Introduction
As a type of malignant epithelial tumor, gastric
cancer is derived from the glandular epithelium of the gastric
mucosa (1). Gastric cancer has high
diffusivity, such as spread to lungs, liver and bones (2). This cancer type is prevalent in men of
developing countries (3,4), and ~8.5% of cancer cases in men is
gastric cancer (5). In 2012,
gastric cancer ranked third after lung and liver cancer, with
700,000 mortalities (6,7). Thus, it is necessary to examine the
molecular mechanisms of gastric cancer and develop therapeutic
schedules.
The molecular mechanisms of gastric cancer have been
previously investigated. For example, as a member of the
BRICHO family, the full-length gene gastrokine 2
(GKN2) is downregulated and associated with gastric cancer
(8). The expression of GKN1
and GKN2 decreased in gastric adenocarcinomas, and their
loss is associated with shorter survival in the intestinal subtype
of gastric adenocarcinomas (9).
Expression of soluble urokinase plasminogen activator receptor
(suPAR) and carbonic anhydrase IX (CA IX) is
associated with the stage and presence of gastric cancer, and the
overexpression of suPAR indicates a poorer prognosis in
patients with gastric cancer (10).
Differentially expressed microseminoprotein (MSMB), Annexin
A10 (ANXA10), Annexin A1 (ANXA1) and prostate stem
cell antigen (PSCA) have been identified in normal and
cancer gastric tissues, and may be used as biomarkers for the
diagnosis and treatment of gastric cancer (11).
Long non-coding RNAs (lncRNAs), having a length
>200 nucleotides, can regulate gene expression during transport,
RNA maturation and protein synthesis (12). Dysregulation of lncRNAs is
associated with many types of human cancer (13). Many differentially expressed
lncRNAs, such as LINC00152 and LINC00261, have been
identified and may act as therapeutic targets and biomarkers in
gastric cancer (12). The
downregulation of lncRNA maternally expressed 3 (MEG3) is
involved with cell proliferation and can be regarded as a poor
prognostic biomarker in gastric cancer (14). Knockdown of lncRNA HOX transcript
antisense RNA (HOTAIR) results in suppression of tumor
invasion and reversal of epithelial-mesenchymal transition process
in gastric cancer cells, suggesting that HOTAIR affects
diagnostics and therapeutics of gastric cancer (15).
In the present study, to examine the molecular
mechanisms of gastric cancer, the expression profile of GSE41476
was downloaded, which involved 3 primary cell culture samples from
gastric cancer tissues, 3 gastric cancer cell lines and 2 normal
tissue samples. The lncRNAs were predicted and differentially
expressed genes (DEGs) were screened. The functions of the DEGs
were analysed using Gene Ontology (GO) and pathway enrichment
analyses. In addition, a search was conducted to determine the
interaction relationships between the DEGs using protein-protein
interaction (PPI) network and modules of the PPI network.
Additionally, lncRNA-DEG pairs were screened, and pathway
enrichment analysis was performed for DEGs co-expressed with each
lncRNA.
Materials and methods
Microarray data
The expression profile of GSE41476 was downloaded
from the Gene Expression Omnibus (GEO, http:/www.ncbi.nlm.nih.gov/geo/), which was based on
the platform of the GPL9115 Illumina Genome Analyzer II (Homo
sapiens). GSE41476 included a collective of 3 primary cell
culture samples from gastric cancer tissues, 3 gastric cancer cell
lines and 2 normal tissue samples.
Sequence alignment
After GSE41476 was downloaded, the SRA format
sequences were translated into FASTQ format, and microarray data
were preprocessed by NGSQC software (16). The ratio of bases with base
sequencing quality <20 was required to be <0.1. The remaining
high quality sequences were compared to human genome 19 (hg19)
using TopHat2 (17). The parameter
was set to - no-discordant - phred64-quals, and the remaining
parameters were set to the default values.
lncRNA prediction
The alignment results were obtained via
transcriptome assembly using Cufflinks software (18). Subsequently, the assembly results
were integrated using Cuffmerge software (19). According to the gene annotation
information of the genome in the UCSC, assembly results that did
not overlap with the extracted arbitrary genes were extracted.
Transcriptions with a length of >200 nt and with ≥2 exons were
screened. According to information obtained from the 29 mammalian
genome alignment, transcriptions with scores <100 were screened
using PhyloCSF software (20).
Moreover, HMMER software was used to compare transcriptions to Pfam
database (21). E-value <1e-5
was used as the cut-off criterion.
DEGs screening
A search through the RefSeq annotation files in UCSC
website identified known lncRNA comments (LNCipedia1.0 database)
and the predicted lncRNA transcription document, DEGs and lncRNAs,
which were screened from the alignment results using Cuffdiff
software (22). The adjusted
p-value of <0.05 and |log fold-change (FC)| >1 were used as
the cut-off criteria.
Functional and pathway enrichment
analyses
As a functional study method, GO analysis is used to
assess large-scale transcriptomic or genomic data (23). The Kyoto Encyclopedia of Genes and
Genomes (KEGG) pathway database shows how molecules or genes act
(24). The GO and KEGG pathway
enrichment analyses were conducted for DEGs. The GO functional
enrichment analysis was mainly focused on the biological process
(BP). P<0.05 was used and ≥2 genes were used as the cut-off
criteria.
PPI network and module analysis
The STRING online software (25) was used to examine the interaction
relationships of the proteins encoded by DEGs, and the combined
score >0.7 was used as the cut-off criterion. The Cytoscape
software (26) was used to
visualize the PPI network. The CFinder software (27) was used to screen modules of the PPI
network, and the parameter k was set to 8.
lncRNA analysis
According to the expression matrix of the
differentially expressed lncRNA and the DEGs, the relationship
between lncRNA and the DEGs was calculated and lncRNA-DEG pairs
were screened. Pearson's correlation of >0.99 was used as the
cut-off criterion.
Results
lncRNA prediction and DEGs analysis
A total of 86 lncRNA transcriptions were obtained,
including 37 transcriptions with an overlap of >50% when
compared with known lncRNAs.
Compared to normal tissue samples, 1,088 upregulated
transcriptions (including 16 known lncRNAs, 1 predicted lncRNAs and
1,071 mRNAs) and 1,537 downregulated transcriptions (including 18
known lncRNAs, 4 predicted lncRNAs and 1,515 mRNAs) were identified
in the gastric cancer samples.
