Introduction
Thyroid cancer has become one of the most common
type of endocrine tumors with a rapid growth of incidence in recent
decades (1). According to the
estimate of the American National Cancer Institute (NCI), 62,980
new cases of thyroid cancer are anticipated in the United States in
2014. Thyroid cancer often occurs in women, and is the fifth
leading cancer type in women (2).
Histologically, over 80% of thyroid cancer is papillary thyroid
carcinoma (PTC), which derives from thyroid follicular cells
(3). As a type of
well-differentiated cancer, PTC is considered treatable. However,
there are ~10% of patients with poor prognosis for recurrence
and/or distant metastasis (4).
Accordingly, it is extremely essential to clarify the mechanisms of
PTC to provide evidence to identify latent biomarkers for early
diagnosis, prognosis and therapies.
As a primary member of the quinone oxidoreductase
(QOR) family, the p53-inducible gene 3 (PIG3 or
TP53I3) was initially identified through the analysis of p53
downstream genes associated with the onset of apoptosis in human
colorectal cancer cell (5). PIG3
can be transactivated by p53 with a p53-response element, i.e., a
polymorphic microsatellite (TGYCC)n (Y=C or T) at its promoter, and
the activation degree of PIG3 was determined by the number of
repeats of the microsatellite (6).
PIG3 is homologous with NADH quinine oxidoreductase 1 (NQO1), and
shows oxidoreductase enzymatic activity, which contributes to the
process of apoptosis induced by p53 through production of the
reactive oxygen species (ROS) (5,7). In
addition, PIG3 is a molecule involved in the DNA damage response
(DDR) pathway by increasing the phosphorylation of checkpoint
kinases including Chk1 and Chk2, and contributing to the
recruitment of other DNA repair components (8).
Although the relationship between PIG3 and various
types of cancer has been previously investigated, the role of PIG3
in cancer remains to be clarified. As one of the downstream
effectors of the important tumor suppressor p53, PIG3 alone is
insufficient to induce apoptosis unless activated and cooperating
with a set of simultaneously activated pro-apoptotic genes induced
by ROS (5). Accumulating evidence
has identified the number of repeats of the microsatellite to be
correlated with the generation of several types of tumors, although
no relationship with increased risk of breast and lung carcinomas
was found (9–11). The aforementioned results raised the
question regarding the reasons for PIG3 rarely being affected in
cancer (12). Recent findings have
demonstrated that PIG3 playd a significant role in cancer cell
survival. The proliferation ability of PIG3-depleted HeLa cells was
found to be decreased despite exhibiting a prolonged progression of
G2-M phases (13). In addition,
PIG3 participates in the malignant development of the disease by
creating a connection between oxidative stress and DDR. In
p53+/+ cancer cells, intense DNA damage induced by
genotoxic/oxidative stress was capable of recruiting a large part
of total PIG3 in the nucleus, but failed to activate ROS-dependent
apoptosis when other pro-apoptotic genes were not expressed under
identical conditions (8,14). This would result in sublethal levels
of ROS which continuously maintain the oxidative stress (15). Thus, the continuous demand for PIG3
as a DDR component remains a positive feedback between PIG3 and DNA
damage, as the accumulation of DNA damage may lead to mutagenesis,
including p53 loss or mutation, which is crucial in carcinogenesis
(16,17).
To the best of our best knowledge, however, the
clinical and functional significance of PIG3 in PTC remains to be
clarified. In this study, we first detected the expression of PIG3
in PTC and normal thyroid tissues and analyzed its clinical
significance in PTC. furthermore, we determined its functional role
in PTC cell proliferation and the role of phosphatidylinositide
3-kinases/protein kinase B/phosphatase and tensin homologue deleted
on chromosome 10 (PI3K/AKT/PTEN) signaling pathway in a series of
assays in vitro. The results showed that PIG3 was aberrantly
overexpressed and plays an oncogenic role in PTC.
Materials and methods
Patients and samples
Consent was provided by all the patients who
participated in the study. The study was approved by the
Institutional Review board of the Taizhou Municipal Hospital. In
total, 16 male and 54 female patients with PTC (age range, 19–70
years) were recruited for this study and underwent surgery at the
Taizhou Municipal Hospital (Zhejian, China) between February and
December, 2013. Tumors and normal thyroid tissues were obtained
during surgery and immediately stored in liquid nitrogen prior to
quantitative PCR and western blot analysis. Matched
paraffin-embedded samples used for immunohistochemistry were kindly
donated by the Department of Pathology. All the cases were
confirmed pathologically and staged on the basis of TNM
classification system.
