Introduction
Chronic lymphocytic leukemia (CLL), the most common
type of adult leukemia in Western countries, characterized by the
accumulation of malignant CD19+CD5+ cells, is
a highly heterogeneous disease with variable prognoses and clinical
course (1–3). Some patients never require treatment
and are indolent, while other patients have aggressive disease and
require intensive treatment soon after diagnosis (4,5).
Traditionally, symptomatic CLL patients can be effectively treated
with fludarabine, glucocorticoids, alkylating agents or monoclonal
antibodies. However, despite these therapeutic regimens, CLL
disease is still incurable. Thus, the discovery of novel, less
toxic and more effective drugs for CLL patients is in urgent need.
Moreover, relapsed or refractory CLL patients have limited
therapeutic treatment options.
To discover new drugs, the use of plant-derived
substances or immunomodulatory drugs was reported to have a
therapeutic effect in CLL treatment. Additionally, new candidates
for CLL therapies include histone deacetylase inhibitors, Bcl-2
inhibitors and proteasome inhibitors have also been developed
(6,7). These latter agents may induce CLL cell
apoptosis partly through pro-apoptotic and anti-apoptotic family
members (6,7). Among these drugs, fludarabine, an
inhibitor of STAT1 activity and DNA synthesis inhibitor, is the
most effective drug for the treatment of CLL disease, especially
for routine treatment failure patients. However, toxic side effects
in the clinic are extremely evident (8). The toxic effects of fludarabine
include immunosuppression marked by a decrease in the
CD4+/CD8+ ratio, and the development of
myelosuppression, opportunistic infections or gastrointestinal
toxicities which include vomiting, nausea and hepatic lesions have
also been reported (8).
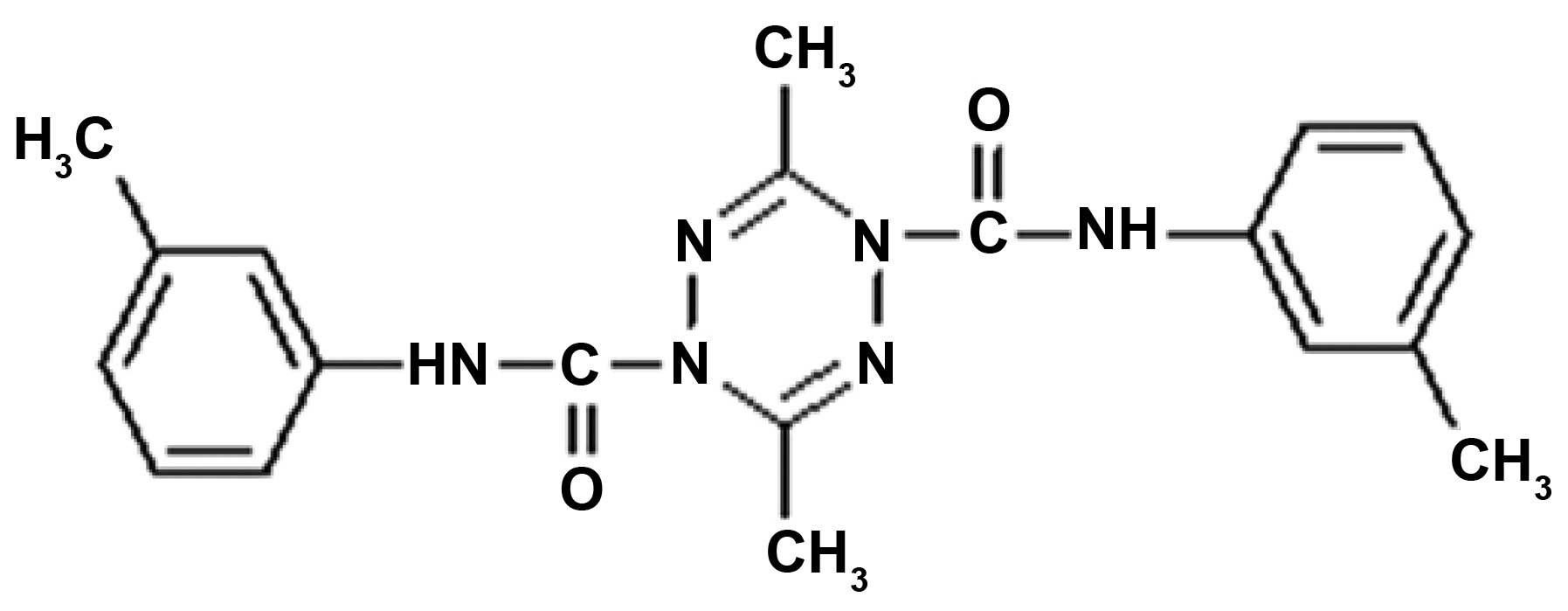
ZGDHu-1
[N,N′-di-(m-methylphenyi)-3,6-dimethyl-1,4-dihydro-1,2,4,5-tetrazine-1,4-dicarboamide]
(Fig. 1) is a tetrazine compound
(9), which has been reported by our
group to exhibit antitumor activity (10). It has been identified as a potential
proteasome inhibitor (11). It was
demonstrated that ZGDHu-1 induces the apoptosis of B lymphocytes
from CLL patients (12). In the
present study, we investigated ZGDHu-1 used alone or combined with
fludarabine in regards to the ex vivo apoptotic effects on
CLL cells and normal lymphocytes derived from peripheral blood. We
examined the apoptosis of CLL cells, loss of mitochondrial membrane
potential (ΔΨ m), phosphatidylserine (PS) translocation across the
plasma membrane (13,14) and accumulation of reactive oxygen
species (ROS) (15). At the same
time, the percentage of active caspase-3-expressing cells,
intracellular Bcl-2 and Bax expression were also investigated.
Subsequently, we also analyzed the correlation between these
apoptotic effects with clinical indices, such as lactate
dehydrogenase (LDH), ZAP-70 or CD38 expression, lymphocyte count,
β2-microglobulin level and Rai classification status.
Materials and methods
Patients
Twenty-five untreated, newly diagnosed CLL patients
were enrolled. CLL diagnosis was carried out according to clinical
examination, morphological and immunological criteria. After
informed consent, peripheral blood cells were obtained from the CLL
patients. The present study was approved by the Zhejiang Provincial
People's Hospital Ethics Committee. Patient characteristics are
summarized in Table I.
 | Table ICharacteristics of the CLL
patients. |
Table I
Characteristics of the CLL
patients.
|
Characteristics | Data |
|---|
| Gender, n |
| Female | 8 |
| Male | 17 |
| Age (years) |
| Mean | 67.2 |
| Range | 58–89 |
| Rai, n |
| 0 | 5 |
| I | 6 |
| II | 8 |
| III | 6 |
| Lymphocyte count
(×109/l) |
| Mean | 21.6 |
| Range | 12.6–73.4 |
| CD38+, n
(%) | 6 (24) |
| ZAP-70+,
n (%) | 8 (32) |
Reagents and instruments
ZGDHu-1 compound (Fig.
1) with a purity of >95% was kindly provided by Dr Wei-xiao
Hu (Pharmaceutical College of Zhejiang university of Technology,
China) as previously reported (12). ZGDHu-1 was dissolved in
dimethylsulfoxide (DMSO) as a stock solution (1 mg/ml) and stored
at −20°C. Antibodies against Bcl-2 (SC-7382), Bax (SC-2774),
caspase-3 (SC-9746), β-actin (SC-4967) for western blot analysis
were purchased from Cell Signaling Technology (Beverly, MA, USA).