Functional and pathway enrichment
analyses
The enriched GO functions for the DEGs are provided
in Table I, including cell
migration (P=8.97E-14), extracellular matrix (P=3.06E-14), positive
regulation of response to stimulus (P=6.66E-16) and single-organism
process (P=1.33E-15).
 | Table IThe top 10 enriched GO functions and
KEGG pathways for the DEGs. |
Table I
The top 10 enriched GO functions and
KEGG pathways for the DEGs.
| Genes | ID | Name | Gene no. | Gene symbol | P-value |
|---|
| GO functions |
| Upregulated | GO:0030198 | Extracellular
matrix organization | 71 | ACTN1,
ADAM17… | 0 |
| GO:0043062 | Extracellular
structure organization | 71 | ADAM9,
ADAMTS2… | 0 |
| GO:0016477 | Cell migration | 110 | AJUBA,
AMOTL1… | 8.97E-14 |
| GO:0031012 | Extracellular
matrix | 422 | ADAMTS1,
CD248… | 3.06E-14 |
| GO:0005515 | Protein
binding | 521 | ABL2,
ABLIM3… | 3.37E-11 |
| Downregulated | GO:0048584 | Positive regulation
of response to stimulus | 189 | A2M,
ADA… | 6.66E-16 |
| GO:0044699 | Single-organism
process | 1017 | EPHX2,
EPN1… | 1.33E-15 |
| GO:0002694 | Regulation of
leukocyte activation | 76 | AIF1,
BCR… | 1.89E-15 |
| GO:0031224 | Intrinsic to
membrane | 565 | A4GNT,
AADAC… | 1.11E-15 |
| GO:0005102 | Receptor
binding | 146 | APLP1,
APOE… | 4.68E-08 |
| KEGG pathways |
| Upregulated | KEGG:4512 | ECM-receptor
interaction | 20 | CD44,
COL1A1… | 1.18E-06 |
| KEGG:4510 | Focal adhesion | 29 | ACTN1,
COL1A1… | 0.000143 |
| KEGG:5146 | Amoebiasis | 18 | RKCA,
SHC3… | 0.000392 |
| KEGG:5144 | Malaria | 11 | CCL2,
CSF3… | 0.000695 |
| KEGG:1040 | Biosynthesis of
unsaturated fatty acids | 6 | PTPLA,
SCD… | 0.00262 |
| Downregulated | KEGG:4514 | Cell adhesion
molecules (CAMs) | 46 | CD2,
CD22… | 1.33E-15 |
| KEGG:5150 | Staphylococcus
aureus infection | 23 | DQA1,
HLA-DQA2… | 2.53E-10 |
| KEGG:4672 | Intestinal immune
network for IgA production | 19 | ICAM2,
ICAM3… | 2.80E-08 |
| KEGG:5340 | Primary
immunodeficiency | 15 | ADA,
BLNK… | 2.40E-07 |
| KEGG:5416 | Viral
myocarditis | 22 | BID,
CD28… | 2.86E-07 |
The enriched KEGG pathways for the DEGs are shown in
Table I, including ECM-receptor
interaction (P=1.18E-06), focal adhesion (P=0.000143), cell
adhesion molecules (CAMs, P=1.33E-15) and Staphylococcus
aureus infection (P=2.53E-10).
PPI network and module analysis
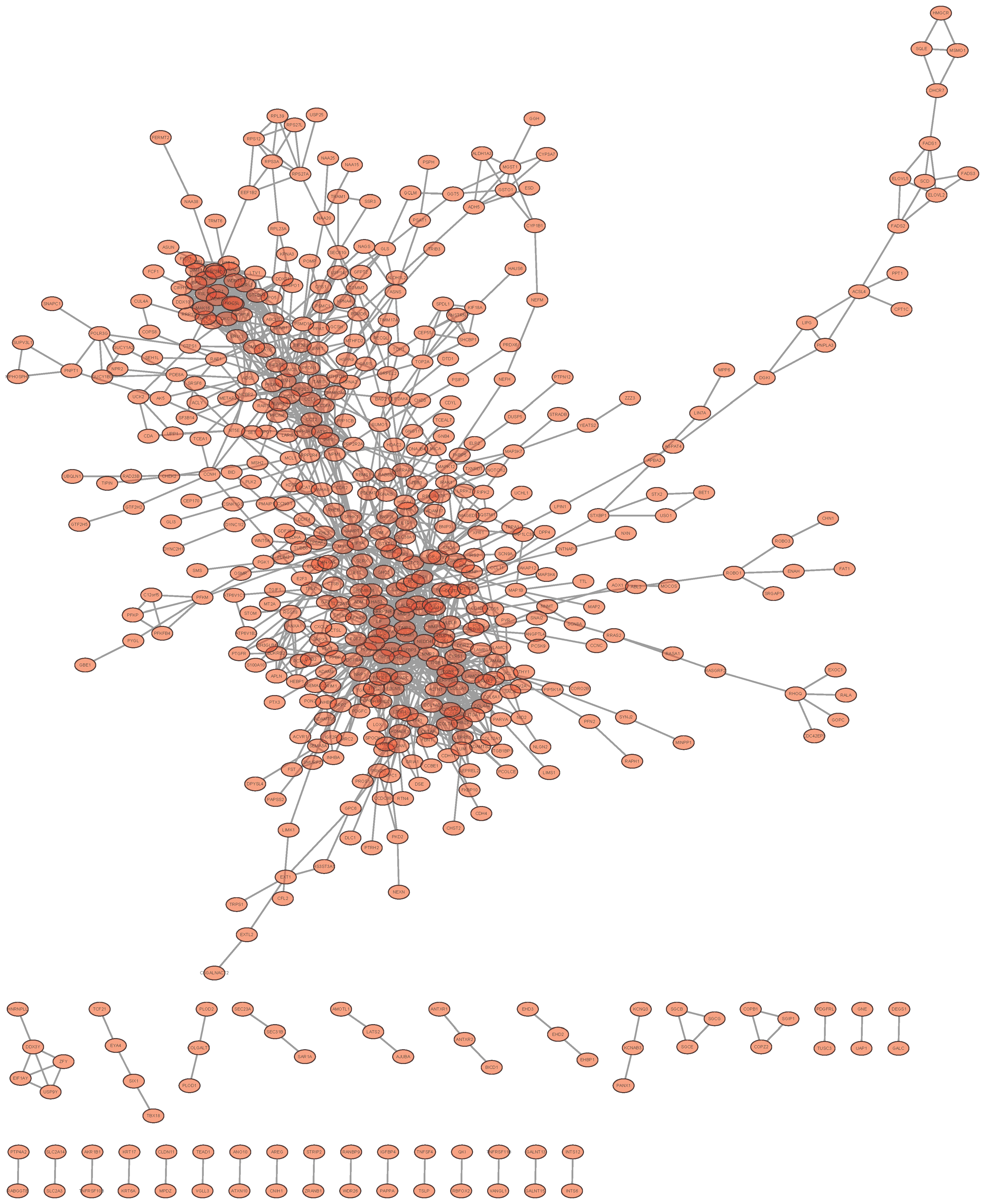
The PPI network of the upregulated genes had 568
nodes and 1,522 interactions (Fig.