Immunohistochemistry
Immunohistochemical (IHC) staining was used to
detect the distribution of PIG3 protein in specimens of PTC and
normal thyroid tissue. The excised samples were formalin-fixed,
paraffin-embedded and blocks were sectioned serially at 4 µm
prior to being examined under a microscope. The slides were
deparaffinized and rehydrated with xylene in a graded series of
ethanol/water concentrations (100, 100, 95, 90, 85 and 75%),
respectively. Antigen retrieval was achieved by immersing the
slides in citrate buffer (pH 6.0). The slides were autoclaved at
120°C for 2 min and then cooled to room temperature. Endogenous
peroxidase activity was blocked with 3% hydrogen peroxide for 10
min at room temperature. After washing with phosphate-buffered
saline (PBS), the samples were treated with 5% goat serum
(ZDR-5118; Zhongshan Golden Bridge Biotechnology Co., Ltd., China)
to block non-special protein binding for 30 min. The slides were
then incubated overnight with primary rabbit polyclonal anti-PIG3
antibody (TA308561; OriGene, Rockville, MD, USA) at a dilution of
1:800 at 4°C in a humid chamber. Negative controls (NC) were
processed by substituting PBS. After incubation at 37°C for 45 min,
the sections were rinsed with PBS and incubated with biotinylated
goat anti-Rb IgG/HRP for 1.5 h at 37°C. After washing with PBS, the
slides were visualized by 3,3′-diaminobenzidine tetrahydrochloride
solution (DAB kit, ZLI-9017; Zhongshan Golden bridge Biotechnology)
and counterstained by Mayer's hematoxylin. After being rinsed with
water for 20 min, the sections were dehydrated with gradient
ethanol sequentially (75, 85, 90, 95, 100 and 100%) and cleared
with xylene. The samples were examined under a microscope (Olympus,
Japan) by pathologists (Z.H.Y and L.H.S) who were blinded to the
clinicopathological data. The semi-quantitative scoring system used
to estimate the expression of PIG3 was combined with staining
intensity and the proportion of positive cells (18). The intensity score was graded as 0
(no staining), 1 (weak), 2 (moderate) and 3 (strong). Then, 2,500
cells in five randomly selected areas (magnification, ×400) were
counted to evaluate the proportion score: the percentage of
positive cells <5, 5–35, 36–70 and >70% were assigned as 0,
1, 2 and 3 points, respectively. Multiplication of the intensity
and proportion scores was employed to determine the final score.
Slides with a score ≤3 were defined as low expression and those
with a score ≥4 were regarded as high expression.
Cell culture
Cell culture reagents were obtained from Gibco
(Carlsbad, CA, USA). Human PTC CGTHW-3 and K1 cells were purchased
from the American Type Culture Collection (ATCC; Manassas, VA,
USA). The two cell lines were cultured in RPMI-1640, supplemented
with 10% fetal bovine serum (FBS) in a modulator incubator chamber
and maintained at 37°C and 5% CO2.
Cell transfection
The RNAi-Mate for cell transfection, small
interfering RNA (siRNA) for human PIG3 (sense, 5′-AAAUG
UUCAGGCUGGAGACUAdTdT-3′ and antisense, 5′-UAGUC
UCCAGCCUGAACAUUUdTdT-3′) and its corresponding NC (sense,
5′-CCUACGCCAAUUUCGUdTdT-3′ and antisense,
5′-ACGAAAUUGGUGGCGUAGGdTdT-3′) were purchased from Shanghai
GenePharma Co., Ltd. (Shanghai, China). When the GTHW-3 and K1
cells grew to 40–60% confluence in 24 h, the medium was removed
from each plate and siRNA/RNAi-Mate complexes incubated in
serum-free Opti-MEM 1 (Gibco) were added to the cells according to
the manufacturer's instructions. The final concentration of siRNA
in each plate was 20 nM. After 6–8 h, the medium was replaced with
RPMI-1640 containing 10% FBS. After being cultured for an
additional 48 or 72 h, the cells were harvested for quantitative
PCR and western blot analysis, respectively.
Cell viability assay
Cells (5,000 CGTHW-3 or 6,500 K1) were seeded in
96-well plates/well and cultured overnight. The two types of cells
were transfected with PIG3 siRNA and NC respectively, and each
experiment was performed in triplicate. After 48 h of transfection,
each well was treated with 10 µl CCK-8 (Beyotime, Jiangsu,
China) solution. The cells were incubated in the modulator
incubator chamber for another 2 h. The optical density (OD) of each
well was determined at 450 nm using a 550 Microplate Reader
(Bio-Rad, Hercules, CA, USA).
Colony formation assay
The CGTHW-3 and K1 cells were transfected with PIG3
siRNA or NC as previously described. After 48 h, the transfected
cells were seeded in 6-well plates at a density of 800 cells/well,
and allowed to form colonies for 10 days with completed medium. The
colonies were fixed in 10% methanol for 15 min and stained with
crystal violet for 25 min at room temperature. The number of
colonies with >50 cells were counted manually. Each experiment
was conducted in triplicate three times.
Quantitative PCR (qPCR)
Total RNA was extracted from thyroid tissues and
cells using TRIzol reagent (Invitrogen-Life Technologies, Carlsbad,
CA, USA). The concentration of total RNA was determined by a UV
spectrophotometer, and all the isolated RNA samples had an
A260/A280 nm ratio of >1.8. Total RNA was reverse transcribed
using a cDNA Reverse Transcription kit (Tiangen Biotechnology,
Beijing, China). As per the manufacturer's instructions, the final
reaction volume was 20 µl. Then, 2.0 µl cDNA was
added to the qPCR reaction system of a total volume of 20
µl, which contained a 9 µl mixture of 2.5X Real
Master mix/20X SYBR solution, 0.5 µl of forward and reverse
primers (10 µM) and 9 µl ddH2O. The
primers used in the qPCR assays to express the investigated
transcripts were produced at Invitrogen-Life Technologies (Table I). GAPDH served as the
reference gene for analysis. The qPCR assays were run in triplicate
on the ABI 7300 Real Time PCR system (Applied Biosystems Life
Technologies, Foster City, CA, USA) under the following cycling
conditions: 95°C for 2 min, 40 cycles of 95°C for 15 sec, 58°C for
30 sec and 68°C for 60 sec. The comparative Ct method was used to
calculate the relative expression of mRNA level.
