Introduction
Chemoresistance is a major cause of cancer treatment
failure. The mechanisms involved in the development of
chemoresistance are complex and not fully understood. Since
conventional chemotherapies target proliferating cells and require
active cycling for induction of apoptosis, it has been proposed
that cells in the quiescent state within tumors are associated with
resistance and cell survival in chemotherapies (1). Moreover, the quiescent nature of cells
is known to be characteristic of cancer stem cells, which have the
ability to self-renew and differentiate to produce heterogeneous
tumor cell lineages (1–3). Increasing evidence indicates that
cancer stem cells are responsible for treatment failure and tumor
recurrence because their quiescent nature is likely to contribute
to the survival in response to chemotherapy (4). Thus, selectively targeting quiescent
cell population including cancer stem cells offers possible way
forward to overcome chemoresistance and improve the clinical
outcomes of cancer patients.
The sphere culture has been proposed to propagate
cells with stem cell properties and has been widely adopted to
study stem cell biology (5–8). It is a relatively easy, rapid and
non-animal-depending model to assess stem cell activity, but the
application of sphere-forming assays for high-throughput screening
is limited due to formation of variable sizes of spheres and lack
of a simple and well-established analytical tool. Moreover, cell
aggregation in spheres can cause misinterpretation. Previously, we
showed that sphere cultures exhibit higher proportions of quiescent
cells and we optimized tumor-sphere cultures for the in
vitro screening of chemotherapeutics against the quiescent cell
population (9).
In this study, we utilized tumorsphere cultures to
identify better ways of eradicating quiescent tumor cell population
in MDA-MB-231 human breast cancer cells. MDA-MB-231 cells are
representative of triple-negative breast tumors, which are
characterized by the absence of estrogen receptor (ER),
progesterone receptor (PR) and human epidermal growth factor
receptor 2 (HER2) (10). This
triple negative tumor subtype is mainly correlated to poor
outcomes, showing the worst overall and disease-free survival rates
due to a lack of effective targeted therapies (11,12).
Thus, cytotoxic chemo-therapies such as doxorubicin/paclitaxel
remain the mainstay of treatment for triple-negative breast cancer,
but resistance is common and can develop rapidly (13). To seek better strategies to overcome
chemoresistance, we analyzed the efficacies of chemotherapeutics,
doxorubicin and paclitaxel, on MDA-MB-231 tumorspheres. Since we
found that the enhanced epidermal growth factor receptor (EGFR)
signaling pathway is characteristic of MDA-MB-231 tumorspheres, we
assessed the combination effects of doxorubicin and lapatinib (a
dual ErbB1/ErbB2 inhibitor) in tumorsphere assays.
Materials and methods
Adherent cell culture
The MDA-MB-231 human breast cancer cell line was
purchased from the Korean Cell Line Bank (Seoul, Korea) and
routinely maintained in DMEM (Welgene, Daegu, Korea) supplemented
with 10% fetal bovine serum (Invitrogen, Carlsbad, CA, USA) and 1%
antibiotic-antimycotic solution (Welgene).
Suspension sphere cultures
The protocol used for tumorsphere culture was as
previously described (5,6,8,14).
Briefly, cells were suspended in serum-free DMEM/F12 (Welgene)
supplemented with 1:50 B27 (Gibco BRL, Grand Island, NY, USA), 10
μg/ml insulin (Welgene), 20 ng/ml recombinant human
epidermal growth factor (EGF; R&D Systems, Minneapolis, MN,
USA), 10 ng/ml recombinant human fibroblast growth factor (FGF;
R&D Systems), and 1% antibiotic-antimycotic solution (Welgene)
and cultured in non-adherent plates.
Cell kinetic assay
To examine cell proliferation rates, MDA-MB-231
cells were plated at different concentrations (3,000–20,000
cells/well) into 96-well plates under non-adherent (see above), or
monolayer culture conditions. After 4 days, premixed cell
proliferation reagent WST-8 (Dojindo Laboratories, Kumamoto, Japan)
was added to each well and the absorbance of the water-soluble
formazan produced by viable cells was measured at 450 nm according
to the manufacturer’s instructions.
Cytotoxicity assay
To compare the chemo-sensitivity of cells in the TS
and 2D culture systems, several chemotherapeutics including
doxorubicin (Sigma, St. Louis, MO, USA), paclitaxel (Sigma),
lapatinib (a dual ErbB1/ErbB2 inhibitor; LC Laboratories, Woburn,
MA, USA), U0126 (a MEK inhibitor; LC Laboratories), or LY294002 (a
PI3K/AKT inhibitor; LC Laboratories) were added into cells grown in
either adherent or non-adherent 96-well plates and cell viabilities
were measured 3 days later. To examine the combinational effects of
doxorubicin and lapatinib, cells were treated with doxorubicin in a
range of 0.2–1 µM in the presence of 5 µM lapatinib.
Flow cytometry analysis: cell cycle
analysis and doxorubicin uptake
For cell cycle analysis, cells grown in 2D or TS
culture for 4 days were trypsinized after washing with PBS,
centrifuged at 1,000 rpm for 3 min and fixed in cold 70% ethanol.
After centrifugation, the cells were washed with PBS containing 2%
FBS and stained in the dark with 20 µg/ml propidium iodide (Sigma)
and 200 µg/ml RNase A (Sigma) for 30 min at room temperature. The
cells were analyzed by FACS Calibur II flow cytometry (Becton
Dickinson Biosciences, San Jose, CA, USA). To measure intracellular
doxorubicin accumulations in cells grown in 2D or tumorsphere
cultures, cells were seeded into adherent or non-adherent 6-well
plates for 3 days. Cells were treated with 0.5 µM doxorubicin for
30 min, trypsinized, and washed twice with PBS containing 2% FBS.