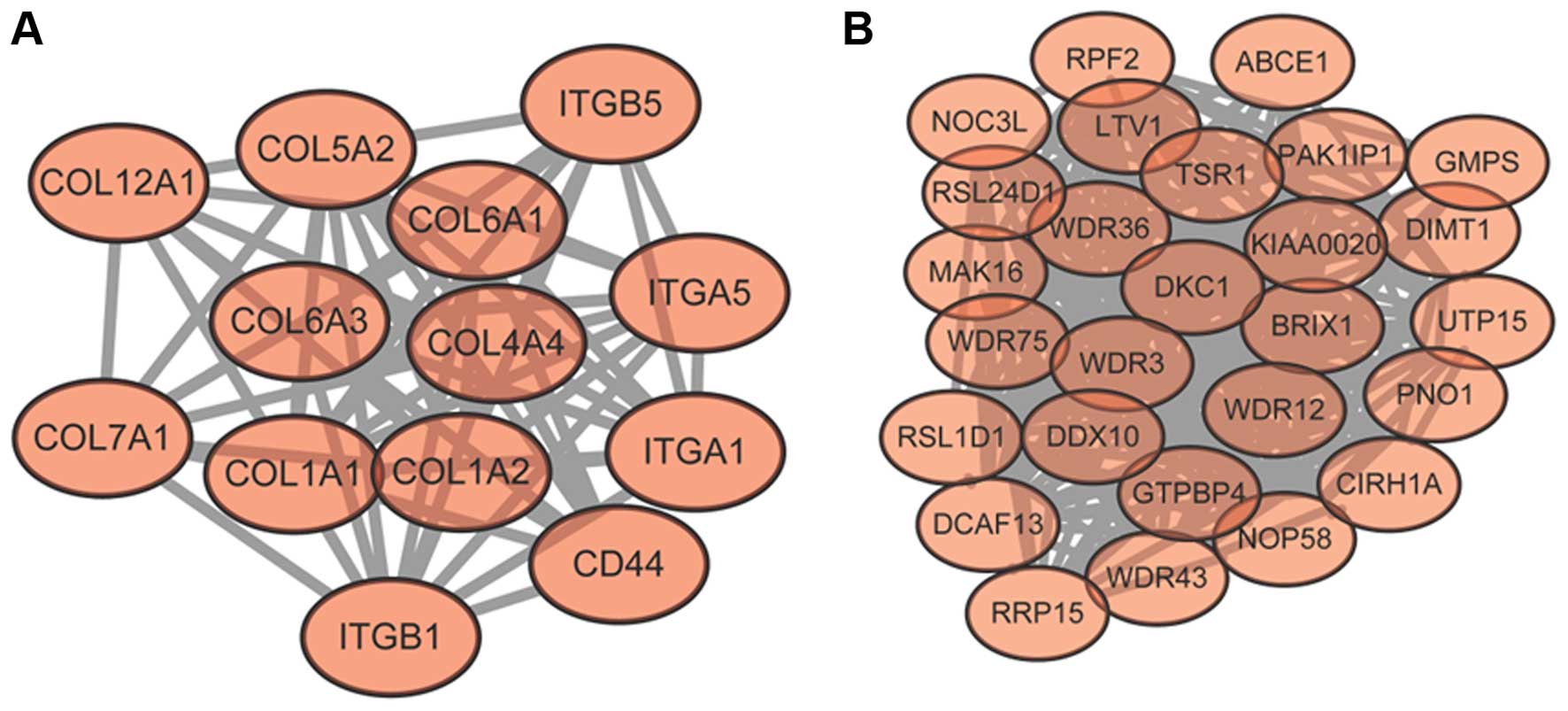
1). Module A (Fig. 2A) and
module B (Fig. 2B) were obtained
from the PPI network. Module A had 13 nodes and 65 interactions.
The enriched GO functions for DEGs in module A are provided in
Table II, including collagen
catabolic process (P=2.22E-16), multicellular organismal catabolic
process (P=6.66E-16) and extracellular matrix disassembly
(P=1.33E-15). The enriched KEGG pathways for DEGs in module A are
also listed in Table II, including
ECM-receptor interaction [P=0, which involved proteins encoded by
collagen (COL) genes such as collagen, type I, α 1
(COL1A1)], collagen, type I, α 2 (COL1A2) and
collagen, type IV, α 4 (COL4A4), as well as proteins encoded
by integrin (ITG) genes such as integrin, α 1
(ITGA1), integrin, α 5 (ITGA5) and integrin, β 1
(ITGB1), focal adhesion (P=1.05E-13, which also involved
proteins encoded by the COL and ITG genes) and
protein digestion and absorption (P=5.49E-11). Proteins encoded by
COL genes interacted with those of the ITG genes,
such as COL1A1-ITGA5 and COL1A2-ITGB1. Module B had 27 nodes and
261 interactions. The enriched GO functions for DEGs in module B
are provided in Table II,
including ribonucleoprotein complex biogenesis (P=2.22E-16), rRNA
processing (P=1.17E-11) and cellular component biogenesis
(P=2.36E-07). The enriched KEGG pathway for DEGs in module B was
ribosome biogenesis in eukaryotes (P=5.55E-16) (Table II).
 | Table IIThe top 10 enriched GO functions and
KEGG pathways for DEGs in module A and B of the PPI network for the
upregulated DEGs. |
Table II
The top 10 enriched GO functions and
KEGG pathways for DEGs in module A and B of the PPI network for the
upregulated DEGs.