 | Table IPrimer sequences used for quantitative
PCR. |
Table I
Primer sequences used for quantitative
PCR.
| Genes | Forward | Reverse |
|---|
| PIG3 |
5′-AGCCGGGCCAGGAGTAAGTAAC-3′ |
5′-GCCGAAGAGGATCAGGCAAAT-3′ |
| p53 | 5′-GGC CCA CTT CAC
CGT ACT AA-3′ | 5′-GTG GTT CTT TCA
AGG CCAGATGT-3′ |
| GAPDH | 5′-CATCAGCAA
TGCCTCCTGCAC-3′ |
5′-TGAGTCCTTCCACGATACCAA AGTT-3′ |
Western blot analysis
Rabbit antibodies of anti-PI3K, anti-AKT,
anti-P-AKT, anti-P53, anti-PTEN and anti-GAPDH were purchased from
Cell Signaling Technology (Beverly, MA, USA) and anti-PIG3 was
purchased from OriGene. The total protein of each sample was
extracted using T-PER solution (Thermo Fisher Scientific, Waltham,
MA, USA) according to the manufacturer's instructions and then
quantified with BSA standard methodology. An equivalent amount of
protein was loaded and fractionated by SDS-polyacrylamide gel
electrophoresis, and electrotransferred onto PVDF membranes. After
blocking with 5% non-fat milk, the membranes were blotted with each
of primary antibodies (1:1,000 dilution) overnight at 4°C. The
membranes were treated with a horseradish peroxidase-conjugated
secondary antibody at a dilution of 1:10,000 for 1.5 h at room
temperature. The blots were visualized using ECL Plus Western
blotting Detection reagents (Beyotime) and scanned in ImageQuant
LAS 4000 Mini (GE Healthcare, Pittsburgh, PA, USA).
Statistical analysis
Experiments were conducted independently three
times. SPSS 17.0 software was used for the statistical analysis.
