Introduction
Fascin-1 (FSCN1, also known as fascin) is a key
actin-bundling protein that organizes F-actin into well-ordered,
tightly packed, parallel bundles in cells (1–3).
Fascin is widely expressed in mesenchymal tissues and cells in the
nervous system. However, regulation of the fascin-actin interaction
is complex and involves components of the extracellular matrix,
peptide factors and other actin-binding proteins. Accumulating
evidence suggests that the expression of fascin is increased in
various types of cancer, including breast, lung, liver and colon
(4,5). Notably, cancer cells that express high
levels of fascin exhibit aggressive characteristics including a
high migratory phenotype and invasiveness, suggesting a positive
correlation between fascin overexpression and the aggressive
behavior of cancer cells (4–9).
Retinoic acid (RA) is a critical regulator of
differentiation, proliferation and apoptosis in various cell types.
The RA-metabolizing enzyme CYP26A1 has been shown to promote cell
survival and contribute to the oncogenic potential of breast
carcinoma cells, suggesting that this protein has an oncogenic
function (10). Consistent with
this finding, enhanced RA metabolism has been observed in various
types of cancer and elevated levels of CYP26A1 have been reported
in a number of cancer cell types (11–13).
The above-mentioned studies suggested that CYP26A1 confers unique
cell-survival properties on cells and modulates the expression of a
variety of genes to favor cell survival. We performed a series of
preliminary experiments using high-resolution oligonucleotide-based
microarray analyses on CYP26A1-overexpressing breast carcinoma
cells. These studies identified a number of genes that drive the
cells into the oncogenic state. In the present study, we focused on
fascin as a potential downstream target of CYP26A1. Fascin
primarily promotes cell motility and invasiveness; therefore, its
potential role in CYP26A1-mediated cancer aggressiveness is
consistent with previous findings demonstrating that the enhanced
expression of CYP26A1 is associated with increased cell motility
and invasiveness (10).
The relevance of increased fascin activity in human
breast cancer remains unclear. In the present study, we
demonstrated that fascin expression increases significantly in
response to constitutive CYP26A1 overexpression. Small-interfering
RNA (siRNA)-mediated suppression of fascin inhibited the malignant
behavior of CYP26A1-expressing breast carcinoma cells. The results
suggested that CYP26A1-mediated malignant behavior may be
partially, albeit significantly responsible for the elevated
expression of fascin.
Materials and methods
Cell culture and transfection
The MCF-7, MDA-MB-231, ZR75-1 and T47-D breast
cancer cell lines were obtained from a local distributor (Summit
Pharmaceuticals International, Tokyo, Japan) of the American Type
Culture Collection (ATCC; Manassas, VA, USA). The AC2M2 breast
cancer cell line was generously provided by Dr Bruce Elliott
(Cancer Research Institute, Queen’s University, Kingston, ON,
Canada) (14). The cells were
maintained in Dulbecco’s modified Eagle’s medium (DMEM; Sigma, St.
Louis, MO, USA) supplemented with 10% fetal bovine serum (FBS;
Invitrogen, Carlsbad, CA, USA), 100 U/ml penicillin and 100
µg/ml streptomycin (Sigma).
The cells were transfected with the pcDNA3.1(-)
expression vector (Invitrogen) containing the full-length CYP26A1
cDNA and a vector encoding fascin-specific or control siRNA (both
from OriGene, Rockvsille, MD, USA) using FuGENE® 6
(Roche, Basel, Switzerland). Transfected clones were selected using
0.8-mg/ml G418 for CYP26A1 and 1.2-µg/ml puromycin for
fascin. After >14 days of selection, drug-resistant clones were
selected and screened for CYP26A1 and fascin expression.
Preliminary experiments revealed that the phenotypes of at least
three independent clones of transfectants were similar, but not
identical. Therefore, cell populations were mixed to establish
stable transfected cell lines, avoid possible clonal variation and
exclude the possibility that the cloning procedures were selected
for a specific phenotype. Empty vector-transfected cells were used
as controls.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted using a TRIzol®
reagent and RT-PCR was performed using an RT-PCR kit (Superscript
II) (both from Invitrogen). Samples were incubated at 42°C for 50
min and the reactions were terminated by incubation at 70°C for 15
min, according to the manufacturer’s instructions. The cDNA was
incubated with 0.5 units of Taq DNA polymerase (Takara,
Shiga, Japan) to amplify the human CYP26A1, fascin
and GAPDH genes. The cycling conditions used were: 20–40
cycles of 30 sec at 96°C, 30 sec at 58°C and 1 min at 72°C,
followed by a final elongation time of 7 min at 72°C. The PCR
primers used were: CYP26A1, 5′-GCCTCTCTAACCTGCACGAC-3′ and
5′-GCTCTTCTCGCACTTTCTGG-3′; fascin, 5′-AGGACGAGCTCTTTGCTCTG-3′ and
5′-TGCCTGTGGAGTCTTTGATG-3′; and GAPDH, 5′-ACCACAGTCCATGCCATCAC-3′
and 5′-TCCACCACCCTGTTGCTGTA-3′. The expression of each gene of
interest was analyzed using cycling parameters that had been
optimized previously to produce expression linearity, allowing the
semi-quantitative analysis of signal intensity. PCR reactions were
repeated in three independent experiments to ensure that the
quantified expression was reproducible. Densitometric analysis of
bands on the agarose gels was performed using ImageJ software
(National Institutes of Health, Bethesda, MD, USA).
Western blotting
Aliquots of whole cell lysates (20 µg) were
separated on 12% sodium dodecyl sulfate (SDS)-polyacryl amide gels
and electroblotted onto nitrocellulose membranes. The membranes
were then immunoblotted with antibodies against fascin (Dako,
Glostrup, Denmark), CYP26A1, p16INK4A,
p21Waf1/Cip1, p27Kip1 (all from Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA), p53 (Novocastra,
Newcastle, UK), membrane type-1 matrix metalloproteinase (MMP-14;
Abcam, Cambridge, UK) and β-actin (Sigma). The membranes were
incubated with the appropriate peroxidase-labeled secondary
antibodies (Dako) and bands were visualized using enhanced
chemiluminescence (GE Healthcare, Buckingham, UK).
