Introduction
Of all gynecological malignancies, ovarian carcinoma
accounts for the highest number of cases of mortality in Europe
(1), the United States (2), and China (3). The current standard of care includes
the combination of radical surgery and platinum-based chemotherapy.
Among patients who have early-stage cancers, 90% can be cured using
current therapies; however, this percentage declines substantially
in patients with advanced disease (4). Approximately 30% of patients with
advanced-stage ovarian carcinoma survive 5 years after the initial
diagnosis. Despite prolific drug development, the treatment of
ovarian carcinoma is confronted with difficulties, such as
metastatic bulky disease burden and stagnant mortality rates
(5). Thus, new methods for the
early detection of ovarian carcinoma and effective treatment are
important.
Targeted therapy is a new treatment strategy that
aims to increase tumor selectivity while decreasing the toxic
effects on healthy cells. Targeted therapies use specific molecules
in tumor tissues as carriers of tumor-targeting drugs to improve
anticancer drug delivery, drug-targeting specificity, and safety
(6,7). Various antigens and receptors in
carcinoma cells that differ from normal cells have been found.
Numerous targets exist in ovarian carcinoma cells. The receptors
include VEGFR, ER-α, ErbB, EGFR, and IGF-1R, among others (8–10). The
antigens include CA-125, TAG-72, PEM and Lewis-Y, among others
(11,12). Various monoclonal antibodies
directed toward specific antigens in patients with ovarian cancer
have been reviewed and discussed (13). However, the effect of clinical
ovarian cancer treatment is not ideal (14). The lack of an efficient targeting
carrier system presents an obstacle to its efficacy.
Peptides have displayed traits that are suitable for
diagnosis and targeted treatment. These traits include efficient
tissue targeting and low toxicity (15–20).
Phage display is a technique that fuses random peptides to the
protein coat of a bacteriophage in a manner that makes them
accessible to target ligands. The DNA that encodes the peptide
sequence is protected within the virion (21). This technology has facilitated
significant developments that can be used in the long term. The
identified ‘homing’ peptides are promising alternatives to the
currently used biomolecules for targeting metastatic cells due to
their rapid blood clearance, increased diffusion and tissue
penetration, non-immunogenic nature, and ease of synthesis
(19).
The goal of this research was to identify a specific
peptide sequence that binds to ovarian cancer cells for the further
development of targeted treatment by screening a library of
phage-displayed peptides in vivo. These peptides can
simulate the body environment and maintain the native conformation
of various types of ligands on the tissues of interest. In the
initial step, a 7-mer library was injected into nude mice through a
vein. After three rounds of screening, phages were enriched.
Through the detection of the target phage and extraction of the
phage DNA test sequence, the specific-binding peptide was derived.
The position, distribution and targeting effect of OSTP were also
verified in vitro and in vivo. However, differences
exist between humans and mice. Thus, human pathologic specimens
were used to test the affinity.
Materials and methods
Cell lines
Human ovarian cancer A2780 cells were provided by
the Huazhong University of Science and Technology. Human
osteosarcoma MG63 cells were preserved in our laboratory. All cell
lines were maintained in complete RPMI-1640 medium containing 10%
fetal bovine serum.
Tissue specimens
The study adhered to the laws of China regarding
research and the guidelines approved by the Ethics Committee of the
University of South China. Archival paraffin-embedded,
formalin-fixed specimens of oophoroma tissues, ovarian cystadenoma
tissues, other ovarian tumor tissues, ovarian normal tissues, and
uterine tissues were obtained from the Pathological Diagnostic
Center and The First Affiliated Hospital of the University of South
China. All tissue samples were collected at initial diagnosis from
January 2009 through December 2012 before treatment, including
chemotherapy or radiation.
Construction of the mouse models
Four-week-old BALB/c nu/nu mice were obtained from
Beijing Vital River Laboratories. A single dose of 1×107
A2780 cells was injected s.c. into the posterior trunk to induce
tumor formation.
Peptide library screening, FliTrx clone
binding and peptide synthesis
A random phage 7-mer peptide display library, FliTrx
(new England Biolabs), was screened. Tumor-targeting FliTrx clones
were isolated from the FliTrx library using combined in vivo
screening according to the manufacturer’s instructions.
Quantification of binding selectivity was determined by cell-based
ELISA. A2780 cells were cultured and plated into a 96-well plate
(1×104 cells/well) the day before use. Cells were
washed, incubated in serum-free RPMi-1640 medium at 37°C for 2 h,
and then fixed in 4% paraformaldehyde in phosphate-buffered saline
(PBS) for 15 min. Cells were washed thrice with Tris-buffered
saline Tween-20 (TBST) and blocked with blocking buffer (TBST
contained 3% BSA) at 37°C for 1 h. The randomly selected amplified
phage clones were each added into the cells at 1012
pfu/well, and the plate was incubated at 37°C for 1.5 h.
