Introduction
Tumor angiogenesis, one of the key steps in tumor
growth and metastasis, is the process wherein new blood vessels
form from the existing vasculature to penetrate into tumors to
supply oxygen and essential nutrients (1,2).
Tumors induce the growth of new blood vessels by secretion of
various growth factors including vascular endothelial growth factor
(VEGF), which is a very specific mitogen for vascular endothelial
cells (3,4). VEGF expressed by various cancer cells
triggers tumor angiogenesis by binding to its receptor, mainly
vascular endothelial growth factor receptor 2 (VEGFR2) (5). Stimulation of VEGFR2 results in the
activation of several downstream signaling pathways, including the
mitogen-associated protein kinase (MAPK)/extracellular
signal-regulated kinase (ERK) pathway and the phosphoinositide
3-kinase (PI3K)/AKT/endothelial nitric oxide synthase (eNOS)
pathway, to induce endothelial cell proliferation, migration and
permeabilization (6,7). Since angiogenesis is one of the
critical steps in cancer biology, the inhibition of tumor
angiogenesis is a promising approach for cancer chemoprevention and
therapy, and the potential of anti-angiogenic drugs to control
tumor growth and metastasis is currently under investigation
(8).
The proliferation of endothelial cells is an
essential step for angiogenesis and is directly induced by
progression of the cell cycle (9).
The cell cycle can be divided in G0/G1, S, G2 and M phases which
are regulated by various checkpoint protein families including
cyclins, cyclin-dependent kinases (CDKs) and CDK inhibitors
(10). CDK-cyclin complex
activation occurs to progress the checkpoint system. Specifically,
CDK2/cyclin E and CDK4/cyclin D1 complexes are essential for G1/S
transition and are inactivated by CDK inhibitors, such as p21 and
p27 (11).
Juniperus chinensis (J. chinensis), a
well-known folk remedy in Korea, has been reported to exhibit
antitumor, antimicrobial and diuretic properties. Previously, we
demonstrated that widdrol, a natural sesquiterpene from J.
chinensis, induces cell cycle arrest and apoptosis in human
colon adenocarcinoma HT29 cells in vitro (12,13).
However, the anti-angiogenic activity of widdrol has yet to be
fully determined. In the present study, we investigated the
anti-angiogenic efficacy of widdrol and its underlying molecular
mechanisms of action using human umbilical vein endothelial cells
(HUVECs) and tumor xenograft mice.
Materials and methods
Chemicals
Widdrol was isolated from J. chinensis as
previously described (12). Widdrol
was dissolved in dimethyl sulfoxide (DMSO) for the in vitro
studies and dissolved in a solution containing 10% ethanol and
Cremophor EL (both from Sigma-Aldrich, St. Louis, MO, USA) in
distilled water for the in vivo experiment.
Animals
Female BALB/c nude mice (BALB/c-nu) were obtained
from Japan SLC Inc. (Shizuoka, Japan), and used at 6–8 weeks of
age. The animals were housed in microisolator cages under standard
conditions for humidity, room temperature and dark-light cycles.
The animal experiments were performed in compliance with the
Dong-Eui University Animal Care guideline (approval no.
R2013-001).
Cell lines and culture
HUVECs purchased from Clonetics (Walkersville, MD,
USA) were maintained in EBM-2 medium containing 2% fetal bovine
serum (FBS), angiogenic growth factors and 1 µg/ml GA-1000
(gentamicin, amphotericin-B) of EGM-2 BulletKit (Lonza,
Walkersville, MD, USA) under standard culture conditions (37°C and
5% CO2). To examine the effect of widdrol on
VEGF-related signaling, HUVECs were cultured with EBM-2 medium
containing 0.5% FBS and 100 ng/ml of VEGF (Sigma-Aldrich). Human
colon adenocarcinoma HT29 cells, were obtained from the American
Type Culture Collection (ATCC; Manassas, VA, USA). HT29 cells were
cultured in Dulbecco's modified Eagle's medium (DMEM) containing
10% FBS and penicillin/streptomycin under standard culture
conditions.
Endothelial cell growth and death
assays
Cell viability was assessed using a water-soluble
tetrazolium salt (WST) assay using the EZ-Cytox Cell Viability
Assay kit (Daeil Lab, Seoul, Korea). EZ-Cytox assay reagent (10
µl) was added to each cell culture well, and the mixture was
incubated for 30 min at 37°C. The absorbance was measured at 450 nm
using a plate reader (Beckman Coulter, Fullerton, CA, USA). A
trypan blue exclusion assay was performed as previously described
(12). The cells were treated with
widdrol, trypsinized, and stained with trypan blue. Viable cells
were counted with a hemocytometer.
Flow cytometric analysis of cell
cycle
Cell cycle analysis was performed using a CycleTest
DNA reagent kit (Becton-Dickinson, San Jose, CA, USA) according to
the manufacturer's instructions. Flow cytometry was conducted (Cell
Lab Quanta SC; Beckman Coulter) and the relative DNA content was
determined using the FlowJo software (Tree Star, Inc., Ashland, OR,
USA).
Western blot analysis
For western blot analysis, 30–50 µg/ml of
proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel
electrophoresis and blotted onto nitrocellulose membranes. Blots
were incubated at 4°C overnight with specific primary antibodies
followed by horseradish peroxidase-conjugated secondary antibodies
and visualized by an enhanced chemiluminescence detection system
(FluorChem®FC2; Alpha Innotech, San Leandro, CA, USA)
using Western Blotting Luminol reagent (Santa Cruz Biotechnology,
Dallas, TX, USA). CDK2, CDK4, cyclin D1 and E, p53, p27, AKT,
ERK1/2, actin primary antibodies and peroxidase-conjugated
secondary antibodies were purchased from Santa Cruz Biotechnology.
