Introduction
Lung cancer is the leading cause of global
cancer-related mortality, resulting in over a million deaths
annually (1). Surgery, chemotherapy
and radiotherapy are the major treatments for human non-small cell
lung cancer (NSCLC). However, the response rate of chemotherapy for
patients with advanced NSCLC is only 20–35% with a median survival
of 10–12 months (2,3). Although light has been shed on the
genomic mutation of human lung cancer (4,5), the
mechanisms involved in the proliferation and migration of lung
cancer remain to be elucidated.
Aquaporins (AQPs) are a family of small (~30
kDa/monomer) membrane transport proteins that assemble in membranes
as tetramers and act primarily as water-selective pores,
facilitating osmotically driven water transport across cell plasma
membranes (6,7). It has been reported that AQP was
expressed in a variety of human tumors, which in some cases was
correlated with tumor grade (8–10). AQP
has also been suggested to be of diagnostic and prognostic value
(11,12). Previous findings demonstrated that
AQPs were involved in angiogenesis, tumor cell migration and
proliferation (13–15). These results suggested that AQPs
play a crucial role in tumor biology.
AQP1 is a member of the AQP family, and has been
shown to be involved in cell migration in several cell types such
as keratocytes (16), epithelial
cells of the kidney (13), and
gastric epithelial cells (17),
B16F10 melanoma, and breast cancer cells (18). However, the function of AQP1 in lung
cancer remains largely unclear. Whether AQP1 facilitates
proliferation and the metastastic potential of lung cancer remains
to be determined. In the present study, we successfully used RNA
interference (RNAi) technology to silence the expression of AQP1 in
lung adenocarcinoma LTEP-A2 and LLC cell lines. The in vitro
experiments demonstrated that the proliferation and migration of
cancer cells was reduced in AQP1-siRNA-transfected LTEP-A2 and LLC
cells. In addition, AQP1 knockdown partly inhibited the
proliferation and migration of lung cancer cells in MMP-2 and
MMP-9-dependent manner.
Materials and methods
Cell culture
LLC and LTEP-A2 cell lines were cultured in
RPMI-1640 supplemented with 10% Hyclone fetal bovine serum (FBS;
ThermoFisher Scientific, Fremont, CA, USA) in an atmosphere of 5%
CO2 at 37°C. The cells were grown in 75-cm2
culture flasks and harvested in a solution of trypsin-EDTA at the
logarithmic growth phase.
Specific siRNAs and transfection
One scrambled non-targeting siRNA (used for a
negative control, mock) and one fluorescent siRNA were designed and
produced by GenePharma Co., Ltd. (Shanghai, China). The sequences
used were: SiAQP1, 5′-GACCCGCTCGGACTTACT-3′ (sense) and
5′-CTTCTGGACCCATGCTCT-3′ (antisense); mock,
5′-UUCUCCGAACGUGUCACGUTT-3′ (sense) and 5′-ACGUGACACGUUCGGAGAATT-3′
(antisense). The 25-, 50- and 100-nM siRNAs were transfected into
culture cells with Lipofectamine 2000 reagent (Invitrogen-life
Technologies, Carlsbad, CA, USA) according to the manufacturer's
instructions. The cells were collected 24, 48, 72 or 96 h after
transfection for analysis. LTEP-A2 and LLC cells treated or
untreated only with Lipo fectamine 2000 reagent served as
controls.
Reverse transcription-PCR
Total RNA was isolated from transfected cells using
TRIzol reagent (Invitrogen-Life Technologies) according to the
manufacturer's instructions. Briefly, 1 µg total cell RNA
was reverse-transcribed by a First Strand cDNA Synthesis kit
(Amersham, Buckinghamshire, UK). Primers used for PCR amplification
of AQP1 were: forward, 5′-CTT ACC TCC AGG ACC CTT CC-3′ and
reverse, 5′-TAG CTC ATC CAC ACG TGC TC-3′, with an annealing
temperature of 60°C. The conditions used for PCR were: 94°C for 30
sec, 58°C for 30 sec, 72°C for 40 sec; 30 cycles and 72°C for 5 min
for the final extension. The PCR products were separated on 1%
agarose gel, visualized under UV and photographed. The result was
analyzed using Quantity One 4.6.2 software for the optical density
(OD).
Cell proliferation assay
Cell proliferative activities were examined using
CCK-8. LTEP-A2 and LLC cells were seeded in 96-well plates
(1×103 cells/well). Following treatment, CCK-8 was added
to each well according to the manufacturer's instructions and
incubated for 4 h at 37°C. The OD value of each well was measured
using a microplate reader (Spectra Thermo, Mannedorf, Switzerland)
with a test wavelength of 450 nm.
Western blot analysis
Total protein was extracted from the cells using a
RIPA kit (Pierce, Rockford, IL, USA). Protein was electrophoresed
on a polyacrylamide gel and transferred to Hybond-C nitrocellulose
membranes. The membranes were incubated with anti-AQP1 (Chemicon,
Temecula, CA, USA) and MMP-2, MMP-9, TGF-β and epidermal growth
factor receptor (EGFR) (all from Cell Signaling Technology) at
1:1,000 dilution at 37°C for 2 h, and then with
peroxidase-conjugated goat anti-rabbit IgG at room temperature for
1 h. GAPDH was used as an internal control. Protein was visualized
using enhanced chemiluminescence (ECL) methods. The membranes were
washed three times and then exposed to X-ray film. Western blot
analysis and quantification analysis of blots were performed as
previously described (19).
Immunofluorescence
The cells were fixed in 4% paraformaldehyde and
embedded in paraffin or OCT for paraffin or frozen sections,
respectively. Immunofluorescence (IF) staining of cells was
performed as previously described (18). Anti-AQP1 primary antibody (1:100;
Chemicon) and rabbit anti-mouse IgG antibody conjugated to Cy3
(1:100; Molecular Probes, Eugene, OR, USA) were used for IF
staining.