Subsequently, unbound phage was removed by washing the plate thrice
with TBST. To detect phage binging to the cells, the wells were
incubated for 1 h with 100 μl/well of mouse anti-M13
antibody (dilution, 1:5,000 in the blocking buffer). After washing
the plate thrice with TBST, 100 μl of HRP-conjugated sheep
anti-mouse ig was added to each well (dilution, 1:5,000 in the
blocking buffer). Subsequently, color development was induced by
adding 100 μl/well of freshly prepared diaminobenzidine
solution and then incubating the plate for 5 min at 37°C. The
plates were read on an automated ELISA plate reader at an
absorbance of 490 nm. Triplicate determinations were performed at
each data point. Selectivity was determined using the following
formula: P/N (the positive phage OD/control phage OD) >2.1. The
ELiSA-positive phage was expanded, and the single-stranded DNA
clone sequence was detected. According to the base sequence, the
short peptide amino acid sequence was obtained. The OSTP peptide
and the control peptide NSTP, the amino acid of which was screened
from another research, were detected. Then, 5-FAM was coupled at
the NH2 terminus. The peptides were synthesized by
Shanghai Sangong Co., and then purified by high-performance liquid
chromatography. The sequence and structure of the peptides were
confirmed by mass spectrometry.
Cell fluorescence staining
Ovarian cancer A2780 cells and osteosarcoma MG63
cells were cultured at the logarithmic phase for use in the
experiment. After pancreatic enzyme digestion and heavy suspension,
the cells were adjusted to 2.5×105/ml and then placed
into 6 orifice plates for culture. In the experimental group, 2
μl (4.9 mg/ml) of 5-FAM-OSTP was added, whereas in the
control group, 2 μl of 50% DMSO solvent agent was added. The
cells were continuously cultured for 16 h while avoiding light. PBS
was used to wash the plates. The cover glasses were removed when
the cells were dry. The cells were then fixed with acetone for 15
min, washed, and exposed to antagonize fluorescence quenching agent
to seal the pieces. The cells were examined for fluorescence by
using laser scanning confocal microscopy (Olympus). Experiments
were repeated thrice. The fluorescence distribution of the
incubated A2780 cells was observed in different periods, and images
were captured.
Tumor targeting
Tumor-bearing mice were used for targeting
experiments. The tumors grew to a size of 1.0 to 2.0
cm3. OSTP (980 μg) was injected into the tail
vein and allowed to circulate for 15 min. The control group was
injected with NSTP (980 μg) and 20% DMSO (980 μg).
The mice were perfused with PBS through the left ventricle to
remove blood and unbound peptides. Tumors and control organs were
excised, and frozen sections were prepared and examined for
fluorescence using laser scanning confocal microscopy.
Quantification of the imaging results was accomplished using
Image-Pro Plus 6.0.
Affinity of OSTP to ovarian cancer
tissues
Human ovarian cancer samples, human ovarian
cystadenoma samples, other ovarian tumor samples, human normal
ovarian samples, and human uterine samples were sectioned serially
at 2 μm. After drying at 60°C from 30 to 60 min, the slides
were transferred to xylene I for 20 min, xylene II for 10 min, 100%
ethanol for 5 min, 95% ethanol for 5 min, and then 85% ethanol for
5 min to be deparaffinized and rehydrated. The slides were then
shaken and washed twice for 5 min with ddH2O and twice
for 5 min with PBS. Antigen was retrieved by soaking the slides in
pH 6.0 citric acid solution. The slides were blocked in BSA for 30
min at 37°C in a chamber and then in 100 μl of 5-FAM-OSTP
(2.0 mg/ml) for 1 h at 37°C in a chamber kept away from light and
sealed with a Na2CO3 and glycerine solution.
The slides were then observed, and images were captured under a
fluorescence microscope. The same tissue slides were diagnosed
through H&E staining.
Statistical analysis
Values are expressed as mean ± SD. The significance
of the difference from the respective controls for each
experimental test condition was assayed using the Student’s t-test
for each paired experiment. A p-value of <0.05 or 0.01 was
considered as indicative of a statistically significant result.
Results
Identification of a peptide that
specifically binds to ovarian cancer cells
Targeting peptides were selected in vivo by
screening the FliTrx library with the ovarian carcinoma A2780 cells
for three rounds. After each round of screening, the FliTrx
concentration in the tumor tissues was significantly increased,
whereas that in the control tissues was reduced. After the third
round, 10 individual FliTrx clones were selected. To confirm the
specific binding of the selected phages to A2780 cells, 10
independent phage clones were randomly selected for testing using
cell-based ELISA. To calculate selectivity, the binding of each
phage to the A2780 cells was compared with the original library
locus coeruleus. The phage optical density ratio of >2.1
indicated that specific binding to the A2780 cells had occurred.