Primary antibodies against p21, p-AKT, p-ERK1/2, VEGFR2, p-VEGFR2,
eNOS, p-eNOS, focal adhesion kinase (FAK) and p-FAK were purchased
from Cell Signaling Technology (Beverly, CA, USA).
Tube formation assay
The tube formation assay was performed as previously
described (14). Briefly, HUVECs
were seeded on Matrigel-coated plates (BD Biosciences, San Jose,
CA, USA) in EBM-2 medium containing 0.5% FBS, and treated with
widdrol. After 6 and 24 h of incubation, the tube formation was
observed under a phase contrast microscope. For the quantitative
data, tube formation was scored by counting the number of branch
points of tubular structure.
Wound-healing assay
The wound-healing assay was performed as previously
described (14). The wounded
monolayers of HUVECs were recorded by photography and then
incubated for 16 h in fresh EBM-2 medium supplemented with 0.5% FBS
in the presence of DMSO or various concentrations of widdrol. After
incubation, images of cells were captured and the migrated cells
were counted from three randomly selected fields. Inhibition
percentage was expressed as a percentage relative to the vehicle
control (100%).
In vivo tumor xenograft study
HT29 cells (5×106) were implanted
subcutaneously into the lateral flanks of female athymic
(BALB/c-nu) mice. When the tumor mass was palpable, widdrol (10 and
50 mg/kg) or adriamycin (2 mg/kg; Sigma-Aldrich), as a positive
control (15), was administered to
the mice (n=5 each group) intravenously three times a week for 15
days. Simultaneously, all the mice were weighed and the tumor
volume was measured and calculated using the formula: tumor volume
(mm3) = [length × (width)2] × π/6. After 19
days of injection, the mice were sacrificed and the tumors were
removed and weighed. Tumor paraffin blocks were sectioned at
5-µm thickness and stained with hematoxylin and eosin
(H&E). Histological changes were observed under a light
microscope (Eclipse C; Nikon, Tokyo, Japan). For immunofluorescence
staining, the slides were incubated with the antibodies against
CD31/PECAM1 and VEGFR2, followed by incubation with Alexa-594- and
Alexa-488-labeled secondary antibodies, respectively. The nuclei
were counterstained with 4′,6-diamidino-2-phenylindole (DAPI;
Sigma-Aldrich). Positive signals were photographed under a
fluorescence microscope (Axio Scope A; Carl Zeiss, Jena, Germany).
The primary antibodies were purchased from Cell Signaling
Technology and fluorescent dye-tagged secondary antibodies were
from Santa Cruz Biotechnology.
Statistical analysis
The data were presented as the mean ± SD from at
least three independent experiments. Statistical comparisons
between groups were performed by the SPSS program followed by a
Student's t-test. P<0.05 was considered statistically
significant.
Results
Widdrol inhibits the VEGF-induced
proliferation and cell cycle progression of HUVECs
Since angiogenesis primarily requires the
proliferation of endothelial cells initiated by growth factors, we
evaluated the inhibitory effect of widdrol on the VEGF-induced
proliferation of HUVECs. As shown in Fig. 1A, the proliferation of HUVECs
stimulated by VEGF was decreased by widdrol at concentrations
>10 µg/ml. The number of viable cells was decreased by
widdrol in a dose-dependent manner using the trypan blue exclusion
assay (Fig. 1B). To demonstrate the
mechanism of inhibition of HUVEC proliferation by widdrol, the
effect of widdrol on progression of the cell cycle in HUVECs was
subsequently examined. Compared to the untreated control, HUVECs
treated with 20 µg/ml of widdrol showed a marked increase in
G1 phase from 61.9 to 85.7%, indicating that widdrol induces G1
arrest of HUVECs (Fig. 1C and
Table I). Widdrol-mediated G1
arrest was associated with the upregulation of CDK inhibitors, p21
and p27, and the downregulation of G1/S transition-related
proteins, CDK2 and cyclin E (Fig.
1D). These results suggested that widdrol inhibited the
proliferation of HUVECs by blocking the cell cycle progression
associated with the increase of p21 expression and the inactivation
of the CDK2/cyclin E complex.
