Introduction
Colorectal neoplasia is one of the most common
malignancies in the Western world. Despite evidence that the 5-year
survival from colorectal cancer is 90% when diagnosed at an early
stage, <40% of cases are diagnosed when the cancer is localized
(1,2). Although the specific underlying
mechanisms of action in cancer progression are just beginning to be
unraveled, it is believed that colorectal cancer results from a
combination of genetic and environmental factors, including the
expression of several inherited susceptibility genes for colorectal
cancer (3), ethnicity and family
history (4), and different dietary
(5), and lifestyle factors
(6). The reported molecular and
biochemical mechanisms that may contribute to the phenotypic
changes that favor carcinogenesis, and to the development and
progression of the cancer include inhibited apoptosis, enhanced
tumor cell proliferation (7),
increased invasiveness, perturbed cell adhesion (8), promotion of angiogenesis (9) and inhibited immune surveillance
(10).
Krüppel-like factors (KLFs) belong to a class of
zinc finger-containing transcription factors that share homology
with the general transcription factor Sp1 (11). The members of this family, which
contains over 26 identified members, are characterized by a highly
conserved C-terminus containing three zinc finger DNA-binding
domains (12). Emerging evidence
has suggested that KLFs may be critical factors for tumor
development, growth and metastasis, and several KLF members,
including KLF4 (13), KLF5
(14) and KLF11 (15), have been reported to be associated
with the oncogenesis of various types of human cancer. KLF8 binds
to GC boxes at a number of gene promoters and regulates their
transcription. Previous findings have demonstrated that KLF8 is
highly expressed in several types of human cancer, including breast
(16) and renal carcinomas
(17), and that this protein plays
an important role in oncogenic transformation. However, the role of
KLF8 in the proliferation, differentiation and apoptosis of
colorectal cells remains elusive.
In the present study, we first showed that KLF8 was
highly expressed in colorectal cancer tissues and cell lines. We
then demonstrated that KLF8 repression significantly induced
differentiation and apoptosis, inhibited tumorigenesis and tumor
growth, and strongly enhanced the antitumor activity of
5-fluorouracil (FU). In vivo, lentivirus-mediated shRNA
knockdown of KLF8 suppressed the growth of tumor xenografts in nude
mice. Therefore, KLF8 knockdown is considered a potential
therapeutic target for treating colorectal cancer.
Materials and methods
Chemicals, tissue specimens and cell
lines
Sodium butyrate (NaB), 5-fluorouracil (5-FU) and
U-0126 (a selective MAP kinase inhibitor) were purchased from Sigma
(St. Louis, MO, USA), and Rhodamine-phallotoxin was purchased from
Molecular Probes (Eugene, OR, USA). Goat anti-human KLF8 and GAPDH
antibodies were purchased from Aviva Systems Biology (San Diego,
CA, USA). For western blot analyses, 14 pairs of colorectal cancer
and adjacent normal tissues (>5 cm from the margin of the tumor)
were obtained from patients by surgical resection in the Nanfang
Hospital (Guangzhou, China). Informed consent was obtained from all
individual participants included in the present study. The Medical
Ethics Committee of Nanfang Hospital approved the use of tissue
specimens in the present study (permit no. NFYY-2013-27). The
tissue specimens were snap-frozen in liquid N2 and stored at −70°C
until use. The tissues were embedded in paraffin, sliced and then
subjected to histopathologic review using immunohistochemistry
(8). Tissues in which >10% of
cancer cells were positively stained were considered positive.
The HCT116, HT29, LoVo, SW620 and SW480 colorectal
cancer cell lines and the human embryonic kidney 293 (HEK293) cell
line were obtained from ATCC (Rockville, MD, USA) and cultured in
RPMI-1640 medium as previously described (7).
RNA isolation and reverse
transcription-PCR analysis
Total RNA was isolated using TRI reagent (Sigma)
(7) and then reversed transcribed
using SuperScript II (Invitrogen-Life Technologies, Carlsbad, CA,
USA) according to the manufacturer's instructions. The primer
sequences used were: carcinoembryonic antigen (CEA) forward,
5′-AACCCTTCATCACCAGCAAC-3′ and reverse, 5′-CAGGAGAGGCTGAGGTTCAC-3′;
E-cadherin forward, 5′-TGCCCAGAAAATGAAAAAGG-3′ and E-reverse,
5′-GTGTATGTGGCAATGCGTTC-3′; and GAPDH forward,
5′-GTCAACGGATTTGGTCGTATTG-3′ and reverse,
5′-CTCCTGGAAGATGGTGATGGG-3′. The expected sizes of the PCR products
were 340, 200 and 204 bp for CEA, E-cadherin and GAPDH,
respectively.
Western blot analysis
Total protein lysates were prepared and submitted to
western blotting. The blots were probed with primary antibody
followed by horseradish peroxidase-conjugated secondary antibody.
Antigen-antibody complexes were visualized using the enhanced
chemiluminescence system (Amersham Biosciences, Little Chalfont,
UK).
Assay of anchorage-independent and
-dependent cell growth
Scrambled siRNA (src siRNA) and
KLF8-siRNA-transfected cells were plated in triplicate in plates
containing 0.35% agar on top of a 0.7% agar base (7). Colonies were scored using Coomassie
blue staining, and only colonies containing ≥50 cells were
considered viable. The cell proliferation reagent WST-1, a
ready-to-use colorimetric assay (Roche Diagnostics), was also
used.
Apoptosis assay
Apoptosis was detected in cells using the Annexin
V-FITC kit according to the manufacturer's instructions (18) (Trevigen, Inc., Gaithersburg, MD,
USA) followed by flow cytometry using WinMDI 2.9. Apoptosis was
also analyzed by staining cell nuclei with Hoechst 33258 and
examining the cells under a fluorescence microscope. The activities
of caspases 3, 8 and 9 were determined using the ApoAlert caspase
colorimetric assay kit according to the manufacturer's instructions
(18) (Clontech, Mountain View, CA,
USA).
Immunofluorescence
For F-actin staining (7), the cells were fixed on coverslips and
incubated with Rhodamine-conjugated phallotoxin (5 U/ml; Molecular
Probes) in phosphate-buffered saline (PBS) at room temperature. In
addition, the nuclei were stained with 1 µg/ml Hoechst
33258. The coverslips were then washed, mounted and visualized
using a Zeiss Axioskop fluorescence microscope.
siRNA transfection in vitro
The siRNA duplexes consisted of 21 base pairs with a
2-base deoxynucleotide overhang (Proligo, Singapore). The sequence
of the KLF8-siRNA: (NM_007250 sense strand, CGAUAUGGAUAAACUCAUATT)
was identical to that employed in a previous study (19), and src siRNA,
(5′-TTCTCCGAACGTGTCACGT-3′), which did not target any genes, was
used as the negative control. The cells were transfected with siRNA
duplexes using oligofectamine (Invitrogen-Life Technologies)
according to the manufacturer's instructions.