Immunohistochemistry
The breast cancer tissue microarray slide
(SuperBioChips Laboratories, Seoul, Korea) was deparaffinized, and
antigen retrieval was performed by microwave heating (500 W) in
citrate buffer for 15 min. The slides were then incubated overnight
at 4°C with monoclonal primary antibodies against fascin (1:100
dilution, clone no. 55K-2; Dako) or CYP26A1 (1:50 dilution, clone
no. F27 P6 A1; Santa Cruz Biotechnology, Inc.). The sections were
then incubated with EnVision™ (Dako) for 30 min at room
temperature. Positive staining was graded according to the
percentage of the stained tumor cells: 3+ (strong), staining in
>50% of cells; 2+ (moderate), staining in 25-49% of cells, 1+
(weak), staining in 5–24% of cells, 0 (negative), no or faint
staining in <5% of the cells. Fascin expression was considered
positive with staining grades of 2+ or 3+ and negative with 0 or
1+. The data were confirmed in independent duplicate analyses. The
original immunohistochemical findings of the microarray array
analysis are available on request.
Senescence-associated β-galactosidase
(SA-β-gal) staining
The MCF-7 cells were exposed to mild oxidative
stress by incubation with 10 µM H2O2
for up to 6 days in the presence or absence of 1 µM
all-trans retinoic acid (atRA; Sigma) and senescent cells
were visualized using SA-β-gal staining (15).
Cell cycle analysis by flow
cytometry
The MCF-7 cells were subjected to mild oxidative
stress with 10 µM H2O2 for 6 days
prior to being harvested by centrifugation and permeabilized with
70% ethanol for 10 min. After being washed with phosphate-buffered
saline (PBS), the cells were treated with PBS containing 100
µg/ml DNase free-RNase A (Invitrogen) at 37°C for 30 min.
The cells were then suspended in PBS containing 40 µg/ml
propidium iodide solution for 15 min. The DNA content was analyzed
using FACSCalibur™ (Becton-Dickinson, Franklin Lakes, NJ, USA).
Apoptosis induction
Oxidative stress-induced apoptosis was stimulated by
incubating cells in 0–100 µM H2O2 for
24 h. Anoikis (anchorage-dependent apoptosis) was then induced to
prevent the cells from adhering to the culture dishes in the
presence or absence of 1 µM atRA. In some experiments,
inhibitors of mitogen-activated protein kinase (MAPK; PD98059, 50
µM), p38 MAPK (SB203580, 20 µM) or
phosphoinositide-3-kinase (PI3K; LY294002, 20 µM) were added
(all from Sigma).
Terminal deoxynucleotidyl
transferase-mediated nick end-labeling (TUNEL) and cell
proliferation assays
Apoptosis was assessed in the MCF-7 cells cultured
on collagen-coated glass coverslips using a TUNEL assay. Apoptotic
cells were visualized using an In Situ Cell Death Detection kit
(Roche). The procedure was also performed without terminal
deoxynucleotidyl transferase as a negative control. To examine the
cell proliferation rate, the cells were manually counted every 24 h
up to day 6 after an equal number of cells were plated. In
addition, DNA synthesis in the cells was assessed using Ki-67
labeling. For immunohistochemistry, the cells on a coverslip were
incubated with a primary antibody against Ki-67 and then incubated
with EnVision™ (both from Dako) for 30 min at room temperature. The
results were confirmed in triplicate independent analyses.
Colony forming assays in two- (2-D) and
three-dimensional (3-D) cultures
The MCF-7 cells (5×105 cells/well) were
plated in 6-well plates 24 h prior to exposure to a lethal dose of
oxidative stress (500 µM H2O2) for 24
h. The small number of cells that survived was then cultured for
>7 days to form individual colonies. Soft agar assays were
performed in 6-cm dishes to assess colony formation in three
dimensions. Breast carcinoma cells (2.5×103) were
uniformly suspended in 6 ml of 0.33% agarose gel with DMEM
supplemented with 5% FBS, and then overlaid onto a base layer of 1%
agarose gel. The plates were incubated for at least 3 weeks and
cell clusters >50 µm in diameter were defined as
positive.
Invasion assay
A cell suspension (5×105 cells of
MDA-MB-231 in 0.5 ml of DMEM with 0.5% FBS) was added to 24-well
cell culture inserts with 8-µm pores that had been
pre-coated with Matrigel™ matrix (both from Becton-Dickinson). DMEM
containing 10% FBS was added to the lower chamber to create a
chemotactic gradient. The number of the invasive cells was then
estimated after 48 h of incubation. The lower surface of the upper
chamber was wiped with a cotton swab and any cells that passed
through the filters onto the lower surface of the cell culture
inserts were quantified.
Gelatin zymography
The gelatinolytic activities of MMPs were determined
in MDA-MB-231 cell supernatants. Aliquots of conditioned media were
separated by electrophoresis under non-reducing conditions without
heating in 0.1% gelatin-containing 9% polyacrylamide gels. The gels
were washed with 2.5% Triton™ X-100 for 1 h to remove the SDS, and
were then incubated overnight at 37°C in a Tris-based buffer. The
gels were then stained for 30 min with 0.5% Coomassie brilliant
blue. Clear bands appeared on the blue background in the areas with
gelatinolytic activity.
Wound-healing assay
The MDA-MB-231 cells were grown to confluence and a
scratch was made through the cell monolayer using a pipette tip.
After being washed twice with PBS, fresh media were added and the
wounded monolayer was photographed overtime.
Statistical analysis
The cells or colonies of interest for each assay
were counted under low magnification (×100) in 10 randomly selected
fields using light microscopy. Data are presented as means ±
standard deviation (SD) from at least three independent
experiments, each performed in triplicate wells. Statistical
differences were analyzed using the Student’s t-tests and data were
considered to indicate a statistically significant result when
P<0.05.
