Introduction
Multiple myeloma (MM) is a plasma cell malignancy
and the second most common hematologic malignancy. Although
numerous promising new drugs are currently being tested in MM, the
disease remains incurable because most patients eventually relapse
or become refractory to treatments. A comprehensive knowledge of
the tumor-suppressor genes and signaling pathways should pinpoint
additional molecular targets. Additionally, the development of new
therapeutic agents is required (1–3).
Protocadherin-10 (PCDH10) belongs to the δ2 subgroup
of the protocadherin subfamily (4,5).
Cadherins are important in calcium-dependent homophilic cell-cell
adhesion and are involved in the establishment of cell polarity,
cell-sorting, cell differentiation, proliferation, survival and
migration (6–8). The human PCDH10 gene is located
at 4q28.3 and is involved as a tumor-suppressor gene. The promoter
methylation and down-regulation of PCDH10 gene expression has been
demonstrated in various human cancer types including lymphoma, as
well as gastric, prostate, bladder, colorectal and cervical cancer
(9–12). In previous studies, we found that
PCDH10 is broadly expressed in normal adults, but almost
undetectable in ΜΜ tissues and cell lines due to the promoter
methylation of PCDH10. The ectopic expression of the PCDH10
gene suppressed tumor cell growth, survival, invasion and migration
(13,14). PCDH10 has been closely correlated
with poor prognosis of colorectal, gastric, prostate and bladder
cancer (15–17).
However, little is known regarding the specific
mechanism of PCDH10 which function as an important tumor
suppressor, although it has been reported that the PCDH-X/Y
gene and PCDHGC3, which also belong to the protocadherin families,
are closely connected with the Wnt/β-catenin pathway and influence
the progression of cancers including prostate cancer, Wilms tumor
and colon cancer (18–20). However, the relationship between
PCDH10 and Wnt/β-catenin pathway in MM remains unclear.
The Wnt/β-catenin signaling pathway is known to
promote cell proliferation, survival and invasion through
β-catenin/TCF-mediated transcription in various types of cancer
(21,22). The molecular genetics underlying
Wnt/β-catenin activation in cancer centers on mutations in genes of
the Wnt/β-catenin pathway that enable β-catenin nuclear
translocation and drive oncogenic Wnt transcription. However,
coactivators for β-catenin activation have been identified as an
alternate pathway in MM, which lacks known mutations of the Wnt
pathway genes. Of note, the human BCL-9 gene, which was
first identified by cloning the t(1;14)(q21;q32) translocation from
a patient with B-cell acute lymphoblastic leukemia, has been
identified as a critical coactivator of β-catenin activation in
association with LEF/TCF family members. It has been confirmed that
BCL-9 possesses a potent transcription activation domain, which is
crucial for BCL-9 to promote β-catenin translocation and aberrant
transcription of Wnt target genes, which in turn promotes tumor
cell proliferation, disease progression and drug resistance
(23–25). Those findings emphasize the
importance of this pathway and BCL-9 for identification of
appropriate target drugs.
In the present study, it was found that PCDH10
suppressed MM cell proliferation and cell cycle progression via the
negative modulation of Wnt/β-catenin/BCL-9 signaling. As a result,
we provide a proof-of-concept for the potential translation of
PCDH10 as a novel therapeutic agent to target the oncogenic
Wnt/β-catenin/BCL-9 pathway in MM and other cancer types with
deregulated Wnt activity.
Materials and methods
Construction of the expression
plasmids
The plasmid pcDNA3.1(+)/TP53 was constructed by
subcloning the full-length wild-type copy of the tumor protein 53
gene (TP53) from the plasmid pC53-SN (a gift from Bert
Vogelstein) into the pcDNA3.1(+) vector. pcDNA3.1(+)/PCDH10 was
constructed by subcloning into the same vector the full-length
PCDH10 gene, amplified by PCR from the clone KIAA1400 (a
gift from the Kazusa DNA Research Institute, Japan) using the
AccuPrime Pfx DNA polymerase (Life Technologies, Grand Island, NY,
USA). The plasmid sequences and the orientation of the cloned
fragments were confirmed by sequencing.
Cell cultures and transfection
KM3 and RPMI-8226 MM cell lines were kindly provided
by Dr Jian Hou (The Second Military Medical University, Shanghai,
China). The cell lines were routinely maintained in RPMI-1640
medium supplemented with 10% fetal bovine serum (both from Gibco
BRL, Rockville, MD, USA), in 5% CO2 in humidified air at
37°C. For stable transfection, the cells were plated into 6-well
plates and kept in antibiotic-free medium for 24 h prior to
transfection. The cells were then transfected with the
pcDNA3.1(+)/PCDH10 plasmid or the empty vector (2 µg each)
using Lipofectamine 2000 (Invitrogen-Life Technologies, Carlsbad,
CA, USA) according to the manufacturer’s instructions. After 48 h,
the cells were transferred to new plates selected with G418
(Sigma-Aldrich, St. Louis, MO, USA) (0.4 mg/ml) for 21 days. The
expression of PCDH10 in the resistant cells was confirmed by RT-PCR
and western blot analysis.
Determination of appropriate
concentrations of Licl
To determine the appropriate concentration of Licl,
RPMI-8226 and KM3 cells (106/ml) were cultured in
serum-free medium for 12 h prior to treatment with Licl at doses of
0, 5, 10, 15 and 20 µM/ml. After 48 h, RT-PCR was used to
detect the expression of β-catenin in cells with different
concentrations of Licl and determine the applicable concentration
of Licl.
