Introduction
Breast cancer (BCa) is the leading cause of
cancer-related mortality in women worldwide (1). The incidence of BCa in Taiwan has
increased from 6.23/100,000 in 1970 to 23.76 in 2000. In a 2005
report, breast cancer was the second most frequent cancer in women
with an incident rate of 42.3/100,000 (2). Cases in men are extremely rare and the
ratio of males to females is 0.3:100. The Bureau of Health
Promotion Data from 1998 to 2002 indicate that 5-year survival for
all stages was 78.37%. The 5- and 10-year survival rates were 98
and 95% for stage 0; 96 and 89% for stage I; 90 and 82% for stage
II; 65 and 53% for stage III; and 22 and 10% for stage IV (3). Triple-negative breast cancer (TNBC)
accounts for 15–25% of the breast cancer cases. This subtype of BCa
refers to any type of breast cancer that does not express the genes
for estrogen (ER) and progesterone (PR) receptors, and Her2/neu
(4). It is thought to be more
aggressive and to respond poorly to hormone therapy, and is more
difficult to treat since there is no receptor target to be
antagonized. The risk of relapse in TNBC is also much higher for
the first 3–5 years.
The Bcl2-like 12 (Bcl2L12) gene was
identified and cloned by Scorilas et al (5) in 2001, and is a proline-rich (PxxP)
protein and a newly identified member of the Bcl2 family,
containing a highly conserved BH2 domain, a BH3-like motif and a
proline-rich region. Currently, two splicing variants of the
Bcl2L12 gene are known: one consisting of seven coding exons
and producing a 334-amino acid protein with a molecular mass of
36.8 kDa, and another resulting from alternative splicing, leading
to a protein of 176 amino acids, a splice variant known as Bcl2L12A
which lacks exon 3 (143 bp) (5).
Expression of the full-length mRNA transcript has been observed in
many tissues, including breast, thymus, prostate, fetal liver,
colon, placenta, pancreas, small intestine, spinal cord, kidney and
bone marrow, whereas Bcl2L12A is mainly expressed in fetal liver,
spinal cord and skeletal muscle (5). Bcl2L12 and Bcl2L12A are localized
within the nucleus (6). The
biological role of Bcl2L12 is not yet completely understood and
remains paradoxical. In previous studies, Bcl2L12 and Bcl2L12A
exhibited pro-apoptotic activity in BCa and gastric cancer
(7–9). A 3-fold increase of Bcl2L12 levels was
demonstrated in non-cancerous compared to cancerous stomach tissues
(9). In BCa, the two proteins are
highly expressed in normal breast tissue, and Bcl2L12 has been
identified as a favorable prognostic marker. Knockdown of its
expression leads to cisplatin-resistance in the MDA-MB-231 BCa cell
line (7). In nasopharyngeal cancer,
the Bcl2L12 expression status was also found to be positively
associated with distant metastases and to be an unfavorable and
independent prognostic indicator of short-term relapse. Bcl2L12
mRNA expression may thus constitute a novel biomarker for the
prediction of short-term relapse in nasopharyngeal carcinoma. By
contrast, Bcl2L12 and Bcl2L12A are ubiquitously overexpressed in
primary human GBMs and may be associated with resistance to
chemotherapeutic agent-induced apoptosis, which is an important
hallmark of this disease (10).
Furthermore, Bcl2L12 plays an anti-apoptotic role in GBM and blocks
post-mitochondrial apoptotic signaling by inhibiting effectors
caspase-3 and -7 (11–13). Besides that, Bcl2L12 attenuates
endogenous p53-directed transcriptomic changes after genotoxic
stress and inhibits p53-dependent DNA damage-induced apoptosis
(10). The anti-apoptotic role of
Bcl2L12 and Bcl2L12A was found to be regulated by GSK3β in
glioblastoma and was inhibited by LiCl (14). ERβ5 was observed to interact with
Bcl2L12 in a novel estrogen-independent molecular pathway that
promotes cisplatin and/or doxorubicin-induced in vitro
apoptosis of the MCF-7 and MDA-MD-231 BCa cell lines (15). Taken together, the roles of Bcl2L12
and its short variant in BCa remain largely unknown and
contradictory. Moreover, it is also unclear whether Bcl2L12 and
Bcl2L12A mRNA can be used as biomarkers for Bca progression and/or
a subtype of BCa. Therefore, in this study we screened and analyzed
the expression of Bcl212 and Bcl2L12A mRNA in clinical specimens to
address these issues.
Materials and methods
Tissue collection
A total of 106 paraffin-embedded BCa tissues and 1
flesh tumor tissue were collected with the permission of the
institutional Review Board of Kaohsiung Armed Forces general
hospital in 2013. The expression profile of Bcl2L12 and Bcl2L12A
was assessed and analyzed to determine whether they were
correlated. The clinicopathological characteristics of these tumors
are shown in Table I. There were
102 (96.23%) invasive and 4 (3.77%) non-invasive tumors. The
invasive tumor group included ductal (n=89, 87.25%), lobular (n=4,
3.92%), papillary (n=1, 0.98%) carcinomas, medullary carcinomas,
atypical (n=1, 0.98%) and mucinous adenocarcinoma (n=7, 6.86%). The
non-invasive tumors included ductal carcinoma in situ (CIS)
(n=2, 50.0%) and intraductal papillary carcinoma (n=2, 50.0%).
Histological grades were classified into the low (grade I and II,
n=97, 91.5%) and high (grade III, n=8, 8.5%) grade groups. HER2/neu
protein expression was negative (0 to +1) in 56 specimens and
positive in 49 (+2 to +3). In TNM staging for tumor size or direct
extent of the primary tumor, T2 stage (n=58) predominated followed
by the T1 stage (n=31), T3 stage (n=3), T4 stage (n=3) and CIS
(Tis, n=2). Regarding the spread to regional lymph nodes, tumor
cells were absent from regional lymph nodes in 64 (N0) and regional
lymph node metastasis was present in 36 (N1). For distant
metastasis, samples were grouped into no distant metastasis (n=95)
and metastasis to distant organs (n=5).
 | Table IClinicopathological characteristics of
BCa specimens. |
Table I
Clinicopathological characteristics of
BCa specimens.
| Clinical diagnostic
BCa type | | Total |
|---|
| Invasive tumors | 102 | 106a |
| Invasive ductal
carcinoma | 89 | |
| Invasive lobular
carcinoma | 4 | |
| Invasive papillary
carcinoma | 1 | |
| Medullary carcinoma,
atypical | 1 | |
| Mucinous
adenocarcinoma | 7 | |
| Non-invasive
tumors | 4 | |
| Ductal CIS | 2 | |
| Intraductal
papillary carcinoma | 2 | |
| Histological
grade | | 105 |
| Low (I and II) | 97 | |
| High (III) | 8 | |
| HER2/neu IHC | | 105 |
| Negative (0 to
+1) | 56 | |
| Positive (+2 to
+3) | 49 | |
| Lymph node
metastasis | | 104b |
| Negative | 60 | |
| Positive | 34 | |
| TNM staging | | 100c |
| T1 : 31 | n0 : 64 | m0 : 95 | | |
| T2 : 58 | n1a : 17 | m1 : 5 | | |
| T3 : 5 | n2a : 9 | | | |
| T4 : 3 | n3a : 10 | | | |
| Tis : 2 | | | | |
| Tx : 1 | | | | |
RNA extraction
Total RNA was prepared from paraffin-embedded breast
cancerous or normal tissue using a PureLink FFPE Total RNA
isolation kit (Invitrogen-Life Technologies, Carlsbad, CA, USA).
