Introduction
Pancreatic ductal adenocarcinoma (PDAC) is an
aggressive disease that frequently presents in an advanced stage
with distant metastases due to a late diagnosis. Furthermore, few
advances have been achieved in terms of therapeutic strategies for
PDAC, thus patient survival remains at very low levels (4% at 5
years after diagnosis) (1). For
these reasons, it is essential to make progress in understanding
the biological behavior of the disease, in parallel with making
headway in its diagnosis and therapeutic strategies.
G-protein-coupled receptors (GPCRs) comprise an
extensive family of cell-surface receptors involved in several
cellular actions, and are the targets of approximately half of the
medications available nowadays (2,3).
Glucagon-like peptide 1 (GLP-1) receptor (GLP-1R), a member of the
GPCR family, is involved in the so-called GLP-1-based therapies for
the treatment of type 2 diabetes mellitus. It has been reported
that treatment with the GLP-1R agonist exendin-4 did not affect the
proliferation or survival of human pancreatic cancer cell lines
(4), while other authors reported
that treatment with the GLP-1R agonist liraglutide had an
antitumorigenic effect on human pancreatic cancer cells in
vitro and in vivo (5,6).
Regardless of these studies, continuing controversies have arisen
that associate GLP-1-based therapies with high risks for the
development of pancreatitis and pancreatic malignancies (7–14).
GLP-1R is a regulatory peptide receptor, and
regulatory peptide receptors can be expressed in diverse human
cancers (15), and thus are
considered useful for diagnostic imaging (due to the high receptor
density in tumor tissues) and for radiation therapy when possible;
both notable and promising clinical strategies.
We recently discussed the possible relationship
between GLP-1R and the malignant behavior of pancreatic
neuroendocrine tumors (PNETs), and the use of GLP-1R for the
diagnosis of these tumors, as well as in therapeutic strategies for
hormonal syndrome and in advanced cases with metastases (16). Despite studies concerning the
effects of GLP-1 agonists on pancreatic cancer cells (4–6), to
our knowledge, there is no information regarding the activity of
GLP-1R itself in pancreatic cancer cells. The aims of the present
study were to assess GLP-1R expression in primary and metastatic
sites of PDAC by immunohistochemical analysis and to examine
whether GLP-1R knockdown affected pancreatic cancer cell
function.
Materials and methods
Pancreatic tissues and
immunohistochemical staining
The study was approved by the Ethics Committee of
Kyushu University (Fukuoka, Japan) and was conducted according to
the Ethical Guidelines for Human Genome/Gene Research enacted by
the Japanese Government and the Helsinki Declaration. Tissue
samples of pancreatic cancer were obtained from 48 patients who
underwent surgery for PDAC at Kyushu University Hospital between
2000 and 2009. Paraffin-embedded sections from the tissue samples
were deparaffinized in xylene and rehydrated in a graded ethanol
series. After endogenous peroxidase activity was blocked by
incubation with 3% hydrogen peroxidase in methanol for 30 min,
antigen retrieval was achieved by microwaving the sections in
citrate buffer for 20 min, and the samples were then incubated
overnight at 4°C with a mouse monoclonal anti-human GLP-1R antibody
(clone 419208; dilution, 1:100; R&D Systems, Minneapolis, MN,
USA). For immunohistochemical labeling, we employed a
peroxidase-labeled polymer (EnVision™+ System-HRP Labeled Polymer
Anti-Mouse; Dako, Carpinteria, CA, USA) and used
3,3′-diaminobenzidine tetrahydrochloride (Sigma-Aldrich, St. Louis,
MO, USA) as a chromogen. Finally, the sections were counterstained
with hematoxylin. Negative control staining without the primary
antibody was performed to confirm the antibody specificity.
Evaluation of GLP-1R expression
There is general agreement that islets of Langerhans
have a high density of GLP-1R (5,13,17–19).