| Modules | ID | Name | Gene no. | Gene symbol | P-value |
|---|
| GO functions |
| A | GO:0030574 | Collagen catabolic
process | 8 | COL5A2,
COL4A4… | 2.22E-16 |
| GO:0044243 | Multicellular
organismal catabolic process | 8 | COL4A4,
COL1A2… | 6.66E-16 |
| GO:0022617 | Extracellular
matrix disassembly | 8 | COL1A2,
COL1A1… | 1.33E-15 |
| GO:0005581 | Collagen | 8 | COL5A2,
COL4A4 | 4.44E-16 |
| GO:0005201 | Extracellular
matrix structural constituent | 5 | COL1A1,
COL12A1… | 4.23E-09 |
| B | GO:0022613 | Ribonucleoprotein
complex biogenesis | 11 | BRIX1,
TSR1… | 2.22E-16 |
| GO:0006364 | rRNA
processing | 7 | WDR12,
NOP58… | 1.17E-11 |
| GO:0044085 | Cellular component
biogenesis | 11 | BRIX1,
TSR1… | 2.36E-07 |
| GO:0031981 | Nuclear lumen | 21 | BRIX1,
TSR1… | 1.33E-15 |
| GO:0003723 | RNA binding | 7 | PNO1,
DKC1… | 4.48E-06 |
| KEGG pathways |
| A | KEGG:4512 | ECM-receptor
interaction | 11 | COL5A2,
COL4A4, COL1A2… | 0 |
| KEGG:4510 | Focal adhesion | 10 | COL5A2,
COL4A4, COL1A2… | 1.05E-13 |
| KEGG:4974 | Protein digestion
and absorption | 7 | COL5A2,
COL4A4, COL1A2… | 5.49E-11 |
| KEGG:5412 | Arrhythmogenic
right ventricular cardiomyopathy (ARVC) | 4 | ITGB1,
ITGA1, ITGA5, ITGB5 | 1.07E-05 |
| KEGG:5410 | Hypertrophic
cardiomyopathy (HCM) | 4 | ITGB1,
ITGA1, ITGA5, ITGB5 | 1.69E-05 |
| KEGG:5414 | Dilated
cardiomyopathy | 4 | ITGB1,
ITGA1, ITGA5, ITGB5 | 2.33E-05 |
| KEGG:5146 | Amoebiasis | 4 | COL5A2,
COL4A4, COL1A2, COL1A1 | 4.45E-05 |
| KEGG:5131 | Shigellosis | 3 | ITGB1,
ITGA5, CD44 | 0.000219795 |
| KEGG:4640 | Hematopoietic cell
lineage | 3 | ITGB1,
ITGA5, CD44 | 0.000649577 |
| B | KEGG:3008 | Ribosome biogenesis
in eukaryotes | 9 | NOP58,
GTPBP4, WDR3, WDR75, UTP15,
WDR36, DKC1, CIRH1A, WDR43 | 5.55E-16 |
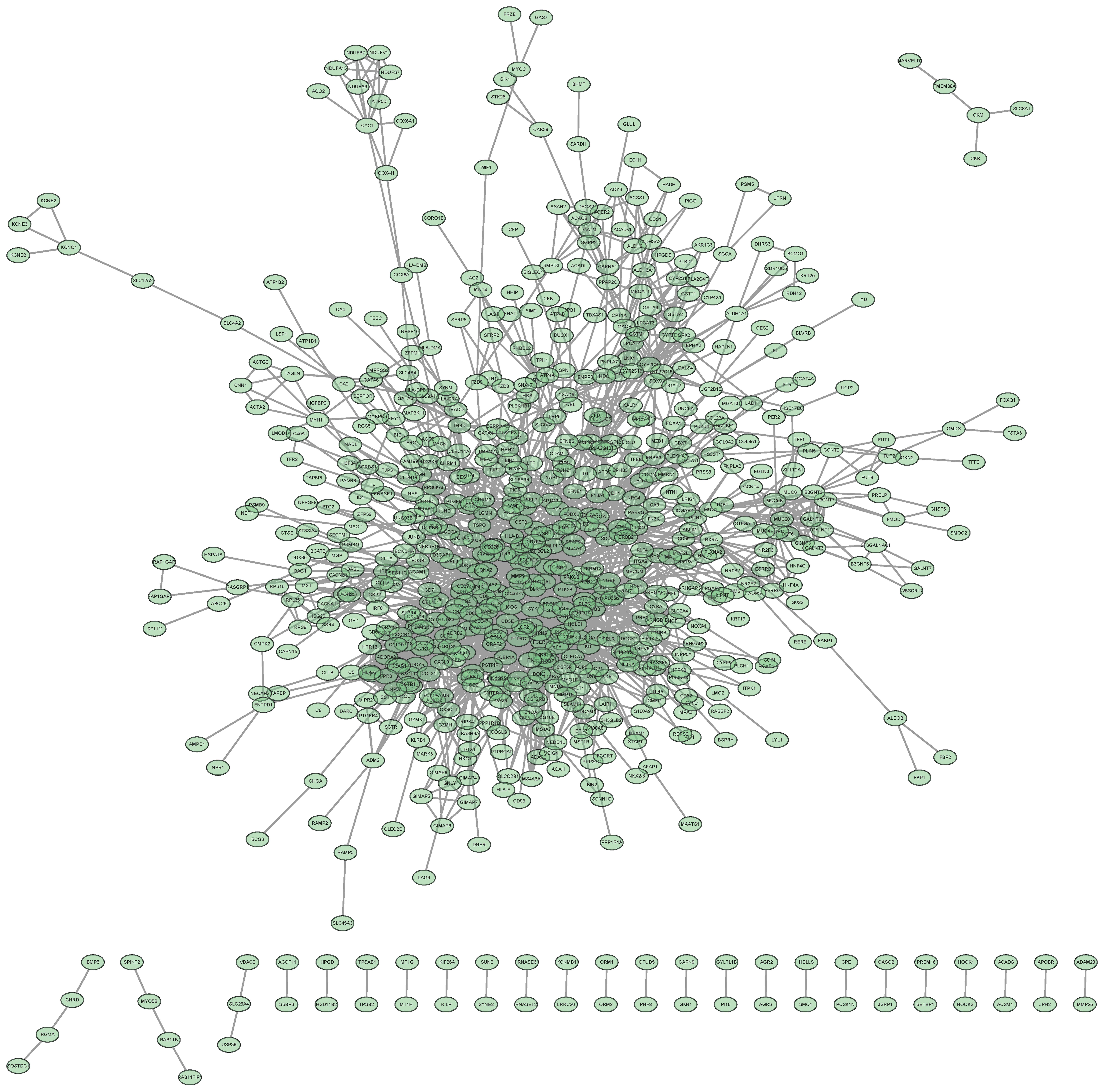
The PPI network of the downregulated genes had 734
nodes and 2345 interactions (Fig.
