Introduction
Malignant mesothelioma is a rare asbestos-induced
aggressive cancer that arises from the mesothelial cells lining the
pleural, peritoneal and pericardial cavities (1,2).
Conventional therapies for this malignancy include surgical
resection, chemotherapy and irradiation; however, these measures
are generally non-curative (2–4).
Consequently, novel therapeutic paradigms are urgently required for
the effective treatment of this aggressive and currently incurable
malignancy.
Recent advances in understanding the regulation of
physiological and pathological angiogenesis have revealed the
processes by which tumors elicit an angiogenic response and have
underscored their requirement for angiogenesis to sustain growth
and metastasis (5). Proteins such
as angiostatin (6–8) and endostatin (8,9) or
vascular endothelial growth factor (VEGF) antagonists such as
truncated forms of VEGF receptors (VEGFR) (10–12)
have shown antitumor effects in preclinical cancer models.
Preclinical studies have shown that angiogenesis has a key role in
the biology of malignant mesothelioma (13,14).
Previous studies also have shown that mesothelioma cells highly
express VEGF and its receptors, VEGFR-1/Flt-1 and VEGFR-2/Flk-1.
VEGF stimulates mesothelioma cells growth in vitro in a
dose-dependent manner and this growth has been shown to be
inhibited by anti-VEGF antibodies (15). Mesothelioma patients have among the
highest circulating VEGF levels of any solid tumor and high VEGF
levels are a poor prognostic factor in this disease (16,17).
Hence, anti-angiogenic therapies could be effective in the
treatment of patients with malignant mesothelioma.
Bevacizumab (Avastin®), a VEGF-blocking
monoclonal antibody, is widely used to treat metastatic colon and
non-small cell lung cancer and ocular vascular proliferative
disorders (18–20). Systemic administration of
bevacizumab has been tested as a therapeutic for malignant pleural
mesothelioma. Two non-randomized phase II trials of bevacizumab as
maintenance strategy after six cycles of platinum-pemetrexed plus
bevacizumab induction did not demonstrate an improvement in median
progression-free survival compared with that of historical controls
treated with pemetrexed/platinum combinations (21,22).
In a double-blind, placebo-controlled, randomized phase II trial,
the addition of bevacizumab to gemcitabine-cisplatin followed by
bevacizumab did not improve progression-free or overall survival
rate in previously untreated patients with malignant pleural
mesothelioma. These limited successes in clinical trials for
mesothelioma are probably caused by the inability to achieve
long-term and sustained therapeutic levels of anti-angiogenic
protein at the tumor via systemic administration of bevacizumab,
unlike the application of bevacizumab for the treatment of macular
degeneration in which bevacizumab is injected directly into the eye
(18). Therefore, persistent in
situ production of anti-angiogenic factors via gene therapy
would be an ideal strategy. We speculated that continuous
production of anti-angiogenic factors at the tumors (in
situ) by gene therapy would achieve maximum therapeutic effect
of anti-angiogenic therapies.
In the present study, we investigated whether
anti-angiogenic factors such as angiostatin, endostatin and the
soluble form of VEGFR-2 (sFlk1) were able to inhibit endothelial
cell proliferation via lentivirus-mediated gene transfer into
MSTO-211H human malignant mesothelioma cells in culture. We also
examined whether a dual-agent strategy could yield greater
therapeutic benefit.
Materials and methods
Cell lines
Human malignant pleural mesothelioma MSTO-211H cells
were obtained from American Type Culture Collection (ATCC,
Manassas, VA, USA) and grown in Roswell Park Memorial institute
(RPMI) 1640 medium (Nacalai Tesque, Kyoto, Japan) supplemented with
10% heat-inactivated fetal calf serum (FCS; HyClone, Logan, UT,
USA). Normal human umbilical vein endothelial cells (HUVEC) and
their specific media, EGM-2, were purchased from Lonza Japan, inc.
(Tokyo, Japan). The cells were cultured in humidified 5%
CO2 at 37°C.
Vector plasmids and virus production
Self-inactivating lentivirus vectors that
individually expressed angiostatin (LV-A) and endostatin (LV-E)
have been described previously as Sin-Ang (8) and Sin-End (8), respectively. The lentivirus vector,
LV-F, was generated by cloning sFlk1 (provided by J. Folkman,
Children’s Hospital, boston, MA, USA) into the Sin-GFP (8) vector construct. Each lentivirus vector
contains an EGFP marker gene linked to the transgene expression
cassette via an internal ribosome entry site. The lentivirus vector
expressing EGFP alone (LV-C), which was described previously as
Sin-Ang (8), was used as a control.
The preparations of lentivirus vectors pseudotyped with vesicular
stomatitis virus G were produced by transient cotransfection of
293T cells, as described previously (23,24).
The titers of these vectors were determined by fluorescent protein
expression by using a FACSCalibur flow cytometer (Becton-Dickinson
Japan, Tokyo, Japan) and expressed in terms of transducing
units/milliliter.
Transduction of MSTO-211H mesothelioma
cells with lentivirus vectors
MSTO-211H cells were cultured in growth medium to
30–50% confluency at the time of transduction (1×106
cells/well in 6-well plates). Then, the cells were incubated with
lentiviral vectors (LV-A, LV-E, LV-F or LV-C) at a multiplicity of
infection of 10 for 72 h; the cells were then subcultured three
times to amplify by conventional culture methods and named MSTO-A,
MSTO-E, MSTO-F cells and MSTO-C, respectively.
