Introduction
Breast cancer is the most common type of cancer in
the world with more than 1.3 million patients and a mortality rate
of ~450,000 deaths/year (1). The
high incidence of disease is suggestive of slow progress in the
prevention programs. In the treatment of women with localized
disease, mortality rates have been improved, but the median
survival time in the metastatic setting is only 25 months (2). Systemic chemotherapy as treatment of
choice for most cancer types and its relatively modest improvement
in survival associated with significant toxicity, highlighted the
need over the past decade to develop targeted therapies. Targeted
drugs can restore the deregulated signalling transduction pathway
in which a mutated gene and its encoded protein is involved
(3). Targeted therapy represents
the major hope against cancer and a substantial step towards
personalized medicine, since this type of drugs is mostly active in
cancer cells without affecting normal healthy cells. However, the
efficacy of these single signal transduction pathway inhibitors is
in most cases modest, thus the progress against cancer is slow and
it is translated into a few weeks of survival prolongation.
Elucidation of the mechanisms beyond intrinsic and acquired tumor
resistance to therapies is hampered by high inter-patient and
intratumor heterogeneity (4–7).
Consequently, a patient-specifc experimental approach is needed to
analyze individual responses to therapies. Based on comprehensive
gene expression profiling, breast tumors are classified into at
least three major subtypes: luminal, human epidermal growth factor
receptor 2+ (HER2+) and basal-like (8,9), with
different risk factors for incidence, response to treatment,
disease progression and preferential organ sites of metastases
(10–12). Antitumor drugs, presently used in
the clinical practice, have been previously characterized in the
two-dimensional in vitro culture and in vivo
xenograft tumor models. A broad analysis on investigational drugs,
done at the National Cancer Institute, pointed out at a poor
correlation between preclinical data from in vitro models
and tumor xenografts and phase II efficacy data leading to the
conclusion that only compounds that are successful in a large
number of different models are likely to be effective in the clinic
(13). Thus, frequent failures in
drug development can be explained by the fact that the existing
preclinical models do not represent the complexity (heterogeneity)
that is typical of human tumors. We have, therefore, recently
explored tissue slice technology via the Krumdieck tissue slicer,
by preserving the tissues in the three-dimensional structure, it
allows the setting up of a powerful and representative ex
vivo tumor model. Moreover, taking into account the notion that
cancer cell lines, passaged in vitro for years, may not
reflect the biology of in vivo tumors, we compared the
activity of docetaxel (small molecule) and trastuzumab (large
molecule), both commonly used for breast cancer therapy, in cancer
cell lines and organotypic tissue slices.
Materials and methods
Materials
The primary antibodies used in the present study
were monoclonal rabbit anti-human Ki-67 antigen (NB600-1252; Novus
Biologicals, Littleton, CO, USA) and cleaved caspase-3 (#9664; Cell
Signaling Technology, Danvers, MA, USA). The secondary antibody was
biotinylated goat anti-rabbit (#E0432; Dako, Denmark).
Ethics statement
All studies were performed in accordance with the
'Directive 2010/63/UE' on the protection of animals used for
scientific purposes, made effective in Italy by the Legislative
Decree 4 March 2014, n. 26, and applying the principles of 3Rs
(i.e., to replace, reduce and refine). Mice were purchased from
Harlan Laboratories (Udine, Italy). All procedures performed on the
animals were approved by the Animal Welfare Body and authorized by
the Italian Ministry of Health, 46/2014-PR. At the end of the
treatment period and before necropsy, mice were euthanized by
compressed CO2 gas in a cylinder as indicated in the
American Veterinary Medical Association (AVMA) Panel on Euthanasia
according to the 1998 UKCCCR Guidelines for the Welfare of Animals
in Experimental Neoplasia.
Tumor xenograft in mammary fat pad
MCF-7 breast tumor cells were injected into the
abdominal fat pad of SCID Beige (7×106 cells/100
µl/mouse), 24 h after the subcutaneous implantation of
estrogen pellets containing 17β-estradiol (0.72 mg/pellet, 60-day
release/mouse) (#SE-121; Innovative Research of America, Sarasota,
FL, USA). Tumor lesions were measured with a Vernier caliper twice
a week to reach a volume of around 300 mm3 prior to
collection for tissue slice preparation.
For in vivo drug efficacy evaluation,
tumor-bearing mice were also treated with docetaxel (Sigma-Aldrich,
St. Louis, MO, USA) at 15 mg/10 ml/kg i.p. (q7dx3, once a week for
3 weeks) and trastuzumab at 10 mg/10 ml/kg i.p. (q7dx3) (Herceptin;
Roche S.p.A., Milan, Italy). Tumor lesions were measured with a
Vernier caliper twice a week to reach a volume of around 300
mm3 before to be collected for analysis of marker
proliferation.
Tumor explantation
Mice were sacrificed, tumors explanted and
immediately cut using the Vibratome VT1200 (Leica, Germany) to
obtain 200 µM thick slices of the whole tumors.
Tissue slice maintaining conditions
To set the best experimental conditions, tumor
slices were maintained in floating normoxic conditions for a
maximum of 4 days using a modular incubator chamber 37°C in
6-multiwell plates, using Dulbecco's modified Eagle's medium (DMEM)
with 10% FBS, a mix of penicillin-streptomycin and 1% L-glutamine
as culture medium. Medium change was performed every 24 h.