3). In addition, 6 modules (module A–F) were obtained from the
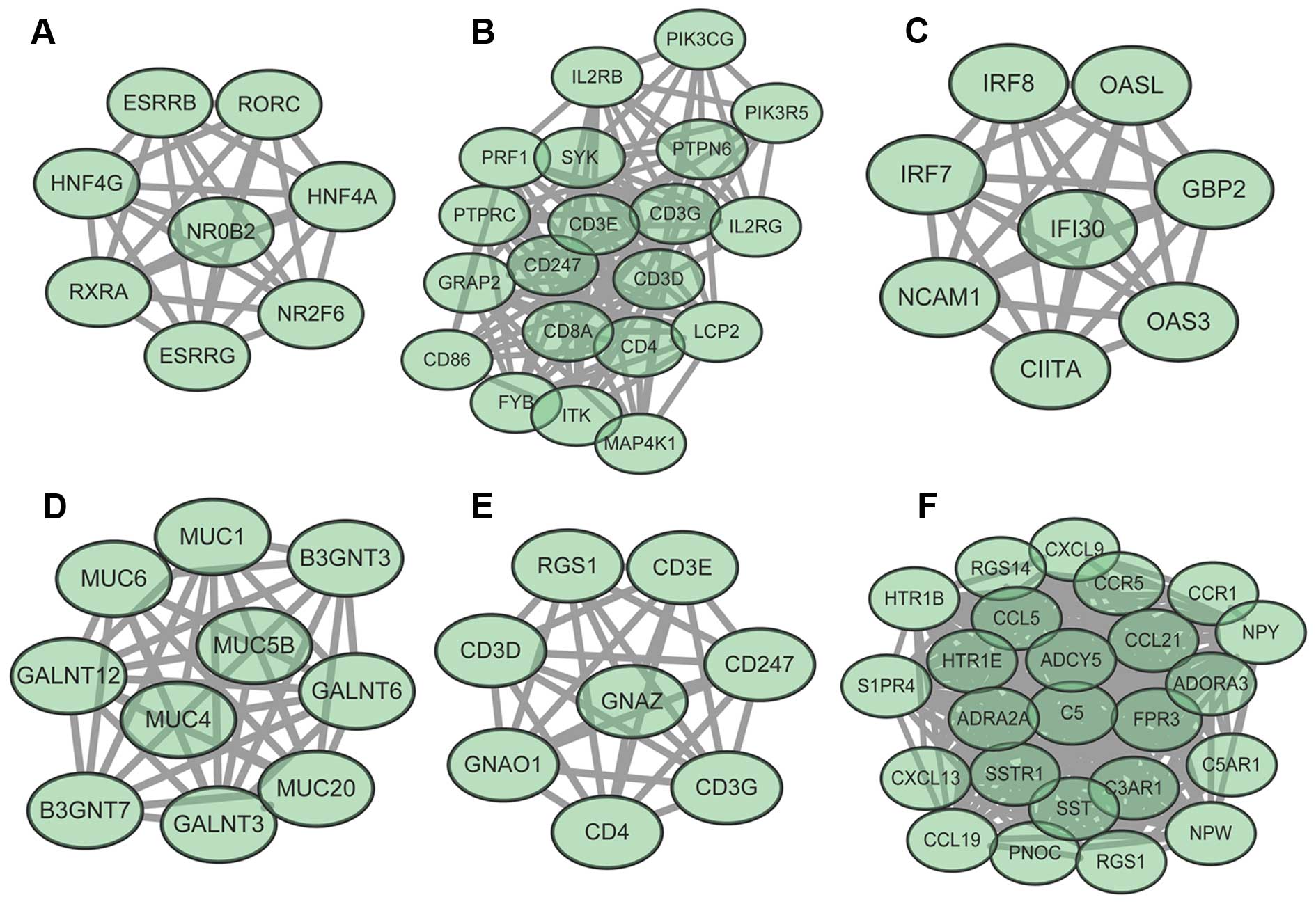
PPI network (Fig. 4). Module A had
8 nodes and 28 interactions. The enriched GO functions for DEGs in
module A included transcription initiation from RNA polymerase II
promoter (P=8.88E-16) and DNA-dependent transcription, initiation
(P=2.66E-15). The enriched KEGG pathways for DEGs in module A
included maturity onset diabetes of the young (P=0.000173) and bile
secretion (P=0.001409). Module B had 20 nodes and 122 interactions.
The enriched GO functions for DEGs in module B included activation
of immune response (P=2.22E-16) and leukocyte activation
(P=4.44E-16). The enriched KEGG pathways for DEGs in module B
included T-cell receptor signaling pathway [P=0, which involved
phosphoinositide-3-kinase, catalytic, γ polypeptide (PIK3CG) and
phosphoinositide-3-kinase, regulatory subunit 5 (PIK3R5)] and
primary immunodeficiency (P=7.35E-10). Specifically, PIK3CG had an
interaction relationship with PIK3R5. Module C had 8 nodes and 28
interactions. The enriched GO functions for DEGs in module C
included cytokine-mediated signaling pathway (P=6.98E-14) and cell
response to cytokine stimulus (P=5.20E-13). The enriched KEGG
pathways for DEGs in module C included antigen processing and
presentation (P=0.001613) and hepatitis C (P=0.004944). Module D
had 10 nodes and 44 interactions. The enriched GO functions for
DEGs in module D included single-organism carbohydrate metabolic
process (P=8.44E-15) and keratan sulfate biosynthetic process
(P=0.000153). The enriched KEGG pathways for DEGs in module D
included mucin-type O-glycan biosynthesis (P=2.39E-06) and
metabolic pathways (P=0.014715). Module E had 8 nodes and 28
interactions. The enriched GO functions for DEGs in module E
included T-cell costimulation (P=1.20E-10). The enriched KEGG
pathways for DEGs in module E included T-cell receptor signaling
pathway (P=3.92E-08). Module F had 24 nodes and 273 interactions.
The enriched GO functions for DEGs in module F included signal
transduction (P=1.46E-13). The enriched KEGG pathways for DEGs in
module F included neuroactive ligand-receptor interaction
(P=9.26E-08) (Table III).
 | Table IIIThe top 10 enriched GO functions and
KEGG pathways for DEGs in module A, B, C, D, E and F of the PPI
network for the downregulated DEGs. |
Table III
The top 10 enriched GO functions and
KEGG pathways for DEGs in module A, B, C, D, E and F of the PPI
network for the downregulated DEGs.