Semi-quantitative reverse transcription
PCR (RT-PCR) and quantitative RT-PCR (RT-qPCR)
Total RNA was isolated from cells using TRIzol
reagent and reverse transcribed using an RT reagent kit (Takara
Bio, Inc., Shiga, Japan) according to the manufacturer’s
instructions. RT-PCR was performed as described previously to
amplify the mRNA expression level of PCDH10. RT-qPCR was performed
to specify the expression level of the relative target genes
according to the manufacturer’s instructions which was amplified
with SYBR-Green real-time PCR master mix (Takara). Relative
expression level of target genes was normalized according to
β-actin. The primer sequences are shown in Table I.
 | Table IThe relative gene primer
sequences. |
Table I
The relative gene primer
sequences.
| Gene | Forward primer | Reverse primer |
|---|
| PCDH10 | ACTGCTATCAGGTT
GCCTG | GTCTGT CAACTAGAT
AGCTG |
| β-catenin |
TGGTGACAGGGAAGACATCA |
CCATAGTGAAGGCGAACTGC |
| c-Myc |
GAGACAGATCAGCAACAACCGA |
CTGCTTGGACGGACAGGATG |
| Cyclin
D1 |
TTCGTTGCCCTCTGTGCCA |
GAAGCGTGTGAGGCGGTAGTAG |
| BCL-9 |
CCAACTTGCCATCAATGAATAA |
GGCATCTGATTGGAGTGAGAA |
| β-actin |
CCACGAACTACCTTCAACTCC |
GTGATCTCCTTCTGCATCCTGT |
Protein extraction and western blot
analysis
The cells were harvested in lysis buffer
supplemented with protease and phosphatase inhibitors, following
the manufacturer’s instructions. The total protein was extracted
using the M-PER mammalian protein extraction reagent (Pierce,
Rockford, IL, USA). Extraction of nuclear proteins was performed
using BeyoECL Plus nuclear and cytoplasmic protein extraction kits
(Beyotime Institute of Biotechnology, Jiangsu, China). Protein
concentrations were determined by the bicinchoninic acid (BCA)
method using the BCA protein assay reagent kit (Pierce). The
gel-separated proteins (50–80 µg of protein/lane) were then
electrophoretically transferred onto polyvinylidene fluoride (PVDF)
membranes (Bio-Rad Laboratories, Hercules, CA, USA). After blocking
with 5% BSA for 1.5 h, the membranes were incubated overnight at
4°C with the respective primary antibodies including anti-PCDH10
(1:2000), anti-TBP (1:10000), anti-c-Myc (1:10000), anti-BCL-9
(1:1000) (all from Abcam, Cambridge, MA, USA), anti-β-catenin,
anti-GSK3β, anti-p-GSK3β (Tyr216), anti-cyclin D1, anti-AKT and
anti-β-actin (1:1000; Cell Signaling Technology, Danvers, MA, USA).
The secondary horseradish peroxidase-conjugated antibody was then
incubated at room temperature for 1–2 h. The bands were visualized
using enhanced chemiluminescence (ECL; Beyotime Institute of
Biotechnology).
Cell proliferation assay
Stably transfected clones of RPMI-8226 and KM3 cells
expressing PCDH10 were selected and multiplied as previously
described (16). Cell proliferation
was analyzed by using the Cell Counting Assay Kit-8 (CCK-8)
(Sigma-Aldrich) according to the manufacturer’s instructions.
Briefly, the cells were seeded in 96-well plates and incubated in
10% CCK-8 diluted in normal culture media at 37°C for 2 h.
Proliferation rates were determined at 0, 24, 48, 72 and 96 h,
respectively. The absorbance (A) at 450 nm was measured using a
spectrophotometer (Bio-Rad, Richmond, CA, USA). For Wnt treatment,
the cells were pretreated with Licl for 48 h to activate
Wnt/β-catenin signaling (27).
Experiments were performed at least three times with representative
data presented.
Cell cycle analysis
The cells were cultured in RPMI-1640 medium and 10%
FBS with or without Licl. These cells were collected and fixed in
ice-cold 70% ethanol for 5 h. The cell cycle profiles were assayed
using an Elite ESP flow cytometer and data were analyzed with the
Cell Quest software (BD Biosciences, Bedford, MA, USA).
Luciferase assay
The cells were initially cultured in serum-free
medium for 3 h and seeded in 24-well plates at a density of
2×105 cells and transfected with TOPflash or FOPflash
reporter plasmids (Millipore, Temecula, CA, USA) as well as
pRL-SV40 to normalize for transfection efficiency. FOPflash is a
negative control for TOPflash containing mutated TCF-binding sites.
Transfection was achieved by using Lipofectamine 2000
(Invitrogen-Life Technologies) according to the manufacturer’s
instructions. Luciferase samples were assayed after 48 h using a
Dual Luciferase Reporter Assay system (Promega, Madison, WI, USA).
Experiments were performed at least three times in triplicate.
Immunofluorescence assay
RPMI-8226 cells were applied onto ice-cold
microscope slides, fixed with 10% paraformal-dehyde solution at
room temperature for 30 min and washed gently with PBS. The cells
were permeabilized in 1% Triton X-100 and followed by incubation in
10% normal goat serum for 1 h at room temperature for 1 h. After
gently removing the blocking solution, the cells were incubated
with anti-β-cat (1:100) followed by staining with phylloidin dye
Alexa Flour-488 goat anti-rabbit anti-IgG (1:200; Proteintech,
Chicago, USA) for 1.5 h. Nuclear staining with propidium iodide
(PI) for 5 min was performed before the cells were imaged for th
elocalization of β-catenin (28).
Stained slides were viewed under a fluorescence microscope at a
magnification of ×400(Carl Zeiss Micro Imagine, Axio Observer ZI,
Germany).
Statistical analysis
Data were presented as the mean ± standard deviation
(SD) from three independent experiments. Statistical analysis was
conducted using the Student’s t-tests. P<0.05 was considered to
indicate statistically significant differences. Data quantification
and statistical analysis were performed using the SPSS 18.0
software (IBM, Armonk, NY, USA) and GraphPad Prism 5 software (San
Diego, CA, USA).