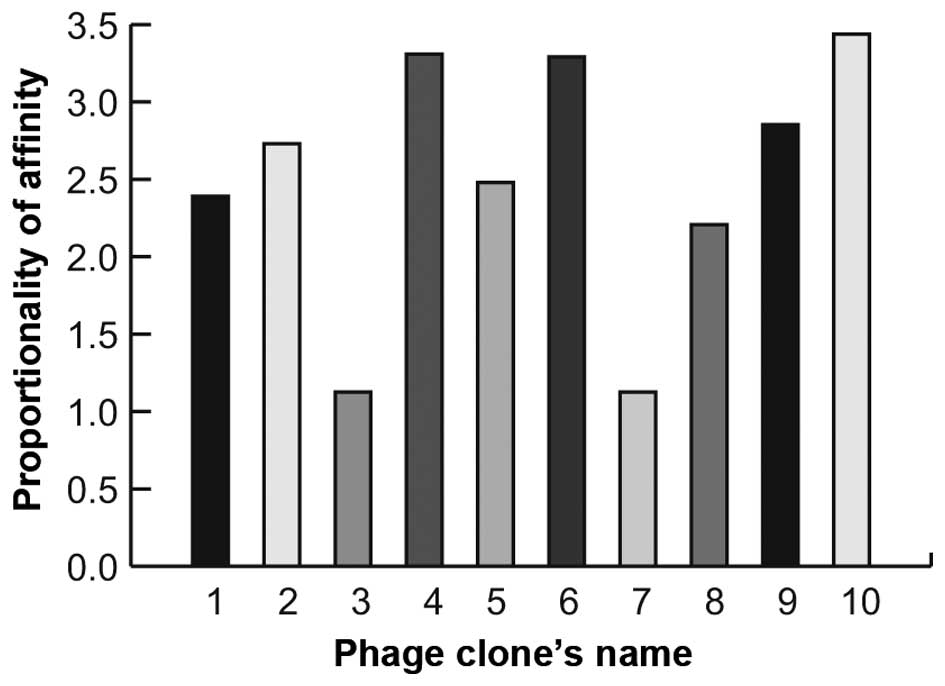
The results showed that 8 phage clones apparently possessed the
most specific binding capability (Fig.
1). The peptide-encoding inserts of these clones were
sequenced. One of the peptide sequences (PHLATLF) appeared 8 times
in the selected clones.
OSTP specifically binds to ovarian cancer
cells in vitro
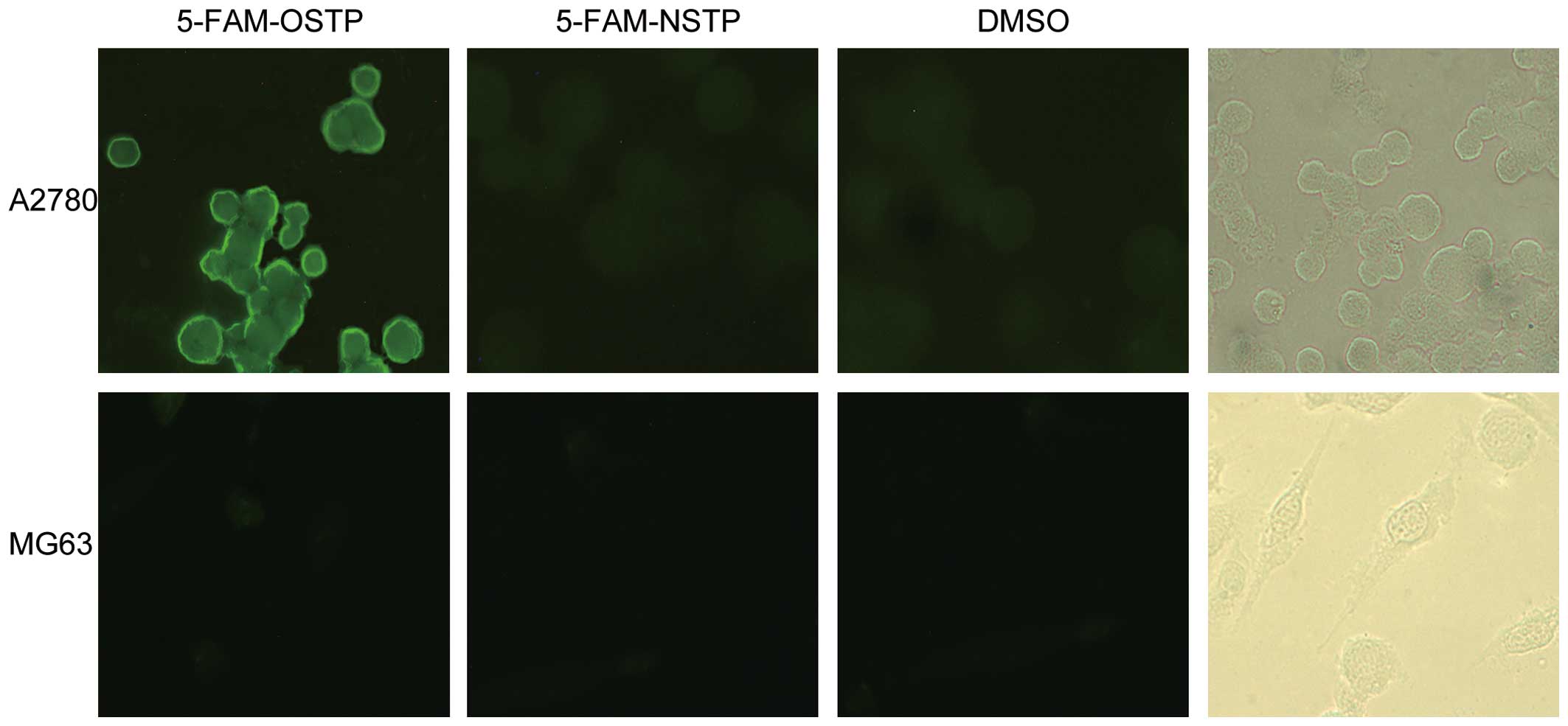
OSTP and NSTP were synthesized and labeled by 5-FAM
from Shanghai Shenggong Co. (purity >95%). The solvent control
group of the ovarian cancer A2780 cells did not produce
fluorescence (the background is dark). MG63 cells also did not
produce spontaneous fluorescence (Fig.