For this reason, and similar to a previous study (13), we used GLP-1R expression in these
islets as an internal positive control for the immunohistochemical
analysis. GLP-1R expression was evaluated as previously described
(16,20). Briefly, samples were analyzed using
an intensity score (IS) that compared the levels of staining in
tumor cells and islets of Langerhans (Fig. 1A and E) and graded them as follows:
0, no staining (Fig. 1A); 1, weak
staining (Fig. 1B); 2, moderate
staining (Fig. 1C); and 3, strong
staining (Fig. 1D). A proportional
score (PS) was also used to grade the samples as follows: 0,
staining in <10% of the area; 1, staining in ≥10% but <30% of
the area; 2, staining in ≥30% but <50% of the area; 3, staining
in ≥50% but <80% of the area; and 4, staining in ≥80% of the
area. A final score (FS) rated the samples for GLP-1R expression as
follows: GLP-1R-negative (FS 1), IS × PS = 0–3; and GLP-1R-positive
(FS 2), IS × PS = 4–12. All samples were independently evaluated by
two investigators (A.I.C. and K.S.) without knowledge of the
records of each case.
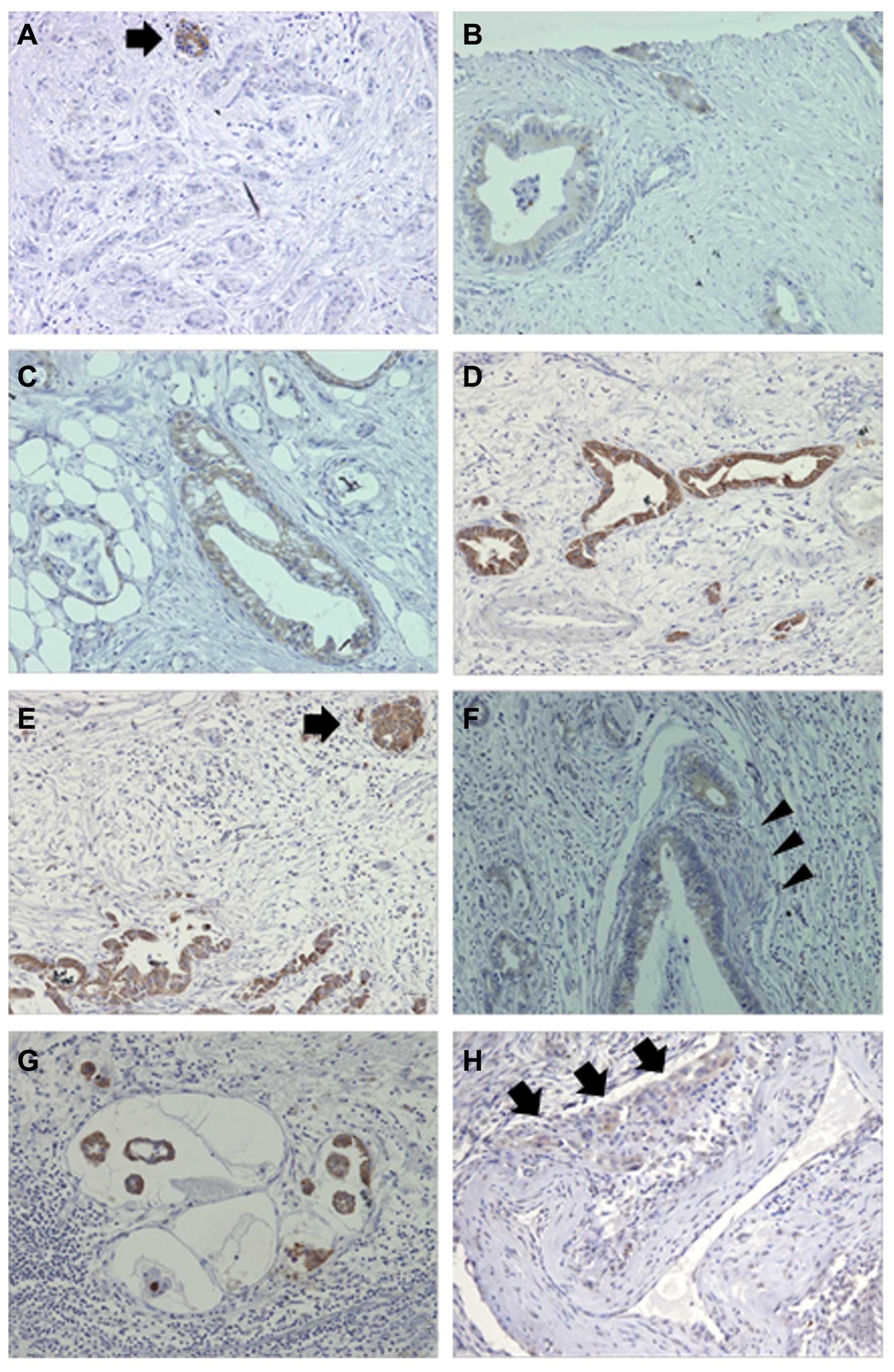 | Figure 1Immunohistochemical assessment of
GLP-1R expression in pancreatic ductal adenocarcinoma. (A and E)
Islets of Langerhans (arrow) were used as an internal positive
control adjacent to tumor cells with (A) negative and (E) strong
staining of GLP-1R. (A–D) Representative micrographs of
immunohistochemical staining in PDAC specimens showing (A)
negative, (B) weak, (C) moderate and (D) strong GLP-1R staining.
(F–H) GLP-1R-positive tumor cells are also found in sites of
perineural invasion (F, arrowheads indicate a nerve), lymphatic
vessel invasion (G) and vascular invasion (H, arrows indicate