After resuspending in PBS containing 2% FBS, the cells were
analyzed by FACSCalibur II flow cytometry (Becton Dickinson
Biosciences).
RNA extraction, RT-PCR and quantitative
real-time PCR
Cells cultured under 2D and TS conditions were
treated with 0.3 µM doxorubicin, 5 µM LY294002 or 10 µM SB203580 (a
p38 inhibitor; LC Laboratories) for 3 days, and harvested for RNA
isolation. Total RNA was extracted using the easy-BLUE™ Total RNA
Extraction kit (iNtRON Biotechnology Inc., Sungnam, Korea) and cDNA
was synthesized with reverse transcriptase (Takara, Shiga, Japan).
RT-PCR for cyclin D1, MDR-1, and GAPDH were conducted as previously
described (15). Densitometric
analysis was performed using Scion Image software (Scion Co.,
Frederick, MD, USA). The utilized primer sequences for the RT-PCR
reactions were as follows: Cyclin D1 (forward)
5′-AGCTCCTGTGCTGCGAAGT GGAAAC-3′ and Cyclin D1 (reverse)
5′-AGTGTTCAAT GAAATCGTGCGGGG-3′; MDR-1 (forward) 5′-GCC
TGGCAGCTGGAAGACAAATACACAAAATT-3′ and MDR-1 (reverse)
5′-CAGACAGCAGCTGACAGTCCAA GAACAGGACT-3′; GAPDH (forward)
5′-ATCCCATCAC CATCTTCCAG-3′ and GAPDH (reverse) 5′-TTCTAGACG
GCAGGTCAGGT-3′. The real-time PCR reactions for multi-drug
resistance-associated protein-1 (MRP-1) and GAPDH were performed
using QuantiMix SYBR green kit (Philekorea, Daejeon, Korea) in Eco
Real-time PCR (Illumina, San Diego, CA, USA). mRNA expression level
of MRP-1 was calculated after normalizing with GAPDH. The utilized
primer sequences for the real-time PCR reactions were as follows:
MRP-1 (forward) 5′-GCGAGTGTCTCCCTCAAACG-3′ and MPR-1 (reverse)
5′-TCCTCACGGTGATGCTGTTC-3′; GAPDH (forward)
5′-CTGCTCCTCCTGTTCGACAGT-3 and GAPDH (reverse)
5′-CCGTTGACTCCGACCTTCAC-3′.
Western blotting
Cells grown in 2D or TS conditions were lysed with
RIPA buffer (50 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate,
0.1% SDS, 50 mM Tris-HCl, pH 7.5 and 2 mM EDTA). Phosphatase and
protease inhibitor cocktails (GenDepot, Barker, TX, USA) were added
immediately before use. Lysates were cleared of debris at 13,000
rpm for 10 min, and protein concentrations were determined using
bicinchoninic acid reagent (Sigma). Equal amounts of protein were
separated by SDS-PAGE and transferred to polyvinylidene fluoride
(PVDF) membranes, which were then blocked with 5% non-fat skim milk
in 1X TBS-0.1% Tween-20 (TTBS) for 2 h and incubated with a primary
antibody (EGFR, p-EGFR, AKT, p-AKT, ERK 1/2, p-ERK 1/2, p38, p-p38
or GAPDH; Cell Signaling, Beverly, MA, USA) overnight.
