Introduction
The epothilones are macrolide compounds originally
isolated from a culture broth of the myxobacterium Sorangium
cellulosum in 1987 (1). The
epothilones have strong antitumor activities against human types of
cancers (2) with a similar mode of
action as the taxanes. Although both epothilones and taxanes are
microtubule stabilizers, the epothilones have significant antitumor
activities against taxane-resistant human cancers (3–7). This
characteristic makes epothilones a hotspot for development of novel
cancer therapeutics and brings hope for the patients who are
refractory to taxane treatment (8–11).
Ixabepilone (aza-epothilone B; Ixempra®; Bristol-Myers
Squibb, Princeton, NJ, USA) has been evaluated in clinical trials
(12–19) and was approved by the FDA to treat
metastatic and advanced breast cancers that are refractory to other
types of chemotherapy (20).
UTD1 is an epothilone analog generated by genetic
engineering of the epothilone biosynthetic gene cluster. UTD1 has
demonstrated high activities in vitro and in vivo
against a broad range of tumors, including paclitaxel-sensitive
tumors as well as paclitaxel-resistant human carcinoma models (Qiu
et al, unpublished data). UTD1 is now under clinical
investigation (21). However, the
effect of UTD1 on important intracellular signaling pathways
related to its anticancer mechanism remains to be addressed.
p53 protein is a well known tumor suppressor that
coordinates cellular signals to mediate cell cycle arrest,
apoptosis and differentiation (22), and plays critical roles in cancer
development (23). p53 keeps its
basic level under normal conditions due to the p53/MDM2-negative
feedback control. When cancer develops, the post-transcriptional
modification of p53 is induced, which keeps p53 away from
ubiquitination by MDM2 and leads to its accumulation in the
cells.
p53 plays two quite different roles at different
cellular localizations: as a transcription factor in the nucleus
and as a pro-apoptotic protein in the cytoplasm, respectively. As a
transcription factor, p53 binds to DNA and induces transcription of
downstream molecules in response to several stress signals, such as
growth arrest, apoptosis, senescence and DNA repair (24). The post-transcriptional modification
of p53, by ATM (ATR), CHK2 (CHK1), cell cycle checkpoint and other
upstream proteins, directly controls its DNA binding and
transcriptional activities. Both p21 and bax are
target genes of p53, and it is generally considered that p21 and
Bax are markers of p53-induced cell cycle arrest and apoptosis
(25–27). In addition to its transcriptional
activity, p53 also acts as a BH3-only protein that moves to the
mitochondria, and contributes to the mitochondrial outer membrane
permeabilization (MOMP) by protein-protein interaction with Bcl2
family members (28–31), and then induces the release of
cytochrome c from mitochondria to trigger apoptosis.
It has been previously demonstrated that Taxol
treatment led to accumulation of p53 in a series of cancer cell
lines. However, the pro- or anti-apoptotic role of p53 in
microtubule inhibitor-induced cell death has been debatable to
date, or whether mitotic blockage triggers a p53-independent
apoptotic pathway (32–34).
In the present study, we investigated the role of
p53 in the UTD1-induced cell death in the MCF-7 cell line which
expresses wild-type p53 and the HT29 cell line which expresses
mutant p53. We found that HT29 cells were more resistant to
UTD1-induced cell death than MCF-7 cells. UTD1 induced aneuploid
cells in the MCF-7 and HT29 cell lines at low concentrations, and
induced G2-M cell cycle arrest at high concentrations.
Our results demonstrated that p53 was localized to different
cellular sites and played distinct roles in these two effects of
the epothilone analog. Its transcriptional activity was activated
in induction of aneuploid cells, and its protein level was
accumulated and enriched in the cytoplasm to cause G2-M
cell cycle arrest.
Materials and methods
Compound
UTD1 was provided by Biostar Technologies, Ltd.
(Beijing, China). UTD1 is an epothilone derivative generated by
genetic manipulation of the epothilone biosynthetic gene cluster.
It is now under phase III clinical trials.
Cell culture
MCF-7 and HT29 cells were cultured in Dulbecco's
modified Eagle's medium (DMEM) (HyClone, Logan, UT, USA),
supplemented with 10% fetal bovine serum (Gibco, Grand Island, NY,
USA), in a 5% CO2 humidified atmosphere at 37°C.
Phosphate-buffered saline (PBS) containing 0.25% trypsin and 0.02%
EDTA (Sigma, St. Louis, MO, USA) was used to harvest the cells. For
various tests, 3×103, 3×104 and
1.2×106 cells were cultured in 96-, 24- and 6-well
plates with drug-free medium, respectively. After 24 h, the cells
were treated with various concentrations of UTD1. In all the
experiments, cells were treated with UTD1 at the indicated
concentrations and times, starting from a stock solution of 100
μmol/l in dimethylsulfoxide (DMSO) (Sigma).
Flow cytometry
The cells treated with various concentrations of
UTD1 for 24 or 48 h were collected and washed twice, and then fixed
with 70% ethanol at −20°C overnight. The cells were rehydrated in
PBS and re-suspended in PBS containing 100 μg/ml RNase
(Sigma) and 10 μg/ml propidium iodide. The cells were
analyzed by flow cytometry with a FACScan (Becton-Dickinson
Biosciences, San Jose, CA, USA). Data were analyzed with ModFit LT
software.
Immunoblotting
The cells treated with various concentrations of
UTD1 for 24 or 48 h were lysed in RIPA buffer containing a
proteinase inhibitor. The protein extract (30 μg) from each
sample, as determined by BCA protein assay (Thermo Fisher
Scientific, USA), was heated with loading buffer for 5 min at 95°C,
and then electrophoresed through a 12% polyacrylamide-SDS gel and
electroblotted onto nitrocellulose membranes in transfer buffer (50
mM Tris, 100 mM glycine and 20% methanol) for 2 h at 100 V. The
following antibodies were used in the immunoblot analysis:
anti-β-tubulin, anti-p53 (DO-1), anti-p21 (F-5) (from Santa Cruz
Biotechnology); anti-caspase 7 (M45) and anti-Bax (C62) (from
Bioworld Technology Biotechnology, USA).