Construction and transfection of
lentiviral vectors containing KLF8 short hairpin RNA
To investigate the effects of siRNA-induced
downregulation of KLF8 expression on in vivo tumor growth, a
KLF8-RNAi lentiviral vector (pGCSIL-KLF8shRNA) was constructed
(Shanghai GeneChem Co., Ltd., Shanghai, China) (20). A GFP-lentiviral vector (pGCSIL-GFP)
was used as a negative control. Double-stranded oligonucleotides
encoding human KLF8-vshRNA
(CCGGCTAGCATGCTACAAGCTCCA-ATTCAAGAGATTGGAGCTTGTAGCATGCTAGTTTTTG)
were inserted into the short hairpin RNA (shRNA) expression vector
pGCSIL (Shanghai GeneChem Co., Ltd.), and the identities of the
clone were verified by sequencing.
A recombinant lentiviral vector was produced by
co-transfecting HEK293T cells with the lentiviral expression vector
and the packaging plasmid mix using Lipofectamine™ 2000 according
to the manufacturer's instructions (19). Infectious lentiviral particles were
harvested at 48 h post-transfection and then filtered through
0.45-µm cellulose acetate filters. The virus was
concentrated, and the titer was determined by serial dilution on
293T cells.
For the lentiviral transduction, LoVo cells were
subcultured at 1×105 cells/well in 6-well culture plates
and then transduced with KLF8-siRNA-expressing (KLF8-siRNA) or src
siRNA-expressing lentivirus at a multiplicity of infection (MOI) of
50. The cells were collected 72 h after infection, and the
transduction efficiency was evaluated by counting the percentage of
GFP-positive cells.
Xenograft tumor model
The present study was carried out strictly in
accordance with the recommendations in the Guide for the IACUC
(Institutional Animal Care and Use Committee), and the protocol was
approved by the Committee on the Ethics of Animal Experiments of
Nanfang Hospital (permit no. NFYY-2013-36). Surgeries were
performed under sodium pentobarbital anesthesia, and all efforts
were made to minimize suffering. After the surgery, the nude mice
were euthanized by sodium pentobarbital anesthesia.
The antitumor effects due to transfection of the
KLF8-siRNA were evaluated in vivo using nude mice xenograft
models (21). Five- to six-week-old
female BALB/c nude mice were bred under pathogen-free conditions at
the Southern Medical University (Guangzhou, China), and all the
animal studies were approved by the Southern Medical University
Animal Care and Use Committee. Lenti-src-shRNA- and
lenti-KLF8-shRNA-infected LoVo cells were harvested during the
exponential growth phase, washed twice in PBS and resuspended in
PBS at a density of 5×107 cells/ml. The cell suspension
(0.1 ml; 5×106 cells) was then subcutaneously injected
into the right flank of each nude mouse. LoVo cells infected with
lenti-src-shRNA were injected into groups a and c, respectively.
Lenti-KLF8-shRNA-infected LoVo cells were injected into group b and
d. Fourteen days after the subcutaneous LoVo cell injection, 5-FU
was injected into mouse groups c and d through intraperitoneal
injection using a 10-ml micro-syringe (Hamilton, Reno, NV, USA),
with the dose of 50 µg/kg/time, once/two days, 12 times.
Five mice were included in each group (for each transduced cell
line), and the tumor volumes were calculated as: V = (4/3)
R12R2, where R1 is radius 1, R2 is radius 2 and
R1<R2. The mice were then sacrificed, and the tumors were
dissected, snap-frozen in liquid N2 and stored at −70°C on day 42
after inoculation for western blotting.
Statistical analysis
Results obtained from in vitro and in
vivo experiments were presented as means ± SD and statistically
evaluated using the standard two-tailed Student's t-test or one-way
ANOVA. If equal variances were not assumed, the Satterthwaite's
t-test or Dunnett's T3 test were used to analyze the quantified
data. The results were considered significant when P<0.05.
Results
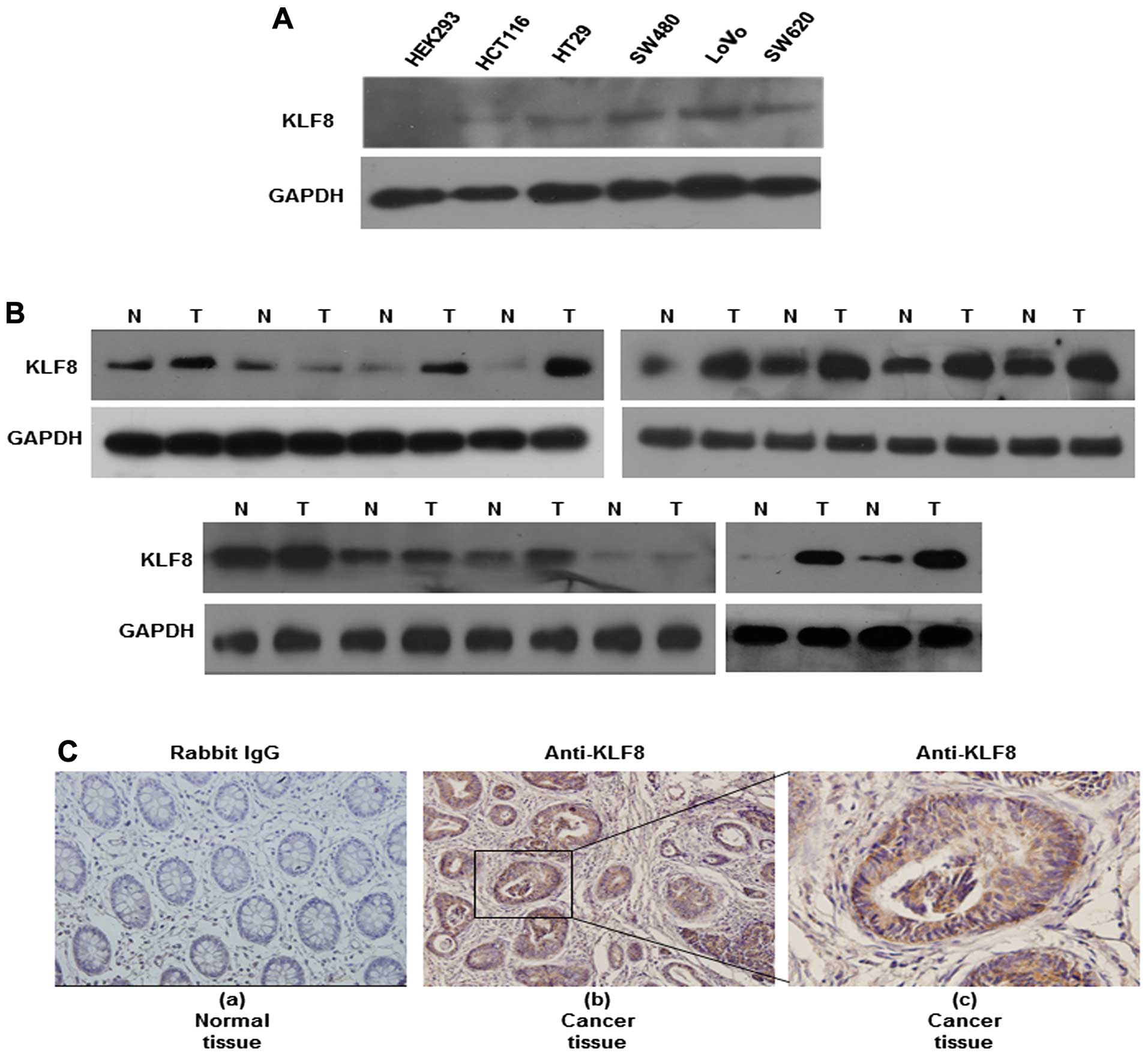
Colon cancer tissues express higher
levels of KLF8 protein than normal tissues
We first showed that KLF8 protein expression was
significantly increased in the HCT116, HT29, LoVo, SW620 and SW480
cancer cell lines, compared with that in the human HEK293
epithelial cell line. We then measured KLF8 expression in matched
normal (N) and cancerous (T) colon tissues by western blotting. Of
the 14 cancerous tissues, 10 expressed higher levels of KLF8 than
the normal tissues (Fig. 1B). KLF8
expression in tissue specimens collected from non-cancerous or
cancerous colons was also measured in situ using
immunohistochemistry. We found nuclear-specific KLF8 protein
expression in the carcinoma cells of all the colorectal cancer
samples. However, KLF8 was not expressed in the control tissue, as
exemplified in Fig. 1C (b and c).