HRP-conjugated secondary antirabbit antibody (Santa Cruz
Biotechnology, Santa Cruz, CA, USA) diluted 1:5,000 was incubated
with blots for 1 h at room temperature. Blots were developed using
Luminescent Image Analyzer LAS-4000 (Fujifilm, Tokyo, Japan).
Statistical analysis
Statistical significance was determined using the
Student’s t-test. All experiments were conducted in triplicates,
and results are presented as mean ± SD. P-values of <0.05 were
considered significant.
Results
Quiescence in tumorspheres generated from
MDA-MB-231 cells
First, we cultured MDA-MB-231 breast cancer cells in
non-adherent culture condition for 4 days to test their ability to
form tumorspheres. As previously reported (14), tumorspheres generated from
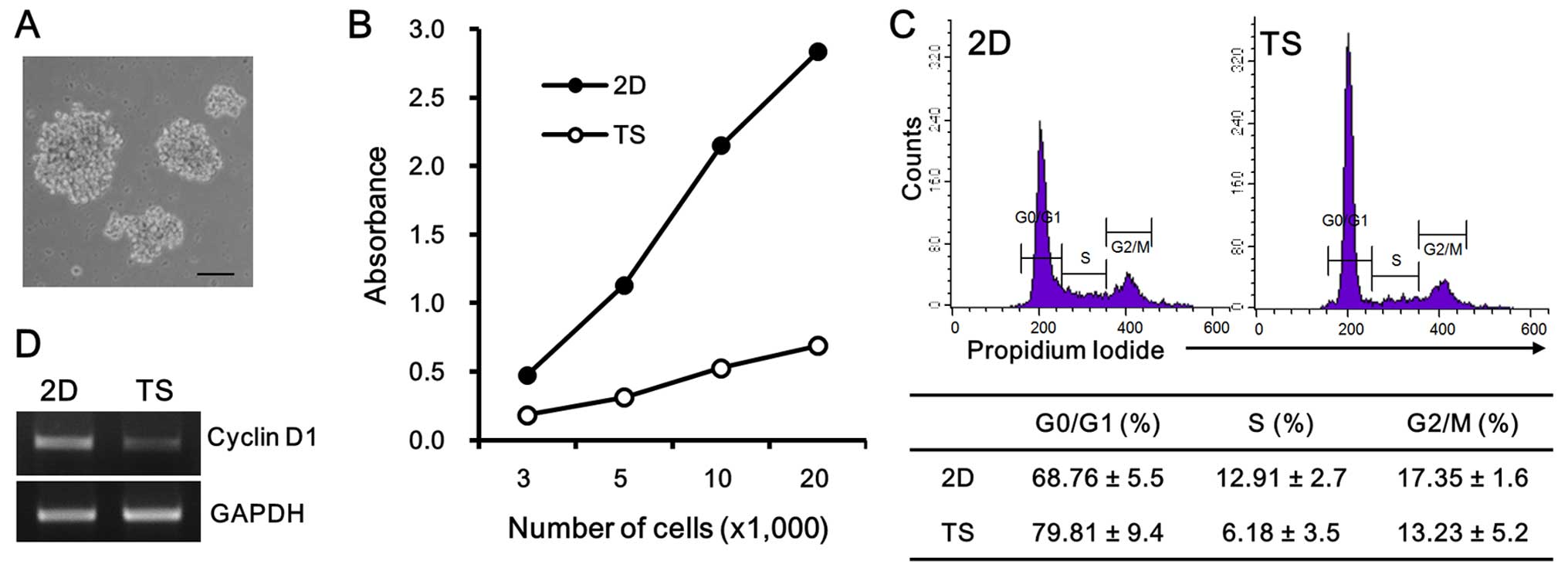
MDA-MB-231 cells exhibited much looser structures (Fig. 1A) than those derived from MCF-7
cells (data not shown). To evaluate the cell growth rates of
tumorspheres and monolayer cultures, cells were plated at different
concentrations (3,000–20,000 cells/well) into 96-well plates, in
either non-adherent plates, or regular tissue culture plates. After
4 days of 2D or TS culture, cell viabilities were assayed by
measuring WST-8 absorbance. The overall WST-8 readings of
suspension cultures were at least three times less than those of 2D
cultures, seeded with the same cell numbers (Fig. 1B). The cell cycle analysis revealed
that this slow cell growth rate in tumorspheres correlated with the
accumulation of cells at the G0/G1 phase, showing that 79.81±9.4%
cell population in tumorspheres was in the G0/G1 phase, whereas
68.76±5.5% of the 2D cultured cell population was in the G0/G1
phase (Fig. 1C). Moreover, the mRNA
expression of cyclin D1, which is the first regulatory protein to
drive the G1/S phase transition, was also decreased in tumorspheres
(Fig. 1D). Taken together, these
results confirmed that the TS culture condition enhanced the
quiescent MDA-MB 231 cell population as compared with the 2D
culture condition.
Chemoresistance of tumorspheres to
doxorubicin and paclitaxel
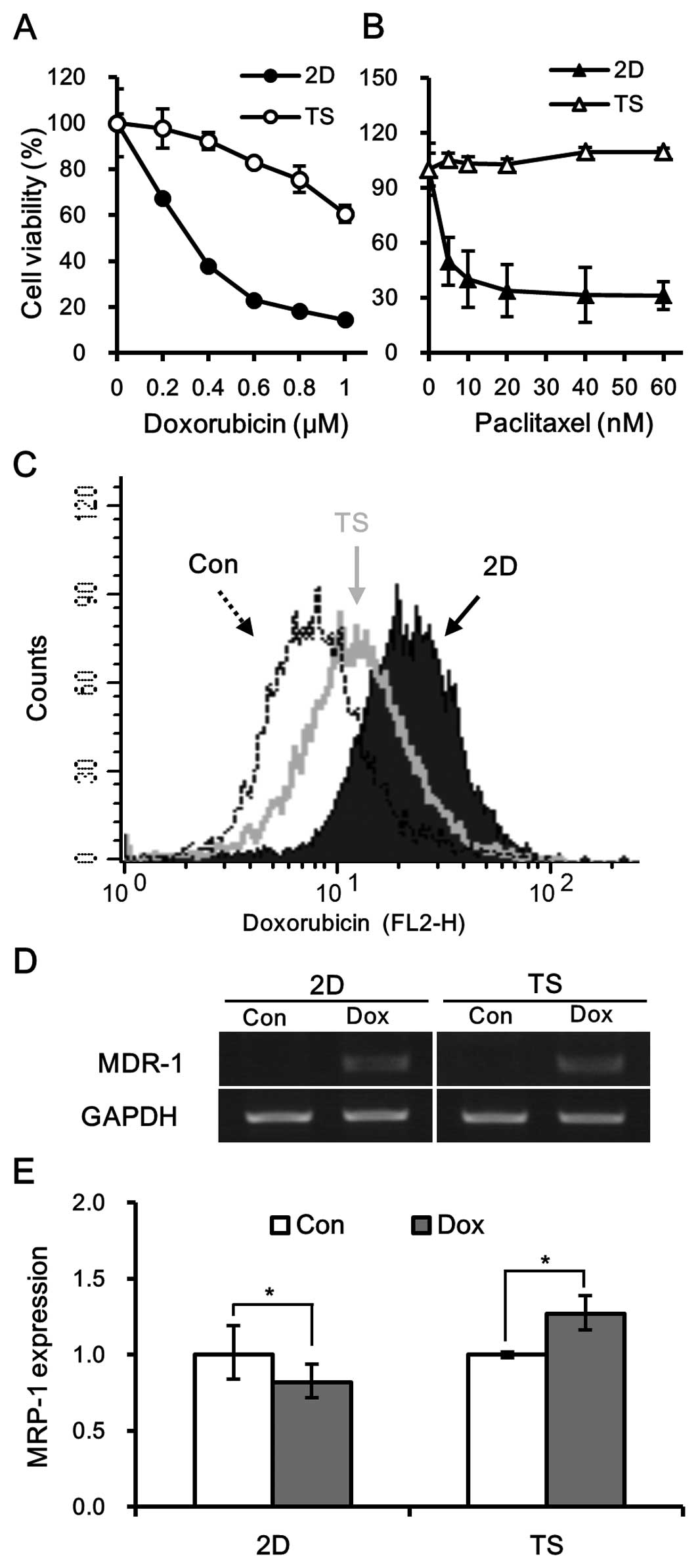
To investigate whether culturing cells as spheres
affects chemo-sensitivity, MDA-MB-231 culture under TS or 2D
conditions were exposed to different concentrations of doxorubicin
(0.2–1 µM) or paclitaxel (5–60 nM) for 3 days and then assessed for
cell viability. It was found that tumorspheres were resistant to
both doxorubicin and paclitaxel (Fig.