Results
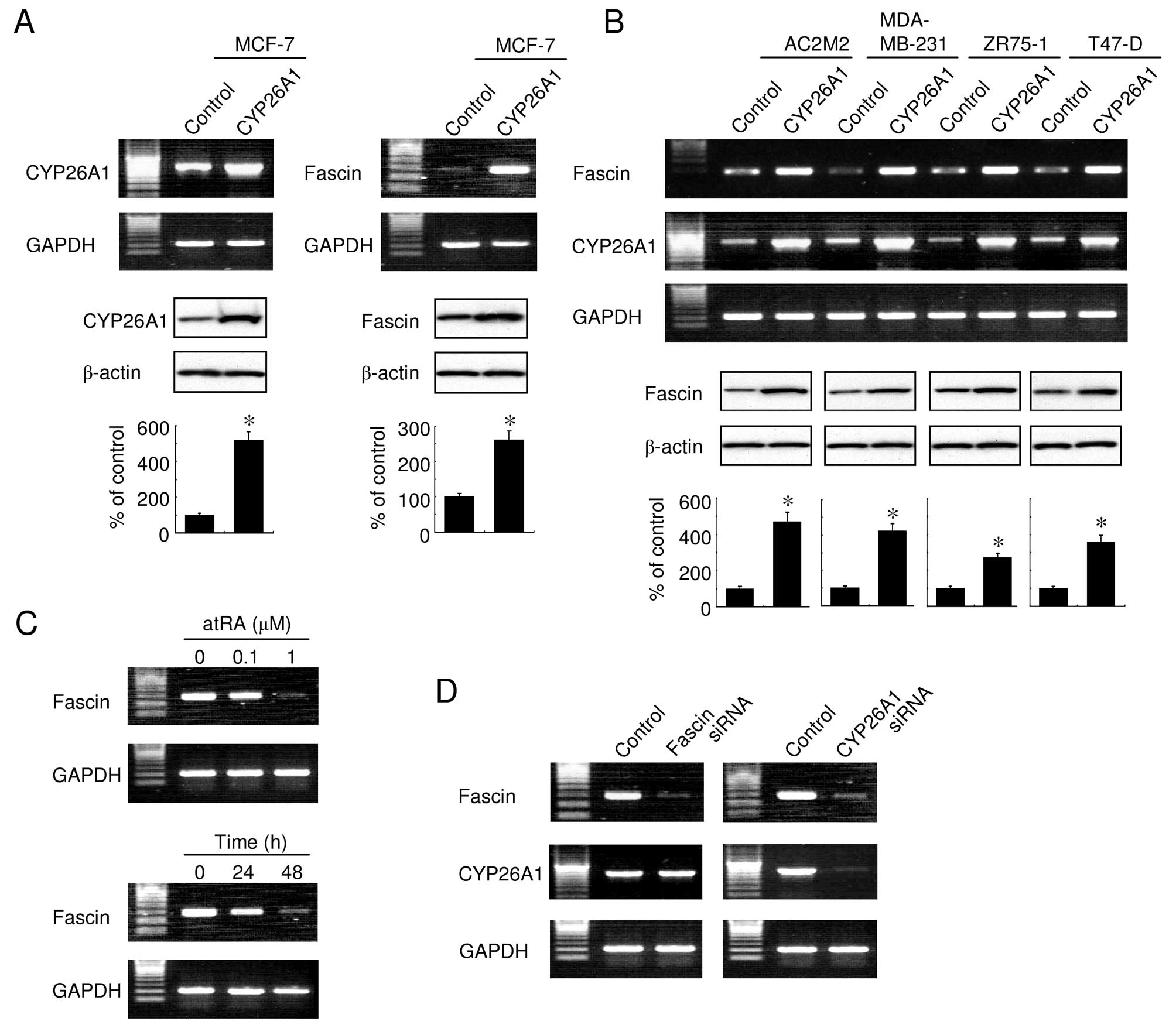
Upregulation of fascin in response to
CYP26A1 overexpression
We performed a series of preliminary experiments on
CYP26A1-overexpressing breast carcinoma cells, and identified a
number of genes that drive the cells into the oncogenic state. The
results confirmed that the CYP26A1-expressing cells expressed
elevated levels of fascin. As expected, fascin was constitutively
expressed in the MCF-7 cells and its expression was elevated in
CYP26A1-transfected cells (Fig.
1A). The fascin expression was also significantly upregulated
in other CYP26A1-overexpressing breast carcinoma cells (Fig. 1B), suggesting that its effect on
fascin occurred in a number of cell lines. In addition, treatment
with atRA downregulated the expression of fascin in a dose- and
time-dependent manner (Fig. 1C).
Conversely, fascin had no effect on the CYP26A1 expression, whereas
the siRNA-mediated knockdown of CYP26A1 downregulated the
expression of fascin (Fig. 1D).
These results suggested that fascin expression was modulated by the
intracellular RA levels, regulated by the expression of
CYP26A1.
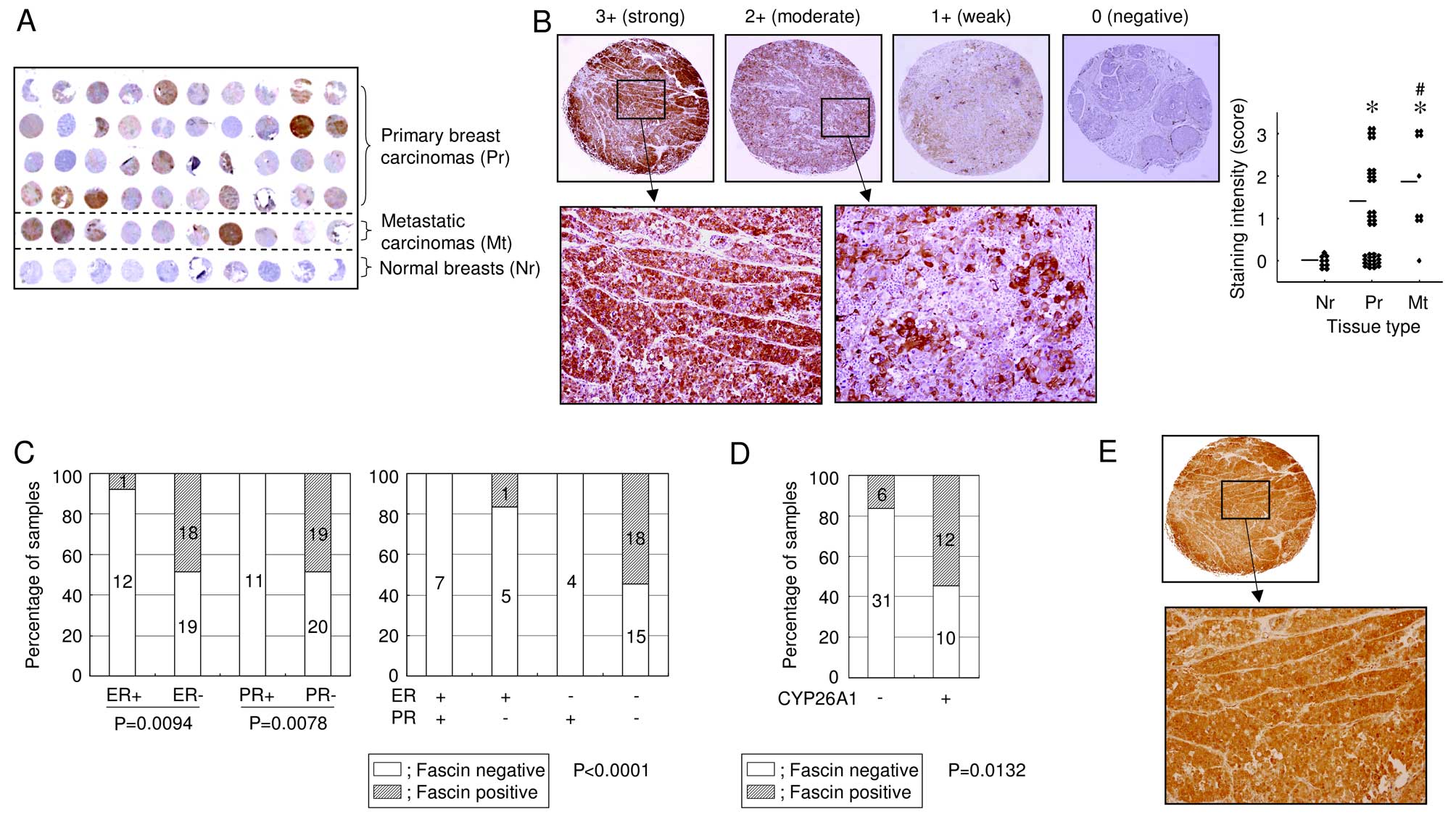
Fascin overexpression in primary breast
carcinomas
The expression of fascin in breast carcinomas was
also investigated using tissue microarray. Although no positive
reactivity was detected in the ducts and lobules of normal breast
tissues, strong cytoplasmic staining for fascin was observed in 19
of 50 (38%) breast carcinoma samples (Fig. 2A). In addition, metastatic
carcinomas had a significantly higher expression of fascin, when
compared with non-metastatic primary carcinomas (Fig. 2B), suggesting a potential role for
fascin in tumor progression. Notably, fascin was upregulated in
hormone receptor-negative cancers (Fig.