Results
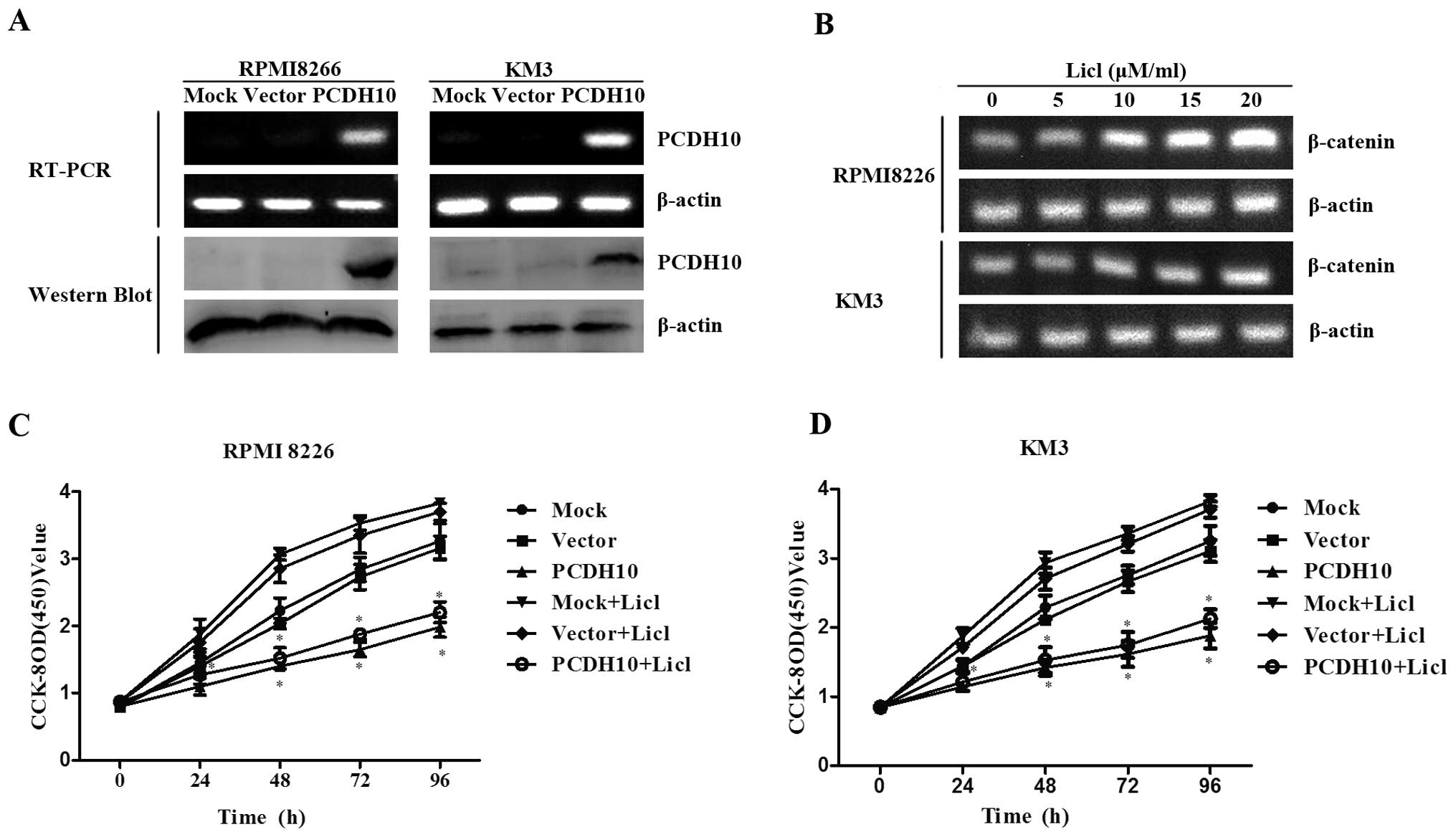
Restoration of PCDH10 successfully
inhibits MM cell proliferation
To assess the effect of PCDH10 on MM cell growth,
the RPMI-8226 and KM3 cell lines were transfected with the
full-length PCDH10 or empty vector. After selection in
G418-supplemented medium for three weeks, the stable expression of
PCDH10 was confirmed by RT-PCR and western blot analysis (Fig. 1A).
To identify the effect of PCDH10 on cell
proliferation, we used Licl, an inhibitor of GSK3β to stimulate the
activation of Wnt signaling. It was observed that the expression of
β-catenin was increased in a dose-dependent manner. β-catenin was
increased significantly at the dose of 15 and 20 µM/ml in
RPMI-8226 and KM3 cells, respectively. Thus, 15 and 20 µM/ml
were used as the appropriate concentrations of Licl and applied to
stimulate RPMI-8226 and KM3 cells, respectively, in the subsequent
investigations (Fig. 1B).
Using the CCK-8 assay, the cell proliferation
capacity was determined in RPMI-8226 and KM3 cells transfected with
PCDH10 or empty vector, with non-transfected cells as control. We
found that the growth of cells transfected with PCDH10 was