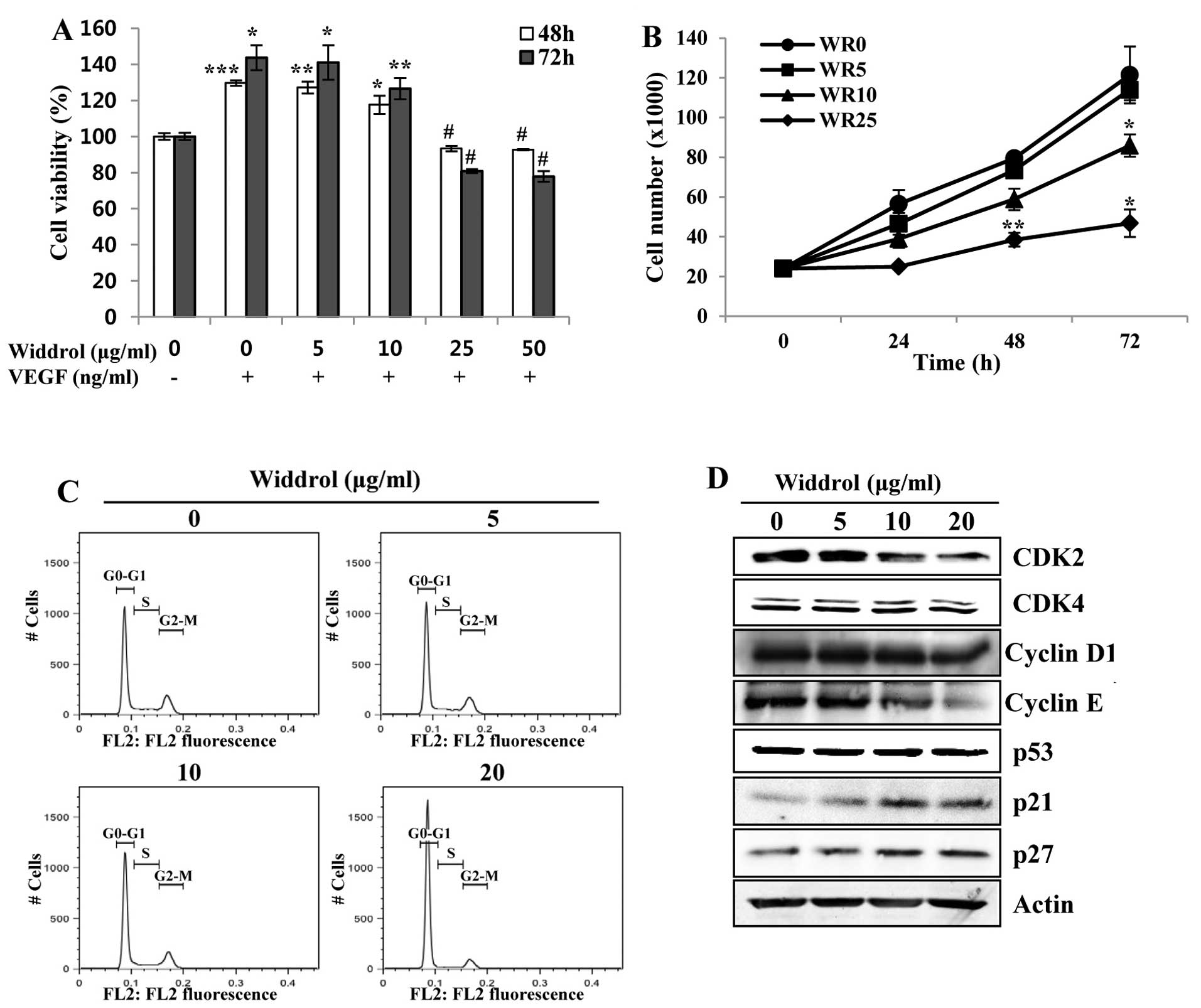 | Figure 1Effects of widdrol on VEGF-induced
proliferation and cell cycle progression of HUVECs. (A) HUVECs in
EBM-2 medium containing 0.5% FBS were treated with or without VEGF
(100 ng/ml) and DMSO (0.1%) or various concentrations of widdrol
for 48 or 72 h. After incubation, cell viability was determined by
the WST assay. Data are presented as percentages of the vehicle
control as mean ± SD of three independent experiments.
*P<0.05, **P<0.01, ***P<0.005 vs.
control group. #P<0.05 vs. VEGF alone treatment. (B)
For the trypan blue exclusion assay, the cells were treated with
various concentrations of widdrol (WR) for the indicated
time-points, harvested and stained with trypan blue, and viable
cells were counted. Data are represented as mean ± SD of three
independent experiments. *P<0.05,
**P<0.01 vs. control group. (C) Cell cycle analysis.
After widdrol treatment for 24 h, the cells were hypotonically
lysed and incubated in propidium iodide (PI) solution. Flow
cytometry was conducted and the relative DNA content was
determined. (D) Western blot analysis. Cells were treated with
widdrol for 24 h and lysed in extraction buffer, followed by
western blot analysis using primary antibodies against G1/S
transition-related proteins. VEGF, vascular endothelial growth
factor; HUVECs, human umbilical vein endothelial cells; DMSO,
dimethyl sulfoxide; WST, water-soluble tetrazolium salt; FBS, fetal
bovine serum. |
 | Table ICell cycle distribution of HUVECs
after widdrol treatment. |
Table I
Cell cycle distribution of HUVECs
after widdrol treatment.
| Phase | Widdrol
(µg/ml)
|
|---|
| 0 | 5 | 10 | 20 |
|---|