Fludarabine, DMSO,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT),
dihydrorhodamine-123 (DHR), broad spectrum caspase inhibitor
benzyloxycarbonyl-Val-Ala-Asp-fluoromethylketone (Z-VAD-fmk) and
Ficoll-Hypaque liquid were purchased from Sigma-Aldrich (St. Louis,
MO, USA) as previously reported (12). The apoptosis kit of propidium iodide
(PI), Annexin V and the IntraPrep™ permeabilization kit were all
from Immunotech Company (Marseille, France). JC-1
(5,5′,6,6′-tetrachloro-1,1′,3,3′-tetrethyl benzimidalyl
carbocyanine iodide) was purchased from BioTeam Inc. Company as
previously reported (12). CLL
cells were stained with the following mAbs: anti-CD19-PerCP CY 5.5
(ID3) and anti-CD5-APC (53.-7.3) (both from BD Pharmingen, San
Diego, CA, USA), anti-ZAP-70 Alexa Fluor®488 (SBZAP) and
anti-CD38-FITC (T16; both from Immunotech), anti-active
caspase-3-PE (C92-605; BD Pharmingen), anti-Bax-FITC (SC20067;
Santa Cruz Biotechnology, CA, USA); and anti-Bcl-2-PE (Bcl-2/100;
BD Pharmingen). Cells were analyzed with Navios FACS (Beckman
Coulter, Miami, FL, USA).
Cell viability assay
Cell viability was analyzed with an MTT assay as
previously described (16). Cells
(at a density of 5×105/ml) were incubated with ZGDHu-1
(50, 100, 150, 200 and 250 ng/ml) alone or in combination with
fludarabine (1 µg/ml) on 96-well plates for 72 h. The
control group was incubated only with drug-free medium with 0.05%
DMSO solution (v/v). Then, MTT (5 mg/ml) was added to each well and
incubated for 4 h at 37°C, the medium was aspirated and then 150
µl DMSO solution was added to each well. The plate was then
measured by the M680 microplate reader (Bio-Rad, Hercules, CA, USA)
at a reference 630 nm wavelength and a test 570 nm wavelength. All
experiments were performed in triplicate and repeated at least
three times. The cell viability was expressed as a percentage of
the DMSO-treated control samples as previously reported (12).
Lymphocyte purification and culture
EDTA-K2 anticoagulant blood samples were obtained
from the CLL patients and healthy controls during a routine
diagnosis at the Zhejiang Provincial People's Hospital. B
lymphocytes were isolated immediately by using Ficoll gradient
centrifugation. After a 1-h incubation at 37°C, in a 5%
CO2 condition, adhesive mononuclear cells were removed.
The non-adherent lymphocytes were washed with Hank's solution
(Biochrom, Berlin, Germany). Then the T lymphocytes were removed
using anti-CD3 Dynabeads® (Dynal, Merseyside, UK). The B
lymphocytes were further purified by flow cytometric sorting based
on CD19 antibody staining (Immunotech, Coulter, USA) as previously
reported (12). B lymphocytes were
then counted in a Neubauer Counting Chamber with trypan blue to
exclude dead cells. The cells were then resuspended in RPMI-1640
medium with 10% fetal bovine serum (FBS), 2 mM glutamine, 100 U/ml
penicillin G and 0.1 mg/ml streptomycin (Sigma-Aldrich) in 75
cm3 fasks at a density of 1–4×106/ml and
cultured at 37°C in 5% CO2 as previously reported
(12). The cells were further
divided into 4 groups. The first group served as the control
without any treatment. The second group was treated with 100 ng/ml
ZGDHu-1 for 0–5 days. The third group was treated with fludarabine
(1 µg/ml) for 0–5 days. The fourth group was treated with
both ZGDHu-1 (100 ng/ml) and fludarabine (1 µg/ml) for 0–5
days. At the end of each time-point, the cells were harvested for
FACS analysis or lysed for western blot analyses.
FACS
CLL cells were stained with the following mAbs:
anti-CD19-PerCP CY 5.5 (ID3), anti-CD5-APC (53.-7.3), anti-ZAP-70
Alexa Fluor® 488, anti-CD38-FITC (T16), anti-active
caspase-3-PE (C92-605), anti-Bax-FITC (SC20067) and anti-Bcl-2-PE
(Bcl-2/100). The stained cells were analyzed with NAVIOS FACS. Then
10,000 cells for each sample were counted and then CD19 and CD5
antibody staining was carried out and the CD19+
CD5+ double-positive cell population was gated for the
following analysis. For the apoptosis detection, the percentage of
Annexin V-positive (+) and PI-negative (−) cells was detected using
the Annexin V kit. The mitochondrial potential (ΔΨ m) was measured
with JC-1 dye. The intracellular accumulation of ROS was assessed
with the fluorescent dye DHR. The percentage of active
caspase-3-positive cells in the control cells was calculated. The
Bcl-2 and Bax expression was examined for each sample. Then the
Bcl-2/Bax ratio for the CD19+CD5+ cells was
calculated. The CD19+CD5− populations were
regarded as non-leukemic cells. To determine the frequency of
prognostic factors, the percentage of CD38 and ZAP-70 was detected
for each sample. Patients were defined as ZAP-70-positive when the
ZAP-70 expression was >20% in leukemic cells. Patients were
defined as CD38-positive, when the CD38 expression was at least 20%
in leukemic cells.
Western blot analysis
The treated CLL and control cells were collected and
lysed in buffer contained 20 mM Tris-HCl (pH 7.5), 1 mM EDTA, 150
mM NaCl, 50 mM NaF, 10 mM sodium pyrophosphate, 1 mM sodium
orthovanadate, 2 mM phenylmethylsulfonyl fluoride, 0.5% Triton-X
and protease inhibitor cocktail (Pierce, Rockford, IL, USA) on ice
for 30 min. Then, the same amount of proteins was loaded and
separated by SDS-PAGE gel, transferred to nitrocellulose membranes
at 100 V for 2 h and then blocked with PBS solution with 5% non-fat
milk for 1 h. After PBS washing, the membrane was incubated with
the primary monoclonal antibodies against human Bcl-2, Bax and
caspase-3 (1:1,000 dilution) for 1 h, respectively. The β-actin
expression was used as the control. Then, the rabbit anti-mouse IgG
antibodies (1:1,000 dilution) were used as the secondary antibody.
The ECL kit (Pierce) and the GDS-8000 imaging system (UVP, Upland,
CA, USA) were used for the visualization of the immunoreactive
bands.
Evaluation of the combination index
A combination index (CI) calculated based on the
Chou-Talalay method was used to evaluate the synergism between
ZGDHu-1 and fludarabine (2,17–19).
The following formula was used: CI = (sum of single agent
treatment/specific apoptosis of combined treatment). The percentage
of specific apoptosis was examined by using the following formula:
Specific apoptosis = (drug induced apoptosis - spontaneous
apoptosis)/(100 - spontaneous apoptosis) × 100%. CI <1, CI =1
and C >1 were regarded as synergistic, additive or
infra-additive, respectively (20,21).
The percentage of active caspase-3 cells was estimated for these
populations. In the present study, 7 of the 25 patients for whom
the non-leukemic cells were higher than 10% in the peripheral blood
were analyzed.
Statistical analysis
Data from individual experiments are presented as
mean ± SD. The statistical analysis was carried out using SPSS10.0
software. The Wilcoxon test was used for two dependent variables;
Mann-Whitney U and Spearman's tests were used for two independent
group and two variable correlations, respectively. P<0.05 was
considered to indicate a statistically significant result.