Subsequently, Teflon supporting and normoxia were
evaluated. Tumor slices were maintained at 37°C and 5%
CO2 (normoxic conditions) for a maximum of 4 days on
organotypic Teflon inserts (#PICM01250 MilliCell; Millipore,
Billerica, MA, USA) in 6-multiwell plates, using DMEM (#D5921;
Sigma-Aldrich) with 10% FBS, 1% L-glutamine, a mix of
penicillin-streptomycin as culture medium. Medium change was
performed every 24 h.
Cell viability determination in tissue
slices
The viability of tumor slices was evaluated using
the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
(MTT) (#M5655; Sigma-Aldrich) under normoxia (21% O2)
after a maximum of 3 days of incubation. Moreover, the integrity of
the slices was assessed by hematoxylin and eosin (H&E)
staining.
Immunohistochemistry
Immunochemistry analysis was performed by employing
paraffin sections. Tissue slices of 200 µm were
paraffin-embedded (horizontal orientation). The sections were
incubated with primary antibodies overnight. After washing,
secondary antibodies were applied at 1:200 dilutions for 30 min.
Images were acquired using a Nikon microscope (Eclipse 80i, Nikon,
Japan) with a Nikon digital camera (DXM1200F). H&E staining was
performed to examine the extent of the slice integrity.
Drug treatment of slice cultures
Slices obtained from three mice were evaluated in
triplicate for MTT assay 2 or 3 days after slicing. For treatment
of slices, docetaxel (Sigma-Aldrich) and trastuzumab (Herceptin)
were used. The concentrations were chosen on the basis of results
obtained from tumor cell growth inhibition assay. Before testing
docetaxel in its final concentrations of 200-20 nM, it was
dissolved in dimethyl sulfoxide (DMSO) and diluted in culture
medium. Trastuzumab was tested at the concentrations of 20-0.02
µg/ml. The incubation period for treatment was up to 2 days.
At the end of the treatment, tissue slices were incubated with 5
mg/ml of MTT at 37°C for 1 h, harvested and precipitated-salt
extracted by incubation with 0.1 M HCl-isopropyl alcohol at room
temperature for 40 min. Viability values were determined by