Immunofluorescence
The cells were plated onto glass coverslips in
6-well plates. The next day, the cells were treated with UTD1 for
24 h, and then washed with PBS and fixed with 3% paraformaldehyde
for 30 min at room temperature. The coverslips were then washed
with PBS and blocked by PBS containing 1% BSA and 0.3% Triton for 1
h. The cells were then dyed using Hoechst 33258 (Sigma) and
MitoTracker Red CMXRos (Invitrogen, USA) for 30 min and incubated
with anti-p53 antibody (Alexa Fluor 488 conjugate; Cell
Signaling).
Transcriptional activity analysis
PathDetect® In Vivo Signal
Transduction Pathway cis-Reporting Systems containing
p53-Luc, pAP-1-Luc and pNF-κB-Luc were purchased from Stratagene
(USA). Lipofectamine™ 2000 was from Invitrogen, and
Dual-Glo® Luciferase Assay System and pRL-TK were from
Promega (USA). Experiments were performed according to the
manufacturer's protocols.
Proliferation analysis
The cells were treated with various concentrations
of UTD1 for 48 and 72 h, and then proliferation was evaluated by an
MTT (Sigma) assay. IC50 values were then calculated.
Results
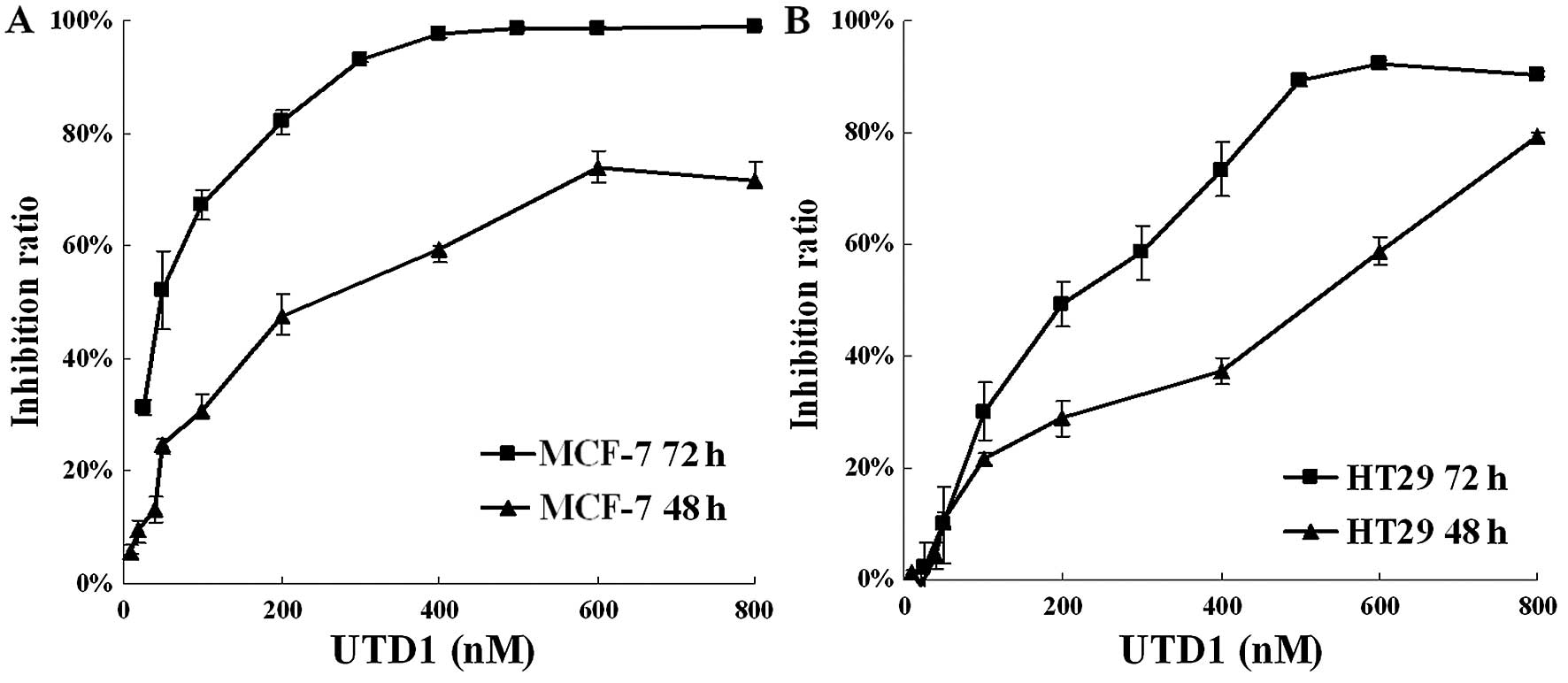
UTD1 inhibits the growth of MCF-7 and
HT29 cells and is a strong promoter of tubulin polymerization
The inhibitory effects of UTD1 on proliferation of
the wild-type and mutant p53 cell lines and tubulin polymerization
were investigated. Breast cancer cell line MCF-7 and colon cancer
cell line HT29 were treated with various concentrations of UTD1 for
48 and 72 h, respectively. As shown in Fig. 1, the IC50 values for the
cytotoxicity of UTD1 on MCF-7 (Fig.
