Introduction
Dicer is a 219-kDa protein that functions to cleave
double-stranded RNA (1). It is a
key component of RNAi pathways and aids in the biogenesis of miRNAs
and siRNAs, rendering it a major regulatory molecule with
wide-reaching pleiotropic effects (1). Dicer function can be linked to several
cell processes and its dysregulation and altered expression has
been studied in several malignancies (1). It affects several hallmarks of cancer
including proliferation, evasion of cell death, and migration and
invasion and it has been shown to play a role as an oncogene or
tumor suppressor depending on the context. In support of an
oncogenic role for Dicer, it has been shown to be upregulated in
prostate and colorectal cancers (2,3).
Furthermore, inhibition of Dicer expression was shown to attenuate
prostate cancer cell growth by promoting cell cycle arrest and
induction of apoptosis (4).
Downregulation of Dicer in ovarian, lung, nasopharyngeal and kidney
cancers supports its role as a tumor suppressor (5–8). In
ovarian cancer, it was demonstrated that the knockdown of Dicer led
to increased cell proliferation and migration (9).
The status of Dicer in urothelial cell carcinoma of
the bladder (UCCB) has been studied; however, the reported results
are inconsistent. In a study by Catto et al, it was
demonstrated that Dicer mRNA expression was downregulated 7.4-fold
in UCCB tissues as compared to normal tissues (10). These data were supported in a study
by Wu et al which assessed Dicer expression at the mRNA and
protein levels (11). In contrast
to these studies, Han et al demonstrated using qPCR on 40
UCCB samples and matched controls that Dicer was upregulated in
this disease (12).
The function of Dicer in UCCB has also been
investigated. Current data suggest an oncogenic role for this
protein despite studies showing that it has a downregulated
expression pattern. It was demonstrated that siRNA-mediated
knockdown of Dicer inhibited proliferation of the T24 UCCB cell
line (13). In addition to
analyzing the expression of Dicer, Han et al showed that
inhibition of Dicer attenuated proliferation and induced apoptosis
in T24 and 5637 UCCB cells (12).
In contrast to this suggested oncogenic role, it has been shown
that Dicer negatively regulates migration and invasion in other
contexts such as ovarian cancer (9,14).
This observation suggests that Dicer serves as a tumor suppressor
that regulates the metastatic cascade. However, whether Dicer
affects migration and invasion in UCCB remains to be
investigated.
In the present study, we aimed to gain a better
understanding of the role Dicer plays in UCCB. We hypothesized that
Dicer has a dual nature in this disease possessing attributes of
both an oncogene and a tumor suppressor. We investigated Dicer
expression in UCCB tissues and we found that the attenuation of
Dicer protein levels led to a decrease in cell viability in part
through induction of apoptosis. Additionally, we found that a
decrease in Dicer expression promoted a mesenchymal phenotype and
increased invasion and the expression of matrix metalloproteinase-2
(MMP-2). We also showed that several miRNAs shown to be negatively
associated with an invasive phenotype were downregulated when Dicer
was knocked down. Thus, we demonstrated novel functions for Dicer
in UCCB. The present study aimed to reconcile contradictions in the
field by suggesting that the downregulation of Dicer may portend a
more invasive and therefore metastatic phenotype.
Materials and methods
Tissues, cell lines and
transfections
UCCB tissue samples and normal tissue control
samples were obtained from the UC Davis Cancer Center
Biorepository. SV-HUC-1, T24, TCCSUP, J82 and UM-UC-3 cells were
generous gifts from Dr Sweeney and Dr De Vere White (UC Davis). The
cell lines were cultured in 10% fetal bovine serum (FBS) RPMI-1640
supplemented with glutamine, penicillin and streptomycin at 37°C
and 5% CO2. Transfections were carried out using
Lipofectamine RNAiMAX (Invitrogen-Life Technologies, Carlsbad, CA,
USA) at an oligonucleotide concentration of 50 nM. siRNAs used
were: ON-TARGET plus control pool (non-targeting), and Dicer
(custom sequence, AAGGCUUACCUUCUCC AGGCUUU) (both from Dharmacon,
Lafayette, CO, USA).
Cell viability assays
T24, TCCSUP, J82 and UM-UC-3 cells were plated at
10,000–25,000 cells/well in 24-well plates. The cells were
transfected with either control non-targeting scrambled
oligonucleotides or Dicer targeting siRNA and viability was
assessed at 48 and 96 h using Cell Counting Kit-8 (CCK-8) viability
detection reagent (Dojindo). Conditions were plated in triplicate.
Data were presented as mean ± standard deviation.
Western blotting
Whole cell lysates were subjected to sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and
transferred to 0.2-µm nitrocellulose membranes. The
membranes were blocked in 5% milk in 0.1% Tween-phosphate-buffered
saline (PBS-T) and were allowed to incubate with primary antibody
overnight at 4°C. The following day, the membranes were incubated
with secondary antibody conjugated to horseradish peroxidase (HRP),
developed using the WesternBright Sirius kit (Advansta, Menlo Park,
CA, USA) and visualized with a Konica Minolta SRX-101A developer or
a Li-Cor C-DiGit Scanner. Tubulin, actin or Ponceau S stain served
as loading controls. The antibodies used were: poly(ADP-ribose)
polymerase (PARP), E-cadherin, N-cadherin, MMP-2 (Cell Signaling
Technology, Inc., Danvers, MA, USA), Dicer (Abcam, Cambridge, UK),
tubulin (Thermo Fisher Scientific, Rockford, IL, USA) and actin
(Millipore, Billerica, MA, USA).
Quantitative PCR (qPCR)
RNA was extracted using either the RNeasy kit
(Qiagen, Hilden, Germany) or the mirVana miRNA Isolation kit
(Ambion-Life Technologies, Carlsbad, CA, USA) and reverse
transcribed into cDNA using either the QuantiTect reverse
transcription kit or the miScript II RT kit (both from Qiagen).
qPCR was performed using the KAPA SYBR FAST Universal qPCR kit
(Kapa Biosystems) or the miScript PCR kit (Qiagen) on a ViiA 7
Real-Time PCR System (Applied Biosystems). Data were analyzed using
the efficiency-corrected ΔCt method. The primers used were:
miR-205, miR-31, miR-200a, miR-200b, miR-200c, miR-148a, miR-149
and miR-106b miScript primer assays (Qiagen); HPRT, F-GCCAGA
CTTTGTTGGATTTG and R-CTCTCATCTTAGGCTTTG TATTTTG; Dicer,
F-TCCACGAGTCACAATCAACACGG and R-GGGTTCTGCATTTAGGAGCTAGATGAG.