significantly suppressed even when the cells were treated with Licl
compared to the control groups (P<0.05, Fig. 1C and D), indicating that PCDH10 can
functionally antagonize MM cell proliferation.
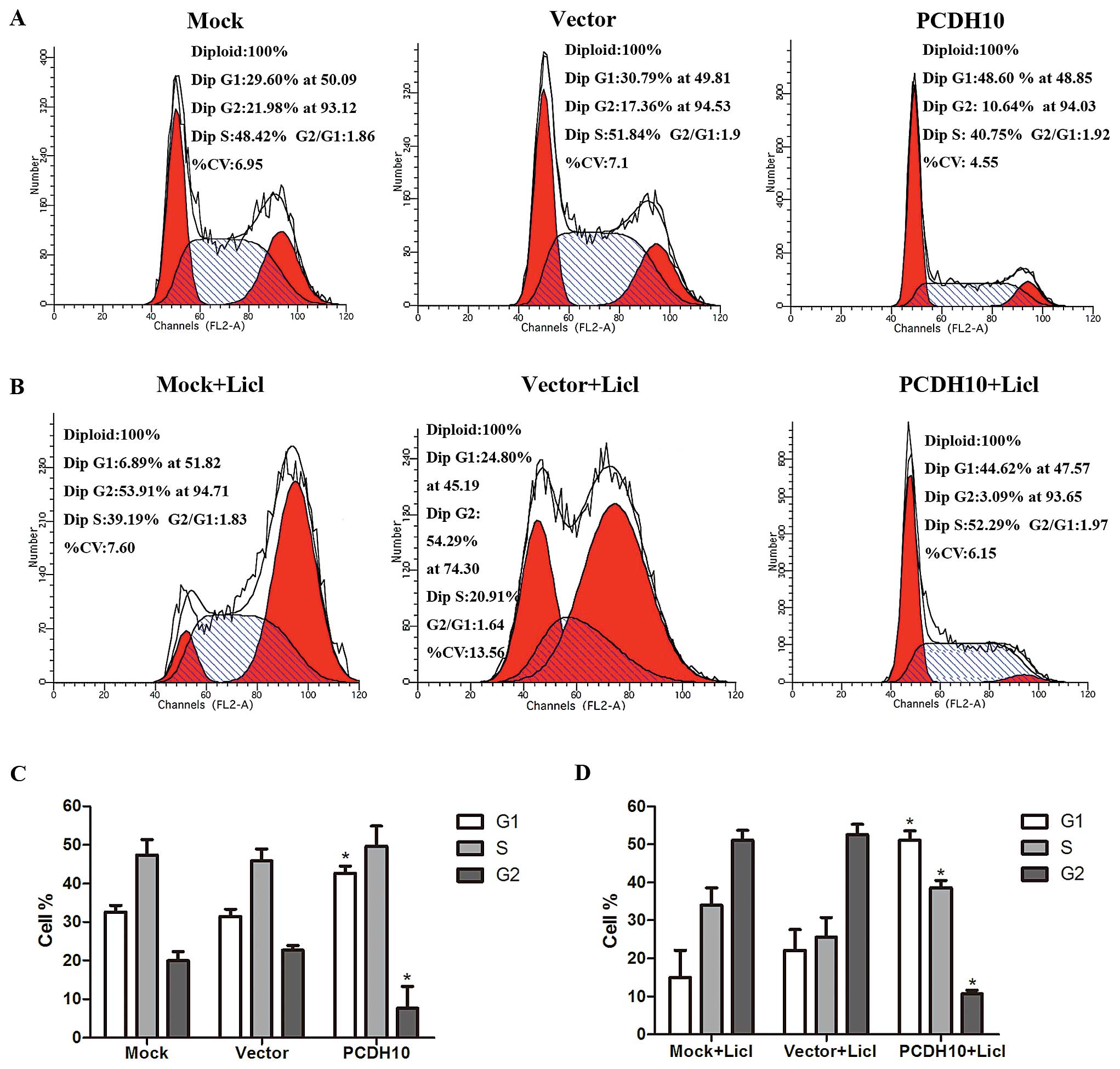
PCDH10 induces cell cycle arrest at the
G1 phase in MM cells
To examine the mechanism regarding how PCDH10 blocks
the cell proliferation capacity, we investigated the cell cycle
distribution by flow cytometry in RPMI-8226 cells. It was shown
that G1 phase was markedly increased in the cells transfected with
PCDH10, while G2 phase was decreased compared with the control
groups (P<0.05, Fig. 2A and C).
The result was more notable when the cells were exposed to Licl
(P<0.05, Fig. 2B and D). There
was no statistical difference of the alteration in S phase in cells
without Licl, but increased after the treatment of Licl
(P<0.05). Collectively, our results demonstrated that PCDH10
exerted its inhibitory activity by the G1 phase retardant.
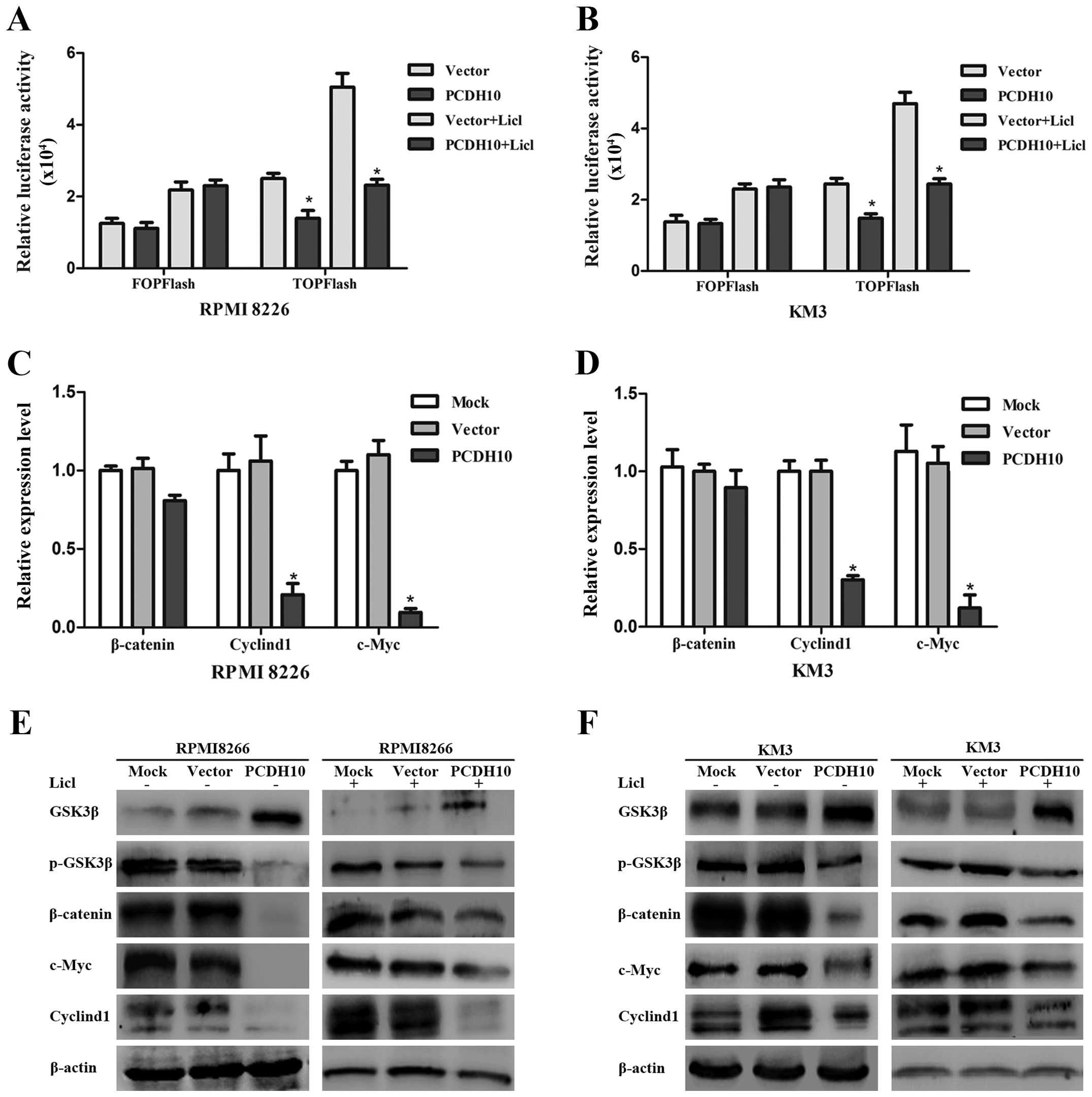
PCDH10 evidently hampers the Wnt
signaling in MM cells
The Wnt/β-catenin pathway is frequently activated in
various types of cancer and is involved in cancer cell
proliferation, survival and invasion (29,30).