1A) and HT29 cells (Fig. 1B)
were 390 and 525 nM at 48 h, 51 and 187 nM at 72 h, indicating a
strong growth inhibitory activity towards human cancer cells for
UTD1; and cells with wild-type p53 were more sensitive. Therefore,
we chose 50 nM (IC50 for MCF-7), 200 nM (IC50
for HT29) and 800 nM (>90% inhibition for both cell lines) of
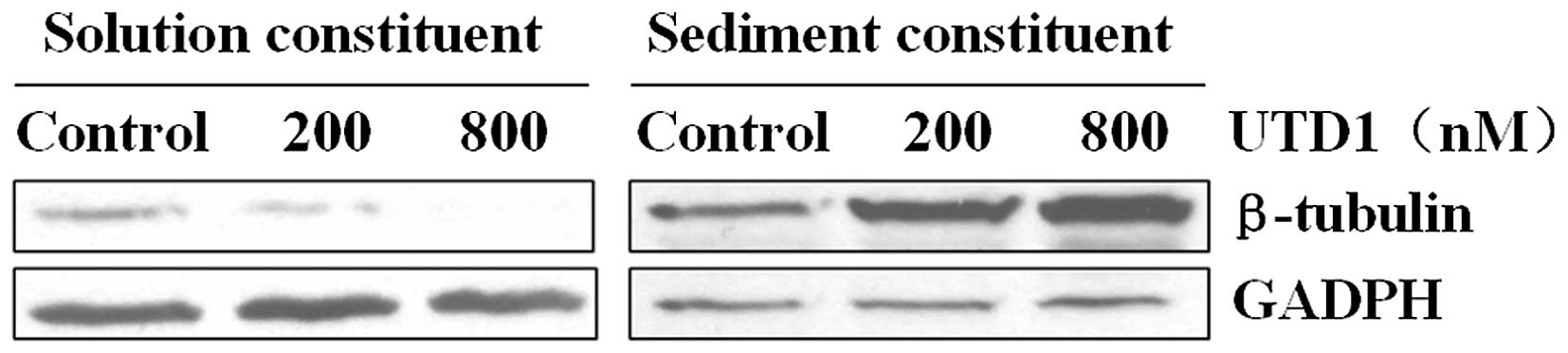
UTD1 in the subsequent experiments. We also confirmed that UTD1
promoted tubulin polymerization and enhanced microtubule stability
in vitro similar to other epothilones, as shown by a
decrease in soluble tubulin and an increase in polymerized tubulin
in the UTD1-treated cells (Fig.
2).
UTD1 transforms MCF-7 and HT29 cells into
aneuploid cells and induces G2-M cell cycle arrest at
different concentrations
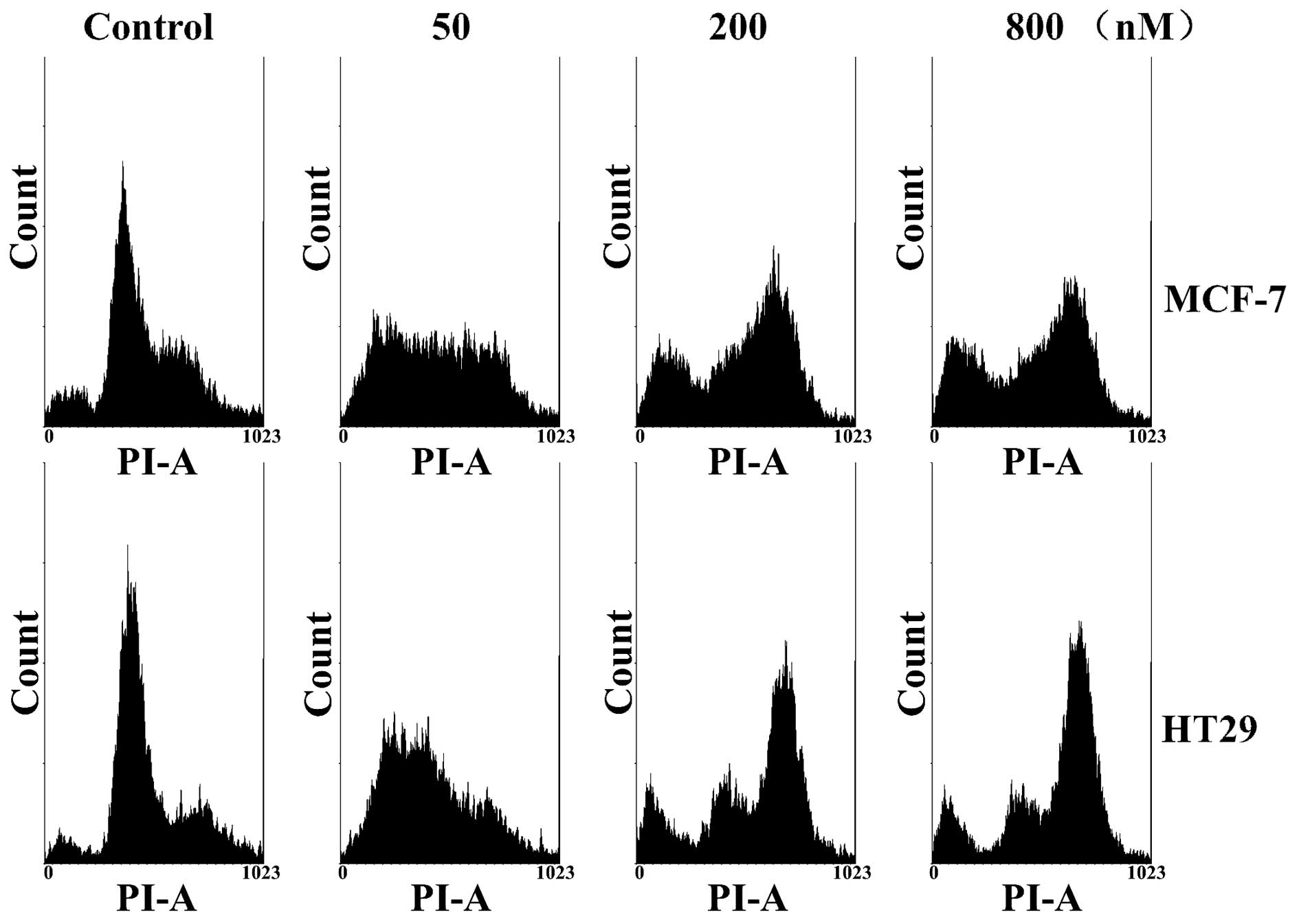
In analyzing UTD1-treated MCF-7 and HT29 cells by
flow cytometry, we observed that both cancer cell lines were
transformed into aneuploid cells after treatment with 50 nM of UTD1
for 24 h, and were blocked at G2/M of the cell cycle at
higher concentrations of UTD1 (Fig.
