Introduction
Cancer is a serious health issue that accounts for
millions of deaths. In general, localized cancer can be resected by
surgery, yet metastatic cancer requires systemic treatment with
chemotherapy (1). However, the
genetic cytotoxic side-effects and resistance of chemotherapeutic
agents used in the clinic have motivated scientists to identify and
develop new drugs to replace the currently used arsenal of agents.
Different strategies based on tumor cell characteristics, the
targeting of specific enzymes or transcription factors, have been
proposed in drug development. It is known that neoplastic cells
have a higher requirement for iron due to their significantly
elevated expression of the transferrin receptor 1 and
ribonucleotide reductase (2). In
addition, cancer cells take up more copper (Cu) than normal cells
and use this metal for angiogenesis and metastasis (3). Due to the crucial roles of these
metals, the development of novel Fe and Cu chelators has become a
promising anticancer strategy (4).
Hydrazones have been intensively investigated in
view of their potential application as anticancer, antiviral,
antibacterial and antifungal agents. Pyridinecarboxylic acid
hydrazide and pyridinecarboxaldehyde are widely used in the
preparation of hydrazone or Schiff base for their potent metal
chelating ability. Pyridoxaldehyde isonicotinoyl hydrazone has been
extensively evaluated as an antiproliferative agent and iron
chelator (5,6). In contrast, these hydrazones have
versatile ability in metal. For instance, the metal complex of
N-heteroaromatic hydrazones of 2-pyridinecarboxaldehyde often
exhibits greater biological activity compared to the corresponding
free ligands, with copper(II) complexes being the most active among
all the tested complexes (7).
However, the underlying mechanisms of the hydrazones and their
metal complexes in regards to their anticancer activity are poorly
understood. Concerning iron chelators, one hypothesis frequently
cited is the inhibition of ribonucleotide reductase, which contains
iron at its catalytic center (8).
Chelators also exert cytotoxic effects via redox cycling of bound
metals and attendant production of free radicals (9,10).
Thus, they are involved in an apoptotic response by activating the
mitochondrial pathway (11).
The excellent biological activities of the
hydrazones and their metal complexes prompted us to probe their
mechanisms of action. As an extended study, the hydrazone of
2-pyridinecarboxaldehyde 2-pyridinecarboxylic acid hydrazone (PPAH)
was prepared and its tumor proliferation inhibition was evaluated.
In view of a possible synergistic effect in combination with metal
ions, the inhibitory effect on cell proliferation of PPAH in the
presence of copper ions was also investigated. Beyond our
expectation, the mixture of PPAH with copper displayed excellent
proliferation inhibition, which motivated us to probe the
underlying mechanism. Thus, the composition of the active species,
cell cycle analysis, RT-PCR, comet assay and topoisomerase (Top)
inhibition assay were performed. The results revealed that PPAH
formed a complex with copper and acted as a novel Top inhibitor for
both type I and II, which is rarely observed for metal
complexes.
Materials and methods
All reactants and solvents were AR grade. MTT,
ethidium bromide (EB), RPMI-1640 medium and agarose were purchased
from Sigma.
Preparation of PPAH
2-Pyridinecarboxylic acid hydrazide was prepared
based on a previously reported procedure (12). PPAH was made by refluxing an equal
molar ratio of 2-pyridinecarboxylic acid hydrazide and
2-pyridinecarboxaldehyde in ethanol, and the reaction process was
monitored by TLC. Pure PPAH was isolated by removing the solvent
and re-crystallization in acetonitrile. The 1HNMR
(DMSO-d6, Bruker 400 MHz) of PPAH: 1HNMR: 12.54(s,
H(N-H)), 8.73(d), 8.70(s, (CHO)), 8.62(d), 8.15(d), 8.06(t),
8.0(d), 7.90(t), 7.70(t), 7.43(t). 13CNMR(ppm): 161.26,
158.36, 149.97, 149.86, 149.78, 149.03, 138.55, 137.35, 127.65,
124.92, 123.37, 120.45. M/Z: 227.2734 (M+H)+. The
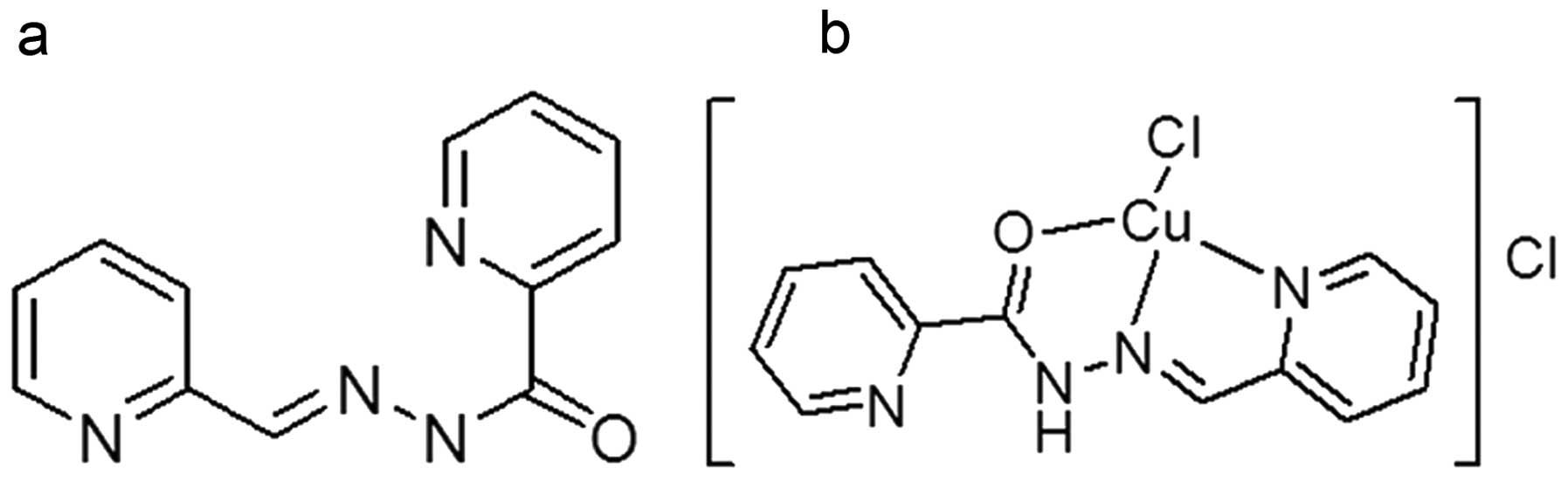
structures of PPAH and its copper complex are shown in Fig. 1.
Determination of the molar ratio of PPAH
to copper(II)
The molar ratio of PPAH/copper(II) was determined by
spectral methods as previously described (13). Briefly, the stock solutions of 1 mM
PPAH in 15% dimethylsulfoxide (DMSO) and 1 mM CuCl2 in
water were prepared. Next, 0.2 ml of the CuCl2 solution
was added to a series of 5-ml volumetric flasks, and then 40, 80,
120, 160, 200, 240, 280, 320, 360 or 400 µl of PPAH was
transferred to each flask, respectively. Finally, water was added
(5 ml). After mixing and equilibrium, the spectra were recorded on
a Shimadzu UV-2450 spectrophotometer.
