Introduction
Colorectal cancer (CRC) is the third most common
gastrointestinal malignancy worldwide, among which colon carcinoma
is more frequent than rectal cancer (1). In developing countries, CRC is
becoming increasingly prevalent, particularly in China (2). Currently, CRC is the fourth most
lethal type of tumor in China (3),
causing ~715,000 new cases and 70,000 deaths annually (4). The prognosis of advanced CRC remains
poor, and the estimated 5-year survival rate remains unsatisfactory
due to metastasis, which leads to poor outcomes (5). Currently, conventional treatments,
including radical surgery and adjuvant therapies, such as
chemotherapy and radiation therapy, have been widely applied to the
treatment of CRC; however, the 5-year survival rate remains low,
and significant adverse events remain unresolved issues (6). Accordingly, there is an urgent need to
develop novel strategies to manage CRC. Today, tumor immunotherapy
for colon cancer is extremely appealing, and cancer vaccines have
become an attractive therapeutic option that have the potential to
control metastatic disease and prolong the time to recurrence
without causing significant side-effects.
Antigen-85A (Ag85A) is a major protein secreted by
Mycobacterium spp. that participates in the synthesis of
mycolic acid in cell walls (7) and
stimulates massive Th1 cell proliferation and cytokine production
in humans or mice infected with mycobacteria (8). Mice vaccinated with Ag85A DNA exhibit
elevated IL-2, interferon (IFN)-γ and IgG2a production, as well as
increased cytotoxic T lymphocyte (CTL) activity in response to BCG
proteins, of which Ag85A is a major component (9,10).
Numerous studies have shown that vaccination with recombinant
Ag85A-DNA induces a vigorous immune response that protects against
tuberculosis in mice (11,12). We have previously demonstrated that
an orally administered Ag85A DNA vaccine induced systemic and
mucosal immunity by inducing Th1 type immune responses to provide
protection against Mycobacterium tuberculosis infection
(13). In light of the role of
Ag85A in inducing cellular immune responses, efforts are being made
to apply single Ag85A or recombinant Ag85A DNA vaccines to the
treatment of tumors. One study has shown that a recombinant Ag85A
and GM-CSF DNA vaccine enhanced antitumor immunity against melanoma
in mice (14). Our previous study
indicated a robust therapeutic effect on bladder cancer through a
dendritic cell vaccine designed to evoke immune responses against
Ag85A (15).
CD226 is a transmembrane glycoprotein and member of
the immunoglobulin superfamily that is constitutively expressed by
the majority of T cells, natural killer (NK) cells,
monocytes/macrophages, platelets and megakaryocytes, as well as by
a subset of B cells (16). During T
cell priming, CD226-mediated co-stimulatory signals can skew
CD4+ T cell differentiation towards the Th1 cell pathway
(17). Additionally, the CD226
molecule itself is an important activated receptor on the surface
of NK cells, and is one of the main molecules involved in tumor
recognition and NK cell-mediated cytotoxicity (18). Our recent study indicated that CD226
could be used as a genetic adjuvant to enhance both systemic and
mucosal immune effects induced by an Ag85A DNA vaccine in normal
mice (19). To optimize
immunotherapy against colon carcinoma, we developed a tumor cell
vaccine expressing Ag85A and CD226 and investigated its anti-colon
carcinoma efficacy in BALB/c mice.
Materials and methods
Mice and cell lines
Female BALB/c mice (6- to 8-weeks old) were
purchased from Liao Ning Chang Sheng Biotechnology Co. (Benxi,
China) and housed in pathogen-free conditions. The present study
was carried out in strict accordance with the recommendations in
the Guide for the Care and Use of Laboratory Animals of the
National Institutes of Health. The protocol was approved by the
Ethics Committee of the Animal Experiments of China Medical
University. All surgery was performed under sodium pentobarbital
anesthesia, and all efforts were made to minimize suffering. Yac-1
and Colon 26 cell lines were obtained from the Institute of
Biochemistry and Cell Biology (Chinese Academy of Sciences,
Shanghai, China), and cultured at 37°C in 5% CO2 in a
humidified atmosphere in complete RPMI-1640 medium (Thermo
Scientific, Waltham, MA, USA), supplemented with 10% fetal bovine
serum (FBS; Cell Culture Technologies, Tokyo, Japan), 100 U/ml
penicillin G sodium and 100 µg/ml streptomycin sulfate (BI
Biological Industries, Kibbutz Beit-Haemek, Israel).
Plasmids and primers
The pcDNA3.1-Ag85A and pcDNA3.1-CD226 plasmids were
constructed in our laboratory. The CD226-PCR2.1-ToPo plasmid was a
gift from Professor Shibuya (University of Tsukuba, Tsukuba,
Japan). Recombinant plasmid pcDNA3.1-Ag85A-CD226 was constructed as
follows. The CD226 gene was first amplified from plasmid
CD226-PCR2.1-ToPo using a regular PCR routine. The amplicon was
then inserted into pcDNA3.1-Ag85A to derive the recombinant plasmid
pcDNA3.1-Ag85A-CD226. After transformation into E. coli
DH5α, the recombinant plasmid DNA was prepared and characterized by
digestion using restriction enzymes and sequence analysis. The
polymerase chain reaction (PCR) primer sequences were as follows:
CD226 forward, 5′-ATAAG
AATGCGGCCGCATGGCTTATGTTACTTGGCTTTTGG-3′ and reverse,
5′-GCCTAGCGTCTAGATCGAGGTCTTGGTTTTGGTCTTC-3′; Ag85A forward,
5′-TTTCGCGGATCCAGATGTTTTCCCGGCC-3′ and reverse,
5′-CTGTTCGGAATTCGGCGCCCTGGG-3′; β-actin forward,
5′-TTCTTGGGTATGGAATCCTGTG-3′ and reverse,
5′-GAGGAGCAATGATCTTGATCTT-3′.
Stable transfection and construction of
tumor cell vaccines
Colon 26 cells were cultured in complete media in
6-well plates. The recombinant plasmids pcDNA3.1-Ag85A-CD226,
pcDNA3.1-Ag85A or pcDNA3.1-CD226, or a mock plasmid, pcDNA3.1 were
transfected into Colon 26 cells at ~80% confluency with
Lipofectamine™ 2000 reagent (Invitrogen, Carlsbad, CA, USA)
according to the manufacturer's protocol. The stably expressing
Ag85A or CD226 cell lines were selected in RPMI-1640
media containing 800 µg/ml G418 (Invitrogen). After 21 days,
G418-resistant clones were selected, and cells expressing Ag85A
were termed 'Colon 26/Ag85A cells', cells expressing CD226 were
termed 'Colon 26/CD226 cells', cells expressing Ag85A and CD226
were termed 'Colon 26/Ag85A-CD226 cells', cells transfected with
mock plasmid pcDNA3.1 were termed 'Colon 26/pcDNA3.1 cells' and
untransfected Colon 26 cells were used as an additional control.
Total RNA and total cell proteins from each transfected cell were
collected and extracted. The expression levels of CD226 and
Ag85A mRNA were characterized by reverse transcriptase
(RT)-PCR, and the protein expression levels of CD226 and Ag85A were
characterized by western blotting using goat anti-mouse DNAM-1 and
mouse anti-Ag85 (both from Santa Cruz Biotechnology, Santa Cruz,
CA, USA), respectively.