Tumor cell migration and invasion
assays
Assays to measure tumor cell migration were
performed in a modified Boyden chamber (Transwell; Corning Costar,
Tewksbury, MA, USA) containing a gelatin-coated polycarbonate
membrane filter (8-µm pore size). BioCoat Matrigel (BD
Biosciences, Bedford, MA, USA), which reconstitutes the basal
membrane, was used to assess cell invasion. The degree of tumor
cell migration and invasion was evaluated according to the
manufacturer's instructions (25).
Non-migrated cells were removed from the upper side of the membrane
by scrubbing and migrated cells from the lower side of the
membrane. Cell counting was accomplished by Coomassie blue staining
and the cells were visualized under a microscope (Leica,
Germany).
Wound-healing assay
Cells were cultured to a confluent monolayer,
synchronized in 1% FBS for 24 h, and wounded by removing
~400-µm wide strips of cells across the well with a standard
200-µl pipette tip. Wounded monolayers were washed twice
with PBS to remove non-adherent cells. Wound healing was quantified
by Image Plus software as a mean percentage of the remaining
cell-free area, compared with the initial wound area (18).
Statistical analysis
Statistical analysis was performed using SPSS 10.0
software. Values are presented as mean ± SD. Statistical analysis
was performed using one-way analysis of variance (ANOVA) and the
LSD post-hoc multiple comparison test. P-values were two-sided and
differences at P<0.05 were considered to indicate statistical
significance.
Results
Localization of AQP1 in LTEP-A2 and LLC
cells
The AQP1 expression and localization of LTEP-A2 and
LLC cells was investigated by immunofluorescence microscopy and
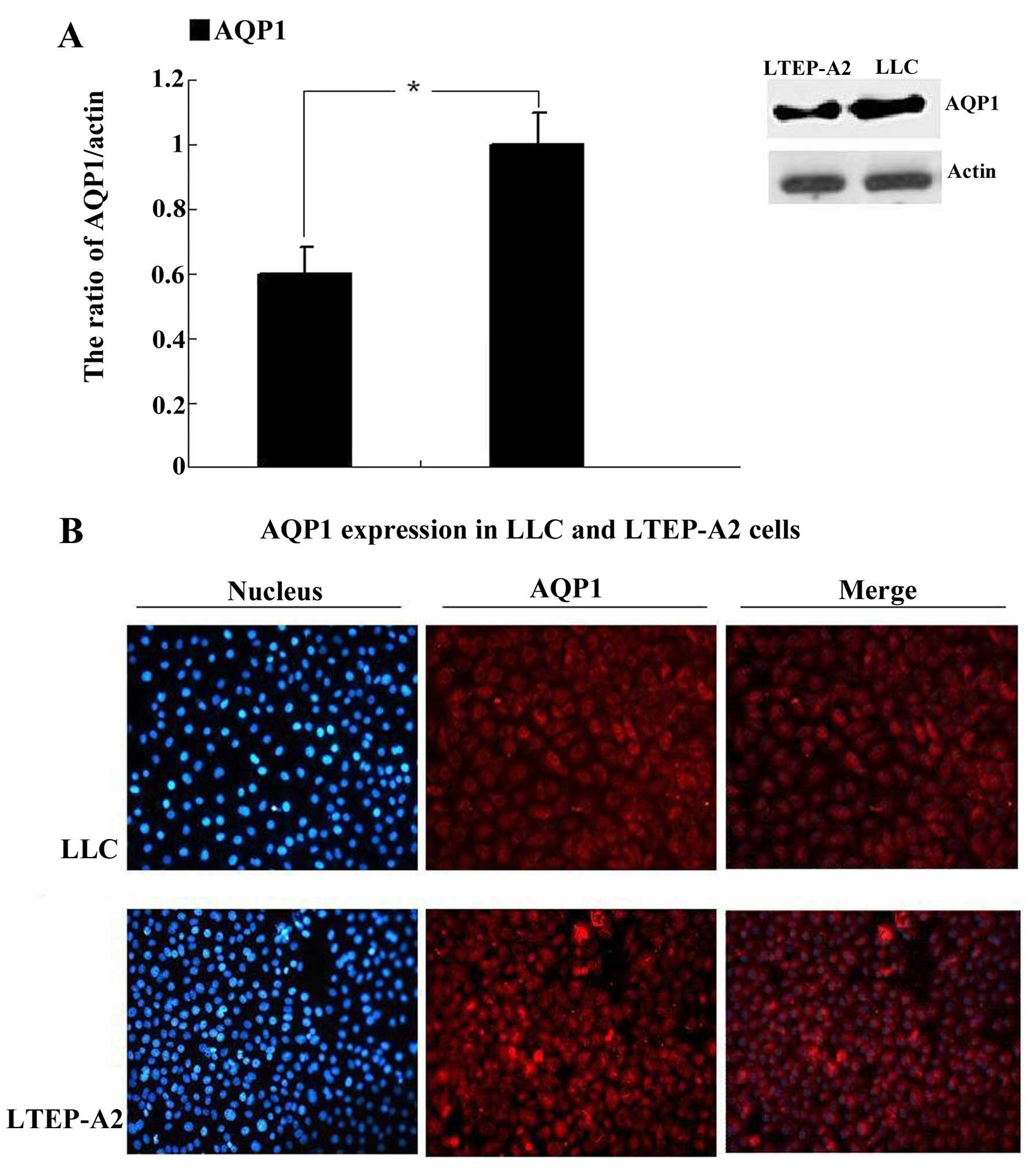
western blot analysis. Western blot analysis revealed that AQP1 was
expressed in LLC and LTEP-A2 cell lines (Fig. 1A). Compared to the LTEP-A2 cell
line, the expression of AQP1 was more prominent in the LLC cell
line. Fig. 1B shows that AQP1 (red)
was detected in the cytosol and membrane in the two cell lines.