dividing the optical density of the formazan at 570 nm by the dry
weight of the explants.
Growth inhibition tumor cell assay
MTT assay was performed for evaluation of the cell
viability within 3 days of culture. Tumor cells were seeded 3,000
cells/well. upon 24 h, tumor cells were treated with different
concentrations of trastuzumab (200-20 µg/ml) and docetaxel
(500-0.5 nM) for 3 days. At the end of treatment, the medium was
discarded, cells were washed twice with PBS, and replaced by
MTT-containing medium. The plates were incubated at 37°C for 4 h.
Then the MTT solution was discarded and without washing, DMSO was
added to dissolve the formazan formed. After 15 min incubation,
cell plates were transferred to the microplate reader (Victor,
Wallac) and the absorbance at 570 nm was measured. The results were
expressed as percent of drug-treated viable cells in comparison
with vehicle-treated cells. The IC50 values were
calculated using four-parameter fit by ALLFIT program.
Statistical analysis
All data are expressed as the mean value ± standard
error (SE) and differences between groups were analyzed using
Mann-Whitney U test. Mean values are considered significantly
different at P<0.05.
Results
In vitro and in vivo antitumor activity
of docetaxel and trastuzumab
In order to evaluate the in vitro and in
vivo antitumor activity of docetaxel and trastuzumab, standard
human MCF-7 cell culture and a tumor xenograft study in nude mice
were carried out. These tumor cells were first of all analysed for
ERBB2 membrane expression by FACS analysis with 97.6% of tumor
cells positive for ERBB2-conjugated antibody (R&S, FAB 11299)
(data not shown). Tumor cell proliferation was tested by MTT assay
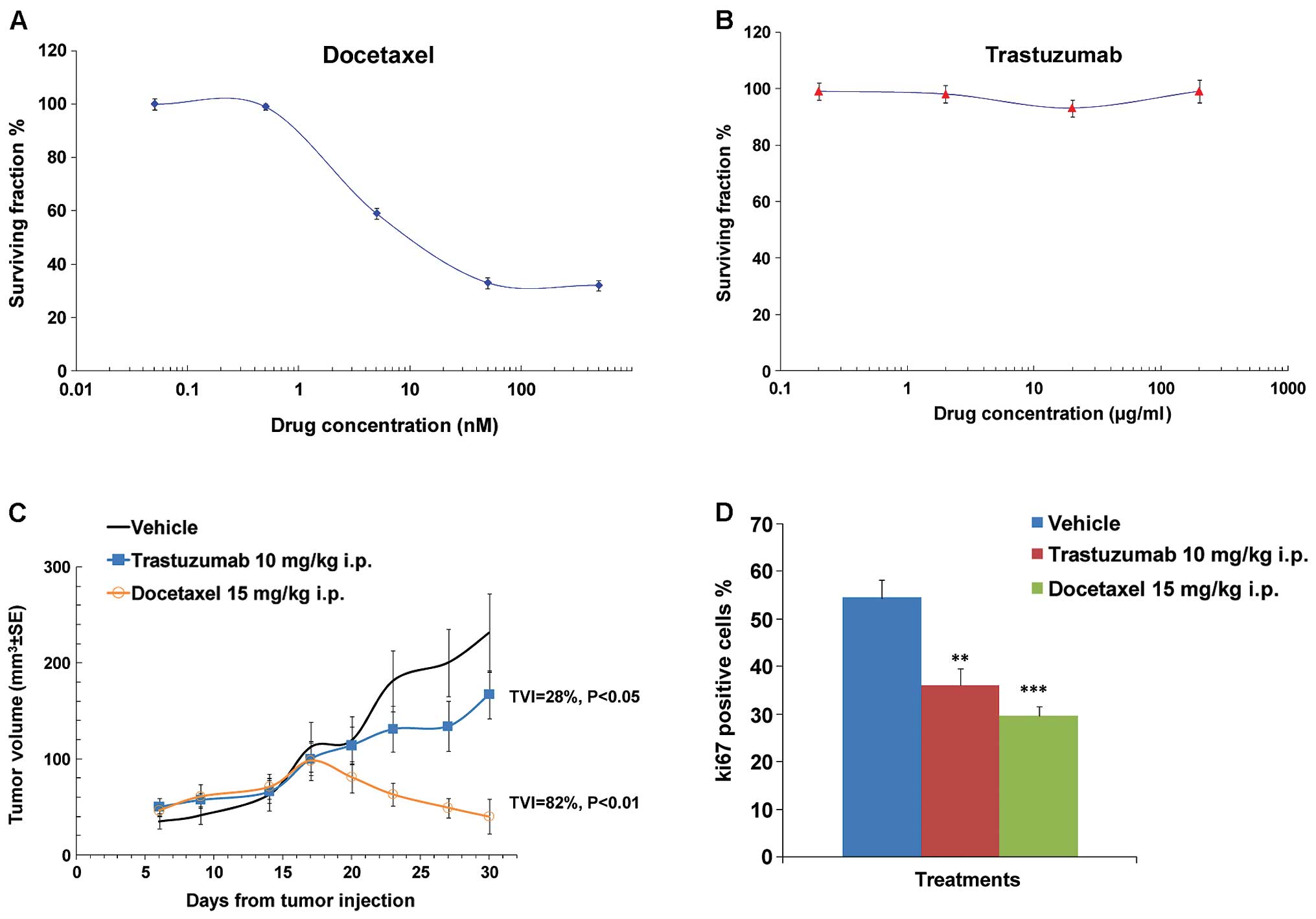
after 3 days of culture showing a dose-dependent inhibitory effect
of docetaxel but not trastuzumab, resulting in an IC50
of 7±0.1 nM and >200 µg/ml, respectively (Fig. 1A and B). Consistently with in
vitro data, docetaxel strongly inhibited the growth of the
MCF-7 xenografts (TVI=82%; P<0.01) whereas trastuzumab exhibited