Tumor vaccine-based immunotherapy in a
murine model
BALB/c mice were randomly divided into five groups,
with nine mice per group. Each mouse was subcutaneously (s.c.)
inoculated with 1×106 Colon 26 cells in the flank and 3
days later mice in each group were immunized with 1×107
Colon 26, Colon 26/pcDNA3.1, Colon 26/CD226, Colon 26/Ag85A or
Colon 26/Ag85A-CD226 cells, previously inactivated by treatment
with 100 µg/ml mitomycin C (Inalco SPA, Milan, Italy) three
times at weekly intervals in the same flank that was initially
challenged with Colon 26 cells. The five groups of mice included a
normal control, pcDNA3.1, Ag85A, CD226 and Ag85A-CD226 group. Tumor
growth was monitored by observing the onset of subcutaneous tumors
and measuring two perpendicular tumor diameters using Vernier
calipers. After onset, the tumor volume, tumor weight and percent
survival (over a 70 day period) were evaluated. Tumor volume (V)
was calculated using the formula: V (mm3) = 0.523 × L ×
W2, in which L (mm) and W (mm) indicated the length and
width of the tumor, respectively. One week after the final
immunization, the mice were euthanized and sera, splenocytes and
peripheral lymph node cells were isolated. Tumors were removed by
dissection and fixed in 10% formalin.
Histopathological analysis
The formalin-fixed tissues were embedded in paraffin
and then sectioned for hematoxylin and eosin (H&E) staining and
immunohistochemical analysis of tumors using a SABC-AP (rat IgG)
immunohistochemical staining kit (Boster, Wuhan, China) according
to the manufacturer's protocol. Sections were washed with
phosphate-buffered saline (PBS) twice for 5 min/wash. After
blocking with 0.1 mg/ml BSA for 20 min at room temperature, the
sections were incubated with monoclonal rat anti-mouse CD8
(LifeSpan BioSciences, Seattle, WA, USA) or monoclonal rat
anti-mouse CD4 (Santa Cruz Biotechnology) at 4°C overnight.
Sections were washed three times with PBS and then incubated with
biotin-rabbit anti-rat IgG for 20 min at 37°C. Subsequently, the
sections were incubated with SABC-AP secondary antibody for 20 min
at 37°C. Slides were viewed using a microscope at a magnification
of ×400; 10 high-power fields containing the positive cells were
randomly selected, and the average number of positive cells was
determined by counting. Tumor samples were analyzed to detect the
ratio of apoptotic cells by the terminal deoxynucleotidyl
transferase-mediated dUTP-biotin nick end-labeling (TUNEL) method,
using an apoptosis In Situ Detection kit (Wako Pure
Chemical, Osaka, Japan), according to the manufacturer's
instructions. TUNEL-positive cells were stained by DAB. The
frequency of DAB-positive cells is shown, and the average
percentage of DAB-positive staining was quantified from 10 randomly
selected high-power fields (magnification, ×400) of each section
using ImmunoRatio or blinded manual cell counting (20).
CTL and NK cell CFSE/PI cytotoxicity
assays using flow cytometry
The NK cell cytotoxicity assay was preformed as
follows, and was based on protocols described elsewhere (21,22).
Briefly, Yac-1 cells (used as target cells) in the logarithmic
growth phase (2×107 cells/ml) were resus-pended and
labeled with 2.5 µM fluorescein-based dye 5-(and
-6)-carboxyfluorescein diacetate succinimidyl ester (CFSE;
eBioscience, San Diego, CA, USA), and then incubated in RPMI-1640
medium (Thermo Scientific) containing 10% FBS at 37°C under 5%
CO2 for 15 min in a 1 ml volume. After the incubation, 1
ml FCS was added to stop the reaction. The cell suspensions were
centrifuged for 5 min at 400 × g, washed twice with PBS and
resuspended in RPMI-1640 at a final concentration of
2×105 cells/ml. Splenocytes (used as effector cells)
were purified from the immunized mice 1 week after the third
immunization, enriched from the splenic homogenate using a Ficoll
gradient (Sigma) and resuspended at a final concentration of
1×106 cells/ml.
The effector (E) and target (T) cells were added
together to yield an E:T ratio of 5:1, and were incubated at 37°C
under 5% CO2 for 4 h. After incubation, the tubes were
mixed gently, placed on ice and 4 µl propidium iodide (PI; 1
µg/l, final concentration; Sigma-Aldrich, St. Louis, MO,
USA) was added to each tube for 10–15 min. Finally, the samples
were analyzed by flow-cytometry within 60 min. All samples were
analyzed on a FACSCalibur (Becton-Dickinson, San Diego, CA, USA),
and flow cytometry data were analyzed using WINMDI 2.9 software.
During data acquisition, a 'live gate' was set on the CFSE-stained
target cell population using an FL1-histogram, and the CFSE-stained
target cells were further gated on the PI-stained target cell
population using an FL3-histogram.
The percentage of specific target cell death
(cytotoxicity) was expressed as: {dead target cells in the sample
(%) - spontaneously dead target cells (%)]/100% - spontaneously
dead target cells (%)} × 100. Control tubes containing target or
effector cells alone were also assayed to facilitate the gating and
marker settings. The fraction of target cells that spontaneously
died was determined based on the number of target cells labeled
with CFSE and incubated without effector cells for 4 h.
The CTL cytotoxicity assay was preformed as follows.
Briefly, 5×106 murine splenocytes (used as effector
cells) were resuspended in 10% RPMI-1640 medium in 24-well plates,
and then were incubated for 72 h with 5×104 Colon 26
cells inactivated with mitomycin C (100 µg/ml).
Additionally, 10 ng rIL-2 (R&D Systems, Minneapolis, MN, USA)
was added to all wells except for the control samples with target
cells alone. Cells were isolated from the splenic homogenate using
a Ficoll gradient, and then were rinsed extensively with complete
RPMI-1640 (1×106 cells/ml, final concentration) and used
in the CTL cytotoxicity assay. Colon 26 cells (2×105
cells/ml, final concentration) were used as target cells. The
operation process and analysis methods were the same as for the NK
cytotoxicity assay.
ELISA assay analysis
At 0, 1, 2, 3 and 4 weeks after immunization, some
mice were euthanized and splenocytes were isolated. Splenocytes
(2×107 cells/ml) purified from the immunized mice were
stimulated with 5 µg/ml ConA in 24-well plates and were
incubated for 72 h with 2×104 Colon 26 cells inactivated
with mitomycin C (100 µg/ml). The levels of IFN-γ in the
culture supernatants were determined using enzyme-linked
immunoabsorbent assay (R&D Systems) according to the
manufacturer's instructions. Absorbance was measured at 450 nm
using a microplate reader (Thermo), and the concentration of IFN-γ
was calculated using the software SoftMax Pro 4.3.1 LS.
Intracellular cytokine staining
Splenocytes and peripheral lymph node cells
(2×106 cells/ml) were isolated from the immunized mice.