Fig. 1C shows representative images
of KLF8 expression in non-cancerous and cancerous specimens. These
findings demonstrated that KLF8 was overexpressed in colorectal
cancer.
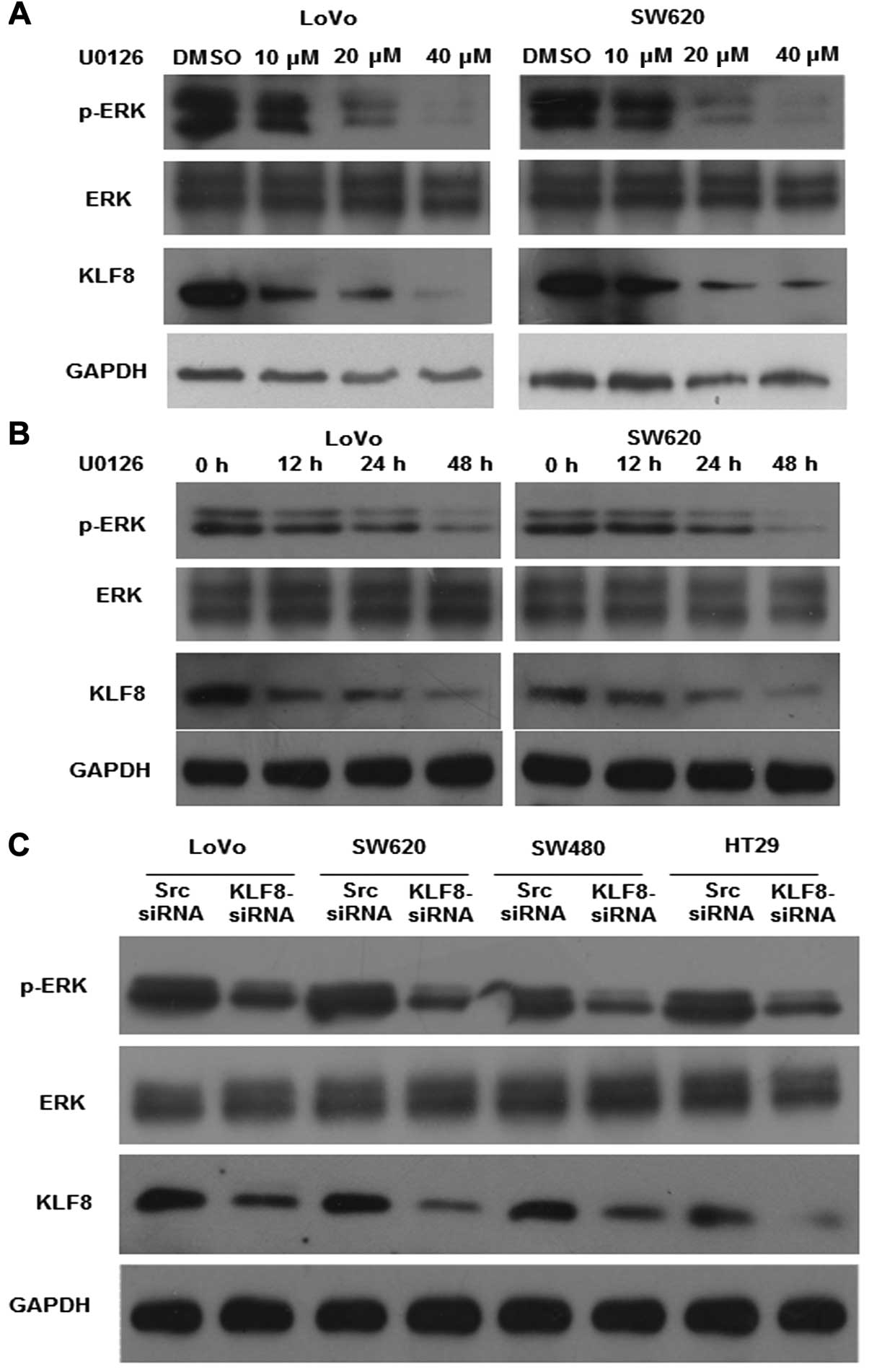
Inhibition of the constitutive activation
of ERK1/2 downregulates KLF8 expression
Previous studies have indicated that activation of
the MAPK/ERK pathway is involved in the regulation of cell
proliferation in colorectal cancer (2,21).
Therefore, we analyzed the effect of ERK inhibition on KLF8
expression after incubating LoVo and SW620 cells with U0126, a
specific MEK/ERK activation inhibitor, for 48 h. The results showed
that the expression of pERK1/2 and KLF8 was significantly
downregulated in a dose- and time-dependent manner after U0126
treatment (Fig. 2A and B).