| Modules | ID | Name | Gene no. | Gene symbol | P-value |
|---|
| GO functions |
| A | GO:0006367 | Transcription
initiation from RNA polymerase II promoter | 8 | ESRRG,
ESRRB… | 8.88E-16 |
| GO:0006352 | DNA-dependent
transcription, initiation | 8 | NR0B2,
HNF4G… | 2.66E-15 |
| B | GO:0002253 | Activation of
immune response | 13 | CD3D,
CD3E… | 2.22E-16 |
| GO:0045321 | Leukocyte
activation | 14 | CD3D,
CD3E… | 4.44E-16 |
| C | GO:0019221 | Cytokine-mediated
signaling pathway | 8 | IRF7,
CIITA… | 6.98E-14 |
| GO:0071345 | Cellular response
to cytokine stimulus | 8 | IRF7,
CIITA… | 5.20E-13 |
| D | GO:0044723 | Single-organism
carbohydrate metabolic process | 10 | GALNT12,
MUC1… | 8.44E-15 |
| GO:0018146 | Keratan sulfate
biosynthetic process | 2 | B3GNT7,
B3GNT3 | 0.000153 |
| E | GO:0031295 | T-cell
costimulation | 5 | CD4,
CD247, CD3D, CD3E, CD3G | 1.20E-10 |
| F | GO:0007165 | Signal
transduction | 24 | CCL5,
ADORA3… | 1.46E-13 |
| KEGG pathways |
| A | KEGG: 04950 | Maturity onset
diabetes of the young | 2 | HNF4G,
HNF4A | 0.000173 |
| KEGG:04976 | Bile secretion | 2 | NR0B2,
RXRA | 0.001409 |
| B | KEGG:4660 | T-cell receptor
signaling pathway | 13 | CD3D,
CD3E, CD3G… | 0 |
| KEGG:5340 | Primary
immunodeficiency | 6 | CD3D,
CD3E, CD4… | 7.35E-10 |
| C | KEGG:4612 | Antigen processing
and presentation | 2 | CIITA,
IFI30 | 0.001613 |
| KEGG:5160 | Hepatitis C | 2 | IRF7,
OAS3 | 0.004944 |
| D | KEGG:512 | Mucin-type O-glycan
biosynthesis | 3 | COL5A2,
COL4A4, COL1A2, COL1A1 | 2.39E-06 |
| KEGG:1100 | Metabolic
pathways | 4 | ITGB1,
ITGA5, CD44 | 0.014715 |
| E | KEGG:4660 | T-cell receptor
signaling pathway | 5 | CD4,
CD247, CD3D, CD3E, CD3G | 3.92E-08 |
| F | KEGG:4080 | Neuroactive
ligand-receptor interaction | 9 | ADORA3,
C5AR1, C3AR1… | 9.26E-08 |
lncRNA analysis
After lncRNA-DEG pairs, such as
TCONS-00068220-IL7, were screened, KEGG pathway enrichment
analysis was conducted for DEGs co-expressed with each lncRNA.
Proteins encoded with co-expressed DEGs of TCONS_00068220
were enriched in cancer-related pathways, such as bladder cancer,
CAMs, chemokine signaling pathway and natural killer cell-mediated
cytotoxicity (Table IV) (28).
 | Table IVThe top 10 enriched KEGG pathways for
the DEGs co-expressed with lncRNA TCONS_00068220. |
Table IV
The top 10 enriched KEGG pathways for
the DEGs co-expressed with lncRNA TCONS_00068220.
| ID | Name | Gene no. | Gene symbol | P-value |
|---|
| KEGG:4514 | Cell adhesion
molecules | 15 | CD2,
CD28… | 3.10E-06 |
| KEGG:4062 | Chemokine signaling
pathway | 15 | CCL5,
CCR1… | 0.00020233 |
| KEGG:5219 | Bladder cancer | 5 | MMP9,
MYC… | 0.005609503 |
| KEGG:4650 | Natural killer
cell-mediated cytotoxicity | 8 | BID,
ICAM2… | 0.033796553 |
| KEGG:4672 | Intestinal immune
network for IgA production | 8 | CD28,
ICOSLG… | 4.17E-05 |
| KEGG:5150 | Staphylococcus
aureus infection | 8 | C1QB,
C1QC… | 0.000114261 |
| KEGG:5140 | Leishmaniasis | 9 | ITGB1,
MAPK12… | 0.000142588 |
| KEGG:4940 | Type I diabetes
mellitus | 7 | CD28,
PRF1… | 0.000149447 |
| KEGG:5330 | Allograft
rejection | 6 | CD28,
HLA-DMB… | 0.000460506 |
| KEGG:5416 | Viral
myocarditis | 8 | BID,
CD28… | 0.000623762 |
Discussion
In the present study, 86 lncRNA transcriptions were
obtained, including 37 transcriptions with an overlap of >50%
when compared with known lncRNAs. Additionally, 1,088 upregulated
and 1,537 downregulated transcriptions were screened. The functions
of cell migration, positive regulation of response to stimulus and
single-organism process were enriched for the DEGs.
At the tumor periphery of scirrhous gastric
carcinoma, collagen biosynthesis is increased and may contribute to
the invasion of tumor cells (29).
Collagen IV can play a role in cell adhesion, as well as tumor
metastasis and invasion (30–32).
It is reported that integrin (ITG) α2β1 can be used as a candidate
target molecule involved in the prevention of gastric cancer
peritoneal dissemination (33).
Integrin α6β4 is a suppressor and can be a biomarker for peritoneal
dissemination in gastric cancer (34). In module A of the PPI network for
the upregulated genes, the enriched KEGG pathways for DEGs included
ECM-receptor interaction and focal adhesion, both of which involved
proteins encoded by COL and ITG genes. ECM-receptor
interaction and focal adhesion are associated with cancer
metastasis and aggression (35,36),
and can represent some molecular differences in gastric cancer
(37). These observations may
indicate that the COL and ITG genes were associated
with gastric cancer. In module A of the PPI network for proteins
encoded by the upregulated genes, proteins encoded by COL
genes were able to interact with those of ITG genes,
suggesting that COL genes are involved in gastric cancer
through the regulation of ITG genes.
There is a high mutation probability of
phosphoinositide-3-kinase, catalytic, α (PIK3CA) in human
cancers and it is a potential therapy target for various tumors
(38). The mutations of
PIK3CA can lead to the attenuation of apoptosis and assist
tumor invasion (39). The
phosphatidylinositol 3-kinase (PI3K) pathway is of great alteration
frequency in gastric tumors and can be used as a therapeutic target
in gastric cancer (40). In module
B of the PPI network for the downregulated genes, the enriched KEGG
pathways for DEGs included the T-cell receptor signaling pathway,
which involved proteins encoded by PIK3CG and PIK3R5.
It has been reported that the T-cell receptor signaling pathway
plays a role in gastric cancer (41). The abovementioned findings showed
that PIK3CG and PIK3R5 may be associated with gastric
cancer. In module B of the PPI network for proteins encoded by the
downregulated genes, PIK3CG also had an interaction relationship
with PIK3R5, indicating that PIK3CG may be involved in
gastric cancer by mediating PIK3R5.
The interleukin-8 (IL8) promoter polymorphism
plays a role in atrophic gastritis and gastric cancer (42). Serum IL6 is correlated with
the progression of gastric cancer and may be used as a biomarker
for monitoring the treatment and response of patients with gastric
cancer (43). Recombinant human
IL7 can retard tumor growth and induce complete regression
(44). By activating p53 and
inhibiting cell proliferation, lncRNA TCONS-00090092-MEG3
may act as a putative tumor-suppressor gene (45–47).