In vitro growth curves
The MSTO-C, MSTO-A, MSTO-E and MSTO-F cells were
seeded in triplicate at 5×103/well in 12-well culture
plates. Cells in triplicate wells were harvested daily by
trypsinization and the number of viable cells was determined by the
trypan blue exclusion assay (Sigma-Aldrich Japan, Tokyo,
Japan).
Sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) and western blot analysis
To confirm the production and secretion of peptides
after lentivirus infection, MSTO-C, MSTO-A, MSTO-E and MSTO-F cells
were plated on two 10-cm dishes at a density of
5×106/dish. The next day, the media were replaced with
10 ml of serum-free media and incubated for 48 h. The conditioned
media were collected and concentrated 20-fold on a Centricon YM-10
column (Merck-Millipore Japan, Tokyo, Japan); Ten microliters of
each sample was subjected to SDS-PAGE using 5–20% linear gradient
gels (e-PAGEL; ATTO, Tokyo, Japan) and proteins were transferred to
polyvinylidene fluoride membranes (Immobilon-P; Merck-Millipore
Japan). For western blot analysis, rabbit anti-mouse angiostatin
ab2904 (1:1000), rabbit anti-mouse endostatin ab58774 (1:250) or
rabbit anti-human VEGF receptor 2 (Flk1) ab39638 (1:250) (Abcam,
Cambridge, UK) were used as the primary antibodies and
peroxidase-conjugated goat anti-rabbit antibody A6154 (1:2,000)
(Sigma-Aldrich Japan) was used as the secondary antibody.
Chemiluminescent detection of bound antibodies was performed by
using the ECL system (ImmunoStar; Wako, Osaka, Japan).
Co-culture assay
MSTO-C, MSTO-A, MSTO-E and MSTO-F cells were
harvested at near confluence using 0.05% trypsin/EDTA solution and
were subsequently counted. Then, 1×105 cells were seeded
into Transwell chambers for 12-well culture plates with a 0.4
µm pore-size (Corning Costar, Cambridge, MA). HUVEC cells
(5×103) were plated on 12-well plates with the
appropriate culture medium. Following 24 h the transwell chambers
were assembled on the 12-well plates for co-culture. After 70 h,
the cells were collected by trypsinization and counted.
To assess the combination effect of anti-angiogenic
factors, MSTO-C, MSTO-A, MSTO-E and MSTO-F cells were harvested at
near confluence; the cells were subsequently counted and mixed with
each other at a ratio of 1:1 to create the following cell mixtures:
MSTO-C/C, C/A, C/E, C/F, A/E, A/F and E/F. These mixed cells
(1×105) were seeded in Transwell chambers. HUVEC cells
(5×103) were plated on 12-well plates with the
appropriate culture medium. The Transwell chambers were then
assembled on the 12-well plates for co-culture 24 h later. After 70
h, the cells were collected by trypsinization and counted.
Subcutaneous tumor models
MSTO-C, MSTO-A, MSTO-E and MSTO-F cells were
harvested at near confluence using 0.05% trypsin/EDTA solution; the
cells were subsequently counted and mixed with each other at a
ratio of 1:1 to create the following cell mixtures: MSTO-C/C, C/A,
C/E, A/E, A/F and E/F. One million of the mixed cells in 100
µl of Ca2+- and Mg2+-free Hank’s
balanced salt solution were injected subcutaneously on the dorsal
flank of 6- to 7-week-old female BALB/c-nu/nu (nude) mice (Charles
River Japan, Inc., Yokohama, Japan) (n=10/group). The mice were
observed closely and tumors were measured by using a caliper every
week over 12 weeks. Tumor volume was calculated as:
a×b2×0.5, where a and b were
the large and small diameters, respectively.
Statistical analysis
The results were presented as the mean ± standard
deviation (SD). The statistical significance of differences was
calculated by using Student’s t-test and a P-value of <0.05 was
considered statistically significant.