Invasion and migration assays
Invasion and migration were assessed using Transwell
assays with or without Matrigel membrane coating, respectively
(Cell Biolabs, Inc., San Diego, CA, USA; CBA-101-C). Cell seeding
was normalized using a parallel viability assay as described below.
Briefly, the cells were plated and then treated 24 h later with a
control non-targeting oligonucleotide or Dicer targeting siRNA.
After 48 h, 10% FBS containing RPMI-1640 was placed in the bottom
chambers of the wells and the cells were seeded into the top
chambers in media with no serum. Twenty-four hours later,
invaded/migrated cells were removed, lysed and read using a
fluorescent plate reader at 480/520 nm. Invasion/migration was
normalized to cell viability from a parallel assay utilizing the
cells from the same suspensions used to seed the invasion/migration
assays. Viability was read using the CCK-8 reagent as previously
described. The conditions were carried out in triplicate. Data were
presented as mean ± standard deviation.
Statistical analysis
Data were presented as mean ± standard deviation. A
two-tailed, two-sampled equal variance Student's t-test was used to
assess the differences between the samples. P≤0.05 was considered
to indicate a statistically significant result.
Results
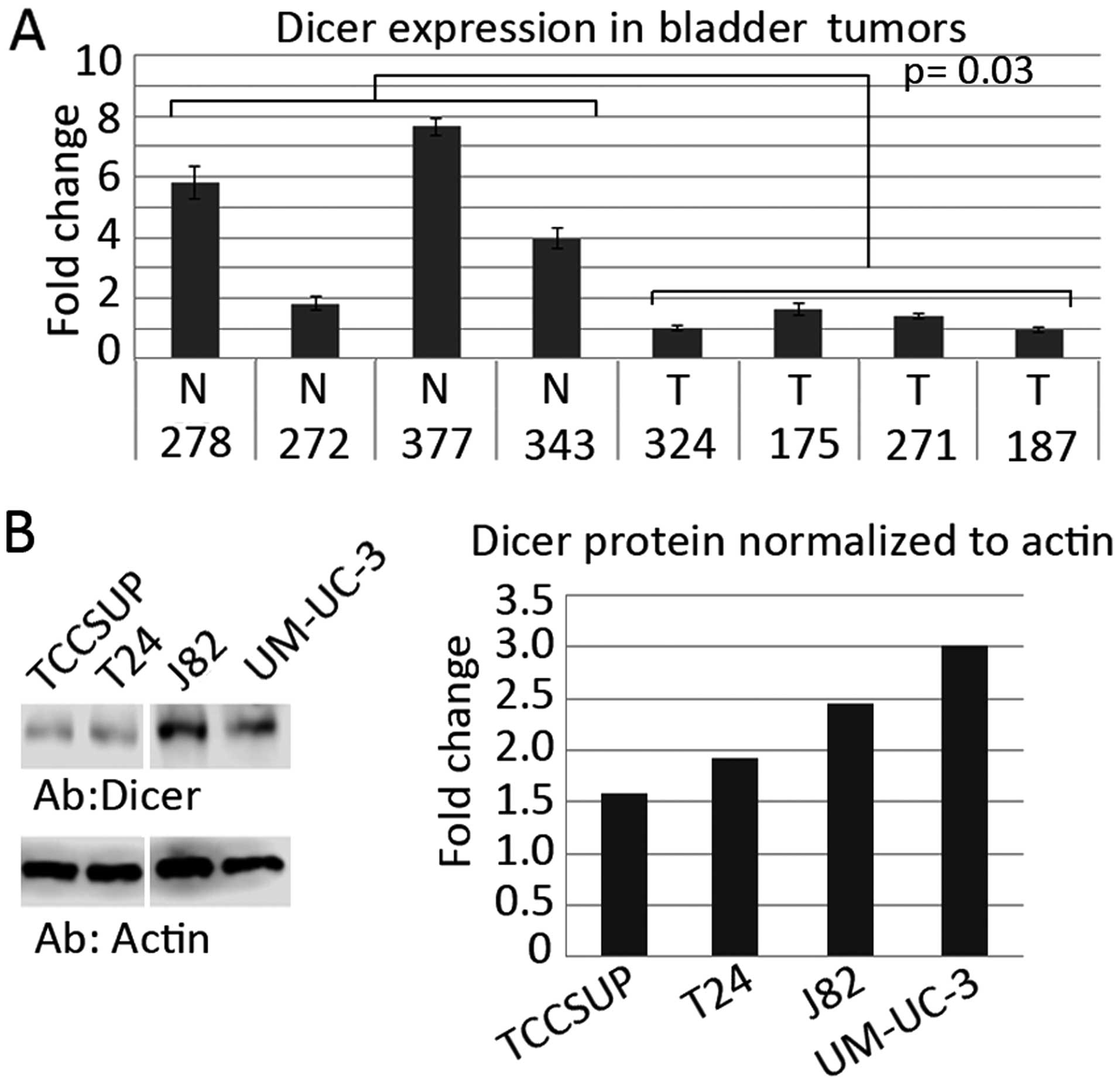
Expression of Dicer is downregulated in
UCCB tumor tissues
Previous studies have demonstrated that Dicer
expression is altered in the context of UCCB, albeit the nature of
this expression alteration is unclear (10–12).
Using qPCR, we examined the expression of Dicer in UCCB tumor
samples. We found a statistically significant decrease in Dicer
expression in tumor tissues as compared to normal tissues (Fig. 1A). Thus, we hypothesized that Dicer
may have an unidentified tumor-suppressor role in this disease.
Additionally, we performed western blot analysis to define the
Dicer protein levels in the T24, TCCSUP, J82 and UM-UC-3 UCCB cell
lines and we found that Dicer expression was variable (Fig. 1B).