2C). Eighteen of 33 (55%) tumors that were negative for the
estrogen receptor (ER) and progesterone receptor (PR) were
fascin-positive, but fascin was negative (0%) in all tumors that
were ER- and PR-positive. A positive correlation between fascin and
CYP26A1 expression was observed in 12 of 22 (55%) CYP26A1-positive
tumors, whereas only 6 of 37 (16%) CYP26A1-negative tumors were
stained positive for fascin (P=0.0132; Fig. 2D and E). These data provide further
evidence of a correlation between CYP26A1 and fascin. Although a
larger cohort study of breast cancers showed that the CYP26A1
expression was significantly associated with patient survival
(16), fascin expression did not
correlate with the patient age, gender, tumor histology, the pTNM
stage, the primary tumor status, the number of lymph nodes involved
or the p53 and HER2 expression levels.
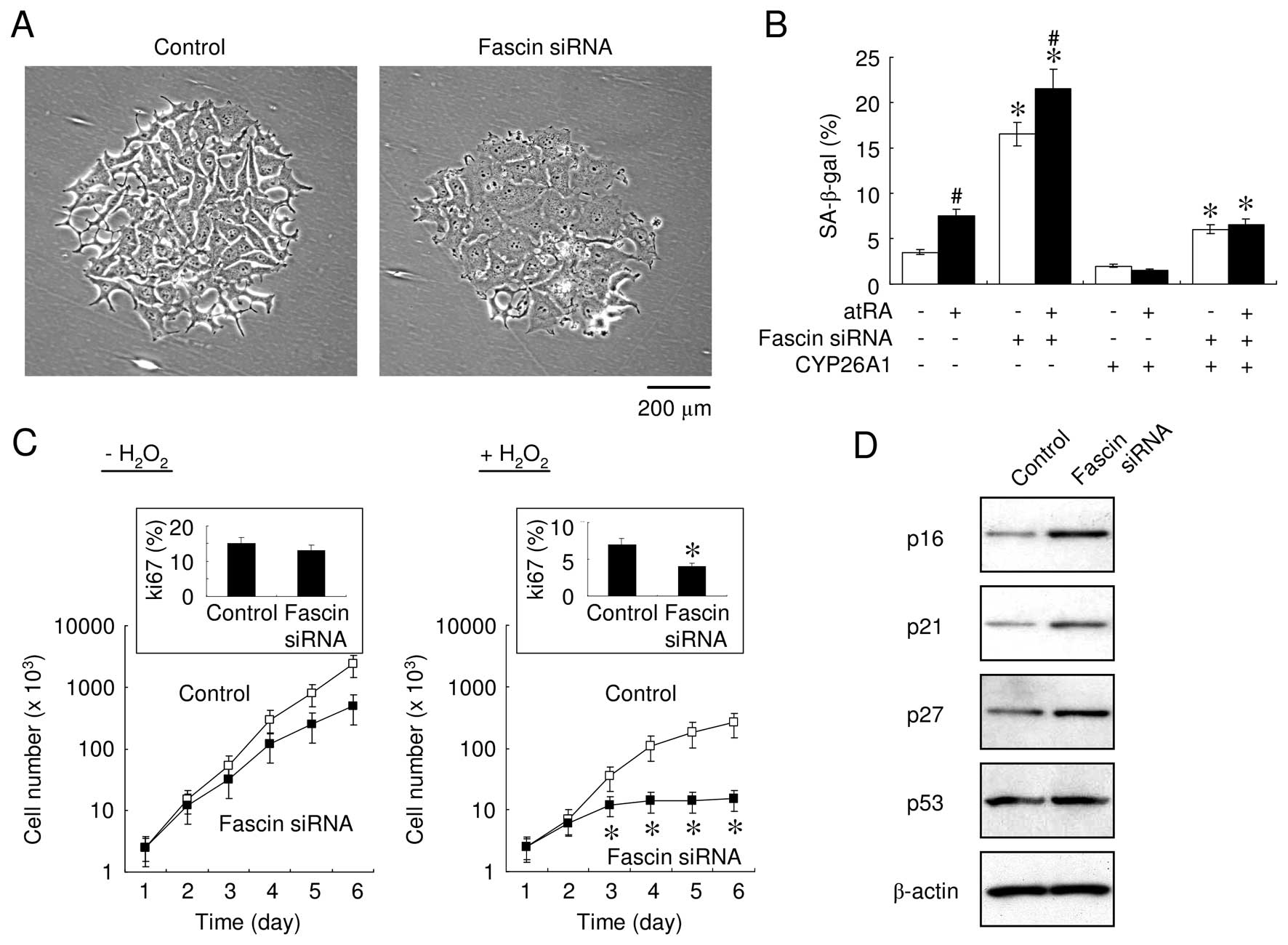
Suppression of fascin induces premature
senescence
The effect of fascin on cell senescence was assessed
since premature senescence is an intrinsic fail-safe mechanism
against carcinogenic insults (15,17).
