathological T-factor, TNM stage and
distant metastasis in the RCC cases. Our previous study showed no
association between PCA-1/ALKBH3 expression and distant metastasis
in pancreatic cancer (25).
Therefore, in contrast to pancreatic cancer, the present study
suggests that PCA-1/ALKBH3 plays a critical role in tumor
metastasis as well as progression in human RCC. Importantly,
patients with high PCA-1/ALKBH3 expression had a significantly
poorer prognosis than patients with low PCA-1/ALKBH3 expression.
Furthermore, we also evaluated PCA-1/ALKBH3 expression in 34
metastatic patients. Notably, although most metastatic patients in
the present study were classified as an intermediate risk by the
MSKCC classification, there was a significant difference in the
cancer-specific survival rate between the PCA-1/ALKBH3 high and low
group. These clinical data suggest that PCA-1/ALKBH3 may be
functionally important in RCC. However, to confirm our preliminary
findings, further large-scale studies are required.
Next, we examined the biological roles of
PCA-1/ALKBH3 in RCC using siRNA method. Our data indicated that
siRNA-mediated knockdown of PCA-1/ALKBH3 resulted in the
significant reduction of human renal cancer cell growth. This was
consistent with previous data on pancreatic and lung cancer
(25,26). The data suggest that PCA-1/ALKBH3
significantly contributes to tumor progression in several human
types of cancers. Furthermore, we used the PARP-cleavage assay in
order to evaluate the effect of PCA-1/ALKBH3 blockade on the
apoptosis of renal cancer cell. We found that inhibition of
PCA-1/ALKBH3 induced mitochondrial pathway-mediated apoptosis in
the RCC cells. This was also consistent with previous studies on
prostate and pancreatic cancer cells (12,13,25).
However, in contrast, PCA-1/ALKBH3 knockdown suppressed cell
proliferation through p21/p27 mediated cell cycle arrest, yet did
not induce apoptosis in human lung cancer cells (26). Other mechanisms may be involved in
tumors associated with PCA-1/ALKBH3 expression. We previously
reported that PCA-1/ALKBH3 silencing downregulated VEGF expression
and inhibited angiogen-esis in pancreatic cancer (25). Similarly, angiogenesis was
suppressed by PCA-1/ALKBH3 gene silencing in urothelial carcinoma
(35). Therefore, we evaluated the
correlation between VEGF expression and PCA-1/ALKBH3 in RCC.
However, there was no difference in VEGF expression between the RCC
cells treated with PCA-1/ALKBH3 siRNA and those with control RNA
(data not shown). Taken together, PCA-1/ALKBH3 contributes to tumor
progression through distinct mechanisms in each type of human
cancer.
Finally, we found that preoperative CRP levels were
positively associated with expression of PCA-1/ALKBH3. In contrast,
there were no significant correlations of PCA-1/ALKBH3 expression
with other factors including Hb, LDH or corrected Ca level. CRP is
well known as an indicator of systemic inflammatory response.
Previous studies showed that increased CRP levels predict poor
survival in patients with both localized and metastatic RCC
(36–38). We confirmed that the patients with a
high CRP level had a significantly poorer prognosis than patients
with a normal CRP level in the present study (data not shown). More
recently, studies found that CRP plays a functionally important
role in the proliferation of tumor cells through various mechanisms
including the protection of cancer cells from apoptosis (39,40).
Although the underlying mechanism of the positive association
between CRP level and PCA-1/ALKBH3 is still unrevealed, further
studies are warranted to clarify the fundamental tumor biology and
to explore a novel therapeutic strategy.
In conclusion, we demonstrated for the first time
that PCA-1/ALKBH3 tumor expression has a significant impact on
patients with RCC. Since our data suggest that PCA-1/ALKBH3 may
play a functionally important role in RCC, it may be a novel
therapeutic target as well as a useful marker for human RCC.
References
|
1
|
Motzer RJ, Mazumdar M, Bacik J, Berg W,
Amsterdam A and Ferrara J: Survival and prognostic stratification
of 670 patients with advanced renal cell carcinoma. J Clin Oncol.
17:2530–2540. 1999.PubMed/NCBI
|
|
2
|
Cohen HT and McGovern FJ: Renal-cell
carcinoma. N Engl J Med. 353:2477–2490. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang JC, Sherry RM, Steinberg SM, Topalian
SL, Schwartzentruber DJ, Hwu P, Seipp CA, Rogers-Freezer L, Morton
KE, White DE, et al: Randomized study of high-dose and low-dose
interleukin-2 in patients with metastatic renal cancer. J Clin
Oncol. 21:3127–3132. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Motzer RJ, Escudier B, Oudard S, Hutson
TE, Porta C, Bracarda S, Grünwald V, Thompson JA, Figlin RA,
Hollaender N, et al RECORD-1 Study Group: Efficacy of everolimus in
advanced renal cell carcinoma: A double-blind, randomised,
placebo-controlled phase III trial. Lancet. 372:449–456. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Motzer RJ, Hutson TE, Tomczak P,
Michaelson MD, Bukowski RM, Oudard S, Negrier S, Szczylik C, Pili
R, Bjarnason GA, et al: Overall survival and updated results for
sunitinib compared with interferon alfa in patients with metastatic
renal cell carcinoma. J Clin Oncol. 27:3584–3590. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rini BI, Escudier B, Tomczak P, Kaprin A,
Szczylik C, Hutson TE, Michaelson MD, Gorbunova VA, Gore ME,
Rusakov IG, et al: Comparative effectiveness of axitinib versus
sorafenib in advanced renal cell carcinoma (AXIS): A randomised
phase 3 trial. Lancet. 378:1931–1939. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Escudier B, Eisen T, Stadler WM, Szczylik
C, Oudard S, Staehler M, Negrier S, Chevreau C, Desai AA, Rolland
F, et al: Sorafenib for treatment of renal cell carcinoma: Final
efficacy and safety results of the phase III treatment approaches
in renal cancer global evaluation trial. J Clin Oncol.