2). However, the ovarian cancer A2780 cell cytoplasm and
membrane produced bright yellow-green fluorescence after incubation
with 5-FAM-OSTP. The results confirmed that 5-FAM-OSTP has specific
combining capability with ovarian cancer A2780 cells, and the
binding sites are mainly located on the cell membrane.
OSTP targets ovarian cancer tumors in
vivo
First, 5-FAM-OSTP (980 μg), 5-FAM-nSTP (980
μg), and 20% DMSO (980 μg) were injected into the
tail vein of the mice and allowed to circulate for 15 min in each
group. Second, tumors and control organs were excised and processed
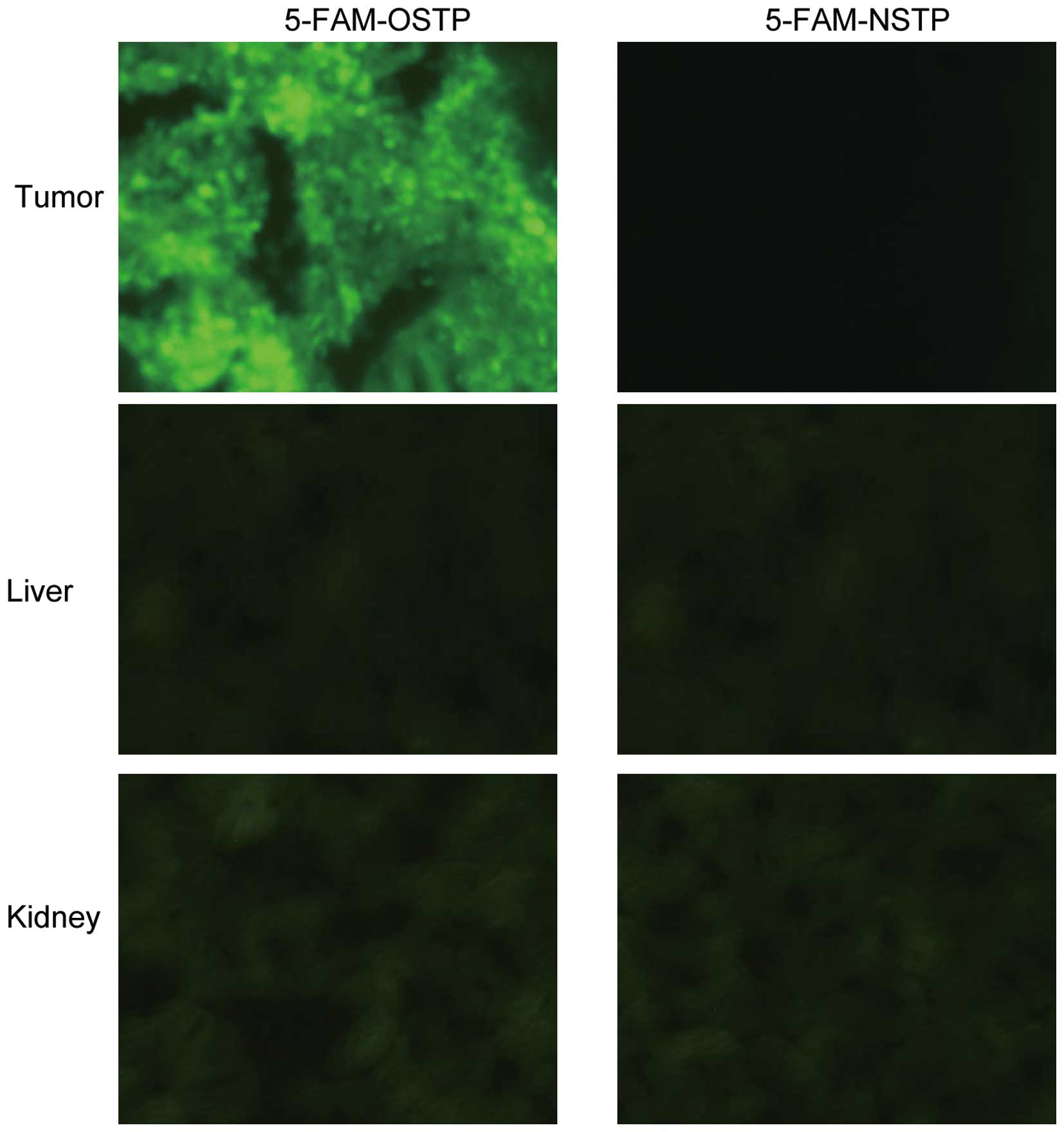
for frozen sectioning. The 5-FAM-OSTP specifically targeted tumors.