Cell culture and cytotoxicity assay
The stock solution of PPAH (10 mM) was prepared in
25% DMSO, and was diluted to the required concentration with water
when used. The copper complex was made by mixing PPAH with a high
concentration of CuCl2 based on 1:1 molar ratio and
diluted to the required concentration with water. The human
colorectal carcinoma cell line (HCT-116) and liver carcinoma cells
(HepG2) were cultured in RPMI-1640 medium supplemented with 10%
fetal calf serum (FCS) and antibiotics. The cells collected during
the exponential phase of growth (1×104/ml) were seeded
equivalently into 96-well plates, and 1 µl of PPAH or its
copper complex at varied concentrations was added after the cells
adhered. Following a 48-h incubation at 37°C in a humidified
atmosphere of 5% CO2, 10 µl MTT solution (1
mg/ml) was added to each well, followed by further incubation for 4
h. The cell culture was removed by aspiration, and 100
µl/well of DMSO was added to dissolve the formazan crystals.
The measurement of the absorbance of the solution that was related
to the number of living cells was performed on a microplate reader
(MK3; Thermo Scientific) at 570 nm. Percent growth inhibition was
defined as the percentage of the decrease in absorbance to the
appropriate absorbance for each cell line. The same assay was
performed in triplet.
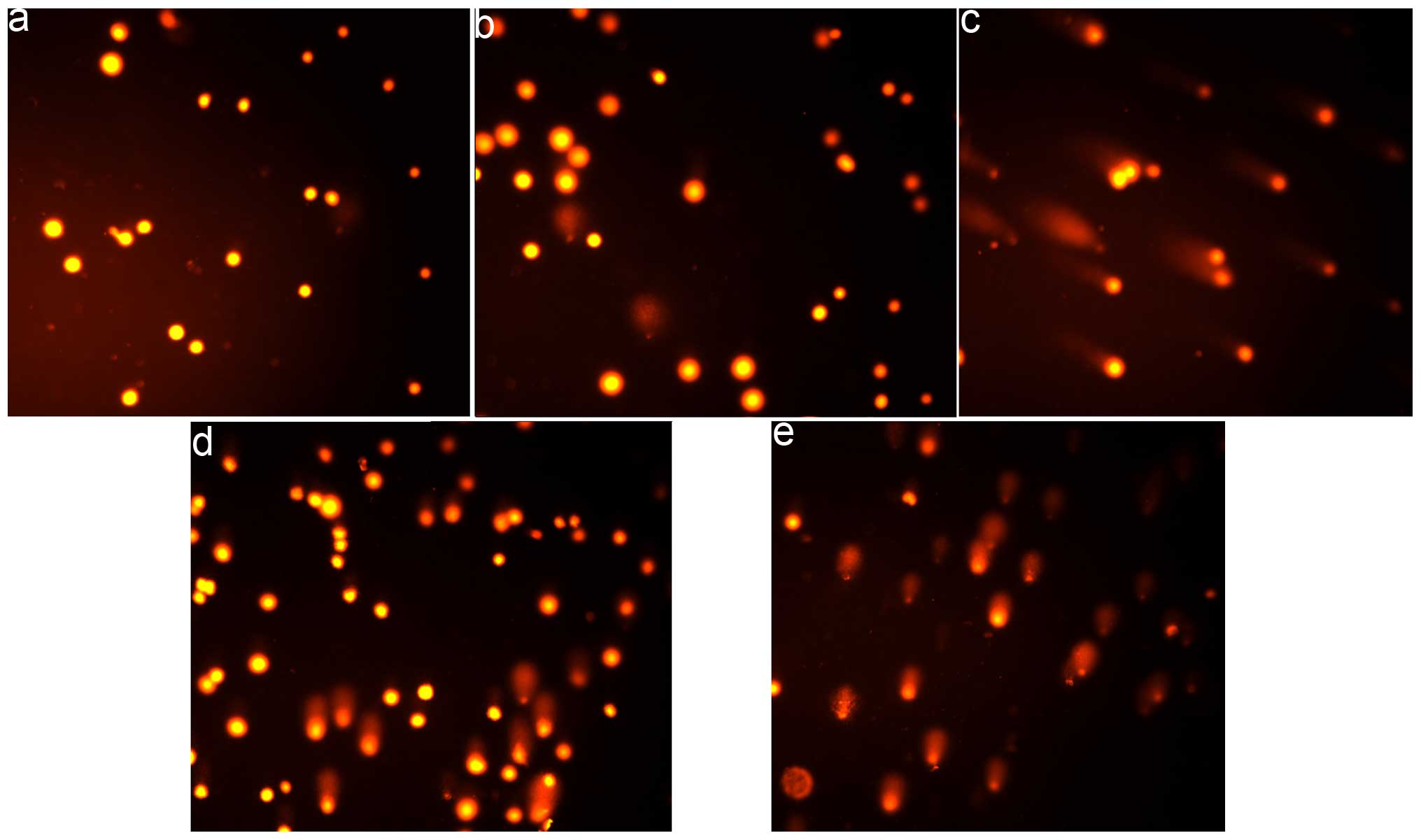
Comet assay
The comet assay was adapted as previously described
(14). HepG2 cells were treated
with or without the investigated compounds (40 and 80 µM for
PPAH or 2.5 and 5 µM for the PPAH-Cu complex) followed by a
48-h incubation in a humidified atmosphere of 5% CO2.
The cells were harvested by centrifugation after trypsinization and
then embedded in 0.5% low melting point agarose at a final
concentration of 1×104 cells/ml. A 20-µl aliquot
of this cellular suspension was then spread onto duplicate frosted
slides that had previously been covered with 1% normal melting
point agarose as a basal layer. The slides were allowed to solidify
for 10 min at 4°C before being placed in lysis buffer for 1 h [2.5
M NaCl, 0.1 M ethylenediaminetetraacetic acid (EDTA), 0.01 M Tris,
1% Triton X-100, 10% DMSO, pH 10.0). After lysis, the slides were
transferred into alkaline buffer for 40 min (0.001 M EDTA, 0.3 M
NaOH, pH >13.0) to allow the DNA to unwind before migration at
0.66 V/cm and 300 mA for 30 min. All these steps were performed in
the dark. After neutralization in 0.4 M Tris-HCl pH 7.4, the slides
were stored at 4°C until analysis following 24 h. Before analysis,
the slides were stained with EB (20 µg/ml) and covered with
a coverslip. Images was captured using fluorescence microscopy.