By contrast, KLF8 knockdown affected the activation
of ERK. After confirming the efficiency of siRNA knockdown by
western blotting (Fig. 2C), the
expression of pERK1/2 was found to be significantly reduced after
KLF8 knockdown. However, no change in the expression of ERK1/2 was
observed. These findings suggested that KLF8 inhibition is
important in ERK activation in colorectal cancer.
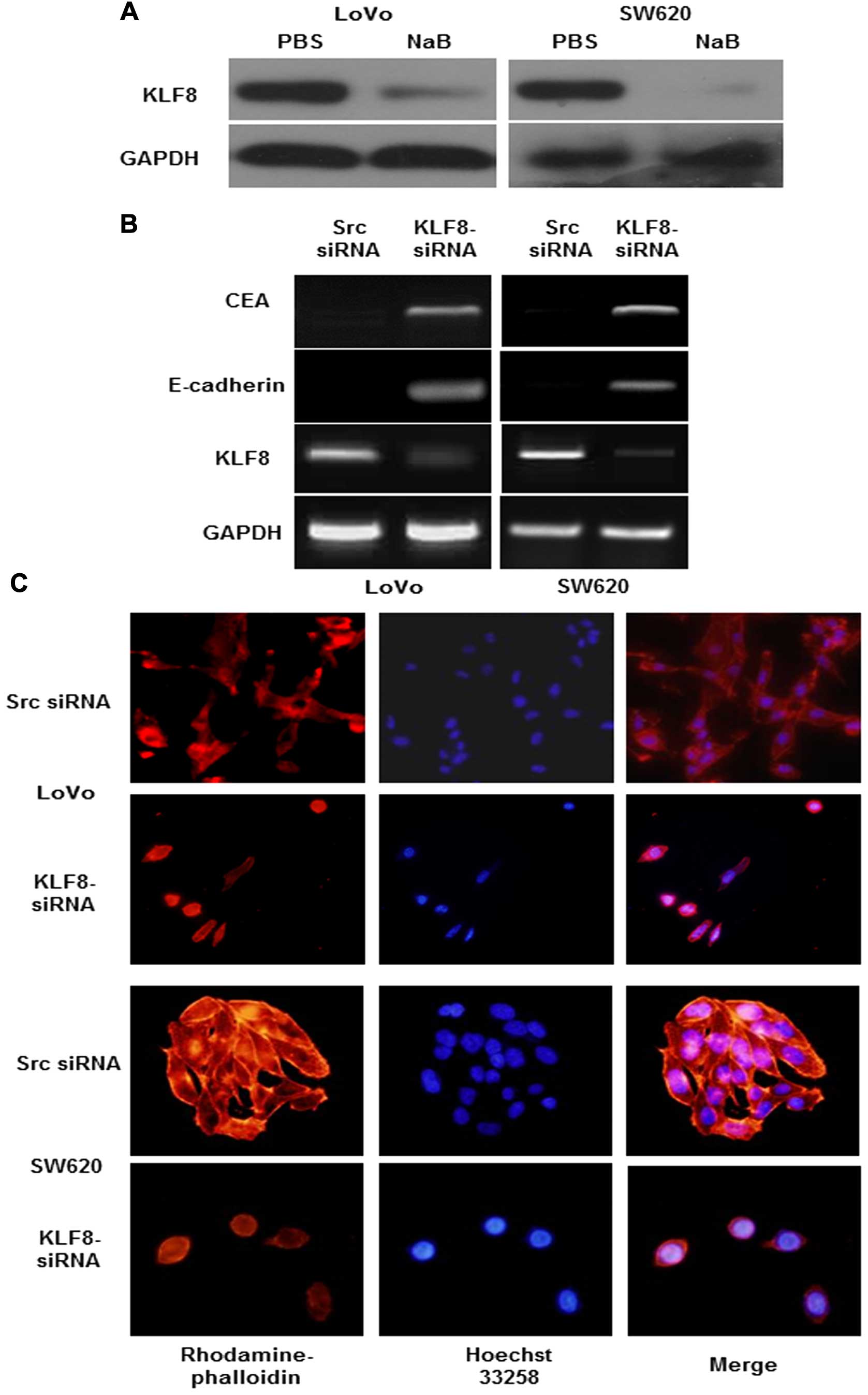
Suppression of KLF8-induced cell
differentiation in cancer cells
To examine the effect of KLF8 on cell
differentiation, we first investigated the effects of
pro-differentiation agents on KLF8 expression (22). LoVo and SW620 cells were treated
with NaB, which significantly reduced the expression of KLF8
(Fig. 3A). Using RT-PCR we showed
that, KLF8 knockdown markedly increased the expression of the
differentiation markers CEA and E-cadherin (7,23)
(Fig. 3B). In addition, cell
morphology was observed using phalloidin staining to detect F-actin
localization (7,24). Diffuse and generally uniform
distribution throughout the cytoplasm of F-actin transfected with
KLF8-siRNA was exhibited. However, src siRNA transfected into a
human colon cancer cell line presented by multiple clumps of
apparently aggregate size at the rim zone of the protrusion,
requiring actin polymerization (Fig.
3C). Thus, the above findings suggested that knockdown of KLF8
induced differentiation of colorectal cancer cells.