Results
Effects of ZGDHu-1 and fludarabine on the
viability of CLL cells
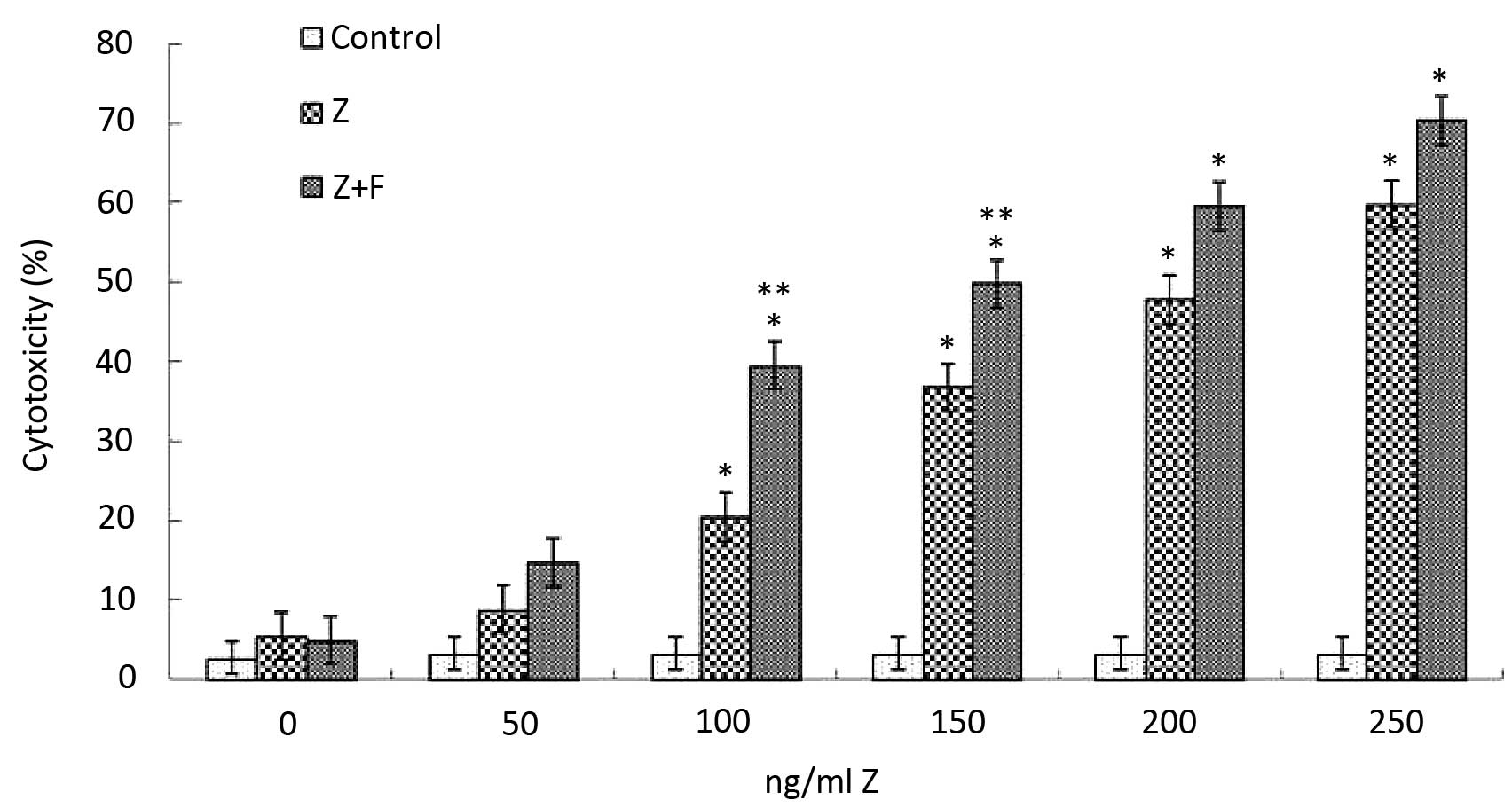
To investigate the synergistic effect of fludarabine
and ZGDHu-1, we used the classical MTT assay to evaluate the
cytotoxic effects of the two drugs. Firstly, we screened the
minimal fludarabine cytotoxic concentration. After a series of
preliminary studies with the aim to reduce the in vitro
cytotoxicity above controls at a mean level of <10% after a
3-day treatment, a 1 µg/ml concentration of fludarabine was
regarded as being non-cytotoxic. Consistent with previous studies
(22), fludarabine did not show
significant cytotoxicity at the concentration of 1 µg/ml
compared to the controls (5.24±1.33%). But when concentrations of 2
and 2.5 µg/ml (30.5±8.05%; 41.7±7.25%, respectively) were
used, its cytotoxicity was significantly increased (Fig. 2). Furthermore, ZGDHu-1 treatment
also caused an increase in the cytotoxicity to CLL cells in a
dose-dependent manner; 5.8±1.34% at 50 ng/ml to 47.8±8.74% at 250
ng/ml on day 3 (Fig. 2). We then
choose the optimal concentrations of fludarabine (1 µg/ml)
and ZGDHu-1 (100 ng/ml). Notably, the minimal synergistic cytotoxic
concentration was observed at ZGDHu-1 (100 ng/ml) and fludarabine
(1 µg/ml), which was slightly increased when compare with
ZGDHu-1 (100 ng/ml) and fludarabine (1 µg/ml) alone
(5.24±1.33% for fludarabine; 20.5±4.56% for ZGDHu-1; 39.5±7.45% for
fludarabine + ZGDHu-1). Thus, in the following synergistic effect
studies, the treatment duration was set at 3 days at a
concentration of 100 ng/ml for ZGDHu-1 and 1 µg/ml for
fludarabine.
Combination of ZGDHu-1 and fludarabine
induces the apoptosis of CLL cells
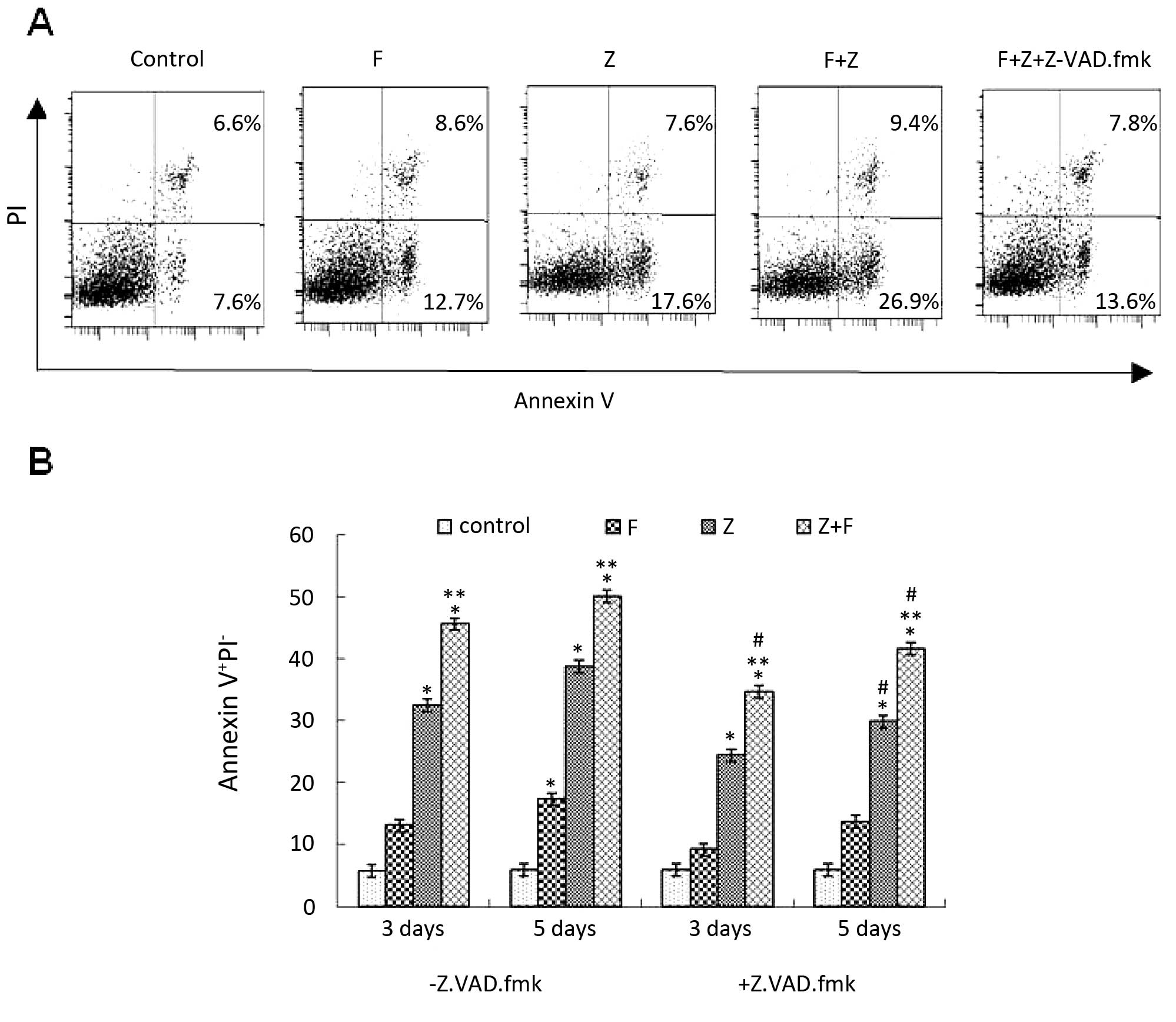
To better support the MTT assay in regards to cell
death triggered by ZGDHu-1 alone and in combination with
fludarabine, CLL cells were evaluated for apoptosis by FACS
analysis following Annexin-V and PI staining. As shown in Fig. 3, the apoptotic cell population was
significantly increased after the CLL cells were treated with
ZGDHu-1 and/or fludarabine on day 3 compared to the control (no
treatment). Notably, the combination of ZGDHu-1 and fludarabine
significantly increased the apoptotic population when compared to
this population in the cells treated with ZGDHu-1 or fludarabine
alone. To further investigate whether ZGDHu-1 and fludarabine
induced CLL cell apoptosis through the caspase-dependent pathways,
we treated CLL cells with ZGDHu-1 and/or fludarabine in the
presence of the broad spectrum caspase inhibitor, Z-VAD-fmk. We
found that the apoptotic cell population was significantly reduced
after pretreatment with Z-VAD-fmk (Fig.