2A and B). In fact, the IC50 of doxorubicin for
tumorspheres was at least three fold higher than its
IC50 for 2D cultured cells (Fig. 2A). Similarly, tumorspheres exhibited
significant resistance to paclitaxel with an IC50 value
ten times that of 2D cultured cells (Fig. 2B).
We further analyzed intracellular doxorubicin
accumulation using flow cytometry (16). Cells grown from either monolayer or
tumorsphere cultures were treated with 0.5 µM doxorubicin for 30
min and then doxorubicin fluorescence was analyzed by flow
cytometry. The treatment of 2D cultured cells with doxorubicin for
30 min caused a right-shift of fluorescence intensity of
doxorubicin as compared with untreated MDA-MB-231 cells, confirming
the intracellular accumulation of doxorubicin within cells. On the
other hand, the fluorescence intensity of tumorspheres was lower
than that of monolayer cultured cells (Fig. 2C), implying that the decreased
accumulation of intracellular doxorubicin may contribute to
chemoresistance of tumorspheres.
It has been well established that the intracellular
accumulation of doxorubicin is associated with the expression of
the ATP-binding cassette (ABC) family of drug transporters
(17), and therefore, we examined
the mRNA expression levels of multidrug resistance (MDR-1) and
MRP-1. It was observed that the expression of MDR-1 was similarly
induced by doxorubicin in TS and 2D cultured cells (Fig. 2D). However, mRNA expression of MRP-1
was induced in tumorspheres by doxorubicin but not in monolayer
cultures (Fig. 2E), suggesting that
MRP-1, and not MDR-1, was involved in the chemoresistance
demonstrated by tumorspheres.
Enhanced EGFR signaling in
tumorspheres
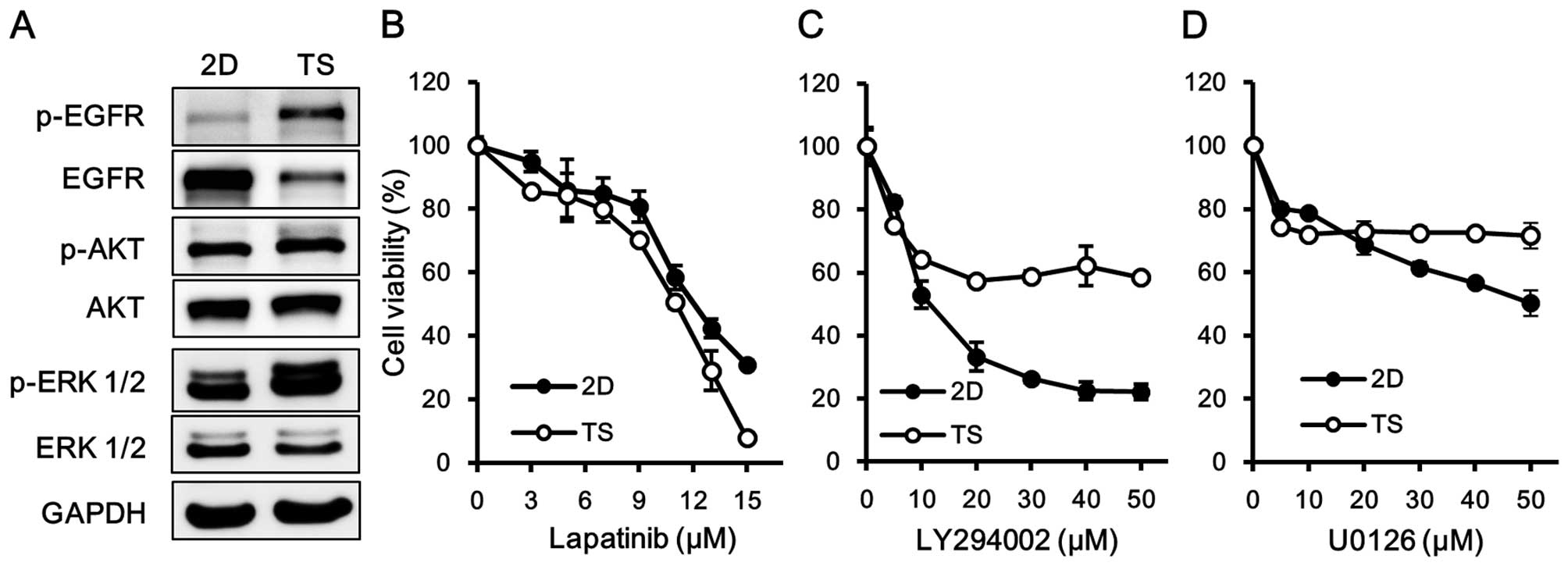
To elucidate the molecular mechanisms responsible
for the chemoresistance of tumorspheres, we first analyzed which
signaling pathways are upregulated in the tumorspheres. Western
blot analysis showed that the EGFR signaling pathway was more
activated in TS than in 2D cultured cells (Fig. 3A). Furthermore, the phosphorylation
of EGFR in tumorspheres was associated with concomitant increases
in the phosphorylations of ERK1/2 and AKT.