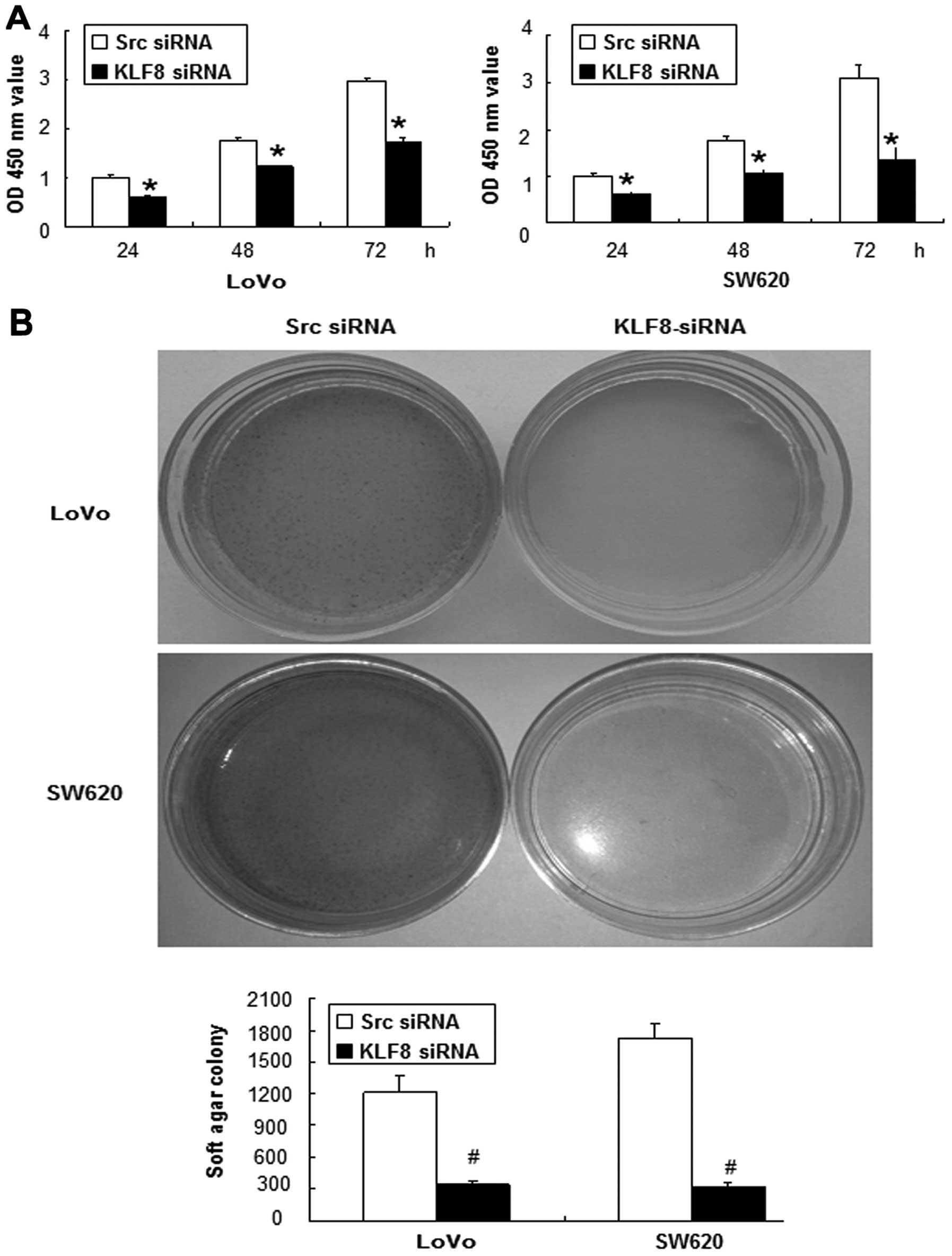
Knockdown of KLF8 inhibits
anchorage-dependent and anchorage-independent growth
We assessed the effect of KLF8 repression on the
growth characteristics of colorectal cancer cells using the WST-1
assay (25). We showed that the
growth rates of cells transfected with the LoVo-KLF8-siRNA after
culture for 24, 48 and 72 h, were 0.6±0.05, 1.22±0.01 and
1.72±0.09%, respectively, whereas those of LoVo-src siRNA cells
were 1±0.05, 1.74±0.07 and 2.97±0.05%, respectively (Fig. 4A). Significant differences between
the growth rates of LoVo-KLF8-siRNA- and LoVo-src siRNA-transfected
cells were found at all three time points (p<0.05), and similar
results were observed for SW620 cells (Fig. 4A).
Anchorage-independent growth is a pivotal
characteristic of malignant transformation (26). Therefore, a soft-agar assay was
carried out to identify the function of KLF8. As expected, KLF8
repression for 14 days significantly inhibited the colony-forming
ability of LoVo and SW620 cells (Fig.
4B).
These results showed that KLF8 knockdown inhibited
anchorage-dependent and -independent growth of colorectal cancer
cells.
Repression of KLF8 induces apoptosis and
sensitizes cancer cells to chemotherapeutic 5-FU
To investigate the mechanism by which KLF8 knockdown
induces growth suppression, an apoptosis assay was performed using
Annexin V-FITC and PI double staining followed by flow cytometry.
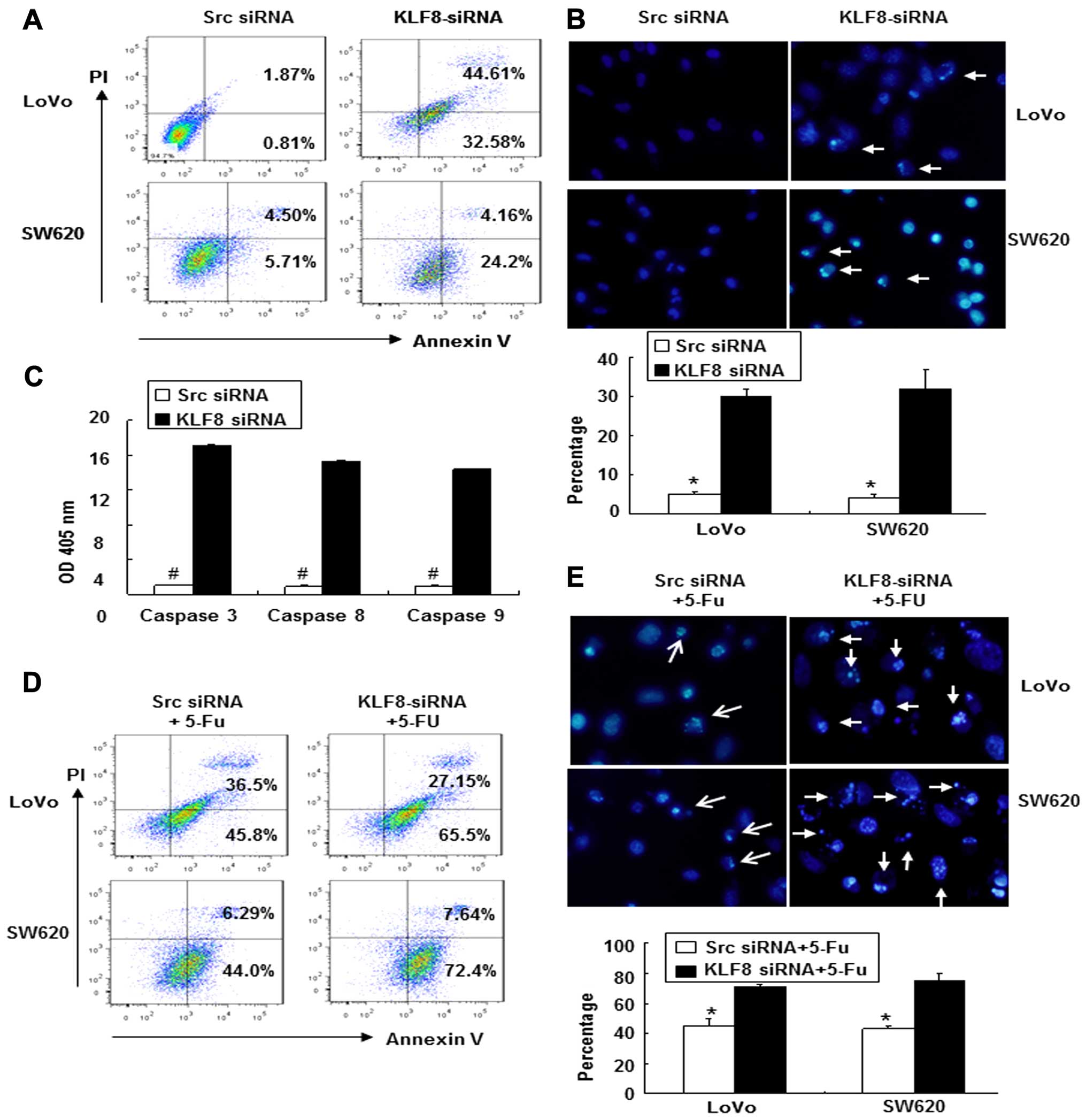
As shown in Fig. 5A, the percentage
of apoptotic cells was significantly increased from 0.81% in src
siRNA-transfected LoVo cells to 32.58% in KLF8-siRNA-transfected
LoVo cells, and similar results were found for SW620 cells (from
5.71 to 24.2%).