3A and B). These data indicate that ZGDHu-1 induced cell
apoptosis through the caspase-dependent pathway.
Effect of the combination of ZGDHu-1 and
fludarabine on the mitochondrial pathway through the change in
ROS
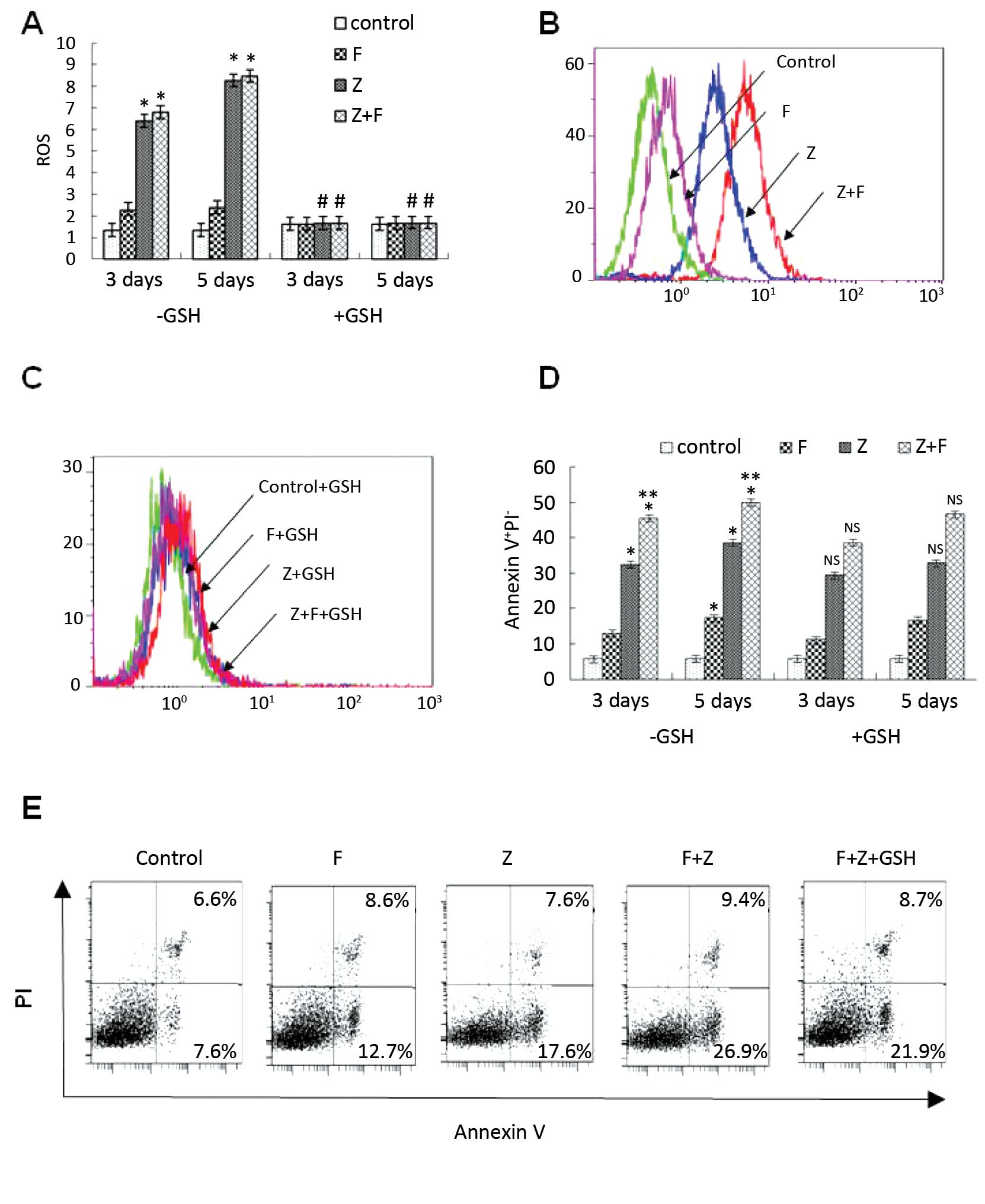
As we know, ROS play an important role in the
mitochondrial pathway during cell apoptosis (15). The ROS level in CLL cells was
examined following treatment with ZGDHu-1 alone or in combination
with fludarabine. In the present study, the ROS level was detected
by DHR staining and FACS method. As shown in Fig. 4A and B, following ZGDHu-1 treatment
alone and in combination with fludarabine on day 3 and 5, the ROS
levels were significantly increased (all P<0.05). However,
treatment with fludarabine for 3 or 5 days did not significantly
increase the ROS level in the CLL cells. Additionally, the ROS
scavenger glutathione (GSH) was also investigated as to whether it
could suppress the apoptosis of CLL cells mediated by ZGDHu-1 or
the combination of ZGDHu-1 and fludarabine. Pretreatment with GSH
(100 µM) for 2 h significantly blocked ZGDHu-1-induced ROS
generation (Fig. 4 A and C,
P<0.05). In contrast, GSH did not inhibit the pro-apoptotic
effects of ZGDHu-1 on the CLL cells (Fig. 4D and E). Overall, these results
suggest that ZGDHu-1 may induce the apoptosis of CLL cells by
altering the ROS level.
