Introduction
Gastric cancer is the second most common cancer
worldwide next to lung cancer (1),
and is a major public health issue in Korea. Currently, the
available treatments for gastric cancer are inadequate. With
advances in recent techniques, the overall 5-year survival rate of
gastric cancer patients ranges from 10 to 30% (2,3).
However, gastric cancer patients in advanced stages have limited
treatment options. Hence, there is an urgency to identify novel
therapeutic agents that can reduce the mortality of cancer patients
with few side effects.
Over the past few years, flavonoids from dietary
sources have attracted interest in preventing cancer with low
toxicity. Flavonoids are abundantly present in fresh fruits and
vegetables and have various health benefits (4,5).
Korean Citrus platymamma hort. ex Tanaka (Byungkyul in
Korean), a member of the Rutaceae family, has been used in
traditional herbal medicines in Korea. It contains abundant
flavonoids which have been reported to have various properties that
regulate the inflammatory response and halt carcinogenesis and
cancer progression (6). It is also
speculated that the intake of flavonoids reduces the risk of most
types of cancer (7,8). Our previous studies indicated that
flavonoids isolated from Korean Citrus aurantium L.
effectively inhibited the proliferation of various cancer cells by
inducing cell cycle arrest and apoptosis and suppressed
inflammatory mediators in L6 skeletal muscle cells (9–12).
However, the anticancer effects and the related mechanisms of
flavonoids from C. platymamma (FCP) have not yet been
elucidated.
Apoptosis is a critical cell death mechanism with a
distinctive phenotype and plays an important role in the mechanism
of chemotherapies against various types of carcinoma (13). Apoptosis signaling pathways mainly
function through two major pathways (the intrinsic pathway -
mitochondria-mediated apoptosis and the extrinsic pathway - death
receptor-mediated apoptosis) (14,15).
In addition, anticancer agents activate the PI3K/Akt signaling
pathway which is critical in regulating cell proliferation and
apoptosis (16). In addition,
mitogen-activated protein kinases (MAPKs) such as extracellular
signal-related kinase 1/2 (ERK1/2), c-Jun N-terminal kinase (JNK),
and p38 mitogen-activated protein kinases (p38 MAPKs) are also
involved in survival, proliferation and apoptosis (17). Thus, apoptosis plays crucial roles
in the anticancer properties of many anticancer molecules by
preventing or controlling abnormal cell development (18).
Based on the above evidence, in the present study,
we investigated the anticancer activity and the related mechanism
of FCP in AGS cells. The present study provides new insight for
understanding the mechanism of the anticancer effects of FCP in AGS
cells.
Materials and methods
Isolation of flavonoids from Korean
Citrus platymamma Hort. ex Tanaka
The fruit of C. platymamma hort. ex Tanaka
was obtained from the Animal Bio-Resources Bank (Jinju, Korea). The
flavonoids were isolated, and high-performance liquid
chromatography (HPLC) was performed at the Department of Chemistry,
Gyeongsang National University by Professor Sung Chul Shin as
described previously (19). The FCP
samples were stored at −70°C until further use.
Materials and chemicals
RPMI-1640 medium, fetal bovine serum (FBS) and
antibiotics (penicillin/streptomycin) were purchased from Gibco
(BRL Life Technologies, Grand Island, NY, USA).
3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide (MTT)
was obtained from Sigma-Aldrich (St. Louis, MO, USA). Antibodies to
Bcl-xL, Bax, caspase-3, -6, -8 and -9, cleaved caspase-3, poly(AdP
ribose) polymerase (PARP), cleaved PARP, p-Akt, JNK, p-JNK, p38,
p-p38, ERK1/2 and p-ERK1/2 were purchased from Cell Signaling
Technology (Danvers, MA, USA). The Akt and β-actin antibodies were
obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA) and
Millipore (Billerica, MA, USA), respectively. Horseradish
peroxidase (HRP)-coupled goat anti-mouse IgG and anti-rabbit IgG
were purchased from Enzo Life Sciences. Muse™ Cell Cycle kit and
Annexin V and Dead Cell kit were purchased from Millipore.
Materials and chemicals used for electrophoresis were obtained from
Bio-Rad (Hercules, CA, USA).
Cell culture and viability assay
Human gastric cancer AGS cells were obtained from
the Korean Cell Line Bank (Seoul, Korea). The AGS cells were
cultured in RPMI-1640 medium supplemented with 10% (v/v)
heat-inactivated FBS and 1% penicillin/streptomycin in a humidified
atmosphere with 5% CO2 at 37°C. To assess the effect of
FCP on AGS cell growth, the cells were seeded at 10×104
cells/ml in a 12-well plate and were treated with FCP at various
concentrations (25, 50, 75, 100, 125 and 150 µg/ml). After
24 h of incubation at 37°C, 100 µl of MTT (0.5 mg/ml) was
subsequently added to each well and incubated for 3 h at 37°C. The
culture medium was then removed, and 500 µl of dimethyl
sulfoxide (DMSO) was added to each well to dissolve the formazan
crystals. After mixing, absorbance was measured at 540 nm using an
enzyme-linked immunosorbent assay (ELISA) plate reader
(Bio-Rad).
Cell cycle distribution and analysis of
cell apoptosis
The AGS cells were incubated without or with FCP at
concentrations of 75 and 150 µg/ml for 24 h at 37°C, and the
cells were collected, washed with cold PBS, and then centrifuged.
The pellet was fixed in cold 70% ethanol (v/v) for 3 h at −20°C.
The cells were washed once with PBS, and 200 µl was
transferred to fresh tube. Muse Cell Cycle kit reagent (200
µl) was added to each tube and incubation was carried out
for 30 min at room temperature in the dark. For analysis of
apoptosis, the pellet was resuspended in 1 ml media and 100
µl was transferred to a new tube. Then, 100 µl of
Muse Annexin V and Dead Cell kit reagent was added to each tube and
incubation was carried out for 20 min at room temperature in the
dark. Then, stained samples were analyzed using a Muse™ Mini FACS
machine (Millipore).
Morphological change and DAPI fluorescent
staining
The AGS cells were treated with the indicated
concentrations of FCP for 24 h at 37°C, and the cells were washed
with cold PBS and fixed with 37% formaldehyde (1:4 dilution with
95% ethanol) for 10 min at room temperature. The fixed cells were
washed with PBS and stained with a 4′,6-diamidino-2-phenylindole
(DAPI, Vectashield H-1500; Vector Laboratories, Inc., Burlingame,
CA, USA). The nuclear morphology of the cells was examined by
fluorescence microscopy(x400 magnification; Leica, Germany).