Cells were incubated for 72 h with 5×104 Colon 26 cells
inactivated with mitomycin C (100 µg/ml) and then stimulated
with 50 ng/ml PMA and 1 µg/ml ionomycin (Sigma) in the
presence of 2 µM monensin (BD Biosciences, San Jose, CA,
USA) for 5 h. Cells were harvested and stained with Fcγ
receptor-blocking mAb (CD16/32; BD Biosciences), and then stained
with fluorescein isothiocyanate (FITC)-conjugated rat anti-mouse
CD4, PE-conjugated rat anti-mouse CD8 and PE/Cy7-conjugated rat
anti-mouse CD3 (all from Biolegend, San Diego, CA, USA) at 4°C for
30 min. FITC mouse IgG2a, κ; PE rat IgG2a, κ; and PE/Cy7 rat IgG2b,
κ (Biolegend) were used as isotype controls. Cells were washed with
PBS three times, fixed with fixation buffer at 4°C for 20 min,
permeabilized with the appropriate solution at room temperature for
20 min, and then stained with APC-conjugated rat anti-mouse IFN-γ;
APC-rat IgG1, κ (both from Biolegend) was used as an isotype
control. The percentage of IFN-γ-secreting CD4+ and
CD8+ T cells was determined using a FACSCalibur flow
cytometer (Becton-Dickinson) and WINMDI 2.9 software.
Statistical analysis
Results are expressed as means ± SD. The statistical
significance of differences between groups was analyzed by
two-tailed independent Student's t-tests. The one-way ANOVA
analysis was performed when more than two groups was compared.
Analyses were performed using GraphPad Prism 5.0 statistical
software package (Graph Pad Inc., USA). In all instances, p<0.05
was considered to indicate a statistically significant result.
Results
Expression of CD226 and Ag85A in Colon 26
cells
The recombinant pcDNA3.1-Ag85A-CD226 plasmid was
constructed and characterized by endonuclease digestion and DNA
sequencing (data not shown). The expression of Ag85A and CD226 in
the pcDNA3.1-Ag85A-CD226 plasmid was confirmed by RT-PCR and
western blotting after stable transfection of the recombinant
plasmid into Colon 26 cells. Both CD226 and Ag85A were successfully
expressed at both the mRNA and protein levels in the Colon 26 cells
(Fig. 1A and B).
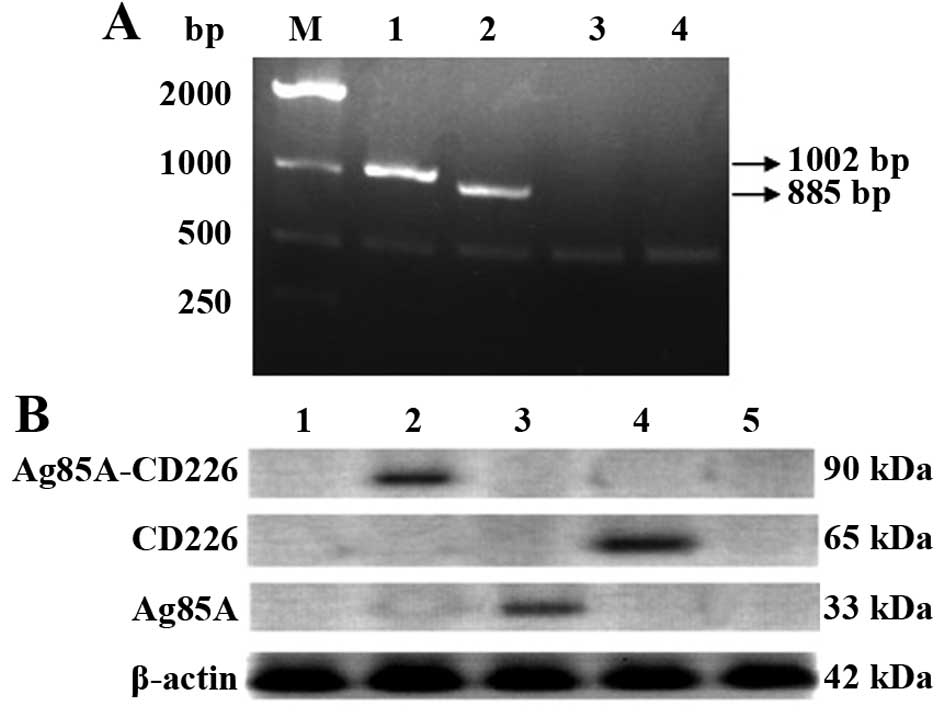 | Figure 1Characterization of the mRNA and
protein levels of CD226 and Ag85A in Colon 26 cells. (A)
Characterization of the expression of CD226 and Ag85A
mRNA. M, DNA molecular ladder; lane 1, CD226 or
β-actin mRNA expression in Colon 26 cells transfected with
pcDNA3.1-Ag85A-CD226; lane 2, Ag85A or β-actin mRNA
expression in Colon 26 cells transfected with pcDNA3.1-Ag85A-CD226;
lane 3, mRNA in Colon 26 cells transfected with pcDNA3.1; lane 4,
mRNA in untransfected Colon 26 cells. (B) Characterization of
protein expression levels in Colon 26 cells transfected with
different recombinant plasmids among total cellular protein
lysates. Lane 1, proteins in untransfected Colon 26 cells; lane 2,
fusion protein Ag85A-CD226 or β-actin protein expression in Colon
26 cells transfected with pcDNA3.1-Ag85A-CD226; lane 3, Ag85A or
β-actin protein expression in Colon 26 cells transfected with
pcDNA3.1-Ag85A; lane 4, CD226 or β-actin protein expression in
Colon 26 cells transfected with pcDNA3.1-CD226; lane 5, proteins in
Colon 26 cells transfected with pcDNA3.1. |
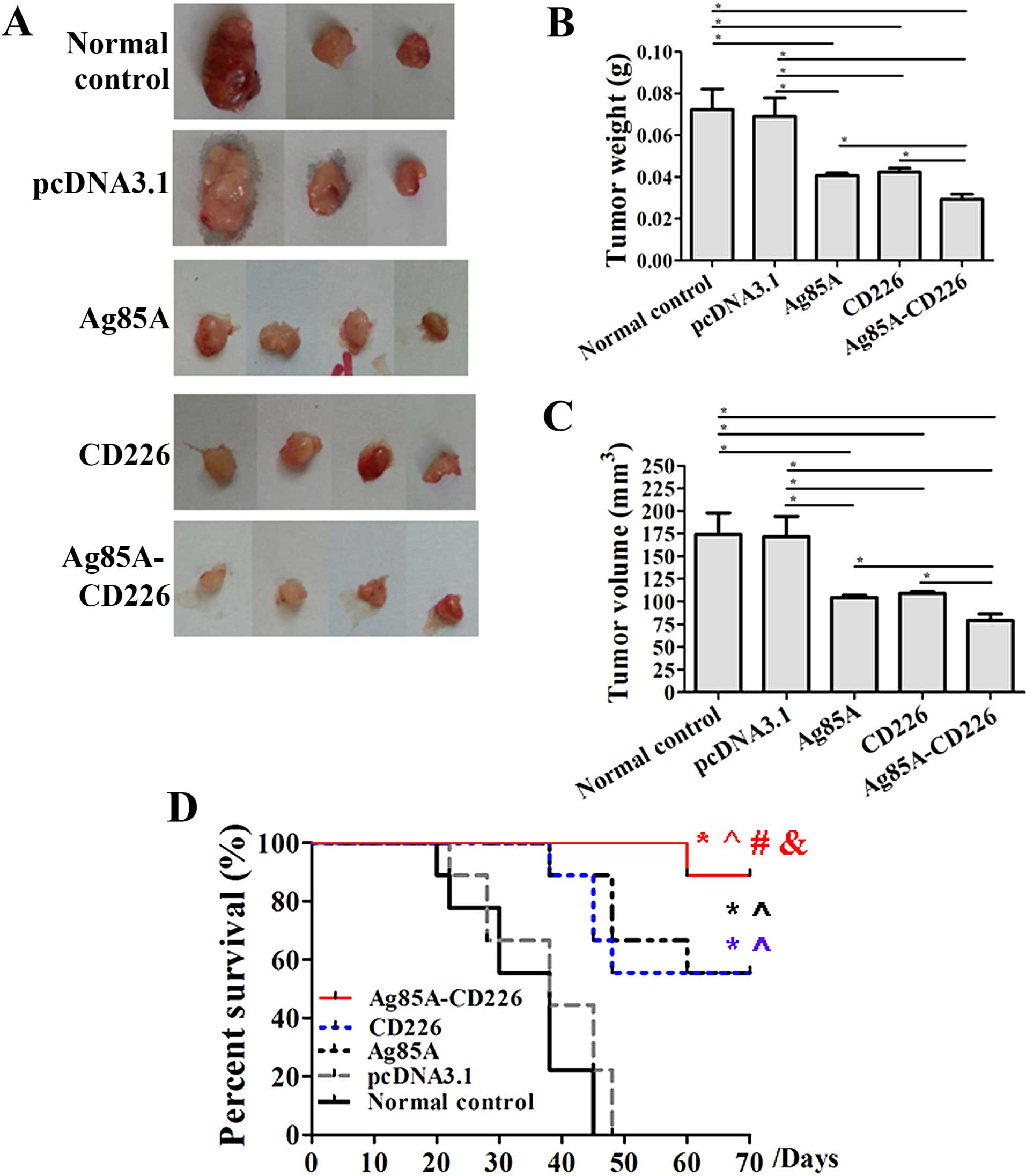