The differences in the IHC staining in thyroid tissues and the
relationship between protein expression levels and
clinicopathological characteristics were calculated using the
χ2 test. The qPCR data, western blot data, colony
formation and CCK-8 data were recorded as numeric data and
presentedas the mean ± standard deviation (SD). The Student's
t-test and one-way ANOVA analysis were used to compare the means
between 2–3 groups, respectively. P<0.05 was regarded as
statistically significant.
Results
PIG3 is highly expressed in PTC
tissues
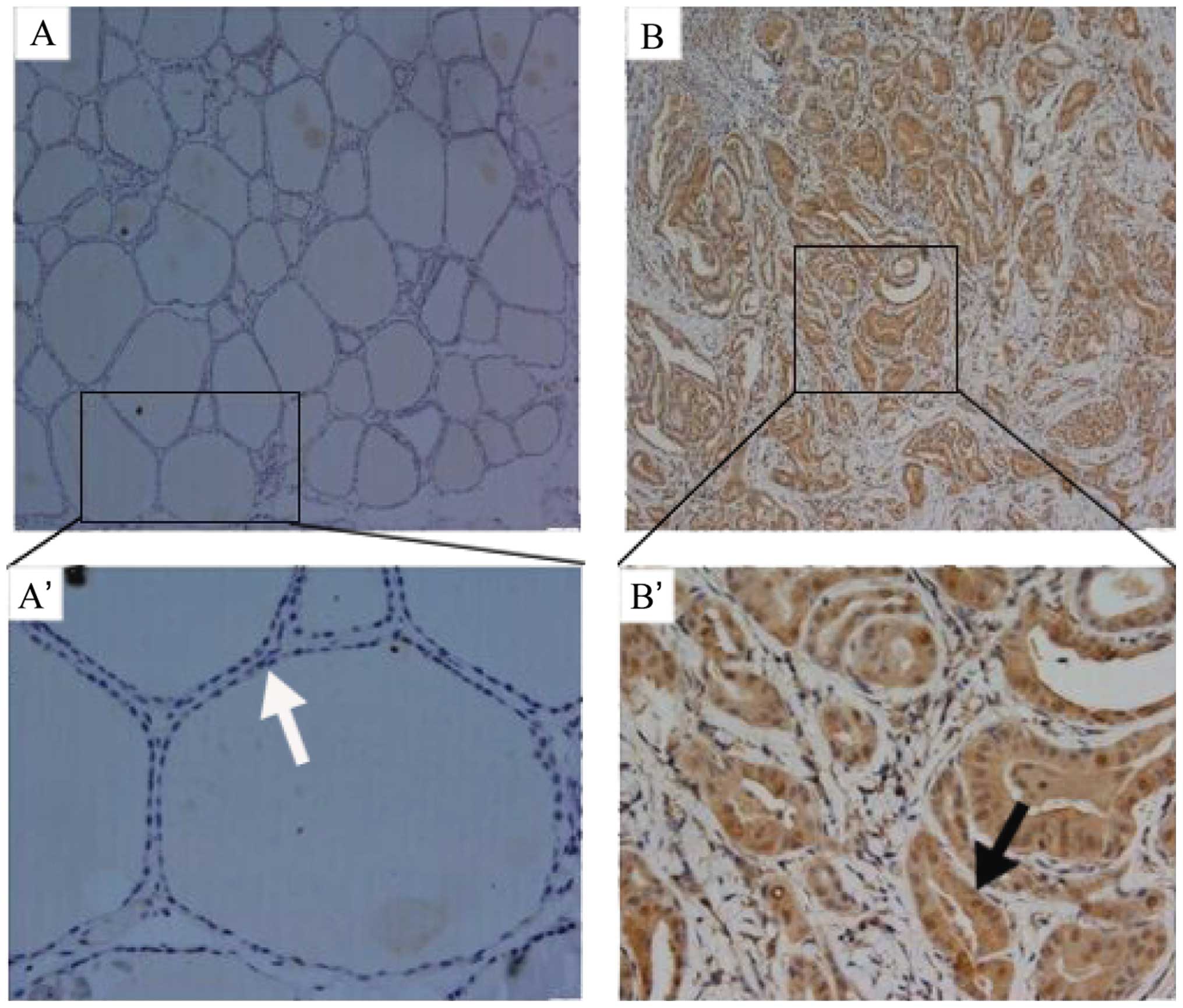
IHC was performed to detect the expression of PIG3
in PTC and normal thyroid tissues. The PIG3 protein foci were
distributed on the nuclear and cytoplasm in PTC and exhibited a
higher expression with total immunoreactive positive rate of 74%
(52/70) (Fig. 1B–B′), which was
significantly higher than the 32% (21/65) in normal thyroid tissues
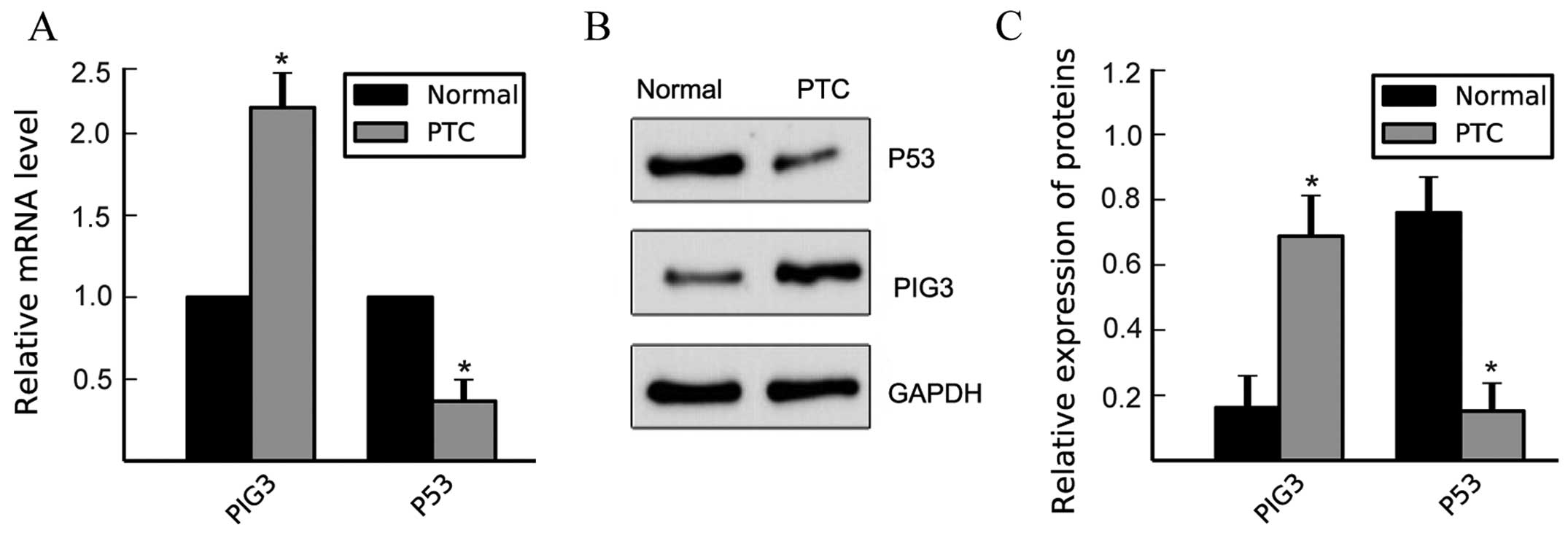
(Fig. 1A–A′; P<0.001; Table II). qPCR and western blotting
confirmed that PIG3 expression was consistent with the IHC results
at the mRNA and protein level, respectively, in PTC (Fig. 2; P<0.01).
 | Table IIExpression of PIG3 in PTC and in
normal thyroid tissue. |
Table II
Expression of PIG3 in PTC and in
normal thyroid tissue.
| Groups | Positive | Negative | χ2 | P-value |
|---|
| PTC tissue | 52 | 18 | | |
| Normal thyroid
tissue | 21 | 34 | 16.525 | 0.000a |
The relationship between PIG3 expression and the
clinicopathologic parameters of the 70 cases of PTC were assessed.