RT-PCR
Total RNA was extracted from the cells following
treatment with the investigated agents for 24 (or 48 h) using
TRIzol reagent (Sangon, Shanghai, China) according to the
manufacturer's protocol. Two micrograms of total RNA were used for
reverse transcription in a total volume of 20 µl with the
M-MLV reverse transcriptase system (LifeFeng Biological Technology
Corporation, Shanghai, China). Two microliters of cDNA were
subsequently amplified in a total volume of 20 µl using the
2X Taq PCR kit (LifeFeng Biological Technology Corporation)
following the conditions recommended by the manufacturer. The sense
and antisense primers (synthesized by Shanghai Generay Biological
Engineering Corporation, Co., Shanghai, China) for β-actin were:
5′-ACACTGTGCCC ATCTACGAGG-3′ and 5′-CGGACTCGTCATACTCCTG CT-3′ (615
bp) used as an internal control; the sense and antisense primers
for caspase 3 were: 5′-GAAGCGAATC AATGGACTCTGG-3′ and
5′-ACATCACGCATCAATTCCA CAA-3′ (241 bp); the sense and antisense
primers for caspase 8 were: 5′-AAGTTCCTGAGCCTGGACTACAT-3′ and
5′-ATTT GAGCCCTGCCTGGTGTCT-3′ (227 bp); the sense and antisense
primers for p53 were: 5′-GTCTACCTCCCGCCAT AA-3′ and
5′-CATCTCCCAAACATCCCT-3′ (316 bp); the sense and antisense primers
for bcl were: 5′-TTACCAAGCAG CCGAAGA-3′ and
5′-TCCCTCCTTTACATTCACAA-3′ (309 bp); the sense and antisense
primers for bax were: 5′-TTT TGCTTCAGGGTTTCATC-3′ and
5′-GGCCTTGAGCACCA GTTT-3′ (299 bp); the sense and antisense primers
for cyclin A were: 5′-TTAGGGAAATGGAGGTTA-3′ and 5′-CAGAAAG
TATTGGGTAAGAA-3′, respectively. RT-PCR was performed on a Nexus
Gradient Mastercycler (Eppendorf). The cycling conditions were:
94°C for 2 min, followed by 30 cycles of 94°C for 30 sec, 53–56°C
for 30 sec, and 72°C for 1 min, and a final extension of 72°C for
10 min. The PCR products were separated on 1.5% agarose gel and
visualized by EB staining. The data were acquired with a Tocan 360
gel imager (version 3.2.1 software) (Shanghai Tiancheng Technology
Co., Ltd., China).
Cell cycle analysis
HepG2 cells (1×105) were seeded in a
6-well plate and incubated for 24 h at 37°C (5% CO2),
the medium was replaced with fresh medium, supplemented or not
(control) with the agents (40 and 80 µM for PPAH, or 2.5 and
5 µM for the PPAH-Cu complex). After 24 h of incubation, the
cells were harvested with trypsin, followed by washing with
phosphate-buffered saline (PBS), fixed in 70% ethanol and stored at
−20°C. The cellular nuclear DNA was stained with propidium iodide
(PI). Briefly, after removing the 70% ethanol, the cells were
washed with PBS and then suspended in 0.5 ml PBS containing 50
µg/ml PI and 100 µg/ml RNase. The cell suspension was
incubated at 37°C for 30 min. DNA flow cytometry was performed in
duplicate with a FACSCalibur flow cytometer (Becton-Dickinson,
USA). For each sample 10,000 events were collected, and fluorescent
signal intensity was recorded and analyzed by CellQuest and ModiFit
(Becton-Dickinson).
DNA Top activity assay
The nuclear extract from HepG2 cells was prepared as
previously described (15). The
nuclear extract (0.4 µg) was added to the Top reaction
mixture containing 10 mM Tris-HCl (pH 7.5), 1 mM EDTA, 150 mM NaCl,
0.1% bovine serum albumin (BSA), 5% glycerol and 0.4 µg
pUC18 and 1 µl (or 2 or 3 µl) test agent (0.5 mM for
PPAH or 0.3 mM for PPAH-Cu complex in water) at a final volume of
20 µl. Following incubation at 37°C for 30 min, the reaction
was terminated by adding 5 µl of stopping buffer (10% SDS,
0.025% bromophenol blue and 5% glycerol). The reaction products
were analyzed by electrophoresis on 1% agarose gel using a TBE
buffer with 0.1% SDS (89 mM Tris-HCl, 89 mM boric acid and 62 mM
EDTA) at 45 V for 3 h, stained by EB (0.5 µg/ml) and
photographed using a short wavelength UV lamp on a Tocan 360 gel
scanner. The assay was conducted in duplicates.
Results
Determination of the molar ratio of
PPAH/copper(II)
PPAH has potential coordination atoms. Thus, in the
presence of copper ions, a new species may be formed as a copper
complex. To determine the molar ratio of PPAH to copper ions, the
absorption spectra of varied concentrations of PPAH in water at a
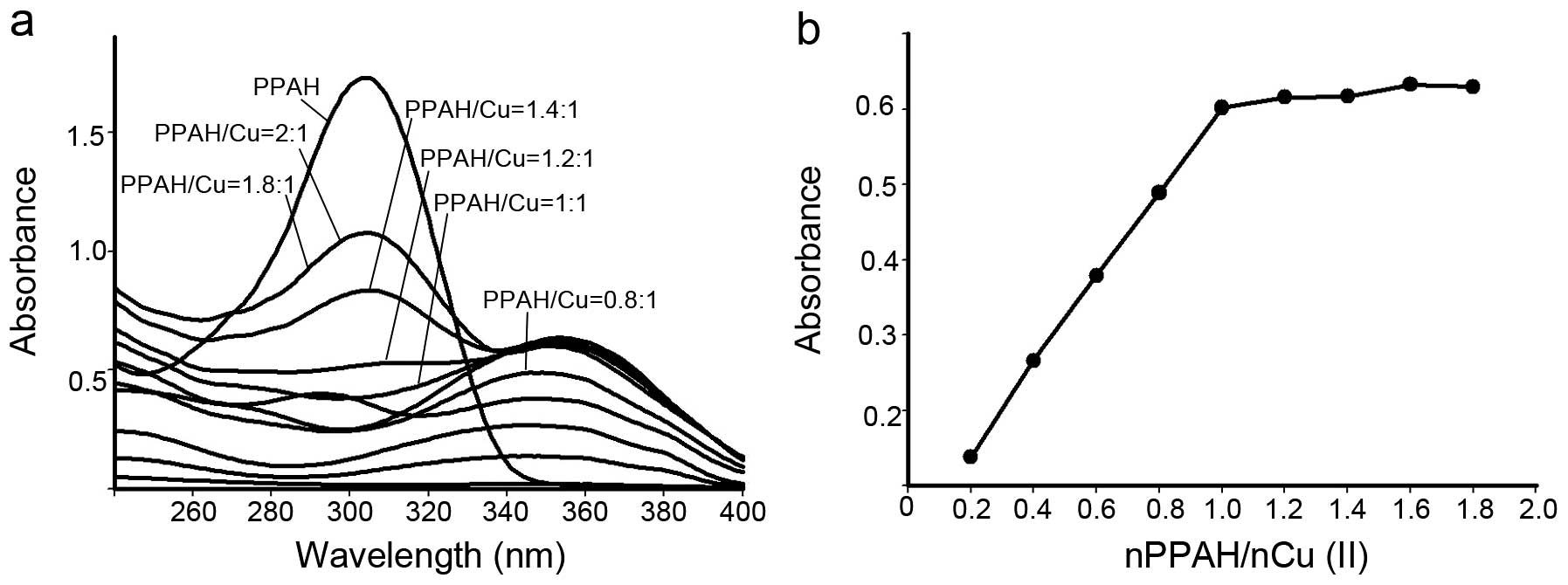
fixed copper concentration were recorded. As shown in Fig. 2, the copper(II) solution had no
absorption in the investigated range of wavelength (240–400 nm).