| G0/G1 | 61.9 | 63.0 | 67.4 | 85.7 |
| S | 13.4 | 13.2 | 11.1 | 3.4 |
| G2/M | 21.1 | 20.8 | 18.9 | 9.4 |
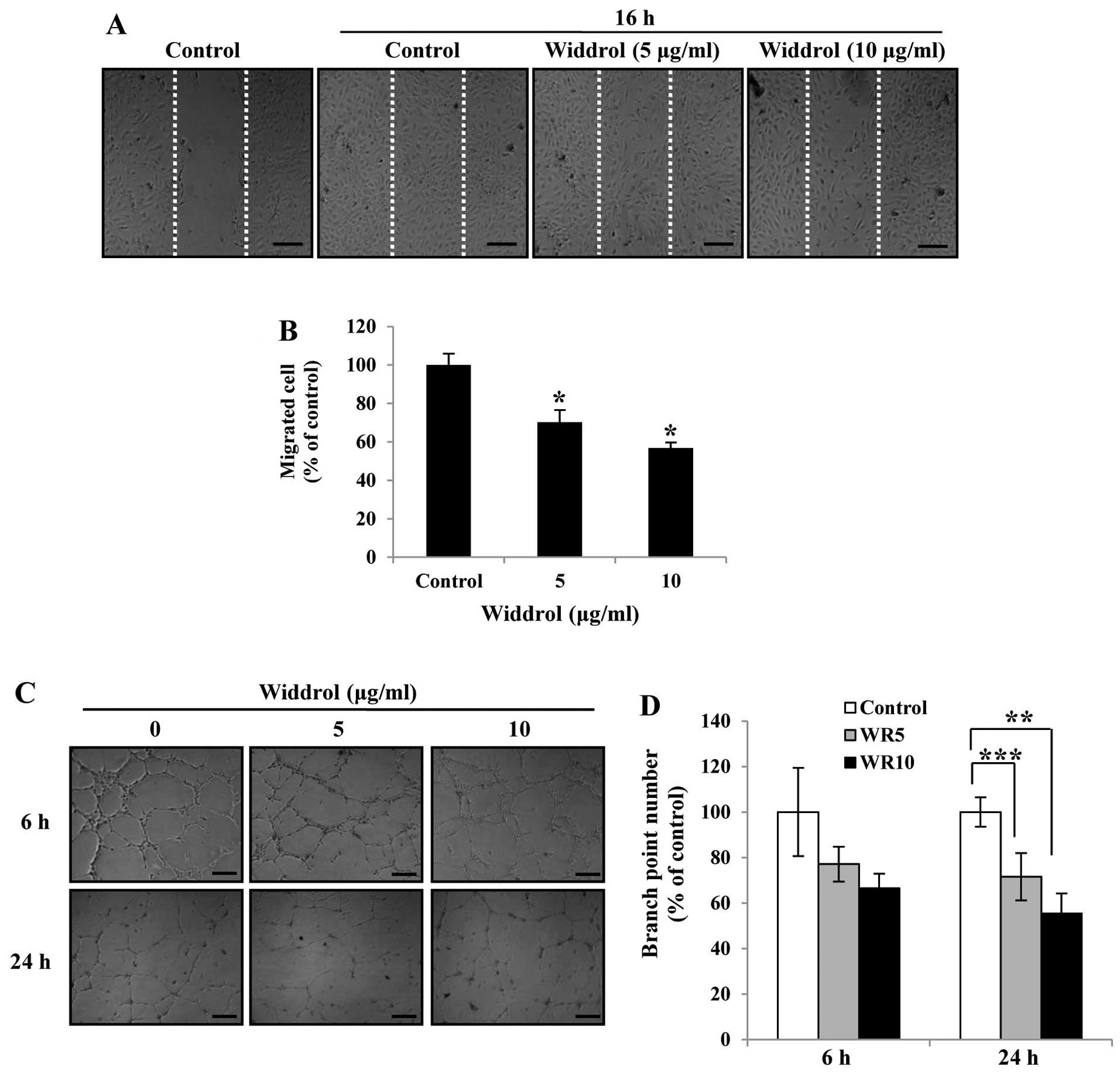
Widdrol inhibits the migration and tube
formation of HUVECs in vitro
To investigate the anti-angiogenic activity of
widdrol in vitro, we carried out wound-healing and tube
formation assays at a non-toxic dose of widdrol. As shown in
Fig. 2A, widdrol effectively
inhibited the cell migration of HUVECs in a dose-dependent manner.
Compared to the control, 10 µg/ml of widdrol treatment for
16 h showed 44% inhibition of cell migration (Fig. 2B). We also found that the tube
formation of HUVECs was suppressed by widdrol treatment, while the
untreated HUVECs formed robust tube-like structures (Fig. 2C). The quantitative data showed that
widdrol inhibited the tube formation of HUVECs in a dose- and
time-dependent manner by 35 and 50% inhibition after 6 and 24 h of
incubation, respectively (Fig. 2D).
These results indicate that widdrol exerts an anti-angiogenic
effect by blocking the endothelial cell migration and tube
formation in vitro.
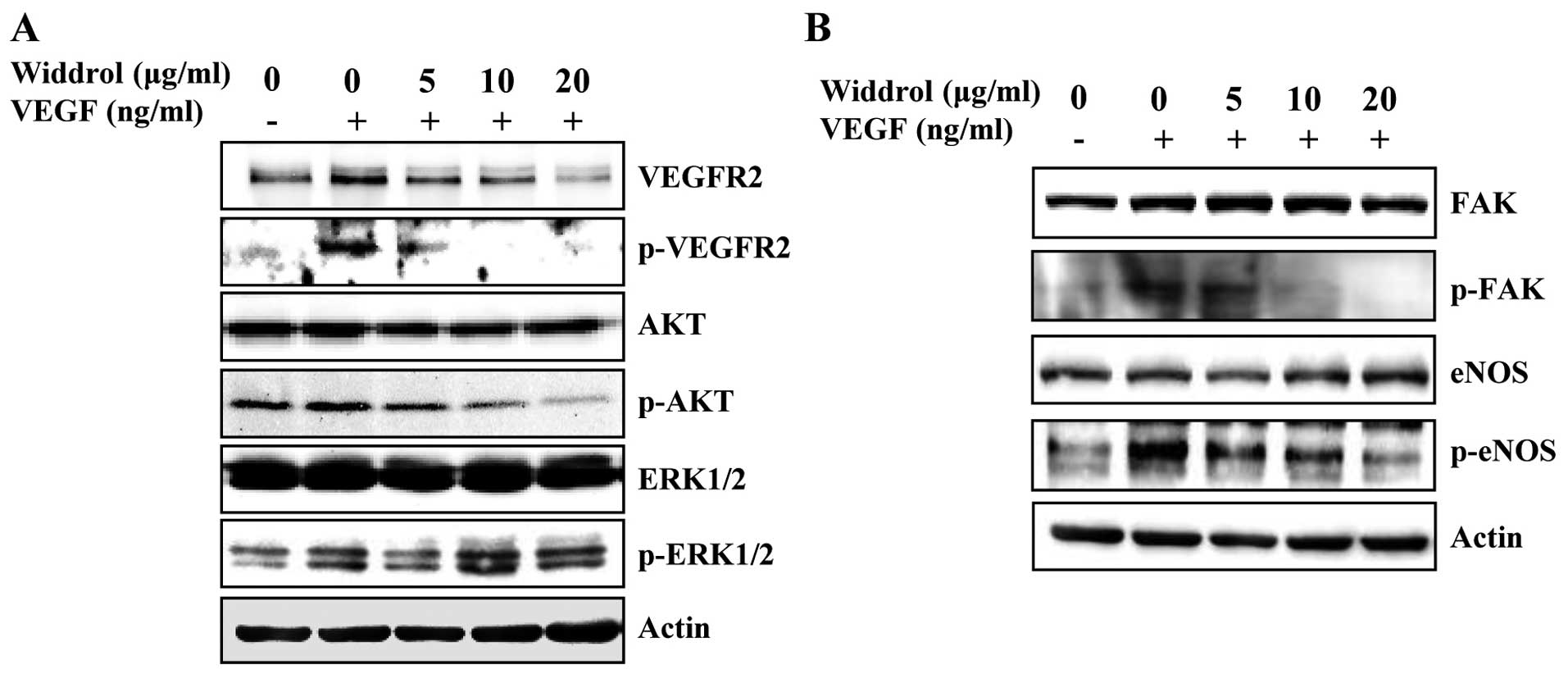
Widdrol suppresses the VEGFR2-mediated
signaling pathway
To determine the molecular mechanism of
widdrol-mediated anti-angiogenesis, the expression and
phosphorylation of VEGFR2 and its downstream molecules was examined
in HUVECs by western blot analysis. As shown in Fig. 3A and B, the VEGF-stimulated
phosphorylation of VEGFR2, AKT and FAK was markedly suppressed by
widdrol in a dose-dependent manner. Although it was slightly
reduced by 5 µg/ml of widdrol, the phosphorylation of ERK1/2
did not show apparent changes in response to widdrol treatment.