Deparaffination, purification and washing were conducted according
to the manufacturer’s instructions. RNA quality was determined by
the ratio of OD260 vs OD280 nm. The RNA concentration was
determined by detecting absorbance at 280 nm on a Tecan infinite
M200 multiscan spectrophotometer (Tecan Group, Bayonne, NJ,
USA).
cDNA synthesis
First-strand cDNA synthesis was carried out using
the ImProm-II Reverse Transcription system (Promega, Madison, WI,
USA). Briefly, up to 1 μg of total RNA was premixed with
Oligo (dT) and random hexamers in a vial, then heated at 70°C for 5
min and chilled at 4°C for 5 min for pre-denaturation.
Subsequently, 4 μl ImProm-II 5X reaction buffer, 25 mM
MgCl2, 1 μl 10 mM dNTP Mix, 20 units ribonuclease
inhibitor and nuclease-free water were added to a 1.5 ml eppendorf
tube. Then, 1 μl ImProm-II Reverse Transcriptase was added
to a final volume of 15 μl and incubated at 25°C for 5 min
for primer annealing, 42°C for 60 min for synthesis, and 70°C for
15 min to inactivate the enzyme. The generated cDNA were stored at
−20°C for quantitative PCR (qPCR).
Quantitative PCR
A total of 106 BCa tissues underwent qPCR. Detection
of mRNA expression levels with respect to endogenous Bcl2L12 and
Bcl2L12A was performed by EZtime 2X SYBR-Green Premix real-time PCR
(Yeastern Biotech, Taipei, Taiwan). The thermal cycling program was
carried out on an IQ5 Real-Time PCR Detection system (Bio-Rad,
Hercules, CA, USA). The increase in fluorescence emission (Rn) was
measured during PCR amplification, and the difference (ΔRn) between
the fluorescence emission of the product and the baseline was
calculated with IQ5 Optical System software (Bio-Rad) and plotted
against the cycle number. Threshold cycle values were then
calculated by determining the point at which the emitted
fluorescence exceeded the threshold, determined as 10-fold the
standard deviation of the baseline of cycles 3–15 (16). The reaction mixture contained 10 ng
cDNA diluted in 2.5 μl diethylpyro-carbonate-treated water
(Applied Biosystems, Foster City, CA, USA), 12.5 μl EZtime
2X SYBR-green Premix PCR and 2 μl gene-specific primers
(final concentration: 50 nmol/l each), in a final reaction volume
of 25 μl. The reaction conditions were set up as follows:
For Bcl2L12 and Bcl2L2A: denaturation of the template and
activation of DNA polymerase at 95°C for 10 min, followed by 45
cycles of 95°C for 20 sec; for denaturation of the PCR products,
58°C for 1 min for primer annealing and 72°C for 15 sec for
extension. The conditions for β-actin were: denaturation of the
template and activation of DNA polymerase at 95°C for 10 min
followed by 45 cycles of 95°C for 10 sec for denaturation of the
PCR products, 58°C for 1 min for primer annealing and 55°C for 15
sec for extension. Each RT-PCR experiment was performed in
duplicate to evaluate data reproducibility. To distinguish the main
PCR products from primer-dimers or other non-specific products, a
melting curve analysis of the PCR products was generated after
amplification by heating the reaction mixtures at 60–95°C with a
heat ramping rate of 0.1°C/sec while continuously acquiring
fluorescence emission data (18).
The melting temperatures (Tm) of the target genes and β-actin
amplicons were expected to be 80 and 85°C respectively, whereas
primer-dimers or other non-specific products were characterized by
a much lower Tm (up to 75.0°C). The calculations and validation of
the comparative CT (2−ΔΔCT) method were used for target
gene mRNA quantification. The application of this method was based
on the hypothesis that the PCR amplification efficiencies of the
target and the reference genes were similar to each other and close
to 100% (17). The prerequisites
for the application of the 2−ΔΔCT method were checked in
a validation experiment, in which CT values of target genes and