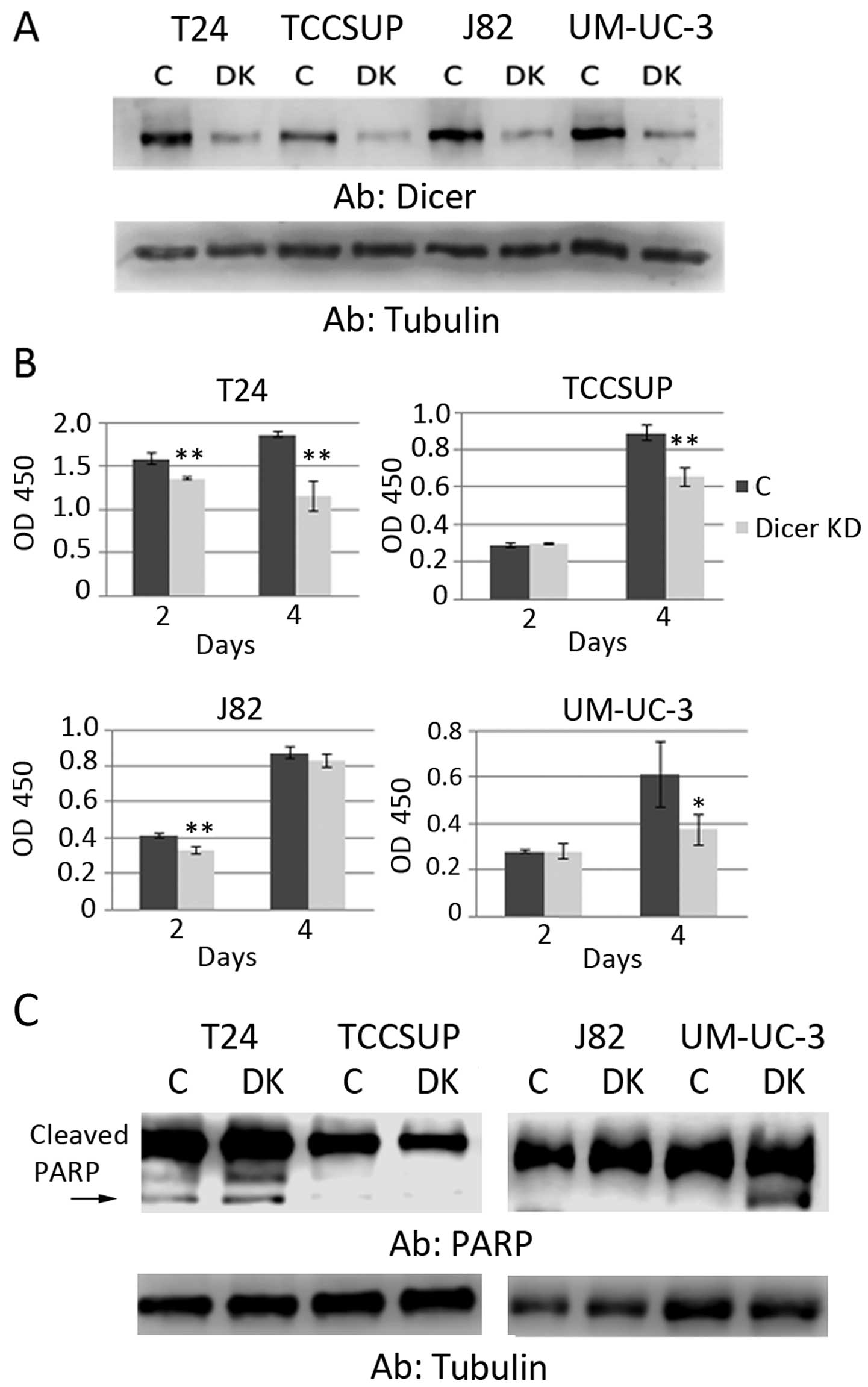
Knockdown of Dicer in UCCB cell lines
inhibits proliferation and induces apoptosis
We aimed to better understand how Dicer functions in
UCCB by knocking down Dicer in the four cell lines mentioned above
and assessing viability (Fig. 2A).
We found that a decreased expression of Dicer led to the
attenuation of cell growth in all the cell lines except for J82
(Fig. 2B). The results are
consistent with previous studies that show that decreased Dicer
impairs proliferation (12,13). The reason for J82 cells being
refractory to this treatment is unclear. However, this result
highlights known heterogeneity found in different tumors. These
data suggest that in certain contexts, Dicer increases cell
viability and plays an oncogenic role.
We assessed whether the decrease in cell viability
in response to Dicer knockdown was due to an increase in apoptosis
(Fig. 2C). Using western blot
analysis to detect cleaved PARP, we demonstrated that a decreased
Dicer expression induced apoptosis in T24 and UM-UC-3 cells. As
with the viability assay above, we observed no change in J82.
Notably, no change was observed in TCCSUP, suggesting that
decreased viability previously noted in this cell line may not be
due to apoptosis in these cells. Alternatively, apoptosis in this
cell line may not result in PARP cleavage.
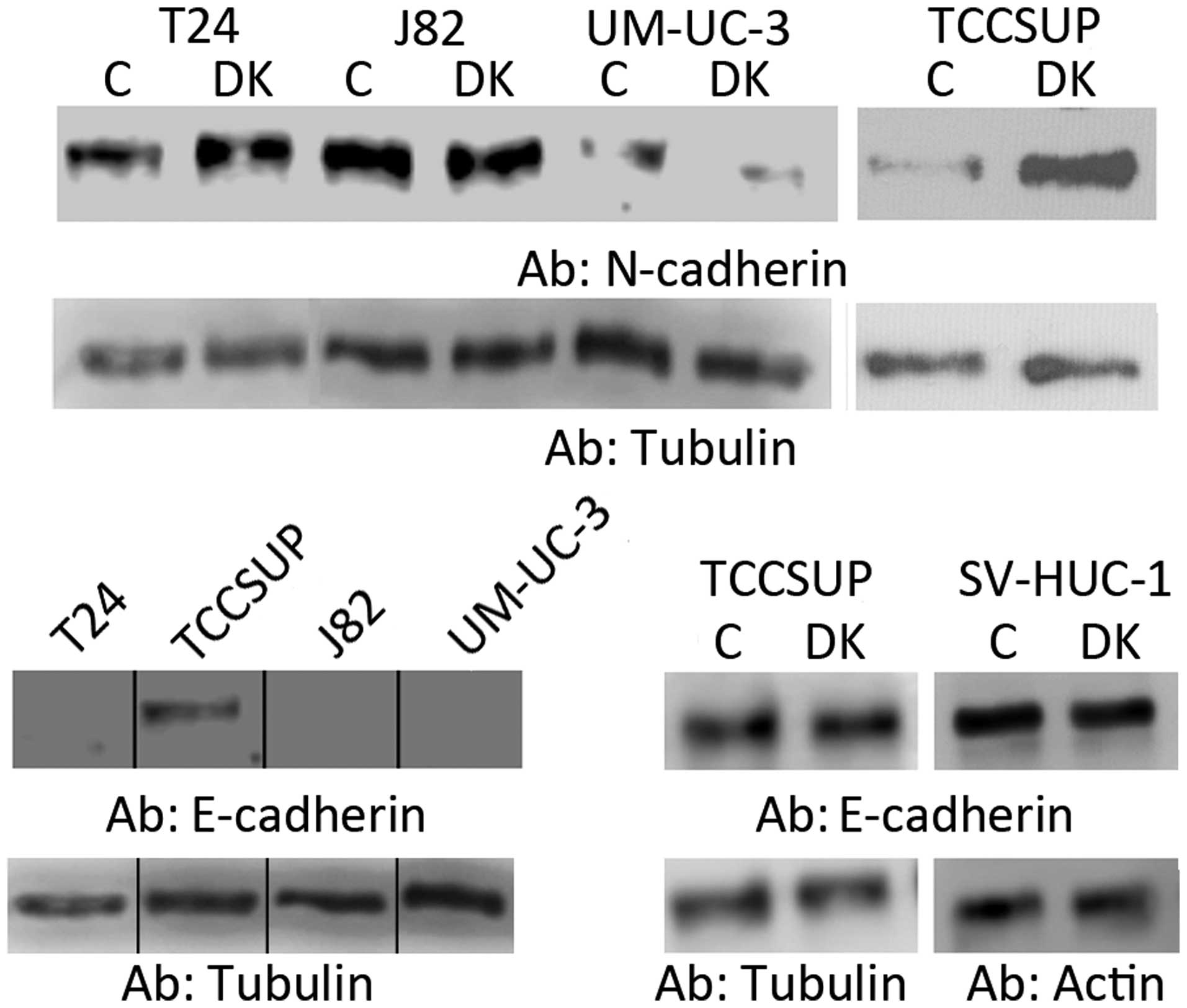
Decreased Dicer expression promotes a
mesenchymal phenotype
Recent evidence has shown that lower levels of Dicer
in breast and ovarian cancers are associated with an
epithelial-to-mesenchymal transition (EMT), a mesenchymal
phenotype, and leads to increased motility (9,15,16).
Since we and other investigators have shown that Dicer can be
downregulated in UCCB, we hypothesized that Dicer may function
similarly in this context and thus is also able to serve a
tumor-suppressor role (10,11). To assess a possible role in EMT for
Dicer, we examined the expression of the mesenchymal marker
N-cadherin and the epithelial marker E-cadherin (Fig. 3). We found that Dicer knockdown led
to an increase in N-cadherin expression in T24 and TCCSUP cells.
Depletion of Dicer did not cause an alteration of N-cadherin
expression in J82 cells. In UM-UC-3 cells, we detected a very low
expression of N-cadherin which appeared to decrease with Dicer
knockdown. E-cadherin was detected in TCCSUP cells but not in T24,
J82 and UM-UC-3 cells. However, E-cadherin did not change in