In the liver and kidney very weak fluorescence was observed,
whereas in other control organs, no visible fluorescence was
observed (Fig. 3). As shown in
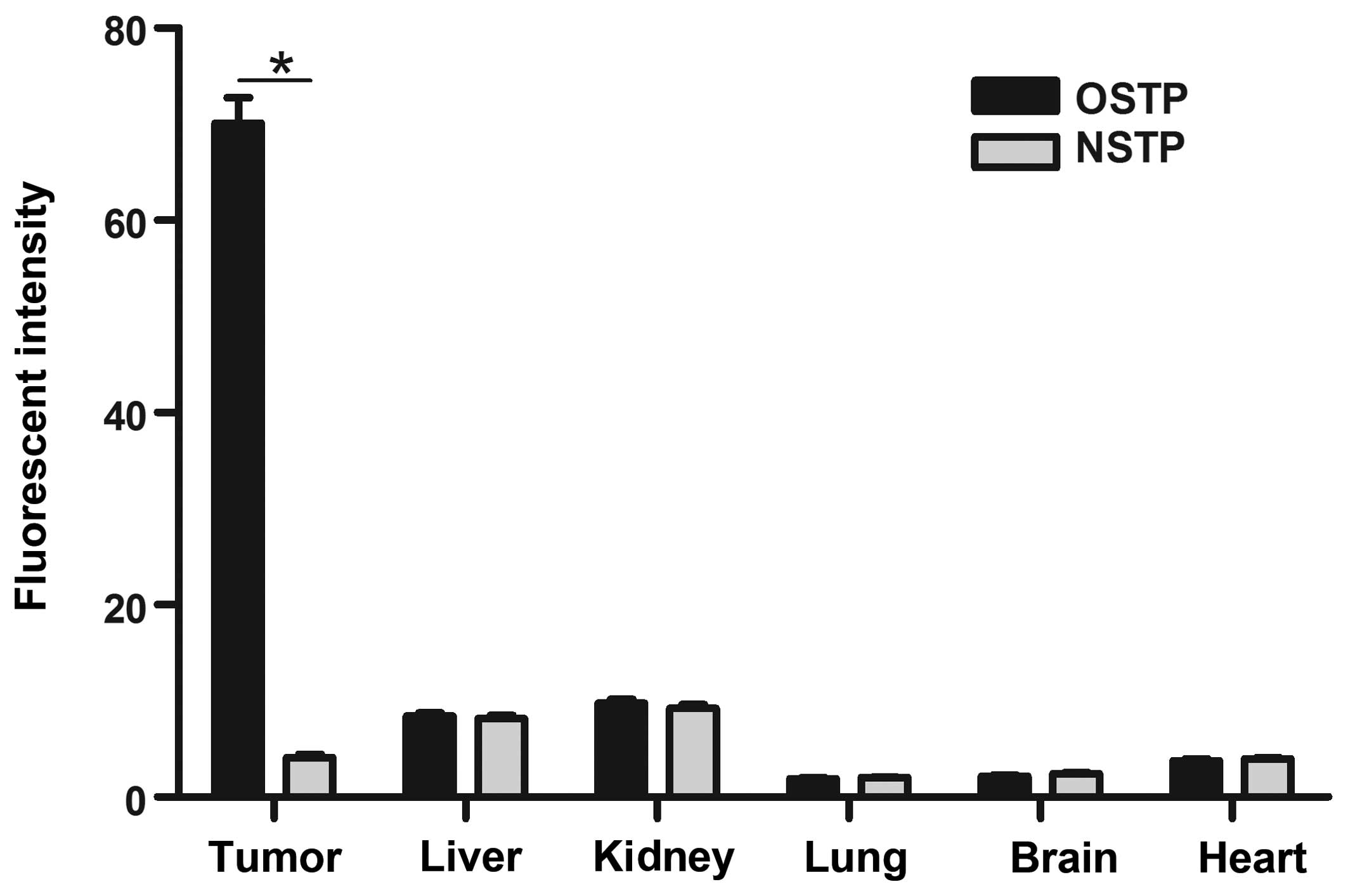
Fig. 4, the results of the analysis
of the average fluorescence intensity of organs using ImageJ
software are shown. Compared with other tissues, the fluorescent
intensity in tumor tissues was significantly higher
(P<0.01).