β-actin were measured in a dilution series of control cDNA over a
106-fold range and then plotted against log cDNA
dilution according to a previous study (18). RT-PCR efficiency (e%) for
amplification of each gene was calculated using the formula: E% =
[−1 + 10 (−1/α)] × 100, where α is the slope of the corresponding
amplification plot (18). β-actin
was used as a reference gene to normalize the PCRs for the amount
of RNA added to the reverse transcription reactions. Normalized
results were expressed in the ratio of target gene mRNA copies to
β-actin mRNA copies (c/c). The results were the average of data
from at least triplicate experiments and were shown as fold
increase ± SEM. All primer pairs with respect to Bcl2L12, Bcl2L12A
and β-actin were designed using a web-based program provided by
GeneScript.com. The primer pairs were qualified and demonstrated:
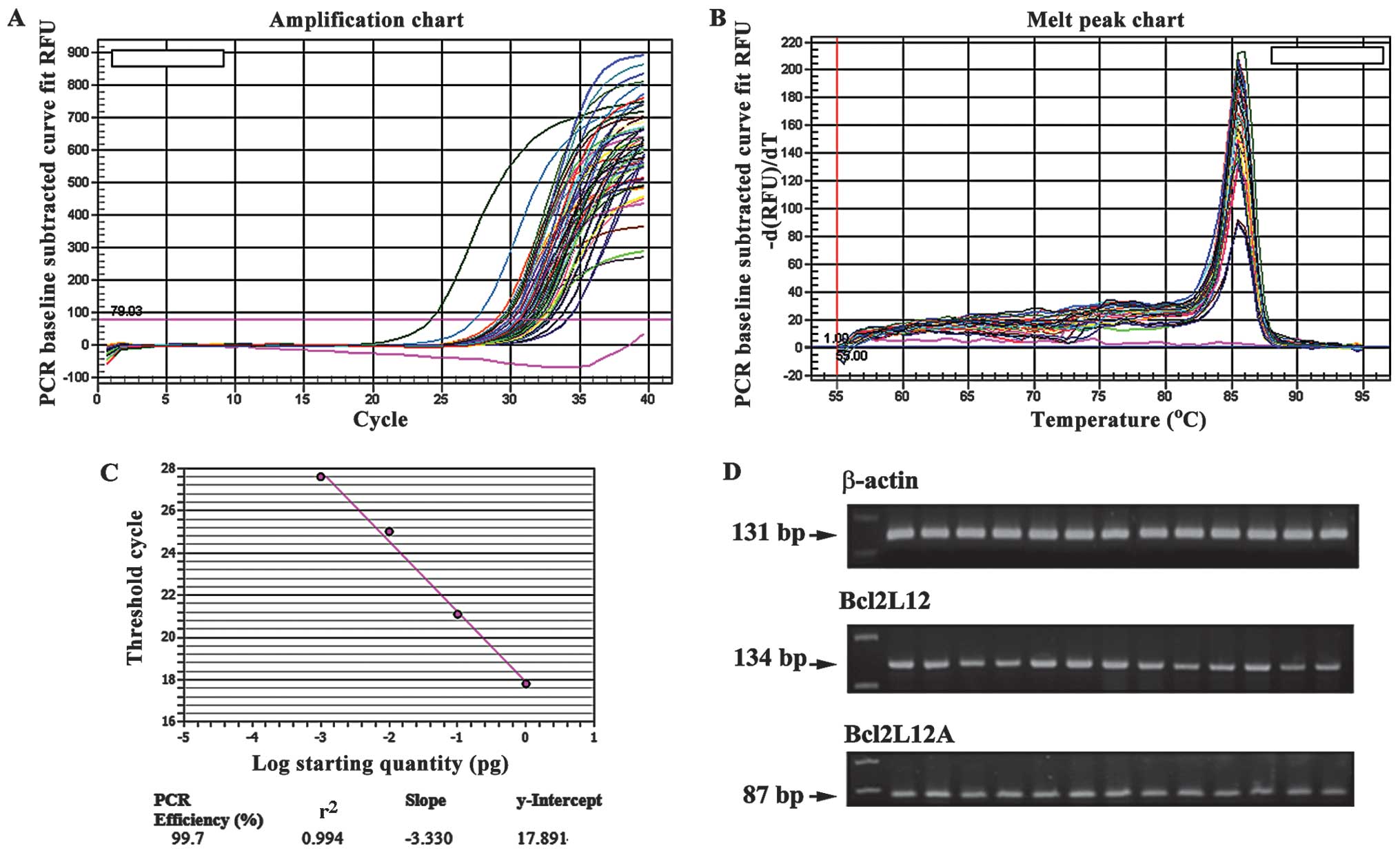
i) high amplification efficiency (≥96%) across a wide range of cDNA
dilutions (see Fig. 3); ii)
specific single products in dissociation curve analysis; and iii)
Tm similar to those predicted by oligonucleotide software. The
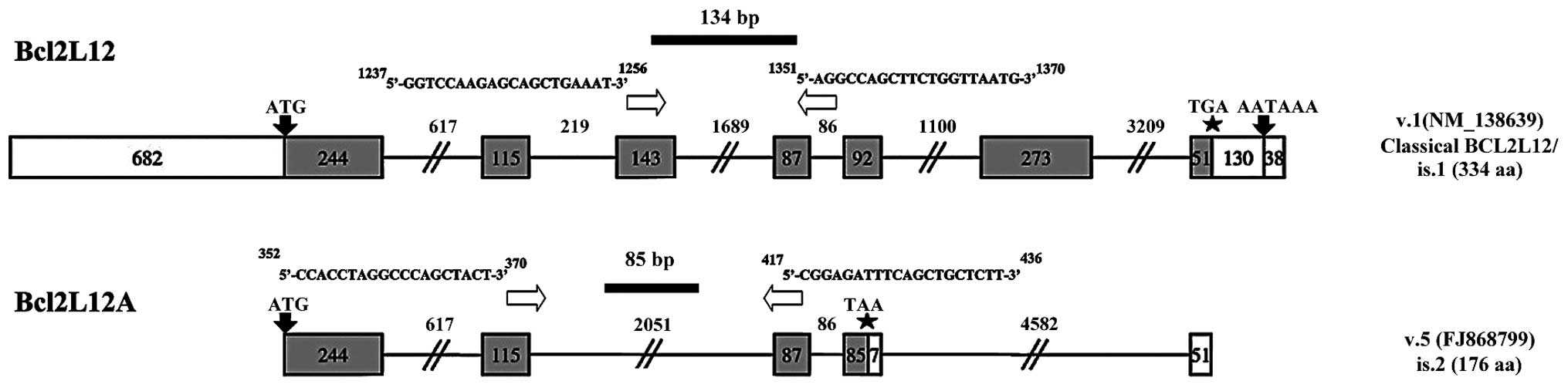
sequences of each primer pair were as follows: Bcl2L12 forward,
5′-GGTCCAA GAGCAGCTGAAAT-3′ and Bcl2L12 and reverse, 5′-AGGCC
AGCTTCTGGTTAATG-3′; Bcl2L12A forward, 5′-CCACCT AGGCCCAGCTACT-3′
and Bcl2L12A and reverse, 5′-CGGA GATTTCAGCTGCTCTT-3′; β-actin
forward, 5′-GACATC CGCAAAGACCTGTA-3′, and β-actin reverse,
5′-GGAGCA ATGATCTTGATCTTCA-3′. The amplification strategy for
Bcl2L12 and Bcl2L12A mRNA is shown in Fig. 1.
Statistical analysis
Statistical procedures were conducted using SPSS
22.0 statistical software (IBM). A Kruskal-Wallis test was applied
to discriminate whether expression levels with respect to Bcl2L12
and Bcl2L12A mRNA were significantly different across different
stage, grade and TNM staging breast cancer groups. The Stepwise and
Enter methods, respectively, were used in linear regression to
determine associated factors for Bcl2L12 and Bcl2L12A mRNA
expression in total, TNBC and non-TNBC samples. The analyzed
variables included diagnostic age, clinical diagnosis type, tumor
size, number of metastastic lymph nodes, TNM staging, staging, Her2
expression, ER status, PR status, histological grade, lymph node
metastasis, invasive status, grade 3, TNBC, Bcl2L12 mRNA and
Bcl2L12A mRNA. The different expression levels of Bcl2L12 and
Bcl2L12A in dichotomous groups were assessed using the independent
sample Student’s t-test. The linear regression formula and plot of
Bcl2L12 against Bcl2L12A were estimated using SigmaPlot 12.0
(Systat Software, Inc., San Jose, CA, USA). P<0.05, β>0.14
and r2>0.14 were considered statistically
significant.
Results
Since the amplification efficiencies of the target
(Bcl2L12 and Bcl2L12A) and reference gene (β-actin) were
approximately equal, the ΔΔCt calculations of our RT-PCR data were
valid. The slopes of the Bcl2L12, Bcl2L12A and β-actin standard
curves were similar (−3.361, −3.341 and −3.440, respectively), and
showed similar efficiencies to the corresponding amplicons (98.4,
99.0 and 98.3%, respectively). To confirm the efficient and
specific amplification, the standard curve and melting curve
analysis was generated for each target gene and the reference gene.
As shown in Fig. 2, Bcl2L12
amplification showed a good amplification efficiency of 99.7 and
r2 number of 0.994 of the standard curve. Checking the
products by gel electrophoresis, the three target gene
amplifications had the expected size bands as shown in Fig. 2D.
The expression levels with respect to Bcl2L12 and
Bcl2L12A mRNA in our samples were estimated for the skewness value
of 3.16–6.58 and kurtosis index of 9.38–44.828 (data not shown),
suggesting that they were unlikely to be normally distributed.