Senescence was assessed by measuring SA-β-gal enzymatic activity.
Since a high level of oxidative stress efficiently induced
apoptosis, we examined the level of oxidative stress required to
induce senescence in the cells, but not to undergo apoptosis in our
preliminary study. When the MCF-7 cells were exposed to mild
oxidative stress that was insufficient to induce apoptosis,
knocking down fascin caused prominent phenotypic alterations
(Fig. 3A), including stimulating an
enlarged, flat morphology with multiple vacuoles and cytoplasmic
protrusions. In addition, the SA-β-gal activity was significantly
increased after transfection with fascin-specific siRNA, in the
presence and absence of atRA (Fig.
3B). These results suggested that atRA and fascin suppression
synergis-tically enhanced cell senescence, since atRA inhibited the
fascin expression (Fig. 1C).
Although the number of senescent cells markedly decreased when
CYP26A1 was overexpressed, fascin suppression significantly
increased the number of the SA-β-gal-positive cells (Fig. 3B). These results suggested that
elevated levels of fascin play a role in the signaling pathways
that mediate the escape from premature senescence.
Downregulation of fascin induces growth
arrest during oxidative stress
To investigate the effect of fascin signaling on
MCF-7 cell proliferation, the cell number was analyzed in a
time-dependent manner. Manual cell counting revealed no significant
differences in the growth and DNA synthesis rates, regardless of
the expression of fascin. However, cells transfected with
fascin-specific siRNA that were exposed to mild oxidative stress
exhibited a significantly decreased cell growth and DNA synthesis
rate as assessed using Ki-67 labeling (Fig. 3C). Consistent with this, the
expression of cyclin-dependent kinase (CDK) inhibitors, including
p16INK4A, p21Waf1/Cip1 and p27Kip1
but not p53, was increased in fascin-suppressed cells during
oxidative stress (Fig. 3D). In
addition, cell cycle analyses indicated that fascin suppression
induced G1 arrest and a concomitant decrease in the S-phase
fractions in response to mild oxidative stress that did not induce
apoptotic cell death (G1-G0, 54.9%; S, 25.4%; and G2-M, 19.7% in
the control cells; G1-G0, 75.1%; S, 7.1%; and G2-M, 17.8%
fascin-suppressed cells), suggesting that fascin-induced cell
senescence was involved in G1/S-phase transition and may sensitize
cells to specific cell-cycle checkpoints. Withdrawal of the
oxidative stress did not release the cells from growth arrest or
premature senescence. The results provided support for the
possibility that fascin signaling is associated with stress-induced
premature senescence.
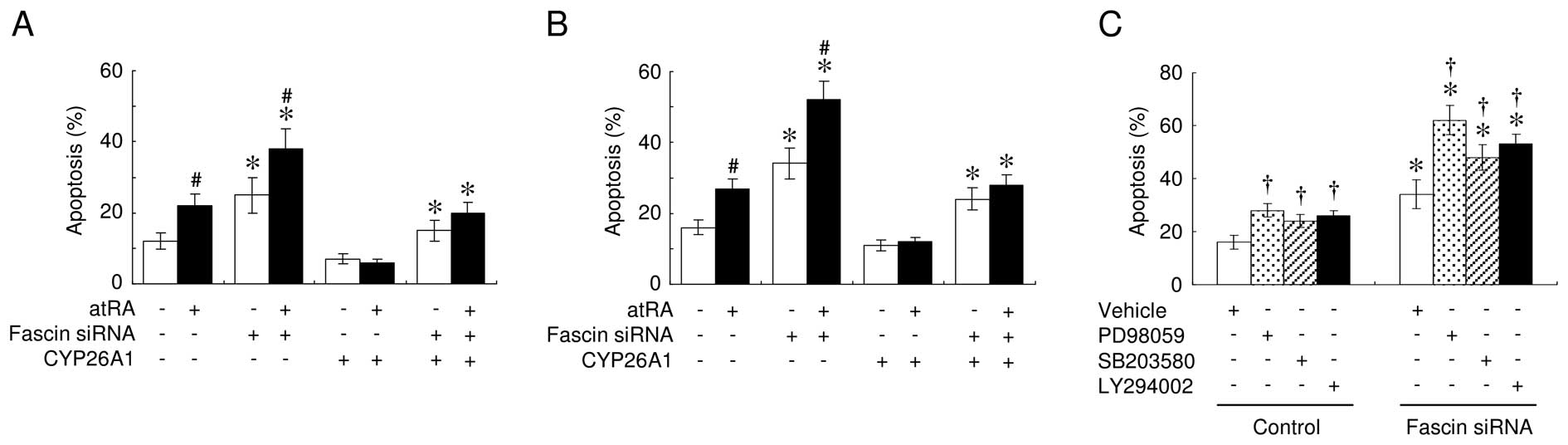
Suppression of fascin promotes apoptosis
and inhibits anchorage-independent growth
To examine the effect of the altered fascin
expression on apoptosis, MCF-7 cells were incubated with various
concentrations of H2O2 for 24 h. The TUNEL
assays consistently revealed that treatment with atRA enhanced the
sensitivity of cells to H2O2-induced cell
death, whereas knocking down fascin expression significantly
increased cell sensitivity to apoptosis (Fig. 4A). Fascin suppression also promoted
atRA-mediated enhanced anoikis (Fig.
4B). Anoikis was also observed in the event of fascin
suppression, even when the cells overexpressed CYP26A1. However,
atRA had no effect on anoikis in CYP26A1-overexpressing cells.
To investigate the signal transduction pathways
involved in the effects of fascin on apoptotic sensitivity, various
kinase inhibitors were used (Fig.
4C). The PD98059 MAPK inhibitor efficiently induced anoikis,
which was increased further when the fascin expression was
suppressed. The p38 MAPK inhibitor SB203580 and the PI3K inhibitor
LY294002 had significant, but much less pronounced, effects
compared to PD98059.