The results indicated that PIG3 expression was positively
associated with TNM grade (Table
III; P<0.05), while no association between PIG3 expression
and age, gender, tumor size and lymphatic metastasis was identified
(Table III; P>0.05).
 | Table IIIRelationship between PIG3 protein
expression and clinicopathological characteristics of PTC. |
Table III
Relationship between PIG3 protein
expression and clinicopathological characteristics of PTC.
| Variables | No. | Positive | Negative | χ2 | P-value |
|---|
| Gender |
| Male | 16 | 11 | 5 | 0.063 | 0.802 |
| female | 54 | 41 | 13 | | |
| Age (years) |
| <45 | 39 | 27 | 12 | 1.178 | 0.278 |
| ≥45 | 31 | 25 | 6 | | |
| Tumor size (cm) |
| ≤2 | 54 | 38 | 16 | 1.105 | 0.293 |
| >2 | 16 | 14 | 2 | | |
| Lymphatic
metastasis |
| Yes | 46 | 35 | 11 | 0.228 | 0.633 |
| No | 24 | 17 | 7 | | |
| TNM stages |
| I+II | 49 | 33 | 16 | 4.117 | 0.042a |
| III+IV | 21 | 19 | 2 | | |
PIG3 has been proven to be one of the downstream
effectors of the important tumor suppressor p53. To investigate
whether the high expression of PIG3 in PTC was induced by p53, we
determined the expression of p53 in PTC and normal thyroid tissues
by qPCR and western blotting. In contrast to PIG3, the expression
of p53 in PTC was lower than normal thyroid tissues at the mRNA and
protein level (Fig. 2;
P<0.01).
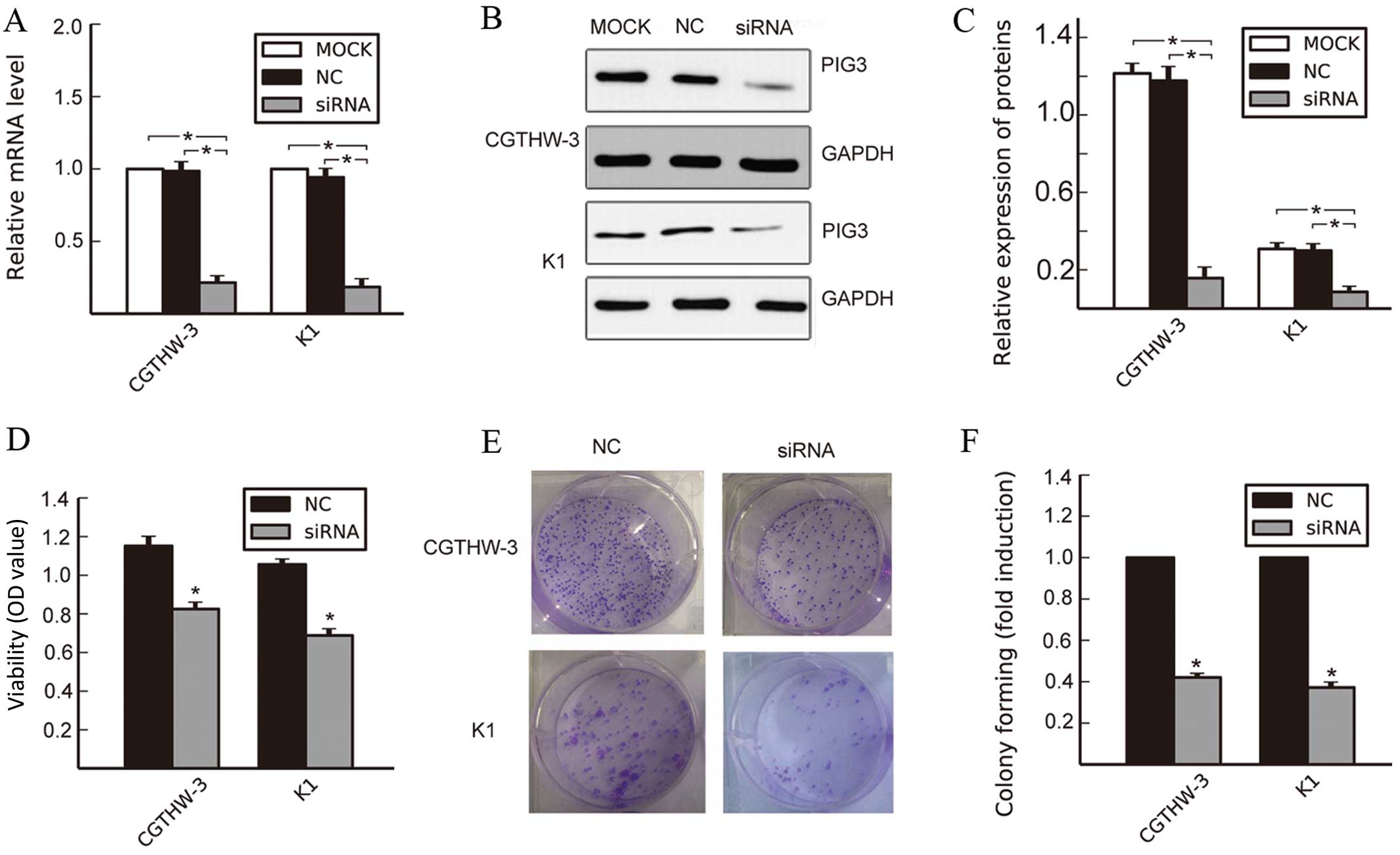
Silencing PIG3 induces anti-proliferative
effect in PTC cell lines
To determine whether the downregulation of PIG3
affected the biological behavior of PTC cell lines, PIG3 siRNA and
the corresponding NC were transfected into CGTHW-3 and K1 cells.
The two types of cells showed a significant decrease in PIG3 mRNA
and protein expression levels in the group of siRNA, compared with
the untreated group (MOCK) and the NC group (Fig. 3A–C; P<0.05).