response to Dicer knockdown in TCCSUP cells. Additionally, Dicer
depletion did not induce the expression of E-cadherin in T24, J82
and UM-UC-3 cells (data not shown). These data indicate that
decreased Dicer expression promotes a more mesenchymal phenotype in
specific cell contexts via the upregulation of N-cadherin. However,
Dicer downregulation failed to promote complete EMT.
Additionally, we determined whether Dicer knockdown
promotes a mesenchymal phenotype in an immortalized non-transformed
cell line. Using SV-HUC-1 cells, we again assessed EMT marker
expression and found that the attenuation of Dicer did not reduce
E-cadherin expression (Fig. 3).
N-cadherin was not detected in these cells and its expression was
not induced by Dicer knockdown (data not shown).
Dicer knockdown increases cell migration
and invasion and MMP-2 expression
A study by Nieman et al demonstrated that
N-cadherin expression is more indicative of a motile and invasive
phenotype rather than a loss of E-cadherin (17). They also showed that a forced
expression of N-cadherin in E-cadherin-expressing cells promoted
motility and invasion despite the presence of E-cadherin (17). Thus, we conclude from our EMT data
that decreased Dicer expression can promote mesenchymal properties
and therefore may promote a more motile and invasive phenotype via
upregulation of N-cadherin in T24 and TCCSUP cells.
Using transwell migration assays, we determined
whether decreased Dicer expression promotes migration and invasion
(Fig. 4A and B). Because we hoped
to augment function, we wanted to choose the line that would allow
us to detect the most change. Based on previous studies of the
invasive propensities of UCCB cell lines, we decided that TCCSUP
cells would be best suited for these studies since they exhibit a
low invasive capability compared to T24 (18). Furthermore, TCCSUP cells exhibited
the most robust induction of N-cadherin expression in response to
Dicer knockdown. We also used SV-HUC-1 in the present study (also
shown to have low invasiveness) to further assess whether Dicer
inhibition regulates motility and whether it may function to
promote malignancy in a non-transformed context (19). Our data demonstrated that Dicer
knockdown increased the migration of TCCSUP cells but only
potentiated a trend towards increased motility in the SV-HUC-1
cells (p=0.06) (Fig. 4A). We
conclude from these studies that Dicer can play a role in
regulating motility of UCCB cells.