a lower but significant antitumor activity (TVI=28%; P<0.05)
(Fig. 1C). Reduction of Ki-67
expression correlated with both docetaxel-dependent and
trastuzumab-dependent antitumor activity (Fig. 1D). Ki-67 is an important marker
predicting recurrence, prognosis and overall survival in breast
cancer patients (14–16). It has also been associated with
positive axillary lymph nodes in most studies (17,18).
Recently, Ki-67 was integrated as a prognostic factor into
molecular typing in prognosis of patients with luminal B breast
cancer (19). Therefore, we
investigated Ki-67 expression in further tumor slice
experiments.
Tumor slice preparation
The set up of ex vivo organotypic cultures of
human breast carcinoma was carried out before drug testing. Tumor
slices of 200 µm were obtained from MCF-7 xenotransplanted
mammary fat pads by the use of a vibratome. Slices were subjected
to histological analysis to identify the best experimental
conditions to preserve the tissue culture viability. The slices
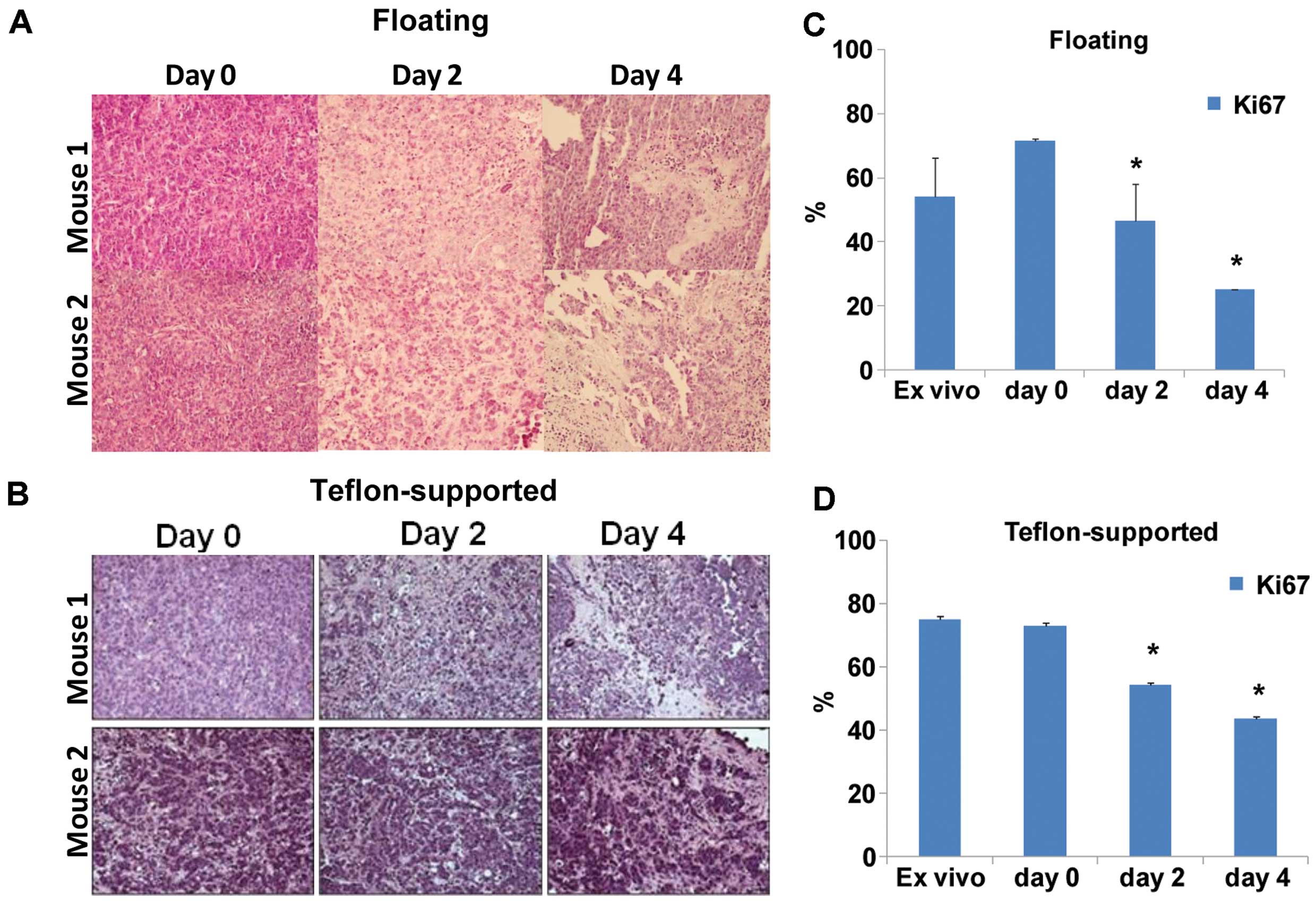
were maintened in normoxia for 2 or 4 days in floating or in
Teflon-supported conditions before analyses. H&E staining of
tumor slices revealed a high qualitative viability in the
Teflon-supported compared to floating condition (Fig. 2A and B). Accordingly, significantly
decreased expression of Ki-67 was found by immunohistochemistry in
floating slices compared to Teflon-supported slices (Fig. 2C and D).
These results indicated that an inert support is
useful to keep slices viable and tumor cells in a proliferative
status, thus indicating that tissue slices represent a
physiologically more relevant model compared to tissue cultures to
study solid cancer. Moreover, despite the lack of blood supply the
viability of Teflon-supported 200 µm thick tissue slices,
maintained in normoxia, suggests an adequate nutrient diffusion
from the culture medium.
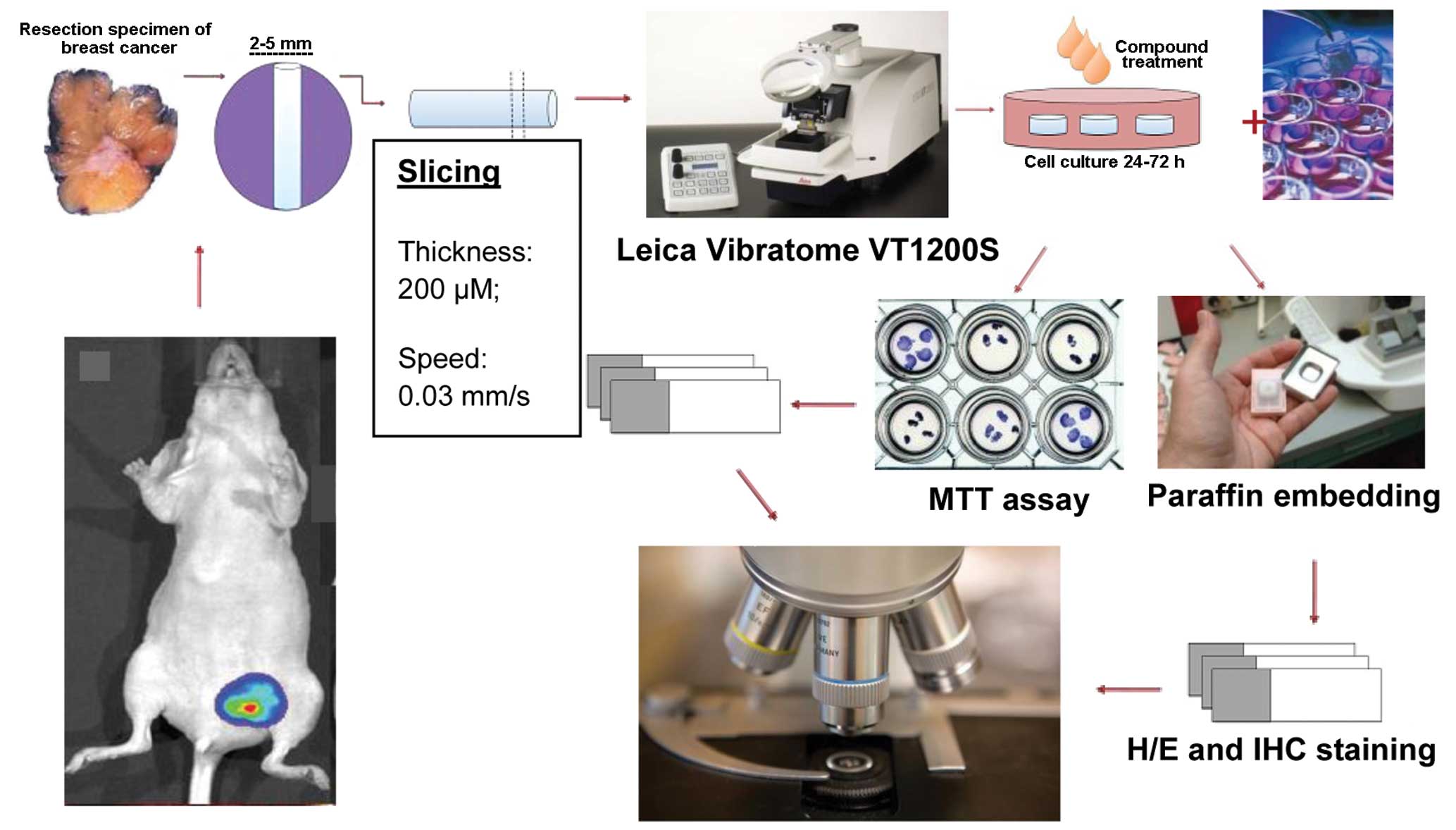
A schematic representation of the procedure to
obtain organotypic breast cancer tissue slices from MCF-7 mammary
fat pad xenotransplant is shown in Fig.