Therefore, a non-parametric statistical method, the Kruskal-Wallis
test, was applied to discriminate whether expression levels with
respect to Bcl2L12 and Bcl2L12A mRNA were significantly different
across categories of stage, grade and TNM staging. As shown in
Table II, there was no significant
difference in the Bcl2L12 and Bcl2L12A mRNA expression for the
categories of stage, grade and TNM staging (P>0.05). This result
suggested that Bcl2L2 and Bcl2L12A mRNA expression were unlikely to
be associated with BCa progression.
 | Table IImRNA expression of Bcl2L12 and its
variant in different BCa groups. |
Table II
mRNA expression of Bcl2L12 and its
variant in different BCa groups.
| Variables | Stage | No. | Mean rank | Kruskal-Wallis
chi-square | df | Asymp. Sig.a |
|---|
| Bcl2L12 | 0 (CIS) | 3 | 89.33 | 12.959 | 7 | 0.073 |
| IA | 24 | 45.42 | | | |
| IIA | 37 | 47.00 | | | |
| IIB | 13 | 46.69 | | | |
| IIIA | 7 | 53.29 | | | |
| IIIC | 9 | 67.67 | | | |
| IV | 4 | 27.75 | | | |
| V | 1 | 54.00 | | | |
| Total | 98 | | | | |
| Bcl2L12A | 0 (CIS) | 3 | 72.33 | 5.072 | 7 | 0.651 |
| IA | 24 | 48.79 | | | |
| IIA | 37 | 45.57 | | | |
| IIB | 13 | 47.62 | | | |
| IIIA | 7 | 51.43 | | | |
| IIIC | 9 | 50.44 | | | |
| IV | 4 | 69.50 | | | |
| V | 1 | 66.00 | | | |
| Total | 98 | | | | |
| Grade | N | Mean rank | Kruskal-Wallis
chi-square | df | Asymp. Sig.a |
| Bcl2L12 | I | 5 | 44.80 | 0.561 | 2 | 0.756 |
| II | 90 | 52.82 | | | |
| III | 8 | 47.25 | | | |
| Total | 103 | | | | |
| Bcl2L12A | I | 5 | 53.80 | 0.028 | 2 | 0.986 |
| II | 90 | 51.82 | | | |
| III | 8 | 52.88 | | | |
| Total | 103 | | | | |
| TNM | N | Mean rank | Kruskal-Wallis
chi-square | df | Asymp. Sig.a |
| Bcl2L12 | T0N0M0 | 3 | 87.67 | 16.616 | 15 | 0.342 |
| T1N0M0 | 24 | 44.79 | | | |
| T1N1M0 | 3 | 38.33 | | | |
| T1N2M0 | 1 | 11.00 | | | |
| T1N2M1 | 1 | 53.00 | | | |
| T1N3M0 | 1 | 67.00 | | | |
| T2N0M0 | 33 | 47.15 | | | |
| T2N1M0 | 10 | 48.10 | | | |
| T2N1M1 | 1 | 27.00 | | | |
| T2N2M0 | 6 | 59.33 | | | |
| T2N3M0 | 6 | 69.50 | | | |
| T2N3M1 | 1 | 34.00 | | | |
| T3N0M0 | 3 | 39.33 | | | |
| T3N1M0 | 1 | 38.00 | | | |
| T3N1M1 | 1 | 16.00 | | | |
| T3N3M0 | 1 | 29.00 | | | |
| Total | 96 | | | | |
| Bcl2L12A | T0N0M0 | 3 | 72.00 | 12.588 | 15 | 0.634 |
| T1N0M0 | 24 | 48.63 | | | |
| T1N1M0 | 3 | 19.00 | | | |
| T1N2M0 | 1 | 18.00 | | | |
| T1N2M1 | 1 | 66.00 | | | |
| T2N0M0 | 33 | 48.88 | | | |
| T2N1M0 | 10 | 44.90 | | | |
| T2N1M1 | 1 | 75.00 | | | |
| T2N2M0 | 6 | 56.50 | | | |
| T2N3M0 | 6 | 46.67 | | | |
| T2N3M1 | 1 | 53.00 | | | |
| T3N0M0 | 3 | 56.67 | | | |
| T3N1M0 | 1 | 11.00 | | | |
| T3N1M1 | 1 | 65.00 | | | |
| T3N3M0 | 1 | 60.00 | | | |
| Total | 96 | | | | |
The correlation between Bcl2L12 and Bcl2L12A mRNA
levels and clinicopathological characteristics was analyzed using
linear regression. As shown in Table
III, when the stepwise method was applied, the Bcl2L12 mRNA
level was highly correlated with the Bcl2L12A mRNA level (β=0.356,
P<0.001), grade 3 tumors (β=0.205, P=0.032) and TNBC status (β=
0.189, P= 0.049) in the total sample. However, grade 3 was no
longer associated with a high Bcl2L12 mRNA expression in the total
sample (β=0.196, P=0.229) when the Enter method was used (Table IV). Alternatively, Bcl2L12 was
relevant to the number of metastatic lymph nodes (β=0.837, P=0.030)
and grade 3 tumors (β=0.904, P=0.016) in the TNBC group (Table IV). Bcl2L12 mRNA was only
correlated with Bcl2L12A mRNA expression in the non-TNBC group (β=
0.473, P<0.001). Conversely, Bcl2L12A was solely associated with
Bcl2L12 mRNA expression in the total sample (Table III, β=0.385, P<0.001) and was
correlated with Bcl2L12 mRNA (Table
III, β=0.384, P<0.001), number of metastatic lymph nodes
(Table III, β=0.505, P<0.001)
and staging (Table III, β=−0.324,
P=0.007) in the non-TNBC group when using the stepwise method.
Nevertheless, when the Enter method was used, Bcl2L12A mRNA was
additionally associated with the number of metastatic lymph nodes
(Table IV, β=0.328, P=0.018) in
the total sample and correlated with Bcl2L12 mRNA (Table IV, β=0.382, P=0.001), number of
metastatic lymph nodes (Table IV,
β= 0.475, P=0.002) and staging (Table
IV, β=−0.406, P=0.032) in the non-TNBC group, but not
correlated with any analyzed variable in TNBC tumors (Table IV, P>0.05). These results
suggested that Bcl2L12 and Bcl2L12A have an unequal impact on TNBC
and non-TNBC. Bcl2L12 has a unique role in TNBC, and a high
expression correlated with high-grade tumor and the number of
metastatic lymph nodes. Bcl2L12A was correlated with Bcl2L12 in
non-TNBC and their association was possibly linked to the severity
of lymph node metastasis. We assessed whether Bcl2L12 and/or
Bcl2L12A were unequally expressed in the dichotomous group of BCa.
As a result, we observed that Bcl2L12, but not Bcl2L12A, mRNA had a
significantly higher expression in the TNBC than that in the
non-TNBC group (Table V, P=0.014)
and was also more highly expressed in grade 3 than non-grade 3
tumors (Table V, P=0.030).
Conversely, in the non-TNBC group, tumors with metastatic lymph
nodes ≥12 were found to have a higher Bcl2L12A expression (Table V, P=0.021) than their counterparts.