After addition of PPAH to the copper solution a new absorption at
~350 nm was noted, which was different from the PPAH absorption
(~310 nm), indicating that a copper complex had formed. To further
determine its composition, a series of solutions was prepared in
which the concentration of CuCl2 was held constant while
that of the PPAH was varied. The absorbance of each solution was
measured (Fig. 2a) and plotted vs.
the molar ratio of Cu(II)/PPAH (Fig.
2b). From Fig. 2, a 1:1 molar
ratio of Cu(II)/PPAH was determined. It was reported that the
coordination geometry of 2-pyridinecarboxaldehyde benzoyl hydrazone
with copper complexes has been described as a tridentate chelator
(16). In view of the similarity of
PPAH to the above ligand in chemistry, the structure of the PPAH
copper complex was tentatively proposed (Fig. 1b).
Proliferation assay
To determine the potential antitumor activity of the
agents, the inhibitory effects on the proliferation of the HepG2
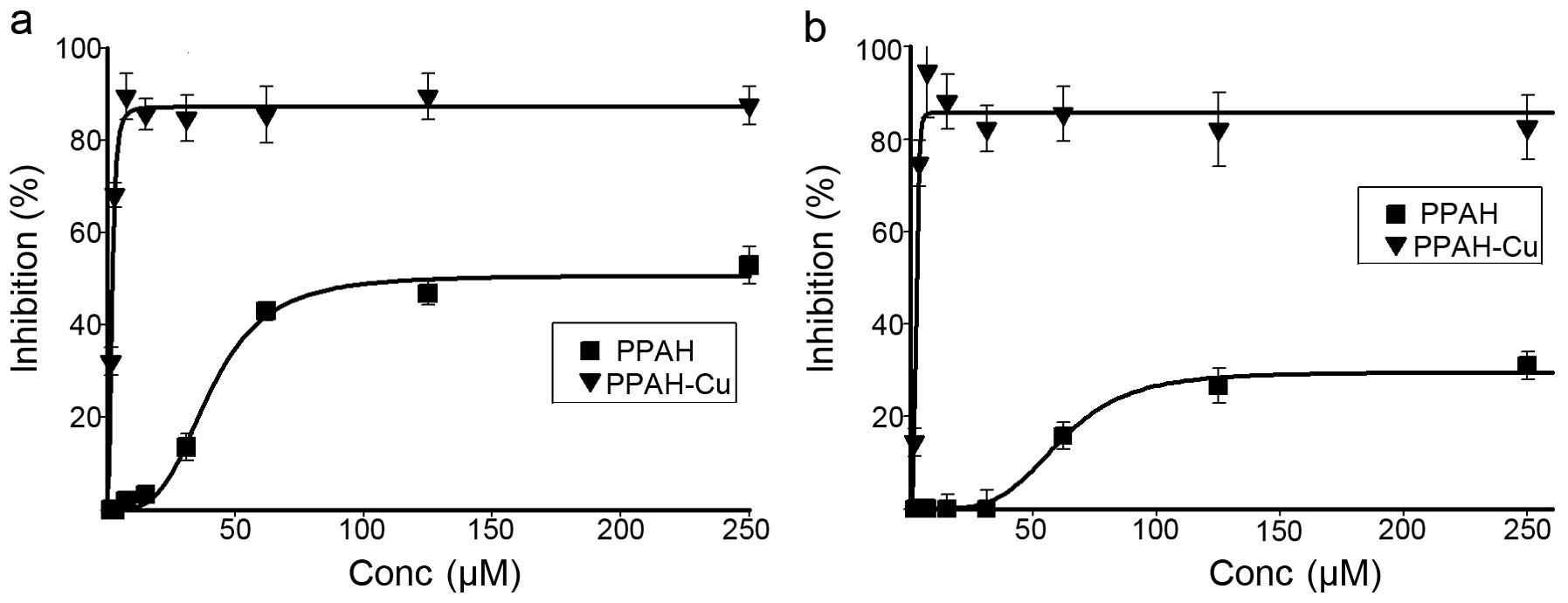
and HCT-116 cell lines was investigated by MTT assay (Fig. 3). For both cell lines, the PPAH
exhibited moderate inhibition of proliferation, i.e. 50% growth
inhibition at ≥60 µM against HCT-116; ~30% growth inhibition
at ≥100 µM against HepG2 (Fig.
3a and b). The copper complex exhibited excellent proliferation
inhibition against the cell lines. The IC50 value of the
copper complex was 2.75±0.30 µM for the HepG2 cells, an
~45-fold increase in proliferation inhibition compared to that of
PPAH. A similar trend against the HCT-116 cell line was also
observed. The IC50 value of the copper complex was
1.90±0.2 µM, an ~30-fold increase, which demonstrated that
the copper complex exhibited excellent antitumor activity.