3.
Evaluation of docetaxel and trastuzumab
antitumor activity on breast cancer tissue slices
Because substantial morphological integrity of
tissues, defined as preservation of general architecture including
epithelial structures and their spatial relationship to stroma, was
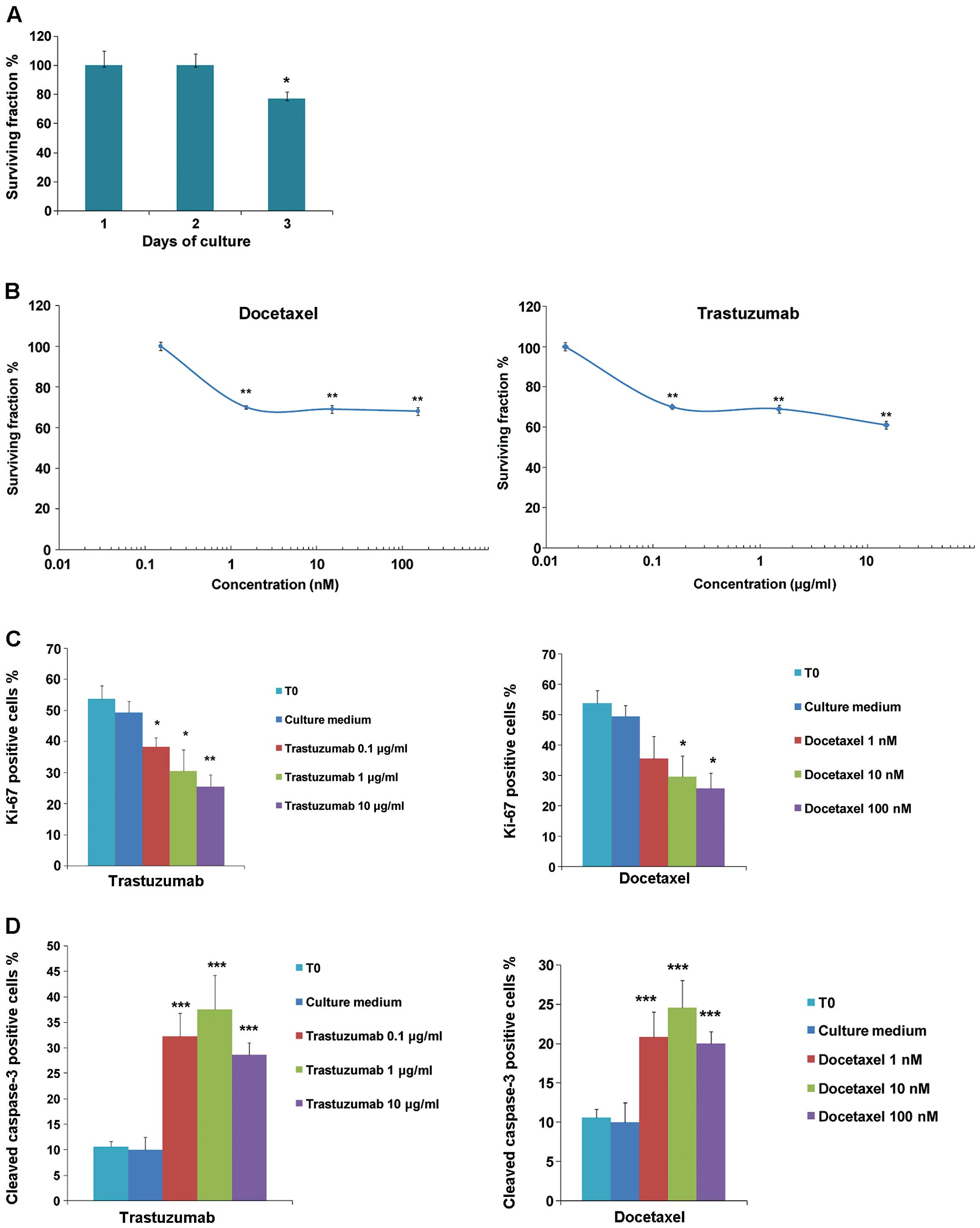
observed in tumor tissue slices up to 2 days of culture (Fig. 2). MTT assay was performed to
quantify viability. Data in Fig. 4A
show similar number of viable cells after 1 or 2 days dropping to
~80% after 3 days of cultivation. Further experiments were then
performed cultivating the tissue slices for 2 days in the presence
of different concentrations of docetaxel or trastuzumab. MTT assay
revealed a significant dose-dependent loss of viable cells upon
treatment with both drugs (Mann-Whitney U test;
**P<0.01) (Fig. 4B).
In agreement with this result, the percentage of proliferating
cells assessed by Ki-67 immunostaining significantly decreased upon
docetaxel or trastuzumab exposure (Fig.