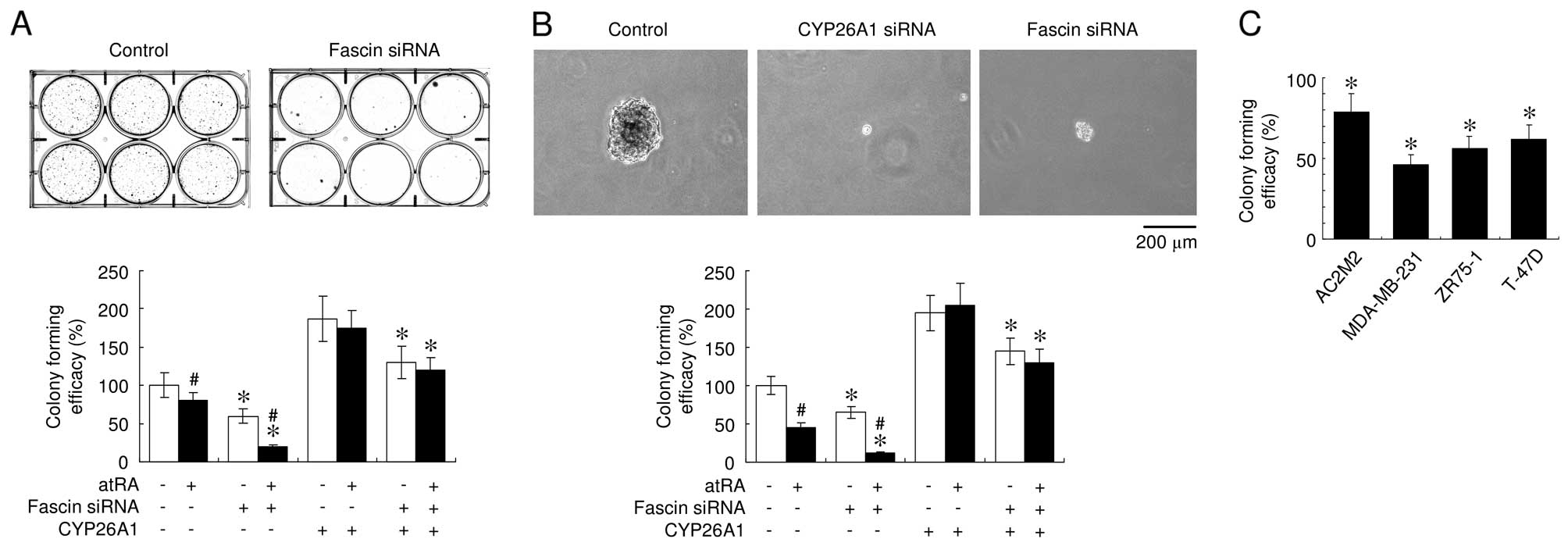
Suppressing fascin expression also significantly
inhibited colony formation, a measure of cell transformation, by
decreasing the number and size of colonies in 2-D and 3-D cultures
(Fig. 5A and B). In addition,
downregulation of the CYP26A1 expression significantly suppressed
colony formation in soft agar (Fig.
5B), supporting the presence of a direct mechanistic link
between CYP26A1 expression and oncogenic behavior. Treatment with
atRA consistently inhibited anchorage-independent growth and
enhanced the effects of fascin downregulation. These observations
suggested that atRA and fascin suppression synergistically inhibit
anchorage-independent growth properties. The knockdown of fascin
expression inhibited the efficacy of colony formation in the
CYP26A1-overexpressing MCF-7 cells. Similarly, fascin suppression
significantly inhibited colony formation in various types of breast
carcinoma cells (Fig. 5C). These
results suggested that fascin expression modulates colony formation
in CYP26A1-expressing cells.
Suppression of fascin decreases cell
invasion and motility
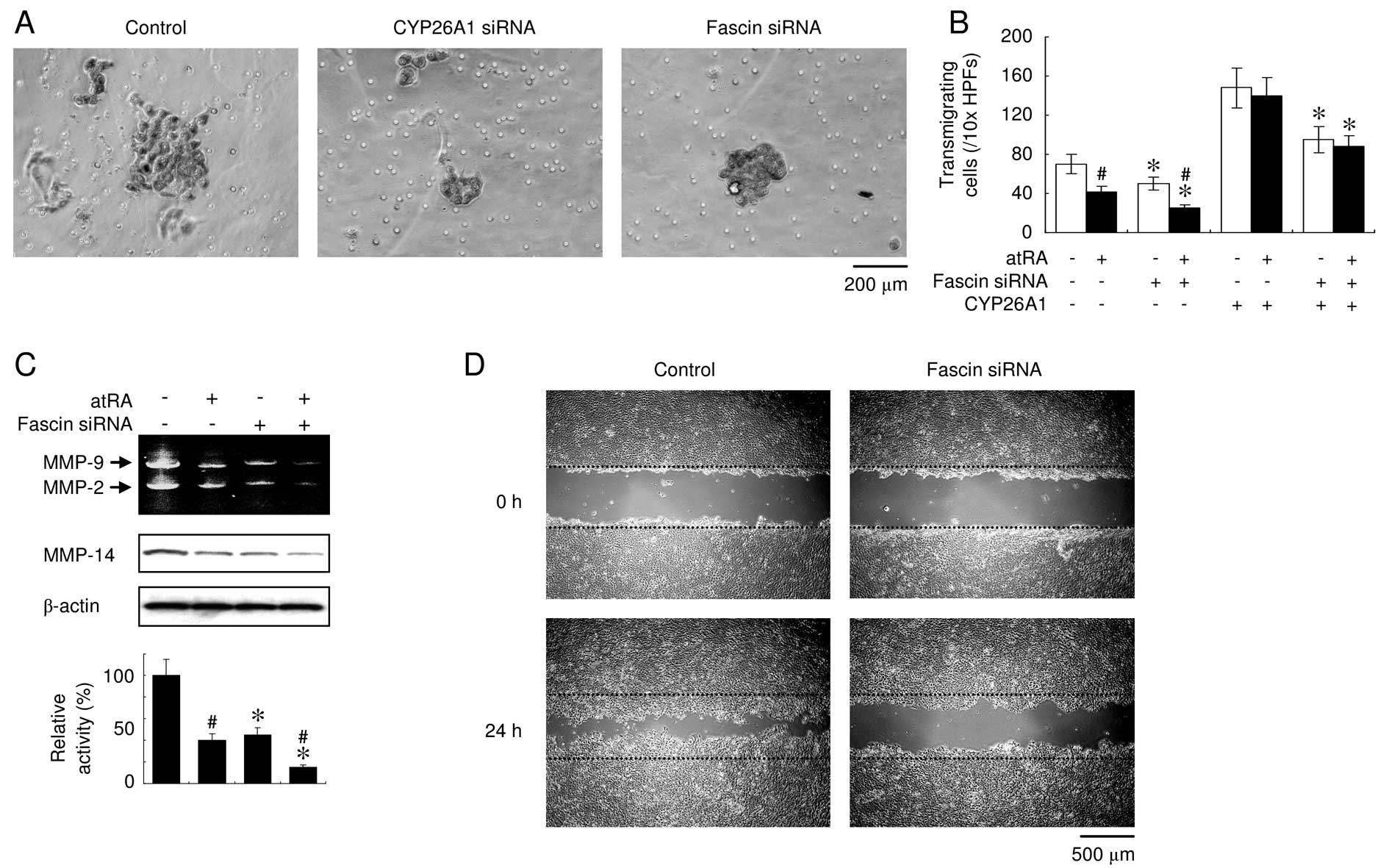
To determine whether fascin modulated cell invasion,
an invasion assay with Matrigel™ was employed using MDA-MB-231
cells, since the MCF-7 cells were not sufficiently invasive.