Results
Transduction of human mesothelioma cells
by lentivirus vectors expressing anti-angiogenic factors
Human malignant pleural mesothelioma MSTO-211H cells
were transduced by using the lentiviral vectors LV-A, LV-E and LV-F
and linked to EGFP marker gene expression via an internal ribosome
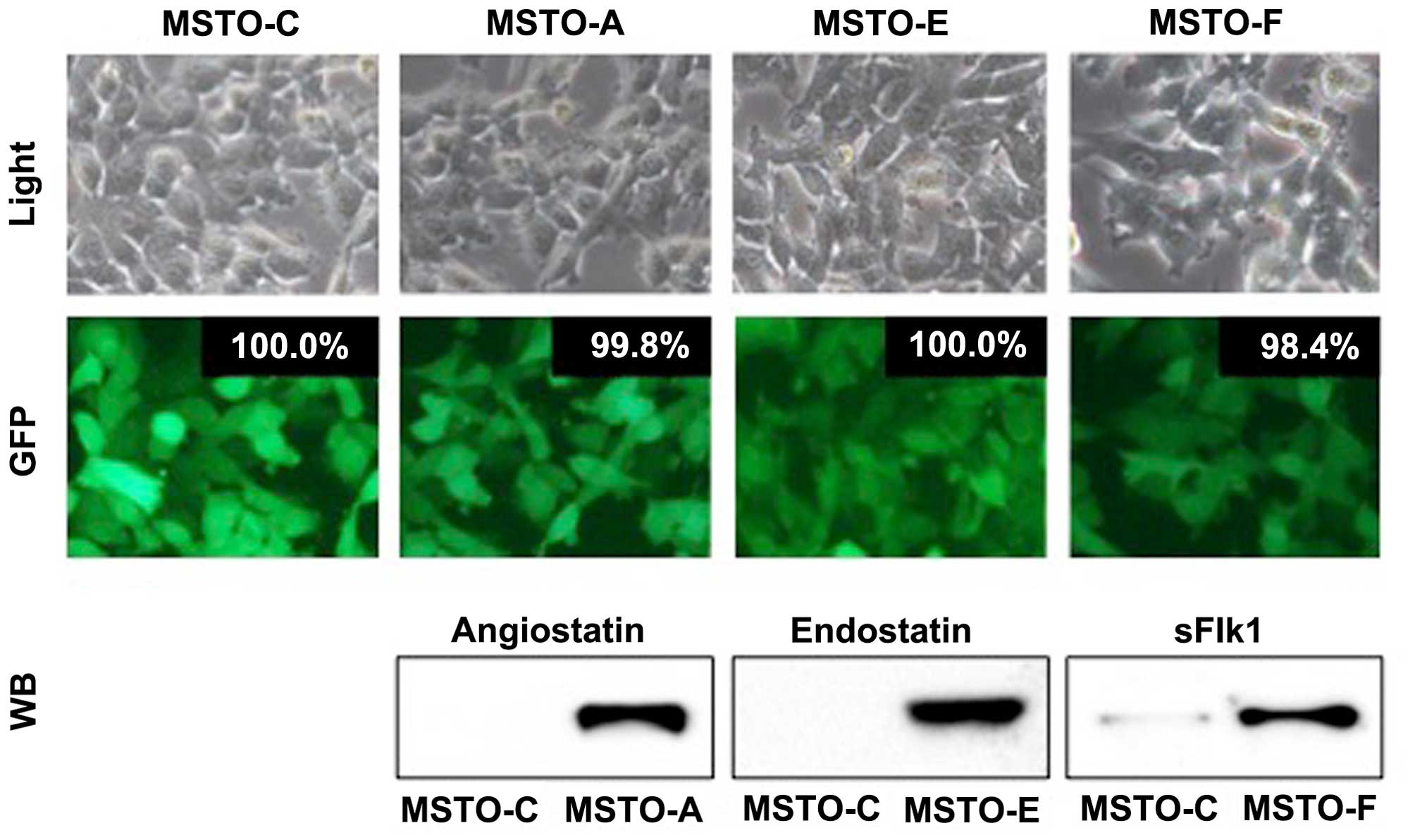
entry site. LV-C was used as the control. As shown in Fig. 1, the resultant cells, MSTO-A,
MSTO-E, MSTO-F and MSTO-C, were nearly 100% EGFP-positive even
after subculturing three times, which indicated highly efficient
and stable transduction of MSTO-211H cells by the lentivirus
vectors. The conditioned media from the lentivirus
vector-transduced cells were concentrated and the corresponding
anti-angiogenic proteins were analyzed by western blot analysis. In
addition, all anti-angiogenic proteins were observed to be stably
expressed and secreted into the cell culture medium after
transduction by their respective vectors.
In vitro biological properties of
MSTO-211H cells expressing anti-angiogenic factors
We investigated whether anti-angiogenic factors have
any biological properties on MSTO-211 cells. Firstly, there was no