Therapeutic efficacy against Colon 26
tumors using a Colon 26/Ag85A-CD226-pcDNA3.1 tumor cell
vaccine
We investigated the therapeutic tumor efficacy of a
tumor vaccine expressing Ag85A and CD226. We detected a noticeable
effect of the antitumor immune therapy in mice immunized with the
Colon 26/Ag85A-CD226 tumor cell vaccine. Under the same
experimental conditions, measurable tumors were detected in all
mice injected with 5×106 inactivated Colon 26 cells on
day 28. We found that the volume and weight of tumors were the
smallest in the Colon 26/Ag85A-CD226 cell vaccine group when
compared with the Colon 26 cell vaccine group, Colon 26/pcDNA3.1
cell vaccine group, Colon 26/Ag85A cell vaccine group or Colon
26/CD226 cell vaccine group on day 28 (Fig. 2A–C). Based on our previous study,
2×106 Colon 26 cells in logarithmic grown phase were
inoculated into BALB/c mice, which usually generated a palpable
tumor in ~7 days. However, the tumor growth was not palpable in all
mice treated with the different tumor cell vaccines until 11 days
into the observation, except for the Colon 26 and Colon 26/pcDNA3.1
cell vaccine groups. Furthermore, the survival time of mice
vaccinated with the Colon 26/Ag85A-CD226 vaccine was the longest,
and the survival time of mice vaccinated with the Colon 26/Ag85A or
Colon 26/CD226 vaccine was significantly longer than that of the
mice vaccinated with the Colon 26/pcDNA3.1 or Colon 26 vaccine
(p<0.01; Fig. 2D). It appeared
that the therapeutic effect of the Colon 26/Ag85A-CD226 tumor cell
vaccine was the strongest in all of the five vaccine groups.
Tumor histopathology analysis
Tumor tissues were fixed and stained. H&E
staining of the tumor sections showed the active growth of tumor
cells, with obvious nuclear division and rich vessels in both the
Colon 26/pcDNA3.1 and Colon 26 tumor cell vaccine groups (Fig. 3A-a and -b). In the Colon 26/CD226
tumor cell vaccine group, tumor cells grew slowly and few
inflammatory cells infiltrated the tumor tissues (Fig. 3A-c). In the Colon 26/Ag85A tumor
cell vaccine group, the infiltration of inflammatory cells was
increased appreciably and central necrosis was noticeable in the
tumor tissues (Fig. 3A-d). In the
Colon 26/Ag85A-CD226 tumor cell vaccine group, massive numbers of
inflammatory cells infiltrating the tumor tissues could be
observed, massive necrosis in tumor tissues was visible and the
tumors had obviously shrunk, experienced ordered rarefaction and
had degenerated (Fig. 3A-e). The
TUNEL staining results indicated that the maximum percentage of
apoptosis could be detected in the samples from the Colon
26/Ag85A-CD226 tumor cell vaccine-treated mice (Fig. 3B and C). The immunohistochemical
staining results indicated that the infiltration of CD4+
or CD8+ T cells increased significantly in the tumor
tissues of the Colon 26/Ag85A-CD226 tumor cell vaccine group
compared with that in other tumor cell vaccine groups (Fig. 3D and E). Histopathology analyses
suggested that the Ag85A and CD226 genes alone or in combination
induced a cellular immune response against colon carcinoma cells,
and that the antitumor effect induced by the Colon 26/Ag85A-CD226
tumor cell vaccine was the strongest.