We next explored the role of the EGFR signaling
pathway in the formation of tumorspheres. Cells were treated with
lapatinib, U0126, or LY294002 to block the EGFR, MAPK, and PI3K/AKT
signaling pathways, respectively, and then cell viabilities were
assessed. Of note, responses to lapatinib were similar for TS and
2D cultured cells (Fig. 3B),
whereas TS cultured cells were less sensitive to U0126 and to
LY29400 (Fig. 3C and D). These
results suggested that the EGFR signaling pathway plays an
important role in mediating the survival of cells in the quiescent
state rather than pathways downstream of EGFR, such as, the MAPK
and PI3K/AKT pathways.
Lapatinib sensitized tumorspheres to
doxorubicin by inhibiting the expression of MRP-1
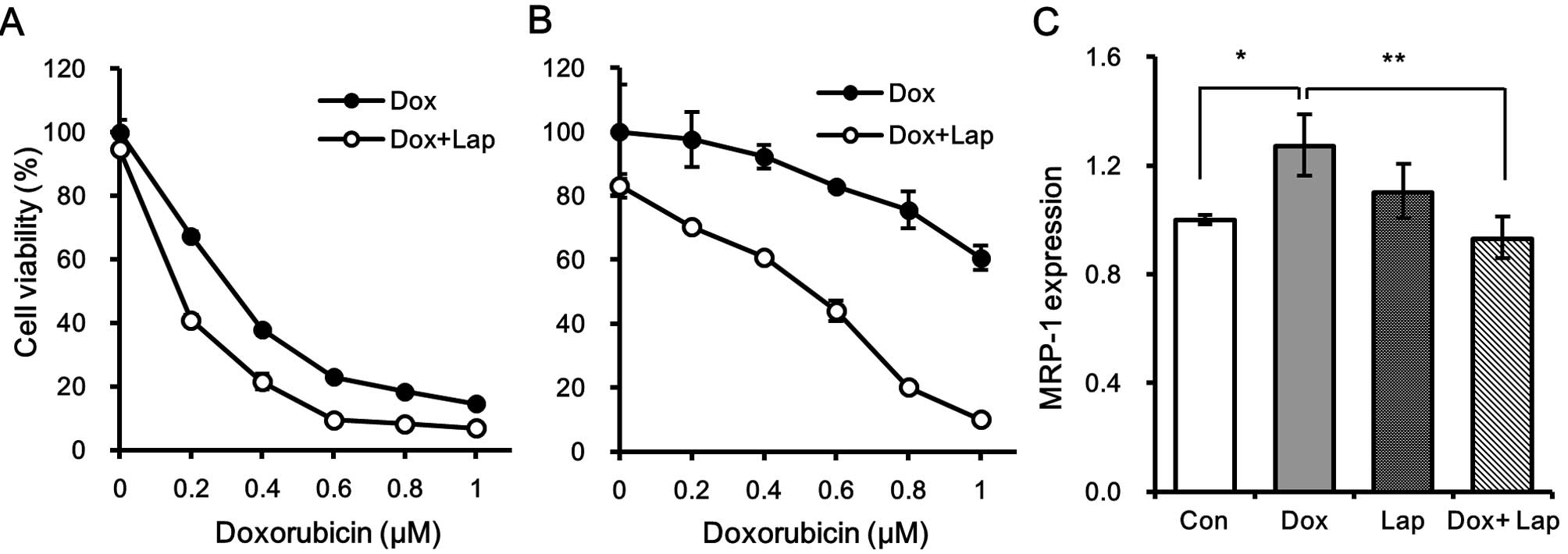
We next examined whether the blockade of EGFR
signaling by lapatinib enhanced cytotoxic effect of doxorubicin on
quiescent MDA-MB-231 cells. Cells were treated with different
concentrations of doxorubicin in the presence of 5 µM lapatinib for
3 days and the cell viability was measured. Noteworthy, the
chemosensitivity of tumorspheres to doxorubicin was more enhanced
by lapatinib than that of 2D cultured cells (Fig. 4A and B). As shown in Fig. 4A, treatment with lapatinib mildly
increased cytotoxicity of doxorubicin in the monolayer culture but
the chemosensitivity of tumorspheres was dramatically increased in
the presence of lapatinib (Fig.
4B). In fact, the 60% cell viability after treatment with 1 µM
of doxorubicin was significantly decreased to <5% when TS
cultured cells were treated with 1 µM doxorubicin in the presence
of 5 µM lapatinib (Fig. 4B).
Since we found that increased MRP-1 expression was
responsible for the chemoresistance of tumorspheres (Fig. 2E), we tested whether treatment with
lapatinib affected the expression of MRP-1 in doxorubicin-treated
tumorspheres. As shown in Fig. 4C,
doxorubicin-induced MRP-1 expression was significantly suppressed
in the presence of lapatinib, suggesting that lapatinib sensitizes
tumorspheres to doxorubicin by inhibiting the expression of
MRP-1.