In the present study, IL7 was co-expressed with
TCONS-00068220. Proteins encoded with co-expressed DEGs of
TCONS_00068220 were enriched in cancer-associated pathways.
Thus, the expression levels of IL7 and TCONS-00068220
may be associated with gastric cancer, and IL7 may function
in gastric cancer by regulating TCONS-00068220.
In conclusion, we have conducted a comprehensive
bioinformatics analysis of genes and lncRNAs that may be associated
with gastric cancer. A total of 86 lncRNA transcriptions were
obtained, as well as 1,088 upregulated and 1,537 down-regulated
transcriptions were screened. COL and ITG genes,
PIK3CG, PIK3R5, IL7 and lncRNA
TCONS-00068220 may be correlated with gastric cancer.
However, investigations are to be conducted to determine the
functional mechanisms of these genes in gastric cancer.
Abbreviations:
|
ANXA1
|
Annexin A1
|
|
BP
|
biological process
|
|
CA IX
|
carbonic anhydrase IX
|
|
DEGs
|
differentially expressed genes
|
|
GKN2
|
gastrokine 2
|
|
GO
|
Gene Ontology
|
|
hg19
|
human genome 19
|
|
HOTAIR
|
HOX transcript antisense RNA
|
|
IL8
|
interleukin-8
|
|
lncRNAs
|
long non-coding RNAs
|
|
KEGG
|
Kyoto Encyclopedia of Genes and
Genomes
|
|
MEG3
|
maternally expressed 3
|
|
PI3K
|
phosphatidylinositol 3-kinase
|
|
PPI
|
protein-protein interaction
|
|
PSCA
|
prostate stem cell antigen
|
|
suPAR
|
soluble urokinase plasminogen
activator receptor
|
References
|
1
|
Kumar V, Abbas AK, Fausto N and Aster JC:
Robbins and Cotran Pathologic Basis of Disease, Professional
Edition: Expert Consult-Online. 8th edition. Elsevier; Health
Sciences, Philadelphia, PA: 2009
|
|
2
|
Ruddon RW: Cancer Biology. 4th edition.
Oxford University Press; New York, NY: 2007
|
|
3
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Organization WHO: Are the number of cancer
cases increasing or decreasing in the world? World Health
Organization; Geneva: 2008
|
|
5
|
Cancer IAfRo: World Cancer Report 2014.
World Health Organization; Geneva: 2014
|
|
6
|
Lozano R, Naghavi M, Foreman K, Lim S,
Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, et
al: Global and regional mortality from 235 causes of death for 20
age groups in 1990 and 2010: a systematic analysis for the Global
Burden of Disease Study 2010. Lancet. 380:2095–2128. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Organization WHO: Global battle against
cancer won't be won with treatment alone effective prevention
measures urgently needed to prevent cancer crisis. International
Agency for Research on Cancer; Geneva: 2014
|
|
8
|
Du JJ, Dou KF, Peng SY, Wang WZ, Wang ZH,
Xiao HS, Guan WX, Liu YB and Gao ZQ: Down-regulated full-length
novel gene GDDR and its effect on gastric cancer. Zhonghua Yi Xue
Za Zhi. 83:1166–1168. 2003.In Chinese. PubMed/NCBI
|
|
9
|
Moss SF, Lee J-W, Sabo E, Rubin AK, Rommel
J, Westley BR, May FE, Gao J, Meitner PA, Tavares R, et al:
Decreased expression of gastrokine 1 and the trefoil factor
interacting protein TFIZ1/GKN2 in gastric cancer: Influence of
tumor histology and relationship to prognosis. Clin Cancer Res.
14:4161–4167. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fidan E, Mentese A, Ozdemir F, Deger O,
Kavgaci H, Caner Karahan S and Aydin F: Diagnostic and prognostic
significance of CA IX and suPAR in gastric cancer. Med Oncol.
30:5402013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Meng LX, Li Q, Xue YJ, Guo RD, Zhang YQ
and Song XY: Identification of gastric cancer-related genes by
multiple high throughput analysis and data mining. Zhonghua Wei
Chang Wai Ke Za Zhi. 10:169–172. 2007.In Chinese. PubMed/NCBI
|
|
12
|
Cao WJ, Wu HL, He BS, Zhang YS and Zhang
ZY: Analysis of long non-coding RNA expression profiles in gastric
cancer. World J Gastroenterol. 19:3658–3664. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ponting CP, Oliver PL and Reik W:
Evolution and functions of long noncoding RNAs. Cell. 136:629–641.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sun M, Xia R, Jin F, Xu T, Liu Z, De W and
Liu X: Downregulated long noncoding RNA MEG3 is associated with
poor prognosis and promotes cell proliferation in gastric cancer.
Tumour Biol. 35:1065–1073. 2014. View Article : Google Scholar
|
|
15
|
Xu ZY, Yu QM, Du YA, Yang LT, Dong RZ,
Huang L, Yu PF and Cheng XD: Knockdown of long non-coding RNA
HOTAIR suppresses tumor invasion and reverses
epithelial-mesenchymal transition in gastric cancer. Int J Biol
Sci. 9:587–597. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dai M, Thompson RC, Maher C,
Contreras-Galindo R, Kaplan MH, Markovitz DM, Omenn G and Meng F:
NGSQC: Cross-platform quality analysis pipeline for deep sequencing
data. BMC Genomics. 11(Suppl 4): S72010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim D, Pertea G, Trapnell C, Pimentel H,
Kelley R and Salzberg SL: TopHat2: Accurate alignment of
transcriptomes in the presence of insertions, deletions and gene
fusions. Genome Biol. 14:R362013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Trapnell C, Roberts A, Goff L, Pertea G,
Kim D, Kelley DR, Pimentel H, Salzberg SL, Rinn JL and Pachter L:
Differential gene and transcript expression analysis of RNA-seq
experiments with TopHat and Cufflinks. Nat Protoc. 7:562–578. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li B, Duan H, Li J, Deng XW, Yin W and Xia
X: Global identification of miRNAs and targets in Populus
euphratica under salt stress. Plant Mol Biol. 81:525–539. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lin MF, Jungreis I and Kellis M: PhyloCSF:
A comparative genomics method to distinguish protein coding and
non-coding regions. Bioinformatics. 27:i275–i282. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bateman A, Coin L, Durbin R, Finn RD,
Hollich V, Griffiths-Jones S, Khanna A, Marshall M, Moxon S,
Sonnhammer EL, et al: The Pfam protein families database. Nucleic
Acids Res. 32:D138–D141. 2004. View Article : Google Scholar :
|
|
22
|
Anders S, Reyes A and Huber W: Detecting
differential usage of exons from RNA-seq data. Genome Res.