DAPI was used to stain the nucleus.
Effective downregulation of AQP1 using
siRNA in LTEP-A2 and LLC cells
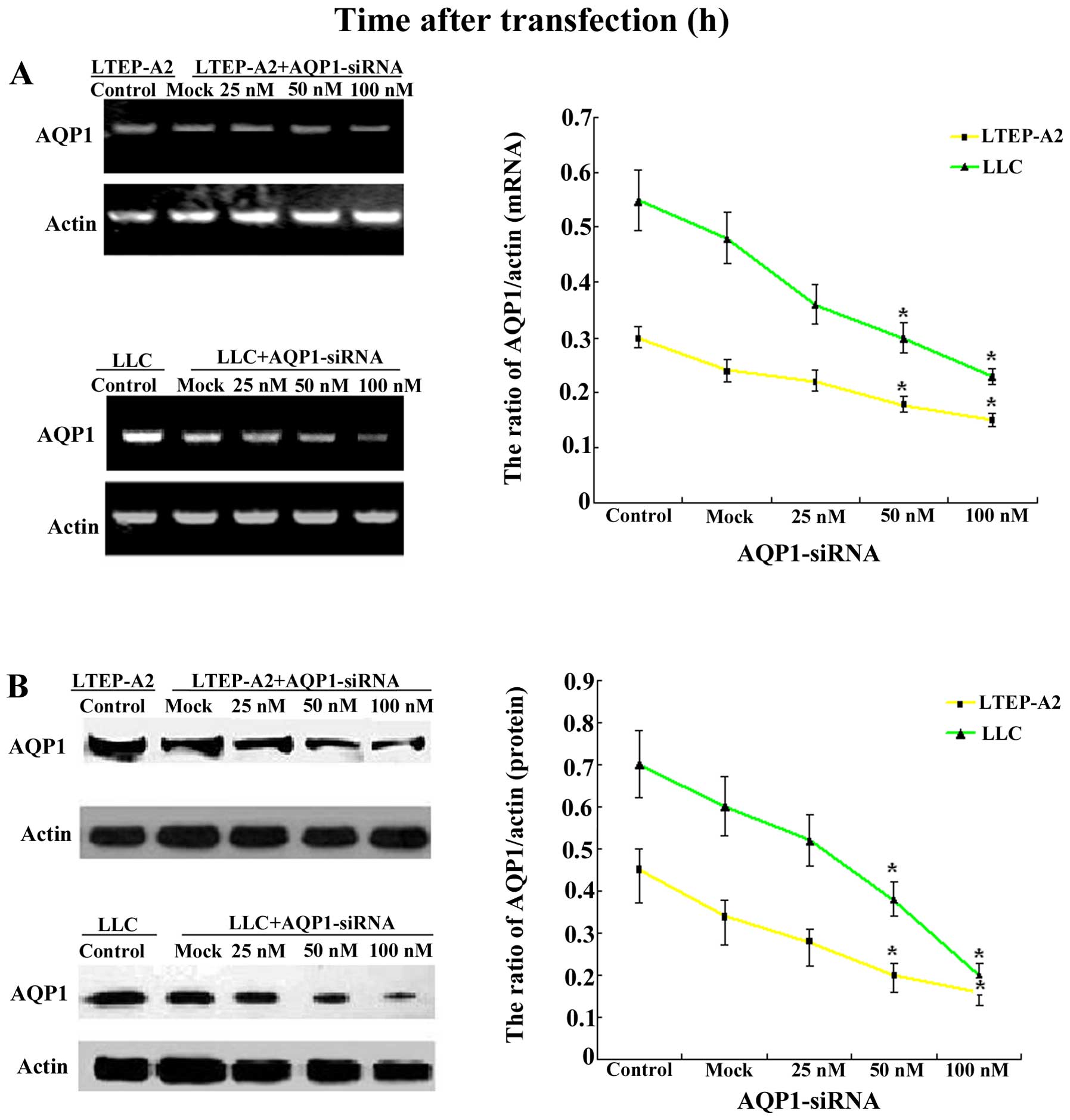
LTEP-A2 and LLC cells were transfected with
AQP1-siRNA and scrambled control siRNA. Treatment of the cells with
AQP1-targeting siRNA (25, 50 and 100 nM SiAQP1) and the scrambled
siRNA (mock, 100 nM) for 96 h resulted in a significant
downregulation of the AQP1 mRNA level and protein expression of
AQP1 at 96 h following transfection with AQP1-siRNA, as determined
by RT-PCR (Fig. 2A) and western
blot analysis (Fig. 2B). As shown
in Fig. 2, the mock did not affect
the expression levels of AQP1. However, compared with the mock, the
expression of AQP1 in the 25, 50 and 100 nM siRNA groups decreased
at the protein and mRNA levels. Specifically, 100 nM siRNA
indicated an 80 and 85% reduction of AQP1 expression in LTEP-A2 and
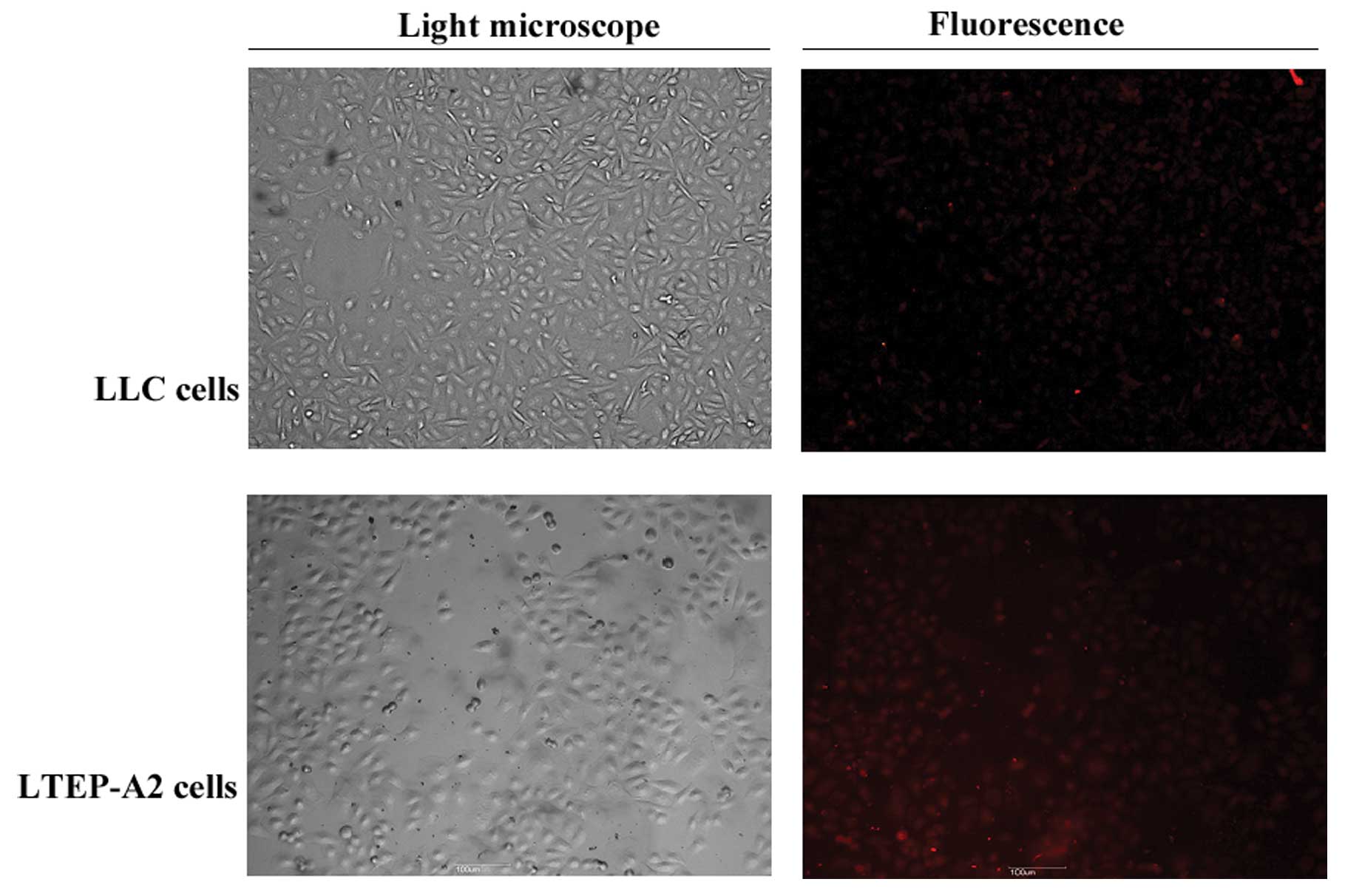
LLC cells, respectively. At the same time, there was minimal
expression of AQP1 (red) in the cells transfected with AQP1-siRNA,
whereas the expression of AQP1 was not altered in the control cells
by immunofluorescence analysis (Fig.
3). The results demonstrated that the AQP1-targeting siRNA was
efficient to knock down the expression of AQP1 in LTEP-A2 and LLC
cells. This downregulation was determined by western blot analysis
using AQP1 antibody and the experiments were repeated three times
(n=3).
Effects of AQP1 downregulation on LTEP-A2
and LLC migration and proliferation
A significant downregulation in the AQP1 mRNA and
protein level occurred at 96 h, and 100 nM siRNA groups exhibited
the largest reduction of AQP1 expression in the LLC and LTEP-A2
cells, compared with the negative control (P<0.01, Fig. 2). siRNA groups (100 nM) were thus
selected to investigate whether a decreased expression of AQP1
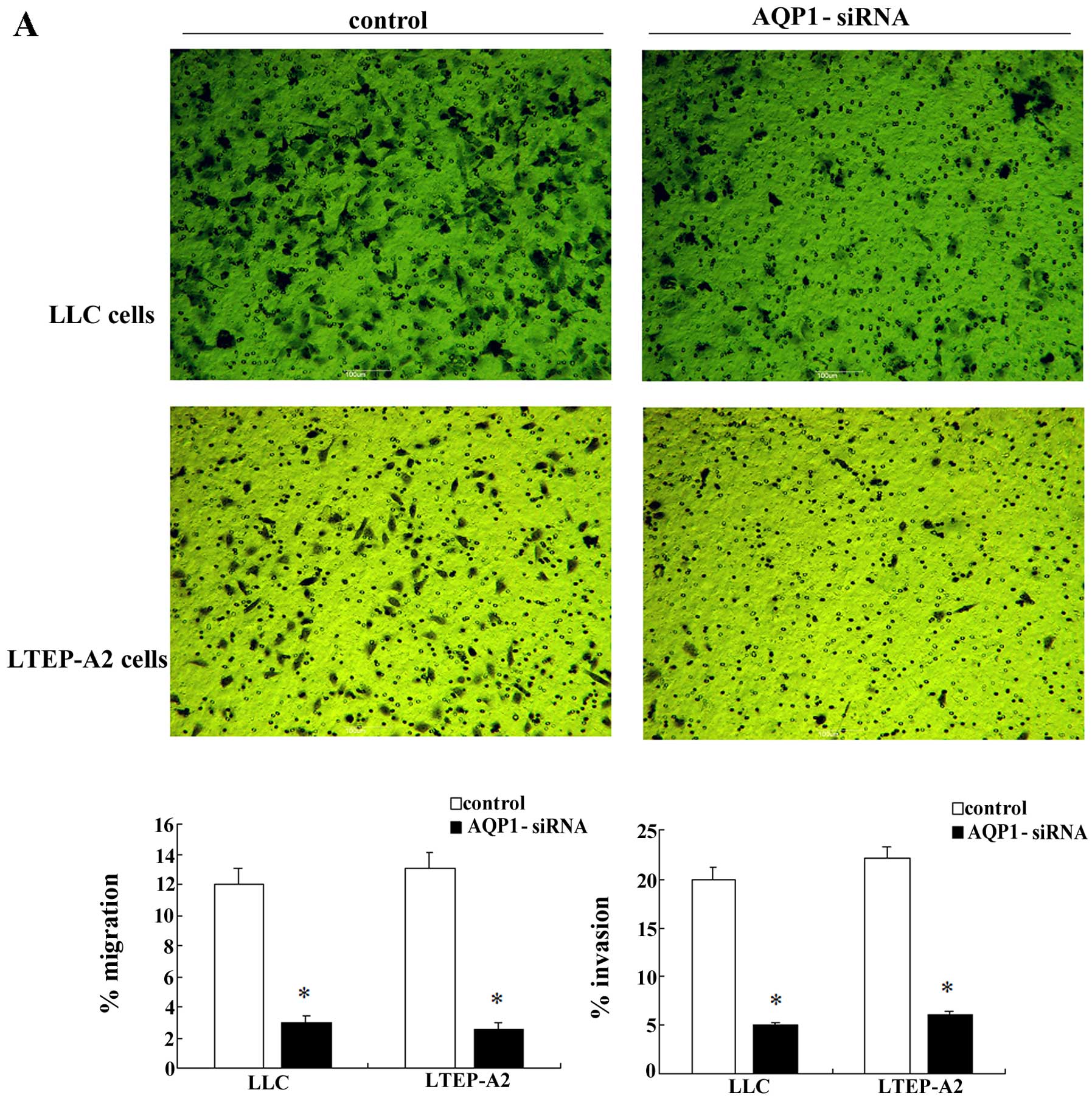