The CIS samples also showed a higher expression of Bcl2L12 and
Bcl2L12A than other samples (Table
V, Bcl2L12: P=0.010; Bcl2L12A: P=0.008). Since, as mentioned
above, Bcl2L12 was correlated with Bcl2L12A expression in the total
sample and non-TNBC group, we plotted the regression line and
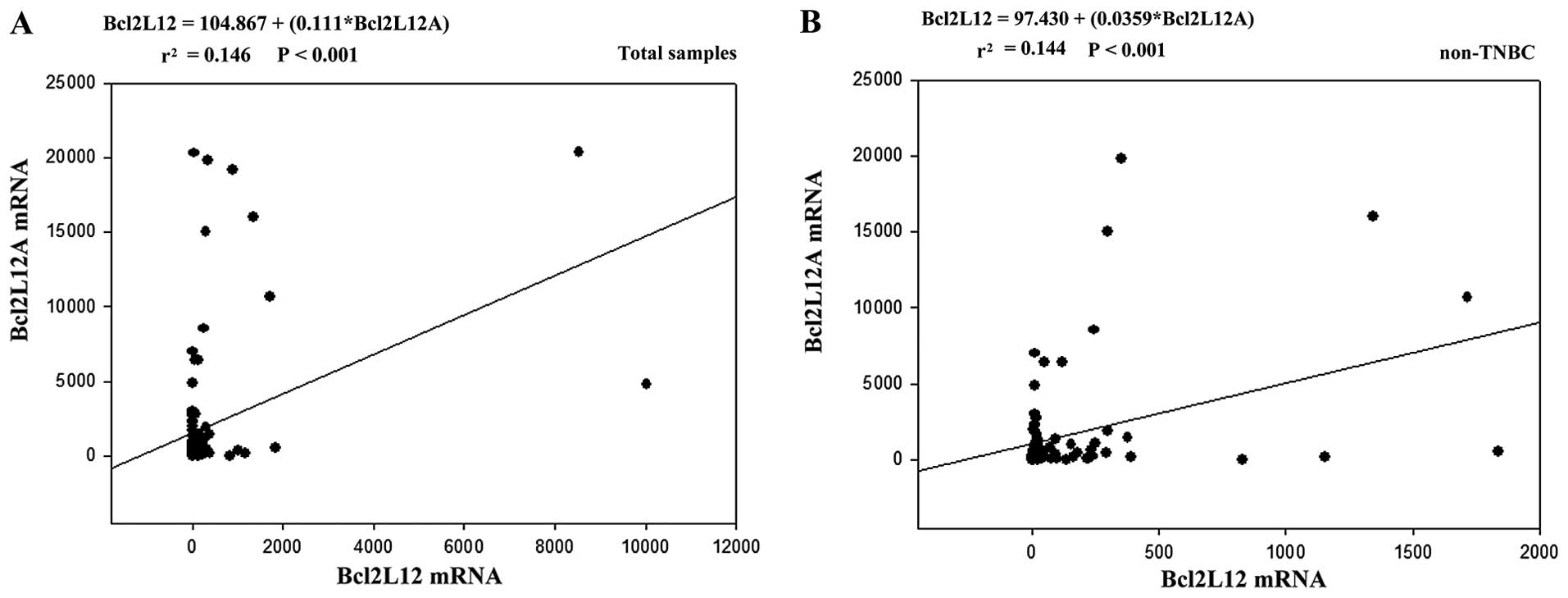
estimated the regression formula as shown in Fig. 3 (total sample: Bcl2L12=104.867
+(0.111*Bcl2L12A); non-TNBC: Bcl2L12=97.430+(0.0359* Bcl2L12A).
According to the result and regression formula, Bcl2L12A mRNA
expression had a 3-fold enhancement in the non-TNBC group compared
to the total sample. In addition, Bcl2L12 was correlated with
Bcl2L12A expression with a low level index (r2=
0.144-0.146, P<0.001). These data indicated that the interplay
between Bcl2L12 and its variant may be linked to non-TNBC
tumors.
 | Table IIIParsimonious results of linear
regression by stepwise method to identify the associated factors
with respect to mRNA expression of Bcl2L12 and its variant. |
Table III
Parsimonious results of linear
regression by stepwise method to identify the associated factors
with respect to mRNA expression of Bcl2L12 and its variant.
| Model factors | Unstandardized
coefficients | Standardized
coefficients | t | Sig. | 95.0% CI for B |
|---|
|
|
|---|
| B | Std. Error | β | Lower bound | Upper bound |
|---|
| 1 | (Constant | −98.068 | 155.768 | | −0.630 | 0.531 | −407.623 | 211.487 |
| Bcl2L12A mRNA | 0.103 | 0.027 | 0.356 | 3.782 | <0.001 | 0.049 | 0.157 |
| Grade 3 | 1060.372 | 485.531 | 0.205 | 2.184 | 0.032 | 95.481 | 2025.263 |
| TNBCa | 617.757 | 309.898 | 0.189 | 1.993 | 0.049 | 1.900 | 1233.614 |
| 2 | (Constant) | 80.170 | 35.256 | | 2.274 | 0.026 | 9.836 | 150.503 |
| Bcl2L12A mRNA | 0.037 | 0.008 | 0.473 | 4.465 | <0.001 | 0.021 | 0.054 |
| 3 | (Constant) | 1707.252 | 474.109 | | 3.601 | 0.001 | 765.351 | 2649.153 |
| Bcl2L12 mRNA | 1.327 | 0.336 | 0.385 | 3.953 | <0.001 | 0.660 | 1.995 |
| 4 | (Constant) | 3231.182 | 1084.556 | | 2.979 | 0.004 | 1066.399 | 5395.964 |
| Bcl2L12 mRNA | 4.865 | 1.236 | 0.384 | 3.936 | <0.001 | 2.398 | 7.332 |
| Number of
metastatic lymph nodes | 381.132 | 88.158 | 0.505 | 4.323 | <0.001 | 205.168 | 557.095 |
| Staging | −142.309 | 51.131 | −0.324 | −2.783 | 0.007 | −244.367 | −40.250 |
 | Table IVResults of linear regression using
the Enter method to identify the associated factors with respect to
mRNA expression of Bcl2L12 and its variant. |
Table IV
Results of linear regression using
the Enter method to identify the associated factors with respect to
mRNA expression of Bcl2L12 and its variant.
| Model factors | Unstandardized
Coefficients | Standardized
Coefficients | t | Sig. | 95.0% CI for B |
|---|
|
|
|---|
| B | Std. Error | β | Lower bound | Upper bound |
|---|
| 1 | (Constant) | 1201.310 | 2207.699 | | 0.544 | 0.588 | −3193.005 | 5595.625 |
| Bcl2L12A mRNA | 0.096 | 0.031 | 0.332 | 3.122 | 0.003 | 0.035 | 0.158 |
| TNBCa | 741.683 | 335.668 | 0.227 | 2.210 | 0.030 | 73.553 | 1409.813 |
| TNM staging | −.309 | 4.092 | −0.017 | −0.075 | 0.940 | −8.453 | 7.836 |
| Grade 3 | 1009.777 | 832.876 | 0.196 | 1.212 | 0.229 | −648.022 | 2667.575 |
| Staging | 8.852 | 26.723 | 0.057 | 0.331 | 0.741 | −44.338 | 62.042 |
| Histological
grade | 455.355 | 627.208 | 0.120 | 0.726 | 0.470 | −793.071 | 1703.782 |
| No. of metastatic
lymph nodes | 8.389 | 41.075 | 0.029 | 0.204 | 0.839 | −73.368 | 90.147 |
| Invasive | −1460.935 | 1134.381 | −0.346 | −1.288 | 0.202 | −3718.863 | 796.993 |
| Lymph node
metastasis | 40.941 | 415.754 | 0.014 | 0.098 | 0.922 | −786.596 | 868.479 |
| Tumor size | −108.108 | 128.346 | −0.153 | −0.842 | 0.402 | −363.574 | 147.359 |
| Clinical diagnosis
type | −193.893 | 313.043 | −0.178 | −0.619 | 0.537 | −816.990 | 429.203 |
| Diagnostic age | −8.884 | 11.252 | −0.083 | −0.789 | 0.432 | −31.281 | 13.514 |
| 2 | (Constant) | 6960.557 | 9841.433 | | 0.707 | 0.499 | −15733.829 | 29654.942 |
| Bcl2L12A mRNA | 0.077 | 0.090 | 0.194 | 0.850 | 0.420 | −0.131 | 0.285 |
| Diagnostic age | 36.151 | 44.194 | 0.203 | 0.818 | 0.437 | −65.761 | 138.063 |
| Clinical diagnosis
type | −1822.850 | 1963.595 | −0.738 | −0.928 | 0.380 | −6350.909 | 2705.209 |
| Tumor size | −1478.243 | 645.415 | −1.366 | −2.290 | 0.051 | −2966.572 | 10.087 |
| No. of metastatic
lymph nodes | 679.731 | 258.063 | 0.837 | 2.634 | 0.030 | 84.636 | 1274.825 |
| TNM staging | 38.209 | 19.514 | 1.120 | 1.958 | 0.086 | −6.789 | 83.208 |
| Staging | −123.668 | 96.783 | −0.436 | −1.278 | 0.237 | −346.851 | 99.514 |
| Her2
expression | 2298.805 | 1374.020 | 0.362 | 1.673 | 0.133 | −869.692 | 5467.301 |
| Histological
grade | 1078.154 | 1614.687 | 0.213 | 0.668 | 0.523 | −2645.320 | 4801.629 |
| Lymph node
metastasis | −4828.536 | 2355.310 | −0.842 | −2.050 | 0.075 | −10259.890 | 602.818 |