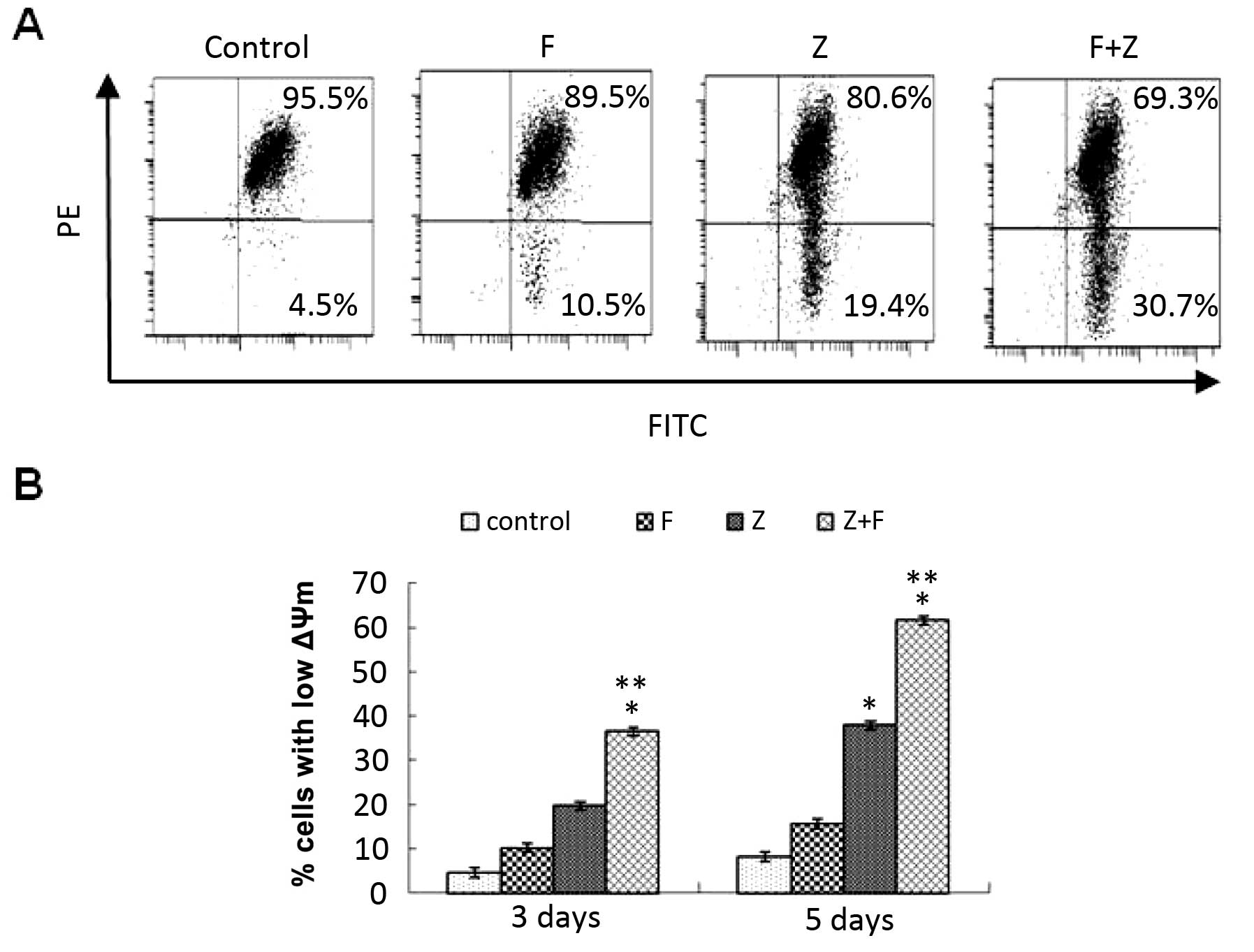
Effect of the combination of ZGDHu-1 and
fludarabine on the mitochondrial pathway through ΔΨm
To further investigate whether ZGDHu-1 induces CLL
cell apoptosis through the mitochondrial pathway, we analyzed the
ΔΨm after ZGDHu-1 and/or fludarabine treatment by FACS analysis.
After the CLL cells were treated with ZGDHu-1 and/or fludarabine on
day 3 and 5, then the CLL cells stained with JC-1 (10
µmol/l) were detected for the ΔΨm level. As shown in
Fig. 5B, the percentage of cells
with low ΔΨm in the ZGDHu-1 + fludarabine-treated culture was
significantly higher than that of the ZGDHu-1 group and the
fludarabine group, indicating that ZGDHu-1 synergistically acts
with fludarabine inducing CLL cell apoptosis through the
mitochondrial pathway.
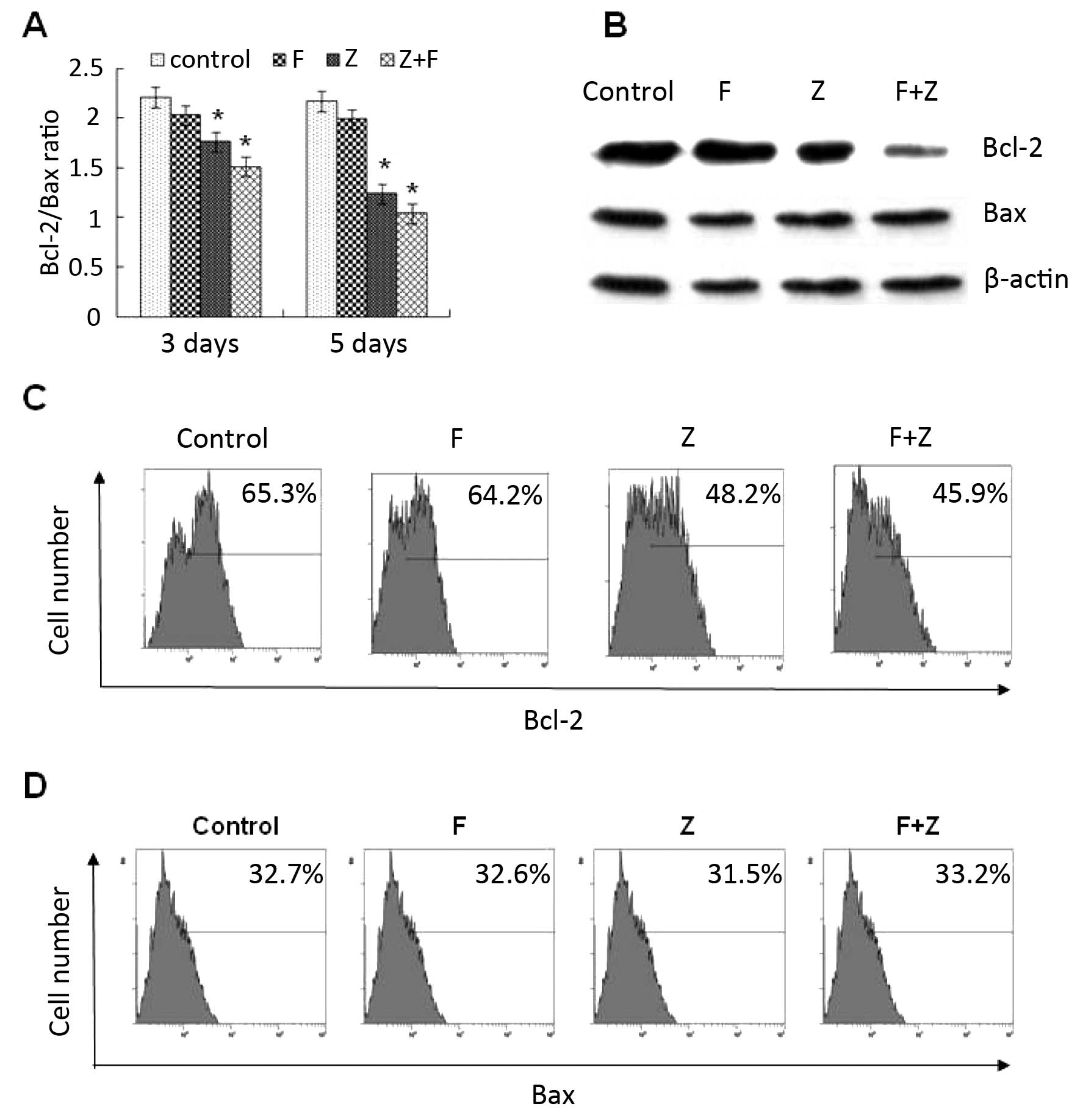
Effect of the combination of ZGDHu-1 and
fludarabine on the mitochondrial pathway through the Bcl-2
family
As known, Bcl-2 family proteins play an important
role in the control of membrane permeability of mitochondria and
caspase activation (23). The
expression levels of anti-apoptotic factor Bcl-2 and pro-apoptotic
Bax factor in CLL cells on day 3 and 5 were detected by FACS
(Fig. 6A) and western blot analysis
(Fig. 6B). Following exposure to
ZGDHu-1 alone and in combination with fludarabine, the Bcl-2
expression was significantly decreased while the Bax expression was
not changed. However, when CLL cells were exposed to fludarabine
alone, the expression of Bcl-2 and Bax was not changed (Fig. 6C and D). Overall, these data suggest
that ZGDHu-1 induces the apoptosis of CLL cells through the
intrinsic mitochondrial pathway, which was different from
fludarabine.