3). This observation demonstrated a concentration-dependent
differential effect of UTD1 on the cell cycle of cancer cells. This
may have been due to abnormal microtubule dynamics, the fact that
cells treated with low concentrations of UTD1 suffered mitotic
disorder, and chromosomes were randomly distributed to daughter
cells which became aneuploid cells. However, higher concentrations
of UTD1 arrested cells at G2/M, preventing mitotic
progression.
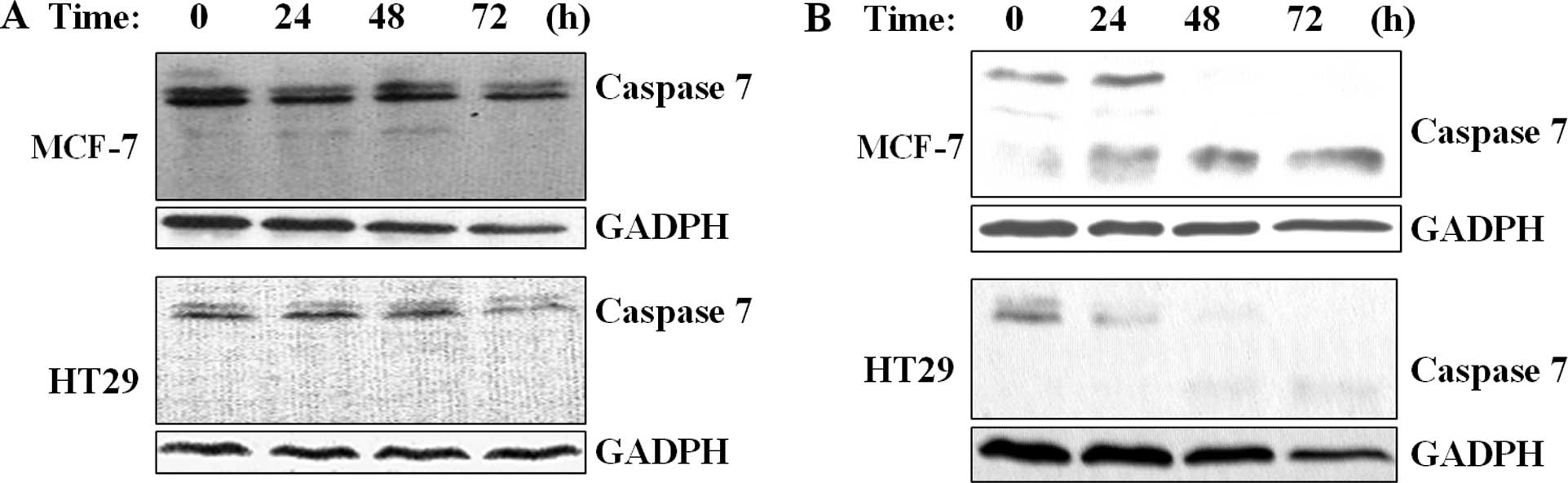
UTD1 induces apoptosis at high but not
low concentrations
To assess whether the epothilone analog induces
apoptosis, cancer cells were treated with different concentrations
of UTD1 and apoptotic markers were analyzed. As shown in Fig. 4, caspase 7 zymogen was degraded in
both the MCF-7 and HT29 cells exposed to 800 nM, yet not at 50 nM
of UTD1. In addition, activated caspase 7 subunit was detected in
the MCF-7 cells. This observation suggests that high concentrations
of UTD1 induce apoptosis in MCF-7 cells but not in the case of low
concentrations (Fig. 4). Cells may
survive at low concentrations of UTD1, but undergo mitotic
disorder.