4C), paralleled by increased percentage of cleaved caspase-3
positive cells (Fig. 4D). In
particular, biomarkers of proliferation and apoptosis used to
quantify effects of trastuzumab and docetaxel were evaluated by
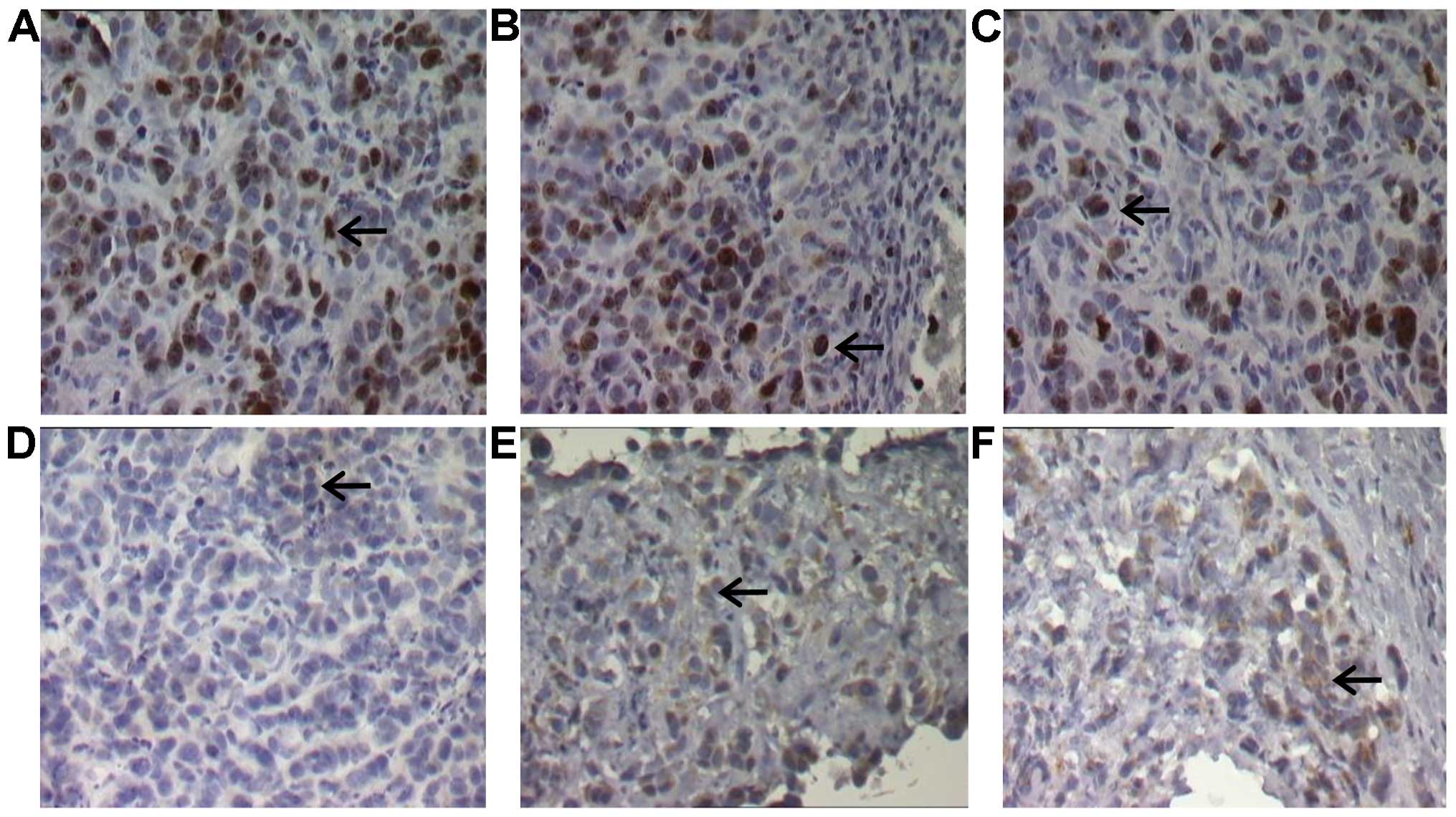
counting numbers of positively stained cells (Fig. 5A–F). Taken together, these data show
that tissue slices can better refect the in vivo
experimental outcome.
Discussion
In the present study we report a method to obtain
organotypic breast cancer tissue slices useful to evaluate the
efficacy of both small and a large molecule drugs. The advantage of
the tissue slice cultures is that the complex cross-talk between
matrix and cells as well as the cellular heterogeneity and
susceptibility to drugs is preserved avoiding potential source of
artefacts deriving from isolation of tumor cells from their
biological environment (20,21).