| Invasive | −9889.988 | 6180.396 | −1.280 | −1.600 | 0.148 | −24142.006 | 4362.030 |
| Grade 3 | 6985.884 | 2306.121 | 0.904 | 3.029 | 0.016 | 1667.960 | 12303.808 |
| 3 | (Constant) | −415.603 | 601.281 | | −0.691 | 0.492 | −1620.114 | 788.909 |
| Bcl2L12A mRNA | 0.035 | 0.010 | 0.450 | 3.409 | 0.001 | 0.015 | 0.056 |
| Diagnostic age | 4.948 | 3.159 | 0.194 | 1.566 | 0.123 | −1.380 | 11.276 |
| Clinical diagnosis
type | 110.386 | 77.675 | 0.476 | 1.421 | 0.161 | −45.215 | 265.987 |
| Tumor size | 68.108 | 34.841 | 0.382 | 1.955 | 0.056 | −1.687 | 137.903 |
| No. of metastatic
lymph nodes | 3.603 | 10.436 | 0.061 | 0.345 | 0.731 | −17.304 | 24.509 |
| TNM staging | −1.482 | 1.025 | −0.352 | −1.447 | 0.154 | −3.535 | 0.570 |
| Staging | 5.746 | 7.202 | 0.166 | 0.798 | 0.428 | −8.681 | 20.172 |
| Her2
expression | −85.187 | 73.381 | −0.141 | −1.161 | 0.251 | −232.186 | 61.812 |
| Histological
grade | 53.824 | 94.210 | 0.090 | 0.571 | 0.570 | −134.902 | 242.550 |
| Lymph node
metastasis | 21.775 | 33.721 | 0.088 | 0.646 | 0.521 | −45.777 | 89.326 |
| Invasive | −168.374 | 217.986 | −0.162 | −0.772 | 0.443 | −605.053 | 268.305 |
| Grade 3 | −57.712 | 99.344 | −0.092 | −0.581 | 0.564 | −256.722 | 141.298 |
| 4 | (Constant) | −13851.468 | 7453.258 | | −1.858 | 0.067 | −28686.807 | 983.872 |
| TNBCa | 1055.147 | 1184.807 | 0.094 | 0.891 | 0.376 | −1303.152 | 3413.446 |
| TNM staging | 17.390 | 13.950 | 0.271 | 1.247 | 0.216 | −10.377 | 45.158 |
| Grade 3 | −3106.166 | 2872.626 | −0.174 | −1.081 | 0.283 | −8823.986 | 2611.653 |
| Staging | −45.721 | 91.915 | −0.086 | −0.497 | 0.620 | −228.674 | 137.232 |
| Histological
grade | 2444.395 | 2148.886 | 0.187 | 1.138 | 0.259 | −1832.856 | 6721.646 |
| No. of metastatic
lymph nodes | 328.809 | 136.518 | 0.328 | 2.409 | 0.018 | 57.077 | 600.542 |
| Invasive | 5633.146 | 3894.765 | 0.387 | 1.446 | 0.152 | −2119.189 | 13385.480 |
| Tumor size | −370.230 | 441.864 | −0.151 | −0.838 | 0.405 | −1249.738 | 509.278 |
| Clinical diagnosis
type | 1825.681 | 1060.586 | 0.484 | 1.721 | 0.089 | −285.363 | 3936.725 |
| Diagnostic age | 41.296 | 38.611 | 0.112 | 1.070 | 0.288 | −35.558 | 118.150 |
| Bcl2L12 mRNA | 1.141 | 0.365 | 0.331 | 3.122 | 0.003 | 0.414 | 1.868 |
| 5 | (Constant) | −16618.637 | 37593.474 | | −0.442 | 0.670 | −103309.343 | 70072.069 |
| Diagnostic age | 137.144 | 165.604 | 0.305 | 0.828 | 0.432 | −244.740 | 519.029 |
| Clinical diagnosis
type | −473.151 | 7749.804 | −0.076 | −0.061 | 0.953 | −18344.230 | 17397.928 |
| Tumor size | −2196.546 | 3016.639 | −0.803 | −0.728 | 0.487 | −9152.928 | 4759.836 |
| No. of metastatic
lymph nodes | −473.285 | 1312.034 | −0.231 | −0.361 | 0.728 | −3498.840 | 2552.270 |
| TNM staging | 47.881 | 87.395 | 0.555 | 0.548 | 0.599 | −153.653 | 249.416 |
| Staging | 143.306 | 395.105 | 0.200 | 0.363 | 0.726 | −767.806 | 1054.419 |
| Her2
expression | −1857.754 | 5951.652 | −0.116 | −0.312 | 0.763 | −15582.287 | 11866.780 |
| Histological
grade | 6187.159 | 5825.695 | 0.484 | 1.062 | 0.319 | −−7246.917 | 19621.235 |
| Lymph node
metastasis | −5737.446 | 10720.572 | −0.396 | −0.535 | 0.607 | −30459.130 | 18984.238 |
| Invasive | −1871.498 | 26626.121 | −0.096 | −0.070 | 0.946 | −63271.444 | 59528.448 |
| Grade 3 | −7020.365 | 12429.037 | −0.360 | −0.565 | 0.588 | −35681.777 | 21641.046 |
| Bcl2L12 mRNA | 1.079 | 1.270 | 0.427 | 0.850 | 0.420 | −1.849 | 4.008 |
| 6 | (Constant) | −1754.655 | 7050.335 | | −0.249 | 0.804 | −15878.173 | 12368.863 |
| Diagnostic age | −29.233 | 37.502 | −0.090 | −0.780 | 0.439 | −104.358 | 45.892 |
| Clinical diagnosis
type | 654.643 | 919.482 | 0.223 | 0.712 | 0.479 | −1187.302 | 2496.587 |
| Tumor size | −179.240 | 419.998 | −0.079 | −0.427 | 0.671 | −1020.597 | 662.117 |
| No. of metastatic
lymph nodes | 358.237 | 112.268 | 0.475 | 3.191 | 0.002 | 133.336 | 583.137 |
| TNM staging | 15.254 | 12.021 | 0.286 | 1.269 | 0.210 | −8.826 | 39.335 |
| Staging | −178.224 | 81.186 | −0.406 | −2.195 | 0.032 | −340.860 | −15.589 |
| Her2
expression | 585.023 | 863.977 | 0.077 | 0.677 | 0.501 | −1145.730 | 2315.776 |
| ER status | −634.557 | 1100.531 | −0.083 | −0.577 | 0.567 | −2839.186 | 1570.071 |
| PR status | 116.982 | 395.094 | 0.037 | 0.296 | 0.768 | −674.485 | 908.450 |
| Histological
grade | 1196.637 | 2555.108 | 0.091 | 0.468 | 0.641 | −3921.859 | 6315.133 |
| Lymph
metastasis | −920.613 | 1157.537 | −0.116 | −0.795 | 0.430 | −3239.437 | 1398.211 |
| Invasive | 2211.404 | 3558.910 | 0.184 | 0.621 | 0.537 | −4917.949 | 9340.756 |
| Grade 3 | 828.127 | 3043.055 | 0.050 | 0.272 | 0.787 | −5267.844 | 6924.098 |
| Bcl2L12 mRNA | 4.843 | 1.421 | 0.382 | 3.409 | 0.001 | 1.997 | 7.689 |
 | Table VThe Student’s t-test was used to
discriminate the difference between dichotomous groups of BCa. |
Table V
The Student’s t-test was used to
discriminate the difference between dichotomous groups of BCa.