Effect of PPAH and its copper complex on
the expression of apoptotic genes
ROS play a crucial role in cell growth and
apoptosis. To reveal the mechanism of action of PPAH and its copper
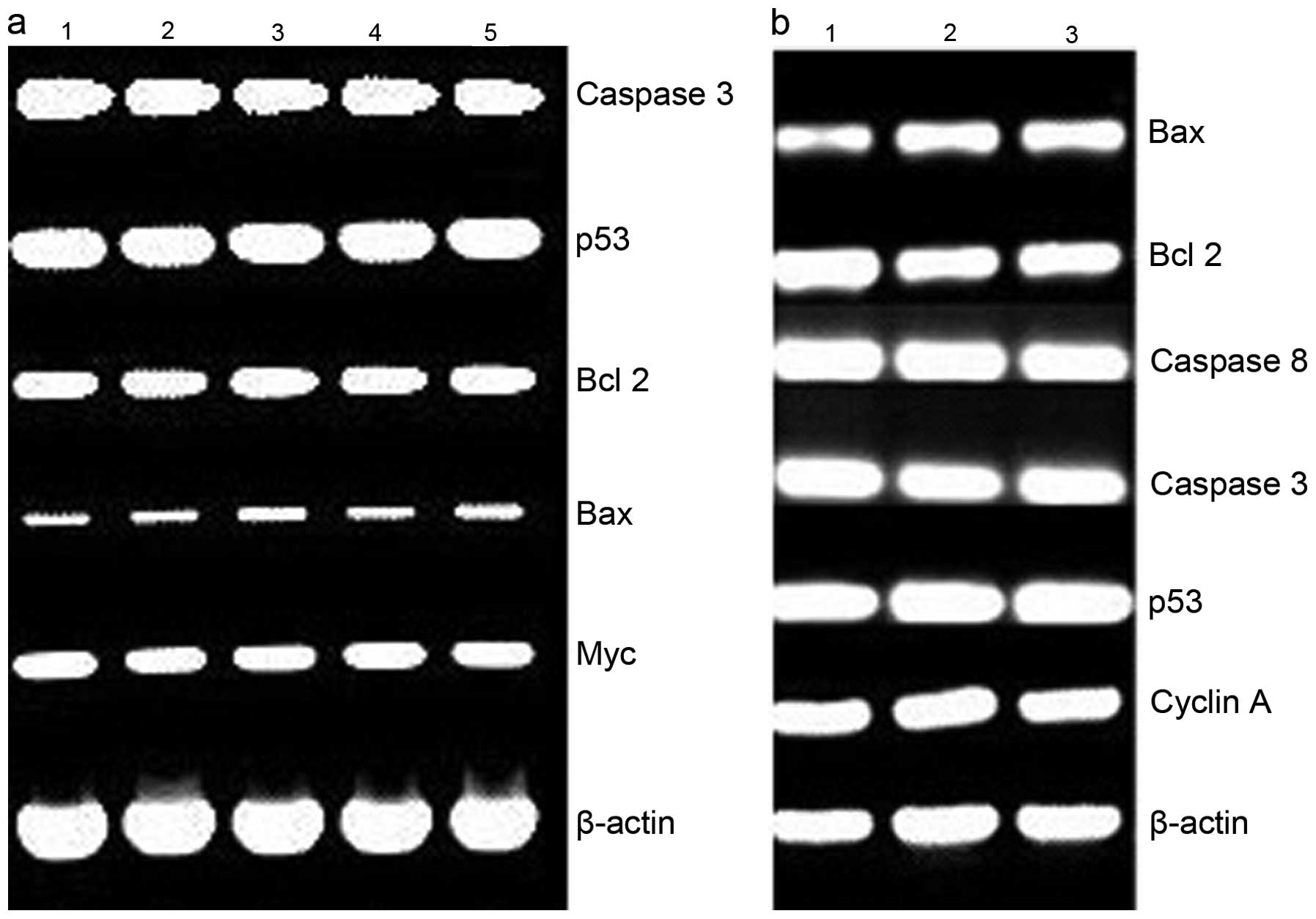
complex, RT-PCR was conducted to determine changes in expression of
apoptotic genes, such as bcl-2, bax, p53, caspase-3 and -8 at the
mRNA level after HepG2 cells were treated with PPAH (Fig. 4a) or its copper complex (Fig. 4b) for 48 h. The investigated genes
were not evidently regulated by the agents. bcl-2 downregulation
and bax upregulation were not observed, which normally occurs in
ROS-related apoptosis. Similar analysis was conducted using the
HCT-116 cell line. Except for PPAH due to its lesser toxicity,
slight changes in the levels of the apoptosis-related genes were
observed in the PPAH-Cu-treated cells. To ascertain whether ROS are
involved in the proliferation inhibition, ROS production was
investigated in vitro. The results indicated that the
PPAH-Cu complex was non-redox active and did not lead to DNA
fragmentation (data not shown). This was also consistent with the
results from the comet assay (Fig.
5). The DNA fragmentation was only observed at a higher
concentration and 48 h exposure to PPAH and its copper complex.
PPAH and its copper complex induce S
phase arrest
As shown above, ROS production was not correlated to
the effect on inhibition of proliferation by PPAH and its copper
complex. Thus, we hypothesized that they may exert their effect via
disturbing the cell cycle. We therefore evaluated the effect of
PPAH and its copper complex on the cell cycle using PI staining and
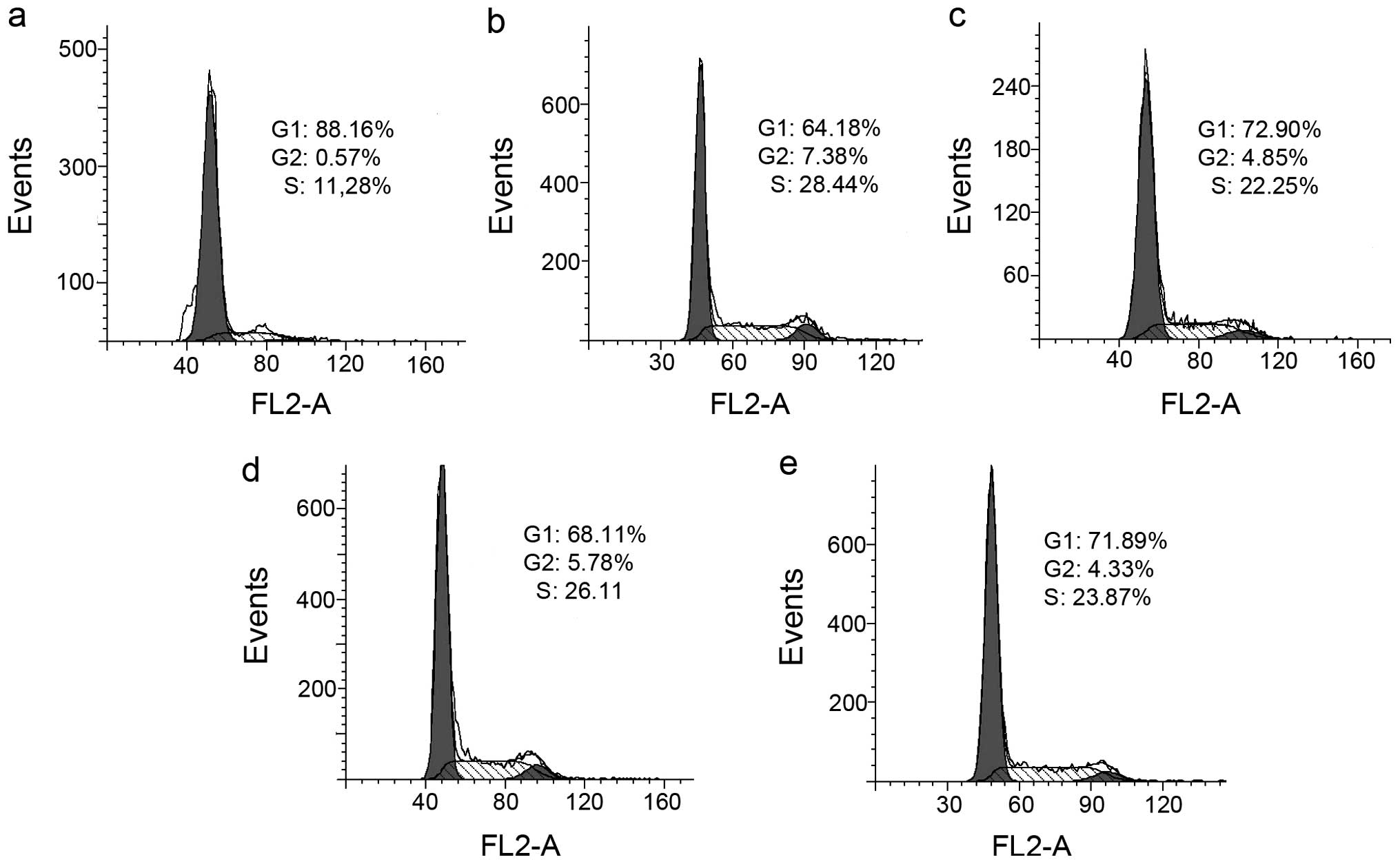
flow cytometry. As shown in Fig. 6,
PPAH caused an accumulation of cells in the S-phase. The percentage
of cells in the S-phase was significantly increased from 11.22 to
28.44 and 22.25% after treatment with 40 and 80 µM PPAH,
respectively. The PPAH-Cu complex had a similar effect when
compared to PPAH; the S-phase population increased from 11.22 to
26.11 (2.5 µM) and 23.87% (2.5 µM), indicating that
the agents have a similar mechanism of action that involves effects
on the cell cycle except with differences at various
concentrations.