Widdrol also inhibited phosphorylation of eNOS, which is downstream
of AKT, in a dose-dependent manner. These results collectively
suggested that widdrol blocks VEGFR2 signaling via AKT and FAK
inactivation, leading to the inhibition of angiogenesis in
HUVECs.
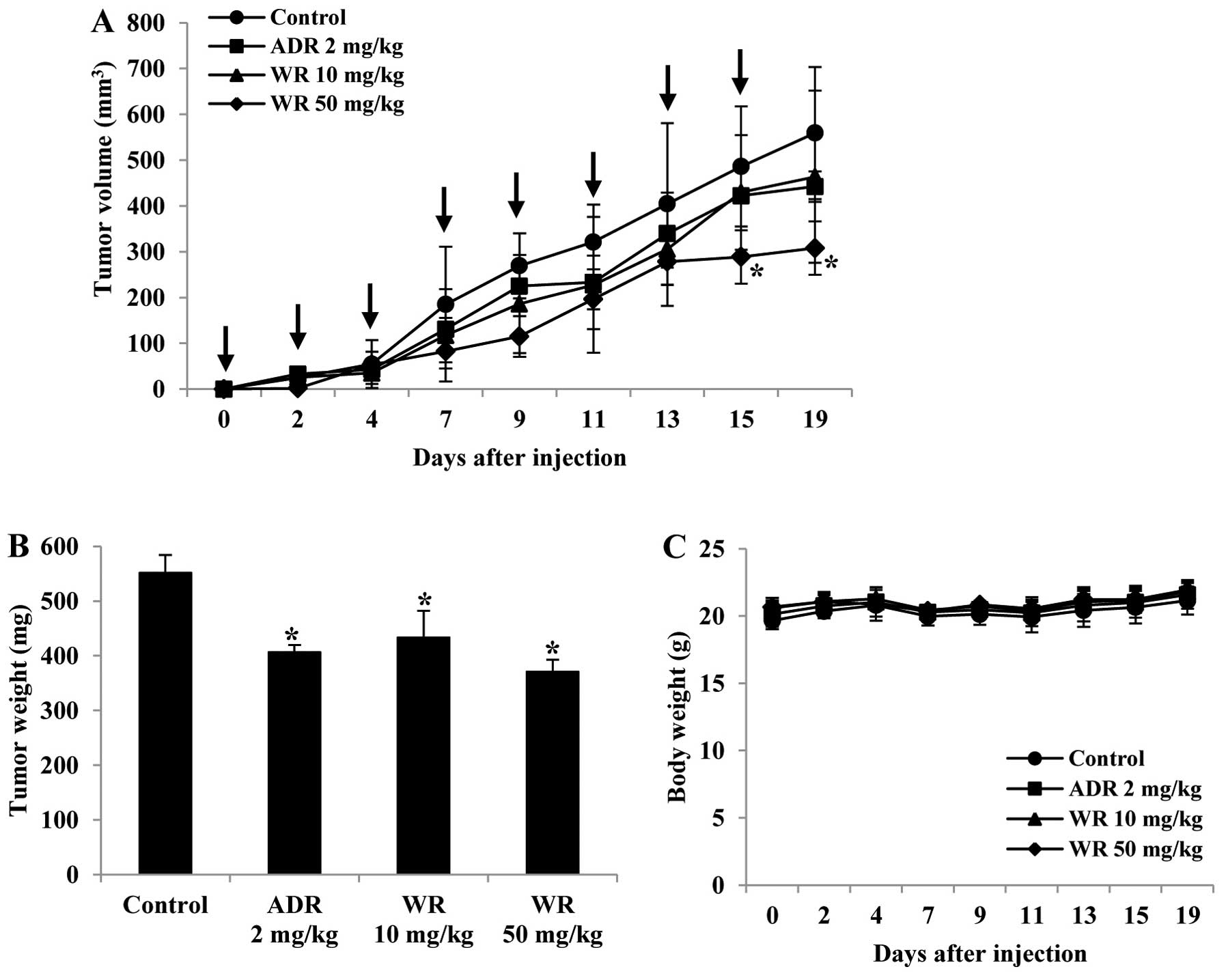
Widdrol inhibits tumor growth in
vivo
In order to evaluate the effect of widdrol on tumor
growth and angiogenesis in vivo, we established the
xenograft model using colon adenocarcinoma HT29 cells. As shown in
Fig. 4A and B, widdrol
significantly reduced tumor volume and weight compared to vehicle
control in a dose-dependent manner. Treatment with 50 mg/kg of
widdrol inhibited tumor growth more effectively than treatment with
adriamycin (2 mg/kg) as a positive control. However, widdrol
treatment did not cause body weight loss, suggesting that widdrol
has an anticancer efficacy in vivo without any apparent
toxicity (Fig. 4C).