To determine whether the inhibitory effect of PCDH10 is connected
with Wnt signaling, a luciferase assay was utilized to detect the
Wnt activity in MM cells transfected with or without PCDH10. It was
revealed that LEF/TCF activity (TOP-Flash) was obviously suppressed
by PCDH10. Moreover, the stimulated TOP-Flash activity of Licl was
also impaired by PCDH10 re-expression (P<0.05) (Fig. 3A and B), confirming that PCDH10 can
effectively inhibit the activity of LEF/TCF.
To verify this, the expression of relative genes in
the Wnt/β-catenin signaling pathway, including GSK3β, pGSK-3β,
β-catenin, cyclin D1 and c-Myc, were determined in RPMI-8226 cells
and KM3 cells. We found that PCDH10 obviously downregulated the
mRNA expression of cyclin D1 and c-Myc (P<0.05) (Fig. 3C and D). For the protein analysis,
the pGSK3β, cyclin D1, and c-Myc were blocked by PCDH10 compared
with the control groups. Conversely, GSK3β was enhanced by PCDH10
overexpression, even in the cells which were supplemented with Licl
(Fig. 3E and F). Of note, the mRNA
level of β-catenin was not inhibited by PCDH10, whereas its protein
expression was blocked effectively. Thus, the forced expression of
PCDH10 significantly hindered the Wnt/β-catenin signaling in the
presence or absence of Licl.
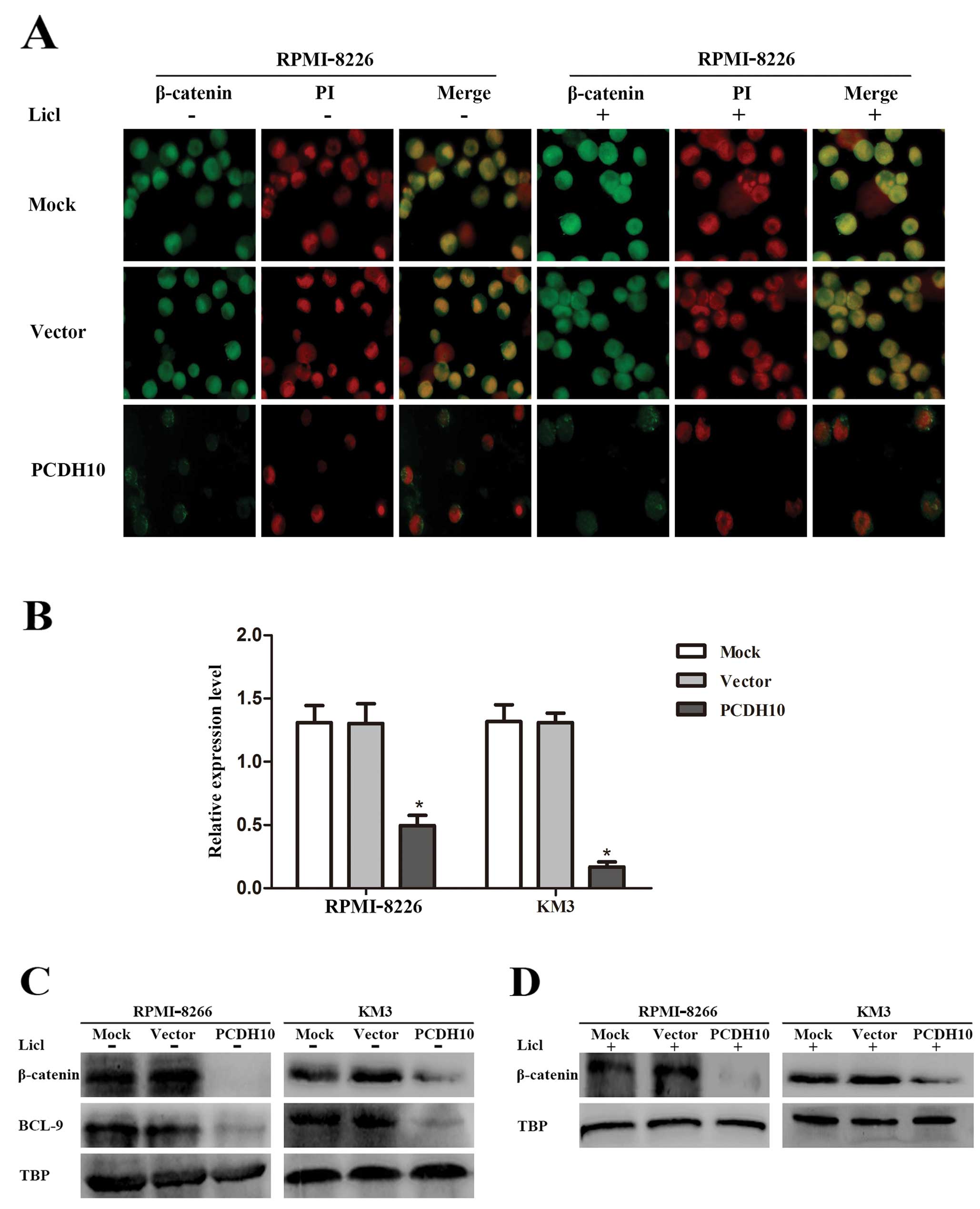
PCDH10 restrains the translocation of
β-catenin and the expression of BCL-9
The canonical Wnt signaling pathway is known to
underlie the pathogenesis of MM by the accumulation and nuclear
localization of β-catenin. Nuclear localization of the β-catenin is
translocated from the cytoplasm into the nucleus to stimulate
Wnt/β-catenin signaling and then to accelerate tumor cell
proliferation (27,30). Since we have confirmed that PCDH10
suppresses MM cell growth by targeting Wnt signaling, we
investigated whether the functional PCDH10 disturbed the nuclear
translocation of β-catenin and its coactivator BCL-9.
Immunofluorescence was utilized in RPMI-8226 cells
supplemented with or without Licl to evaluate the relationship
between PCDH10 and the translocation of β-catenin. Compared to the
control groups, PCDH10 overexpression, not only obviously resulted
in the reduction of nuclear β-catenin, but also arrested β-catenin
in the cytoplasm (Fig. 4A). This
result suggested that the enhanced expression of PCDH10 can hinder
the translocation of β-catenin.