difference in cell morphology among the MSTO-A, MSTO-E, MSTO-F and
MSTO-C cells (Fig. 1). Secondly, no
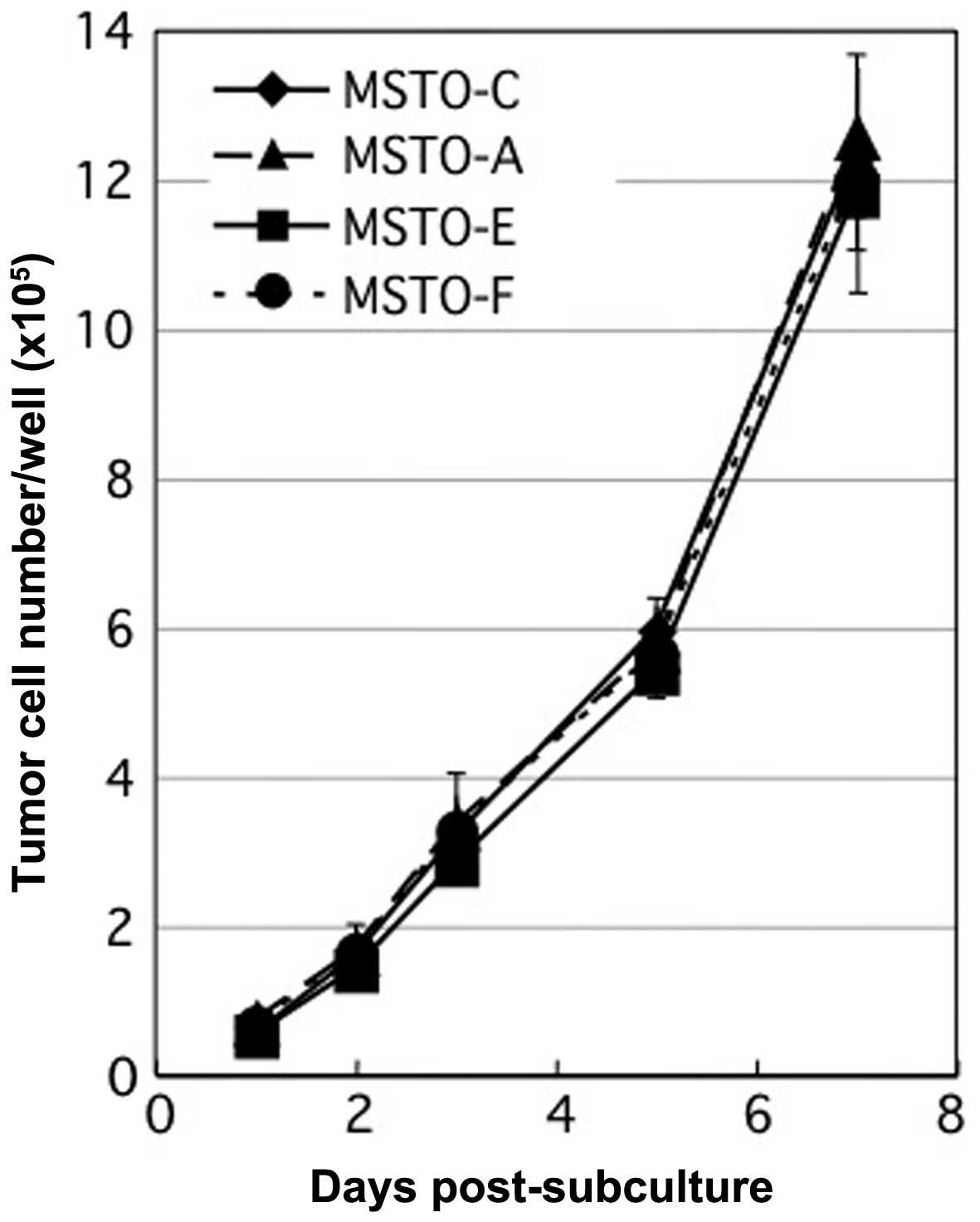
differences were observed in the in vitro growth rates after
7 days among the MSTO-A, MSTO-E, MSTO-F and MSTO-C cells
(P>0.05) (Fig. 2). These results
showed that the expression and secretion of anti-angiogenic factors
in MSTO-211H culture did not affect the morphology and growth of
MSTO-211H by themselves in vitro.
Inhibition of HUVEC growth by co-culture
with transduced MSTO-211H human mesothelioma cells expressing
anti-angiogenic factors
To investigate whether anti-angiogenic factors
secreted from tumor cells inhibit the growth of adjacent
endothelial cells, HUVEC cells were co-cultured with MSTO-C,
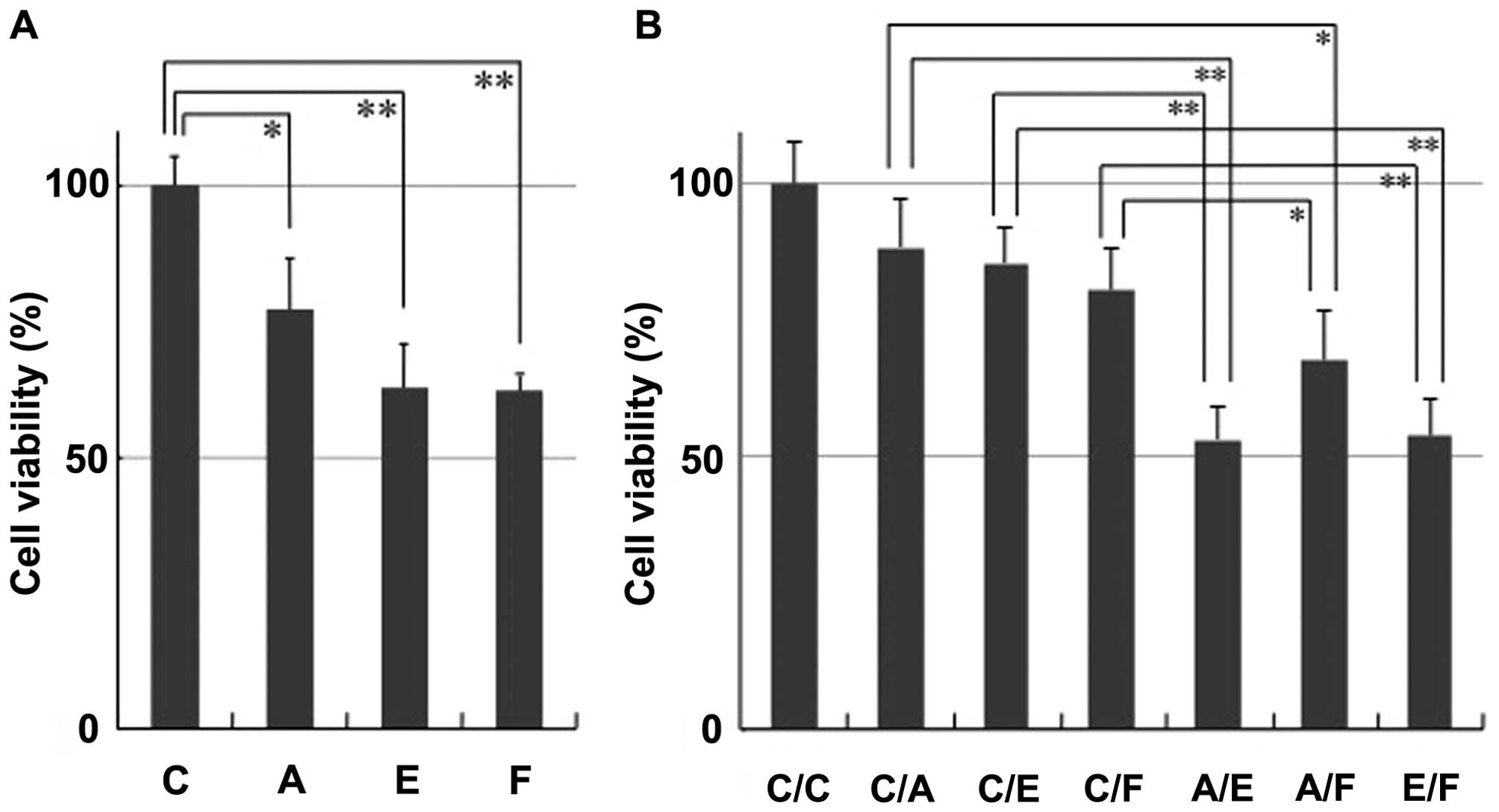
MSTO-A, MSTO-E and MSTO-F cells in a dual-chamber assay (Fig. 3A). Co-culture of HUVEC cells with
either MSTO-A (77.3±11.6%, P= 0.0191), MSTO-E (62.7±8.3%, P=0.0009)
or MSTO-F (62.2±1.5%, P=0.0002) cells resulted in significant
inhibition of HUVEC cell proliferation compared with that of MSTO-C
cells expressing no exogenous anti-angiogenic factors (100.0±5.2%).