Moreover, the presence ofb ECM provides important differentiation
cues, for example, by signalling via Toll-like receptors and
integrins (22–24), thus further supporting the use of
tissue slice method for screening of therapeutics. Organotypic
tissue slice cultures offer a more attractive option compared to
cell line cultures or subcutaneous xenografts both lacking the
complexity of a tumor nested in a mammary gland (25–27).
Tissue slice samples with a thickness between 400–800 µm,
cultured 24 h were previously used to evaluate the transcriptional
effects of 1,25(OH)2D3 in breast cancer
benefitting from the heterogeneous combination of epithelial and
stromal cells that secrete a variety factors affecting the overall
response of tumor cells to the vitamin (28). Tissue slices of 250 µm from
human primary invasive ductal breast tumors were also employed to
test tamoxifen and doxorubicin activity, confirming the complexity
and heterogeneity of breast cancer among patients (29). Primary tissue slices of 400
µm were cultured in the presence of rapamycin, showing that
such a culture method preserves the tumor AKT/mTOR pathway activity
(30). Moreover, slice cultures of
350 µm from head and neck squamous cell carcinoma from
patients were also used to test cisplatin, docetaxel and cetuximab
allowing the design of personalized therapies and investigation of
mechanisms of tumor resistance (31,32).
It is becoming increasingly evident that the development of cancer
and the response to anti-cancer drugs not only depend on genetic
alterations but also on specific interactions between tumor cells
and surrounding tissue components. In invasive breast carcinoma,
differentiated myoepithelial cells and intact basement membranes
are lost and tumor cells are in direct contact with an activated
collagenous tumor stroma (27). To
simulate such conditions either three-dimensional tissue cultures
using several biomatrices or co-culture experiments with tumor
fibroblasts have been performed. However, these systems cannot
mimic the complex tissue architecture and the high degree of
variability seen in individual tumors. Therefore, to evaluate the
activity of docetaxel and trastuzumab antitumor drugs we used
tissue slice cultures starting from orthotopic human breast cancer
xenografts in mice and compared results with in vivo
efficacy. Our data support the use of the patient's tissue slices
as predictive translational model for personalized therapies.
So far, neither in vitro nor ex vivo
testing technologies have gained a significant impact in predicting
clinical efficacy of therapeutic treatments. Present data can be
framed within a widespread effort to identify testing conditions
useful to increase the success rate of drug investigation, thus
facilitating translational medicine.
Here we provide evidence that breast cancer tissue
slice is a more useful model than cell cultures to predict
antitumor efficacy of both small and large molecules. Sensitivity
of breast tumors to anticancer drugs depends upon dynamic
interactions between epithelial tumor cells and their
microenvironment including stromal cells and extracellular matrix.
Moreover, in addition to cancer cell viability, this model improves
the understanding of the impact of a given treatment on stromal and
endothelial cells and the distribution of a biologic, a small
molecule or viral vectors within tumor microenvironment. Another
potential application may include the ex vivo addition of
immune cells for OncoImmunology studies.
In conclusion, tissue slices represent an optimal
tool to investigate therapeutics and further studies to evaluate
this model for novel investigational drugs are warranted.
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rask-Andersen M, Almén MS and Schiöth HB:
Trends in the exploitation of novel drug targets. Nat Rev Drug
Discov. 10:579–590. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Klein CA: Selection and adaptation during
metastatic cancer progression. Nature. 501:365–372. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bedard PL, Hansen AR, Ratain MJ and Siu
LL: Tumour heterogeneity in the clinic. Nature. 501:355–364. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Roukos DH: Beyond HER2 and trastuzumab:
Heterogeneity, systems biology, and cancer origin research may
guide the future for personalized treatment of very early but
aggressive breast cancer. J Clin Oncol. 28:e279–e280; author reply
e282-e283. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Curtis C, Shah SP, Chin SF, Turashvili G,
Rueda OM, Dunning MJ, Speed D, Lynch AG, Samarajiwa S, Yuan Y, et
al METABRIC Group: The genomic and transcriptomic architecture of
2,000 breast tumours reveals novel subgroups. Nature. 486:346–352.
2012.PubMed/NCBI
|
|
8
|
Sørlie T, Perou CM, Tibshirani R, Aas T,
Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey
SS, et al: Gene expression patterns of breast carcinomas
distinguish tumor subclasses with clinical implications. Proc Natl
Acad Sci USA. 98:10869–10874. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Perou CM, Sørlie T, Eisen MB, van de Rijn
M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA,
et al: Molecular portraits of human breast tumours. Nature.
406:747–752. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lehmann BD, Bauer JA, Chen X, Sanders ME,
Chakravarthy AB, Shyr Y and Pietenpol JA: Identifcation of human
triple-negative breast cancer subtypes and preclinical models for
selection of targeted therapies. J Clin Invest. 121:2750–2767.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Prat A, Parker JS, Karginova O, Fan C,
Livasy C, Herschkowitz JI, He X and Perou CM: Phenotypic and
molecular characterization of the claudin-low intrinsic subtype of
breast cancer. Breast Cancer Res. 12:R682010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Russnes HG, Vollan HK, Lingjaerde OC,
Krasnitz A, Lundin P, Naume B, Sørlie T, Borgen E, Rye IH, Langerød
A, et al: Genomic architecture characterizes tumor progression
paths and fate in breast cancer patients. Sci Transl Med. 2:38–47.
