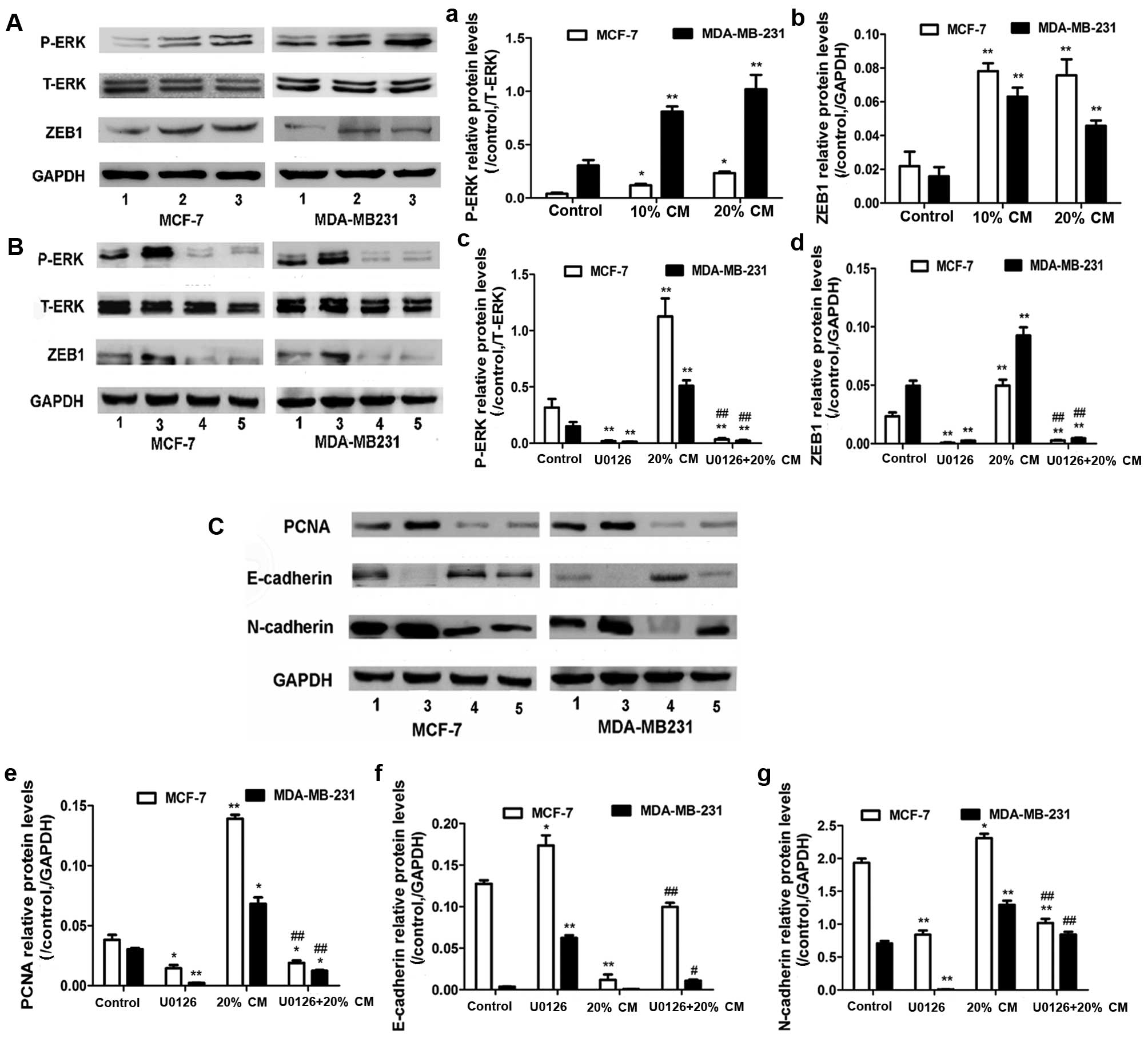
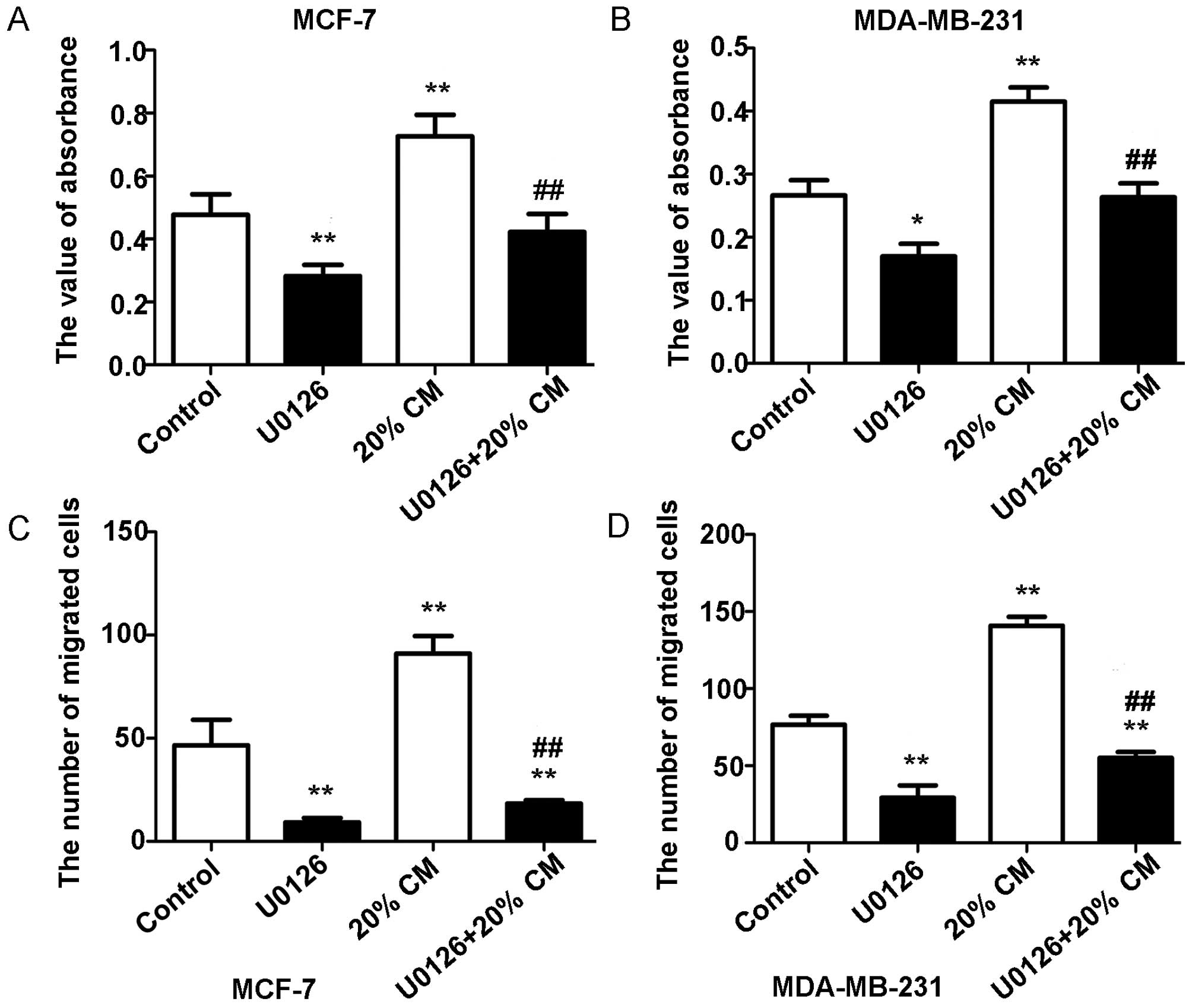
|
1
|
Nelson HD, Zakher B, Cantor A, Fu R,
Griffin J, O'Meara ES, Buist DS, Kerlikowske K, van Ravesteyn NT,
Trentham-Dietz A, et al: Risk factors for breast cancer for women
aged 40 to 49 years: A systematic review and meta-analysis. Ann
intern Med. 156:635–648. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hugosson J, Stranne J and Carlsson SV:
Radical retropubic prostatectomy: A review of outcomes and
side-effects. Acta Oncol. 50(Suppl 1): 92–97. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
De Ruysscher D, Van Meerbeeck J,
Vandecasteele K, Oberije C, Pijls M, Dingemans AM, Reymen B, van
Baardwijk A, Wanders R, Lammering G, et al: Radiation-induced
oesophagitis in lung cancer patients. Is susceptibility for
neutropenia a risk factor? Strahlenther Onkol. 188:564–567. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mbeunkui F and Johann DJ Jr: Cancer and
the tumor microenvironment: A review of an essential relationship.