p53 transcriptional activity is activated
at low concentrations and accumulated at high concentrations of
UTD1
To investigate a possible effect of UTD1 on
transcriptional activity of p53 and various other factors important
for cell apoptosis and survival control, reporter gene assays of
p53, AP-1 and NF-κB were carried out. Due to the low transfection
efficiency of the HT29 cells, only MCF-7 cells were used in this
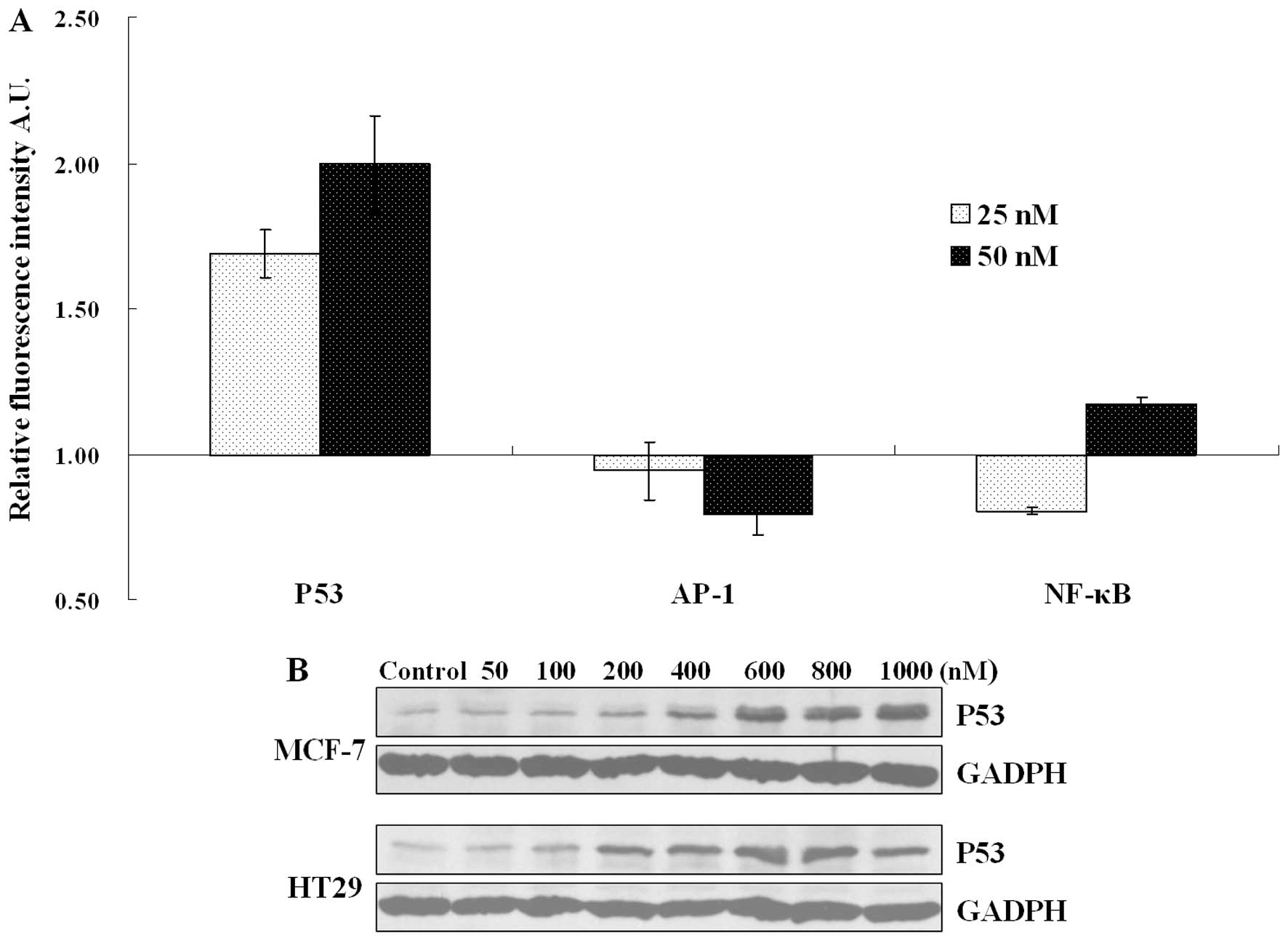
experiment. As shown in Fig. 5A,
after treatment with low concentrations of UTD1 for 24 h,
p53-induced luc fluorescence intensity was markedly increased. By
contrast, AP-1- and NF-κB-induced luc fluorescence intensities were
nearly unchanged. We then further investigated the protein quantity
of p53 in both cell lines treated with UTD1. p53 tended to
accumulate in the UTD1 cells treated with high concentrations, yet
not in the cells treated with low concentrations (Fig. 5B).
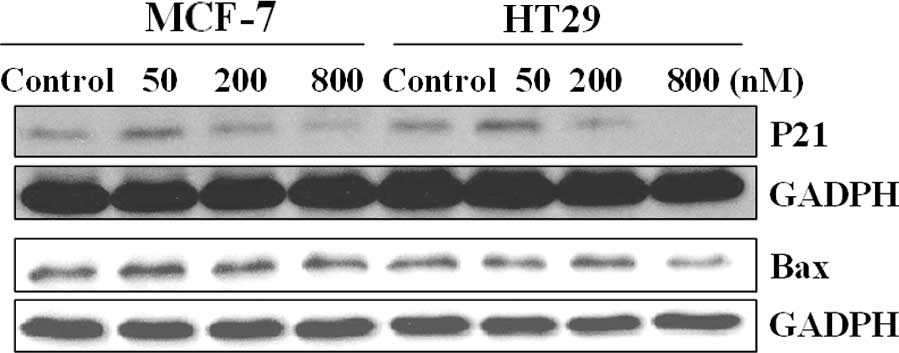
p53 plays an anti-apoptotic role in the
cells treated with low concentrations of UTD1
To clarify the contradiction of the pro- or
anti-apoptotic role of p53 in UTD1-treated cells, the expression
levels of p21 and Bax in these cells were investigated (Fig. 6), as the downstream proteins of p53,
p21 and Bax are considered to be the hallmarks of p53
transcription-dependent activity on cell cycle arrest and
apoptosis, respectively (21). As
shown in Fig. 6, p21 was expressed
at a low concentration of UTD1, and the protein quantity of Bax was
unchanged at both concentrations. This suggests that a low
concentration of UTD1 induced abnormal mitosis resulting in
activation of p21, which in turn blocked the cell cycle at
G2/M. As the transcription factor of p21, p53 appears to
play an anti-apoptotic role in this process. A high concentration
of UTD1 resulted in the accumulation of p53, while the level of
downstream pro-apoptosis protein Bax was not affected, suggesting
that apoptosis induction was p53 transcription-independent or this
effect was Bax-independent.