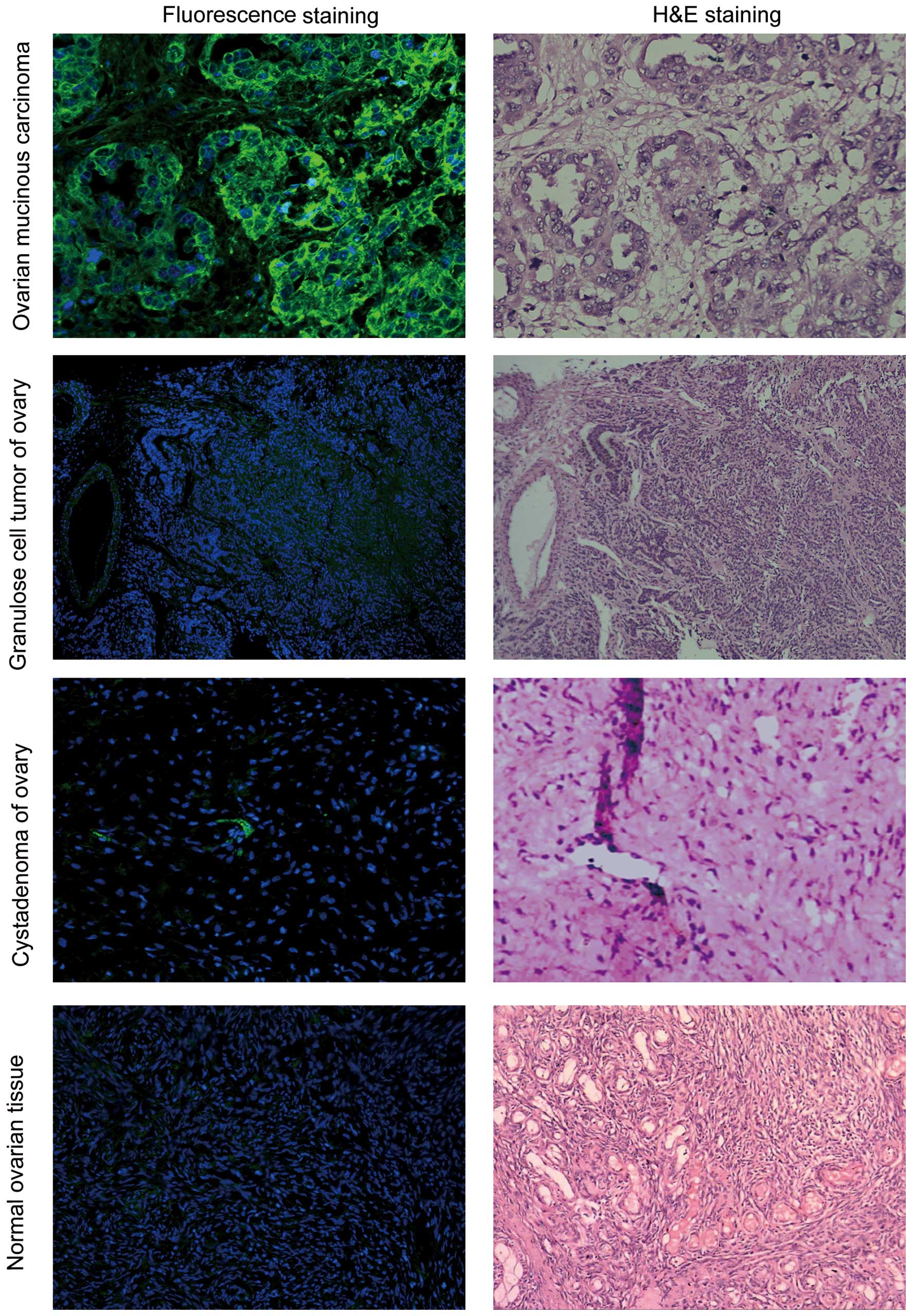
OSTP specifically binds to human ovarian
cancer specimens
Yellow-green fluorescence was observed in the
ovarian cancer tumor tissues. The fluorescence was brighter at the
cell membrane and weaker in the cytoplasm. Very weak background
fluorescence was observed in tumor stroma. 5-FAM-OSTP may
specifically combine with ovarian cancer tissues that express
various antigens. However, 5-FAM-OSTP did not combine with the
endometria and uterine wall. 5-FAM-OSTP did not combine with other
human ovarian tumors, ovarian cystadenoma and normal ovarian
tissues (Fig. 5 and Table I).
 | Table IFluorescent signal of 5-FAM-OSTP in
ovarian carcinoma tissues. |
Table I
Fluorescent signal of 5-FAM-OSTP in
ovarian carcinoma tissues.
| Type of tissue | Total no. | Cases with positive
fluorescence |
|---|
| Ovarian
carcinoma | 38 | 33 |
| Non-epithelial
ovarian malignant tumor | 12 | 2 |
| Cystadenoma of the
ovary | 11 | 1 |
| Normal ovarian | 10 | 1 |
Discussion
Ovarian cancer remains a challenge for clinical
treatment. The disease is incurable for the majority of patients
due to relapse attributed to resistance to chemotherapeutic drugs
(22). The typically used
chemotherapeutic drugs are carboplatin and paclitaxel. Novel drugs
for targeted therapy are needed to reduce drug resistance and
severe side effects, which are mainly caused by the non-cancer cell
specificity of the agents and the insensitivity of cancer cells.
Different avenues have been pursued to achieve this goal (23).
Since 1990, the phage display has been successfully
constructed, and peptide libraries have been widely applied for the
in vivo and in vitro screening for research on target
enzymes, carbohydrates, receptors, antibodies and nucleic acids
(24). A wide variety of
tumor-specific binding peptides have been discovered through the
phage display. These peptides include RGD-4C, NGR, CPRECES and GSL,
which can target tumor vascular endothelium (25-27).
Pasqualini and colleagues (28,29)
were the first to screen peptides that combined specifically to the
brain and kidney. They successfully screened another short peptide
that included the RGD sequence, which can specifically bind to
malignant melanoma and breast cancer integrin α (subunit v). By
using phage display technology, a short peptide binding
specifically to tumor vasculature was selected as a drug carrier of
adriamycin for the targeted therapy of breast tumor-bearing mice
in vivo with superior effects (30). Teesalu et al (31) found that tumor-penetrating peptides
can bind to the NRP-1 protein, thereby significantly improving the
drug penetration capability by increasing tumor accumulation. The
use of targeted peptides has made significant progress in recent
years. Yang et al (19)
detected a novel tumor-homing peptide that specifically targets
metastasis. The specific-binding peptide A54, which was screened
from a phage display library, represents a promising approach for
the development of novel targeted therapeutic strategies against
hepatocellular carcinoma. The affinity peptide was discovered for
targeted detection of dysplasia in Barrett’s esophagus (18). OA02 peptide-targeted polymeric
micelle system was developed for effective paclitaxel delivery in
an ovarian cancer xenograft mouse model (32). Li et al (33) utilized a pro-apoptotic peptide
conjugated to a Toll-like receptor and two mediated
cell-penetrating peptides that target acute myeloid leukemia. A
large number of peptide complexes are under further study. The use
of a phage display for peptide selection shows great potential for
recognizing specific targets.