27:3312–3318. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Escudier B, Pluzanska A, Koralewski P,
Ravaud A, Bracarda S, Szczylik C, Chevreau C, Filipek M, Melichar
B, Bajetta E, et al AVOREN Trial investigators: Bevacizumab plus
interferon alfa-2a for treatment of metastatic renal cell
carcinoma: A randomised, double-blind phase III trial. Lancet.
370:2103–2111. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hudes G, Carducci M, Tomczak P, Dutcher J,
Figlin R, Kapoor A, Staroslawska E, Sosman J, McDermott D, Bodrogi
I, et al: Global ARCC Trial: Temsirolimus, interferon alfa, or both
for advanced renal-cell carcinoma. N Engl J Med. 356:2271–2281.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sternberg CN, Davis ID, Mardiak J,
Szczylik C, Lee E, Wagstaff J, Barrios CH, Salman P, Gladkov OA,
Kavina A, et al: Pazopanib in locally advanced or metastatic renal
cell carcinoma: Results of a randomized phase III trial. J Clin
Oncol. 28:1061–1068. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Albiges L, Choueiri T, Escudier B, Galsky
M, George D, Hofmann F, Lam T, Motzer R, Mulders P, Porta C, et al:
A systematic review of sequencing and combinations of systemic
therapy in metastatic renal cancer. Eur Urol. 67:100–110. 2015.
View Article : Google Scholar
|
|
12
|
Shimada K, Nakamura M, Ishida E, Higuchi
T, Yamamoto H, Tsujikawa K and Konishi N: Prostate cancer antigen-1
contributes to cell survival and invasion though discoidin receptor
1 in human prostate cancer. Cancer Sci. 99:39–45. 2008.
|
|
13
|
Konishi N, Nakamura M, Ishida E, Shimada
K, Mitsui E, Yoshikawa R, Yamamoto H and Tsujikawa K: High
expression of a new marker PCA-1 in human prostate carcinoma. Clin
Cancer Res. 11:5090–5097. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Aas PA, Otterlei M, Falnes PO, Vågbø CB,
Skorpen F, Akbari M, Sundheim O, Bjørås M, Slupphaug G, Seeberg E,
et al: Human and bacterial oxidative demethylases repair alkylation
damage in both RNA and DNA. Nature. 421:859–863. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Falnes PO, Johansen RF and Seeberg E:
AlkB-mediated oxidative demethylation reverses DNA damage in
Escherichia coli. Nature. 419:178–182. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dinglay S, Trewick SC, Lindahl T and
Sedgwick B: Defective processing of methylated single-stranded DNA
by E. coli AlkB mutants. Genes Dev. 14:2097–2105. 2000.PubMed/NCBI
|
|
17
|
Tsujikawa K, Koike K, Kitae K, Shinkawa A,
Arima H, Suzuki T, Tsuchiya M, Makino Y, Furukawa T, Konishi N, et
al: Expression and sub-cellular localization of human ABH family
molecules. J Cell Mol Med. 11:1105–1116. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen B, Liu H, Sun X and Yang CG:
Mechanistic insight into the recognition of single-stranded and
double-stranded DNA substrates by ABH2 and ABH3. Mol Biosyst.
6:2143–2149. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gerken T, Girard CA, Tung YC, Webby CJ,
Saudek V, Hewitson KS, Yeo GS, McDonough MA, Cunliffe S, McNeill
LA, et al: The obesity-associated FTO gene encodes a
2-oxoglutarate-dependent nucleic acid demethylase. Science.
318:1469–1472. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kurowski MA, Bhagwat AS, Papaj G and
Bujnicki JM: Phylogenomic identification of five new human homologs
of the DNA repair enzyme AlkB. BMC Genomics. 4:482003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ougland R, Zhang CM, Liiv A, Johansen RF,
Seeberg E, Hou YM, Remme J and Falnes PO: AlkB restores the
biological function of mRNA and tRNA inactivated by chemical
methylation. Mol Cell. 16:107–116. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sundheim O, Vågbø CB, Bjørås M, Sousa MM,
Talstad V, Aas PA, Drabløs F, Krokan HE, Tainer JA and Slupphaug G:
Human ABH3 structure and key residues for oxidative demeth-ylation
to reverse DNA/RNA damage. EMBO J. 25:3389–3397. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yu B, Edstrom WC, Benach J, Hamuro Y,
Weber PC, Gibney BR and Hunt JF: Crystal structures of catalytic
complexes of the oxidative DNA/RNA repair enzyme AlkB. Nature.