CCK-8 assay was used to examine the effect of
silencing PIG3 on the proliferation of PTC cells. Silencing PIG3
significantly decreased the viability of CGTHW-3 and K1 cells at 48
h after transfection, compared with the NC group (Fig. 3D, P<0.05). The colony formation
assay also showed the effects of PIG3 knockdown on the growth of
PTC cells. Compared with the NC group, the downregulation of PIG3
suppressed the colony formation ability of the two PTC cell types
(Fig. 3E and F, P<0.05). These
data suggested that PIG3 played an important role in promoting the
proliferation of PTC cells.
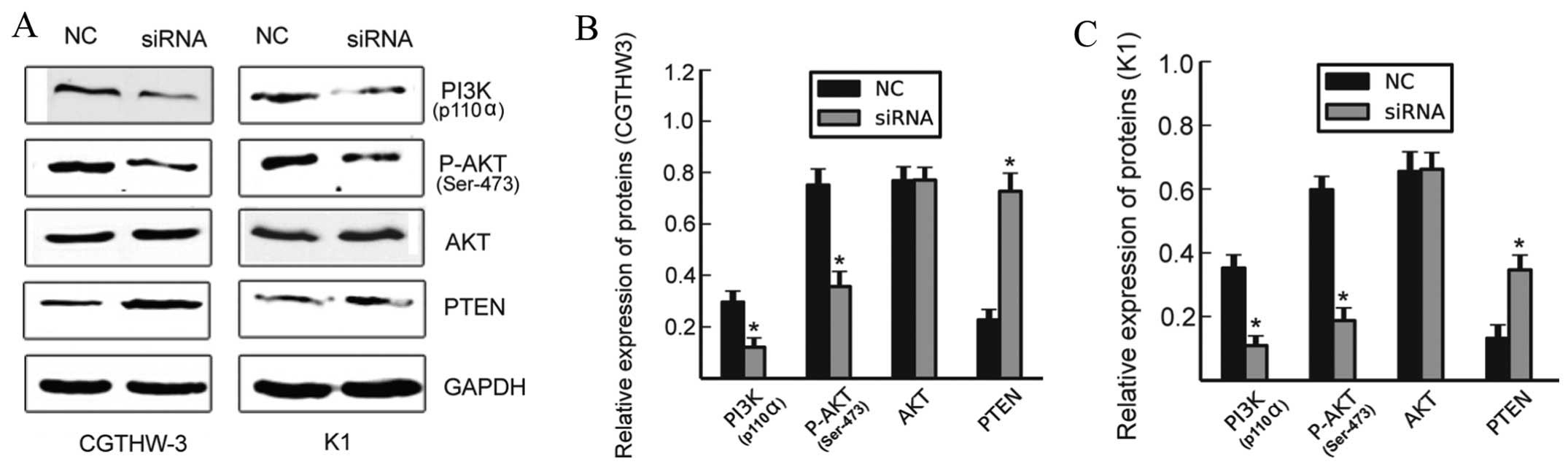
Knockdown of PIG3 suppresses activation
of the PI3K/AKT signaling pathway
PI3K/AKT/PTEN is a classical signaling pathway
involved in many cancer types which enhance malignant cell
proliferation. To identify the exact mechanisms involved in PIG3
promotion of PTC cell proliferation. Activation of the
PI3K/AKT/PTEN signaling pathway was determined by using western
blot analysis after silencing the expression of PIG3 in CGTHW-3 and
K1 cells. The protein expression levels of PTEN were markedly
increased while PI3K p110a was significantly reduced (Fig. 4; P<0.05) and the expression of
AKT exhibited no notable difference between the siRNA and NC
groups. by contrast, the amount of p-AKT markedly decreased in
cells transfected with siRNA (Fig.
4, P<0.05). These data suggested that the downregulation of
PIG3 suppressed the proliferation of PTC cells via regulation of
the PI3K/AKT/PTEN signaling pathway.
Discussion
PIG3 was initially isolated through analysis of the
p53 downstream genes associated with the onset of apoptosis in 1997
(5). Although several studies have
identified an association between PIG3 and human cancers, the role
of PIG3 in cancers remains to be determined. In the present study,
we showed that the expression of PIG3 in PTC was significantly
higher than that in normal thyroid tissues at the mRNA and protein
level. We assessed the relationship between PIG3 expression and
clinicopathological parameters of the 70 cases of PTC. The results
indicated a positive association of PIG3 expression with TNM grade.
Since PIG3 can be mediated by p53 mainly through a microsatellite
(TGYCC)n (Y=C or T) at its promoter and the extent of PIG3
activation was determined by the number of repeats of the
microsatellite (6). The expression
of p53 was also detected in these thyroid tissues. Notably, the
expression of p53 was lower in PTC compared with that in the normal
thyroid tissues. We also demonstrated that PIG3 promoted PTC cell
proliferation by modulating the PI3K/AKT/PTEN signing pathway. Our
results suggest that PIG3 plays an essential role in facilitating
the proliferation of PTC and that the expression of PIG3 is not
associated with p53 in PTC.