Suppression of the constitutive expression of CYP26A1 in the
MDA-MB-231 cells significantly reversed the CYP26A1- mediated
effects, including invasiveness (Fig.
6A). When fascin was knocked down using siRNA, a significantly
decreased invasion was observed and treatment with atRA potentiated
these effects (Fig. 6A and B).
Fascin suppression partially, but significantly decreased the
invasiveness of the CYP26A1- overexpressing cells, whereas atRA had
no effect. Gelatin zymography was then performed to assess MMP-2
and -9. The results of the assay revealed decreases in the
activities of the two MMPs when fascin was downregulated (Fig. 6C). In addition, fascin suppression
resulted in the decreased expression of MMP-14, which is known to
be a pro-invasive marker, and enhanced the atRA-mediated
downregulation of MMP-14. The monolayer wound-healing assay clearly
demonstrated that decreased fascin expression significantly
inhibited cell migration (Fig. 6D).
These observations indicated that fascin suppression inhibited the
cell invasion ability and motility of the CYP26A1-expressing cells
and suggest that atRA has a synergistic impact on fascin via its
suppressive effect.
Discussion
The expression levels of fascin increased
significantly in response to the constitutive overexpression of
CYP26A1 and conversely treatment with atRA downregulated the
expression of fascin, suggesting that fascin expression is
modulated by the intracellular RA status, regulated by the
expression of CYP26A1. In addition, primary breast carcinomas,
particularly hormone receptor-negative carcinomas and
CYP26A1-overexpressing cancers, expressed elevated levels of
fascin. Since tumors expressing ER and PR generally exhibit a less
aggressive phenotype and are associated with an increased
disease-free and overall survival, these data support a potential
correlation between fascin overexpression and aggressive tumor
behavior, and provide evidence of a correlation between CYP26A1 and
fascin. Our observations also showed that siRNA-mediated
suppression of fascin inhibited the malignant behavior of
CYP26A1-expressing breast carcinoma cells. CYP26A1 exerts oncogenic
functions during breast carcinogenesis; therefore, CYP26A1-mediated
oncogenic effects may be at least partially responsible for the
elevated expression of fascin. Nevertheless, it is difficult to
conclude that fascin directly mediates the effect of CYP26A1. Given
that suppressing fascin expression had significant, but partial
effects on the CYP26A1-overexpressing cells, the possibility that
the CYP26A1-mediated oncogenic effects are governed by a regulatory
mechanism that is independent of fascin cannot be excluded.
The present study did not identify how intracellular
RA modulates the expression of fascin. Of note, the bioinformatics
analyses did not identify a putative RA response element in the
fascin promoter, suggesting that atRA does not regulate fascin
expression directly. One possible explanation for this is that atRA
alters the activity of other trans-acting nuclear
transcription factors and/or co-activators. The fascin promoter
contains a number of binding motifs including those for p300 and
CREB-binding protein (CBP). Previous findings have demonstrated
that the atRA-mediated differentiation of F9 embryonic carcinoma
cells was inhibited by a ribozyme directed against p300 mRNA
(18). In addition, fascin
expression was significantly decreased in CBP-depleted NT2 neuronal
cells (19). However, direct
comparisons among cell lines are challenging since various cell
types may exhibit unique activation pathways that may be mutated or
dysregulated. These changes may provide erroneous data with regard
to the sensitivity of cells to atRA. On the other hand, the present
study has clearly demonstrated that the CYP26A1-mediated increase
in fascin expression occurs at the transcriptional level.
Nevertheless, this does not exclude the possibility that fascin is
also regulated by post-transcriptional mechanisms, although the
post-transcriptional regulation of fascin has not been
reported.
CYP26A1- and fascin-mediated cell events may be
mediated via the MAPK signaling pathway (10). Such crosstalk between CYP26A1 and
fascin signaling suggests that CYP26A1 indirectly regulates fascin
expression. Given the complexity of the cell signaling events that
regulate epithelial cell apoptosis, proliferation and
differentiation, it is likely that CYP26A1 and fascin modulate a
variety of signal transduction molecules that regulate tumor
pathology. This is partially supported by the CYP26A1
overexpression-mediated changes in gene-expression, suggesting that
a number of genes that favor cell survival are modulated by
CYP26A1.
Non-cytotoxic cell stress induces premature
senescence, which is characterized by the upregulation of negative
cell cycle regulators (20). The
present findings have shown that fascin suppression induced
premature senescence, which was accompanied by the upregulation of
p16INK4A, p21Waf1/Cip1 and
p27Kip1. Although CDK inhibitors are the key mediators
of growth arrest during premature senescence, whether the
upregulation of CDK inhibitors directly contributes to the
senescence-associated phenotype in fascin-specific siRNA-expressing
cells remains unclear. However, it is possible that the
downregulation of fascin provokes premature senescence via the
enhanced expression of CDK inhibitors. By contrast, no changes in
p53 were detected during premature senescence, suggesting that the
senescence associated with fascin signaling was mediated by
p53-independent mechanisms and/or mutant p53. The functional
requirements for CDK inhibitors in the senescent state appear to be
heterogeneous under each experimental condition (20). For example, a p53-independent
senescence mechanism was suggested to be responsible for
hypermitogenic proto-oncogenic ERBB2 signaling in breast
carcinoma cells (21). Another
study reported SA-β-gal positivity in clinical breast cancer cells,
suggesting that chemotherapy-induced DNA damage induced senescence
in vivo and that SA-β-gal staining was associated with low
levels of p53 (22).