These data demonstrate the ability of anti-angiogenic lentivirus
vectors to mediate anti-proliferative effects on HUVEC cells
indirectly via paracrine secretion from transduced cells.
For assessing the combination effect of
anti-angiogenic factors secreted from tumor cells, HUVEC cells were
co-cultured with transduced cells mixed with MSTO-C, MSTO-A, MSTO-E
and MSTO-F cells at a ratio of 1:1 in a dual-chamber assay
(Fig. 3b). Inhibition effects on
HUVEC cells in co-culture with any of the cells expressing single
anti-angiogenic factors were MSTO-C/A (88.5±8.8%, P= 0.1404),
MSTO-C/E (85.6±6.3%, P=0.0557) or MSTO-C/F (80.6±7.7%, P=0.0266),
and that in co-culture with cells expressing no exogenous
anti-angiogenic factors was MSTO-C/C (100.0±7.6%). These inhibition
effects on HUVEC cells decreased (MSTO-C/A, C/E and C/F) as
compared with those in the previous experiment (MSTO-A, E and F)
(Fig. 3A), probably because of a
decrease in the percentages of MSTO-A, MSTO-E and MSTO-F cells by
mixing with MSTO-C. Notably, any combination of anti-angiogenic
factors resulted in enhanced inhibition (A/E, A/F and E/F; Fig. 3B), which suggested that the
combinatorial anti-angiogenic therapy has the potential for greater
therapeutic efficacy than that of a single-agent regimen.
In vivo combination effect of
anti-angiogenic factors on subcutaneous mesothelioma tumor
growth
To confirm the therapeutic advantage of
combinatorial anti-angiogenic therapy, we examined in vivo
antitumor efficacy in a subcutaneous MSTO xenograft model in
athymic nude mice, which received subcutaneous injection of the
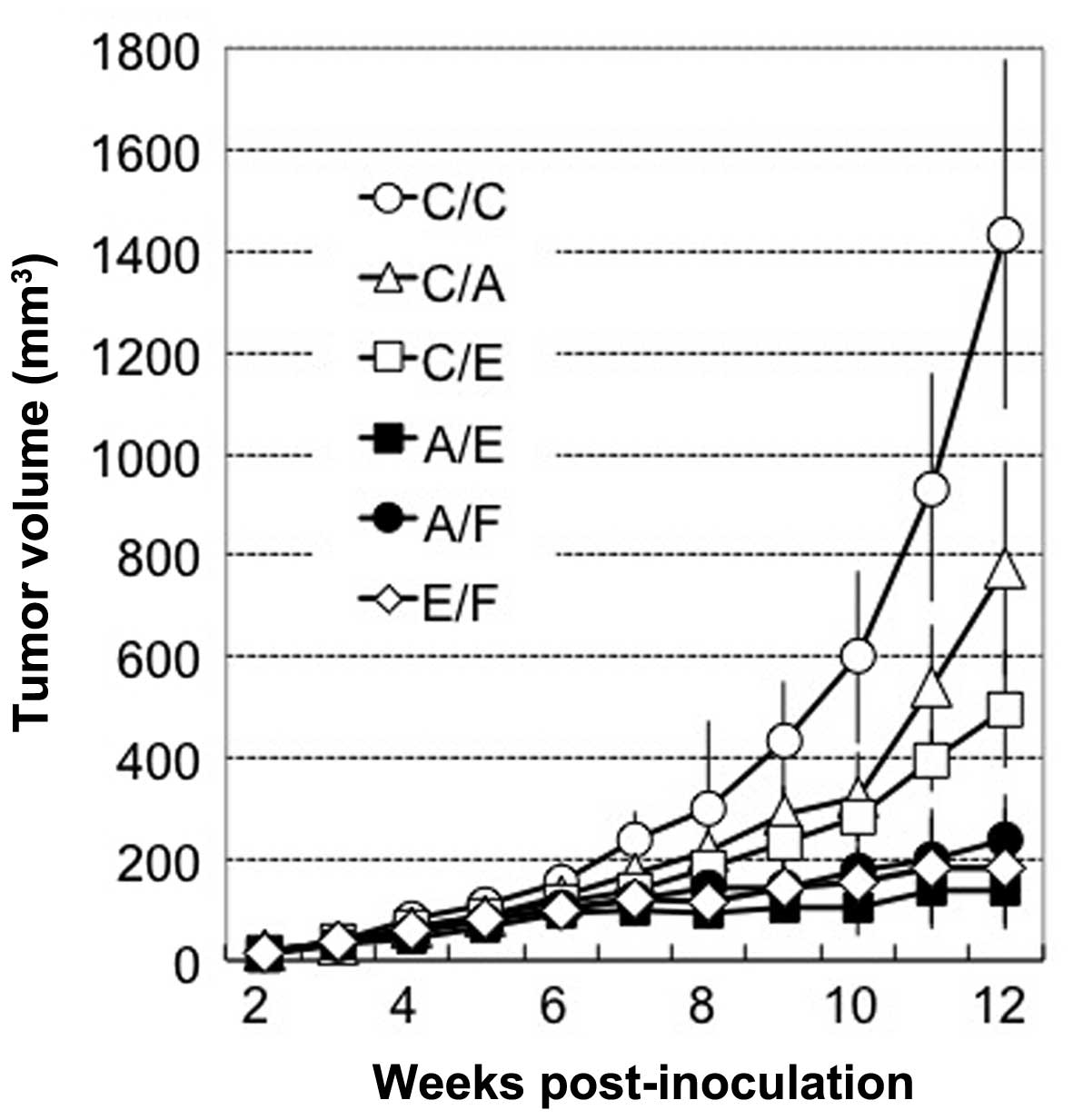
following cell mixtures: MSTO-C/C, C/A, C/E, A/E, A/F and E/F.
Tumors injected with the cells expressing single anti-angiogenic
factors (C/A, C/E) resulted in a significant (46.1–65.4%) reduction
in tumor volume compared with that in the C/C group (P<0.05)
(Fig. 4). This reduction was
unexpectedly high with respect to the in vitro co-culture
data (Fig. 3B), which suggested
that these anti-angiogenic factors possess an indirect effect in
vivo. Furthermore, the combination of any two among MSTO-A,
MSTO-E and MSTO-F significantly enhanced the antitumor efficacy
compared with that of the single anti-angiogenic factor group
(MSTO-C/A and C/F), which indicated the feasibility of the
combinatorial anti-angiogenic therapy in vivo.
Discussion
Tumors elicit an angiogenic response and have been
shown to require angiogenesis to sustain growth and metastasis
(5). For example, VEGF is produced
by a variety of tumors, including malignant mesothelioma and
stimulate neovascularization of tumors (4,25).