In the present study, OSTP with specific affinity to
ovarian cancer in vitro and in vivo was demonstrated
in different assays. Three rounds of screening in nude mice were
conducted. After the third round of screening, the peptide sequence
(PHLTALF) appeared 8 times in ELISA, thus illustrating that the
short peptide has an effective concentration in vivo. A
random peptide library contained the same specific fragment of
phage. Thus, OSTP exhibited tissue-specific affinity. During the
input of the first round of screening, the phage library was
2.0×1011 pfu, thus ensuring that tens of thousands of
short peptide sequences contained the short peptide in the ovarian
cancer cell cytoplasm and membrane, in which some antigens that
specifically bind to OSTP are present. High affinity to tumor
tissues was observed after circling in vivo. The tumor
tissues exhibited a strong fluorescence signal. Meanwhile, the
control organs exhibited no visible fluorescence signal.
Identifying the receptor that binds to OSTP on the
cell surface is important for better understanding of the molecular
properties of OSTP. We are conducting further research to isolate
the OSTP receptor. Affinity chromatography and time-of-flight
delayed extraction MALDI mass spectrometry is being used for this
purpose.
Moreover, OSTP was investigated using clinical
tissue samples of ovarian cancer due to the differences between
human ovarian cancer tissues from the cells cultured in
vitro and simulative animal models. According to the
immunofluorescence technique, meaningful results were obtained.
Immunofluorescence results demonstrated yellow-green fluorescence
in the human ovarian cancer tissues. The fluorescence was mainly
present on the cell membranes, whereas the stroma exhibited only
weak background fluorescence. Ovarian cyst-adenoma, other ovarian
tumors, and contrast uterine tissues did not show an obvious
yellow-green fluorescent signal. The results demonstrated that OSTP
has specificity for human ovarian cancer tissues.
Therefore, OSTP may be applied as a new ovarian
cancer screening biomarker and a new targeting carrier that can be
coupled with chemotherapy drugs. The peptide structure must be
modified to improve stability in vivo and to increase water
solubility so that when combined with a drug, the antitumor
activity of the compound can be detected in ovarian cancer cells.
Moreover, the anticancer therapeutic effect on ovarian
cancer-bearing mice can be confirmed, and the pharmaco-kinetics of
compound cycling in tumor-bearing mice can be studied. OSTP appears
to be a useful tool for targeted treatment and diagnosis of ovarian
cancer.
Acknowledgments
This study was supported by grants from the National
Science Foundation of China (no. 81101988).
References
|
1
|
Gondos A, Bray F, Hakulinen T and Brenner
H: EUNICE Survival Working Group: Trends in cancer survival in 11
European populations from 1990 to 2009: A model-based analysis. Ann
Oncol. 20:564–573. 2009. View Article : Google Scholar
|
|
2
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J,
Murray T and Thun MJ: Cancer statistics, 2008. CA Cancer J Clin.
58:71–96. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang NN, Yan YQ and Gong J: An analysis on
incidence and mortality of ovarian cancer from 2003 to 2007 in
China. China Cancer. 21:401–405. 2012.
|
|
4
|
Yang D, Sun Y, Hu L, Zheng H, JI P, Pecot
CV, Zhao Y, Reynolds S, Cheng H, Rupaimoole R, et al: Integrated
analyses identify a master microRNA regulatory network for the
mesenchymal subtype in serous ovarian cancer. Cancer Cell.
23:186–199. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Coleman RL, Monk BJ, Sood AK and Herzog
TJ: Latest research and treatment of advanced-stage epithelial
ovarian cancer. Nat Rev Clin Oncol. 10:211–224. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhu AX and Hezel AF: Development of
molecularly targeted therapies in biliary tract cancers:
Reassessing the challenges and opportunities. Hepatology.
53:695–704. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Masoumi Moghaddam S, Amini A, Morris DL
and Pourgholami MH: Significance of vascular endothelial growth
factor in growth and peritoneal dissemination of ovarian cancer.