Western blot analysis
For the western blot analysis, the AGS cells were
treated with the indicated concentrations of FCP for the indicated
times at 37°C and the cells were lysed in ice-cold RIPA buffer [1%
(w/w) NP-40, 1% (w/v) sodium deoxycholate, 0.1% (w/v) SDS, 0.15 M
NaCl, 0.01 M sodium phosphate buffer, pH 7.2, 2 mM EDTA, and 50 mM
NaF (as phosphatase inhibitor) and protease inhibitors]. The
protein concentrations were determined using a Bradford assay
(Bio-Rad) method (20). Proteins
were separated by 12% SDS-polyacrylamide gel electrophoresis
(SDS-PAGE) and transferred to a polyvinyldene fluoride (PVDF)
membrane (Immobilon-P, 0.45 µm; Millipore) using the TE 77
Semi-Dry Transfer Unit (GE Healthcare Life Sciences,
Buckinghamshire, UK). The membranes were incubated with the primary
antibodies overnight followed by a conjugated secondary antibody to
peroxidase. Blots were developed under an ECL detection system (GE
Healthcare Life Sciences). The bands were quantitatively analyzed
using the Image J program (http://rsb.info.nih.gov).
Statistical analysis
The statistical analysis was calculated by the
Student's t-test, using SPSS version 10.0 for Windows (SPSS,
Chicago, IL, USA). The results are expressed as the mean ± standard
deviation (SD) of at least three independent experiments. The
statistical significance was accepted as P< 0.05.
Results
Quantitative analysis and
characterization of FCP
The flavonoids were isolated from the fruit of C.
platymamma by using HPLC-MS/MS. Totally 13 peaks were
identified based on the HPLC retention time and the
ultraviolet-visible spectra of standard compounds in a library
(Fig. 1). The flavonoids were
identified according to the peaks of the HPLC chromatogram and the
mass-spectral and quantification data are provided in Table I.
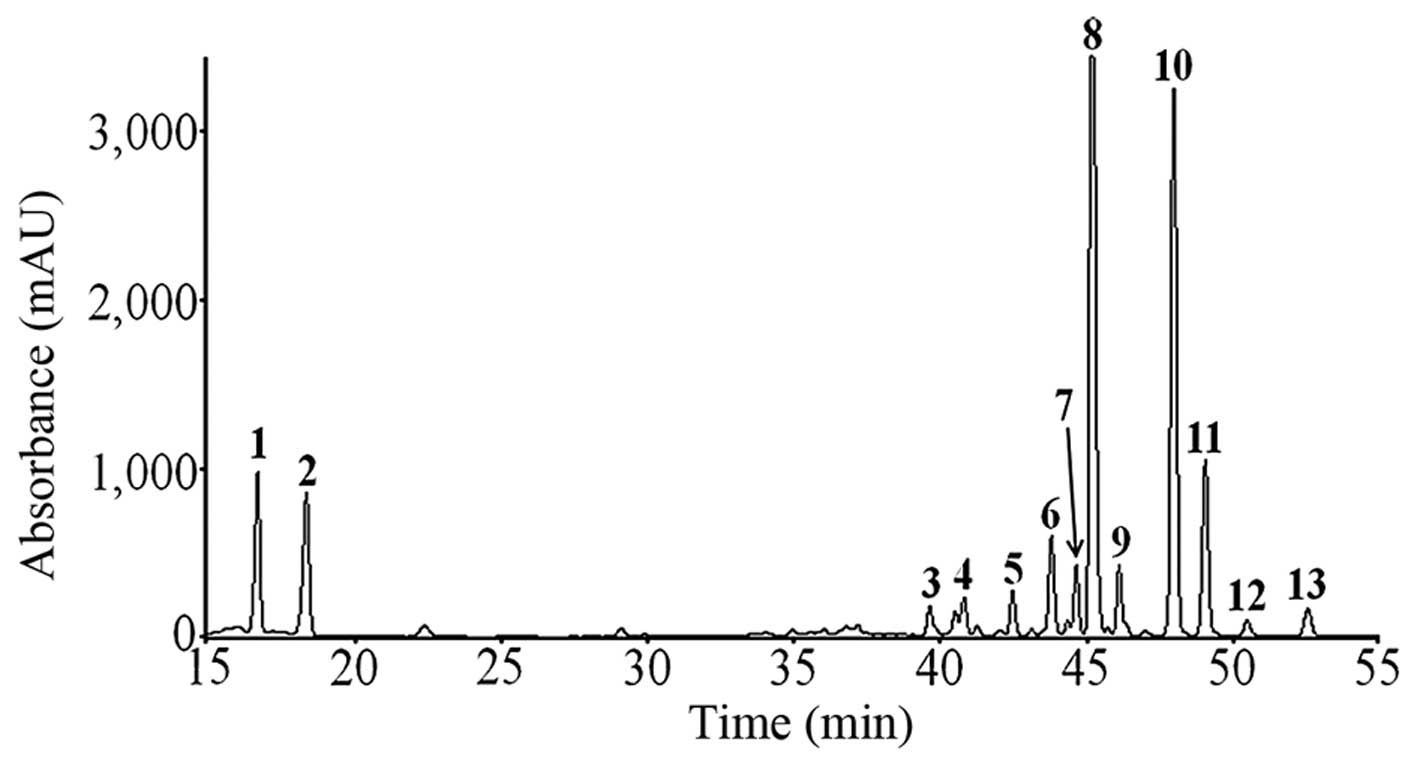 | Figure 1HPLC chromatogram patterns of C.
platymamma at 280 nm. The list of identified flavonoids from
the peaks included (1) naringin,
(2) hesperidin, (3, 4, 11 and 13)
hydroxypentamethoxyflavone, (5)
sinensetin, (6) pectolinarigenin,
(7) dihydroxytetramethoxyflavone,
(8) nobiletin, (9) heptamethoxyflavone, (10) tetramethyl-O-isoscutellarein, and
(12) hydroxyhexatamethoxyflavone.