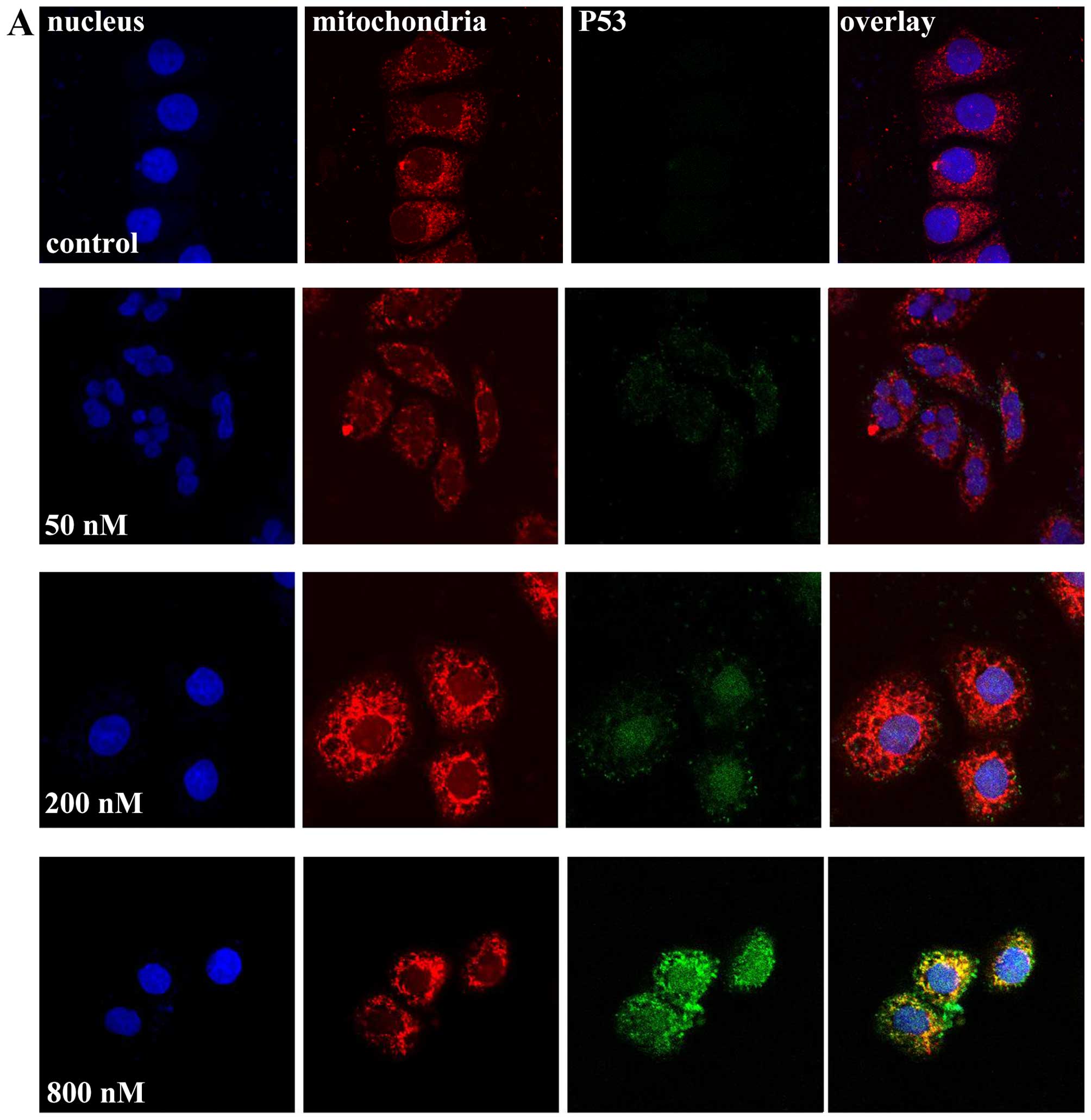
p53 protein is enriched in the cytoplasm
of the cells exposed to a high concentration of UTD1
To explain the contradiction between the high
protein accumulation and low transcriptional activity of p53 in
cells treated with a high concentration of UTD1, we tracked the
localization of p53 by the anti-p53 fluorescent antibody. Hoechst
33258 and MitoTracker Red CMXRos were used to mark the nuclei and
mitochondria, respectively. As shown in Fig. 7, both MCF-7 and HT29 cells were
transformed into aneuploid cells following treatment with a low
concentration of UTD1, with their nucleolus dividing into unequal
sizes quite different from the apoptotic cells. p53 in the control
and low dosage-treated cells was hardly detectable. These
observations were consistent with the results of the flow
cytometric and western blot analyses. Following treament of 200 nM
UTD1, p53 was detectable, locating in the nucleolus. However, when
the concentration of UTD1 was increased to 800 nM, most p53 was
detected in the cytoplasm. These observations indicate that the
cellular localization of p53 is affected by the concentration of
UTD1, which in turn influences the function of this important
tumor-suppressor protein.
Discussion
Epothilones have been widely used in the clinical
development of therapies for diverse types of cancer, yet the
mechanism underlying their antitumor activities, such as apoptosis
induction is not yet fully understood. p53 is well known as a
tumor-suppressor, controlling DNA repair, cell cycle arrest and
apoptosis in a transcriptional-dependent or
transcriptional-independent way, and accumulates in the cells
treated with Taxol. However, inactivation experiments of p53
demonstrated that the apoptosis induced by Taxol is p53-independent
(35). However, the role of p53 in
other microtubule inhibitorinduced cell death remains unclear.
In the present study, we studied the effect of UTD1,
a genetically engineered epothilone analog, on p53 and its
downstream proteins in MCF-7 and HT29 cells. The IC50
values of UTD1 on MCF-7 and HT29 cells were 390 and 525 nM at 48 h,
51 and 187 nM at 72 h, respectively. Notably, different
concentrations of UTD1 led both types of cells to different fates.