affected cell migration and invasion. Fig. 4A shows that, AQP1 downregulation by
siRNA resulted in decreased cell migration at 6 h and reduced tumor
cell invasion at 24 h. Moreover, the wound-healing assay showed
significantly decreased wound closure in the AQP1 gene-silencing
group vs. the mock group after 24-h scratching (Fig. 4B and C). The assays described above
were performed in the presence of 0.04% mitomycin C, an inhibitor
of cell proliferation, and similar results were obtained. To
determine the effect of AQP1 downregulation on cell proliferation,
a CCK-8 assay was performed. A significant decrease in cell
proliferation was observed following AQP1 downregulation as
determined by the CCK-8 proliferation assay (Fig. 4C). There was a significant decrease
in cell proliferation after 48 and 72 h following transfection with
AQP1-siRNA.
Molecular mechanisms of the antitumor
effects by AQP1-siRNA
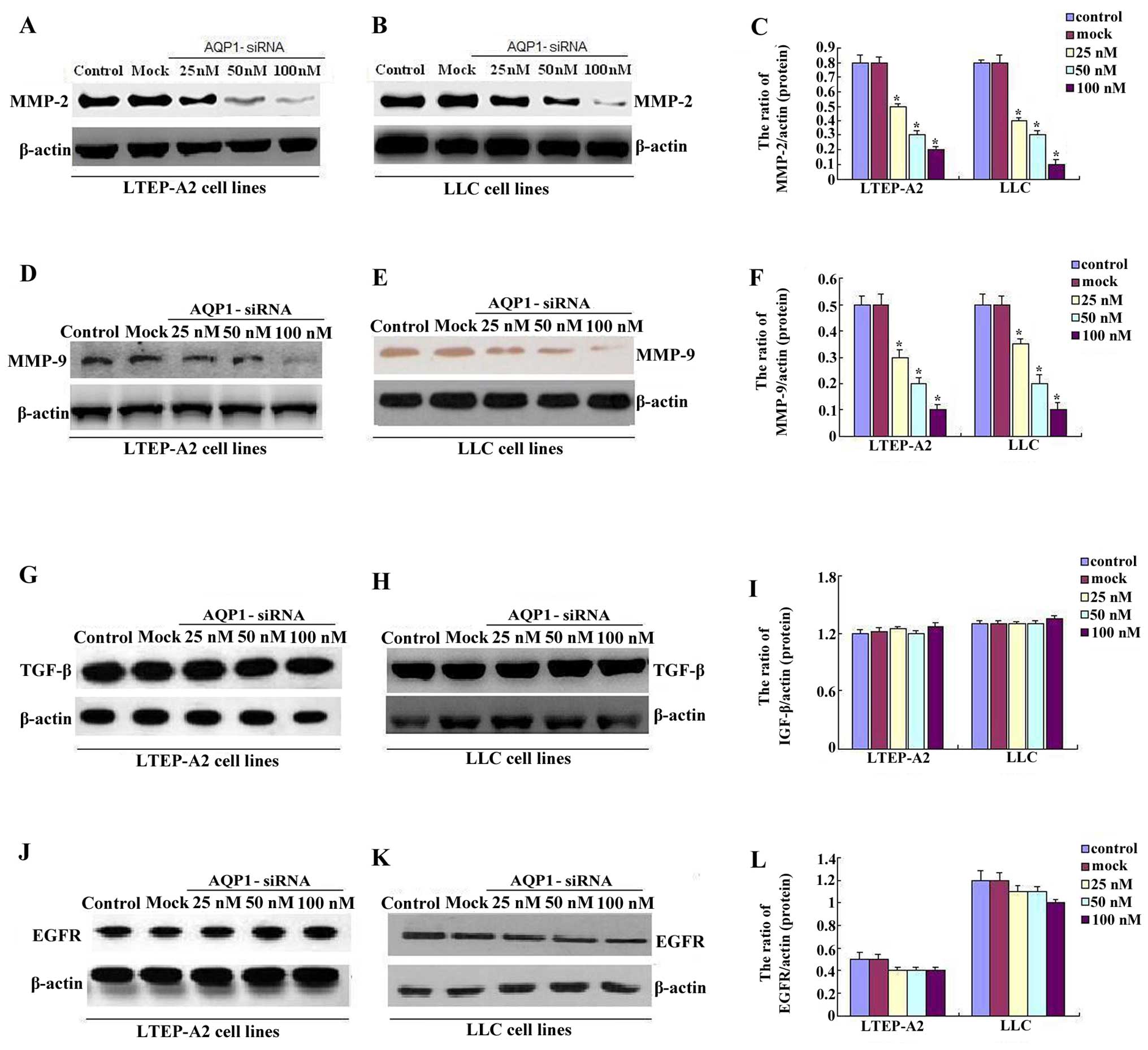
The protein from the transfected cells was extracted
to examine the effects of AQP1-siRNA on certain cytokines and
signaling molecules. After 96 h of transfection and treatment with
high concentrations of 50 and 100 nM siRNA, the protein relative
expression levels of MMP-2/-9 were decreased in LTEP-A2 and LLC
cells, whereas no differences were identified in the control and
mock groups (Fig. 5A–F).
Furthermore, it was found that the higher the doses of siRNA, the
lower the level of MMP-2/-9 expression. The expression of MMP-2/-9
was highest in the 25 nM siRNA group, intermediate in the 50 nM
siRNA group, and lowest in the 100 nM siRNA group. However,
compared to the control and mock groups, transfection with high
concentrations of 50 and 100 nM AQP1-siRNA did not suppress the
expression of TGF-β and EGFR (Fig.
5G–L). These data demonstrated that the knockdown of AQP1 by