HPLC, high-performance liquid chromatography. |
 | Table IList of identified flavonoids from
C. platymamma and the quantification data. |
Table I
List of identified flavonoids from
C. platymamma and the quantification data.
| Compound | RT (min) |
[M-H]−/[M-H]+ | MS/MS | Mean ± SD |
|---|
| 1 | Naringin | 16.93 | 579/− | 459, 313, 271, 193,
151 | 2,483.5±1.6 |
| 2 | Hesperidin | 18.45 | 609/− | 608, 325, 301 | 1,163.2±1.6 |
| 3 |
Hydroxypentamethoxyflavone | 39.88 | /389 | 374, 359, 341,
165 | 2,785.2±10.9 |
| 4 |
Hydroxypentamethoxyflavone | 39.88 | /389 | 374, 359, 341,
165 | 393.4±2.3 |
| 5 | Sinensetin | 42.57 | −/373 | 373, 358, 343, 339,
329, 320, 312, 283, 181, 151 | 384.7±4.2 |
| 6 |
Pectolinarigenin | 43.84 | /313 | 313, 285, 181, 156,
153, 135 | 525.8±13.2 |
| 7 |
Dihydroxytetramethoxyflavone | 44.76 | 375 | 375, 360, 345, 342,
314, 302, 299, 285, 271, 227, 212, 197, 169, 166, 149 | 370.2±4.2 |
| 8 | Nobiletin | 45.32 | −/403 | 388, 373, 355, 327,
211, 165 | 3,911.9±5.5 |
| 9 |
Heptamethoxyflavone | 46.15 | /433 | 418, 403, 385, 211,
165 | 674.5±4.4 |
| 10 |
Tetramethyl-O-isoscutellarein | 47.99 | −/343 | 343, 328, 313, 299,
285, 211, 181, 135, 133 | 3,417.4±11.8 |
| 11 |
Hydroxypentamethoxyflavone | 49.35 | /389 | 374, 359, 341,
165 | 1,258.3±7.7 |
| 12 |
Hydroxyhexatamethoxyflavone | 50.56 | /419 | 404, 389, 373, 361,
343, 328, 315, 283, 227, 165 | 154.5±3.5 |
| 13 |
Hydroxypentamethoxyflavone | 52.67 | 359 | 359, 344, 329, 311,
298, 286, 241, 224, 227, 211, 197, 183, 179, 135 | 258.5±1.7 |
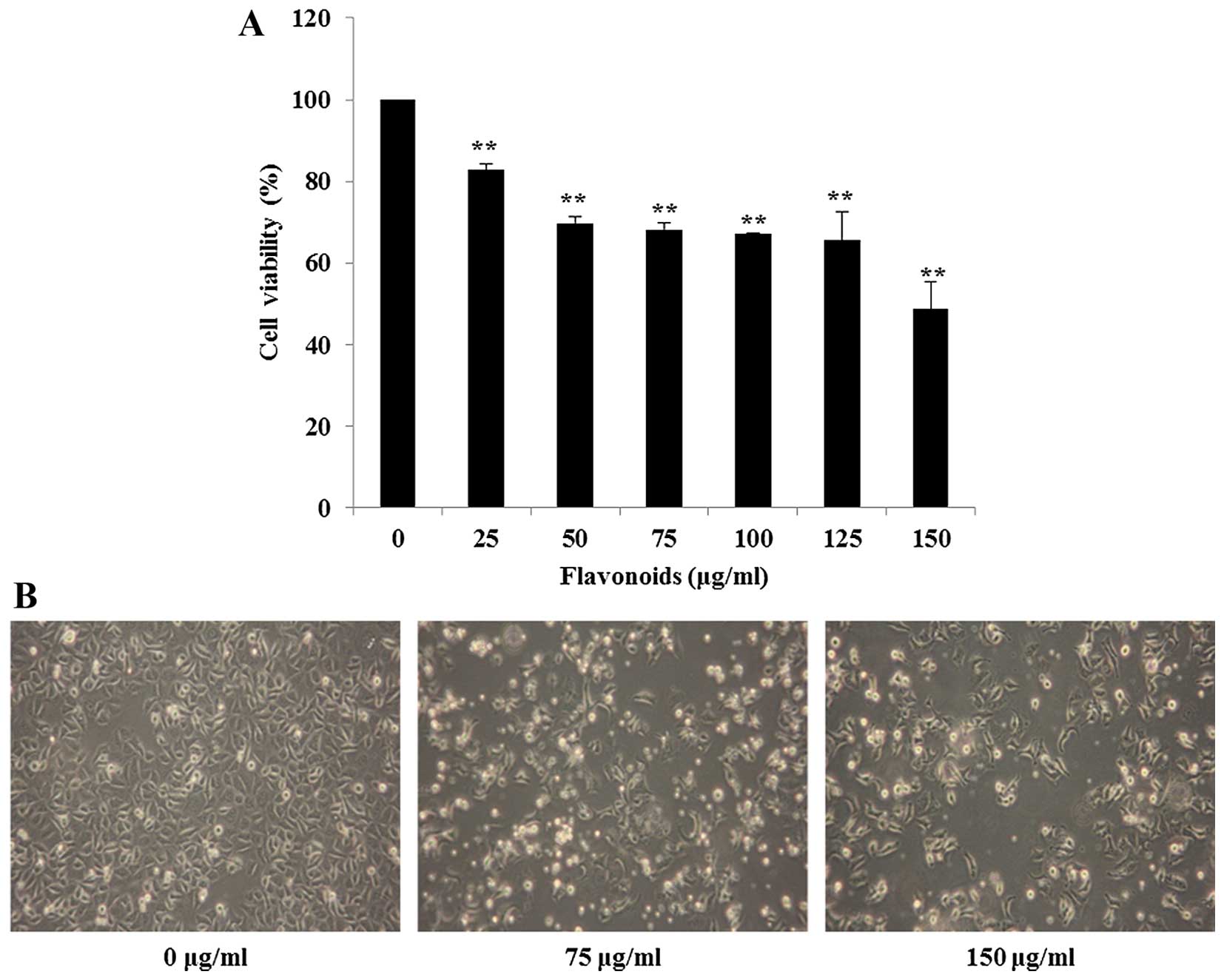
FCP inhibits the growth of AGS cells
To determine the appropriate inhibitory
concentrations of FCP, firstly AGS cells were treated with various
concentrations (0–150 µg/ml) for 24 h and cell viability was
evaluated by MTT assay. As shown in Fig. 2A, FCP showed a dose-dependent
inhibitory effect at 24 h when compared to the control (DMSO only),
and the 50% inhibitory concentration (IC50) value was
~150 µg/ml (P<0.01 for the FCP-treated group compared
with the control). Hence, we used FCP at concentrations of 0, 75
and 150 µg/ml for the subsequent experiments. Microscopic
examination revealed changes in cell shape, such as cell shrinkage
and a decrease in cell numbers was also observed in the FCP-treated
cells (Fig. 2B).