When exposed to a low concentration (50 nM) of UTD1 for 24 h, both
types of cells were transformed into aneuploid cells with their
nucleolus dividing into unequal sizes. The protein level of p53 was
not significantly changed as demonstrated by western blot and
confocal microscopy analyses. However, the transcriptional activity
of p53 in the MCF-7 cells was markedly increased. By contrast, the
transcriptional activities of AP-1 and NF-κB were not affected. The
effect on p53 transcription could be supported by the increased
protein level of p21. When the concentration of UTD1 was increased
to 200 nM, results of the flow cytometry showed that most MCF-7 and
HT29 cells were blocked at G2/M of the cell cycle at the
24-h point, at which the protein level of p53 was upregulated and
mainly located in the nucleus. Yet, upregulated p53 did not result
in an increase in the protein levels of p21 and Bax. Similarly,
treatment with 800 nM UTD1 for 24 h led both types of cells to be
blocked at the G2/M phase of the cell cycle and neither
p21 nor Bax was increased when compared with the control. Notably,
p53 was located in the cytoplasm, yet not in the nucleus which was
quite different from the low-concentration conditions and control
cells.
We speculated that p53 functions as an
anti-apoptotic transcription factor at a low concentration of UTD1
and as a pro-apoptotic protein at a high concentration of UTD1.
At the low concentration, 50 nM UTD1 disturbed
mitosis, yet did not block it completely, thus it transformed MCF-7
and HT29 cells into aneuploid cells and activated the G0/G1
checkpoint and p53. Accumulation of protein is not necessary for
the transcriptional activity of p53, and a basal level of p53 is
sufficient for that. Subsequently p21, but not Bax is
transcribed.
At a high concentration, microtubule dynamics were
totally destroyed and mitosis was completely blocked. We found that
p21 and Bax were not upregulated, and p53 was localized mainly in
the cytoplasm, probably at mitochondria. These findings indicate
that p53 accumulation in the cytoplasm functions as a pro-apoptotic
protein to induce the release of cytochrome c from
mitochondria.
A disorder in mitosis activates more than one
signaling pathways and leads to cell death. This may explain why
cells containing mutant p53 or no p53 can still be blocked at the
G2 phase of the cell cycle rigidly by microtubule
inhibitors and undergo cell death. We also found that MCF-7 cells
were completely inhibited by high concentrations (>500 nM) of
UTD1. The inhibition rate at 72 h was above 99% (Fig. 1A); however, HT29 cells seemed to be
more resistant to this drug treatment with the inhibition rate
never higher than 90% (Fig. 1B).
Another significant distinction is that we did not detect activated
caspase 7 in the cells treated with 800 nM UTD1, suggesting that
high concentrations of UTD1 fail to induce apoptosis in HT29 cells.
This differential effect reflects the difference in p53 status in
these two cancer cell lines.
Acknowledgments
We thank Dr Jinsong Yan of the Dalian Medical
University for advice and expertise with the luminescence
microplate readers.
References
|
1
|
Gerth K, Bedorf N, Höfle G, Irschik H and
Reichenbach H: Epothilons A and B: Antifungal and cytotoxic
compounds from Sorangium cellulosum (Myxobacteria). Production,
physicochemical and biological properties. J Antibiot. 49:560–563.
1996. View Article : Google Scholar
|
|
2
|
Goodin S, Kane MP and Rubin EH:
Epothilones: Mechanism of action and biologic activity. J Clin
Oncol. 22:2015–2025. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee JJ and Swain SM: Development of novel
chemotherapeutic agents to evade the mechanisms of multidrug
resistance (MDR). Semin Oncol. 32(Suppl 7): S22–S26. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee FY, Borzilleri R, Fairchild CR, Kim
SH, Long BH, Reventos-Suarez C, Vite GD, Rose WC and Kramer RA:
BMS-247550: A novel epothilone analog with a mode of action similar
to paclitaxel but possessing superior antitumor efficacy. Clin
Cancer Res. 7:1429–1437. 2001.PubMed/NCBI
|
|
5
|
Fojo AT and Menefee M: Microtubule
targeting agents: Basic mechanisms of multidrug resistance (MDR).
Semin Oncol. 32(Suppl 7): S3–S8. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Orr GA, Verdier-Pinard P, McDaid H and
Horwitz SB: Mechanisms of Taxol resistance related to microtubules.
Oncogene. 22:7280–7295. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bhandari MS and Hussain M: Epothilones and
the next generation of phase III trials for prostate cancer. BJU
Int. 96:296–302. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee JJ and Swain SM: The epothilones:
Translating from the laboratory to the clinic. Clin Cancer Res.
14:1618–1624. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bergstralh DT and Ting JP: Microtubule
stabilizing agents: Their molecular signaling consequences and the
potential for enhancement by drug combination. Cancer Treat Rev.
32:166–179. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Larkin JM and Kaye SB: Epothilones in the
treatment of cancer. Expert Opin Investig Drugs. 15:691–702. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cortes J and Baselga J: Targeting the
microtubules in breast cancer beyond taxanes: The epothilones.
Oncologist. 12:271–280. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Low JA, Wedam SB, Lee JJ, Berman AW,
Brufsky A, Yang SX, Poruchynsky MS, Steinberg SM, Mannan N, Fojo T,
et al: Phase II clinical trial of ixabepilone (BMS-247550), an
epothilone B analog, in metastatic and locally advanced breast
cancer. J Clin Oncol. 23:2726–2734. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Thomas E, Tabernero J, Fornier M, Conté P,
Fumoleau P, Lluch A, Vahdat LT, Bunnell CA, Burris HA, Viens P, et
al: Phase II clinical trial of ixabepilone (BMS-247550), an
epothilone B analog, in patients with taxane-resistant metastatic
breast cancer. J Clin Oncol. 25:3399–3406. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Perez EA, Lerzo G, Pivot X, Thomas E,
Vahdat L, Bosserman L, Viens P, Cai C, Mullaney B, Peck R, et al:
Efficacy and safety of ixabepilone (BMS-247550) in a phase II study
of patients with advanced breast cancer resistant to an
anthracycline, a taxane, and capecitabine. J Clin Oncol.