arterial wall invasion). Original magnifications, ×100 in D and H;
×200 in A, B, C, E, F and G. GLP-1R, glucagon-like peptide 1
(GLP-1) receptor; PDAC, pancreatic ductal adenocarcinoma. |
Cell lines and culture conditions
The following 15 pancreatic cancer cell lines were
used: AsPC-1, KP-2, KP-3, H48N and Panc-1 (generously provided by
Dr H. Iguchi, National Shikoku Cancer Center, Matsuyama, Japan);
HPC-3 (generously provided by Dr K. Yasoshima, Sapporo Medical
University, Hokkaido, Japan); SUIT-2 and MIA PaCa-2 (Japanese
Cancer Resource Bank, Tokyo, Japan); BxPC-3, Capan-1, Capan-2,
CFPAC-1, Hs766T and SW 1990 (American Type Culture Collection
(ATCC; Manassas, VA, USA); and NOR-P1 (21). In addition, we used a primary
culture of normal human pancreatic epithelial cells (CS-PE cells;
Cell System-PE cells; Cell Systems; Applied Cell Biology Research
Institute, Kirkland, WA, USA) and a human immortalized pancreatic
ductal epithelial cell line (HPDE6-E6E7 clone 6; kindly provided by
Dr Ming-Sound Tsao, University of Toronto, Toronto, Canada)
(22). Most cells were cultured in
Dulbecco's modified Eagle's medium (DMEM; Sigma-Aldrich)
supplemented with streptomycin (0.1 g/l), penicillin (0.15 g/l),
NaHCO3 (3.7 g/l) and 10% fetal bovine serum (FBS;
Gibco®, Life Technologies, Grand Island, NY, USA). For
Capan-1 cells, Iscove's modified Dulbecco's medium (IMDM;
Gibco®) supplemented with 20% FBS was used. CS-PE cells
were cultured in CSC Complete Recombinant Medium (Cell Systems
Corporation, Kirkland, WA, USA), and HPDE cells were cultured in
HuMedia-KG2 medium (Kurabo, Osaka, Japan). All the cells were kept
in a humidified atmosphere of 10% CO2/90% air at
37°C.