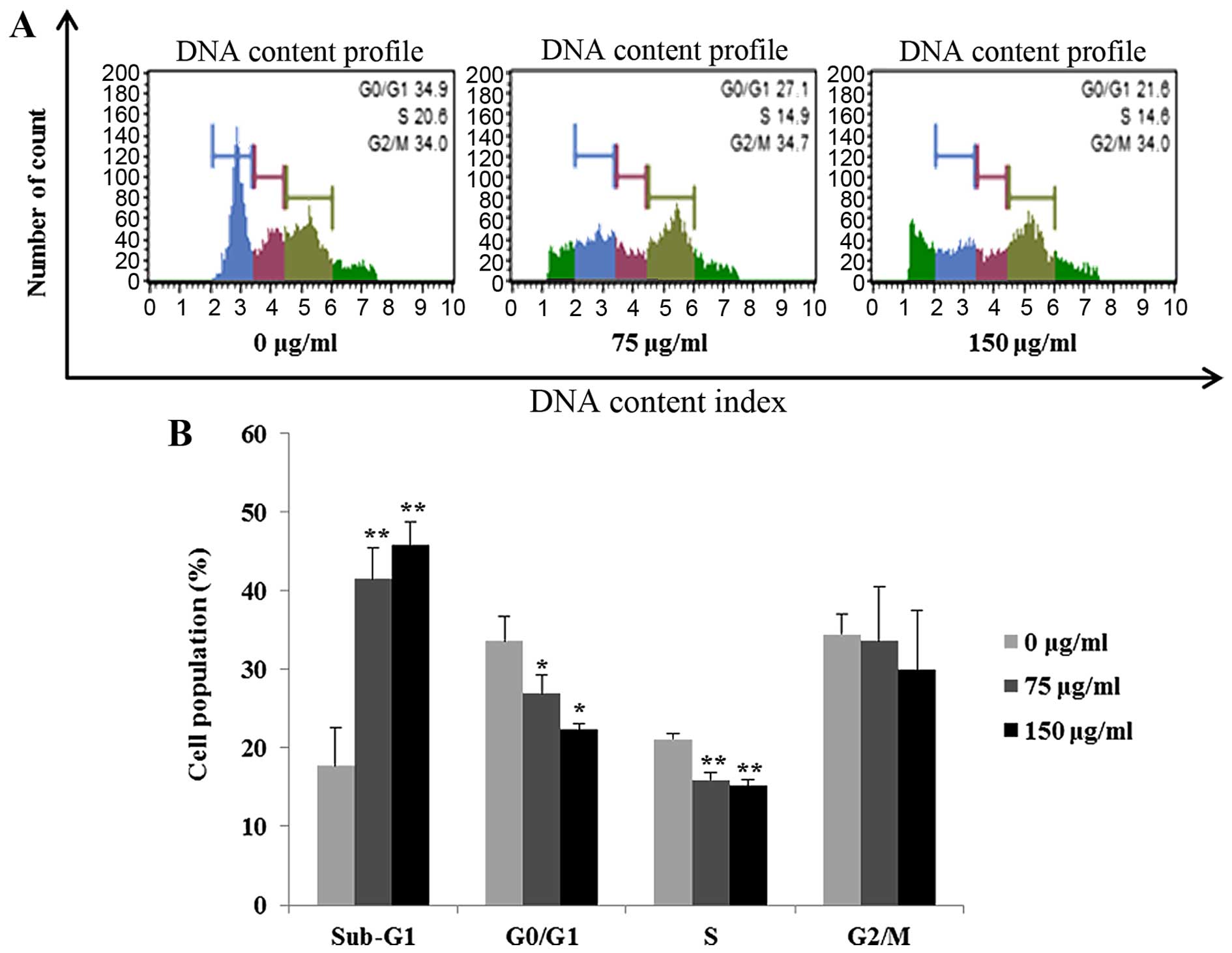
FCP induces apoptosis in AGS cells
Next, flow cytometry was performed to determine cell
cycle distribution and the population of cell death in the
FCP-treated AGS cells. FCP treatment increased the percentage of
the sub-G1 cells (apoptotic cell population) by 18, 41 (P<0.01)
and 45% (P<0.01) at 0, 75 and 150 µg/ml, respectively.
Meanwhile, FCP substantially decreased the G0/G1, S and G2/M
populations (Fig. 3). We also
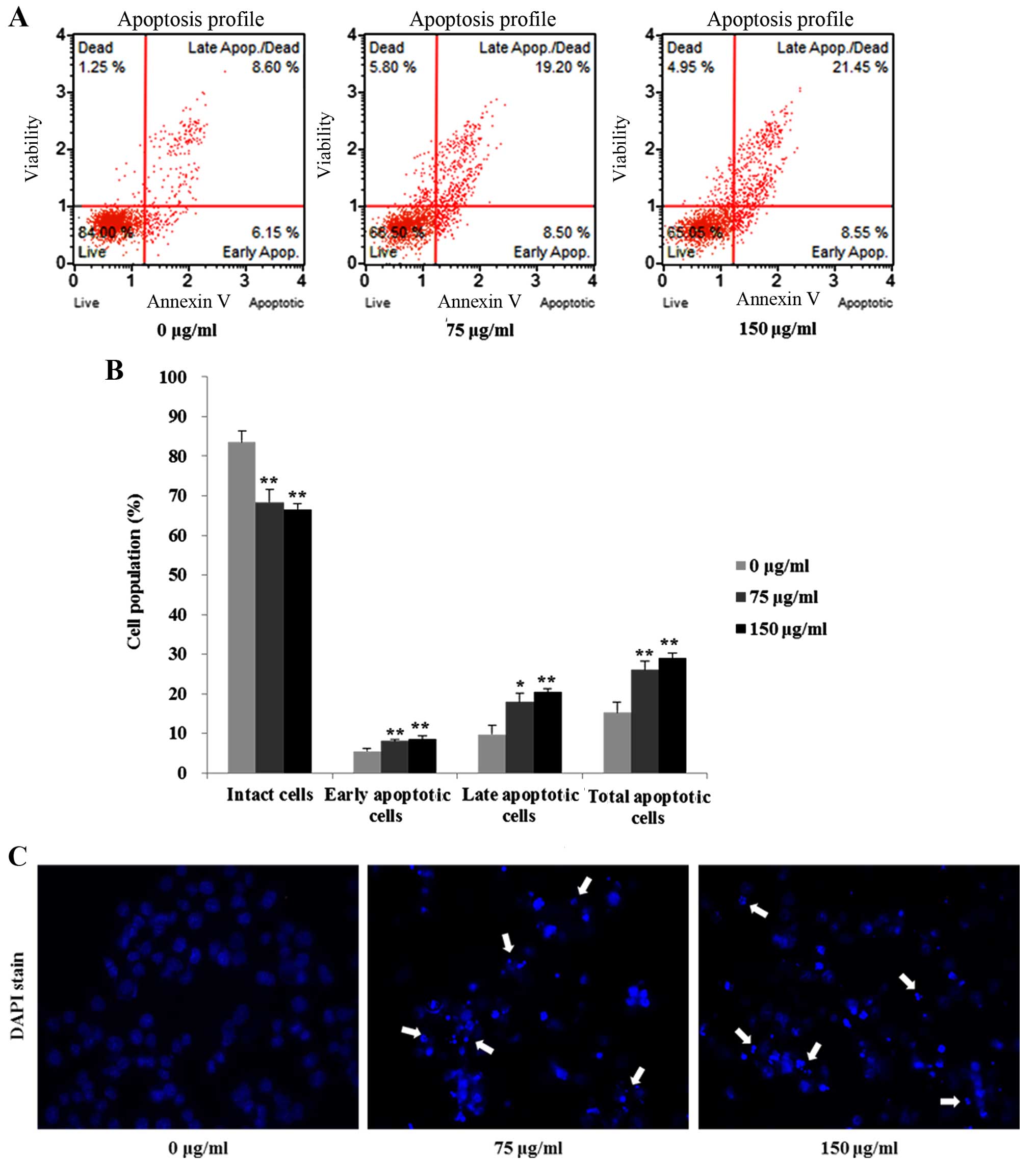
assessed the effect of FCP on the induction of apoptosis in AGS
cells by Annexin V-FITC/PI double-labeled staining and flow
cytometry. As shown in Fig. 4A and
B, FCP significantly increased the early apoptotic cell
proportion and the late apoptotic cell proportion of AGS cells in a
dose-dependent manner. Moreover, apoptotic changes such as nuclear
fragmentation and apoptotic bodies were also observed in the
FCP-treated AGS cells at 75 and 150 µg/ml by Hoechest 33342
staining (Fig. 4C). These results
suggest that FCP could induce cell death in the AGS cells.