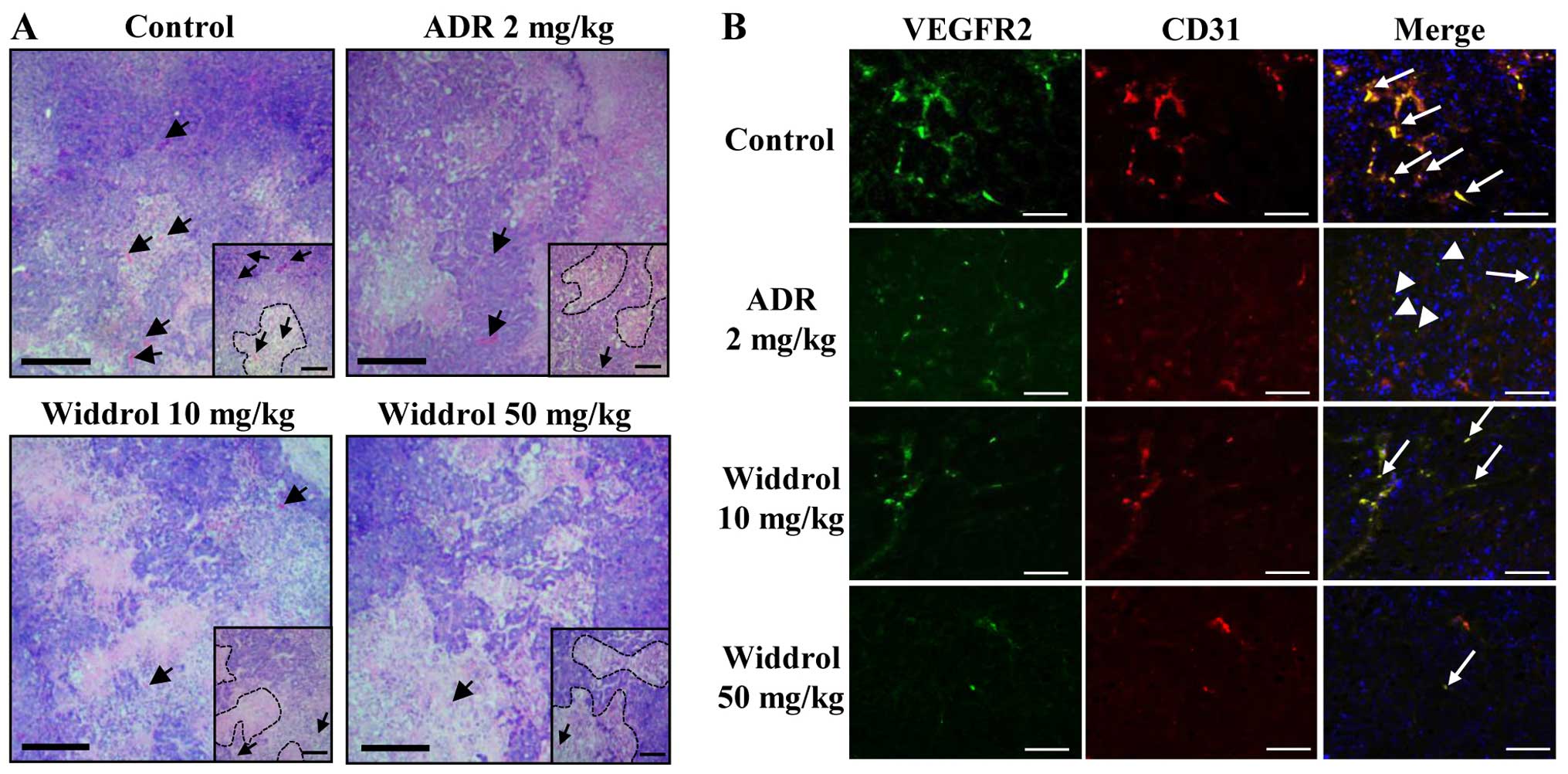
Widdrol changes the histological
structure and regulates angiogenesis in tumor xenograft mice
To examine whether widdrol inhibits angiogenesis
in vivo, the vessel formation in tumor tissue was observed
using histochemical studies. After H&E staining, the
histological changes in tumor were examined. As shown in Fig. 5A, tumor cells of the control group
(with vehicle only) were packed densely in parenchyma (insets) and
the blood vessels were easily observed in stroma (arrow). By
contrast, tumor cells of the widdrol- or adriamycin-treated group
were sparse as compared to those of the control group in parenchyma
and the blood vessels were hardly detected in stroma. For
immunofluorescence staining, the paraffin blocks of tumor were
sectioned and stained with specific antibodies against CD31, a
pan-endothelial cell marker, and VEGFR2. As shown in Fig. 5B, CD31 and VEGFR2 expression was
distinctly detected in control tumor tissues and the CD31-positive
endothelial cells simultaneously expressed VEGFR2 (arrow). Although
CD31-positive cells were significantly decreased, VEGFR2 expression
in the adriamycin-treated group continued to be observed but was
slightly decreased (arrowhead) as compared to the control group.
However, in the widdrol-treated group, CD31 and VEGFR2
double-positive cells were decreased in a dose-dependent manner.