Subsequently, the expression of BCL-9 was evaluated
by RT-qPCR. It was revealed that BCL-9 was highly expressed in
RPMI-8226 and KM3 cells, but not in PCDH10-transfected cells
(P<0.05, Fig. 4B). The result
suggested that PCDH10 was associated with the modulation of BCL-9
to achieve its function. To verify this, western blot analysis was
performed to assess the nuclear localization of β-catenin and
BCL-9. It was also observed that PCDH10 blocked the nuclear
localization of β-catenin and BCL-9 (Fig. 4C and D). These results showed that
PCDH10 exerted an antagonistic effect by negatively affecting the
Wnt/β-catenin/BCL-9 signaling pathway.
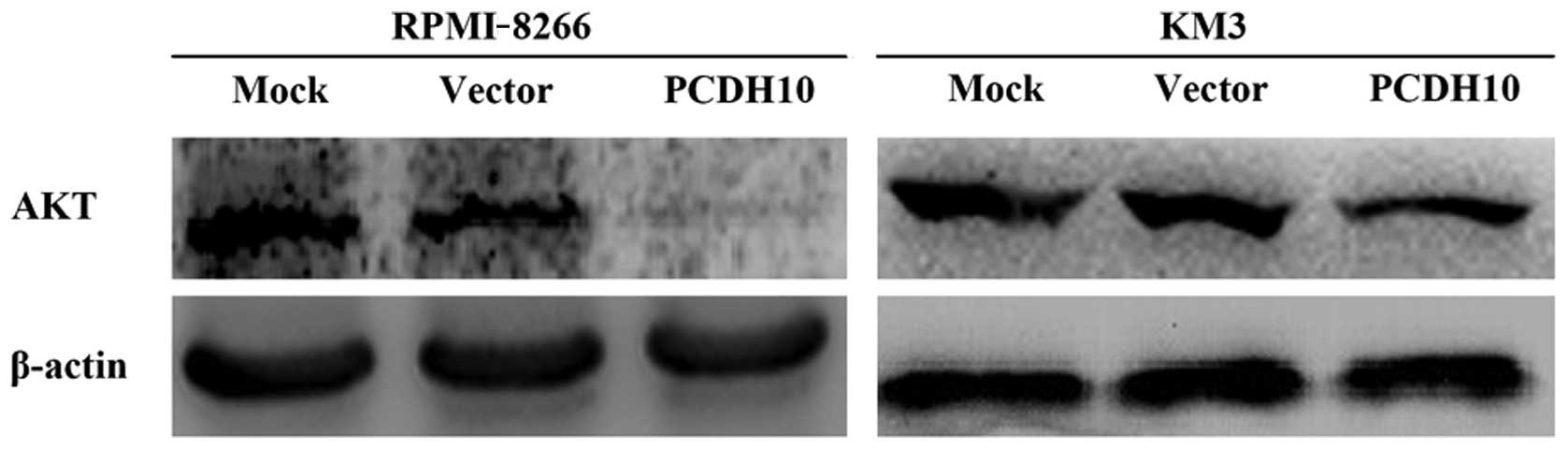
PCDH10 suppresses the activity of
AKT
Since the expression of GSK3β is promoted by the
re-expression of PCDH10, PCDH10 is more involved in intracellular
signaling then adhesion ability (5)
and GSK3β is frequently downregulated by the activity of PI3K/AKT
(33,34). Thus, we hypothesized that PCDH10
increased the expression of GSK3β by obstructing the activity of
AKT.
To confirm this, we detected the protein level of
AKT in RPMI-8226 and KM3 cells. It was shown that PCDH10
successfully suppressed the expression of AKT (Fig. 5). This result suggested that PCDH10
was involved in PI3K/AKT signaling, allowing PCDH10 to upregulate
the expression of GSK3β. However, the accurate mechanism involved
in this process needs to be further elucidated.
Discussion
In the present study, we identified a novel
functional link between PCDH10 and Wnt signaling pathway in MM. It
was confirmed that PCDH10 is a tumor suppressor and it can be a
potential therapeutic tool by targeting Wnt/β-catenin/BCL-9. The
constitutively active canonical Wnt/β-catenin signaling pathway has
been documented in MM. However, MM remains incurable because of
drug resistance, relapse or refractory (28–30).
Therefore, further exploration of novel and more selective
molecular targets is necessary.
In the present study, we determined that PCDH10 can
successfully block MM cell growth, even in the situation of Wnt
stimulation and the antagonistic effect was associated with cell
cycle, which was achieved by G1-phase blockage. Additionally, the
genes that associate with cell proliferation such as cyclin D1 and
c-Myc were also downregulated by PCDH10 overexpression. We also
revealed that the ectopic expression of PCDH10 highly contributed
to the inactivation of LEF/TCF. This result shows that the ectopic
expression of PCDH10 can significantly suppress the expression of
cyclin D1 and c-Myc by arresting the activity of LEF/TCF and
leading to the retardation of MM cell proliferation.
It is known that Wnt signaling results in the
inhibition of GSK3β activity by promoting the phosphorylation of
GSK3β, and then activates the accumulation of β-catenin, which
trans-locates to the nucleus (31,32).
Of note, we found that, PCDH10 restrained the phosphorylation of
GSK3β and the protein expression of β-catenin, but there was no
statistical difference of the alteration in the mRNA expression of
β-catenin. Thus, β-catenin is modulated by PCDH10 mainly at the
protein level rather than via mRNA expression. PCDH10 effectively
obstructed the protein expression of AKT. Furthermore, it has been
shown that the activation of AKT promotes the phosphorylation of
GSK3β and accelerates the development of various types of cancer
(33,34). This allows PCDH10 to upregulate
GSK3β by blocking the activity of AKT, which in turn, stimulates
the phosphorylation of β-catenin but decreases the protein
expression of β-catenin in the cytoplasm and nucleus. Consequently,
the β-catenin-TCF complex, which is crucial to the activation of
Wnt signaling was also obstructed, together with the suppression of
target genes such as cyclin D1 and c-Myc. However, the specific
mechanism involved needs to be further investigated.
β-catenin nuclear translocation has been identified
as a key event that disturbs Wnt/β-catenin signaling and BCL-9, an
extremely essential coactivator of the β-catenin-TCF complex and a
novel therapeutic target (24,25).