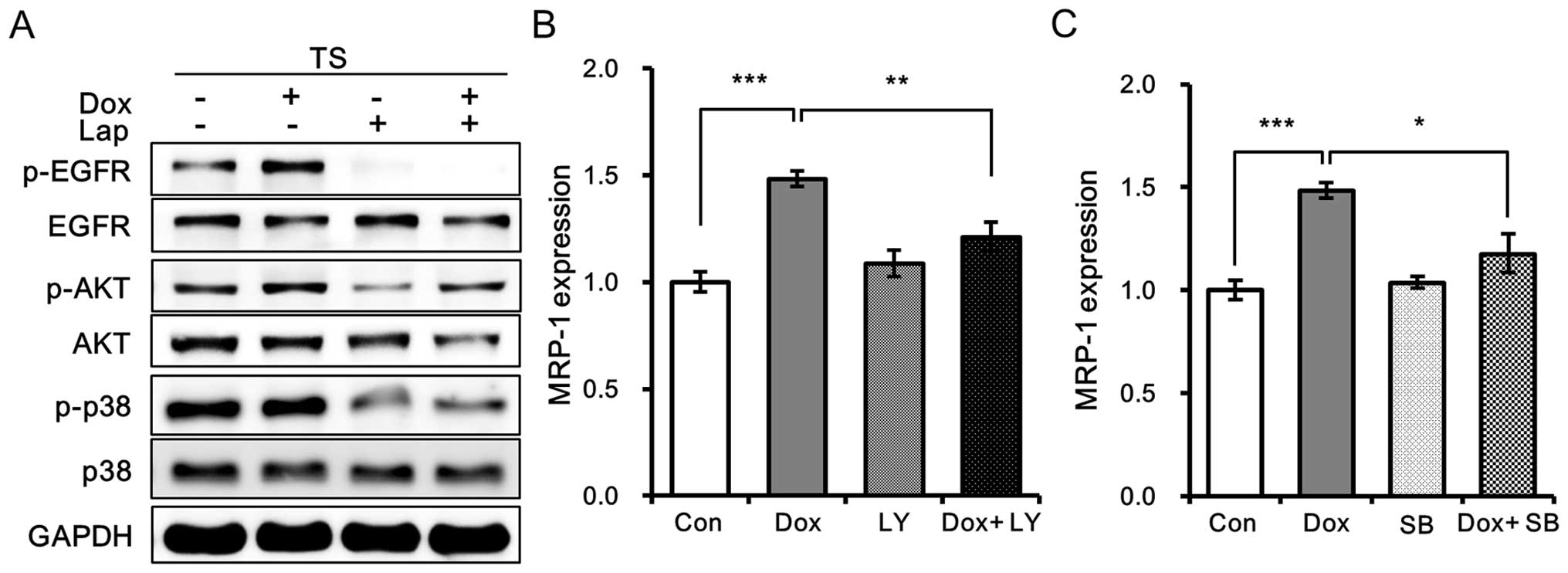
Lapatinib inhibits doxorubicin-induced
MRP-1 expression by inhibiting PI3K/AKT and p38 MAPK signaling
pathways
To obtain more insight into the mechanism underlying
the inhibitory effects of lapatinib against doxorubicin-induced
MRP-1 expression in tumorspheres, we first analyzed the effects of
lapatinib on EGFR and its downstream signaling pathways. Treatment
with lapatinib was found to inhibit the phosphorylation of EGFR and
that of AKT and p38 in doxorubicin-treated tumorspheres (Fig. 5A). Furthermore, blockades of the
PI3K/AKT or p38 MAPK signaling pathways with 5 µM LY294002 or 10 µM
SB203580 remarkably decreased the expression of MRP-1 in
doxorubicin-treated tumorspheres (Fig.
5B and C), indicating the involvement of PI3K/AKT and p38 MAPK
signaling pathways in the expression of MRP-1 in tumorspheres.
These observations suggest that lapatinib inhibits
doxorubicin-induced MRP-1 expression by inhibiting EGFR signaling
and its downstream PI3K/AKT and p38 MAPK signaling pathways.
Discussion
In this study, we utilized tumorsphere cultures to
seek better strategies to overcome chemoresistance based on the
eradication of quiescent cell population in MDA-MB-231 human breast
cancer cells. Tumorsphere culture has been widely adapted to detect
and propagate human breast cancer stem cells in stem cell biology
(5,6,8).
However, several studies reported that the formation of
tumorspheres does not always predict cancer stem cell enrichment
and disagreed on considering it as a suitable in vitro
culturing method for cancer stem cells because the formation of
tumorspheres is influenced by factors such as cell density and
culture duration (14,18,19).
Despite ongoing arguments about the enrichment of cancer stem cells
in tumorspheres, the generation of tumorspheres confers interesting
and unique features such as quiescence (20,21).
Previously, we showed that most cells in tumorsphere cultures are
quiescent, whereas cells in monolayer culture have a high mitotic
index (9). Similar to this study,
the sphere-forming population of hepatoma cells contained a higher
proportion of cells in the G0/G1 phase than the same cells cultured
as monolayers (22). Since the
quiescence is one of the traits in understanding the contribution
of cancer stem cells to chemoresistance, we previously optimized
tumorsphere cultures for in vitro screening methods for
evaluating chemotherapeutics against quiescent cell population
(9).
In the present study, tumorspheres generated from
MDA-MB-231 cells exhibited chemoresistance to both doxorubicin and
paclitaxel. However, we found that the epidermal growth factor
receptor (EGFR) signaling pathway was more activated in TS than in
2D cultured cells and this enhanced activation of EGFR signaling in
tumorspheres mediated survival of cells in quiescent state. EGFR is
a member of the ErbB family of receptors and its activation by
specific ligand binding triggers several signal transduction
cascades, principally the PI3K/AKT and MAPK pathways, leading to
cell proliferation, adhesion, and migration (23,24).
In human tumors, EGFR and the other three members of the EGFR
family, HER2, HER3, and HER4, are often overexpressed or
dysregulated, which promotes tumor growth and/or progression
(23,24). EGFR is frequently overexpressed in
triple-negative breast cancer and is emerging as a therapeutic
target (25). Although the use of
single-agent of tyrosine kinase inhibitors targeting EGFR in
triple-negative breast cancer patients have produced the
disappointing results (26,27), several studies have reported that
cytotoxic chemotherapy in combination with EGFR inhibition has
shown promising results in treatment of breast cancer patients
(28,29). Lapatinib is an orally active small
molecule, which inhibits the tyrosine kinases of EGFR and HER2, and
has been approved by the FDA in combination with other anticancer
agents for the treatment of HER2-positive breast cancers (30). More recent studies reported that
lapatinib enhanced the cytotoxic effect of chemotherapeutics
including paclitaxel, vincristine, and topotecan by inhibiting the
drug efflux function of ABC transporters, such as P-glycoprotein
(P-gp), MRPs, or ABCG2 (BCRP) transporters (31–33).