DNA relaxation inhibition
Various transition metal complexes have Top
inhibition. To determine whether PPAH and its copper complex
recapitulate such activity, pUC18 plasmid DNA was incubated with
nucleic extract in the presence of varied concentrations of the
investigated agents, and reaction products were analyzed by gel
electrophoresis. As shown in Fig.
7, PPAH exhibited very weaker inhibition even at a higher
concentration (Top I, >100 µM; Top II >75 µM).
The PPAH-Cu complex exhibited dual Top inhibition. For type I, a
IC50 value of ~50 µM was noted; while type II
inhibition was initialized at 12.5 µM (Fig. 7b and c). To further reveal the
action mode of the copper complex in Top inhibition, the reaction
mixtures were subjected to electrophoresis on EtB-containing
agarose gel. As shown from the gel (Fig. 7d), the topology was dominated by the
intercalative dye, all closed circular forms of DNA were positively
supercoiled and migrated with a similar rate. The Top II cleavage
complex was identified by comparison with the positive control of
etoposide. However, in our experiment, the Top II cleavage complex
was not observed. This situation may have occurred due to a lower
Top II concentration used in the assay. The mode of Top inhibition
of the PPAH-Cu complex was not clearly identified, but the cleaved
DNA (Fig. 7d) increased with
increasing concentrations of the copper complex implying that there
was the possibility of catalytic or 'poisoner' inhibition.
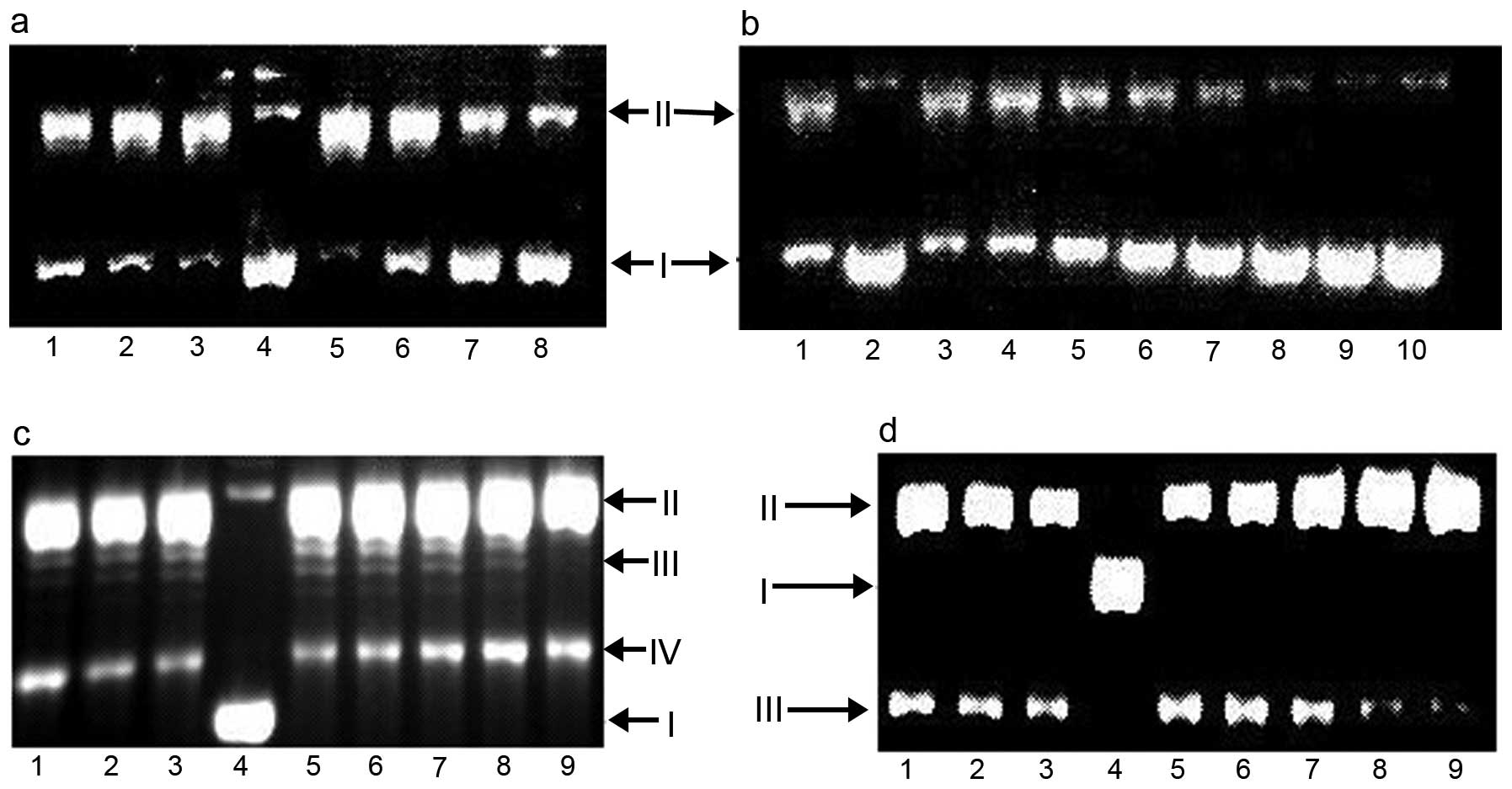 | Figure 7Top inhibition of PPAH and its copper
complex. I, supercoiled DNA; II nicked DNA; III, relaxed DNA. (a)
Lane 1, 150 µM PPAH; lane 2, 100 µM PPAH; lane 3, 50
µM PPAH; lane 4, pUC18; lane 5, pUC18 + NE; lane 6, 30
µM PPAH-Cu; lane 7, 60 µM PPAH-Cu; lane 8, 90
µM PPAH-Cu. (b) Lane 1, no PPAH; lane 2, pUC18; lane 3, 6
µM PPAH-Cu; lane 4, 12.5 µM PPAH-Cu; lane 5, 25
µM PPAH-Cu; lane 6, 50 µM PPAH-Cu; lane 7, 75
µM PPAH-Cu; lane 8, 100 µM PPAH-Cu; lane 9, 125
µM PPAH-Cu; lane 10, 150 µM PPAH-Cu. (c) Lane 1, 75
µM PPAH; lane 2, 50 µM PPAH; lane 3, 25 µM
PPAH; lane 4, pUC18 only; lane 5, pUC18 and without investigated
agent; lane 6, 12.5 µM PPAH-Cu; lane 7, 25 µM
PPAH-Cu; lane 8, 37.5 µM PPAH-Cu; lane 9, etoposide control
(3 mM). (d) Lane 1, 75 µM PPAH; lane 2, 50 µM PPAH;
lane 3, 25 µM PPAH; lane 4, pUC18 only; lane 5, pUC18 and
without investigated agent; lane 6, 12.5 µM PPAH-Cu; lane 7,
25 µM PPAH-Cu; lane 8, 37.5 µM PPAH-Cu; lane 9,
etoposide (3 mM) control, electrophoresis in the presence of EB.