PIG3 has been proven to be one of the downstream
effectors of the important tumor suppressor p53 and may be induced
by p53 under cell stress and control conditions (19). When cooperated with other p53
downstream pro-oxidative genes, PIG3 participated in the process of
apoptosis induced by p53 by producing ROS (5). However, the expression of PIG3 was not
only induced by p53. Previous findings have demonstrated that the
variable number of tandem repeats (VNTRs) of pentanucleotides
(TGYCC)n at the promoter of PIG3 was reported to be correlated with
the generation of squamous cell carcinoma of the head and neck and
invasive bladder cancer (10,11).
In addition, after p53 stimulus was removed from cells, elevated
levels of PIG3 were maintained while p53, MDM2 and p21 protein
levels decreased rapidly (19). The
present study focused on determining the relationship between PIG3
and p53 in PTC and found that PIG3 can be induced by molecules
other than p53, which may identify the molecular mechanisms
involved in PIG3 contribution to carcinogenesis and development in
human cancers.
Sustained proliferation is one of the most
fundamental features of cancer cells (20). Our findings show that silencing PIG3
significantly decreased the viability and colony formation ability
of CGTHW-3 and K1 cells, which is similar to previously obtained
results (13,19). A recent finding showed that the
proliferation capability of HeLa cells decreased when PIG3 was
knocked down despite elongating the G2-M phase (13). Although PIG3 levels were elevated
during p53-mediated growth arrest, the expression of PIG3 remained
relatively constant in cells that resumed cycling when the
expression of p53 was decreased (19). Additionally, the ROS level in cells
and their condition is a crucial regulatory factor that directly
determines the fate of the cells, i.e., proliferation, apoptosis
and migration (21). Under some
conditions when the selective expression of pro-apoptotic genes was
induced by p53, PIG3 could produce sublethal levels of ROS, but
failed to activate ROS-dependent apoptosis, thus playing a vital
role in carcinogenesis (22). The
results indicate that PIG3 potentially plays an oncogenic role in
human cancers.
We determined the exact mechanisms of how PIG3
promoted the proliferation of PTC cells. The activity of the
PI3K/AKT/PTEN signaling pathway was also determined by using siRNA
to silence PIG3 expression. The PI3K/AKT/PTEN signing pathway plays
a pivotal role in cell proliferation, and survival in human cancers
including those deriving in the thyroid gland (23,24).
Previous findings have demonstrated that ROS increases the
expression of PI3K and inactivates PTEN directly, and can mediate
cell survival and proliferation by modulating the PI3K/AKT/PTEN
pathway (25,26). However, the relationship between
PIG3 and the PI3K/AKT/PTEN signaling pathway remains unknown. In
the present study, we found that after knockdown of PIG3 in PTC
cells, the PI3K/AKT pathway was inactivated while the expression of
PTEN was markedly increased. PIG3 silencing suppresses the
activation of PI3K/AKT/PTEN signaling pathway. Our results and
those of previous studies suggest that PIG3 probably regulates the
expression of the PI3K/AKT/PTEN signaling pathway by producing ROS
in PTC. However, a detailed elucidation of the connection between
these molecules remains to be determined in future studies.
In summary, our study has demonstrated that PIG3 is
highly expressed in PTC and may promote the proliferation of PTC
via regulation of the PI3K/AKT/PTEN pathway, which indicates that
PIG3 possibly plays an oncogenic role in PTC and may serve as a new
target for the clinical diagnosis and treatment of PTC. Moreover,
we found that the expression of PIG3 in PTC was not induced by p53.
However, the molecular basis of how PIG3 is activated and how PIG3
regulates the expression of the PI3K/AKT/PTEN signaling pathway in
PTC remain important issues to be investigated.
Acknowledgments
We would like to thank Professor David T. Yew
(Department of Anatomy Chinese University of Hong Kong) for
carefully editing the manuscript and sincerely acknowledge Dr
Huayuan Zhan (Department of Pathology, The Affiliated Municipal
Hospital) for the technical support received for the frozen
sections. This study was supported by the National Natural Science
Foundation of China (no. 81072209).
References
|
1
|
Kweon KH, Lee CR, Jung SJ, Ban EJ, Kang
SW, Jeong JJ, Nam KH, Jo YS, Lee J and Chung WY: Sirt1 induction
confers resistance to etoposide-induced genotoxic apoptosis in
thyroid cancers. Int J Oncol. 45:2065–2075. 2014.PubMed/NCBI
|
|
2
|
Xing Y, Luo DY, Long MY, Zeng SL and Li
HH: High ALDH1A1 expression correlates with poor survival in
papillary thyroid carcinoma. World J Surg Oncol. 12:292014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tang C, Yang L, Wang N, Li L, Xu M, Chen
GG and Liu ZM: High expression of GPER1, EGFR and CXCR1 is
associated with lymph node metastasis in papillary thyroid
carcinoma. Int J Clin Exp Pathol. 7:3213–3223. 2014.PubMed/NCBI
|
|
4
|
Minna E, Romeo P, De Cecco L, Dugo M,
Cassinelli G, Pilotti S, Degl'Innocenti D, Lanzi C, Casalini P,
Pierotti MA, et al: miR-199a-3p displays tumor suppressor functions
in papillary thyroid carcinoma. Oncotarget. 5:2513–2528.
2014.PubMed/NCBI
|
|
5
|
Polyak K, Xia Y, Zweier JL, Kinzler KW and
Vogelstein B: A model for p53-induced apoptosis. Nature.