| Variables | Groups | N | Mean | Std. Error
Mean | t-value | P-value |
|---|
| Bcl2L12 |
Non-TNBC | 79 |
157.3000 |
39.02000 | −2.487 | 0.014 |
| TNBC | 24 |
899.7200 |
531.58000 | | |
|
Bcl2L12A |
Non-TNBC | 79 |
1665.8200 |
412.22000 | −1.371 | 0.174 |
| TNBC | 24 |
3104.3300 |
1346.37000 | | |
| Bcl2L12 | Non-grade 3 | 97 | 245.6278 | 92.84499 | −2.203 | 0.030 |
| Grade 3 | 8 | 1280.0088 | 1250.11850 | | |
| Bcl2L12A | Non-grade 3 | 97 | 1976.7996 | 468.62588 | −0.013 | 0.989 |
| Grade 3 | 8 | 1998.6525 | 882.57020 | | |
| Bcl2L12 | ≥12 metastatic
lymph nodes | 6 | 264.4200 | 124.34000 | 0.989 | 0.326 |
| <12 metastatic
lymph nodes | 65 | 342.9000 | 148.20000 | | |
| Bcl2L12A | ≥12 metastatic
lymph nodes | 6 | 5230.9800 | 3212.87000 | 2.363 | 0.021 |
| <12 metastatic
lymph nodes | 65 | 1484.1300 | 393.26000 | | |
| Bcl2L12 | ≥ Stage IA | 73 | 147.7100 | 38.71000 | −2.654 | 0.010 |
| < Stage IA | 2 | 795.8700 | 546.37000 | | |
| Bcl2L12A | ≥ Stage IA | 73 | 1559.1700 | 397.70000 | −2.710 | 0.008 |
| < Stage IA | 2 | 8538.6600 | 7461.89000 | | |
Discussion
In BCa, Bcl2L12 expression is initially recognized
as a good prognostic marker and is associated with long-term
survival (19). Using a
conventional RT-PCR approach, Bcl2L12 mRNA shows a relatively
higher expression in low stage (I/II) and grade (I/II) tumors. The
chemotherapeutic agent taxol, used to treat the MCF-7 BCa line,
downregulates Bcl2L12A and caspase-9 expression, but causes
elevation of Bax expression. Thomadaki et al (20) suggested that Bcl2L12 and Bc12L12A
were potential biomarkers for predicting patient outcome after
chemotherapy. Antineoplastic agents, such as cisplatin, carboplatin
and doxorubicin have also been tested in different BCa lines.
Bcl2L12 has been found to be associated with BCa chemoresistance
associated with Bcl2 and can be modulated by chemotherapeutic
drugs. The hypothesis of Bcl2L12 as a marker of favorable outcome
in BCa is based on the finding that Bcl2L12 is highly expressed in
low-stage clinical samples (8) and
on acquired resistance to cisplatin consequent to RNAi-based
knockdown of Bcl2L12 in the BCa MDA-MB-231 cancer cell line
(7). Apparently, ectopically
expressed GFP-tagged Bcl2L12 and Bcl2L12A inhibit CHO cell growth.
However, Bcl2L12 is more likely to trigger apoptosis, whereas
Bcl2L12A as a nuclear protein affects phosphorylation of cyclin B1
and interferes with the G2/M transition in the cell cycle to cause
growth arrest (6). Investigation of
the interaction of Bcl2L12 and its variant revealed that HSP70
protected Bcl2L12 and Bcl2L12A from N-terminal ubiquitin-mediated
proteosomal degradation (21). More
recently, in contrast to previous findings on the role of Bcl2L12
in BCa, a putative tumor suppressor of BCa, eRβ5, was found to
interact with Bcl2L12, which may compete with the interaction of
Bcl2L12-caspase-7 and result in sensitization to chemotherapeutic
agent-induced apoptosis (15). This
finding supports the anti-apoptotic role of Bcl2L12in GBM, by
interacting with caspase-7 to antagonize apoptotic activity. Of
note, the use of chemotherapeutic agents to treat cancer cell lines
generally results in the downregulation of Bcl2L12 and activation
of pro-apoptotic members in the Bcl2 protein family, such as Bax.
Thus, the reason for chemotherapeutic agent-associated signaling
downregulating a favorable prognosis marker such as Bcl2L12, should
be investigated. However, Bcl2L12 and/or its variant may have
different impacts on drug response, tumorigenesis and patient
outcome. Bcl2L12 and Bcl2L12A need to be evaluated synchronously to
elucidate their interplay in BCa.
Regarding the correlation between ER and Bcl2L12, a
previous study demonstrated that Bcl2 is more highly expressed in
ER-positive and Bcl2L12-positive BCa (8). A high Bcl2L12 expression is suggested
to be associated with ER and Bcl2 expression. However, in the
present study, we observed that Bcl2L12 mRNA was more abundantly
expressed in TNBC tumors. Furthermore, Bcl2L12A mRNA was elevated
in TNBC compared to non-TNBC tumors although the result was not
statistically significant. In non-TNBC, Bcl2L12A expression was
markedly enhanced, correlated with Bcl2L12 mRNA level and was
associated with the severity of lymph node metastasis. In another
study, Bcl2L12 was found to interact with ERs through ERβ5, but not
ERα, ERβ1 and β2 (15). The
expression profiles of ERβ5, caspase-7 and Bcl2L12 in TNBC and
non-TNBC subtypes should be further investigated.
Despite the lack of knowledge concerning the role of
Bcl2L12 in BCa, the molecular mechanism of Bcl2L12 involvement in
GBM tumorigenesis is better known. Its caspase-inhibiting role is
dependent on negatively regulating p53 transcription and the
subsequent triggering of the αB-crystallin/caspase-3 interaction
(22,23). Recent findings have demonstrated
that αB-crystallin overexpression promoted brain metastasis, while
silencing αB-crystallin inhibited brain metastasis in orthotopic
TNBC (ER/PR/Her2 negative BCa) (24). αB-crystallin promoted the adhesion
of TNBC cells to HBMECs at least in part through an α3β1
integrin-dependent mechanism. These findings suggest a role for
Bcl2L12 in TNBC brain metastasis. In the present study, we also
found that TNBC highly expressed Bcl2L12 and Bcl2L12A. In GBM,
αB-crystallin is known to be a downstream gene of Bcl212 and may be
important for inactivating caspase-3 during tumorigenesis. More
studies are needed to determine whether Bcl2L12 is an upstream
activator of αB-crystallin in promoting distal metastasis of TNBC.