Quantitative real-time reverse
transcription-polymerase chain reaction
Total RNA was extracted from cultured cells using a
High Pure RNA Isolation kit and DNase I (both from Roche
Diagnostics, Mannheim, Germany) according to the manufacturer's
instructions. RNA was quantified by its absorbance at 260 nm and
evaluated for its purity by the 260/280 absorbance ratio using a
NanoDrop 1000 Spectrophotometer (Thermo Scientific, Waltham, MA,
USA). Quantitative real-time reverse transcription-polymerase chain
reaction (qRT-PCR) was performed using a QuantiTect SYBR-Green
RT-PCR kit (Qiagen, Venlo, The Netherlands) and a CFX96 Touch™
Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, CA,
USA). Briefly, each reaction mixture was initially incubated for 30
min at 50°C to allow RT. The PCR initial activation step was
performed at 95°C for 15 min to activate the polymerase, followed
by 40 three-step cycles of 15 min at 94°C, 30 min at 55°C and 30
min at 72°C. The primers (Sigma-Aldrich) used were: GLP-1R forward,
5′-tctgcatcgtggtatccaaa-3′ and GLP-1R reverse,
5′-cttggcaagtctgcatttga-3′; 18S forward, 5′-gtaacccgttgaaccccatt-3′
and 18S reverse, 5′-ccatccaatcggtagtagccg-3′. Data were calculated
as the relative GLP-1R expression levels normalized by the 18S rRNA
levels. All samples were run in duplicate or triplicate at least
three times.
Western blot analysis
Cells were lysed in PRO-PREP™ (iNtRON Biotechnology,
Seongnam, Korea). Cell lysate proteins (5–15 µg) were
fractionated in 4–15% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis gels (Bio-Rad Laboratories) and transferred to
polyvinylidene difluoride membranes (Trans-Blot® Turbo™;
Bio-Rad Laboratories). The membranes were incubated overnight at
4°C with anti-GLP-1R (ab39072; dilution, 1 µg/ml; Abcam,
Cambridge, UK) or anti-β-actin (sc-81178; dilution, 1:2,500)
antibodies, and then probed with secondary antibodies conjugated
with horseradish peroxidase (both from Santa Cruz Biotechnology,
Santa Cruz, CA, USA). Immunoreactive bands were analyzed using an
ECL detection system (GE Healthcare, Buckinghamshire, UK) and
chemiluminescence was detected using a ChemiDoc™ XRS (Bio-Rad
Laboratories).
GLP-1R silencing by small interfering
RNAs
For receptor silencing, we used the following small
interfering RNAs (siRNAs; all purchased from Qiagen): GLP-1R 4
sense, 5′-ggaacuccaacaugaacuatt-3′ and GLP-1R 4 antisense,
5′-uaguucauguuggaguucctg-3′; GLP-1R 5 sense,
5′-ggcucguucgugaaugucatt-3′ and GLP-1R 5 antisense,
5′-ugacauucacgaacgagcctg-3′. A negative control siRNA (siControl;
Qiagen) was used to verify the knockdown specificity. Transfections
of CFPAC-1 cells (up to passage 8) were performed by
electroporation using a Nucleofector™ device (Lonza, Cologne,
Germany) in triplicate. Cells (1.7–2×106) were
centrifuged at 2,000 × g for 1 min, and the supernatant was
discarded. Next, the cells were suspended in 95 µl of Cell
Line Nucleofector™ Solution V (Lonza), and 5 µl of 20
µM GLP-1R siRNA (siGLP-1R 4 or siGLP-1R 5) or siControl was
added. The transfected cells were resuspended in DMEM 10% FBS and
cultured for 48 h before subsequent experiments. Silencing by siRNA
was confirmed by qRT-PCR and western blot analysis.
Cell proliferation assay
Cell proliferation was evaluated by measuring the
fluorescence intensity of propidium iodide (PI) as previously
described (23). Transfected
CFPAC-1 cells were suspended in DMEM without phenol red
supplemented with 10% FBS, and seeded at a density of
2×104 cells/well in 24-well cell culture plates (BD
Falcon, BD Biosciences, Franklin Lakes, NJ, USA). After 24 h of
culture, the medium was changed to fresh serum-free medium for 24 h
of culture under serum starvation. The following day, the medium
was replaced with DMEM without phenol red supplemented with 1% FBS,
and evaluated for cell proliferation by the PI assay. Briefly, PI
(30 µM) and digitonin (600 µM) were added to each
well and the fluorescence intensity was measured using a multimode
microplate reader (Infinite F200; Tecan, Grödig, Austria) with
535-nm excitation and 620-nm emission filters. A separate well with
the same medium but no cells was used to obtain a baseline PI
signal as a control, and the difference between each sample well
and the control well was evaluated. Cell proliferation was defined
relative to the cell number measured at the beginning of the
experiment (day 0). All experiments were performed in triplicate
wells and repeated at least three times.