389:300–305. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Contente A, Dittmer A, Koch MC, Roth J and
Dobbelstein M: A polymorphic microsatellite that mediates induction
of PIG3 by p53. Nat Genet. 30:315–320. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Porté S, Valencia E, Yakovtseva EA, Borràs
E, Shafqat N, Debreczeny JE, Pike AC, Oppermann U, Farrés J, Fita
I, et al: Three-dimensional structure and enzymatic function of
proapoptotic human p53-inducible quinone oxidoreductase PIG3. J
Biol Chem. 284:17194–17205. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee JH, Kang Y, Khare V, Jin ZY, Kang MY,
Yoon Y, Hyun JW, Chung MH, Cho SI, Jun JY, et al: The p53-inducible
gene 3 (PIG3) contributes to early cellular response to DNA damage.
Oncogene. 29:1431–1450. 2010. View Article : Google Scholar
|
|
9
|
Gorgoulis VG, Liloglou T, Sigala F,
Korkolis D, Yannoukakos D, Papalambros E, Asimacopoulos PJ,
Papavassiliou AG and Kotsinas A: Absence of association with cancer
risk and low frequency of alterations at a p53 responsive PIG3 gene
polymorphism in breast and lung carcinomas. Mutat Res. 556:143–150.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ito M, Nishiyama H, Watanabe J, Kawanishi
H, Takahashi T, Kamoto T, Habuchi T and Ogawa O: Association of the
PIG3 promoter polymorphism with invasive bladder cancer in a
Japanese population. Jpn J Clin Oncol. 36:116–120. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guan X, Liu Z, Wang L, Wang LE, Sturgis EM
and Wei Q: Functional repeats (TGYCC)n in the p53-inducible gene 3
(PIG3) promoter and susceptibility to squamous cell carcinoma of
the head and neck. Carcinogenesis. 34:812–817. 2013. View Article : Google Scholar :
|
|
12
|
Kotsinas A, Pateras IS, Galanos PS,
Karamouzis MV, Sfikakis PP and Gorgoulis VG: Why is p53-inducible
gene 3 rarely affected in cancer? Oncogene. 29:52202010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li B, Shang ZF, Yin JJ, Xu QZ, Liu XD,
Wang Y, Zhang SM, Guan H and Zhou PK: PIG3 functions in DNA damage
response through regulating DNA-PKcs homeostasis. Int J biol Sci.
9:425–434. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xie P, Tian C, An L, Nie J, Lu K, Xing G,
Zhang L and He F: Histone methyltransferase protein SETD2 interacts
with p53 and selectively regulates its downstream genes. Cell
Signal. 20:1671–1678. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kryston TB, Georgiev AB, Pissis P and
Georgakilas AG: Role of oxidative stress and DNA damage in human
carcinogenesis. Mutat Res. 711:193–201. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Moreli JB, Santos JH, Rocha CR, Damasceno
DC, Morceli G, Rudge MV, Bevilacqua E and Calderon IM: DNA damage
and its cellular response in mother and fetus exposed to
hyperglycemic environment. Biomed Res Int. 2014:6767582014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Halazonetis TD, Gorgoulis VG and Bartek J:
An oncogene-induced DNA damage model for cancer development.
Science. 319:1352–1355. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chambers JT, Carcangiu ML, Voynick IM and
Schwartz PE: Immunohistochemical evaluation of estrogen and
progesterone receptor content in 183 patients with endometrial
carcinoma. Part II: Correlation between biochemical and
immunohistochemical methods and survival. Am J Clin Pathol.
94:255–260. 1990.PubMed/NCBI
|
|
19
|
Flatt PM, Polyak K, Tang LJ, Scatena CD,
Westfall MD, Rubinstein LA, Yu J, Kinzler KW, Vogelstein B, Hill
DE, et al: p53-dependent expression of PIG3 during proliferation,
genotoxic stress, and reversible growth arrest. Cancer Lett.
156:63–72. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ivanova D, Bakalova R, Lazarova D, Gadjeva
V and Zhelev Z: The impact of reactive oxygen species on anticancer
therapeutic strategies. Adv Clin Exp Med. 22:899–908. 2013.
|
|
22
|
Kotsinas A, Aggarwal V, Tan EJ, Levy B and
Gorgoulis VG: PIG3: A novel link between oxidative stress and DNA
damage response in cancer. Cancer Lett. 327:97–102. 2012.
View Article : Google Scholar
|
|
23
|
Liu R, Liu D, Trink E, Bojdani E, Ning G
and Xing M: The Akt-specific inhibitor MK2206 selectively inhibits
thyroid cancer cells harboring mutations that can activate the
PI3K/Akt pathway. J Clin Endocrinol Metab. 96:E577–E585. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lim HJ, Crowe P and Yang JL: Current
clinical regulation of PI3K/PTEN/Akt/mTOR signalling in treatment
of human cancer. J Cancer Res Clin Oncol. 141:671–689. 2015.
View Article : Google Scholar
|
|
25
|
Korbecki J, Baranowska-Bosiacka I,
Gutowska I and Chlubek D: The effect of reactive oxygen species on
the synthesis of prostanoids from arachidonic acid. J Physiol
Pharmacol. 64:409–421. 2013.PubMed/NCBI
|
|
26
|
Kumar B, Koul S, Khandrika L, Meacham RB
and Koul HK: Oxidative stress is inherent in prostate cancer cells
and is required for aggressive phenotype. Cancer Res. 68:1777–1785.
2008. View Article : Google Scholar : PubMed/NCBI
|