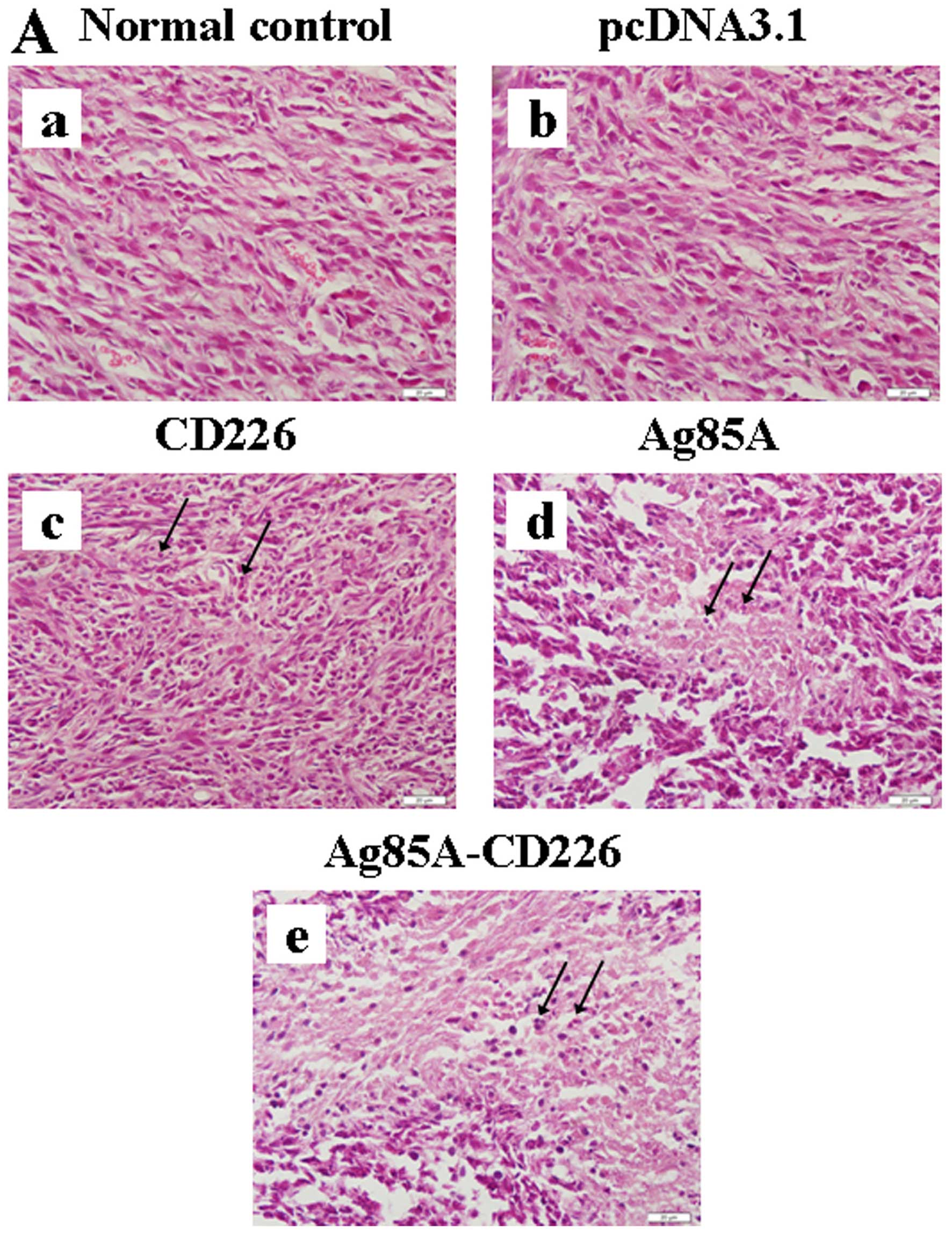 | Figure 3Histopathological analysis of tumor
tissues. (A) Histopathology of the tumor tissues from the BALB/c
mice (H&E, magnification, ×400). (a and b) Histopathological
tumor tissue sections in mice treated with Colon 26 tumor cells or
Colon 26/pcDNA3.1 tumor cells, respectively, wherein the tumor
cells grew actively and rich vessels were observed along with
obvious nuclear division. (c) Histopathological tumor tissue
sections in mice treated with the Colon 26/CD226 tumor cell
vaccine, wherein the tumor cells grew slowly, a small amount of
inflammatory infiltrates could be observed, and vessels in the
tumor tissue were significantly reduced. (d) Histopathological
tumor tissue sections in mice treated with the Colon 26/Ag85A tumor
cell vaccine, wherein the tumor cells grew slowly, few inflammatory
cells could be observed infiltrating the tumor tissue, and there
was evidence of central necrosis in the tumor tissue. (e)
Histopathological tumor tissue sections in mice treated with the
Colon 26/Ag85A-CD226 tumor cell vaccine, wherein the infiltration
of lymphocytes, monocytes and neutrophils increased appreciably and
massive necrosis in tumor tissues was visible, vessels were not
visible and visible connective tissue around tumors and surrounding
tissues appeared. Black arrows indicate inflammatory cells. (B) A
representative DAB immunohistochemistry micrograph for apoptotic
cells in tumor tissues by TUNEL staining (magnification, ×400).
Black arrows indicate examples of DAB-positive nuclei. Scale bar,
20 µm. (C) Percentage of TUNEL-positive tumor cells.
(D1) Immunohistochemical staining for CD4+ T
cells in tumor tissues (magnification, ×400). Black arrows indicate
CD4+ T cells. (E) The absolute number of infiltrating
CD4+ T cells in the tumor was counted under a light
microscope. Scale bar, 20 µm. Data are expressed as means ±
SD (n=5), and one representative of three individual experiments is
shown; *p<0.05, **p<0.01.
(D2) Immunohistochemical staining for CD8+ T
cells in tumor tissues (magnification, ×400). Black arrows indicate
CD8+ T cells. (E) The absolute number of infiltrating
CD4+ or CD8+ T cells in the tumor was counted
under a light microscope. Scale bar, 20 µm. Data are
expressed as means ± SD (n=5), and one representative of three
individual experiments is shown; *p<0.05,
**p<0.01. |
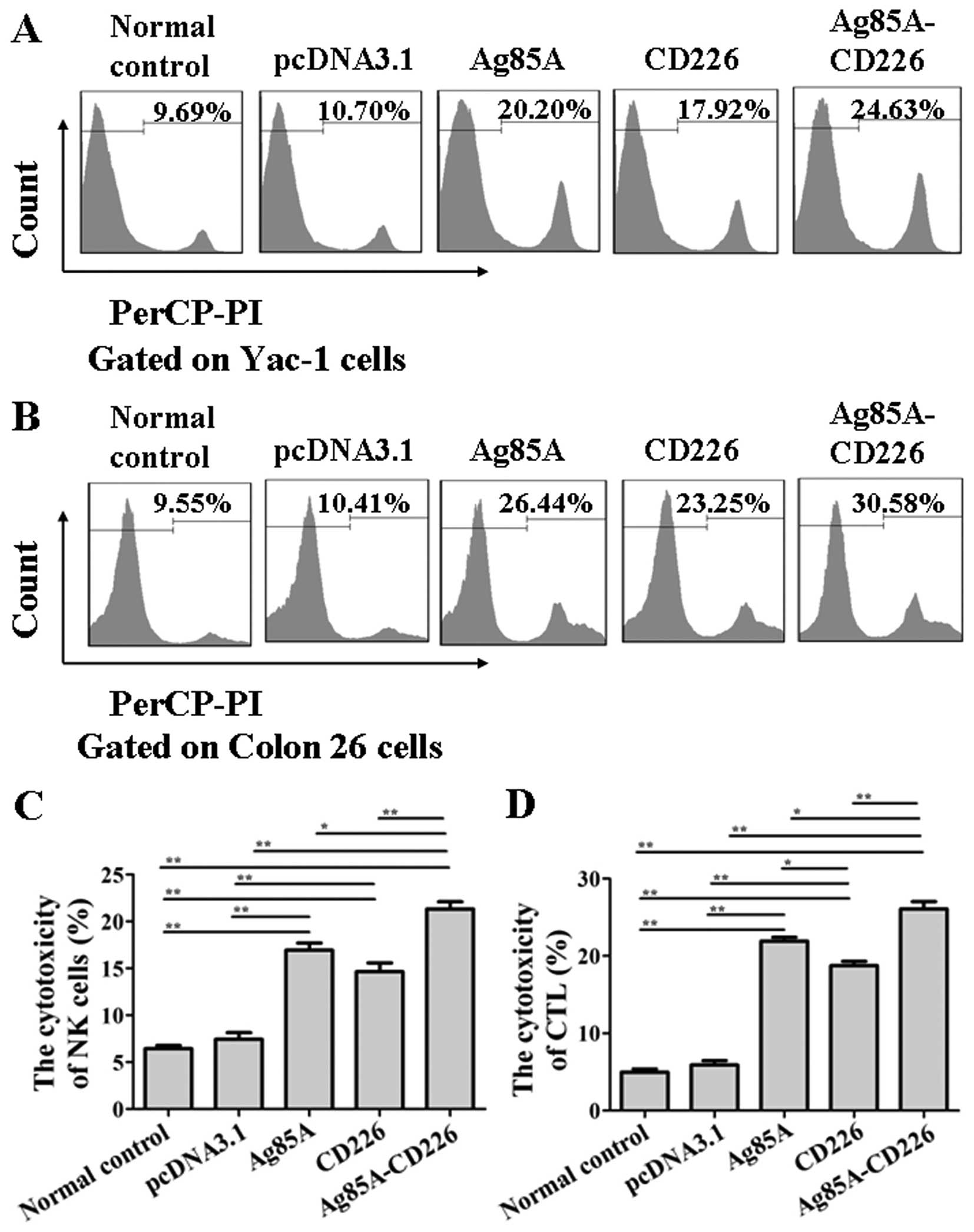
Detection of NK cell and CTL cytotoxicity
in splenocytes
To examine whether the CTL and NK cell cytotoxicity
was enhanced in mice vaccinated with the Colon 26/Ag85A-CD226 tumor
cell vaccine, splenocytes from each group were purified at day 7
after the third immunization. We found that the NK cell
cytotoxicity of splenocytes from the mice immunized with the Colon
26/Ag85A-CD226 tumor cell vaccine was significantly greater than
that of the Colon 26/Ag85A, Colon 26/CD226, Colon 26/pcDNA3.1 or
Colon 26 tumor cell vaccine treated mice (p<0.01; Fig. 4A and C). The experimental results of
CTL cytotoxicity were consistent with that of NK cell cytotoxicity
(p<0.01; Fig. 4B and D). The
findings suggested that the Colon 26/Ag85A-CD226 tumor cell vaccine
induced the generation of the strongest NK cell and CTL
cytotoxicity in mice.