PPAH, 2-pyridinecarboxaldehyde 2-pyridinecarboxylic acid hydrazone;
EB, ethidium bromide. |
Discussion
Pyridoxal isonicotinoyl hydrazone and Dp44mT have
been extensively investigated for the treatment of different types
of cancers and they are also considered as iron chelators as they
disturb the homeostasis of this metal in cells. In terms of the
mechanism, they are involved in iron deprivation and ROS
enhancement (17,18). Although PPAH can enhance ROS
generation and lead to DNA fragmentation in Fenton reaction, its
cytotoxicity was found to be weak. In contrast, the PPAH-Cu complex
displayed excellent antitumor activity. This seemingly
contradictory phenomenon was reported for derivatives of 4-pyridine
carboxylic acid hydrazide; Fe chelation efficacy is not always well
correlated to the ability of a ligand to inhibit proliferation
(19), indicating that ROS
contributed less to their cytotoxicity. Comet assay has been widely
used to evaluate ROS-induced DNA fragmentation. In the present
study, cellular DNA fragmentation was only observed following a
48-h exposure of the agents, demonstrating that ROS were less
involved in the proliferation inhibition. To seek further evidence,
RT-PCR was conducted to clarify whether the ROS were involved in
the cell proliferation inhibition. As expected, the gene expression
of bcl-2, bax, p53 and caspase at the mRNA level was not obviously
changed following exposure of the agents for 24 or 48 h. It has
been demonstrated that Bax and Bcl-2 are apoptosis-related genes
and Bax upregulation and Bcl-2 downregulation are indicative of
apoptosis (20,21). There were no changes in the
investigated genes at the mRNA level which further suggested that
proliferation inhibition by PPAH and its copper complex did not
involve ROS generation.
Copper exhibits numerous biological processes as a
co-factor of enzymes and angiopoiesis. A strategy using copper
depletion therapy has been proposed to prevent the spread of
cancers (22). Copper complexes
have significant anticancer activity (23,24),
yet the underlying mechanism is largely unknown. It has been
reported that copper complexes induce cell death by causing
double-strand DNA breakage and inhibition of Top function (25,26),
thus the PPAH copper complex in the present study may share a
similar mechanism of action. To test the hypothesis, DNA relaxation
assay was carried out. It was found that the PPAH-Cu complex
exhibited dual Top inhibition; type II Top inhibition was initiated
at ~10 µM, while type I Top inhibition appeared to require a
slightly higher dose (~40 µM). In contrast, PPAH had no
obvious inhibitory effect, thus it could be speculated that the
excellent antitumor activity of the PPAH-Cu complex may be closely
correlated to its dual Top inhibition. Top inhibition by metal
complexes has been reported in many studies. Metal complexes
inhibited either Top I or Top II, such as
9-methyl-[1,10]phenanthroline-2-carboxylic acid cobalt complex
(27), oxindolimine copper(II)
complexes (28), organoplatinum(II)
complexes (29) and
Ru(II)-polypyridyl complexes (30).
Yet, metal complexes acting as dual Top inhibitors are few. The
PPAH-copper complex in the present study and ruthenium(II)
anthraquinone complexes are the only two examples (31). It should be noted that the ligands
were all fused aromatic compounds in the above mentioned metal
complex, while in the present study PPAH was a simple pyridine
derivative. There are many modes of interaction between
Topoisomerases and their inhibitors, such as competitive ATP
binding, poisoning DNA-Top covalent complex and allosteric effect
on disturbing unwinding DNA helix. In the present study, the
PPAH-Cu complex and etoposide appeared to possess similar action
causing DNA cleavage; thus, the 'poisoner' of the Top II cleaved
complex for the PPAH-Cu complex may be possible. Top inhibition
normally leads to cell cycle arrest at the G2/M phase, yet S phase
arrest was recently reported (32).
The PPAH-Cu complex induced S-phase arrest at much lower
concentrations that may be indicative of retarded DNA topology
during DNA replication following exposure of the investigated
agents. The PPAH-Cu complex was prepared in water and had excellent
antitumor activity, yet PPAH-Fe2+ did not possess
activity (data not shown), indicating that the geometry of the
ligand induced by metal ions plays an important role in the
inhibitory effect on cell proliferation.
In conclusion, the PPAH-Cu complex had significant
proliferation inhibition compared to PPAH. The RT-PCR and comet
assay indicated that ROS were less involved in the antitumor
activity, yet the DNA relaxation assay provided new insight,
revealing that the PPAH-Cu complex displayed dual Top inhibition in
contrast to PPAH. Thus, the proliferation inhibition of the PPAH-Cu
complex may mainly stem from its dual Top inhibition. Notably, Top
inhibitors normally are coplanar aromatic compounds even in various
metal complexes, and lack of fused aromatic cyclic compound as Top
inhibitor is rare. Thus, the PPAH copper complex in the present
study may be a novel dual Top inhibitor. However the detailed
mechanism, particularly Top inhibition in vivo, and the
interaction model between the copper complex and topoisomerase
require further investigation.
Acknowledgments
This study was supported by grants (132102310250)
from the Science and Technology Department of Henan Province and
(2109901) from the Plan of Health Scientific and Technological
Innovation Talents of Henan Province for S.L.
References
|
1
|
Chen Y and Hu L: Design of anticancer
prodrugs for reductive activation. Med Res Rev. 29:29–64. 2009.
View Article : Google Scholar
|
|
2
|
Chen Z, Zhang D, Yue F, Zheng M, Kovacevic
Z and Richardson DR: The iron chelators Dp44mT and DFO inhibit
TGF-β-induced epithelial-mesenchymal transition via up-regulation
of N-Myc downstream-regulated gene 1 (NDRG1). J Biol Chem.
287:17016–17028. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gupte A and Mumper RJ: Elevated copper and
oxidative stress in cancer cells as a target for cancer treatment.
Cancer Treat Rev. 35:32–46. 2009. View Article : Google Scholar
|
|
4
|
Pahl PM and Horwitz LD: Cell permeable
iron chelators as potential cancer chemotherapeutic agents. Cancer
Invest. 23:683–691. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kalinowski DS, Sharpe PC, Bernhardt PV and
Richardson DR: Structure-activity relationships of novel iron
chelators for the treatment of iron overload disease: The methyl
pyrazinylketone isonicotinoyl hydrazone series. J Med Chem.
51:331–344. 2008. View Article : Google Scholar
|
|
6
|
Chaston TB, Watts RN, Yuan J and
Richardson DR: Potent antitumor activity of novel iron chelators
derived from di-2-pyridylketone isonicotinoyl hydrazone involves
Fenton-derived free radical generation. Clin Cancer Res.