Endothelial cells engaged in angiogenesis express VEGFR-1 and
VEGFR-2; however, they produce only low levels of endogenous VEGF
for an autocrine/paracrine signal required for endothelial cell
survival and vascular homeostasis mainly through intracellular
VEGFR-2 (26,27). In addition, VEGFR-2 appears to play
a more important role in initiating signal transduction pathways
within endothelial cells because of its greater kinase activity and
thus, in activating multiple signaling networks that lead to
increased proliferation, sprouting, migration and tube formation of
endothelial cells (28). Similar to
the case described above, anti-angiogenic factors may function as
an autocrine or paracrine growth factor for endothelial cells or
several types of tumor cells, including mesothelioma. Therefore,
continuous in situ production of anti-angiogenic factors at
the tumors by gene therapy would achieve maximum therapeutic effect
of anti-angiogenic therapies. As expected, in the present study,
even a single factor significantly suppressed vascular endothelial
growth. However, the anti-angiogenic factors, angiostatin,
endostatin and sFlk1, did not affect the morphology and growth of
any MSTO-211H cells producing anti-angiogenic factors (Fig. 1 and 2). This observation is partially
consistent with a previous observation that MSTO-211H expressed
VEGFr-2 at the mRNA level but did not produce detectable VEGFR-2 at
the protein level (29). Therefore,
other growth factor receptors and their signaling pathways may be
much more important for the proliferation of this cell line.
In addition, we also showed that a dual-agent
strategy may yield greater therapeutic benefit. The anti-angiogenic
activities of angiostatin and endostatin interfere with the
αVβ3 integrin-mediated signaling in
endothelial cells (6–9), although sFlk1 uses a different pathway
through VEGF-signaling blockage (10–12).
Because the combination of angiostatin and endostatin that have the
same mechanism of anti-angiogenic action could yield greater
therapeutic benefit than a single factor, the integrin pathway may
not be saturated even by continuous in situ production of a
single factor. Our data suggest that in situ continuous
production of combined anti-angiogenic factors would be absolutely
required at levels that cannot be achieved by oral or intermittent
systemic administration. However, anti-angiogenic gene therapy
targeting the different pathways of endothelial growth factor
signaling should be better for long-term success by presumably
reducing the risk of resistance.
Anti-angiogenic therapy cannot eradicate tumors by
itself and therefore, is a kind of second-line maintenance therapy.