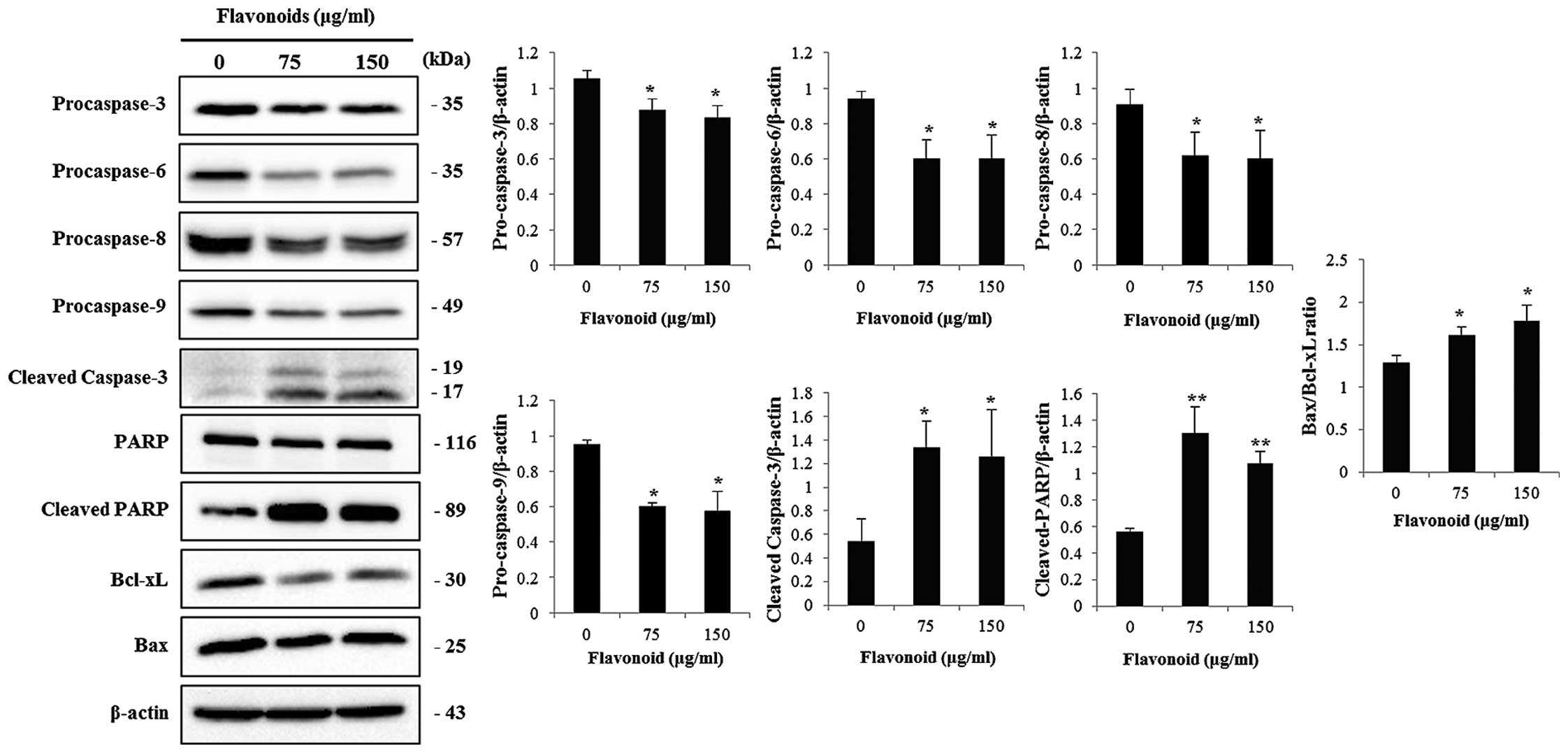
FCP induce caspase activation and
subsequent cleavage of PARP in AGS cells
Western blotting was performed to determine whether
FCP-induced cell death was caspase-dependent. In addition, we
examined the expression of apoptosis-related proteins, such as Bax
and Bcl-2, in the FCP-treated AGS cells. The results showed that
the expression of procaspase-3, -6, -8 and -9 was significantly
decreased while cleaved caspase-3 and cleaved PARP were
significantly increased in a dose-dependent manner (Fig. 5). no significant changes were found
in PARP expression. FCP also increased the Bax/Bcl-xL ratio in the
AGS cells in a dose-dependent manner. These results suggest that
FCP induced caspase-dependent apoptosis in AGS cells.
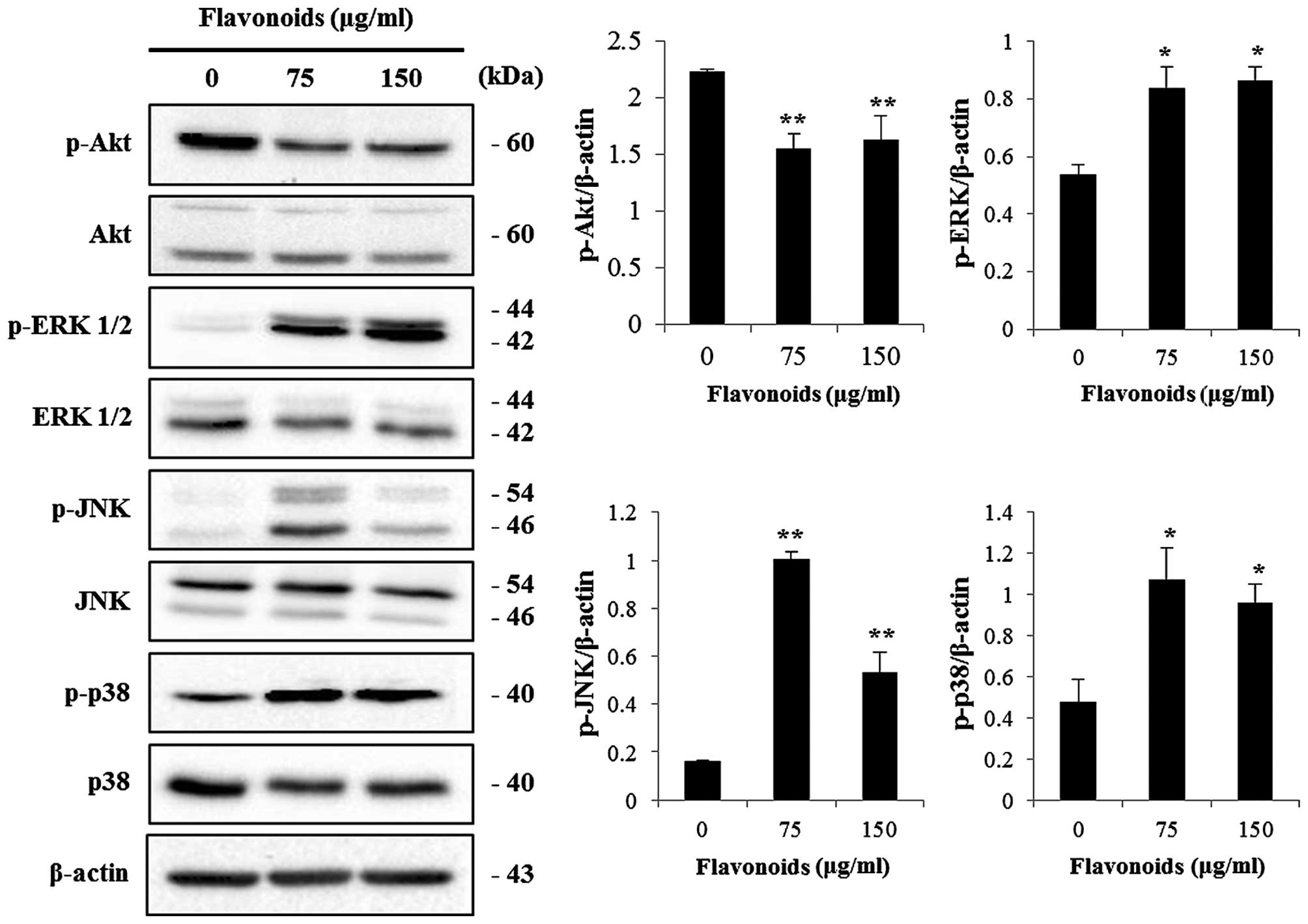
FCP modulates the PI3K/AKT and MAPK
pathways in the AGS cells
The PI3K/AKT and MAPK signaling pathways play an
important role in regulating cell proliferation and apoptosis.