2010.
|
|
13
|
Johnson JI, Decker S, Zaharevitz D,
Rubinstein LV, Venditti JM, Schepartz S, Kalyandrug S, Christian M,
Arbuck S, Hollingshead M, et al: Relationships between drug
activity in NCI preclinical in vitro and in vivo models and early
clinical trials. Br J Cancer. 84:1424–1431. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
de Azambuja E, Cardoso F, de Castro G Jr,
Colozza M, Mano MS, Durbecq V, Sotiriou C, Larsimont D,
Piccart-Gebhart MJ and Paesmans M: Ki-67 as prognostic marker in
early breast cancer: A meta-analysis of published studies involving
12,155 patients. Br J Cancer. 96:1504–1513. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li FY, Wu SG, Zhou J, Sun JY, Lin Q, Lin
HX, Guan XX and He ZY: Prognostic value of Ki-67 in breast cancer
patients with positive axillary lymph nodes: A retrospective cohort
study. PLoS One. 9:e872642014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Stuart-Harris R, Caldas C, Pinder SE and
Pharoah P: Proliferation markers and survival in early breast
cancer: A systematic review and meta-analysis of 85 studies in
32,825 patients. Breast. 17:323–334. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Urruticoechea A, Smith IE and Dowsett M:
Proliferation marker Ki-67 in early breast cancer. J Clin Oncol.
23:7212–7220. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jalava P, Kuopio T, Juntti-Patinen L,
Kotkansalo T, Kronqvist P and Collan Y: Ki67 immunohistochemistry:
a valuable marker in prognostication but with a risk of
misclassification: proliferation subgroups formed based on Ki67
immunoreactivity and standardized mitotic index. Histopathology.
48:674–682. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cheang MC, Chia SK, Voduc D, Gao D, Leung
S, Snider J, Watson M, Davies S, Bernard PS, Parker JS, et al: Ki67
index, HER2 status, and prognosis of patients with luminal B breast
cancer. J Natl Cancer Inst. 101:736–750. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pietras K and Ostman A: Hallmarks of
cancer: Interactions with the tumor stroma. Exp Cell Res.
316:1324–1331. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Moreth K, Brodbeck R, Babelova A, Gretz N,
Spieker T, Zeng-Brouwers J, Pfeilschifter J, Young MF, Schaefer RM
and Schaefer L: The proteoglycan biglycan regulates expression of
the B cell chemoattractant CXCL13 and aggravates murine lupus
nephritis. J Clin Invest. 120:4251–4272. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Merline R, Moreth K, Beckmann J, Nastase
MV, Zeng-Brouwers J, Tralhão JG, Lemarchand P, Pfeilschifter J,
Schaefer RM, Iozzo RV, et al: Signaling by the matrix proteoglycan
decorin controls inflammation and cancer through PDCD4 and
MicroRNA-21. Sci Signal. 4:ra752011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Alghisi GC, Ponsonnet L and Rüegg C: The
integrin antagonist cilengitide activates alphaVbeta3, disrupts
VE-cadherin localization at cell junctions and enhances
permeability in endothelial cells. PLoS One. 4:e44492009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Krumdieck CL, dos Santos JE and Ho KJ: A
new instrument for the rapid preparation of tissue slices. Anal
Biochem. 104:118–123. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hood CJ and Parham DM: A simple method of
tumour culture. Pathol Res Pract. 194:177–181. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
van der Kuip H, Mürdter TE, Sonnenberg M,
McClellan M, Gutzeit S, Gerteis A, Simon W, Fritz P and Aulitzky WE
and Aulitzky WE: Short term culture of breast cancer tissues to
study the activity of the anticancer drug taxol in an intact tumor
environment. BMC Cancer. 6:86–92. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Milani C, Katayama ML, de Lyra EC, Welsh
J, Campos LT, Brentani MM, Maciel MS, Roela RA, del Valle PR, Góes
JC, et al: Transcriptional effects of 1,25 dihydroxyvitamin D(3)
physiological and supra-physiological concentrations in breast
cancer organotypic culture. BMC Cancer. 13:1192013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Holliday DL, Moss MA, Pollock S, Lane S,
Shaaban AM, Millican-Slater R, Nash C, Hanby AM and Speirs V: The
practicalities of using tissue slices as preclinical organotypic
breast cancer models. J Clin Pathol. 66:253–255. 2013. View Article : Google Scholar
|
|
30
|
Grosso SH, Katayama ML, Roela RA, Nonogaki
S, Soares FA, Brentani H, Lima L, Folgueira MA, Waitzberg AF,
Pasini FS, et al: Breast cancer tissue slices as a model for
evaluation of response to rapamycin. Cell Tissue Res. 352:671–684.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gerlach MM, Merz F, Wichmann G, Kubick C,
Wittekind C, Lordick F, Dietz A, Bechmann I and Waitzberg AF: Slice
cultures from head and neck squamous cell carcinoma: A novel test
system for drug susceptibility and mechanisms of resistance. Br J
Cancer. 110:479–488. 2014. View Article : Google Scholar :
|
|
32
|
Goldhirsch A, Ingle JN, Gelber RD, Coates
AS, Thürlimann B and Senn HJ: Panel members: Thresholds for
therapies: Highlights of the St Gallen International Expert
Consensus on the primary therapy of early breast cancer 2009. Ann
Oncol. 20:1319–1329. 2009. View Article : Google Scholar : PubMed/NCBI
|