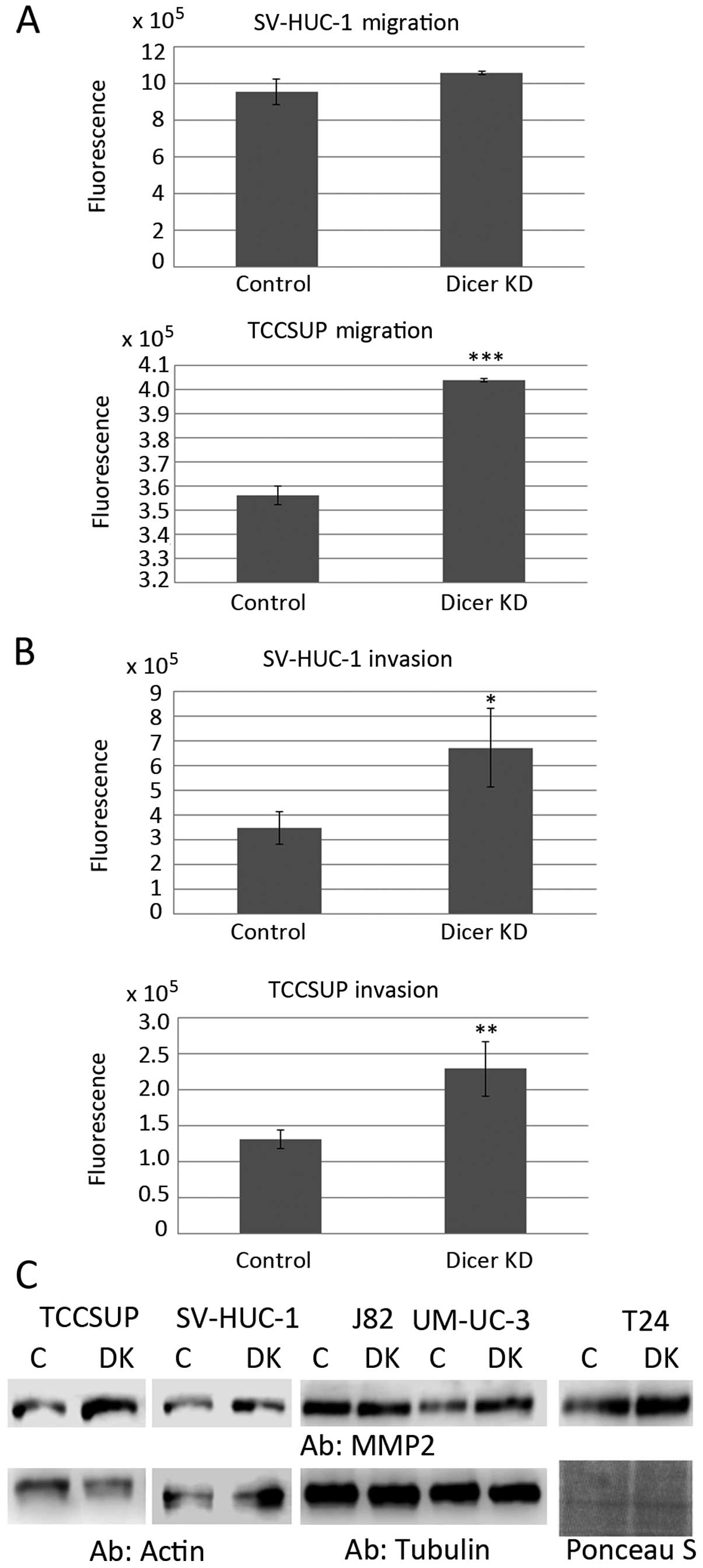 | Figure 4Effects of Dicer knockdown on
migration, invasion and MMP-2 expression. (A) Transwell migration
assays demonstrated the effect of Dicer knockdown on cell motility
in SV-HUC-1 and TCCSUP cells. (B) Matrigel-coated transwell
invasion assays demonstrated the effect of Dicer knockdown on cell
invasion in SV-HUC-1 and TCCSUP cells. (C) Western blot analyses
demonstrate the effect of Dicer knockdown on MMP-2 in TCCSUP,
SV-HUC-1, J82, UM-UC-3 and T24 cells. Actin, tubulin and Ponceau S
served as loading controls. C, control oligonucleotide; DK, siRNA
targeting Dicer; Dicer KD, Dicer knockdown; Ab, antibody used for
western blotting. Error bars are the standard deviation.
*p≤0.05, **p≤0.01, ***p≤0.001.
MMP-2, matrix metalloproteinase-2. |
Studies have also shown that decreased levels of
Dicer led to increased cell invasion (20). Thus, in addition to assessing
migration, we examined whether Dicer regulates the invasive ability
of UCCB cells (Fig. 4B). Using a
matrigel-coated transwell assay, we demonstrated that Dicer
knockdown increases invasion almost 2-fold in the TCCSUP and
SV-HUC-1 cells suggesting that lower levels of Dicer may potentiate
a more metastatic phenotype. Western blot analyses demonstrated
that Dicer knockdown also led to increased levels of MMP-2 in the
two cell lines (Fig. 4C).
Additionally, we showed that Dicer knockdown increased the
expression of MMP-2 in T24 and UM-UC-3 cells, but not in J82 cells
Fig. 4C). It has been shown that
MMP-2 expression and activity are associated with increased stage
and invasiveness of bladder cancer, respectively (21,22).
These data suggest that decreased Dicer may increase the invasive
abilities of UCCB cells and demonstrate a mechanism by which Dicer
plays a tumor-suppressor role.
Decreased Dicer expression leads to
attenuated expression of invasion-associated miRNAs
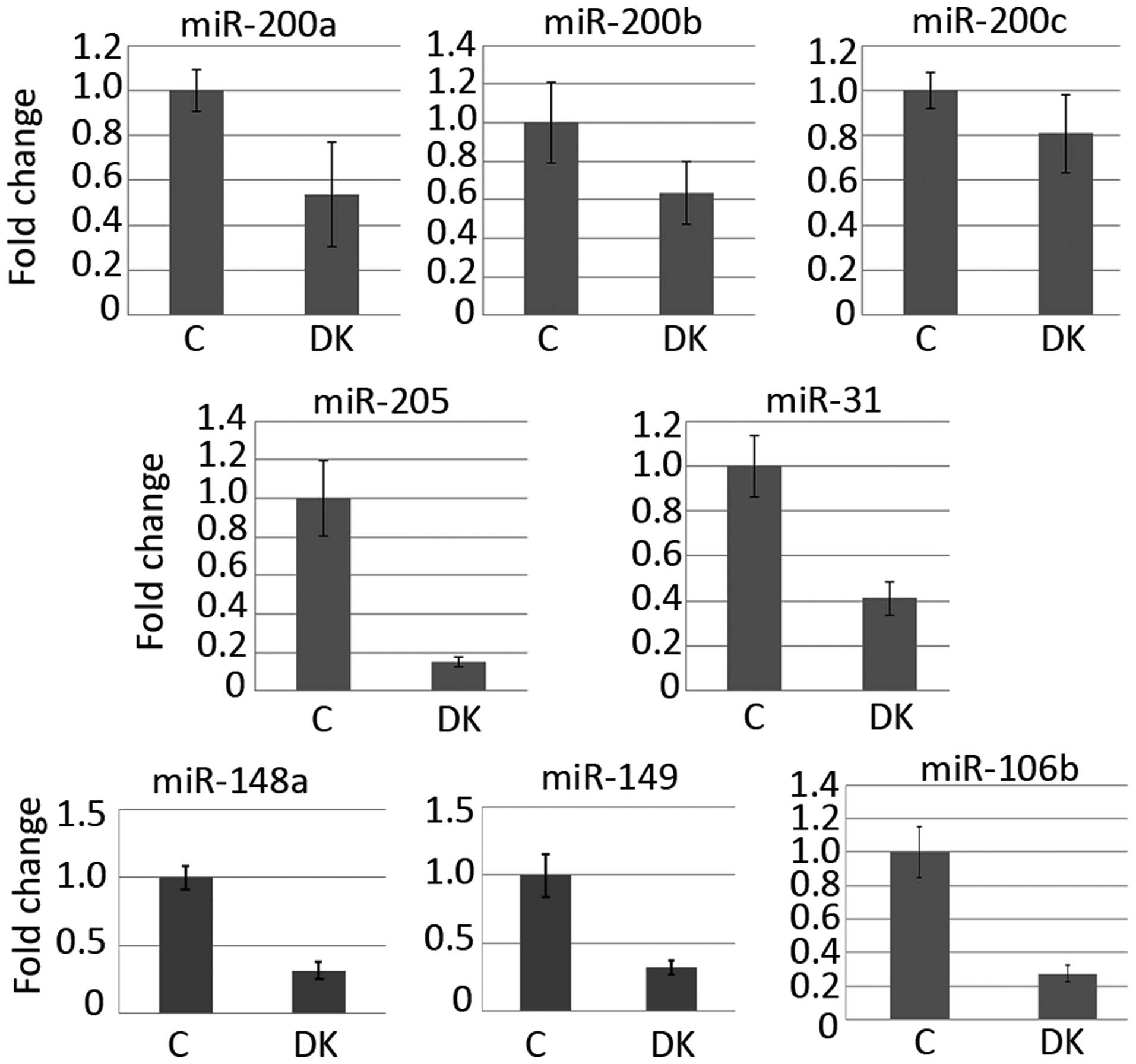
We used qPCR to assess the expression of miRNAs
associated with a motile/invasive phenotype (Fig. 5). Based on Dicer's known functions,
we hypothesized that miRNAs may be ultimately responsible for the
effects observed when Dicer is knocked down. In a study by Wszolek
et al, a panel of invasion-associated miRNAs was determined
using qPCR comparing 31 invasive UCCB lesions to 26 non-invasive
UCCB lesions (23). From this
panel, 5 miRNAs were selected to investigate in response to Dicer
knockdown. We selected the well-characterized miR-200a/b/c in
addition to miR-31, which has been shown to negatively correlate
with UCCB patient progression and mortality, and miR-205 which was
demonstrated to have strong discriminatory power in distinguishing
invasive UCCB tumors from non-invasive UCCB tumors (24–26).
Wzsolek et al showed that the overexpression of these miRNAs
in UM-UC-3 cells attenuated invasion using a transwell
matrigel-coated membrane assay (23). In response to Dicer knockdown, we
found small decreases in the levels of miR-200a/b/c (Fig. 5). However, we found a >5-fold
reduction in miR-205 levels and a ~2-fold decrease in miR-31
expression (Fig. 5). These data
along with the study by Wzsolek suggested that decreased Dicer
expression may potentiate cell invasion via decreased expression of
miRNAs (23). However, we
hypothesized that decreased Dicer could lead to global
downregulation of miRNAs. Using qPCR, we assessed the expression of