Since the activity of AKT is regulated by phosphorylation, we
examined the phosphorylation status of PI3K/AKT and MAPKs by
immunoblotting during the FCP-induced apoptosis in AGS cells. FCP
significantly dephosphorylated AKT at 75 and 150 µg/ml but
no effects were found in total AKT (Fig. 6). Moreover, the phosphorylated forms
of ERK1/2, JNK and p38 MAPK were significantly increased at 75 and
150 µg/ml, but no effects were found on total ERK1/2, JNK
and p38 MAPK (Fig. 6). These
findings suggest that FCP induced the apoptosis in AGS cells by
modulating the PI3K/AKT and MAPK pathways.
Discussion
The present study was designed to determine whether
FCP induces cell death and to further investigate the underlying
mechanisms of the FCP-induced apoptosis of AGS cells. Flavonoids
are naturally occurring botanical polyphenols present in plant
foods and can safely modulate the physiological function and
enhance the anticancer activity against various human cancer cell
lines (21). Furthermore, the
pharmacological activities of FCP against inflammation, allergies,
viruses, cancer, and other ailments have been reported (22). In addition, flavonoids and
polyphenols from various herbal plants such as Scutellaria
baicalensis G., Lonicera japonica T. exhibit
anti-inflammatory and anticancer activities by inducing cell cycle
arrest and apoptosis in various cancer cell lines (23,24).
In addition, monomers such as naringin, nobiletin and hesperetin
exhibit anticancer effects by cell cycle arrest and the apoptosis
pathway in human cancer cell lines (25,26).
In the present study, we investigated the anticancer activity of
FCP on AGS human gastric cancer cells.
Firstly, FCP significantly suppressed the growth of
AGS cells in a dose-dependent manner. Evidence suggests that
apoptosis (type I programmed cell death) is the most popular
underlying mechanism by which various anticancer and
chemopreventive agents including natural compounds exert anticancer
effects (27). Previous studies
have demonstrated that the mechanism of cell apoptosis is through
the caspase signaling pathway. Recently, our studies demonstrated
that cell cycle aberrations often lead to apoptosis in various
cancer cell lines (11,12). Presently, accumulation of sub-G1
phase cells (indication of apoptosis) was found in the FCP-treated
AGS cells in a dose-dependent manner (Fig. 3). Furthermore, apoptosis was
confirmed by FITC-Annexin V and PI double staining (Fig. 4A and B). Similar results have been
reported on the induction of apoptosis in various cancer cell lines
(11,12,28).
In addition, cleaved nuclei and apoptotic bodies were found in the
FCP-treated cells (Fig. 4C). These
results revealed that FCP effectively suppressed the growth of AGS
cells and induced apoptosis.
For the evaluation of their underlying mechanisms,
immunoblotting was performed. The results showed that the
expression of procaspase -3, -6, -8 and -9 was significantly
downregulated in a dose-dependent manner. Caspase-3 is a crucial
executioner caspase that activates cleavage of PARP which results
in apoptosis. In our study, the increased expression of cleaved
caspase-3 simultaneously induced PARP cleavage (Fig. 5). These results indicate that FCP
induce apoptosis in a caspase-3-dependent manner. Moreover, the
Bcl-2 family plays an important role in apoptosis and are apoptotic
regulatory proteins which control the mitochondrial apoptotic
process. The pro-apoptotic and anti-apoptotic proteins of the Bcl-2
family in the cell, determines whether a cell lives or dies
(29). Bcl-xL interacts with the
mitochondrial plasma membrane and protects from other apoptotic
factors, such as Bax and Bak that prevents induced cytochrome
c from the plasma membrane. A previous study demonstrated
that nobiletin induces apoptosis in various tumor cell lines via
inhibition of overexpression of the Bcl family of proteins
(26). The ratio of Bax/Bcl-xL
appears to be a determining factor of apoptosis. In the present
study, Bcl-xL was significantly downregulated, whereas Bax protein
was unchanged while the ratio of Bax/Bcl-xL was upregulated in the
FCP-treated AGS cells (Fig. 5).
Cytochrome c can bind to APAF-1 when it is released from the
mitochondria into the cytosol by increasing the Bax/Bcl-xL ratio,
thus leading to the activation of caspase-3 and finally
apoptosis.
We further examined the phosphorylation status of
PI3K/AKT and MAPKs by immunoblotting to elucidate the molecular
mechanism and pathways involved in FCP-induced apoptosis. We
demonstrated that FCP inhibited the constitutive level of PI3K and
its downstream target AKT (Fig. 6),
which have been reported to regulate cell proliferation and
apoptosis. Similarly to our results, the inhibition of the PI3K/Akt
signaling pathway can induce the apoptosis of various types of
cancer cells (30,31). Moreover, the MAPK signaling pathway
is also involved in survival, proliferation and apoptosis and
consists of three major groups: ERKs, JNKs and the p38 MAPKs
(32). Even though activation of
the ERK1/2 pathway is generally associated with cell proliferation
and survival, it has also been reported to stimulate apoptosis in T
cells through Fas ligand expression (33). In addition, ERK1/2 induces apoptosis
via prevention of the inactivation of a member of the pro-apoptotic
Bcl-2 family, BAD (34). Moreover,
JNK is a downstream kinase of the MAPK family which has been
reported to regulate the expression of receptors such as Fas and
the Fas ligand in apoptosis (35).