Apoptotic induction was further confirmed at the
individual cell level using Hoechst 33258 staining (18). We found that the ratios of cells
that were positive for condensed nuclei were higher in
KLF8-siRNA-transfected LoVo cells (30±2%) compared with src
siRNA-transfected LoVo cells (5±1%, p<0.05) (Fig. 5B). Similar results were observed for
SW620 cells.
The activities of caspases 3, 8 and 9 were then
analyzed after KLF8-siRNA transfection and were normalized to the
OD, which was measured at 405 nm. As shown in Fig. 5C, the activities of caspases 3, 8
and 9 were significantly increased in KLF8-siRNA-transfected cells
compared with src siRNA transfected cells (Fig. 5C, p<0.05).
To assess the role of KLF8-siRNA in
chemotherapy-induced apoptosis, KLF8-siRNA- or src
siRNA-transfected cells were treated with or without 5-FU [50
µg/ml in normal saline (NS)] followed by flow cytometric
analysis. As shown in Fig. 5D, the
apoptotic index of cells treated with KLF8-siRNA + 5-FU was
significantly increased relative to that of the src siRNA control
cells. In addition, apoptotic morphological changes were analyzed
using Hoechst 33258 staining. The ratios of LoVo cells that were
positive for condensed nuclei were higher in cells treated with
KLF8-siRNA + 5-FU compared with the src siRNA + 5-FU control cells
(p<0.05) (Fig. 5E). Similar
results were observed for SW620 cells.
These findings suggested that KLF8-siRNA enhanced
the susceptibility of cancer cells to apoptotic triggers induced by
5-FU.
Suppression of KLF8 sensitizes cancer
cells to 5-FU-induced apoptosis in vivo
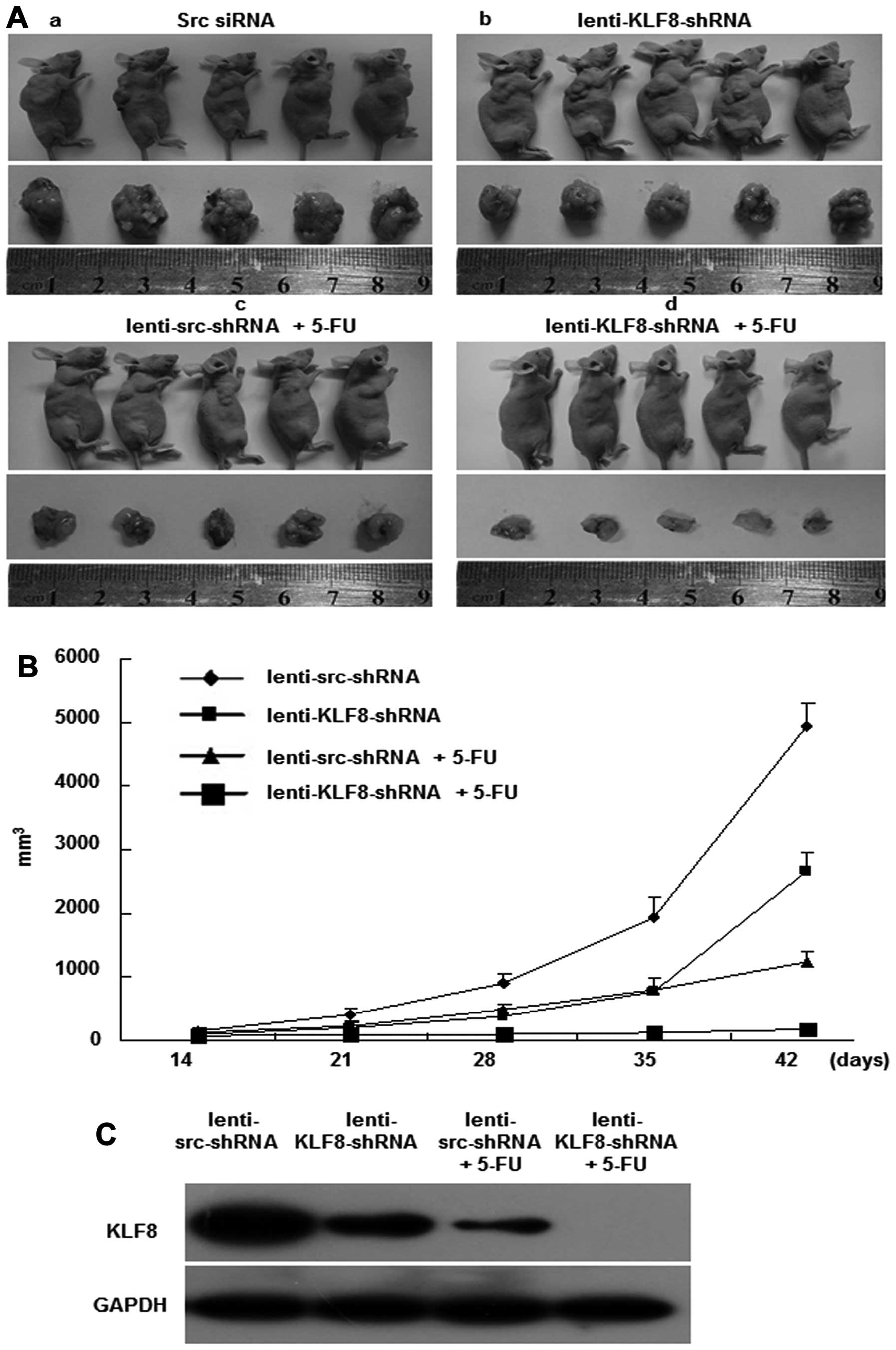
To determine whether KLF8 silencing inhibits tumor
development in vivo, lentivirus-transduced LoVo cells were
subcutaneously injected into the right dorsal flank of nude mice
that were also treated with or without 5-FU (Fig. 6A). As shown in Fig. 6B, the tumor volumes of
LoVo-lenti-KLF8-shRNA-injected mice were markedly smaller than that
of the LoVo-lenti-src-shRNA-injected mice (a and b). Similar
results were found in the 5-FU-treated mice compared with mice not
treated with 5-FU (c and d). More importantly, mice injected with
LoVo-lenti-KLF8-shRNA and 5-FU presented the smallest tumor
nodules. In Fig. 6C, we measured
KLF8 expression in the tumor tissues of four mice groups by western
blotting. Obviously, KLF8 expresses the lowest in tumor tissues of
mice injected with LoVo-lenti-KLF8-shRNA and 5-FU. Therefore,
synergism between the lenti-KLF8-shRNA and 5-FU inhibited LoVo cell
proliferation in vivo.