additional miRNAs (miR-148a, miR-149 and miR-106b) associated with
invasion in other contexts and found these were also downregulated
in response to attenuated Dicer levels (Fig. 5) (27–29).
Our data suggest that pan miRNA expression may be decreased along
with decreased Dicer levels and that the global downregulation of
miRNAs promotes an invasive phenotype.
Discussion
The expression of Dicer in UCCB is unclear and its
function in UCCB tumorigenesis is only partially understood. The
present study investigated the possible bases of differing results
while expanding our current understanding of Dicer in these tumors.
We demonstrated that Dicer expression was decreased in UCCB tumor
tissue using qPCR, a result that is consistent with previous
reports (10,11). However, a study by Han et al
demonstrated an increased expression of Dicer in UCCB (12). We hypothesized that Dicer expression
is variable in UCCB tumors and may function in distinct ways.
Two prior reports indicate that Dicer is oncogenic
(12,13). Our data are consistent with these
findings demonstrating that attenuation of Dicer in UCCB cell lines
results in decreased viability. However, J82 cells proved
refractory to this treatment. We also showed that Dicer knockdown
induced cell death in T24 and UM-UC-3 cells, but this was not
evident in J82 or TCCSUP cells. Our results emphasize the
heterogeneity of cancer and demonstrate that the importance of
Dicer in cellular viability varies with context. Collectively,
these data suggest that Dicer plays an oncogenic role by promoting
cell viability and inhibiting cell death. However, our and previous
studies have demonstrated the downregulation of Dicer in UCCB,
suggesting that Dicer also functions as a tumor suppressor
(10,11). We resolved this contradiction by
assessing the role of Dicer in regulating motility and
invasion.
Several lines of evidence have shown that Dicer is
involved in elements of the metastatic cascade (9,15,16).
We hypothesized that Dicer downregulation in UCCB may promote EMT
and increase migration and invasion. Our data demonstrates that
Dicer knockdown promoted a mesenchymal phenotype by inducing
N-cadherin expression in T24 and TCCSUP cells. While decreased
Dicer expression failed to promote complete EMT, a previous study
has shown that the expression of N-cadherin is more indicative of a
motile and invasive phenotype (17).
We next assessed migration and found that Dicer
knockdown led to an increase in migration in TCCSUP cells. A trend
towards enhancement of migration in SV-HUC-1 cells was identified.
These data suggest that in the context of UCCB, Dicer is able to
enhance migratory propensity. Notably, we found that Dicer
knockdown enhanced invasion almost 2-fold in TCCSUP and SV-HUC-1
cells. This phenotypic change was accompanied by an increase in
MMP-2 protein expression, suggesting a potential mechanism by which
Dicer alters invasiveness. Additionally, decreased Dicer levels led
to an increase in MMP-2 in T24 and UM-UC-3 cells, indicating this
effect may be widespread in UCCB. We did not detect this change in
J82 cells, which were an outlier throughout the present study. J82
cells may have evolved mechanisms that reduce their dependence on
Dicer. Our data suggest that decreased Dicer expression may promote
a more malignant phenotype by promoting motility and invasion.