Therefore, long-term and combination treatment regimens will be
essential in a clinical setting. In the present study, we used
lentivirus-based vectors that allowed permanent integration and
hence, the potential for stable expression of the delivered
transgene. Alternatively, retroviral replicating vectors (RRVs)
would be feasible in combination with RRV-mediated prodrug
activator gene therapy against malignant mesothelioma because they
can keep producing anti-angiogenic factors while a tumor exists
(30,31). As a combination, oncolytic viruses
such as adenovirus and measles that can selectively kill tumor
cells would be feasible because they can keep producing
anti-angiogenic factors while viruses exist in the mesothelioma
tumors (32–34).
In conclusion, continuous in situ production
of anti-angiogenic factors at tumors by combinatorial
anti-angiogenic gene therapy targeting the same and/or different
pathways of endothelial growth factor signaling has the potential
for greater therapeutic efficacy than that of a single-agent
regimen.
Acknowledgments
We thank Atsuko Tamamoto, Nobutaka Okamura and
members of the Joint-use research Facilities of the Hyogo College
of Medicine for their technical assistance; Toshiaki Shichinohe for
a part of the lentiviral constructs and Judah Folkman for sFlk1
plasmid. The present study was supported by a Grant-in-Aid for
researchers, Hyogo College of Medicine and a Grant-in-Aid for
Scientific research from the Ministry of Education, Culture,
Sports, Science and Technology of Japan (25460484).
Abbreviations:
|
VEGF
|
vascular endothelial growth factor
|
|
EGFP
|
enhanced green fluorescent protein
|
|
HUVEC
|
human umbilical vein endothelial
cells
|
|
sFlk1
|
soluble form of vascular endothelial
growth factor receptor 2
|
|
LV-A
|
lentivirus vector expressing
angiostatin
|
|
LV-E
|
lentivirus vector expressing
endostatin
|
|
LV-F
|
lentivirus vector expressing sFlk1
|
|
LV-C
|
lentivirus vector expressing EGFP
alone
|
References
|
1
|
Ismail-Khan R, Robinson LA, Williams CC
Jr, Garrett CR, Bepler G and Simon GR: Malignant pleural
mesothelioma: A comprehensive review. Cancer Control. 13:255–263.
2006.PubMed/NCBI
|
|
2
|
Tsao AS, Wistuba I, Roth JA and Kindler
HL: Malignant pleural mesothelioma. J Clin Oncol. 27:2081–2090.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
van der Most RG, Robinson BW and Nelson
DJ: Gene therapy for malignant mesothelioma: Beyond the infant
years. Cancer Gene Ther. 13:897–904. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kotova S, Wong RM and Cameron RB: New and
emerging therapeutic options for malignant pleural mesothelioma:
Review of early clinical trials. Cancer Manag Res. 7:51–63.
2015.PubMed/NCBI
|
|
5
|
Weis SM and Cheresh DA: Tumor
angiogenesis: Molecular pathways and therapeutic targets. Nat Med.
17:1359–1370. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Redlitz A, Daum G and Sage EH: Angiostatin
diminishes activation of the mitogen-activated protein kinases
ERK-1 and ERK-2 in human dermal microvascular endothelial cells. J
Vasc Res. 36:28–34. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
O’reilly MS, Holmgren L, Shing Y, Chen C,
Rosenthal RA, Moses M, Lane WS, Cao Y, Sage EH and Folkman J:
Angiostatin: A novel angiogenesis inhibitor that mediates the
suppression of metastases by a Lewis lung carcinoma. Cell.
79:315–328. 1994. View Article : Google Scholar
|
|
8
|
Shichinohe T, Bochner BH, Mizutani K,
Nishida M, Hegerich-Gilliam S, Naldini L and Kasahara N:
Development of lentiviral vectors for antiangiogenic gene delivery.
Cancer Gene Ther. 8:879–889. 2001. View Article : Google Scholar
|
|
9
|
O’reilly MS, Boehm T, Shing Y, Fukai N,
Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR and Folkman J:
Endostatin: An endogenous inhibitor of angiogenesis and tumor
growth. Cell. 88:277–285. 1997. View Article : Google Scholar
|
|
10
|
Kuo CJ, Farnebo F, Yu EY, Christofferson
R, Swearingen RA, Carter R, von Recum HA, Yuan J, Kamihara J, Flynn
E, et al: Comparative evaluation of the antitumor activity of
antiangiogenic proteins delivered by gene transfer. Proc Natl Acad
Sci USA. 98:4605–4610. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Szentirmai O, Baker CH, Bullain SS, Lin N,
Takahashi M, Folkman J, Mulligan RC and Carter BS: Successful
inhibition of intracranial human glioblastoma multiforme xenograft
growth via systemic adenoviral delivery of soluble endostatin and
soluble vascular endothelial growth factor receptor-2: Laboratory
investigation. J Neurosurg. 108:979–988. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu Y, Li ZY, Zhao X, Kan B and Wei YQ:
Inhibition of ovarian tumor growth by gene therapy with recombinant
soluble vascular endothelial growth factor receptor 2. Hum Gene
Ther. 17:941–948. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zucali PA, Ceresoli GL, De Vincenzo F,
Simonelli M, Lorenzi E, Gianoncelli L and Santoro A: Advances in
the biology of malignant pleural mesothelioma. Cancer Treat Rev.