Compared to the control group, the treatment of 50 mg/kg widdrol
induced a marked decrease of CD31 and VEGFR2 expression in tumor
tissues. These results indicated that widdrol induces histological
changes of tumor and effectively inhibits tumor angiogenesis with
the suppression of CD31 and VEGFR2 expression in colon tumor
xenograft mice.
Discussion
Angiogenesis is a complex multistep process
including endothelial cell proliferation, migration and capillary
formation, and plays a critical role in tumor growth and metastasis
(16,17). Endothelial cells are activated by
various angiogenic stimuli including VEGF, one of the most potent
pro-angiogenic factors, to release nitric oxide (NO) and
degradative enzymes allowing them to migrate and penetrate into the
tumor mass or tissues, resulting in neovascularization (18–20).
It has been reported that the signaling events of VEGF mainly occur
via VEGFR2 and regulating VEGFR2 activity can modulate
angiogenesis, making VEGFR2 an attractive target for antitumor
drugs (21–23). It also has been suggested that
downstream of VEGFR2, such as ERK, PI3K/AKT and FAK pathway, can be
a potential target for therapeutics because it plays a critical
role in regulating tumor angiogenesis (24,25).
The anticancer efficacy of natural products, such as flavonoids and
terpenes, has been examined and it has been demonstrated that
various natural compounds can inhibit angiogenesis in vitro
and in vivo (26–28). In vitro anticancer activity
of widdrol has been reported (12,13),
however, the anti-angiogenic activity of widdrol has yet to be
fully elucidated.
In the present study, we found that the expression
of CDK2 and cyclin E was decreased, whereas the expression of p21
was increased by widdrol in HUVECs, resulting in the inhibition of
cell proliferation by cell cycle arrest. Widdrol effectively
suppressed the phosphorylation of VEGFR2, AKT and FAK, leading to
blocking of cell migration and tube formation of HUVECs. We also
found that widdrol inhibited the phosphorylation of eNOS, a
downstream molecule of AKT, requiring further studies to ascertain
the widdrol-mediated inhibition of endothelial NO production.
Widdrol successfully inhibited tumor growth accompanied by a
decrease of CD31 and VEGFR2 double-positive endothelial cells,
indicating that tumor angiogenesis is suppressed by widdrol.
Similar to our finding, Xiao et al showed that phenethyl
isothiocyanate, a constituent of cruciferous vegetables, exerted an
anti-angiogenic effect in HUVECs associated with the downregulation
of VEGFR2 protein levels and inactivation of AKT (29). It has been reported that
catunaregin, a marine compound, showed anti-angiogenic effects and
significantly decreased the phosphorylation of AKT and eNOS
(30).
Moreover, recent findings have revealed that VEGF
promotes endothelial cell proliferation via ERK and AKT pathways
and is associated with the increase of cyclin A, D1 and E
expression as well as the decrease of CDK inhibitor expression
(31,32). Based on these studies, our data
suggest that the inhibition of VEGFR2 signaling via the AKT pathway
using widdrol leads to inactivation of the CDK/cyclin complex and
induction of CDK inhibitor expression, followed by cell cycle
arrest and proliferation inhibition.
In summary, to the best of our knowledge, our
observations firstly reveal the anti-angiogenic efficacy of widdrol
and suggest the mechanisms of widdrol-mediated anti-angiogenesis.
Widdrol suppressed endothelial cell proliferation by cell cycle
arrest and inhibited angiogenesis by targeting VEGFR2 signaling by
blocking AKT and FAK activation. In tumor xenograft mice, the
reduction of tumor growth and a decrease of vascular structure were
observed by widdrol treatment. Therefore, our findings suggest that
widdrol possesses potential antitumor activities by inhibiting
tumor angiogenesis, suggesting that widdrol may act as an
anti-angiogenic agent and may contribute to anticancer drug
development.
Acknowledgments
This study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF) funded by the Ministry of Science, ICT and Future Planning
(2012R1A1A3011936).
References
|
1
|
Folkman J: Tumor angiogenesis: Therapeutic
implications. N Engl J Med. 285:1182–1186. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kerbel RS: Tumor angiogenesis: Past,
present and the near future. Carcinogenesis. 21:505–515. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Alon T, Hemo I, Itin A, Pe'er J, Stone J
and Keshet E: Vascular endothelial growth factor acts as a survival
factor for newly formed retinal vessels and has implications for
retinopathy of prematurity. Nat Med. 1:1024–1028. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ferrara N: Vascular endothelial growth
factor: Molecular and biological aspects. Curr Top Microbiol
Immunol. 237:1–30. 1999.PubMed/NCBI
|
|
5
|
Dvorak HF, Brown LF, Detmar M and Dvorak
AM: Vascular permeability factor/vascular endothelial growth
factor, microvascular hyperpermeability, and angiogenesis. Am J
Pathol. 146:1029–1039. 1995.PubMed/NCBI
|
|
6
|
Dimmeler S, Fleming I, Fisslthaler B,
Hermann C, Busse R and Zeiher AM: Activation of nitric oxide
synthase in endothelial cells by Akt-dependent phosphorylation.
Nature. 399:601–605. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Olsson AK, Dimberg A, Kreuger J and
Claesson-Welsh L: VEGF receptor signalling - in control of vascular
function. Nat Rev Mol Cell Biol. 7:359–371. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ferrara N and Kerbel RS: Angiogenesis as a
therapeutic target. Nature. 438:967–974. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zheng X, Li A, Zhao L, Zhou T, Shen Q, Cui
Q and Qin X: Key role of microRNA-15a in the KLF4 suppressions of
proliferation and angiogenesis in endothelial and vascular smooth
muscle cells. Biochem Biophys Res Commun. 437:625–631. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Roberts JM, Koff A, Polyak K, Firpo E,
Collins S, Ohtsubo M and Massagué J: Cyclins, Cdks, and cyclin
kinase inhibitors. Cold Spring Harb Symp Quant Biol. 59:31–38.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sherr CJ and Roberts JM: CDK inhibitors:
Positive and negative regulators of G1-phase progression. Genes
Dev. 13:1501–1512. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kwon HJ, Hong YK, Park C, Choi YH, Yun HJ,
Lee EW and Kim BW: Widdrol induces cell cycle arrest, associated
with MCM down-regulation, in human colon adenocarcinoma cells.