The present findings show that the downregulation of
Dicer led to decreased levels of key miRNAs known to be negatively
associated with invasion in UCCB. However, Dicer knockdown may also
affect other miRNAs and could lead to a general downregulation of
miRNAs. We assessed the levels of additional miRNAs and found
decreased levels of these. This led us to hypothesize that the
overall outcome of a widespread downregulation of miRNAs promotes
an invasive phenotype in this disease. Consistent with this
hypothesis, several studies have correlated the downregulation of
Dicer with metastasis and metastatic potential (5,16,30,31). A
study by Luo et al in nasopharyngeal cancer showed that
lower levels of Dicer led to an overall decrease of most miRNAs
(32). This was associated with
increased invasion and mobility (32). Iliou et al demonstrated that
Dicer impairment led to increased colon cancer cell metastasis and
this was associated with a decreased expression of key EMT/invasion
miRNAs (miR-200 family, miR-34a, miR-126 and miR-335) (33).
The present study in conjunction with other studies
suggests a dual nature for Dicer. It has been postulated that Dicer
serves different roles in different contexts and we hypothesized
that as cancer progresses, the requirement for Dicer and miRNA
expression varies. It is thought that tumors are very heterogeneous
and that the invasive edge of a tumor can be quite different than
the bulk of the tumor (34). The
varying levels of Dicer potentiate different phenotypes at
strategic time-points during tumor formation regulating robust
proliferation and invasive capacity. Zhang et al showed that
Dicer promoted the proliferation of prostate cancer cells (35). However, despite this attribute and
an increased expression of Dicer in this disease, it was also shown
that relatively lower levels of Dicer promoted a more motile and
invasive phenotype (35). In the
study by Iliou et al, it was demonstrated that Dicer
impairment led to an increase in stemness and an increased capacity
for tumor initiation suggesting that Dicer plays a role in cancer
stem cells and may be involved in seeding metastases (33). Our data along with previous
literature suggest a complex and multifaceted role for Dicer in
cancer.
In conclusion, the present study has demonstrated
that Dicer has a dual nature in UCCB as it plays the role of an
oncogene by promoting proliferation and viability, and the role of
a tumor suppressor by inhibiting motility and invasion. We
hypothesized that the requirement for Dicer and its role in
tumorigenesis may be different at distinct stages of the
disease.
Abbreviations:
|
SDS-PAGE
|
sodium dodecyl sulfate-polyacrylamide
gel electrophoresis
|
|
PBS-T
|
0.1% Tween-phosphate-buffered
saline
|
|
HRP
|
horseradish peroxidase
|
|
PARP
|
poly(ADP-ribose) polymerase
|
|
qPCR
|
quantitative polymerase chain
reaction
|
|
FBS
|
fetal bovine serum
|
|
siRNA
|
small-interfering RNA
|
Acknowledgments
We thank Zachary Caudle for his contributions to the
present study. The present study is based on a study supported in
part by the US Department of Veterans Affairs, Office of Research
and Development, Biomedical Laboratory Research Program (VA Merit
grant BX001079 to M.M.). The contents of this manuscript do not
represent the views of the Department of Veterans Affairs or the
United States Government.
References
|
1
|
Foulkes WD, Priest JR and Duchaine TF:
DICER1: Mutations, microRNAs and mechanisms. Nat Rev Cancer.
14:662–672. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chiosea S, Jelezcova E, Chandran U,
Acquafondata M, McHale T, Sobol RW and Dhir R: Up-regulation of
Dicer, a component of the microRNA machinery, in prostate
adenocarcinoma. Am J Pathol. 169:1812–1820. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Faber C, Horst D, Hlubek F and Kirchner T:
Overexpression of Dicer predicts poor survival in colorectal
cancer. Eur J Cancer. 47:1414–1419. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bian XJ, Zhang GM, Gu CY, Cai Y, Wang CF,
Shen YJ, Zhu Y, Zhang HL, Dai B and Ye DW: Down-regulation of Dicer
and Ago2 is associated with cell proliferation and apoptosis in
prostate cancer. Tumour Biol. 35:11571–11578. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ma X, Fan Y, Gao Y, Zhang Y, Huang Q, Ai
Q, Ni D, Chen W, Zhang P, Song E, et al: Dicer is down-regulated in
clear cell renal cell carcinoma and in vitro Dicer knockdown
enhances malignant phenotype transformation. Urol Oncol.
32:46.e9–e17. 2014. View Article : Google Scholar
|
|
6
|
Karube Y, Tanaka H, Osada H, Tomida S,
Tatematsu Y, Yanagisawa K, Yatabe Y, Takamizawa J, Miyoshi S,
Mitsudomi T, et al: Reduced expression of Dicer associated with
poor prognosis in lung cancer patients. Cancer Sci. 96:111–115.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guo X, Liao Q, Chen P, Li X, Xiong W, Ma
J, Li X, Luo Z, Tang H, Deng M, et al: The microRNA-processing
enzymes: Drosha and Dicer can predict prognosis of nasopharyngeal
carcinoma. J Cancer Res Clin Oncol. 138:49–56. 2012. View Article : Google Scholar
|
|
8
|
Merritt WM, Lin YG, Han LY, Kamat AA,
Spannuth WA, Schmandt R, Urbauer D, Pennacchio LA, Cheng JF, Nick
AM, et al: Dicer, Drosha, and outcomes in patients with ovarian
cancer. N Engl J Med. 359:2641–2650. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kuang Y, Cai J, Li D, Han Q, Cao J and
Wang Z: Repression of Dicer is associated with invasive phenotype
and chemoresistance in ovarian cancer. Oncol Lett. 5:1149–1154.
2013.PubMed/NCBI
|
|
10
|
Catto JW, Miah S, Owen HC, Bryant H, Myers
K, Dudziec E, Larré S, Milo M, Rehman I, Rosario DJ, et al:
Distinct microRNA alterations characterize high- and low-grade
bladder cancer. Cancer Res. 69:8472–8481. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu D, Tao J, Xu B, Li P, Lu Q and Zhang W:
Downregulation of Dicer, a component of the microRNA machinery, in
bladder cancer. Mol Med Rep. 5:695–699. 2012.
|
|
12
|
Han Y, Liu Y, Gui Y and Cai Z: Inducing
cell proliferation inhibition and apoptosis via silencing Dicer,
Drosha, and Exportin 5 in urothelial carcinoma of the bladder. J
Surg Oncol. 107:201–205. 2013. View Article : Google Scholar
|
|
13
|
Tao J, Wu D, Li P, Xu B, Lu Q and Zhang W:
microRNA-18a, a member of the oncogenic miR-17-92 cluster, targets
Dicer and suppresses cell proliferation in bladder cancer T24
cells. Mol Med Rep. 5:167–172. 2012.