ABC transporters have been linked to the development
of resistance to anticancer drugs as they are involved in the
ATP-dependent efflux of xenobiotics or chemotherapeutics from cells
and tissues (34,35). Consistent with other studies, our
results also show that blockade of the EGFR signaling pathway by
lapatinib significantly increased the anticancer activities of
doxorubicin on quiescent MDA-MB-231 cells by inhibiting the
expression of MRP-1. However, the inhibition of MRP-1 expression
may be insufficient to increase the anticancer activity of
doxorubicin, because we observed that, unlike lapatinib, the
blockade of the PI3K/AKT or p38 MAPK signaling pathways with
LY294002 or SB203580, respectively, did not increase the cytotoxic
effect of doxorubicin on tumorspheres although they were able to
suppress the expression of MRP-1 in tumorspheres. These
observations suggest that the synergistic effects of lapatinib and
doxorubicin may arise from the inhibition of MRP-1 expression and
the inhibition of EGFR-mediated survival signaling pathways.
In summary, we propose that although EGFR inhibition
alone does not represent an effective therapeutic approach to
triple-negative breast cancer, treatment with lapatinib in
combination with cytotoxic chemotherapy may provide a useful
approach to improve clinical responses by eradicating the quiescent
cell population.
Abbreviations:
|
TS
|
tumorsphere
|
|
2D
|
two dimensional
|
|
Lap
|
lapatinib
|
|
Dox
|
doxorubicin
|
|
ABC
|
ATP-binding cassette
|
|
MRP-1
|
multidrug resistance-associated
protein-1
|
|
MDR-1
|
multidrug resistance protein-1
|
|
BCRP
|
breast cancer resistance protein
|
|
HER2
|
human epidermal growth factor receptor
2
|
|
ER
|
estrogen receptor
|
|
PR
|
progesterone receptor
|
|
EGFR
|
epidermal growth factor receptor
|
|
PI3K
|
phosphoinositol-3 kinase
|
|
MAPK
|
mitogen-activated protein kinase
|
|
AKT
|
protein kinase B
|
|
ERK
|
extracellular signal-regulated
kinase
|
|
GAPDH
|
glyceraldehyde-3-phosphate
dehydrogenase
|
Acknowledgments
This study was supported by a grant from the
National R&D Program for Cancer Control, Ministry of Health
& Welfare, Republic of Korea (no. 1320060).
References
|
1
|
Moore N and Lyle S: Quiescent,
slow-cycling stem cell populations in cancer: A review of the
evidence and discussion of significance. J Oncol.
pii:3960762011.
|
|
2
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tirino V, Desiderio V, Paino F, De Rosa A,
Papaccio F, La Noce M, Laino L, De Francesco F and Papaccio G:
Cancer stem cells in solid tumors: An overview and new approaches
for their isolation and characterization. FASEB J. 27:13–24. 2013.
View Article : Google Scholar
|
|
4
|
Li L and Bhatia R: Stem cell quiescence.
Clin Cancer Res. 17:4936–4941. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dontu G, Abdallah WM, Foley JM, Jackson
KW, Clarke MF, Kawamura MJ and Wicha MS: In vitro propagation and
transcriptional profiling of human mammary stem/progenitor cells.
Genes Dev. 17:1253–1270. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Farnie G, Clarke RB, Spence K, Pinnock N,
Brennan K, Anderson NG and Bundred NJ: Novel cell culture technique
for primary ductal carcinoma in situ: Role of Notch and epidermal
growth factor receptor signaling pathways. J Natl Cancer Inst.
99:616–627. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Grimshaw MJ, Cooper L, Papazisis K,
Coleman JA, Bohnenkamp HR, Chiapero-Stanke L, Taylor-Papadimitriou
J and Burchell JM: Mammosphere culture of metastatic breast cancer
cells enriches for tumorigenic breast cancer cells. Breast Cancer
Res. 10:R522008. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ponti D, Costa A, Zaffaroni N, Pratesi G,
Petrangolini G, Coradini D, Pilotti S, Pierotti MA and Daidone MG:
Isolation and in vitro propagation of tumorigenic breast cancer
cells with stem/progenitor cell properties. Cancer Res.
65:5506–5511. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim S and Alexander CM: Tumorsphere assay
provides more accurate prediction of in vivo responses to
chemotherapeutics. Biotechnol Lett. 36:481–488. 2014. View Article : Google Scholar :
|
|
10
|
Reis-Filho JS and Tutt AN: Triple negative
tumours: A critical review. Histopathology. 52:108–118. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lund MJ, Trivers KF, Porter PL, Coates RJ,
Leyland-Jones B, Brawley OW, Flagg EW, O’Regan RM, Gabram SG and
Eley JW: Race and triple negative threats to breast cancer
survival: A population-based study in Atlanta, GA. Breast Cancer
Res Treat. 113:357–370. 2009. View Article : Google Scholar
|
|
12
|
Sorlie T, Tibshirani R, Parker J, Hastie
T, Marron JS, Nobel A, Deng S, Johnsen H, Pesich R, Geisler S, et
al: Repeated observation of breast tumor subtypes in independent
gene expression data sets. Proc Natl Acad Sci USA. 100:8418–8423.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Isakoff SJ: Triple-negative breast cancer:
Role of specific chemotherapy agents. Cancer J. 16:53–61. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shaw FL, Harrison H, Spence K, Ablett MP,
Simões BM, Farnie G and Clarke RB: A detailed mammosphere assay
protocol for the quantification of breast stem cell activity. J
Mammary Gland Biol Neoplasia. 17:111–117. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim S, Chun SY, Lee DH, Lee KS and Nam KS:
Mineral-enriched deep-sea water inhibits the metastatic potential
of human breast cancer cell lines. Int J Oncol. 43:1691–1700.