439:879–884. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hausinger RP:
FeII/alpha-ketoglutarate-dependent hydroxylases and related
enzymes. Crit Rev Biochem Mol Biol. 39:21–68. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yamato I, Sho M, Shimada K, Hotta K, Ueda
Y, Yasuda S, Shigi N, Konishi N, Tsujikawa K and Nakajima Y:
PCA-1/ALKBH3 contributes to pancreatic cancer by supporting
apoptotic resistance and angiogenesis. Cancer Res. 72:4829–4839.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tasaki M, Shimada K, Kimura H, Tsujikawa K
and Konishi N: ALKBH3, a human AlkB homologue, contributes to cell
survival in human non-small-cell lung cancer. Br J Cancer.
104:700–706. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guinan P, Sobin LH, Algaba F, Badellino F,
Kameyama S, MacLennan G and Novick A; Union International Contre le
Cancer (UICC) and the American Joint Committee on Cancer (AJCC):
TNM staging of renal cell carcinoma: Workgroup No 3. Cancer.
80:992–993. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fuhrman SA, Lasky LC and Limas C:
Prognostic significance of morphologic parameters in renal cell
carcinoma. Am J Surg Pathol. 6:655–663. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Motzer RJ, Bacik J, Murphy BA, Russo P and
Mazumdar M: Interferon-alfa as a comparative treatment for clinical
trials of new therapies against advanced renal cell carcinoma. J
Clin Oncol. 20:289–296. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shimada K, Nakamura M, Anai S, De Velasco
M, Tanaka M, Tsujikawa K, Ouji Y and Konishi N: A novel human AlkB
homologue, ALKBH8, contributes to human bladder cancer progression.
Cancer Res. 69:3157–3164. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu SS, Xu W, Liu S, Chen B, Wang XL, Wang
Y, Liu SF and Wu JQ: Down-regulation of ALKBH2 increases cisplatin
sensitivity in H1299 lung cancer cells. Acta Pharmacol Sin.
32:393–398. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gao W, Li L, Xu P, Fang J, Xiao S and Chen
S: Frequent down-regulation of hABH2 in gastric cancer and its
involvement in growth of cancer cells. J Gastroenterol Hepatol.
26:577–584. 2011. View Article : Google Scholar
|
|
33
|
Neta G, Brenner AV, Sturgis EM, Pfeiffer
RM, Hutchinson AA, Aschebrook-Kilfoy B, Yeager M, Xu L, Wheeler W,
Abend M, et al: Common genetic variants related to genomic
integrity and risk of papillary thyroid cancer. Carcinogenesis.
32:1231–1237. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Calvo JA, Meira LB, Lee CY, Moroski-Erkul
CA, Abolhassani N, Taghizadeh K, Eichinger LW, Muthupalani S,
Nordstrand LM, Klungland A, et al: DNA repair is indispensable for
survival after acute inflammation. J Clin Invest. 122:2680–2689.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shimada K, Fujii T, Tsujikawa K, Anai S,
Fujimoto K and Konishi N: ALKBH3 contributes to survival and
angiogenesis of human urothelial carcinoma cells through NADPH
oxidase and tweak/Fn14/VEGF signals. Clin Cancer Res. 18:5247–5255.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Casamassima A, Picciariello M, Quaranta M,
Berardino R, Ranieri C, Paradiso A, Lorusso V and Guida M:
C-reactive protein: A biomarker of survival in patients with
metastatic renal cell carcinoma treated with subcutaneous
interleukin-2 based immunotherapy. J Urol. 173:52–55. 2005.
View Article : Google Scholar
|
|
37
|
Lamb GW, McMillan DC, Ramsey S and
Aitchison M: The relationship between the preoperative systemic
inflammatory response and cancer-specific survival in patients
undergoing potentially curative resection for renal clear cell
cancer. Br J Cancer. 94:781–784. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Karakiewicz PI, Hutterer GC, Trinh QD,
Jeldres C, Perrotte P, Gallina A, Tostain J and Patard JJ:
C-reactive protein is an informative predictor of renal cell
carcinoma-specific mortality: A European study of 313 patients.
Cancer. 110:1241–1247. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yang J, Wezeman M, Zhang X, Lin P, Wang M,
Qian J, Wan B, Kwak LW, Yu L and Yi Q: Human C-reactive protein
binds activating Fcgamma receptors and protects myeloma tumor cells
from apoptosis. Cancer Cell. 12:252–265. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Secchiero P, Rimondi E, di Iasio MG,
Agnoletto C, Melloni E, Volpi I and Zauli G: C-Reactive protein
downregulates TRAIL expression in human peripheral monocytes via an
Egr-1-dependent pathway. Clin Cancer Res. 19:1949–1959. 2013.
View Article : Google Scholar : PubMed/NCBI
|