Thus, our results have shown that, a high Bcl2L12 and Bcl2L12A mRNA
expression was not associated with BCa progression, but that
Bcl2L12 mRNA was correlated with high-grade BCa and the TNBC
subtype. In addition, the interplay between Bcl2L12 and its variant
may be associated with high lymph node metastasis in non-TNBC
tumors.
Acknowledgments
The authors would like to thank Gary Mawyer for
language editing, the Department of Pathology of KAFGH for helping
collect the tissue specimens and accessing the medical reviews and
Dr Kao Wei-Tsung for providing the facilities and laboratory space
to perform the molecular biology experiments. This study was
supported by OPD research grant no. 102-29 from Kaohsiung Armed
Forces General Hospital.
References
|
1
|
Anderson BO and Jakesz R: Breast cancer
issues in developing countries: An overview of the Breast Health
Global Initiative. World J Surg. 32:2578–2585. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Annual Reports of the Department of
Health, the Executive Yuan, Republic of China (Taiwan), 2005
|
|
3
|
Leong SP, Shen ZZ, Liu TJ, Agarwal G,
Tajima T, Paik NS, Sandelin K, Derossis A, Cody H and Foulkes WD:
Is breast cancer the same disease in Asian and Western countries?
World J Surg. 34:2308–2324. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Foulkes WD, Smith IE and Reis-Filho JS:
Triple-negative breast cancer. N Engl J Med. 363:1938–1948. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Scorilas A, Kyriakopoulou L, Yousef GM,
Ashworth LK, Kwamie A and Diamandis EP: Molecular cloning, physical
mapping, and expression analysis of a novel gene, BCL2L12, encoding
a proline-rich protein with a highly conserved BH2 domain of the
Bcl-2 family. Genomics. 72:217–221. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hong Y, Yang J, Chi Y, Wang W, Wu W, Yun
X, Kong X and Gu J: BCL2L12A localizes to the cell nucleus and
induces growth inhibition through G2/M arrest in CHO cells. Mol
Cell Biochem. 333:323–330. 2010. View Article : Google Scholar
|
|
7
|
Hong Y, Yang J, Wu W, Wang W, Kong X, Wang
Y, Yun X, Zong H, Wei Y, Zhang S, et al: Knockdown of BCL2L12 leads
to cisplatin resistance in MDA-MB-231 breast cancer cells. Biochim
Biophys Acta. 1782:649–657. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Thomadaki H, Talieri M and Scorilas A:
Prognostic value of the apoptosis related genes BCL2 and BCL2L12 in
breast cancer. Cancer Lett. 247:48–55. 2007. View Article : Google Scholar
|
|
9
|
Florou D, Papadopoulos IN and Scorilas A:
Molecular analysis and prognostic impact of the novel apoptotic
gene BCL2L12 in gastric cancer. Biochem Biophys Res Commun.
391:214–218. 2010. View Article : Google Scholar
|
|
10
|
Stegh AH, Brennan C, Mahoney JA, Forloney
KL, Jenq HT, Luciano JP, Protopopov A, Chin L and Depinho RA:
Glioma oncoprotein Bcl2L12 inhibits the p53 tumor suppressor. Genes
Dev. 24:2194–2204. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stegh AH, Kesari S, Mahoney JE, Jenq HT,
Forloney KL, Protopopov A, Louis DN, Chin L and DePinho RA:
Bcl2L12-mediated inhibition of effector caspase-3 and caspase-7 via
distinct mechanisms in glioblastoma. Proc Natl Acad Sci USA.
105:10703–10708. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Stegh AH, Chin L, Louis DN and DePinho RA:
What drives intense apoptosis resistance and propensity for
necrosis in glioblastoma? A role for Bcl2L12 as a multifunctional
cell death regulator. Cell Cycle. 7:2833–2839. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Stegh AH, Kim H, Bachoo RM, Forloney KL,
Zhang J, Schulze H, Park K, Hannon GJ, Yuan J, Louis DN, et al:
Bcl2L12 inhibits post-mitochondrial apoptosis signaling in
glioblastoma. Genes Dev. 21:98–111. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chou CH, Chou AK, Lin CC, Chen WJ, Wei CC,
Yang MC, Hsu CM, Lung FW, Loh JK, Howng SL, et al: GSK3β regulates
Bcl2L12 and Bcl2L12A anti-apoptosis signaling in glioblastoma and
is inhibited by LiCl. Cell Cycle. 11:532–542. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee MT, Ho SM, Tarapore P, Chung I and
Leung YK: Estrogen receptor β isoform 5 confers sensitivity of
breast cancer cell lines to chemotherapeutic agent-induced
apoptosis through interaction with Bcl2L12. Neoplasia.
15:1262–1271. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Giulietti A, Overbergh L, Valckx D,
Decallonne B, Bouillon R and Mathieu C: An overview of real-time
quantitative PCR: Applications to quantify cytokine gene
expression. Methods. 25:386–401. 2001. View Article : Google Scholar
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2[−ΔΔC(T)] method. Methods. 25:402–408. 2001. View Article : Google Scholar
|
|
18
|
Fendri A, Kontos CK, Khabir A,
Mokdad-Gargouri R and Scorilas A: BCL2L12 is a novel biomarker for
the prediction of short-term relapse in nasopharyngeal carcinoma.
Mol Med. 17:163–171. 2011. View Article : Google Scholar :
|
|
19
|
Talieri M, Diamandis EP, Katsaros N,
Gourgiotis D and Scorilas A: Expression of BCL2L12, a new member of
apoptosis-related genes, in breast tumors. Thromb Haemost.
89:1081–1088. 2003.PubMed/NCBI
|
|
20
|
Thomadaki H, Talieri M and Scorilas A:
Treatment of MCF-7 cells with taxol and etoposide induces distinct
alterations in the expression of apoptosis-related genes BCL2,
BCL2L12, BAX, CASPASe-9 and FAS. Biol Chem. 387:1081–1086. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang J, Hong Y, Wang W, Wu W, Chi Y, Zong
H, Kong X, Wei Y, Yun X, Cheng C, et al: HSP70 protects BCL2L12 and
BCL2L12A from N-terminal ubiquitination-mediated proteasomal
degradation. FEBS Lett. 583:1409–1414. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Stegh AH, Kesari S, Mahoney JE, Jenq HT,
Forloney KL, Protopopov A, Louis DN, Chin L and DePinho RA:
Bcl2L12-mediated inhibition of effector caspase-3 and caspase-7 via
distinct mechanisms in glioblastoma. Proc Natl Acad Sci USA.
105:10703–10708. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Stegh AH, Chin L, Louis DN and DePinho RA:
What drives intense apoptosis resistance and propensity for
necrosis in glioblastoma? A role for Bcl2L12 as a multifunctional
cell death regulator. Cell Cycle. 7:2833–2839. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Malin D, Strekalova E, Petrovic V, Deal
AM, Al Ahmad A, Adamo B, Miller CR, Ugolkov A, Livasy C, Fritchie
K, et al: α-crystallin: a novel regulator of breast cancer
metastasis to the brain. Clin Cancer Res. 20:56–67. 2014.
View Article : Google Scholar :
|