Cancer Lett. 290:96–103. 2010. View Article : Google Scholar
|
|
13
|
Kwon HJ, Lee EW, Hong YK, Yun HJ and Kim
BW: Widdrol from Juniperus chinensis induces apoptosis in human
colon adenocarcinoma HT29 cells. Biotechnol Bioprocess Eng.
15:167–172. 2010. View Article : Google Scholar
|
|
14
|
Martínez-Poveda B, Muñoz-Chápuli R,
Rodríguez-Nieto S, Quintela JM, Fernández A, Medina MA and Quesada
AR: IB05204, a dichloropyridodithienotriazine, inhibits
angiogenesis in vitro and in vivo. Mol Cancer Ther. 6:2675–2685.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Argov M, Kashi R, Peer D and Margalit R:
Treatment of resistant human colon cancer xenografts by a
fluoxetine-doxorubicin combination enhances therapeutic responses
comparable to an aggressive bevacizumab regimen. Cancer Lett.
274:118–125. 2009. View Article : Google Scholar
|
|
16
|
Folkman J: Role of angiogenesis in tumor
growth and metastasis. Semin Oncol. 29(Suppl 16): 15–18. 2002.
View Article : Google Scholar
|
|
17
|
He D, Jin J, Zheng Y, Bruce IC, Tam S and
Ma X: Anti-angiogenesis effect of trichosanthin and the underlying
mechanism. Biochem Biophys Res Commun. 430:735–740. 2013.
View Article : Google Scholar
|
|
18
|
Rössler J, Taylor M, Geoerger B, Farace F,
Lagodny J, Peschka-Süss R, Niemeyer CM and Vassal G: Angiogenesis
as a target in neuroblastoma. Eur J Cancer. 44:1645–1656. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shizukuda Y, Tang S, Yokota R and Ware JA:
Vascular endothelial growth factor-induced endothelial cell
migration and proliferation depend on a nitric oxide-mediated
decrease in protein kinase Cdelta activity. Circ Res. 85:247–256.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Taimeh Z, Loughran J, Birks EJ and Bolli
R: Vascular endothelial growth factor in heart failure. Nat Rev
Cardiol. 10:519–530. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Eskens FA and Verweij J: The clinical
toxicity profile of vascular endothelial growth factor (VEGF) and
vascular endothelial growth factor receptor (VEGFR) targeting
angiogenesis inhibitors; a review. Eur J Cancer. 42:3127–3139.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Saraswati S, Kanaujia PK, Kumar S, Kumar R
and Alhaider AA: Tylophorine, a phenanthraindolizidine alkaloid
isolated from Tylophora indica exerts antiangiogenic and antitumor
activity by targeting vascular endothelial growth factor receptor
2-mediated angiogenesis. Mol Cancer. 12:822013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Takahashi S: Vascular endothelial growth
factor (VEGF), VEGF receptors and their inhibitors for
antiangiogenic tumor therapy. Biol Pharm Bull. 34:1785–1788. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cheng JQ, Lindsley CW, Cheng GZ, Yang H
and Nicosia SV: The Akt/PKB pathway: Molecular target for cancer
drug discovery. Oncogene. 24:7482–7492. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jiang BH and Liu LZ: AKT signaling in
regulating angiogenesis. Curr Cancer Drug Targets. 8:19–26. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yue GG, Chan BC, Kwok HF, Wong YL, Leung
HW, Ji CJ, Fung KP, Leung PC, Tan NH and Lau CB: Anti-angiogenesis
and immunomodulatory activities of an anti-tumor sesquiterpene
bigelovin isolated from Inula helianthus-aquatica. Eur J Med Chem.
59:243–252. 2013. View Article : Google Scholar
|
|
27
|
Hong SW, Jung KH, Lee HS, Son MK, Yan HH,
Kang NS, Lee J and Hong SS: SB365, Pulsatilla saponin D, targets
c-Met and exerts antiangiogenic and antitumor activities.
Carcinogenesis. 34:2156–2169. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang Y, Ma W and Zheng W: Deguelin, a
novel anti-tumorigenic agent targeting apoptosis, cell cycle arrest
and anti-angiogenesis for cancer chemoprevention. Mol Clin Oncol.
1:215–219. 2013.
|
|
29
|
Xiao D and Singh SV: Phenethyl
isothiocyanate inhibits angiogenesis in vitro and ex vivo. Cancer
Res. 67:2239–2246. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu JX, Luo MQ, Xia M, Wu Q, Long SM, Hu
Y, Gao GC, Yao XL, He M, Su H, et al: Marine compound catunaregin
inhibits angiogenesis through the modulation of phosphorylation of
akt and eNOS in vivo and in vitro. Mar Drugs. 12:2790–2801. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Favot L, Keravis T and Lugnier C:
Modulation of VEGF-induced endothelial cell cycle protein
expression through cyclic AMP hydrolysis by PDE2 and PDE4. Thromb
Haemost. 92:634–645. 2004.PubMed/NCBI
|
|
32
|
Shi F, Wang YC, Zhao TZ, Zhang S, Du TY,
Yang CB, Li YH and Sun XQ: Effects of simulated microgravity on
human umbilical vein endothelial cell angiogenesis and role of the
PI3K-Akt-eNOS signal pathway. PLoS One. 7:e403652012. View Article : Google Scholar : PubMed/NCBI
|