Taken together, these data indicate that targeting
KLF8 with lenti-KLF8-shRNA exerted an inhibitory effect on
tumorigenesis in vivo. Furthermore, inhibition of KLF8 has a
synergistic effect with 5-FU treatment in the therapy of colorectal
cancer.
Discussion
In the present study, we characterized the role of
KLF8 in the cell growth and differentiation of colorectal cancer.
We found that KLF8 is overexpressed in most colorectal cancer cell
lines and cancerous tissues. Furthermore, we demonstrated the
antitumor efficacy of KLF8 inhibition in cells and xenograft
models, indicating that lenti-KLF8-shRNA is a novel and potent
therapeutic or 5-FU-co-therapeutic agent for colorectal cancer.
KLF8 is a GT-box (CACCC)-binding, dual transcription
factor that has critical roles in the regulation of cell cycle
progression and in oncogenic transformation (16,17).
KLF8 is expressed at low levels in normal human epithelial cells
but is highly overexpressed in several types of cancer cell lines
that were established from human patients, including ovarian,
breast, gastric and renal carcinoma cells (16,17,27,28).
However, to the best of our knowledge, few studies have focused on
KLF8 expression in colorectal cancer. In the present study, KLF8
was expressed at higher levels in colorectal cancer than in normal
tissues, and specific KLF8 knockdown in colorectal cancer cells
significantly inhibited -dependent and anchorage-independent
growth. These results demonstrate that KLF8 is important in CRC
cell proliferation and transformation.
ERK1/2 is a member of the mitogen-activated protein
kinase (MAPK) family and is known to influence cell growth,
migration and invasion of various types of cancer (2,15,29).
However, the mechanism by which KLF8 affects ERK1/2 activation has
yet to be determined. We found that U0126, a specific MEK/ERK
activation inhibitor, strongly suppressed the expression of KLF8,
suggesting that KLF8 is one of the major downstream effectors of
ERK signaling. Furthermore, KLF8 suppression markedly inhibited
constitutive ERK activation. Thus, the expression levels of pERK
and KLF8 are tightly correlated during cancer progression.
F-actin has been found to reflect
differentiation-related changes in cells undergoing tumorigenesis
(7,30). In agreement with this, we identified
KLF8 as a mediator of NaB-induced pro-differentiation of colorectal
cancer. KLF8 suppression increased the expression of two
differentiation markers for colorectal epithelial cells, CEA and
E-cadherin. Moreover, our finding that KLF8 suppression induced the
maturation of F-actin filaments in cancer cells suggests the
involvement of KLF8 in the dedifferentiation of cancer cells. Taken
together, our data show that KLF8 suppression increases the
differentiation of cancer cells.
Apoptosis is the primary means by which radiotherapy
and most chemotherapy modalities kill cancer cells (18,26).
Our findings revealed that KLF8-siRNA directly induced apoptosis
in vitro and that the combination of lenti-KLF8-shRNA and
5-FU treatment provided a synergistic antitumor growth effect in
vivo. Although a few studies have shown the antitumor effect of
KLF8 inhibition in different types of cancer, to the best of our
knowledge, no reports have shown the therapeutic value of the
lenti-KLF8-shRNA in colorectal cancer.
In conclusion, the present study shows that higher
levels of KLF8 are expressed in colorectal cancer tissues compared
with normal tissues. In addition, KLF8 suppression induces cell
differentiation, inhibits cell growth and promotes apoptosis in
colorectal cancer cells in vitro. Furthermore,
lenti-KLF8-shRNA hinders tumor progression and outright regression
when combined with 5-FU in vivo. Thus, targeting of KLF8 by
RNA interference may have a promising role in the management of
colorectal cancer.
Acknowledgments
The present study was supported by grants from the
National Natural Science Funds of China (nos. 81172057 and
81272761), and the 'President Foundation of Nanfang Hospital,
Southern Medical University' (nos. 2012B009 and 2013Z007) and by
high-level, topic-matching funds of Nanfang Hospital (nos. 201347
and G201227).
Abbreviations:
|
KLF
|
Krüppel-like factors
|
|
EMT
|
epithelial-to-mesenchymal
transition
|
|
NaB
|
sodium butyrate
|
|
HEK293
|
human embryonic kidney 293
|
|
ATCC
|
American Type Culture Collection
|
|
FBS
|
fetal bovine serum
|
|
src siRNA
|
scrambled siRNA
|
|
ERK
|
extracellular signal-regulated protein
kinases
|
References
|
1
|
Brenner H, Kloor M and Pox CP: Colorectal
cancer. Lancet. 383:1490–1502. 2014. View Article : Google Scholar
|
|
2
|
Yu LF, Wang J, Zou B, Lin MC, Wu YL, Xia
HH, Sun YW, Gu Q, He H, Lam SK, et al: XAF1 mediates apoptosis
through an extracellular signal-regulated kinase pathway in colon
cancer. Cancer. 109:1996–2003. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kopelovich L: Heritable colorectal cancer
and cancer genes: Systemic expressions. Mol Carcinog. 8:3–6. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ponce NA, Tsui J, Knight SJ, Afable-Munsuz
A, Ladabaum U, Hiatt RA and Haas JS: Disparities in cancer
screening in individuals with a family history of breast or
colorectal cancer. Cancer. 118:1656–1663. 2012. View Article : Google Scholar :
|
|
5
|
Doubeni CA, Major JM, Laiyemo AO,
Schootman M, Zauber AG, Hollenbeck AR, Sinha R and Allison J:
Contribution of behavioral risk factors and obesity to
socioeconomic differences in colorectal cancer incidence. J Natl
Cancer Inst. 104:1353–1362. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fernandez E, La Vecchia C, Talamini R and
Negri E: Joint effects of family history and adult life dietary
risk factors on colorectal cancer risk. Epidemiology. 13:360–363.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang J, Yang Y, Xia HH, Gu Q, Lin MC,
Jiang B, Peng Y, Li G, An X, Zhang Y, et al: Suppression of FHL2
expression induces cell differentiation and inhibits gastric and
colon carcinogenesis. Gastroenterology. 132:1066–1076. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang W, Jiang B, Guo Z, Sardet C, Zou B,
Lam CS, Li J, He M, Lan HY, Pang R, et al: Four-and-a-half LIM
protein 2 promotes invasive potential and epithelial-mesenchymal
transition in colon cancer. Carcinogenesis. 31:1220–1229. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Warren RS, Yuan H, Matli MR, Gillett NA
and Ferrara N: Regulation by vascular endothelial growth factor of
human colon cancer tumorigenesis in a mouse model of experimental
liver metastasis. J Clin Invest. 95:1789–1797. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Furue H, Matsuo K, Kumimoto H, Hiraki A,
Suzuki T, Yatabe Y, Komori K, Kanemitsu Y, Hirai T, Kato T, et al:
Decreased risk of colorectal cancer with the high natural killer
cell activity NKG2D genotype in Japanese. Carcinogenesis.