JNK is also involved in the intrinsic apoptosis pathway where
activated JNK modulates the expression of pro-apoptotic proteins
such as Bid and Bax and stimulates the release of cytochrome
c from mitochondria into the cytosol (36). Activated JNK also downregulates the
expression of Bcl-2, an anti-apoptotic protein (37). It has also been demonstrated that
activated p38 MAPK stimulates apoptosis in various cell lines in
response to a variety of stimuli (38). Similar expression patterns were
observed in our experiments. Phosphorylated forms of ERK1/2, JNK
and p38 MAPK were increased in the FCP-treated AGS cells (Fig. 6). These results revealed that
PI3K/AKT and MAPKs are involved in the apoptosis induced by FCP in
AGS cells.
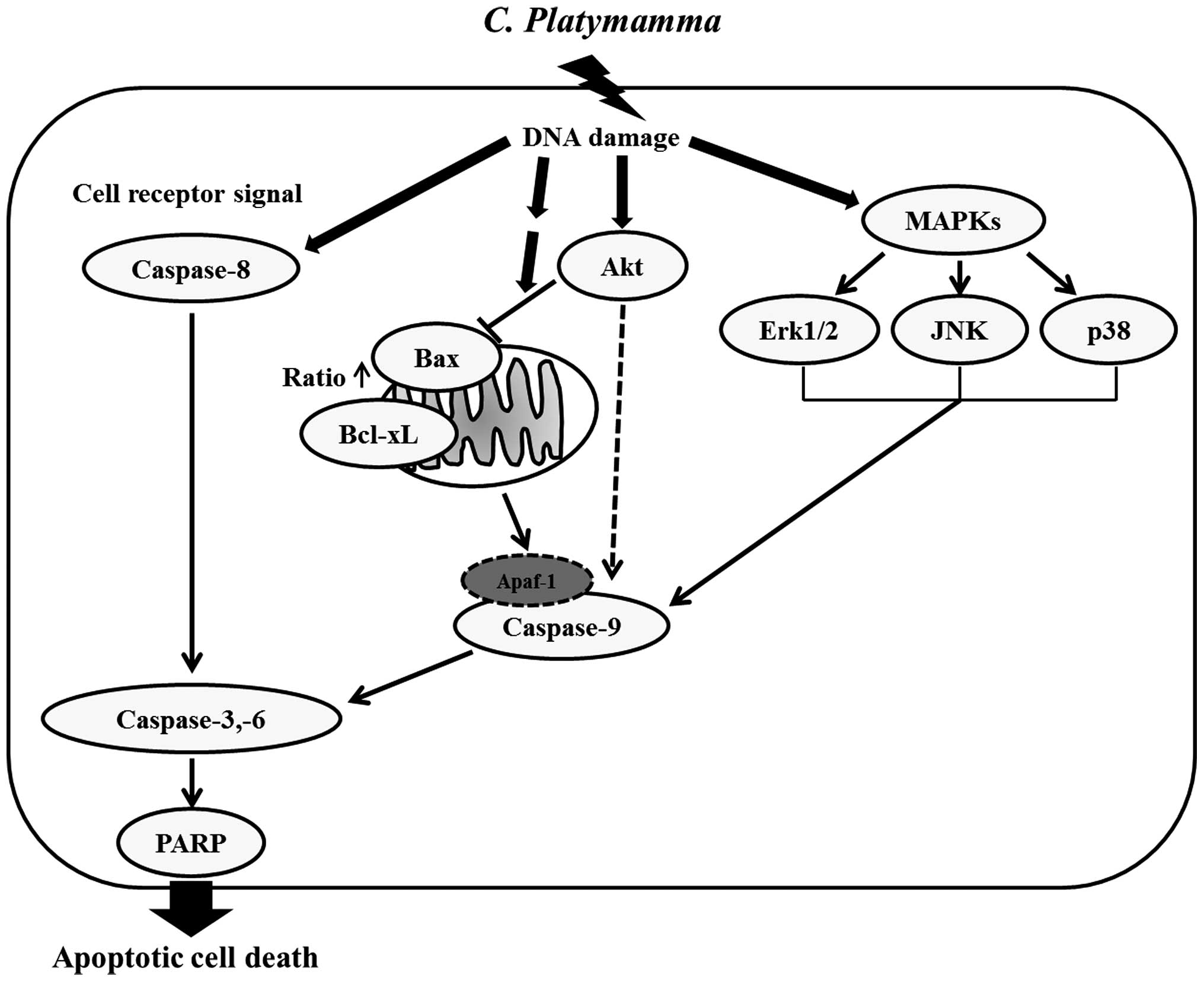
In conclusion, we demonstrated that FCP suppressed
cell viability and induced caspase-dependent cell death in the AGS
cells. The induction of apoptosis triggered in the FCP-treated AGS
cells was modulated by the PI3K/AKT and MAPK signaling pathways
(Fig. 7). To our knowledge, this is
the first study to elucidate the anticancer properties of FCP in
AGS cells. Thus, FCP may be a potential chemotherapeutic agent for
the treatment of human gastric cancer.
Acknowledgments
The present study was supported by a grant from the
National Research Foundation (NRF) of Korea funded by the Ministry
of Science, ICT and Future Planning (nos. 2012M3A9B8019303 and
2012R1A2A2A06045015) and the National R&D Program for Cancer
Control, Ministry for Health, Welfare and Family Affairs, Republic
of Korea (no. 0820050).
References
|
1
|
Crew KD and Neugut AI: Epidemiology of
gastric cancer. World J Gastroenterol. 12:354–362. 2006.PubMed/NCBI
|
|
2
|
Green D, Ponce de Leon S, Leon-Rodriguez E
and Sosa-Sanchez R: Adenocarcinoma of the stomach: Univariate and
multivariate analysis of factors associated with survival. Am J
Clin Oncol. 25:84–89. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Harrison LE, Karpeh MS and Brennan MF:
Extended lymphadenectomy is associated with a survival benefit for
node-negative gastric cancer. J Gastrointest Surg. 2:126–131. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Moufida S and Marzouk B: Biochemical
characterization of blood orange, sweet orange, lemon, bergamot and
bitter orange. Phytochemistry. 62:1283–1289. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Acunzo J, Katsogiannou M and Rocchi P:
Small heat shock proteins HSP27 (HspB1), αB-crystallin (HspB5) and
HSP22 (HspB8) as regulators of cell death. Int J Biochem Cell Biol.
44:1622–1631. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Havsteen B: Flavonoids, a class of natural
products of high pharmacological potency. Biochem Pharmacol.
32:1141–1148. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rossi M, Garavello W, Talamini R, La
Vecchia C, Franceschi S, Lagiou P, Zambon P, Dal Maso L, Bosetti C
and Negri SE: Flavonoids and risk of squamous cell esophageal
cancer. Int J Cancer. 120:1560–1564. 2007. View Article : Google Scholar
|
|
8
|
Theodoratou E, Kyle J, Cetnarskyj R,
Farrington SM, Tenesa A, Barnetson R, Porteous M, Dunlop M and
Campbell H: Dietary flavonoids and the risk of colorectal cancer.
Cancer Epidemiol Biomarkers Prev. 16:684–693. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Alaiya AA, Franzén B, Auer G and Linder S:
Cancer proteomics: From identification of novel markers to creation
of artifical learning models for tumor classification.