Our results show that, the ectopic expression of PCDH10 can hinder
the protein expression of β-catenin and restrain its nuclear
translocation as well as the expression of BCL-9. This result
suggests that PCDH10 is directly involves in the activation of
Wnt/β-catenin transcriptional activity, which is mediated by the
β-catenin-TCF complex and its coactivator BCL-9. BCL-9 is broadly
associated with MM cell proliferation, survival, migration and drug
resistance. More importantly, BCL-9 regulates Wnt target genes that
control transition and stem cell-like behavior with a negligible
effect on the homeostatic role of Wnt signaling in mammalian
(24,27). These results suggest that the
strategies employed on the restoration of PCDH10 can be a potential
therapy to refractory and recurrent patients without a particular
effect on normal tissues.
Recent findings have shown that, detection of PCDH10
methylation can identify high risk of biochemical recurrence and
evaluate the prognosis of patients with gastric, colorectal and
prostatic cancer (15–17). Additionally, c-Myc contributes to
drug resistance in cancer chemotherapy (35,36)
while PCDH10 markedly downregulated the expression of c-Myc. These
findings suggest promising utilization of PCDH10 in the clinic.
Therefore, the role of PCDH10 should be examined in clinical trials
with regard to cancer progression, drug resistance and relapse in
MM patients.
In conclusion, the deficiency of the tumor
suppressor PCDH10 is a frequent pathogenetic event in MM, while
restoration of PCDH10 successfully inhibits MM cell growth by
obstructing Wnt signaling. In the present study, we provide solid
evidence for the concept that PCDH10 restrains MM cell
proliferation by the negative regulation of the Wnt/β-catenin/BCL-9
signaling pathway, which is broadly involved in cell proliferation,
survival, drug resistance and relapse. Taken together, our results
provide novel insights into the potential for clinical translation
of strategies using PCDH10 as a novel selective therapeutic tool.
Furture studies should focus on the restoration of PCDH10 and
identify its application in combination with therapeutic drugs.
Acknowledgments
We would like to thank Dr Qian Tao (State Key
Laboratory in Oncology in South China/Cancer Epigenetics
Laboratory, Hong Kong Cancer Institute and Li Ka Shing Institute of
Health Sciences, Chinese University of Hong Kong, Hong Kong) for
their kind guidance and Dr Jian Hou (the Second Military Medical
University, Shanghai, China) for providing the MM cells. We would
also like to thank the Laboratory Research Center in the First
Affiliated Hospital of Chongqing Medical University for their
technical assistance.
References
|
1
|
de la Puente P, Muz B, Azab F, Luderer M
and Azab AK: Molecularly targeted therapies in multiple myeloma.
Leukemia Res Treat. 2014:9765672014.
|
|
2
|
Kawano Y, Fujiwara S, Wada N, Izaki M,
Yuki H, Okuno Y, Iyama K, Yamasaki H, Sakai A, Mitsuya H, et al:
Multiple myeloma cells expressing low levels of CD138 have an
immature phenotype and reduced sensitivity to lenalidomide. Int J
Oncol. 41:876–884. 2012.PubMed/NCBI
|
|
3
|
McCubrey JA, Steelman LS, Bertrand FE,
Davis NM, Abrams SL, Montalto G, D’Assoro AB, Libra M, Nicoletti F,
Maestro R, et al: Multifaceted roles of GSK-3 and Wnt/β-catenin in
hematopoiesis and leukemogenesis: Opportunities for therapeutic
intervention. Leukemia. 28:15–33. 2014. View Article : Google Scholar :
|
|
4
|
Schmeel LC, Schmeel FC, Kim Y, Endo T, Lu
D and Schmidt-Wolf IG: Targeting the Wnt/beta-catenin pathway in
multiple myeloma. Anticancer Res. 33:4719–4726. 2013.PubMed/NCBI
|
|
5
|
Redies C, Vanhalst K and Roy F:
delta-Protocadherins: Unique structures and functions. Cell Mol
Life Sci. 62:2840–2852. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vanhalst K, Kools P, Staes K, van Roy F
and Redies C: delta-Protocadherins: A gene family expressed
differentially in the mouse brain. Cell Mol Life Sci. 62:1247–1259.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Angst BD, Marcozzi C and Magee AI: The
cadherin superfamily: Diversity in form and function. J Cell Sci.
114:629–641. 2001.PubMed/NCBI
|
|
8
|
Nakao S, Platek A, Hirano S and Takeichi
M: Contact-dependent promotion of cell migration by the
OL-protocadherin-Nap1 interaction. J Cell Biol. 182:395–410. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jao TM, Tsai MH, Lio HY, Weng WT, Chen CC,
Tzeng ST, Chang CY, Lai YC, Yen SJ, Yu SL, et al: Protocadherin 10
suppresses tumorigenesis and metastasis in colorectal cancer and
its genetic loss predicts adverse prognosis. Int J Cancer.
135:2593–2603. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ma JG, He ZK, Ma JH, Li WP and Sun G:
Downregulation of protocadherin-10 expression correlates with
malignant behaviour and poor prognosis in human bladder cancer. J
Int Med Res. 41:38–47. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Narayan G, Xie D, Freddy AJ, Ishdorj G, Do
C, Satwani P, Liyanage H, Clark L, Kisselev S, Nandula SV, et al:
PCDH10 promoter hypermethylation is frequent in most histologic
subtypes of mature lymphoid malignancies and occurs early in
lymphoma-genesis. Genes Chromosomes Cancer. 52:1030–1041. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhong X, Zhu Y, Mao J, Zhang J and Zheng
S: Frequent epigenetic silencing of PCDH10 by methylation in human
colorectal cancer. J Cancer Res Clin Oncol. 139:485–490. 2013.
View Article : Google Scholar
|
|
13
|
Li Y, Yang ZS, Song JJ, Liu Q and Chen JB:
Protocadherin-10 is involved in angiogenesis and methylation
correlated with multiple myeloma. Int J Mol Med. 29:704–710.
2012.PubMed/NCBI
|
|
14
|
Li Z, Yang Z, Peng X, Li Y, Liu Q and Chen
J: Nuclear factor-κB is involved in the protocadherin-10-mediated
pro-apoptotic effect in multiple myeloma. Mol Med Rep. 10:832–838.