|
|
14
|
Zeng S, Yang J, Zhao J, Liu Q, Rong M, Guo
Z and Gao W: Silencing Dicer expression enhances cellular
proliferative and invasive capacities in human tongue squamous cell
carcinoma. Oncol Rep. 31:867–873. 2014.
|
|
15
|
Moyret-Lalle C, Ruiz E and Puisieux A:
Epithelial-mesenchymal transition transcription factors and miRNAs:
'Plastic surgeons' of breast cancer. World J Clin Oncol. 5:311–322.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Grelier G, Voirin N, Ay AS, Cox DG,
Chabaud S, Treilleux I, Léon-Goddard S, Rimokh R, Mikaelian I,
Venoux C, et al: Prognostic value of Dicer expression in human
breast cancers and association with the mesenchymal phenotype. Br J
Cancer. 101:673–683. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nieman MT, Prudoff RS, Johnson KR and
Wheelock MJ: N-cadherin promotes motility in human breast cancer
cells regardless of their E-cadherin expression. J Cell Biol.
147:631–644. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kariko K, Malkowicz S, Li W, Kuo A and
Barnathan E: Invasive neoplastic uroepithelial cells express
high-levels of urokinase receptor and plasminogen receptor,
alpha-enolase. Int J Oncol. 3:1089–1095. 1993.PubMed/NCBI
|
|
19
|
Wu X, Obata T, Khan Q, Highshaw RA, De
Vere White R and Sweeney C: The phosphatidylinositol-3 kinase
pathway regulates bladder cancer cell invasion. BJU Int.
93:143–150. 2004. View Article : Google Scholar
|
|
20
|
Su X, Chakravarti D, Cho MS, Liu L, Gi YJ,
Lin YL, Leung ML, El-Naggar A, Creighton CJ, Suraokar MB, et al:
TAp63 suppresses metastasis through coordinate regulation of Dicer
and miRNAs. Nature. 467:986–990. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vasala K, Pääkkö P and
Turpeenniemi-Hujanen T: Matrix metal-loproteinase-2 immunoreactive
protein as a prognostic marker in bladder cancer. Urology.
62:952–957. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Papathoma AS, Petraki C, Grigorakis A,
Papakonstantinou H, Karavana V, Stefanakis S, Sotsiou F and Pintzas
A: Prognostic significance of matrix metalloproteinases 2 and 9 in
bladder cancer. Anticancer Res. 20:2009–2013. 2000.PubMed/NCBI
|
|
23
|
Wszolek MF, Rieger-Christ KM, Kenney PA,
Gould JJ, Silva Neto B, Lavoie AK, Logvinenko T, Libertino JA and
Summerhayes IC: A MicroRNA expression profile defining the invasive
bladder tumor phenotype. Urol Oncol. 29:794–801. e12011. View Article : Google Scholar
|
|
24
|
Wang S, Li Q, Wang K, Dai Y, Yang J, Xue
S, Han F, Zhang Q, Liu J and Wu W: Decreased expression of
microRNA-31 associates with aggressive tumor progression and poor
prognosis in patients with bladder cancer. Clin Transl Oncol.
15:849–854. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Neely LA, Rieger-Christ KM, Neto BS,
Eroshkin A, Garver J, Patel S, Phung NA, McLaughlin S, Libertino
JA, Whitney D, et al: A microRNA expression ratio defining the
invasive phenotype in bladder tumors. Urol Oncol. 28:39–48. 2010.
View Article : Google Scholar
|
|
26
|
Mongroo PS and Rustgi AK: The role of the
miR-200 family in epithelial-mesenchymal transition. Cancer Biol
Ther. 10:219–222. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang SL and Liu L: microRNA-148a inhibits
hepatocellular carcinoma cell invasion by targeting
sphingosine-1-phosphate receptor 1. Exp Ther Med. 9:579–584.
2015.PubMed/NCBI
|
|
28
|
Pan SJ, Zhan SK, Pei BG, Sun QF, Bian LG
and Sun BM: MicroRNA-149 inhibits proliferation and invasion of
glioma cells via blockade of AKT1 signaling. Int J Immunopathol
Pharmacol. 25:871–881. 2012.
|
|
29
|
Ni X, Xia T, Zhao Y, Zhou W, Wu N, Liu X,
Ding Q, Zha X, Sha J and Wang S: Downregulation of miR-106b induced
breast cancer cell invasion and motility in association with
overexpression of matrix metalloproteinase 2. Cancer Sci.
105:18–25. 2014. View Article : Google Scholar
|
|
30
|
Khoshnaw SM, Rakha EA, Abdel-Fatah TM,
Nolan CC, Hodi Z, Macmillan DR, Ellis IO and Green AR: Loss of
Dicer expression is associated with breast cancer progression and
recurrence. Breast Cancer Res Treat. 135:403–413. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Faggad A, Kasajima A, Weichert W,
Stenzinger A, Elwali NE, Dietel M and Denkert C: Down-regulation of
the microRNA processing enzyme Dicer is a prognostic factor in
human colorectal cancer. Histopathology. 61:552–561.
2012.PubMed/NCBI
|
|
32
|
Luo Z, Dai Y, Zhang L, Jiang C, Li Z, Yang
J, McCarthy JB, She X, Zhang W, Ma J, et al: miR-18a promotes
malignant progression by impairing microRNA biogenesis in
nasopharyngeal carcinoma. Carcinogenesis. 34:415–425. 2013.
View Article : Google Scholar
|
|
33
|
Iliou MS, da Silva-Diz V, Carmona FJ,
Ramalho-Carvalho J, Heyn H, Villanueva A, Muñoz P and Esteller M:
Impaired DICER1 function promotes stemness and metastasis in colon
cancer. Oncogene. 33:4003–4015. 2014. View Article : Google Scholar :
|
|
34
|
Tzamali E, Grekas G, Marias K and Sakkalis
V: Exploring the competition between proliferative and invasive
cancer phenotypes in a continuous spatial model. PLoS One.
9:e1031912014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang B, Chen H, Zhang L, Dakhova O, Zhang
Y, Lewis MT, Creighton CJ, Ittmann MM and Xin L: A dosage-dependent
pleiotropic role of Dicer in prostate cancer growth and metastasis.
Oncogene. 33:3099–3108. 2014. View Article : Google Scholar :
|