22:2008–2017. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hulsegge I, Kommadath A and Smits MA:
Globaltest and GOEAST: Two different approaches for Gene Ontology
analysis. BMC Proc. 3(Suppl 4): S102009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kanehisa M and Goto S: KEGG: Kyoto
encyclopedia of genes and genomes. Nucleic Acids Res. 28:27–30.
2000. View Article : Google Scholar
|
|
25
|
Franceschini A, Szklarczyk D, Frankild S,
Kuhn M, Simonovic M, Roth A, Lin J, Minguez P, Bork P, von Mering
C, et al: STRING v9.1: Protein-protein interaction networks, with
increased coverage and integration. Nucleic Acids Res. 41(D1):
D808–D815. 2013. View Article : Google Scholar :
|
|
26
|
Saito R, Smoot ME, Ono K, Ruscheinski J,
Wang PL, Lotia S, Pico AR, Bader GD and Ideker T: A travel guide to
Cytoscape plugins. Nat Methods. 9:1069–1076. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Adamcsek B, Palla G, Farkas IJ, Derényi I
and Vicsek T: CFinder: Locating cliques and overlapping modules in
biological networks. Bioinformatics. 22:1021–1023. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schink M, Tröger W, Dabidian A, Goyert A,
Scheuerecker H, Meyer J, Fischer IU and Glaser F: Mistletoe extract
reduces the surgical suppression of natural killer cell activity in
cancer patients. a randomized phase III trial. Forsch
Komplementmed. 14:9–17. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Matsui H, Kubochi K, Okazaki I, Yoshino K,
Ishibiki K and Kitajima M: Collagen biosynthesis in gastric cancer:
Immunohistochemical analysis of prolyl 4-hydroxylase. J Surg Oncol.
70:239–246. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang ZN and Xu HM: Relationship between
collagen IV expression and biological behavior of gastric cancer.
World J Gastroenterol. 6:438–439. 2000.
|
|
31
|
David L, Nesland JM, Holm R and
Sobrinho-Simões M: Expression of laminin, collagen IV, fibronectin,
and type IV, collagenase in gastric carcinoma. An
immunohistochemical study of 87 patients. Cancer. 73:518–527. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Guszczyn T and Sobolewski K: Deregulation
of collagen metabolism in human stomach cancer. Pathobiology.
71:308–313. 2004. View Article : Google Scholar
|
|
33
|
Kawamura T, Endo Y, Yonemura Y, Nojima N,
Fujita H, Fujimura T, Obata T, Yamaguchi T and Sasaki T:
Significance of integrin α2/β1 in peritoneal dissemination of a
human gastric cancer xenograft model. Int J Oncol. 18:809–815.
2001.PubMed/NCBI
|
|
34
|
Ishii Y, Ochiai A, Yamada T, Akimoto S,
Yanagihara K, Kitajima M and Hirohashi S: Integrin α6β4 as a
suppressor and a predictive marker for peritoneal dissemination in
human gastric cancer. Gastroenterology. 118:497–506. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chiang AC and Massagué J: Molecular basis
of metastasis. N Engl J Med. 359:2814–2823. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Joshi B, Strugnell SS, Goetz JG, Kojic LD,
Cox ME, Griffith OL, Chan SK, Jones SJ, Leung SP, Masoudi H, et al:
Phosphorylated caveolin-1 regulates Rho/ROCK-dependent focal
adhesion dynamics and tumor cell migration and invasion. Cancer
Res. 68:8210–8220. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yang S, Shin J, Park KH, Jeung H-C, Rha
SY, Noh SH, Yang WI and Chung HC: Molecular basis of the
differences between normal and tumor tissues of gastric cancer.
Biochim Biophys Acta. 1772:1033–1040. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Barbi S, Cataldo I, De Manzoni G, Bersani
S, Lamba S, Mattuzzi S, Bardelli A and Scarpa A: The analysis of
PIK3CA mutations in gastric carcinoma and metanalysis of literature
suggest that exon-selectivity is a signature of cancer type. J Exp
Clin Cancer Res. Apr 16–2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Samuels Y, Diaz LA Jr, Schmidt-Kittler O,
Cummins JM, Delong L, Cheong I, Rago C, Huso DL, Lengauer C,
Kinzler KW, et al: Mutant PIK3CA promotes cell growth and invasion
of human cancer cells. Cancer Cell. 7:561–573. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tran TN, Brettingham-Moore K, Duong CP,
Mitchell C, Clemons NJ and Phillips WA: Molecular changes in the
phosphatidylinositide 3-kinase (PI3K) pathway are common in gastric
cancer. J Surg Oncol. 108:113–120. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cho HJ, Baek KE, Park SM, Kim IK, Choi YL,
Cho HJ, Nam IK, Hwang EM, Park JY, Han JY, et al: RhoGDI2
expression is associated with tumor growth and malignant
progression of gastric cancer. Clin Cancer Res. 15:2612–2619. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Taguchi A, Ohmiya N, Shirai K, Mabuchi N,
Itoh A, Hirooka Y, Niwa Y and Goto H: Interleukin-8 promoter
polymorphism increases the risk of atrophic gastritis and gastric
cancer in Japan. Cancer Epidemiol Biomarkers Prev. 14:2487–2493.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wu CW, Wang SR, Chao MF, Wu TC, Lui WY,
P'eng FK and Chi CW: Serum interleukin-6 levels reflect disease
status of gastric cancer. Am J Gastroenterol. 91:1417–1422.
1996.PubMed/NCBI
|
|
44
|
Vicari A, de Moraes MC, Gombert JM, Dy M,
Penit C, Papiernik M and Herbelin A: Interleukin 7 induces
preferential expansion of V beta
8.2+CD4−8− and V beta
8.2+CD4+8− murine thymocytes
positively selected by class I molecules. J Exp Med. 180:653–661.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang X, Rice K, Wang Y, Chen W, Zhong Y,
Nakayama Y, Zhou Y and Klibanski A: Maternally expressed gene 3
(MEG3) noncoding ribonucleic acid: Isoform structure, expression,
and functions. Endocrinology. 151:939–947. 2010. View Article : Google Scholar :
|
|
46
|
Balik V, Srovnal J, Sulla I, Kalita O,
Foltanova T, Vaverka M, Hrabalek L and Hajduch M: MEG3: A novel
long noncoding potentially tumour-suppressing RNA in meningiomas. J
Neurooncol. 112:1–8. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Qin R, Chen Z, Ding Y, Hao J, Hu J and Guo
F: Long non-coding RNA MEG3 inhibits the proliferation of cervical
carcinoma cells through the induction of cell cycle arrest and
apoptosis. Neoplasma. 60:486–492. 2013. View Article : Google Scholar : PubMed/NCBI
|