Cancer Chemother Pharmacol. 63:571–582. 2009. View Article : Google Scholar
|
|
6
|
Orimo A, Gupta PB, Sgroi DC,
Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL
and Weinberg RA: Stromal fibroblasts present in invasive human
breast carcinomas promote tumor growth and angiogenesis through
elevated SDF-1/CxCL12 secretion. Cell. 121:335–348. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Karnoub AE, Dash AB, Vo AP, Sullivan A,
Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R and Weinberg
RA: Mesenchymal stem cells within tumour stroma promote breast
cancer metastasis. Nature. 449:557–563. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Whiteside TL: The tumor microenvironment
and its role in promoting tumor growth. Oncogene. 27:5904–5912.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shinagawa K, Kitadai Y, Tanaka M, Sumida
T, Kodama M, Higashi Y, Tanaka S, Yasui W and Chayama K:
Mesenchymal stem cells enhance growth and metastasis of colon
cancer. Int J Cancer. 127:2323–2333. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yu JM, Jun ES, Bae YC and Jung JS:
Mesenchymal stem cells derived from human adipose tissues favor
tumor cell growth in vivo. Stem Cells Dev. 17:463–473. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Khakoo AY, Pati S, Anderson SA, Reid W,
Elshal MF, Rovira II, Nguyen AT, Malide D, Combs CA, Hall G, et al:
Human mesenchymal stem cells exert potent antitumorigenic effects
in a model of Kaposi's sarcoma. J Exp Med. 203:1235–1247. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee JK, Park SR, Jung BK, Jeon YK, Lee YS,
Kim MK, Kim YG, Jang JY and Kim CW: Exosomes derived from
mesenchymal stem cells suppress angiogenesis by down-regulating
VEGF expression in breast cancer cells. PLoS One. 8:e842562013.
View Article : Google Scholar
|
|
13
|
Gauthaman K, Yee FC, Cheyyatraivendran S,
Biswas A, Choolani M and Bongso A: Human umbilical cord Wharton's
jelly stem cell (hWJSC) extracts inhibit cancer cell growth in
vitro. J Cell Biochem. 113:2027–2039. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rao MS and Mattson MP: Stem cells and
aging: Expanding the possibilities. Mech Ageing Dev. 122:713–734.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Secco M, Zucconi E, Vieira NM, Fogaça LL,
Cerqueira A, Carvalho MD, Jazedje T, Okamoto OK, Muotri AR and Zatz
M: Multipotent stem cells from umbilical cord: Cord is richer than
blood! Stem Cells. 26:146–150. 2008. View Article : Google Scholar
|
|
16
|
Baksh D, Yao R and Tuan RS: Comparison of
proliferative and multilineage differentiation potential of human
mesenchymal stem cells derived from umbilical cord and bone marrow.
Stem Cells. 25:1384–1392. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ayuzawa R, Doi C, Rachakatla RS, Pyle MM,
Maurya DK, Troyer D and Tamura M: Naïve human umbilical cord matrix
derived stem cells significantly attenuate growth of human breast
cancer cells in vitro and in vivo. Cancer Lett. 280:31–37. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu WT, Bian ZY, Fan QM, Li G and Tang TT:
Human mesenchymal stem cells (hMSCs) target osteosarcoma and
promote its growth and pulmonary metastasis. Cancer Lett.
281:32–41. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rachakatla RS, Pyle MM, Ayuzawa R, Edwards
SM, Marini FC, Weiss ML, Tamura M and Troyer D: Combination
treatment of human umbilical cord matrix stem cell-based
interferon-beta gene therapy and 5-fluorouracil significantly
reduces growth of metastatic human breast cancer in SCID mouse
lungs. Cancer Invest. 26:662–670. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ganta C, Chiyo D, Ayuzawa R, Rachakatla R,
Pyle M, Andrews G, Weiss M, Tamura M and Troyer D: Rat umbilical
cord stem cells completely abolish rat mammary carcinomas with no
evidence of metastasis or recurrence 100 days post-tumor cell
inoculation. Cancer Res. 69:1815–1820. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Moodley Y, Atienza D, Manuelpillai U,
Samuel CS, Tchongue J, Ilancheran S, Boyd R and Trounson A: Human
umbilical cord mesenchymal stem cells reduce fibrosis of
bleomycin-induced lung injury. Am J Pathol. 175:303–313. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Caldas-Lopes E, Cerchietti L, Ahn JH,
Clement CC, Robles AI, Rodina A, Moulick K, Taldone T, Gozman A,
Guo Y, et al: Hsp90 inhibitor PU-H71, a multimodal inhibitor of
malignancy, induces complete responses in triple-negative breast
cancer models. Proc Natl Acad Sci USA. 106:8368–8373. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Soule HD, Vazguez J, Long A, Albert S and
Brennan M: A human cell line from a pleural effusion derived from a
breast carcinoma. J Natl Cancer Inst. 51:1409–1416. 1973.PubMed/NCBI
|
|
24
|
Lau MT, So WK and Leung PC: Fibroblast
growth factor 2 induces E-cadherin down-regulation via
PI3K/Akt/mTOR and MAPK/ERK signaling in ovarian cancer cells. PLoS
One. 8:e590832013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Graham TR, Zhau HE, Odero-Marah VA,
Osunkoya AO, Kimbro KS, Tighiouart M, Liu T, Simons JW and O'Regan
RM: Insulin-like growth factor-I-dependent up-regulation of ZEB1
drives epithelial-to-mesenchymal transition in human prostate
cancer cells. Cancer Res. 68:2479–2488. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Qiao C, Xu W, Zhu W, Hu J, Qian H, Yin Q,
Jiang R, Yan Y, Mao F and Yang H: Human mesenchymal stem cells
isolated from the umbilical cord. Cell Biol Int. 32:8–15. 2008.