29:316–320. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guo Z, Zhang W, Xia G, Niu L, Zhang Y,
Wang X, Zhang Y, Jiang B and Wang J: Sp1 upregulates the four and
half lim 2 (FHL2) expression in gastrointestinal cancers through
transcription regulation. Mol Carcinog. 49:826–836. 2010.PubMed/NCBI
|
|
12
|
Lee PL, Gelbart T, West C, Adams M,
Blackstone R and Beutler E: Three genes encoding zinc finger
proteins on human chromosome 6p21.3: Members of a new subclass of
the Krüppel gene family containing the conserved SCAN box domain.
Genomics. 43:191–201. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Le Magnen C, Bubendorf L, Ruiz C, Zlobec
I, Bachmann A, Heberer M, Spagnoli GC, Wyler S and Mengus C: Klf4
transcription factor is expressed in the cytoplasm of prostate
cancer cells. Eur J Cancer. 49:955–963. 2013. View Article : Google Scholar
|
|
14
|
Yang Y, Nakagawa H, Tetreault MP, Billig
J, Victor N, Goyal A, Sepulveda AR and Katz JP: Loss of
transcription factor KLF5 in the context of p53 ablation drives
invasive progression of human squamous cell cancer. Cancer Res.
71:6475–6484. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ellenrieder V, Buck A, Harth A, Jungert K,
Buchholz M, Adler G, Urrutia R and Gress TM: KLF11 mediates a
critical mechanism in TGF-beta signaling that is inactivated by
Erk-MAPK in pancreatic cancer cells. Gastroenterology. 127:607–620.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang X, Zheng M, Liu G, Xia W,
McKeown-Longo PJ, Hung MC and Zhao J: Krüppel-like factor 8 induces
epithelial to mesenchymal transition and epithelial cell invasion.
Cancer Res. 67:7184–7193. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fu WJ, Li JC, Wu XY, Yang ZB, Mo ZN, Huang
JW, Xia GW, Ding Q, Liu KD and Zhu HG: Small interference RNA
targeting Krüppel-like factor 8 inhibits the renal carcinoma 786-0
cells growth in vitro and in vivo. J Cancer Res Clin Oncol.
136:1255–1265. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu Y, Guo Z, Zhang D, Zhang W, Yan Q, Shi
X, Zhang M, Zhao Y, Zhang Y, Jiang B, et al: A novel colon cancer
gene therapy using rAAV mediated expression of human shRNA-FHL2.
Int J Oncol. 43:1618–1626. 2013.PubMed/NCBI
|
|
19
|
Li JC, Yang XR, Sun HX, Xu Y, Zhou J, Qiu
SJ, Ke AW, Cui YH, Wang ZJ, Wang WM, et al: Up-regulation of
Krüppel-like factor 8 promotes tumor invasion and indicates poor
prognosis for hepatocellular carcinoma. Gastroenterology.
139:2146–2157.e12. 2010. View Article : Google Scholar
|
|
20
|
Wang TB, Hu BG, Liu DW, Shi HP and Dong
WG: The influence of lentivirus-mediated CXCR4 RNA interference on
hepatic metastasis of colorectal cancer. Int J Oncol. 44:1861–1869.
2014.PubMed/NCBI
|
|
21
|
Dent P: Multi-kinase modulation for colon
cancer therapy. Cancer Biol Ther. 14:877–878. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Orchel A, Dzierzewicz Z, Parfiniewicz B,
Weglarz L and Wilczok T: Butyrate-induced differentiation of colon
cancer cells is PKC and JNK dependent. Dig Dis Sci. 50:490–498.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bajenova O, Chaika N, Tolkunova E,
Davydov-Sinitsyn A, Gapon S, Thomas P and O'Brien S:
Carcinoembryonic antigen promotes colorectal cancer progression by
targeting adherens junction complexes. Exp Cell Res. 324:115–123.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yan H, Wang X, Niu J, Wang Y, Wang P and
Liu Q: Anti-cancer effect and the underlying mechanisms of
gypenosides on human colorectal cancer SW-480 cells. PLoS One.
9:e956092014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yu J, Qiao L, Zimmermann L, Ebert MP,
Zhang H, Lin W, Röcken C, Malfertheiner P and Farrell GC:
Troglitazone inhibits tumor growth in hepatocellular carcinoma in
vitro and in vivo. Hepatology. 43:134–143. 2006. View Article : Google Scholar
|
|
26
|
Tu SP, Liston P, Cui JT, Lin MC, Jiang XH,
Yang Y, Gu Q, Jiang SH, Lum CT, Kung HF, et al: Restoration of XAF1
expression induces apoptosis and inhibits tumor growth in gastric
cancer. Int J Cancer. 125:688–697. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schnell O, Romagna A, Jaehnert I, Albrecht
V, Eigenbrod S, Juerchott K, Kretzschmar H, Tonn JC and Schichor C:
Krüppel-like factor 8 (KLF8) is expressed in gliomas of different
WHO grades and is essential for tumor cell proliferation. PLoS One.
7:e304292012. View Article : Google Scholar
|
|
28
|
Lu H, Wang X, Urvalek AM, Li T, Xie H, Yu
L and Zhao J: Transformation of human ovarian surface epithelial
cells by Krüppel-like factor 8. Oncogene. 33:10–18. 2014.
View Article : Google Scholar :
|
|
29
|
Bromberg KD, Kluger HM, Delaunay A, Abbas
S, DiVito KA, Krajewski S and Ronai Z: Increased expression of the
E3 ubiquitin ligase RNF5 is associated with decreased survival in
breast cancer. Cancer Res. 67:8172–8179. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Franovic A, Holterman CE, Payette J and
Lee S: Human cancers converge at the HIF-2alpha oncogenic axis.
Proc Natl Acad Sci USA. 106:21306–21311. 2009. View Article : Google Scholar : PubMed/NCBI
|