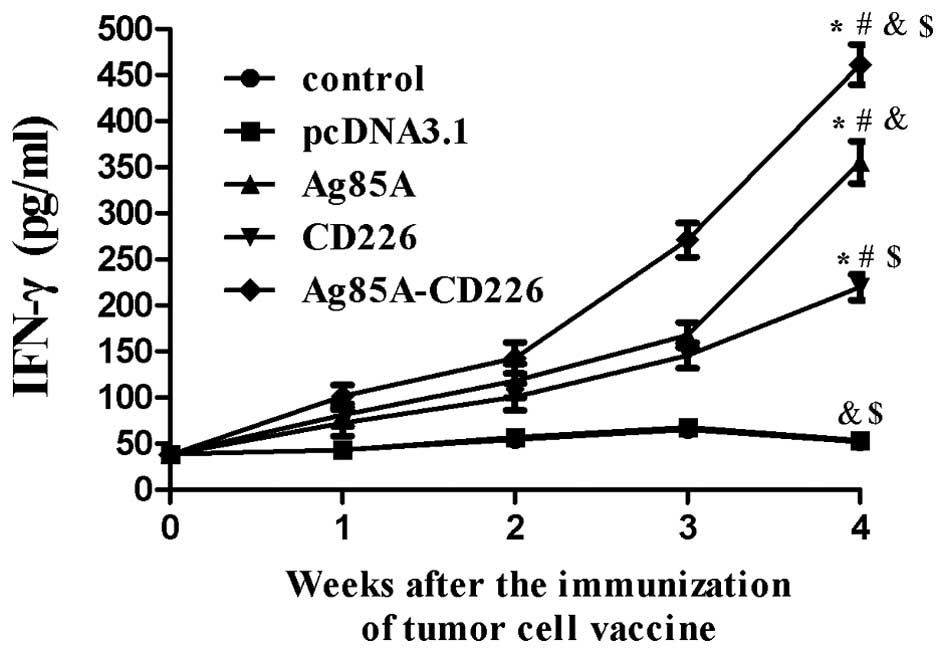
IFN-γ levels in splenocytes immunized
with the Colon 26/Ag85A-CD226 tumor cell vaccine
We found that IFN-γ levels in splenocytes gradually
increased from 1 to 4 weeks of immunization in the mice treated
with the various tumor cell vaccines (Fig. 5). The IFN-γ levels in splenocytes
from mice immunized with the Colon 26/Ag85A-CD226 or Colon 26/Ag85A
tumor cell vaccines increased slowly in the first 2 or 3 weeks,
respectively, of immunization and then increased rapidly
thereafter. IFN-γ levels in splenocytes from mice immunized with
the Colon 26/CD226 tumor cell vaccine increased slowly throughout
the entire 4 weeks of immunization. In the mice immunized with the
Colon 26/pcDNA3.1 or Colon 26 tumor cell vaccines, IFN-γ levels
increased slowly in the first 3 weeks of immunization, and then
showed a trend to decline. Finally, the IFN-γ levels in splenocytes
from mice immunized with the Colon 26/Ag85A-CD226 tumor cell
vaccine were significantly higher than those in mice treated with
the Colon 26/Ag85A, Colon 26/CD226, Colon 26/pcDNA3.1 or Colon 26
tumor cell vaccines (p<0.01) at the 4th week. The findings
suggest that the Colon 26/Ag85A-CD226 tumor vaccine stimulates the
IFN-γ production at higher levels compared with the Colon 26/Ag85A
or Colon 26/CD226 tumor cell vaccines.