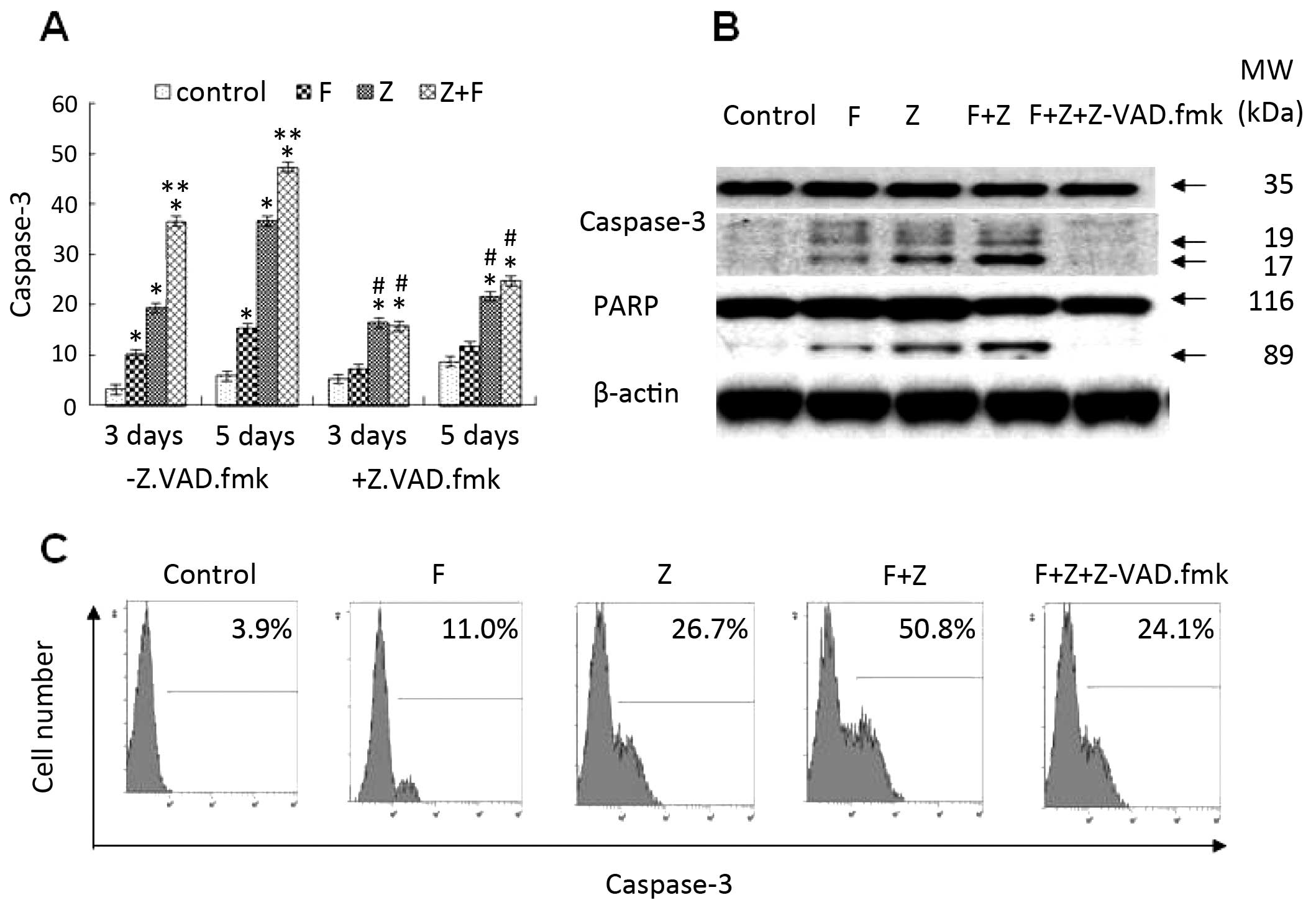
Combination of ZGDHu-1 and fludarabine
synergistically increases caspase-3 activity in CLL cells
Caspase activation, which is a key event in
apoptosis and the downstream signaling pathway of the mitochondrial
pathway, also plays an important role in fludarabine-induced
cytotoxicity (24). Among the
caspase family members, caspase-3 serves as an effector caspase,
causing cleavage of a variety of proteins including polyADP-ribose
polymerase (PARP), a well-known caspase substrate. Our previous
study indicated that caspase-3 is involved in the apoptotic effect
induced by ZGDHu-1 in CLL cells (12). In the present study, the minimal
caspase-3 activation was observed with fludarabine (1 µg/ml)
treatment (Fig. 7). When CLL cells
were incubated with a combination of ZGDHu-1 and fludarabine,
caspase-3 activation exhibited a 2-fold increase on day 3 compared
to the CLL cells treated either with fludarabine or ZGDHu-1 alone
(P<0.01, Fig. 7A). Furthermore,
cleavage of PARP also exhibited a 2-fold increase on day 3 compared
to that in the CLL cells treated either with fludarabine or ZGDHu-1
alone (Fig. 7B). When CLL cells
were exposed to ZGDHu-1 or ZGDHu-1 + fludarabine in the presence of
the broad spectrum caspase inhibitor Z-VAD-fmk, Z-VAD-fmk
significantly blocked ZGDHu-1-induced caspase-3 activation as well
as the cleavage of PARP (Fig. 7B).
Moreover, Z-VAD-fmk pre-treatment also partially attenuated the
ZGDHu-1-induced apoptotic effects on the CLL cells by PS
externalization (Fig. 3A and B),
but did not affect the ΔΨ m or the Bcl-2/Bax ratio (data not
shown). Subsequently, to evaluate the combined effect of ZGDHu-1
and fludarabine more precisely, the combination index (CI) was
calculated where a CI <1 indicates a synergistic effect.
Twenty-two patients in the ZGDHu-1 + fludarabine group of the 25
analyzed patients had a synergistic effect (Table II). In addition, CI =1 was noted in
one patient and CI >1 in two patients, representing an additive
or infra-additive effect, respectively. Overall, our data suggest
that there is a synergistic effect between these two drugs.
 | Table IICombination indices (CIs) of 25 CLL
patients treated with ZGDHu-1 and fludarabine. |
Table II
Combination indices (CIs) of 25 CLL
patients treated with ZGDHu-1 and fludarabine.