2013.PubMed/NCBI
|
|
16
|
Karukstis KK, Thompson EH, Whiles JA and
Rosenfeld RJ: Deciphering the fluorescence signature of daunomycin
and doxorubicin. Biophys Chem. 73:249–263. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shen F, Chu S, Bence AK, Bailey B, Xue X,
Erickson PA, Montrose MH, Beck WT and Erickson LC: Quantitation of
doxorubicin uptake, efflux, and modulation of multidrug resistance
(MDR) in MDR human cancer cells. J Pharmacol Exp Ther. 324:95–102.
2008. View Article : Google Scholar
|
|
18
|
Calvet CY, André FM and Mir LM: The
culture of cancer cell lines as tumorspheres does not
systematically result in cancer stem cell enrichment. PLoS One.
9:e896442014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pastrana E, Silva-Vargas V and Doetsch F:
Eyes wide open: A critical review of sphere-formation as an assay
for stem cells. Cell Stem Cell. 8:486–498. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guttilla IK, Phoenix KN, Hong X, Tirnauer
JS, Claffey KP and White BA: Prolonged mammosphere culture of MCF-7
cells induces an EMT and repression of the estrogen receptor by
microRNAs. Breast Cancer Res Treat. 132:75–85. 2012. View Article : Google Scholar
|
|
21
|
Manuel Iglesias J, Beloqui I,
Garcia-Garcia F, Leis O, Vazquez-Martin A, Eguiara A, Cufi S, Pavon
A, Menendez JA, Dopazo J, et al: Mammosphere formation in breast
carcinoma cell lines depends upon expression of E-cadherin. PLoS
One. 8:e772812013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Uchida Y, Tanaka S, Aihara A, Adikrisna R,
Yoshitake K, Matsumura S, Mitsunori Y, Murakata A, Noguchi N, Irie
T, et al: Analogy between sphere forming ability and stemness of
human hepatoma cells. Oncol Rep. 24:1147–1151. 2010.PubMed/NCBI
|
|
23
|
Grant S, Qiao L and Dent P: Roles of ERBB
family receptor tyrosine kinases, and downstream signaling
pathways, in the control of cell growth and survival. Front Biosci.
7:d376–d389. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Normanno N, De Luca A, Bianco C, Strizzi
L, Mancino M, Maiello MR, Carotenuto A, De Feo G, Caponigro F and
Salomon DS: Epidermal growth factor receptor (EGFR) signaling in
cancer. Gene. 366:2–16. 2006. View Article : Google Scholar
|
|
25
|
Corkery B, Crown J, Clynes M and O’Donovan
N: Epidermal growth factor receptor as a potential therapeutic
target in triple-negative breast cancer. Ann Oncol. 20:862–867.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Baselga J, Albanell J, Ruiz A, Lluch A,
Gascón P, Guillém V, González S, Sauleda S, Marimón I, Tabernero
JM, et al: Phase II and tumor pharmacodynamic study of gefitinib in
patients with advanced breast cancer. J Clin Oncol. 23:5323–5333.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dickler MN, Cobleigh MA, Miller KD, Klein
PM and Winer EP: Efficacy and safety of erlotinib in patients with
locally advanced or metastatic breast cancer. Breast Cancer Res
Treat. 115:115–121. 2009. View Article : Google Scholar
|
|
28
|
Coley HM, Shotton CF, Ajose-Adeogun A,
Modjtahedi H and Thomas H: Receptor tyrosine kinase (RTK)
inhibition is effective in chemosensitising EGFR-expressing drug
resistant human ovarian cancer cell lines when used in combination
with cytotoxic agents. Biochem Pharmacol. 72:941–948. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Molina JR, Kaufmann SH, Reid JM, Rubin SD,
Gálvez-Peralta M, Friedman R, Flatten KS, Koch KM, Gilmer TM,
Mullin RJ, et al: Evaluation of lapatinib and topotecan combination
therapy: Tissue culture, murine xenograft, and phase I clinical
trial data. Clin Cancer Res. 14:7900–7908. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mukherjee A, Dhadda AS, Shehata M and Chan
S: Lapatinib: A tyrosine kinase inhibitor with a clinical role in
breast cancer. Expert Opin Pharmacother. 8:2189–2204. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dai CL, Tiwari AK, Wu CP, Su XD, Wang SR,
Liu DG, Ashby CR Jr, Huang Y, Robey RW, Liang YJ, et al: Lapatinib
(Tykerb, GW572016) reverses multidrug resistance in cancer cells by
inhibiting the activity of ATP-binding cassette subfamily B member
1 and G member 2. Cancer Res. 68:7905–7914. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Perry J, Ghazaly E, Kitromilidou C,
McGrowder EH, Joel S and Powles T: A synergistic interaction
between lapatinib and chemotherapy agents in a panel of cell lines
is due to the inhibition of the efflux pump BCRP. Mol Cancer Ther.
9:3322–3329. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shukla S, Chen ZS and Ambudkar SV:
Tyrosine kinase inhibitors as modulators of ABC
transporter-mediated drug resistance. Drug Resist Updat. 15:70–80.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dean M: ABC transporters, drug resistance,
and cancer stem cells. J Mammary Gland Biol Neoplasia. 14:3–9.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Higgins CF: ABC transporters: From
microorganisms to man. Annu Rev Cell Biol. 8:67–113. 1992.
View Article : Google Scholar : PubMed/NCBI
|