Cancer Metastasis Rev. 31:143–162. 2012. View Article : Google Scholar :
|
|
8
|
Ferrara N, Gerber HP and LeCouter J: The
biology of VEGF and its receptors. Nat Med. 9:669–676. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bookman MA, Darcy KM, Clarke-Pearson D,
Boothby RA and Horowitz IR: Evaluation of monoclonal humanized
anti-HER2 antibody, trastuzumab, in patients with recurrent or
refractory ovarian or primary peritoneal carcinoma with
overexpression of HER2: A phase ii trial of the Gynecologic
Oncology Group. J Clin Oncol. 21:283–290. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tebbutt N, Pedersen MW and Johns TG:
Targeting the ERBB family in cancer: Couples therapy. Nat Rev
Cancer. 13:663–673. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Morotti M, Valenzano Menada M, Venturini
PL, Mammoliti S and Ferrero S: Pemetrexed disodium in ovarian
cancer treatment. Expert Opin Investig Drugs. 21:437–449. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Oei AL, Sweep FC, Thomas CM, Boerman OC
and Massuger LF: The use of monoclonal antibodies for the treatment
of epithelial ovarian cancer (Review). Int J Oncol. 32:1145–1157.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Leone Roberti Maggiore U, Bellati F,
Ruscito I, Gasparri ML, Alessandri F, Venturini PL and Ferrero S:
Monoclonal antibodies therapies for ovarian cancer. Expert Opin
Biol Ther. 13:739–764. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Siegel R, Ward E, Brawley O and Jemal A:
Cancer statistics, 2011: The impact of eliminating socioeconomic
and racial disparities on premature cancer deaths. CA Cancer J
Clin. 61:212–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Corso S, Ghiso E, Cepero V, Sierra JR,
Migliore C, Bertotti A, Trusolino L, Comoglio PM and Giordano S:
Activation of HER family members in gastric carcinoma cells
mediates resistance to MET inhibition. Mol Cancer. 9:1212010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Smith GP: Filamentous fusion phage: Novel
expression vectors that display cloned antigens on the virion
surface. Science. 228:1315–1317. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang N, Thuraisingam T, Fallavollita L,
Ding A, Radzioch D and Brodt P: The secretory leukocyte protease
inhibitor is a type 1 insulin-like growth factor receptor-regulated
protein that protects against liver metastasis by attenuating the
host proinflammatory response. Cancer Res. 66:3062–3070. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li M, Anastassiades CP, Joshi B, Komarck
CM, Piraka C, Elmunzer BJ, Turgeon DK, Johnson TD, Appelman H, Beer
DG, et al: Affinity peptide for targeted detection of dysplasia in
Barrett’s esophagus. Gastroenterology. 139:1472–1480. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang W, Luo D, Wang S, Wang R, Chen R, Liu
Y, Zhu T, Ma X, Liu R, Xu G, et al: TMTP1, a novel tumor-homing
peptide specifically targeting metastasis. Clin Cancer Res.
14:5494–5502. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sugahara KN, Teesalu T, Karmali PP,
Kotamraju VR, Agemy L, Girard OM, Hanahan D, Mattrey RF and
Ruoslahti E: Tissue-penetrating delivery of compounds and
nanoparticles into tumors. Cancer Cell. 16:510–520. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Noren KA and Noren CJ: Construction of
high-complexity combinatorial phage display peptide libraries.
Methods. 23:169–178. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bamias A, Pignata S and Pujade-Lauraine E:
Angiogenesis: A promising therapeutic target for ovarian cancer.
Crit Rev Oncol Hematol. 84:314–326. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vosjan MJ, Vercammen J, Kolkman JA,
Stigter-van Walsum M, Revets H and van Dongen GA: Nanobodies
targeting the hepatocyte growth factor: Potential new drugs for
molecular cancer therapy. Mol Cancer Ther. 11:1017–1025. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zwick MB, Shen J and Scott JK:
Phage-displayed peptide libraries. Curr Opin Biotechnol. 9:427–436.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ferrara N and Kerbel RS: Angiogenesis as a
therapeutic target. Nature. 438:967–974. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Brooks PC, Montgomery AM, Rosenfeld M,
Reisfeld RA, Hu T, Klier G and Cheresh DA: Integrin alpha v beta 3
antagonists promote tumor regression by inducing apoptosis of
angiogenic blood vessels. Cell. 79:1157–1164. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li XB, Schluesener HJ and Xu SQ: Molecular
addresses of tumors: Selection by in vivo phage display. Arch
Immunol Ther Exp (Warsz). 54:177–181. 2006. View Article : Google Scholar
|
|
28
|
Pasqualini R and Ruoslahti E: Organ
targeting in vivo using phage display peptide libraries. Nature.
380:364–366. 1996. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pasqualini R, Koivunen E and Ruoslahti E:
Alpha v integrins as receptors for tumor targeting by circulating
ligands. Nat Biotechnol. 15:542–546. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Arap W, Pasqualini R and Ruoslahti E:
Cancer treatment by targeted drug delivery to tumor vasculature in
a mouse model. Science. 279:377–380. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Teesalu T, Sugahara KN, Kotamraju VR and
Ruoslahti E: C-end rule peptides mediate neuropilin-1-dependent
cell, vascular, and tissue penetration. Proc natl Acad Sci USA.
106:16157–16162. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xiao K, Li Y, Lee JS, Gonik AM, Dong T,
Fung G, Sanchez E, Xing L, Cheng HR, Luo J, et al: ‘OA02’ peptide
facilitates the precise targeting of paclitaxel-loaded micellar
nanoparticles to ovarian cancer in vivo. Cancer Res. 72:2100–2110.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li K, Lv XX, Hua F, Lin H, Sun W, Cao WB,
Fu XM, Xie J, Yu JJ, Li Z, et al: Targeting acute myeloid leukemia
with a proapoptotic peptide conjugated to a toll-like receptor
2-mediated cell-penetrating peptide. Int J Cancer. 134:692–702.
2014. View Article : Google Scholar
|