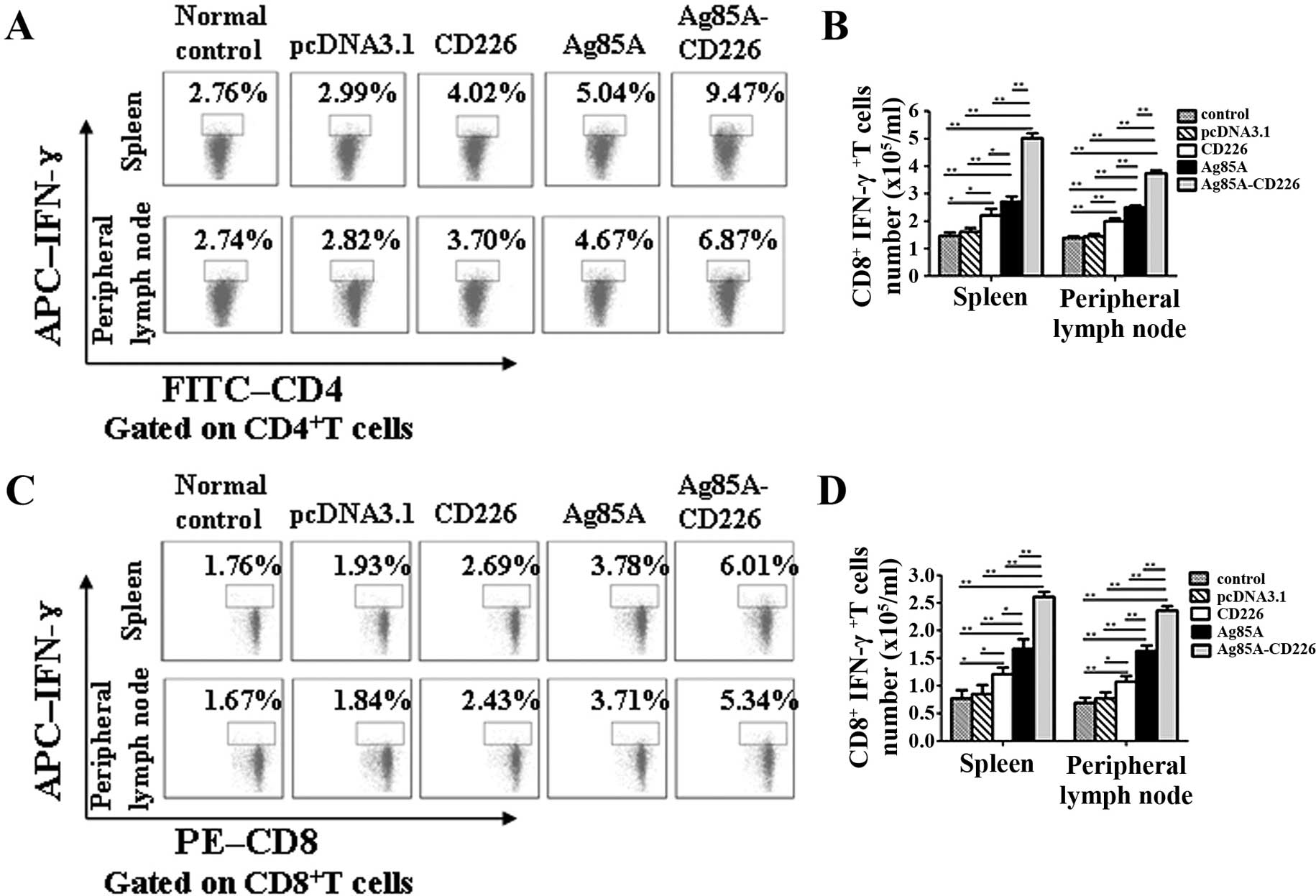
Enhanced Th1 cell-dominated cellular
immunity antitumor responses induced by the tumor cell vaccine
The CD4+IFN-γ+ and
CD8+IFN-γ+ T cells were both most
significantly increased (p<0.01) in the mice immunized with the
Colon 26/Ag85A-CD226 tumor cell vaccine in both splenocytes and
peripheral lymph node cells, followed by the Colon 26/Ag85A
vaccine, the Colon 26/CD226 vaccine, the Colon 26/pcDNA3.1 and the
Colon 26 vaccine (Fig. 6),
indicating that the Colon 26/Ag85A-CD226 tumor cell vaccine
enhanced a Th1 cell-dominated antitumor immune response.
Discussion
In immunotherapy, antitumor vaccines represent one
of the most promising therapeutic strategies. To enhance the immune
response, cancer vaccines are usually administered with adjuvants,
such as co-stimulatory molecules and are often combined with
cytokines, such as granulocyte macrophage colony-stimulating factor
(GM-CSF) or interleukin (IL)-2 (23). Recently, genetically modified cells
have been shown to be one of the most effective cancer vaccine
strategies, which has been applied to various types of cancer in
preclinical models, including some that are being tested in
clinical trials (24–26). The Colon 26 cell vaccine expressing
Ag85A and CD226 in the present study was designed to evaluate the
inclusion of Ag85A as an immune enhancer, yet also takes into
account the role of CD226. As a co-stimulatory and adhesion
molecule in the activation and proliferation of T cells, CD226
promotes the initial differentiation of T to Th1 cells and boosts
CTL cyto-toxicity (17,27). In cancer immunotherapy, the general
concept is that a Th1 cell skewed response directed against a tumor
is favorable. The Th1 cell response leads to the activation of
tumor-specific CTL that are capable of killing or impairing the
proliferation of tumor cells (28).
One study of a colon cancer vaccine has shown that Th1 cell
responses are essential in cancer immunotherapy and indicates the
therapeutic potential of a vaccine (29).
These different therapeutic Colon 26 colon tumor
efficacies in mice indicated that tumor appearance was delayed,
tumor growth was inhibited, tumor volume and weight were decreased
and the survival time of the tumor-bearing mice was extended,
particularly in the Colon 26/Ag85A-CD226 tumor cell vaccine group.
We speculated that the therapeutic effects that we observed in mice
depended mainly on immune responses, in particular an enhanced Th1
cell-dominated cellular immune response. This model is supported by
the enhanced NK cell and CTL cytotoxicity, increased IFN-γ levels
in splenocytes and more abundant CD4+IFN-γ+
and CD8+IFN-γ+ T cells in splenocytes and
peripheral lymph node cells in mice treated with the Colon
26/Ag85A-CD226 tumor cell vaccine. The enhanced non-specific
killing activities of NK cells and tumor-specific killing
activities of CTL led to increased necrosis in tumor tissues.
Furthermore, the tumor infiltrating CD4+ or
CD8+ T cells in tumor tissues led to increased apoptosis
of tumor cells in the Colon 26/Ag85A-CD226 tumor cell vaccine
group. The present study also indicated that the immune responses
induced by the Colon 26/Ag85A-CD226 vaccine were significantly
stronger than Colon 26/Ag85A, and then we speculated that CD226
played a genetic adjuvant role to promote the immune responses
induced by Ag85A in anti-Colon 26 colon carcinoma.
In summary, the present study demonstrated that the
tumor vaccine with co-expression of CD226 and Ag85A induced more
intensive antitumor immunity than the tumor vaccine expressing
Ag85A or CD226 only. Our investigation suggests that Ag85A and
CD226 play a synergistic role in anti-Colon 26 colon carcinoma
immune responses and CD226 could be used as a genetic adjuvant to
enhance the effects of the Ag85A vaccine against tumors. Our
findings establish a new strategy for the development of a novel
vaccine against colon carcinoma.
Acknowledgments
We kindly thank Professor Shibuya in University of
Tsukuba in Japan for providing CD226-PCR2.1-ToPo. This study was
financially supported by the National Natural Science Foundation of
China (no. 31270972), and the Natural Science Foundation of
Liaoning Province of China (no. 2011415052-1).
References
|
1
|
Esteban-Jurado C, Garre P, Vila M, Lozano
JJ, Pristoupilova A, Beltrán S, Abulí A, Muñoz J, Balaguer F, Ocaña
T, et al: New genes emerging for colorectal cancer predisposition.
World J Gastroenterol. 20:1961–1971. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li HZ, Mao WM, Wang XH, Yu CD and Du LB:
Incidence and mortality of cancer in Zhejiang province in 2009.
Zhonghua Yu Fang Yi Xue Za Zhi. 47:592–596. 2013.In Chinese.
PubMed/NCBI
|
|
3
|
Yi N, Xiao MB, Ni WK, Jiang F, Lu CH and
Ni RZ: High expression of peroxiredoxin 4 affects the survival time
of colorectal cancer patients, but is not an independent
unfavorable prognostic factor. Mol Clin Oncol. 2:767–772.