siRNA may inhibit MMP-2/-9, which are important in tumor
progression.
Discussion
Despite advances in medical and surgical treatments,
lung cancer remains the worldwide leading cause of cancer mortality
(20). Due to intrinsic properties
of lung adenocarcinoma in which cells show a high ability to
progress rapidly, it has a poor prognosis in main histological
types of lung cancer (21,22). Therefore, how to effectively inhibit
the proliferative and metastatic biological behavior of lung
adenocarcinoma cells is a key issue to be overcome in order to
improve the outcome.
As mentioned earlier, AQP1 has been found in tumors
of different tissues, such as endometrioid adenocarcinoma and colon
and breast cancer cells (23–25).
Additionally, AQP1 expression in these tumor cells has been
correlated with its metastatic potential (26,27).
For example, Hu and Verkman found that AQP1 facilitated tumor cell
migration and extra invasion across blood vessels in B16F10
melanoma cells (18). However, the
roles of AQP1 in oncogenesis and metastasis have not yet to be
clearly defined in lung cancer.
In the present study, we first used LTEP-A2 and LLC
cell lines to investigate the localization of AQP1. Previous
findings on localization of AQP1 have shown that AQP1 is localized
in the cytosol and cell membrane, which is in concordance with
those of a previous study (28).
Our results demonstrate that the localization of AQP1 in the
cytosol and membrane may be cell-specific or a purely in
vitro phenomenon. Furthermore, we have shown that chemically
synthesized siRNAs specifically targeting AQP1 successfully knocked
down the expression of AQP1 at the protein and mRNA levels from 80
to 85% in human lung adenocarcinoma LTEP-A2 cells and mouse lung
adenocarcinoma LLC cells. The assays as described above were used
to detect the effects on the biological behavior of LTEP-A2 and LLC