2014.PubMed/NCBI
|
|
15
|
Danese E, Minicozzi AM, Benati M,
Montagnana M, Paviati E, Salvagno GL, Gusella M, Pasini F, Guidi GC
and Lippi G: Epigenetic alteration: New insights moving from tissue
to plasma - the example of PCDH10 promoter methylation in
colorectal cancer. Br J Cancer. 109:807–813. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang L, Xie P-G, Lin YL, Ma JG and Li WP:
Aberrant meth-ylation of PCDH10 predicts worse biochemical
recurrence-free survival in patients with prostate cancer after
radical prostatectomy. Med Sci Monit. 20:1363–1368. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Deng J, Liang H, Ying G, Dong Q, Zhang L,
Yu J, Fan D and Hao X: Clinical significance of the methylated
cytosine-phosphate-guanine sites of protocadherin-10 promoter for
evaluating the prognosis of gastric cancer. J Am Coll Surg.
219:904–913. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dallosso AR, Øster B, Greenhough A,
Thorsen K, Curry TJ, Owen C, Hancock AL, Szemes M, Paraskeva C,
Frank M, et al: Long-range epigenetic silencing of chromosome 5q31
proto- cadherins is involved in early and late stages of colorectal
tumori-genesis through modulation of oncogenic pathways. Oncogene.
31:4409–4419. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dallosso AR, Hancock AL, Szemes M,
Moorwood K, Chilukamarri L, Tsai HH, Sarkar A, Barasch J,
Vuononvirta R, Jones C, et al: Frequent long-range epigenetic
silencing of proto-cadherin gene clusters on chromosome 5q31 in
Wilms’ tumor. PLoS Genet. 5:e10007452009. View Article : Google Scholar
|
|
20
|
Yang X, Chen M-W, Terry S, Vacherot F,
Chopin DK, Bemis DL, Kitajewski J, Benson MC, Guo Y and Buttyan R:
A human- and male-specific protocadherin that acts through the wnt
signaling pathway to induce neuroendocrine transdifferentiation of
prostate cancer cells. Cancer Res. 65:5263–5271. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Qiang YW, Walsh K, Yao L, Kedei N,
Blumberg PM, Rubin JS, Shaughnessy J Jr and Rudikoff S: Wnts induce
migration and invasion of myeloma plasma cells. Blood.
106:1786–1793. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Iwai S, Yonekawa A, Harada C, Hamada M,
Katagiri W, Nakazawa M and Yura Y: Involvement of the Wnt-β-catenin
pathway in invasion and migration of oral squamous carcinoma cells.
Int J Oncol. 37:1095–1103. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Willis TG, Zalcberg IR, Coignet LJ,
Wlodarska I, Stul M, Jadayel DM, Bastard C, Treleaven JG, Catovsky
D, Silva ML, et al: Molecular cloning of translocation
t(1;14)(q21;q32) defines a novel gene (BCL9) at chromosome 1q21.
Blood. 91:1873–1881. 1998.PubMed/NCBI
|
|
24
|
Mani M, Carrasco DE, Zhang Y, Takada K,
Gatt ME, Dutta-Simmons J, Ikeda H, Diaz-Griffero F, Pena-Cruz V,
Bertagnolli M, et al: BCL9 promotes tumor progression by conferring
enhanced proliferative, metastatic, and angiogenic properties to
cancer cells. Cancer Res. 69:7577–7586. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Le Baccon P, Leroux D, Dascalescu C, Duley
S, Marais D, Esmenjaud E, Sotto JJ and Callanan M: Novel evidence
of a role for chromosome 1 pericentric heterochromatin in the
pathogenesis of B-cell lymphoma and multiple myeloma. Genes
Chromosomes Cancer. 32:250–264. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao Y, Yang Y, Trovik J, Sun K, Zhou L,
Jiang P, Lau TS, Hoivik EA, Salvesen HB, Sun H, et al: A novel wnt
regulatory axis in endometrioid endometrial cancer. Cancer Res.
74:5103–5117. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao JJ, Lin J, Zhu D, Wang X, Brooks D,
Chen M, Chu ZB, Takada K, Ciccarelli B, Admin S, et al: miR-30–5p
functions as a tumor suppressor and novel therapeutic tool by
targeting the oncogenic Wnt/β-catenin/BCL9 pathway. Cancer Res.
74:1801–1813. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Su J, Zhang A, Shi Z, Ma F, Pu P, Wang T,
Zhang J, Kang C and Zhang Q: MicroRNA-200a suppresses the
Wnt/β-catenin signaling pathway by interacting with β-catenin. Int
J Oncol. 40:1162–1170. 2012.PubMed/NCBI
|
|
29
|
Calaf GM, Alvarado ME and Hei TK: β
catenin is associated with breast cancer progression in vitro. Int
J Oncol. 26:913–921. 2005.PubMed/NCBI
|
|
30
|
Berry WL, Kim TD and Janknecht R:
Stimulation of β-catenin and colon cancer cell growth by the KDM4B
histone demethylase. Int J Oncol. 44:1341–1348. 2014.PubMed/NCBI
|
|
31
|
Ge X and Wang X: Role of Wnt canonical
pathway in hemato-logical malignancies. J Hematol Oncol. 3:332010.
View Article : Google Scholar
|
|
32
|
Liu YZ, Wu K, Huang J, Liu Y, Wang X, Meng
ZJ, Yuan SX, Wang DX, Luo JY, Zuo GW, et al: The PTEN/PI3K/Akt and
Wnt/β-catenin signaling pathways are involved in the inhibitory
effect of resveratrol on human colon cancer cell proliferation. Int
J Oncol. 45:104–112. 2014.PubMed/NCBI
|
|
33
|
Medina M, Garrido JJ and Wandosell FG:
Modulation of GSK-3 as a Therapeutic Strategy on Tau Pathologies.
Front Mol Neurosci. 4:242011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kamarudin MN, Mohd Raflee NA, Hussein SS,
Lo JY, Supriady H and Abdul Kadir H: (R)-(+)-α-lipoic acid
protected NG108–15 cells against H2O2-induced
cell death through PI3K-Akt/GSK-3β pathway and suppression of
NF-κβ-cytokines. Drug Des Devel Ther. 8:1765–1780. 2014.
|
|
35
|
Fauriat C and Olive D: AML drug
resistance: c-Myc comes into play. Blood. 123:3528–3530. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xie C, Pan Y, Hao F, Gao Y, Liu Z, Zhang
X, Xie L, Jiang G, Li Q and Wang E: C-Myc participates in
β-catenin-mediated drug resistance in A549/DDP lung adenocarcinoma
cells. APMIS. 122:1251–1258. 2014. View Article : Google Scholar : PubMed/NCBI
|