View Article : Google Scholar
|
|
27
|
Fong CY, Richards M, Manasi N, Biswas A
and Bongso A: Comparative growth behaviour and characterization of
stem cells from human Wharton's jelly. Reprod Biomed Online.
15:708–718. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhu W, Huang L, Li Y, Qian H, Shan X, Yan
Y, Mao F, Wu X and Xu WR: Mesenchymal stem cell-secreted soluble
signaling molecules potentiate tumor growth. Cell Cycle.
10:3198–3207. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang T, Lee YW, Rui YF, Cheng TY, Jiang
XH and Li G: Bone marrow-derived mesenchymal stem cells promote
growth and angiogenesis of breast and prostate tumors. Stem Cell
Res Ther. 4:702013. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ke CC, Liu RS, Suetsugu A, Kimura H, Ho
JH, Lee OK and Hoffman RM: In vivo fluorescence imaging reveals the
promotion of mammary tumorigenesis by mesenchymal stromal cells.
PLoS One. 8:e696582013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yan XL, Fu CJ, Chen L, Qin JH, Zeng Q,
Yuan HF, Nan X, Chen HX, Zhou JN, Lin YL, et al: Mesenchymal stem
cells from primary breast cancer tissue promote cancer
proliferation and enhance mammosphere formation partially via
EGF/EGFR/Akt pathway. Breast Cancer Res Treat. 132:153–164. 2012.
View Article : Google Scholar
|
|
32
|
Nollet F, Berx G and van Roy F: The role
of the E-cadherin/catenin adhesion complex in the development and
progression of cancer. Mol Cell Biol Res Commun. 2:77–85. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gottardi CJ, Wong E and Gumbiner BM:
E-cadherin suppresses cellular transformation by inhibiting
beta-catenin signaling in an adhesion-independent manner. J Cell
Biol. 153:1049–1060. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yanagisawa M and Anastasiadis PZ: p120
catenin is essential for mesenchymal cadherin-mediated regulation
of cell motility and invasiveness. J Cell Biol. 174:1087–1096.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fierro FA, Sierralta WD, Epuñan MJ and
Minguell JJ: Marrow-derived mesenchymal stem cells: Role in
epithelial tumor cell determination. Clin Exp Metastasis.
21:313–319. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Martin FT, Dwyer RM, Kelly J, Khan S,
Murphy JM, Curran C, Miller N, Hennessy E, Dockery P, Barry FP, et
al: Potential role of mesenchymal stem cells (MSCs) in the breast
tumour microenvironment: Stimulation of epithelial to mesenchymal
transition (EMT). Breast Cancer Res Treat. 124:317–326. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bolós V, Peinado H, Pérez-Moreno MA, Fraga
MF, Esteller M and Cano A: The transcription factor Slug represses
E-cadherin expression and induces epithelial to mesenchymal
transitions: A comparison with Snail and E47 repressors. J Cell
Sci. 116:499–511. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Peinado H, Olmeda D and Cano A: Snail, Zeb
and bHLH factors in tumour progression: An alliance against the
epithelial phenotype? Nat Rev Cancer. 7:415–428. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Strzalka W and Ziemienowicz A:
Proliferating cell nuclear antigen (PCNA): A key factor in DNA
replication and cell cycle regulation. Ann Bot (Lond).
107:1127–1140. 2011. View Article : Google Scholar
|
|
40
|
Sun B, Yu KR, Bhandari DR, Jung JW, Kang
SK and Kang KS: Human umbilical cord blood mesenchymal stem
cell-derived extracellular matrix prohibits metastatic cancer cell
MDA-MB-231 proliferation. Cancer Lett. 296:178–185. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ma Y, Hao X, Zhang S and Zhang J: The in
vitro and in vivo effects of human umbilical cord mesenchymal stem
cells on the growth of breast cancer cells. Breast Cancer Res
Treat. 133:473–485. 2012. View Article : Google Scholar
|