2014.PubMed/NCBI
|
|
4
|
Yang Z, Bai Y, Huo L, Chen H, Huang J, Li
J, Fan X, Yang Z, Wang L and Wang J: Expression of A disintegrin
and metalloprotease 8 is associated with cell growth and poor
survival in colorectal cancer. BMC Cancer. 14:5682014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim J, Huynh R, Abraham I, Kim E and Kumar
RR: Number of lymph nodes examined and its impact on colorectal
cancer staging. Am Surg. 72:902–905. 2006.PubMed/NCBI
|
|
6
|
Jemal A, Center MM, DeSantis C and Ward
EM: Global patterns of cancer incidence and mortality rates and
trends. Cancer Epidemiol Biomarkers Prev. 19:1893–1907. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Belisle JT, Vissa VD, Sievert T, Takayama
K, Brennan PJ and Besra GS: Role of the major antigen of
Mycobacterium tuberculosis in cell wall biogenesis. Science.
276:1420–1422. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huygen K, Content J, Denis O, Montgomery
DL, Yawman AM, Deck RR, DeWitt CM, Orme IM, Baldwin S, D'Souza C,
et al: Immunogenicity and protective efficacy of a tuberculosis DNA
vaccine. Nat Med. 2:893–898. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Borremans M, de Wit L, Volckaert G, Ooms
J, de Bruyn J, Huygen K, van Vooren JP, Stelandre M, Verhofstadt R
and Content J: Cloning, sequence determination, and expression of a
32-kilodalton-protein gene of Mycobacterium tuberculosis. Infect
Immun. 57:3123–3130. 1989.PubMed/NCBI
|
|
10
|
Montgomery DL, Huygen K, Yawman AM, Deck
RR, Dewitt CM, Content J, Liu MA and Ulmer JB: Induction of humoral
and cellular immune responses by vaccination with M. tuberculosis
antigen 85 DNA. Cell Mol Biol (Noisy-le-grand). 43:285–292.
1997.
|
|
11
|
Dou J, Tang Q, Yu F, Yang H, Zhao F, Xu W,
Wang J, Hu W, Hu K and Liou C: Investigation of immunogenic effect
of the BCG priming and Ag85A-GM-CSF boosting in Balb/c mice model.
Immunobiology. 215:133–142. 2010. View Article : Google Scholar
|
|
12
|
Dou J, Wang Y, Yu F, Yang H, Wang J, He X,
Xu W, Chen J and Hu K: Protection against Mycobacterium
tuberculosis challenge in mice by DNA vaccine Ag85A-ESAT-6-IL-21
priming and BCG boosting. Int J Immunogenet. 39:183–190. 2012.
View Article : Google Scholar
|
|
13
|
Wang D, Xu J, Feng Y, Liu Y, Mchenga SS,
Shan F, Sasaki J and Lu C: Liposomal oral DNA vaccine
(mycobacterium DNA) elicits immune response. Vaccine. 28:3134–3142.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu CS, Wu Y, Dou J, Wen P, Zhao FS, Tang
Q, Li JL and Wang YQ: Study of anti-melanoma effect in mice
injected with melanoma cells transfected with the recombinant
expressing Ag85A and GM-CSF. J Southeast Univ. 29:57–61. 2010.
|
|
15
|
Zhang P, Wang J, Wang D, Wang H, Shan F,
Chen L, Hou Y, Wang E and Lu CL: Dendritic cell vaccine modified by
Ag85A gene enhances anti-tumor immunity against bladder cancer. Int
Immunopharmacol. 14:252–260. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shibuya A, Campbell D, Hannum C, Yssel H,
Franz-Bacon K, McClanahan T, Kitamura T, Nicholl J, Sutherland GR,
Lanier LL, et al: DNAM-1, a novel adhesion molecule involved in the
cytolytic function of T lymphocytes. Immunity. 4:573–581. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shirakawa J, Shibuya K and Shibuya A:
Requirement of the serine at residue 329 for lipid raft recruitment
of DNAM-1 (CD226). Int Immunol. 17:217–223. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Seth S, Georgoudaki AM, Chambers BJ, Qiu
Q, Kremmer E, Maier MK, Czeloth N, Ravens I, Foerster R and
Bernhardt G: Heterogeneous expression of the adhesion receptor
CD226 on murine NK and T cells and its function in NK-mediated
killing of immature dendritic cells. J Leukoc Biol. 86:91–101.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li Y, Yang F, Zhu J, Sang L, Han X, Wang
D, Shan F, Li S, Sun X and Lu C: CD226 as a genetic adjuvant to
enhance immune efficacy induced by Ag85A DNA vaccination. Int
Immunopharmacol. 25:10–18. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tuominen VJ, Ruotoistenmäki S, Viitanen A,
Jumppanen M and Isola J: ImmunoRatio: A publicly available web
application for quantitative image analysis of estrogen receptor
(ER), progesterone receptor (PR), and Ki-67. Breast Cancer Res.
12:R562010. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Marcusson-Ståhl M and Cederbrant K: A
flow-cytometric NK-cytotoxicity assay adapted for use in rat
repeated dose toxicity studies. Toxicology. 193:269–279. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhao F, Dou J, Wang J, Chu L, Tang Q, Wang
Y, Li Y, Cao M, Hu W and Hu K: Investigation on the anti-tumor
efficacy by expression of GPI-anchored mIL-21 on the surface of
B16F10 cells in C57BL/6 mice. Immunobiology. 215:89–100. 2010.
View Article : Google Scholar
|
|
23
|
Clive KS, Tyler JA, Clifton GT, Holmes JP,
Mittendorf EA, Ponniah S and Peoples GE: Use of GM-CSF as an
adjuvant with cancer vaccines: Beneficial or detrimental? Expert
Rev Vaccines. 9:519–525. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Miguel A, Herrero MJ, Sendra L, Botella R,
Algás R, Sánchez M and Aliño SF: Comparative antitumor effect among
GM-CSF, IL-12 and GM-CSF+IL-12 genetically modified tumor cell
vaccines. Cancer Gene Ther. 20:576–581. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Olivares J, Kumar P, Yu Y, Maples PB,
Senzer N, Bedell C, Barve M, Tong A, Pappen BO, Kuhn J, et al:
Phase I trial of TGF-beta 2 antisense GM-CSF gene-modified
autologous tumor cell (TAG) vaccine. Clin Cancer Res. 17:183–192.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Agarwalla P, Barnard Z, Fecci P, Dranoff G
and Curry WT Jr: Sequential immunotherapy by vaccination with
GM-CSF-expressing glioma cells and CTLA-4 blockade effectively
treats established murine intracranial tumors. J Immunother.
35:385–389. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Seth S, Qiu Q, Danisch S, Maier MK, Braun
A, Ravens I, Czeloth N, Hyde R, Dittrich-Breiholz O, Förster R, et
al: Intranodal interaction with dendritic cells dynamically
regulates surface expression of the co-stimulatory receptor CD226
protein on murine T cells. J Biol Chem. 286:39153–39163. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Burgdorf SK, Claesson MH, Nielsen HJ and
Rosenberg J: Changes in cytokine and biomarker blood levels in
patients with colorectal cancer during dendritic cell-based
vaccination. Acta Oncol. 48:1157–1164. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nishimura T, Nakui M, Sato M, Iwakabe K,
Kitamura H, Sekimoto M, Ohta A, Koda T and Nishimura S: The
critical role of Th1-dominant immunity in tumor immunology. Cancer
Chemother Pharmacol. 46(Suppl): S52–S61. 2000. View Article : Google Scholar : PubMed/NCBI
|