The present study provides evidence that fascin
signaling may be associated with premature senescence and
apoptosis. It is well accepted that senescence involves aspects of
the apoptotic machinery, and is an antioncogenic fail-safe
mechanism that may function as a natural brake to tumor development
(23). Previously, we suggested
that strategies to decrease CYP26A1 activity may be a significant
method to increase sensitivity of cancer cells to pro-apoptotic
agents (10). By contrast, a number
of reports have successfully demonstrated that DNA-damaging agents
induce cell senescence, even in cells with reduced apoptotic
responsiveness (24). Therefore,
the induction of fascin signaling-associated cell senescence may
have potential as an anticancer mechanism to inhibit oncogenic
activity by inducing cancer cells to exit uncontrolled
proliferation. Given the similarities between the apoptotic and
senescence machineries, the CYP26A1-fascin axis may be an
appropriate therapeutic target for breast cancer.
Acknowledgments
The present study was supported in part by grants
from the Grants-in-Aid for Scientific Research from the Japan
Society for the Promotion of Science and the Smoking Research
Foundation of Japan.
References
|
1
|
Adams JC: Roles of fascin in cell adhesion
and motility. Curr Opin Cell Biol. 16:590–596. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kureishy N, Sapountzi V, Prag S, Anilkumar
N and Adams JC: Fascins, and their roles in cell structure and
function. Bioessays. 24:350–361. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Edwards RA and Bryan J: Fascins, a family
of actin bundling proteins. Cell Motil Cytoskeleton. 32:1–9. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Grothey A, Hashizume R, Ji H, Tubb BE,
Patrick CW Jr, Yu D, Mooney EE and McCrea PD: C-erbB-2/HER-2
upregulates fascin, an actin-bundling protein associated with cell
motility, in human breast cancer cell lines. Oncogene.
19:4864–4875. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Grothey A, Hashizume R, Sahin AA and
McCrea PD: Fascin, an actin-bundling protein associated with cell
motility, is upregulated in hormone receptor negative breast
cancer. Br J Cancer. 83:870–873. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jawhari AU, Buda A, Jenkins M, Shehzad K,
Sarraf C, Noda M, Farthing MJ, Pignatelli M and Adams JC: Fascin,
an actin-bundling protein, modulates colonic epithelial cell
invasiveness and differentiation in vitro. Am J Pathol. 162:69–80.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hashimoto Y, Ito T, Inoue H, Okumura T,
Tanaka E, Tsunoda S, Higashiyama M, Watanabe G, Imamura M and
Shimada Y: Prognostic significance of fascin overexpression in
human esophageal squamous cell carcinoma. Clin Cancer Res.
11:2597–2605. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hwang JH, Smith CA, Salhia B and Rutka JT:
The role of fascin in the migration and invasiveness of malignant
glioma cells. Neoplasia. 10:149–159. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hayashi Y, Osanai M and Lee GH: Fascin-1
expression correlates with repression of E-cadherin expression in
hepatocellular carcinoma cells and augments their invasiveness in
combination with matrix metalloproteinases. Cancer Sci.
102:1228–1235. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Osanai M, Sawada N and Lee GH: Oncogenic
and cell survival properties of the retinoic acid metabolizing
enzyme, CYP26A1. Oncogene. 29:1135–1144. 2010. View Article : Google Scholar
|
|
11
|
Chang CL, Hong E, Lao-Sirieix P and
Fitzgerald RC: A novel role for the retinoic acid-catabolizing
enzyme CYP26A1 in Barrett’s associated adenocarcinoma. Oncogene.
27:2951–2960. 2008. View Article : Google Scholar
|
|
12
|
Shelton DN, Sandoval IT, Eisinger A,
Chidester S, Ratnayake A, Ireland CM and Jones DA: Up-regulation of
CYP26A1 in adenomatous polyposis coli-deficient vertebrates via a
WNT-dependent mechanism: Implications for intestinal cell
differentiation and colon tumor development. Cancer Res.
66:7571–7577. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Brown GT, Cash BG, Blihoghe D, Johansson
P, Alnabulsi A and Murray GI: The expression and prognostic
significance of retinoic acid metabolising enzymes in colorectal
cancer. PLoS One. 9:e907762014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Elliott BE, Maxwell L, Arnold M, Wei WZ
and Miller FR: Expression of epithelial-like markers and class I
major histo-compatibility antigens by a murine carcinoma growing in
the mammary gland and in metastases: Orthotopic site effects.
Cancer Res. 48:7237–7245. 1988.PubMed/NCBI
|
|
15
|
Dimri GP, Lee X, Basile G, Acosta M, Scott
G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O,
et al: A biomarker that identifies senescent human cells in culture
and in aging skin in vivo. Proc Natl Acad Sci USA. 92:9363–9367.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Murray GI, Patimalla S, Stewart KN, Miller
ID and Heys SD: Profiling the expression of cytochrome P450 in
breast cancer. Histopathology. 57:202–211. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ben-Porath I and Weinberg RA: When cells
get stressed: An integrative view of cellular senescence. J Clin
Invest. 113:8–13. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kawasaki H, Eckner R, Yao TP, Taira K,
Chiu R, Livingston DM and Yokoyama KK: Distinct roles of the
co-activators p300 and CBP in retinoic-acid-induced F9-cell
differentiation. Nature. 393:284–289. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Megiorni F, Indovina P, Mora B and
Mazzilli MC: Minor expression of fascin-1 gene (FSCN1) in NTera2
cells depleted of CREB-binding protein. Neurosci Lett. 381:169–174.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
McConnell BB, Starborg M, Brookes S and
Peters G: Inhibitors of cyclin-dependent kinases induce features of
replicative senescence in early passage human diploid fibroblasts.
Curr Biol. 8:351–354. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Trost TM, Lausch EU, Fees SA, Schmitt S,
Enklaar T, Reutzel D, Brixel LR, Schmidtke P, Maringer M, Schiffer
IB, et al: Premature senescence is a primary fail-safe mechanism of
ERBB2-driven tumorigenesis in breast carcinoma cells. Cancer Res.
65:840–849. 2005.PubMed/NCBI
|
|
22
|
te Poele RH, Okorokov AL, Jardine L,
Cummings J and Joel SP: DNA damage is able to induce senescence in
tumor cells in vitro and in vivo. Cancer Res. 62:1876–1883.
2002.PubMed/NCBI
|
|
23
|
Sherr CJ and DePinho RA: Cellular
senescence: Mitotic clock or culture shock? Cell. 102:407–410.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chang BD, Broude EV, Dokmanovic M, Zhu H,
Ruth A, Xuan Y, Kandel ES, Lausch E, Christov K and Roninson IB: A
senescence-like phenotype distinguishes tumor cells that undergo
terminal proliferation arrest after exposure to anticancer agents.
Cancer Res. 59:3761–3767. 1999.PubMed/NCBI
|