cells in vitro. Using the CCK-8 assay, we were able to first
show that the proliferation of AQP1-siRNA-transfected lung
adenocarcinoma cells was significantly inhibited in vitro,
although previous studies have failed to show this finding in
colorectal cancer and B16F10 melanoma cells (29–31).
We hypothesized that the main reason for inhibition of
proliferation in lung adenocarcinoma cells by AQP1-siRNA is that
AQP1 has distinct roles in different cell lines and tissues.
The formation of metastases is the leading cause of
mortality in cancer patients. Establishment of metastasis is a
multistep process involving the dissociation of cancer cells from
the primary tumor, invasion of extracellular matrix, angiogenesis,
intravasation into the vasculature or lymphatic systems, survival
at these sites, extravasation and proliferation at a distant site
(32–34). Downregulation of AQP1 has been shown
to be involved in decreased cell migration in several cell types
including human melanoma cell lines and human microvascular
endothelial, kidney epithelial and gastric epithelial cells.
Overexpression of AQP1 has been reported in proliferating tumor
vessels suggesting its involvement in tumor angiogenesis (15). In the present study, the
downregulation of AQP1 in LTEP-A2 and LLC cells by AQP1 gene
silencing showed significantly decreased migration and invasion.
These in vitro studies provide evidence that AQP1 is
important in the development of lung cancer. However, we did not
observe any significant changes in cell size and morphology
following AQP1 downregulation in the LTEP-A2 and LLC cells.
AQP1 has emerged as an important player in
metastasis. However, previous studies do not provide information
regarding the downstream proteins induced by the inhibition of AQP1
expression via AQP1-siRNA in lung cancer or other cancer cells.