37:543–558. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Remon J, Lianes P, Martínez S, Velasco M,
Querol R and Zanui M: Malignant mesothelioma: New insights into a
rare disease. Cancer Treat Rev. 39:584–591. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Strizzi L, Catalano A, Vianale G, Orecchia
S, Casalini A, Tassi G, Puntoni R, Mutti L and Procopio A: Vascular
endothelial growth factor is an autocrine growth factor in human
malignant mesothelioma. J Pathol. 193:468–475. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kumar-Singh S, Weyler J, Martin MJ,
Vermeulen PB and Van Marck E: Angiogenic cytokines in mesothelioma:
A study of VEGF, FGF-1 and -2, and TGF beta expression. J Pathol.
189:72–78. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yasumitsu A, Tabata C, Tabata R, Hirayama
N, Murakami A, Yamada S, Terada T, Iida S, Tamura K, Fukuoka K, et
al: Clinical significance of serum vascular endothelial growth
factor in malignant pleural mesothelioma. J Thorac Oncol.
5:479–483. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kourlas H and Abrams P: Ranibizumab for
the treatment of neovascular age-related macular degeneration: A
review. Clin Ther. 29:1850–1861. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jenab-Wolcott J and Giantonio BJ:
Bevacizumab: Current indications and future development for
management of solid tumors. Expert Opin Biol Ther. 9:507–517. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Langer C and Soria JC: The role of
anti-epidermal growth factor receptor and anti-vascular endothelial
growth factor therapies in the treatment of non-small-cell lung
cancer. Clin Lung Cancer. 11:82–90. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ceresoli GL, Zucali PA, Mencoboni M, Botta
M, Grossi F, Cortinovis D, Zilembo N, Ripa C, Tiseo M, Favaretto
AG, et al: Phase II study of pemetrexed and carboplatin plus
bevacizumab as first-line therapy in malignant pleural
mesothelioma. Br J Cancer. 109:552–558. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dowell JE, Dunphy FR, Taub RN, Gerber DE,
Ngov L, Yan J, Xie Y and Kindler HL: A multicenter phase ii study
of cisplatin, pemetrexed, and bevacizumab in patients with advanced
malignant mesothelioma. Lung Cancer. 77:567–571. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kubo S and Mitani K: A new hybrid system
capable of efficient lentiviral vector production and stable gene
transfer mediated by a single helper-dependent adenoviral vector. J
Virol. 77:2964–2971. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kubo S, Seleme MC, Soifer HS, Perez JL,
Moran JV, Kazazian HH Jr and Kasahara N: L1 retrotransposition in
non-dividing and primary human somatic cells. Proc Natl Acad Sci
USA. 103:8036–8041. 2006. View Article : Google Scholar
|
|
25
|
Takahashi S: Vascular endothelial growth
factor (VEGF), VEGF receptors and their inhibitors for
antiangiogenic tumor therapy. Biol Pharm Bull. 34:1785–1788. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bougatef F, Quemener C, Kellouche S, Naïmi
B, Podgorniak MP, Millot G, Gabison EE, Calvo F, Dosquet C, Lebbé
C, et al: EMMPRIN promotes angiogenesis through hypoxia-inducible
factor-2alpha-mediated regulation of soluble VEGF isoforms and
their receptor VEGFR-2. Blood. 114:5547–5556. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee S, Chen TT, Barber CL, Jordan MC,
Murdock J, Desai S, Ferrara N, Nagy A, Roos KP and Iruela-Arispe
ML: Autocrine VEGF signaling is required for vascular homeostasis.
Cell. 130:691–703. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Robinson CJ and Stringer SE: The splice
variants of vascular endothelial growth factor (VEGF) and their
receptors. J Cell Sci. 114:853–865. 2001.PubMed/NCBI
|
|
29
|
Li Q, Yano S, Ogino H, Wang W, Uehara H,
Nishioka Y and Sone S: The therapeutic efficacy of anti vascular
endothelial growth factor antibody, bevacizumab, and pemetrexed
against orthotopically implanted human pleural mesothelioma cells
in severe combined immunodeficient mice. Clin Cancer Res.
13:5918–5925. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kawasaki Y, Tamamoto A, Takagi-Kimura M,
Maeyama Y, Yamaoka N, Terada N, Okamura H, Kasahara N and Kubo S:
Replication-competent retrovirus vector-mediated prodrug activator
gene therapy in experimental models of human malignant
mesothelioma. Cancer Gene Ther. 18:571–578. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kubo S, Takagi-Kimura M, Logg CR and
Kasahara N: Highly efficient tumor transduction and antitumor
efficacy in experimental human malignant mesothelioma using
replicating gibbon ape leukemia virus. Cancer Gene Ther.
20:671–677. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kubo S, Kawasaki Y, Yamaoka N, Tagawa M,
Kasahara N, Terada N and Okamura H: Complete regression of human
malignant mesothelioma xenografts following local injection of
midkine promoter-driven oncolytic adenovirus. J Gene Med.
12:681–692. 2010.PubMed/NCBI
|
|
33
|
Takagi-Kimura M, Yamano T, Tagawa M and
Kubo S: Oncolytic virotherapy for osteosarcoma using midkine
promoter-regulated adenoviruses. Cancer Gene Ther. 21:126–132.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Takagi-Kimura M, Yamano T, Tamamoto A,
Okamura N, Okamura H, Hashimoto-Tamaoki T, Tagawa M, Kasahara N and
Kubo S: Enhanced antitumor efficacy of fiber-modified, midkine
promoter-regulated oncolytic adenovirus in human malignant
mesothelioma. Cancer Sci. 104:1433–1439. 2013. View Article : Google Scholar : PubMed/NCBI
|