| Patient no. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
|---|
| CI | 0.8 | 0.9 | 1.1 | 0.5 | 0.4 | 0.6 | 0.7 | 0.8 | 0.7 | 0.9 | 1.0 | 0.6 | 0.7 |
| Patient no. | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | |
|---|
| CI | 0.6 | 0.9 | 1.2 | 0.8 | 0.9 | 0.7 | 0.6 | 0.7 | 0.6 | 0.9 | 0.8 | 0.9 | |
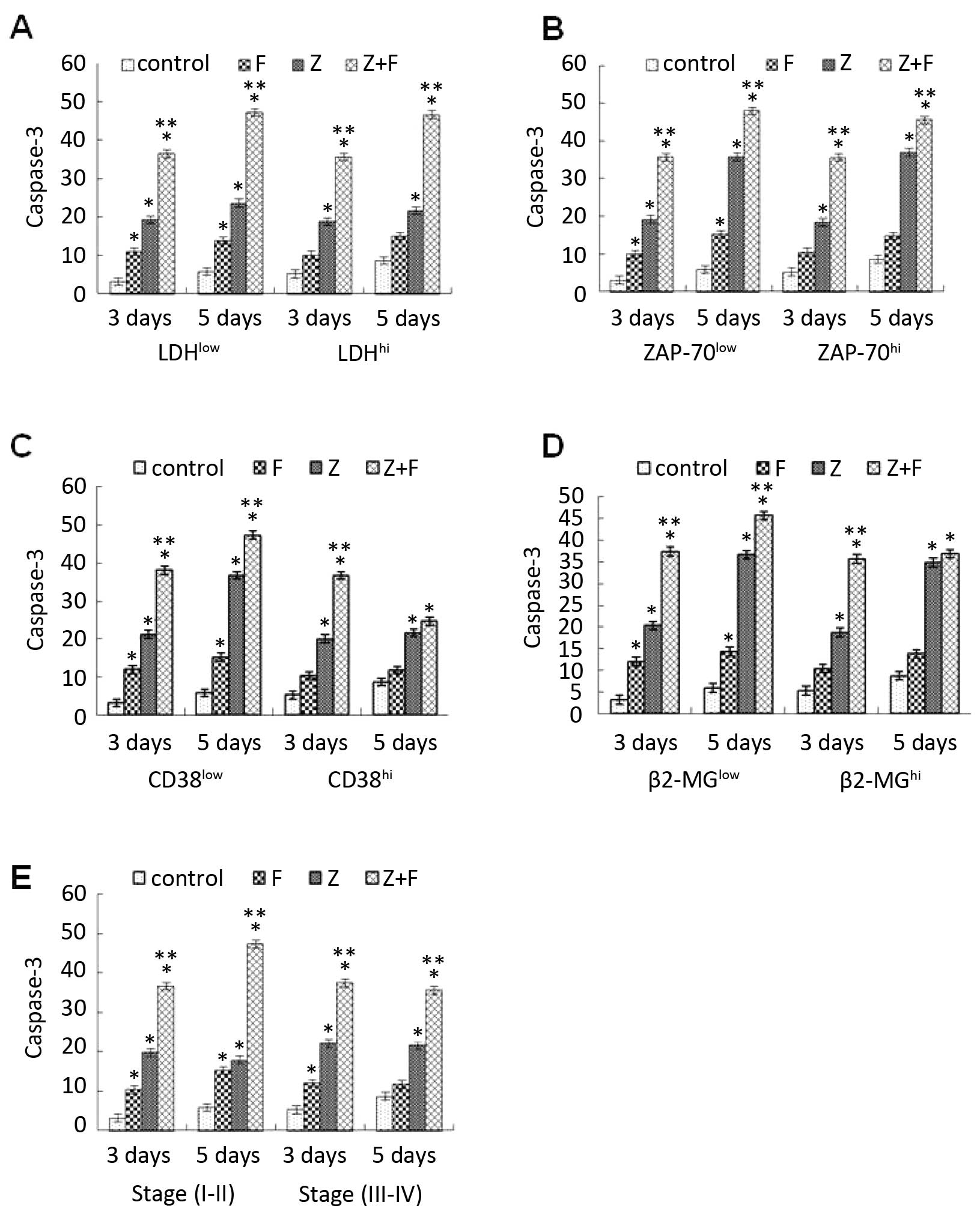
ZGDHu-1-induced apoptosis rate of CLL
cells is independent of ZAP-70 and CD38 expression
Traditionally, high CD38 expression, high ZAP-70
expression, immunoglobulin heavy chain genes (IgVH) gene and
cytogenetic abnormalities (especially deletions of 11q and 17p) are
all associated with the poor prognosis of CLL patients and can
define patients who are in an aggressive status (4,5,25–27).
To assess whether the rate of ZGDHu-1-induced apoptosis is
associated with the prognosis of CLL, the apoptotic parameters in
ZAP-70high vs. ZAP-70low groups and
CD38high vs. CD38low groups were compared.
However, a significant difference in the percentage of
caspase-3-positive CLL cells cultured with ZGDHu-1 between the
ZAP-70high and ZAP-70low groups was not found
(Fig. 8B), also between the
CD38high and CD38low patients (Fig. 8C). Moreover, the rate of apoptosis
caused by ZGDHu-1 as a single agent or in combination with
fludarabine in early (I–II) stage compare to advanced (III–IV)
stages disease was assessed (Fig.
8E). In the present study, there was no significant difference
between these groups. That is to say, the rate of ZGDHu-1-induced
apoptosis was independent of these prognostic markers, such as
lymphocytosis, LDH or the β2-microglobulin level (Fig. 8A and D).
Discussion
CLL is the most common leukemia in Western
countries, characterized by the accumulation of malignant B
lymphocytes following the failure to undergo apoptosis. Despite
huge progress in treatment, it is still an incurable disease.
Currently, purine analogs are widely used for the treatment of CLL
and have achieved a higher remission rate (28,29).
Fludarabine has been shown to have multiple functions such as
interference with DNA synthesis and repair, apoptosis induction and
cell cycle regulation in leukemia cells (30). However, the toxic effect of
fludarabine such as severe opportunistic infections,
myelosuppression and gastrointestinal toxicities including
vomiting, nausea and hepatic lesions have been widely reported
(6). Thus, reducing the fludarabine
toxicity by lowering its dose and exploring new drug are
desperately needed. In the present study, we highlight that the use
of fludarabine in combination with ZGDHu-1 may reduce the
fludarabine dose due to the synergistic effect of the two drugs.
The combination of ZGDHu-1 and fludarabine may be useful for the
maintenance therapy of CLL patients, as it can sensitize CLL cells
to low-dose fludarabine without increasing the risk of long-term
side effects on the immune system or other opportunistic
infections.
ZGDHu-1, a potential proteasome inhibitor (14), showed significant cytotoxicity on
malignant B lymphocytes isolated from CLL patients in a
dose-dependent manner, but not on normal B lymphocytes of healthy
controls. Moreover, ZGDHu-1 may induce the apoptosis of CLL cells
by increasing the mitochondrial membrane permeability, production
of ROS, activation of caspase-3 and a decrease in the Bcl-2/Bax
ratio (15). Moreover, the
pro-apoptotic effect of ZGDHu-1 was tumor-specific and this effect
was not observed on non-leukemic lymphocytes. Furthermore, to the
best of our knowledge, this is the first study to demonstrate that
ZGDHu-1 may increase the percentage of apoptotic cells in
combination with fludarabine and this synergistic effect of ZGDHu-1
with fludarabine was assessed based on the CI. Previous data
demonstrated that ZGDHu-1 may induce the apoptosis of CLL cells
through the mitochondrial pathway. Moreover, in the present study,
the mitochondrial pathway also played an important role in the
combination of ZGDHu-1 with fludarabine. As we know, Bcl-2 is
regarded as a classical marker for the intrinsic apoptosis pathway
and the major anti-apoptotic protein of the Bcl-2 family.
Overexpression of Bcl-2 may inhibit the apoptosis partly by
suppressing ROS generation or by inhibiting the mitochondrial
permeability transition (13–15).
Overall, compared to fludarabine, ZGDHu-1 had no side effects.
Moreover, it had a significant synergistic effect with fludarabine
to induce the apoptosis of CLL cells partly through the
mitochondrial pathway (31).
As we know, caspase-3 can be cleaved into the 17-
and 12-kDa subunits during cleavage and activation (32). In the present study, in CLL cells,
ZGDHu-1 induced the complete cleavage of caspase-3 and this effect
was inhibited by the caspase inhibitor Z-VAD-fmk. In addition,
caspase-3 was activated partly through the intrinsic pathway. Our
results revealed that caspase-3 activation may participate in the
synergistic apoptotic combined effect of ZGDHu-1 and
fludarabine.
Additionally, several prognostic markers, such as
lymphocyte count, LDH elevation, β2-microglobulin, IgVH, ZAP-70 and
CD38, are widely used for CLL patients (4,25). In
the present study, we assessed whether the apoptosis rate caused by
ZGDHu-1 is different between CLL patients with favorable and
unfavorable prognosis. In the present study, the rate of
ZGDHu-1-induced apoptosis of CLL cells was independent of ZAP-70 or
CD38 expression and the clinical Rai classification status.
Furthermore, it did not correlate with lymphocytosis, LDH and
β2-microglobulin. Overall, our data imply that ZGDHu-1 may be
equally effective in CLL patients with both a favorable and poor
prognosis.
In conclusion, the present study indicates that
ZGDHu-1 may be used as a single agent or in combination with
fludarabine for the treatment of CLL patients. One of the
mechanisms of ZGDHu-1 synergism with fludarabine appears to be
associated with the triggering of caspase-3 activation that makes
CLL cells more susceptible to apoptosis. Thus, ZGDHu-1 combined
with fludarabine is a promising treatment strategy for CLL
patients.
Acknowledgments
The present study was supported by the National
Natural Science Foundation (no. 30973568), by funding from the key
platform funded projects from the Zhejiang Province Health Bureau
(no. 2013ZDA005), by the Zhejiang Provincial Program for the
Cultivation of High-Level Innovative Health Talents (no. 2012) and
by the Zhejiang Province Natural Science Fund (no. LY12H16019).