Several MMPs are believed to be important in the process of
angiogenesis, especially MMP-2 and MMP-9. These two MMPs are
involved in lung cancer initiation, invasion, angiogenesis and
metastasis (35–37).
TGF-β and EGFR are crucial in many tumors. TGF-β
supports tumor progression by stimulating the transdifferentiation
of epithelial cancer cells into migratory mesenchymal cells
(38,39) by promoting cell invasion, and
dissemination to distant sites (40), and enhancing angiogenesis (41). EGFR is overexpressed in many
epithelial-derived tumors and its role in the migration and
survival of NSCLC is widely documented (42–44).
Since migration and invasion alter following
treatment with AQP1-siRNA in lung cancer, whether changes of MMPs,
TGF-β and EGFR would occur was determined. Our results show that
MMPs decreased after AQP1-siRNA in the two cell lines. It was found
that the higher the doses of AQP1-siRNA, the lower the level of
MMP-2/-9 expression. The expression of MMP-2/-9 was highest in the
25 nM siRNA group, intermediate in the 50 nM siRNA group and lowest
in the 100 nM siRNA group. However, AQP1 knockdown did not have a
significant effect on the TGF-β and EGFR, a finding that was
consistent with results obtained by Zhang et al (45). These data indicate that AQP1 may
produce a marked effect on tumor growth, vascularization and
metastasis in lung adenocarcinoma by inhibiting MMP-2/-9, which
play an important role in tumor progression.
In summary, to the best of our knowledge, we are
first to report that the ability of lung adenocarcinoma LTEP-A2 and
LLC cell line growth and metastasis is reduced after treatment with
AQP1-siRNA. In addition, the expression of MMP protein decreased
after LTEP-A2 and LLC cells were transfected with AQP1-siRNA,
whereas no changes were observed for TGF-β and EGFR. Our finidngs
may therefore provide evidence for genetic therapy for lung
adenocarcinoma.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (no. 81173390/H2902), the National
Basic Science Program of China (no. 2009CB523000) and the Shanghai
Science and Technology Committee (no. 09XD1400700).
References
|
1
|
Sun S, Schiller JH and Gazdar AF: Lung
cancer in never smokers - a different disease. Nat Rev Cancer.
7:778–790. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Spiro SG and Silvestri GA: One hundred
years of lung cancer. Am J Respir Crit Care Med. 172:523–529. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Spiro SG, Tanner NT, Silvestri GA, Janes
SM, Lim E, Vansteenkiste JF and Pirker R: Lung cancer: Progress in
diagnosis, staging and therapy. Respirology. 15:44–50. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cancer Genome Atlas Research Network:
Comprehensive genomic characterization of squamous cell lung
cancers. Nature. 489:519–525. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cancer Genome Atlas Research Network:
Comprehensive molecular profiling of lung adenocarcinoma. Nature.
511:543–550. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Agre P, King LS, Yasui M, Guggino WB,
Ottersen OP, Fujiyoshi Y, Engel A and Nielsen S: Aquaporin water
channels - from atomic structure to clinical medicine. J Physiol.
542:3–16. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Verkman AS and Mitra AK: Structure and
function of aquaporin water channels. Am J Physiol Renal Physiol.
278:F13–F28. 2000.PubMed/NCBI
|
|
8
|
Mobasheri A, Airley R, Hewitt SM and
Marples D: Heterogeneous expression of the aquaporin 1 (AQP1) water
channel in tumors of the prostate, breast, ovary, colon and lung: A
study using high density multiple human tumor tissue microarrays.
Int J Oncol. 26:1149–1158. 2005.PubMed/NCBI
|
|
9
|
Moon C, Soria JC, Jang SJ, Lee J, Obaidul
Hoque M, Sibony M, Trink B, Chang YS, Sidransky D and Mao L:
Involvement of aquaporins in colorectal carcinogenesis. Oncogene.
22:6699–6703. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Saadoun S, Papadopoulos MC, Davies DC,
Krishna S and Bell BA: Aquaporin-4 expression is increased in
oedematous human brain tumours. J Neurol Neurosurg Psychiatry.
72:262–265. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kafé H, Verbavatz JM, Cochand-Priollet B,
Castagnet P and Vieillefond A: Collecting duct carcinoma: An entity
to be redefined? Virchows Arch. 445:637–640. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mazal PR, Stichenwirth M, Koller A, Blach
S, Haitel A and Susani M: Expression of aquaporins and PAX-2
compared to CD10 and cytokeratin 7 in renal neoplasms: A tissue
microarray study. Mod Pathol. 18:535–540. 2005. View Article : Google Scholar
|
|
13
|
Hara-Chikuma M and Verkman AS: Aquaporin-1
facilitates epithelial cell migration in kidney proximal tubule. J
Am Soc Nephrol. 17:39–45. 2006. View Article : Google Scholar
|
|
14
|
Hara-Chikuma M and Verkman AS: Aquaporin-3
facilitates epidermal cell migration and proliferation during wound
healing. J Mol Med (Berl). 86:221–231. 2008. View Article : Google Scholar
|
|
15
|
Saadoun S, Papadopoulos MC, Hara-Chikuma M
and Verkman AS: Impairment of angiogenesis and cell migration by
targeted aquaporin-1 gene disruption. Nature. 434:786–792. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ruiz-Ederra J and Verkman AS:
Aquaporin-1-facilitated keratocyte migration in cell culture and in
vivo corneal wound healing models. Exp Eye Res. 89:159–165. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hayashi S, Takahashi N, Kurata N,
Yamaguchi A, Matsui H, Kato S and Takeuchi K: Involvement of
aquaporin-1 in gastric epithelial cell migration during wound
repair. Biochem Biophys Res Commun. 386:483–487. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hu J and Verkman AS: Increased migration
and metastatic potential of tumor cells expressing aquaporin water
channels. FASEB J. 20:1892–1894. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Barabutis N and Schally AV: Knocking down
gene expression for growth hormone-releasing hormone inhibits
proliferation of human cancer cell lines. Br J Cancer.