10:7365–7374. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Filipović N, Borrmann H, Todorović T,
Borna M, Spasojević V, Sladić D, Novaković I and Anđelković K:
Copper(II) complexes of N-heteroaromatic hydrazones: Synthesis,
X-ray structure, magnetic behavior, and antibacterial activity.
Inorg Chim Acta. 362:1996–2000. 2009. View Article : Google Scholar
|
|
8
|
Hoyes KP, Hider RC and Porter JB: Cell
cycle synchronization and growth inhibition by
3-hydroxypyridin-4-one iron chelators in leukemia cell lines.
Cancer Res. 52:4591–4599. 1992.PubMed/NCBI
|
|
9
|
Chaston TB and Richardson DR: Interactions
of the pyridine-2-carboxaldehyde isonicotinoyl hydrazone class of
chelators with iron and DNA: Implications for toxicity in the
treatment of iron overload disease. J Biol Inorg Chem. 8:427–438.
2003.PubMed/NCBI
|
|
10
|
Chaston TB, Lovejoy DB, Watts RN and
Richardson DR: Examination of the antiproliferative activity of
iron chelators: Multiple cellular targets and the different
mechanism of action of triapine compared with desferrioxamine and
the potent pyridoxal isonicotinoyl hydrazone analogue 311. Clin
Cancer Res. 9:402–414. 2003.PubMed/NCBI
|
|
11
|
Turner J, Koumenis C, Kute TE, Planalp RP,
Brechbiel MW, Beardsley D, Cody B, Brown KD, Torti FM and Torti SV:
Tachpyridine, a metal chelator, induces G2 cell-cycle
arrest, activates checkpoint kinases, and sensitizes cells to
ionizing radiation. Blood. 106:3191–3199. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fu Y, Zhou S, Liu Y, Yang Y, Sun X and Li
C: The cytotoxicity of benzaldehyde nitrogen mustard-2-pyridine
carboxylic acid hydrazone being involved in topoisomerase IIα
inhibition. BioMed Res Int. 2014:5270422014. View Article : Google Scholar
|
|
13
|
Zheng X, Zhao Y and Zhu BX: Coordination
of the new Schiff base with copper(II) or Iron(III) in solution.
Wuji Huaxue Xuebao. 27:1523–1528. 2011.In Chinese.
|
|
14
|
Singh NP, McCoy MT, Tice RR and Schneider
EL: A simple technique for quantitation of low levels of DNA damage
in individual cells. Exp Cell Res. 175:184–191. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fu Y, Zhang Y, Zhou SF, Liu Y, Wang J,
Wang Y, Lu C and Li C: The effects of substitution of carboxyl with
hydrazide group on position 3 of ciprofloxacin on its antimicrobial
and antitumor activity. Int J Pharm. 9:416–429. 2013. View Article : Google Scholar
|
|
16
|
Banerjee S, Sen S, Basak S, Mitra S,
Hughes DL and Desplanches C: Two new pseudohalide-bridged Cu(II)
complexes with a hydrazone ligand: Syntheses, crystal structures
and magnetic studies. Inorg Chim Acta. 361:2707–2714. 2008.
View Article : Google Scholar
|
|
17
|
Buss JL, Neuzil J and Ponka P: Oxidative
stress mediates toxicity of pyridoxal isonicotinoyl hydrazone
analogs. Arch Biochem Biophys. 421:1–9. 2004. View Article : Google Scholar
|
|
18
|
Jansson PJ, Hawkins CL, Lovejoy DB and
Richardson DR: The iron complex of Dp44mT is redox-active and
induces hydroxyl radical formation: An EPR study. J Inorg Biochem.
104:1224–1228. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Becker E and Richardson DR: Development of
novel aroylhydrazone ligands for iron chelation therapy:
2-pyridylcarboxaldehyde isonicotinoyl hydrazone analogs. J Lab Clin
Med. 134:510–521. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gillardon F, Wickert H and Zimmermann M:
Up-regulation of bax and down-regulation of bcl-2 is associated
with kainate-induced apoptosis in mouse brain. Neurosci Lett.
192:85–88. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Niu G, Yin S, Xie S, Li Y, Nie D, Ma L,
Wang X and Wu Y: Quercetin induces apoptosis by activating
caspase-3 and regulating Bcl-2 and cyclooxygenase-2 pathways in
human HL-60 cells. Acta Biochim Biophys Sin. 43:30–37. 2011.
View Article : Google Scholar
|
|
22
|
Turski ML and Thiele DJ: New roles for
copper metabolism in cell proliferation, signaling, and disease. J
Biol Chem. 284:717–721. 2009. View Article : Google Scholar :
|
|
23
|
Jain S, Cohen J, Ward MM, Kornhauser N,
Chuang E, Cigler T, Moore A, Donovan D, Lam C, Cobham MV, et al:
Tetrathiomolybdate-associated copper depletion decreases
circulating endothelial progenitor cells in women with breast
cancer at high risk of relapse. Ann Oncol. 24:1491–1498. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tardito S and Marchiò L: Copper compounds
in anticancer strategies. Curr Med Chem. 16:1325–1348. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Skrott Z and Cvek B:
Diethyldithiocarbamate complex with copper: The mechanism of action
in cancer cells. Mini Rev Med Chem. 12:1184–1192. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou BB and Elledge SJ: The DNA damage
response: Putting checkpoints in perspective. Nature. 408:433–439.
2000. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ahmad M, Afzal M, Tabassum S, Kalińska B,
Mrozinski J and Bharadwaj PK: Synthesis and structure elucidation
of a cobalt(II) complex as topoisomerase I inhibitor: In vitro DNA
binding, nuclease and RBC hemolysis. Eur J Med Chem. 74:683–693.
2014. View Article : Google Scholar
|
|
28
|
Katkar P, Coletta A, Castelli S, Sabino
GL, Couto RA, Ferreira AM and Desideri A: Effect of oxindolimine
copper(II) and zinc(II) complexes on human topoisomerase I
activity. Metallomics. 6:117–125. 2014. View Article : Google Scholar
|
|
29
|
Wang P, Leung CH, Ma DL, Lu W and Che CM:
Organoplatinum(II) complexes with nucleobase motifs as inhibitors
of human topoisomerase II catalytic activity. Chem Asian J.
5:2271–2280. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gao F, Chao H and Ji LN: DNA binding,
photocleavage, and topoisomerase inhibition of functionalized
ruthenium(II)-polypyridine complexes. Chem Biodivers. 5:1962–1979.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kou JF, Qian C, Wang JQ, Chen X, Wang LL,
Chao H and Ji LN: Chiral ruthenium(II) anthraquinone complexes as
dual inhibitors of topoisomerases I and II. J Biol Inorg Chem.
17:81–96. 2012. View Article : Google Scholar
|
|
32
|
Lin RW, Yang CN, Ku S, Ho CJ, Huang SB,
Yang MC, Chang HW, Lin CM, Hwang J, Chen YL, et al: CFS-1686 causes
cell cycle arrest at intra-S phase by interference of interaction
of topoisomerase 1 with DNA. PLoS One. 9:e1138322014. View Article : Google Scholar : PubMed/NCBI
|