References
|
1
|
Caligaris-Cappio F and Hamblin TJ: B-cell
chronic lymphocytic leukemia: A bird of a different feather. J Clin
Oncol. 17:399–408. 1999.PubMed/NCBI
|
|
2
|
Podhorecka M, Halicka D, Klimek P, Kowal
M, Chocholska S and Dmoszynska A: Simvastatin and purine analogs
have a synergic effect on apoptosis of chronic lymphocytic leukemia
cells. Ann Hematol. 89:1115–1124. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hamblin TJ and Oscier DG: Chronic
lymphocytic leukaemia: The nature of the leukaemic cell. Blood Rev.
11:119–128. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stilgenbauer S: Chronic lymphocytic
leukemia: Genetics for predicting outcome. Hematology (EHA Edur
Program). 2:185–190. 2006.
|
|
5
|
Hamblin TJ, Davis Z, Gardiner A, Oscier DG
and Stevenson FK: Unmutated Ig V(H) genes are associated with a
more aggressive form of chronic lymphocytic leukemia. Blood.
94:1848–1854. 1999.PubMed/NCBI
|
|
6
|
Inoue S, Riley J, Gant TW, Dyer MJ and
Cohen GM: Apoptosis induced by histone deacetylase inhibitors in
leukemic cells is mediated by Bim and Noxa. Leukemia. 21:1773–1782.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vogler M, Butterworth M, Majid A, Walewska
RJ, Sun XM, Dyer MJ and Cohen GM: Concurrent up-regulation of
BCL-XL and BCL2A1 induces approximately 1000-fold resistance to
ABT-737 in chronic lymphocytic leukemia. Blood. 113:4403–4413.
2009. View Article : Google Scholar
|
|
8
|
Morrison VA: Infectious complications in
patients with chronic lymphocytic leukemia: Pathogenesis, spectrum
of infection, and approaches to prophylaxis. Clin Lymphoma Myeloma.
9:365–370. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hu WX, Zhou M, Cai ZB and Yang YZ:
Synthesis of new type antineoplastic drug 3, 6-dimethyl-1,
4-dihydro-s-tetrazine-1, 4-dicarboamide. Patent China: 2004
|
|
10
|
Rao GW and Hu WX: Synthesis, structure
analysis, and antitumor activity of
3,6-disubstituted-1,4-dihydro-1,2,4,5-tetrazine derivatives. Bioorg
Med Chem Lett. 16:3702–3705. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou Y, Lv Y, Xu W and Hu W: Determination
of proteasome activities with fluorogenic kinetic assays and its
application in screening proteasome inhibitor. Chi J Clin Phar
Therap. 10:1127–1133. 2008.
|
|
12
|
Qiu LN, Zhou YL, Wang ZN, Huang Q and Hu
WX: ZGDHu-1 promotes apoptosis of chronic lymphocytic leukemia
cells. Int J Oncol. 41:533–540. 2012.PubMed/NCBI
|
|
13
|
Ly JD, Grubb DR and Lawen A: The
mitochondrial membrane potential (deltapsi(m)) in apoptosis; an
update. Apoptosis. 8:115–128. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kiss T: Apoptosis and its functional
significance in molluscs. Apoptosis. 15:313–321. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Simon HU, Haj-Yehia A and Levi-Schaffer F:
Role of reactive oxygen species (ROS) in apoptosis induction.
Apoptosis. 5:415–418. 2000. View Article : Google Scholar
|
|
16
|
Chou TC: Drug combination studies and
their synergy quantification using the Chou-Talalay method. Cancer
Res. 70:440–446. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shakir FK, Audilet D, Drake AJ III and
Shakir KM: A rapid protein determination by modification of the
Lowry procedure. Anal Biochem. 216:232–233. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
ten Cate B, Samplonius DF, Bijma T, de
Leij LF, Helfrich W and Bremer E: The histone deacetylase inhibitor
valproic acid potently augments gemtuzumab ozogamicin-induced
apoptosis in acute myeloid leukemia cells. Leukemia. 21:248–252.
2007. View Article : Google Scholar
|
|
19
|
Bouzar AB, Boxus M, Defoiche J, Berchem G,
Macallan D, Pettengell R, Willis F, Burny A, Lagneaux L, Bron D, et
al: Valproate synergizes with purine nucleoside analogues to induce
apoptosis of B-chronic lymphocytic leukaemia cells. Br J Haematol.
144:41–52. 2009. View Article : Google Scholar
|
|
20
|
Newman A, Clutterbuck RD, Powles RL,
Catovsky D and Millar JL: A comparison of the effect of the
3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase
inhibitors simvastatin, lovastatin and pravastatin on leukaemic and
normal bone marrow progenitors. Leuk Lymphoma. 24:533–537. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dimitroulakos J, Nohynek D, Backway KL,
Hedley DW, Yeger H, Freedman MH, Minden MD and Penn LZ: Increased
sensitivity of acute myeloid leukemias to lovastatin-induced
apoptosis: A potential therapeutic approach. Blood. 93:1308–1318.
1999.PubMed/NCBI
|
|
22
|
Rao VA and Plunkett W: Activation of a
p53-mediated apoptotic pathway in quiescent lymphocytes after the
inhibition of DNA repair by fludarabine. Clin Cancer Res.
9:3204–3212. 2003.PubMed/NCBI
|
|
23
|
Cory S and Adams JM: The Bcl2 family:
Regulators of the cellular life-or-death switch. Nat Rev Cancer.
2:647–656. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sanhes L, Tang R, Delmer A, DeCaprio JA
and Ajchenbaum-Cymbalista F: Fludarabine-induced apoptosis of B
chronic lymphocytic leukemia cells includes early cleavage of
p27kip1 by caspases. Leukemia. 17:1104–1111. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Crespo M, Bosch F, Villamor N, Bellosillo
B, Colomer D, Rozman M, Marcé S, López-Guillermo A, Campo E and
Montserrat E: ZAP-70 expression as a surrogate for
immunoglobulin-variable-region mutations in chronic lymphocytic
leukemia. N Engl J Med. 348:1764–1775. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Oscier DG, Thompsett A, Zhu D and
Stevenson FK: Differential rates of somatic hypermutation in V(H)
genes among subsets of chronic lymphocytic leukemia defined by
chromosomal abnormalities. Blood. 89:4153–4160. 1997.PubMed/NCBI
|
|
27
|
Genini D, Budihardjo I, Plunkett W, Wang
X, Carrera CJ, Cottam HB, Carson DA and Leoni LM: Nucleotide
requirements for the in vitro activation of the apoptosis
protein-activating factor-1-mediated caspase pathway. J Biol Chem.
275:29–34. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Van den Neste E, Cardoen S, Offner F and
Bontemps F: Old and new insights into the mechanisms of action of
two nucleoside analogs active in lymphoid malignancies: Fludarabine
and cladribine (Review). Int J Oncol. 27:1113–1124. 2005.PubMed/NCBI
|
|
29
|
Robak T: Therapy of chronic lymphocytic
leukemia with purine analogs and monoclonal antibodies. Transfus
Apheresis Sci. 32:33–44. 2005. View Article : Google Scholar
|
|
30
|
Kobylinska A, Bednarek J, Blonski JZ,
Hanausek M, Walaszek Z, Robak T and Kilianska ZM: In vitro
sensitivity of B-cell chronic lymphocytic leukemia to cladribine
and its combinations with mafosfamide and/or mitoxantrone. Oncol
Rep. 16:1389–1395. 2006.PubMed/NCBI
|
|
31
|
Chow KU, Nowak D, Boehrer S, Ruthardt M,
Knau A, Hoelzer D, Mitrou PS and Weidmann E: Synergistic effects of
chemotherapeutic drugs in lymphoma cells are associated with
down-regulation of inhibitor of apoptosis proteins (IAPs),
prostate-apoptosis-response-gene 4 (Par-4), death-associated
protein (Daxx) and with enforced caspase activation. Biochem
Pharmacol. 66:711–724. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nicholson DW, Ali A, Thornberry NA,
Vaillancourt JP, Ding CK, Gallant M, Gareau Y, Griffin PR, Labelle
M, Lazebnik YA, et al: Identification and inhibition of the
ICE/CED-3 protease necessary for mammalian apoptosis. Nature.
376:37–43. 1995. View
Article : Google Scholar : PubMed/NCBI
|