25:3407–3414. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Roché H, Yelle L, Cognetti F, Mauriac L,
Bunnell C, Sparano J, Kerbrat P, Delord JP, Vahdat L, Peck R, et
al: Phase II clinical trial of ixabepilone (BMS-247550), an
epothilone B analog, as first-line therapy in patients with
metastatic breast cancer previously treated with anthracycline
chemotherapy. J Clin Oncol. 25:3415–3420. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Denduluri N, Low JA, Lee JJ, Berman AW,
Walshe JM, Vatas U, Chow CK, Steinberg SM, Yang SX and Swain SM:
Phase II trial of ixabepilone, an epothilone B analog, in patients
with metastatic breast cancer previously untreated with taxanes. J
Clin Oncol. 25:3421–3427. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vahdat LT, Thomas E, Li R, et al: Phase
III trial of ixabepilone plus capecitabine compared to capecitabine
alone in patients withmetastatic breast cancer (MBC) previously
treated or resistant to an anthracycline and resistant to taxanes.
Proc Am Soc Clin Oncol. 25:10062007.
|
|
18
|
Denduluri N, Lee JJ, Walshe J, Berman AW,
Vatas U, Chow CK, Steinberg SM, Cox MC, Low JA and Swain SM: Phase
II trial of ixabepilone, an epothilone B analog, given daily for
three days every three weeks, in metastatic breast cancer. Invest
New Drugs. 25:63–67. 2007. View Article : Google Scholar
|
|
19
|
Lee JJ, Low JA, Croarkin E, Parks R,
Berman AW, Mannan N, Steinberg SM and Swain SM: Changes in
neurologic function tests may predict neurotoxicity caused by
ixabepilone. J Clin Oncol. 24:2084–2091. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Winer E, Gralow J, Diller L, Karlan B,
Loehrer P, Pierce L, Demetri G, Ganz P, Kramer B, Kris M, et al:
Clinical cancer advances 2008: Major research advances in cancer
treatment, prevention, and screening - a report from the American
Society of Clinical Oncology. J Clin Oncol. 27:812–826. 2009.
View Article : Google Scholar :
|
|
21
|
Zhang P, Sun M, Qiu R, Tang L, Dou G and
Xu B: Phase I clinical and pharmacokinetic study of UTD1, a
genetically engineered epothilone analog in patients with advanced
solid tumors. Cancer Chemother Pharmacol. 68:971–978. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vousden KH and Lane DP: p53 in health and
disease. Nat Rev Mol Cell Biol. 8:275–283. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Puca R, Nardinocchi L, Givol D and D'Orazi
G: Regulation of p53 activity by HIPK2: Molecular mechanisms and
therapeutical implications in human cancer cells. Oncogene.
29:4378–4387. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vazquez A, Bond EE, Levine AJ and Bond GL:
The genetics of the p53 pathway, apoptosis and cancer therapy. Nat
Rev Drug Discov. 7:979–987. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen JG, Yang CP, Cammer M and Horwitz SB:
Gene expression and mitotic exit induced by microtubule-stabilizing
drugs. Cancer Res. 63:7891–7899. 2003.PubMed/NCBI
|
|
26
|
Blagosklonny MV: Prolonged mitosis versus
tetraploid checkpoint: How p53 measures the duration of mitosis.
Cell Cycle. 5:971–975. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Blagosklonny MV: Mitotic arrest and cell
fate: Why and how mitotic inhibition of transcription drives
mutually exclusive events. Cell Cycle. 6:70–74. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Marchenko ND, Wolff S, Erster S, Becker K
and Moll UM: Monoubiquitylation promotes mitochondrial p53
translocation. EMBO J. 26:923–934. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Moll UM, Marchenko N and Zhang XK: p53 and
Nur77/TR3 - transcription factors that directly target mitochondria
for cell death induction. Oncogene. 25:4725–4743. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tomita Y, Marchenko N, Erster S,
Nemajerova A, Dehner A, Klein C, Pan H, Kessler H, Pancoska P and
Moll UM: WT p53, but not tumor-derived mutants, bind to Bcl2 via
the DNA binding domain and induce mitochondrial permeabilization. J
Biol Chem. 281:8600–8606. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Leu JI, Dumont P, Hafey M, Murphy ME and
George DL: Mitochondrial p53 activates Bak and causes disruption of
a Bak-Mcl1 complex. Nat Cell Biol. 6:443–450. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bacus SS, Gudkov AV, Lowe M, Lyass L, Yung
Y, Komarov AP, Keyomarsi K, Yarden Y and Seger R: Taxol-induced
apoptosis depends on MAP kinase pathways (ERK and p38) and is
independent of p53. Oncogene. 20:147–155. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Woods CM, Zhu J, McQueney PA, Bollag D and
Lazarides E: Taxol-induced mitotic block triggers rapid onset of a
p53-independent apoptotic pathway. Mol Med. 1:506–526.
1995.PubMed/NCBI
|
|
34
|
Giannakakou P, Robey R, Fojo T and
Blagosklonny MV: Low concentrations of paclitaxel induce cell
type-dependent p53, p21 and G1/G2 arrest instead of mitotic arrest:
Molecular determinants of paclitaxel-induced cytotoxicity.
Oncogene. 20:3806–3813. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lanni JS, Lowe SW, Licitra EJ, Liu JO and
Jacks T: p53-independent apoptosis induced by paclitaxel through an
indirect mechanism. Proc Natl Acad Sci USA. 94:9679–9683. 1997.
View Article : Google Scholar : PubMed/NCBI
|