98:1790–1796. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Janssen-Heijnen ML and Coebergh JW: The
changing epidemiology of lung cancer in Europe. Lung Cancer.
41:245–258. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Devesa SS, Bray F, Vizcaino AP and Parkin
DM: International lung cancer trends by histologic type: male:
female differences diminishing and adenocarcinoma rates rising. Int
J Cancer. 117:294–299. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jiang Y: Aquaporin-1 activity of plasma
membrane affects HT20 colon cancer cell migration. IUBMB Life.
61:1001–1009. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Otterbach F, Callies R, Adamzik M, Kimmig
R, Siffert W, Schmid KW and Bankfalvi A: Aquaporin 1 (AQP1)
expression is a novel characteristic feature of a particularly
aggressive subgroup of basal-like breast carcinomas. Breast Cancer
Res Treat. 120:67–76. 2010. View Article : Google Scholar
|
|
25
|
Pan H, Sun CC, Zhou CY and Huang HF:
Expression of aquaporin-1 in normal, hyperplasic, and carcinomatous
endometria. Int J Gynaecol Obstet. 101:239–244. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hoque MO, Soria JC, Woo J, Lee T, Lee J,
Jang SJ, Upadhyay S, Trink B, Monitto C, Desmaze C, et al:
Aquaporin 1 is overexpressed in lung cancer and stimulates NIH-3T3
cell proliferation and anchorage-independent growth. Am J Pathol.
168:1345–1353. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Andersen P, Villingshøj M, Poulsen HS and
Stockhausen MT: Improved response by co-targeting EGFR/EGFRvIII and
Src family kinases in human cancer cells. Cancer Invest.
27:178–183. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shankardas J, Patil RV and Vishwanatha JK:
Effect of downregulation of aquaporins in human corneal endothelial
and epithelial cell lines. Mol Vis. 16:1538–1548. 2010.PubMed/NCBI
|
|
29
|
Yu JL, May L, Lhotak V, Shahrzad S,
Shirasawa S, Weitz JI, Coomber BL, Mackman N and Rak JW: Oncogenic
events regulate tissue factor expression in colorectal cancer
cells: Implications for tumor progression and angiogenesis. Blood.
105:1734–1741. 2005. View Article : Google Scholar
|
|
30
|
Amarzguioui M, Peng Q, Wiiger MT, Vasovic
V, Babaie E, Holen T, Nesland JM and Prydz H: Ex vivo and in vivo
delivery of anti-tissue factor short interfering RNA inhibits mouse
pulmonary metastasis of B16 melanoma cells. Clin Cancer Res.
12:4055–4061. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang X, Wang M, Amarzguioui M, Liu F,
Fodstad Ø and Prydz H: Downregulation of tissue factor by RNA
interference in human melanoma LOX-L cells reduces pulmonary
metastasis in nude mice. Int J Cancer. 112:994–1002. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Steeg PS: Tumor metastasis: Mechanistic
insights and clinical challenges. Nat Med. 12:895–904. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chambers AF, Groom AC and MacDonald IC:
Dissemination and growth of cancer cells in metastatic sites. Nat
Rev Cancer. 2:563–572. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nguyen DX, Bos PD and Massagué J:
Metastasis: From dissemination to organ-specific colonization. Nat
Rev Cancer. 9:274–284. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yu C, Pan K, Xing D, Liang G, Tan W, Zhang
L and Lin D: Correlation between a single nucleotide polymorphism
in the matrix metalloproteinase-2 promoter and risk of lung cancer.
Cancer Res. 62:6430–6433. 2002.PubMed/NCBI
|
|
36
|
Chetty C, Lakka SS, Bhoopathi P and Rao
JS: MMP-2 alters VEGF expression via alphaVbeta3 integrin-mediated
PI3K/AKT signaling in A549 lung cancer cells. Int J Cancer.
127:1081–1095. 2010. View Article : Google Scholar :
|
|
37
|
Rojiani MV, Alidina J, Esposito N and
Rojiani AM: Expression of MMP-2 correlates with increased
angiogenesis in CNS metastasis of lung carcinoma. Int J Clin Exp
Pathol. 3:775–781. 2010.PubMed/NCBI
|
|
38
|
Xu J, Lamouille S and Derynck R:
TGF-beta-induced epithelial to mesenchymal transition. Cell Res.
19:156–172. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Miyazono K: Transforming growth
factor-beta signaling in epithelial-mesenchymal transition and
progression of cancer. Proc Jpn Acad Ser B Phys Biol Sci.
85:314–323. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Massagué J: TGFbeta in cancer. Cell.
134:215–230. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ten Dijke P, Goumans MJ and Pardali E:
Endoglin in angiogenesis and vascular diseases. Angiogenesis.
11:79–89. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Scagliotti GV, Selvaggi G, Novello S and
Hirsch FR: The biology of epidermal growth factor receptor in lung
cancer. Clin Cancer Res. 10:4227s–4232s. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Nicholson RI, Gee JM and Harper ME: EGFR
and cancer prognosis. Eur J Cancer. 37(Suppl 4): S9–S15. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mendelsohn J and Baselga J: The EGF
receptor family as targets for cancer therapy. Oncogene.
19:6550–6565. 2000. View Article : Google Scholar
|
|
45
|
Zhang Z, Chen Z, Song Y, Zhang P, Hu J and
Bai C: Expression of aquaporin 5 increases proliferation and
metastasis potential of lung cancer. J Pathol. 221:210–220. 2010.
View Article : Google Scholar : PubMed/NCBI
|