Electrophoresis. 21:1210–1217. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim JA, Park HS, Kang SR, Park KI, Lee DH,
Nagappan A, Shin SC, Lee WS, Kim EH and Kim GS: Suppressive effect
of flavonoids from Korean Citrus aurantium L. on the expression of
inflammatory mediators in L6 skeletal muscle cells. Phytother Res.
26:1904–1912. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Park KI, Park HS, Nagappan A, Hong GE, Lee
H, Kang SR, Kim JA, Zhang J, Kim EH, Lee WS, et al: Induction of
the cell cycle arrest and apoptosis by flavonoids isolated from
Korean Citrus aurantium L. in non-small-cell lung cancer cells.
Food Chem. 135:2728–2735. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee DH, Park KI, Park HS, Kang SR,
Nagappan A, Kim JA, Kim EH, Lee WS, Hah YS, Chung HJ, et al:
Flavonoids isolated from Korea Citrus aurantium L. induce G2/M
phase arrest and apoptosis in human gastric cancer AGS cells. Evid
Based Complement Alternat Med. 2012:5159012012. View Article : Google Scholar
|
|
13
|
Han SI, Kim YS and Kim TH: Role of
apoptotic and necrotic cell death under physiologic conditions. BMB
Rep. 41:1–10. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Reed JC, Miyashita T, Takayama S, Wang HG,
Sato T, K rajewski S, Aimé-Sempé C, Bodrug S, Kitada S and Hanada
M: BCL-2 family proteins: Regulators of cell death involved in the
pathogenesis of cancer and resistance to therapy. J Cell Biochem.
60:23–32. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Stennicke HR and Salvesen GS: Properties
of the caspases. Biochim Biophys Acta. 1387:17–31. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kennedy SG, Wagner AJ, Conzen SD, Jordán
J, Bellacosa A, Tsichlis PN and Hay N: The PI 3-kinase/Akt
signaling pathway delivers an anti-apoptotic signal. Genes Dev.
11:701–713. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Widmann C, Gibson S, Jarpe MB and Johnson
GL: Mitogen-activated protein kinase: Conservation of a
three-kinase module from yeast to human. Physiol Rev. 79:143–180.
1999.PubMed/NCBI
|
|
18
|
Hengartner MO: The biochemistry of
apoptosis. Nature. 407:770–776. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee DH, Lee TH, Jung CH and Kim YH:
Wogonin induces apoptosis by activating the AMPK and p53 signaling
pathways in human glioblastoma cells. Cell Signal. 24:2216–2225.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Middleton E Jr, Kandaswami C and
Theoharides TC: The effects of plant flavonoids on mammalian cells:
Implications for inflammation, heart disease, and cancer. Pharmacol
Rev. 52:673–751. 2000.PubMed/NCBI
|
|
22
|
Oh YC, Cho WK, Oh JH, Im GY, Jeong YH,
Yang MC and Ma JY: Fermentation by Lactobacillus enhances
anti-inflammatory effect of Oyaksungisan on LPS-stimulated RAW
264.7 mouse macrophage cells. BMC Complement Altern Med. 12:172012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hong GE, Kim JA, Nagappan A, Yumnam S, Lee
HJ, Kim EH, Lee WS, Shin SC, Park HS and Kim GS: Flavonoids
identified from Korean Scutellaria baicalensis Georgi inhibit
inflammatory signaling by suppressing activation of NF-κB and MAPK
in RAW 264.7 cells. Evid Based Complement Alternat Med.
2013:9120312013. View Article : Google Scholar
|
|
24
|
Park HS, Park KI, Lee DH, Kang SR,
Nagappan A, Kim JA, Kim EH, Lee WS, Shin SC, Hah YS, et al:
Polyphenolic extract isolated from Korean Lonicera japonica Thunb.
induce G2/M cell cycle arrest and apoptosis in HepG2 cells:
Involvements of PI3K/Akt and MAPKs. Food Chem Toxicol.
50:2407–2416. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim DI, Lee SJ, Lee SB, Park K, Kim WJ and
Moon SK: Requirement for Ras/Raf/ERK pathway in naringin-induced
G1-cell-cycle arrest via p21WAF1 expression. Carcinogenesis.
29:1701–1709. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Luo G, Guan X and Zhou L: Apoptotic effect
of citrus fruit extract nobiletin on lung cancer cell line A549 in
vitro and in vivo. Cancer Biol Ther. 7:966–973. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shi Y: Caspase activation: Revisiting the
induced proximity model. Cell. 117:855–858. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nagappan A, Park KI, Park HS, Kim JA, Hong
GE, Kang SR, Lee H, Kim EH, Lee WS, Won CK, et al: Vitamin C
induces apoptosis in AGS cells by down-regulation of 14-3-3σ via a
mitochondrial dependent pathway. Food Chem. 135:1920–1928. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wong WW and Puthalakath H: Bcl-2 family
proteins: The sentinels of the mitochondrial apoptosis pathway.
IUBMB Life. 60:390–397. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hussain AR, Al-Rasheed M, Manogaran PS,
Al-Hussein KA, Platanias LC, Al Kuraya K and Uddin S: Curcumin
induces apoptosis via inhibition of PI3′-kinase/AKT pathway in
acute T cell leukemias. Apoptosis. 11:245–254. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gururajan M, Dasu T, Shahidain S, Jennings
CD, Robertson DA, Rangnekar VM and Bondada S: Spleen tyrosine
kinase (Syk), a novel target of curcumin, is required for B
lymphoma growth. J Immunol. 178:111–121. 2007. View Article : Google Scholar
|
|
32
|
Raman M, Chen W and Cobb MH: Differential
regulation and properties of MAPKs. Oncogene. 26:3100–3112. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
van den Brink MR, Kapeller R, Pratt JC,
Chang JH and Burakoff SJ: The extracellular signal-regulated kinase
pathway is required for activation-induced cell death of T cells. J
Biol Chem. 274:11178–11185. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Basu S, Bayoumy S, Zhang Y, Lozano J and
Kolesnick R: BAD enables ceramide to signal apoptosis via Ras and
Raf-1. J Biol Chem. 273:30419–30426. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Faris M, Kokot N, Latinis K, Kasibhatla S,
Green DR, Koretzky GA and Nel A: The c-Jun N-terminal kinase
cascade plays a role in stress-induced apoptosis in Jurkat cells by
up-regulating Fas ligand expression. J Immunol. 160:134–144.
1998.PubMed/NCBI
|
|
36
|
Dhanasekaran DN and Reddy EP: JNK
signaling in apoptosis. Oncogene. 27:6245–6251. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sinha K, Das J, Pal PB and Sil PC:
Oxidative stress: The mitochondria-dependent and
mitochondria-independent pathways of apoptosis. Arch Toxicol.
87:1157–1180. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kang YJ, Zhou ZX, Wang GW, Buridi A and
Klein JB: Suppression by metallothionein of doxorubicin-induced
cardiomyocyte apoptosis through inhibition of p38 mitogen-activated
protein kinases. J Biol Chem. 275:13690–13698. 2000. View Article : Google Scholar : PubMed/NCBI
|