Invasion and migration assays
Invasion of transfected CFPAC-1 cells was assessed
by counting the numbers of cells that invaded through
Matrigel-coated Transwell chambers with 8-µm pore size
membranes (BD Biosciences). The Transwell inserts were coated with
20 µg/well BD Matrigel™ (BD Biosciences). Each group of
transfected cells (1×105) was suspended in 250 ml of
DMEM containing 1% FBS and placed in the upper chamber. The upper
chamber was then placed in the well of a 24-well culture dish
containing 750 ml of DMEM with 10% FBS. In addition, cellular
migration was assessed using uncoated Transwell inserts. After 24 h
of incubation, the invading and migrating cells were fixed with 70%
ethanol and stained with hematoxylin and eosin (H&E). The cell
numbers were counted under a light microscope in five random fields
at a magnification of ×200 for migration, and in one random field
at a magnification of ×100 for invasion. The results were expressed
as the mean numbers of counted cells/field. Each experiment was
carried out in quadruplicate wells and repeated three times.
Statistical analysis
Statistical analyses were carried out using JMP 10
for Windows software (SAS Institute, Inc., Cary, NC, USA). Survival
was evaluated from the time of surgery, with death as the end
point. The cumulative survival rate was determined by Kaplan-Meier
analysis. In cellular experiments, data are expressed as means ±
SD. Differences between two groups were estimated by the Student's
t-test or the χ2 test. Experiments were repeated at
least three times. Differences were considered significant for
values of P<0.05.
Results
Evaluation of GLP-1R in pancreatic
cancer
Immunohistochemical evaluation of GLP-1R was
performed in 48 PDAC specimens from 48 patients, comprising 22
women and 26 men with a median age of 64 years (range, 36–82 years)
(Table I). The results revealed 23
tumors (48%) with positive-GLP-1R expression and 25 tumors (52%)
with negative-GLP-1R expression (Table
I). A noteworthy element was the presence of GLP-1R-positive
tumor cells in invasive localization areas, such as perineural
invasion, lymph vessel invasion and vascular invasion (Fig. 1F–H).
 | Table IClinicopathological characteristics of
the patients with pancreatic ductal adenocarcinoma and evaluation
of GLP-1R expression (n=48). |
Table I
Clinicopathological characteristics of
the patients with pancreatic ductal adenocarcinoma and evaluation
of GLP-1R expression (n=48).
| Characteristics | Data |
|---|
| Age, median (range),
years | 64 (36–82) |
| Gender |
| Female | 22 (46) |
| Male | 26 (54) |
| pT category |
| pT1 | 1 (2) |
| pT2 | 1 (2) |
| pT3 | 43 (90) |
| pT4 | 3 (6) |
| pN category |
| pN0 | 6 (12) |
| pN1 | 42 (88) |
| UICC stage |
| I | 1 (2) |
| II | 42 (88) |
| III | 3 (6) |
| IV | 2 (4) |
| Histological
grade |
| G1 | 8 (17) |
| G2 | 16 (33) |
| G3 | 24 (50) |
| Lymphatic
invasion |
| Negative | 8 (17) |
| Positive | 40 (83) |
| Vascular
invasion |
| Negative | 8 (17) |
| Positive | 40 (83) |
| Neural
invasion |
| Negative | 5 (10) |
| Positive | 43 (90) |
| Surgical
margin |
| Negative | 29 (60) |
| Positive | 19 (40) |
| GLP-1R
expression |
| Negative | 25 (52) |
| Positive | 23 (48) |
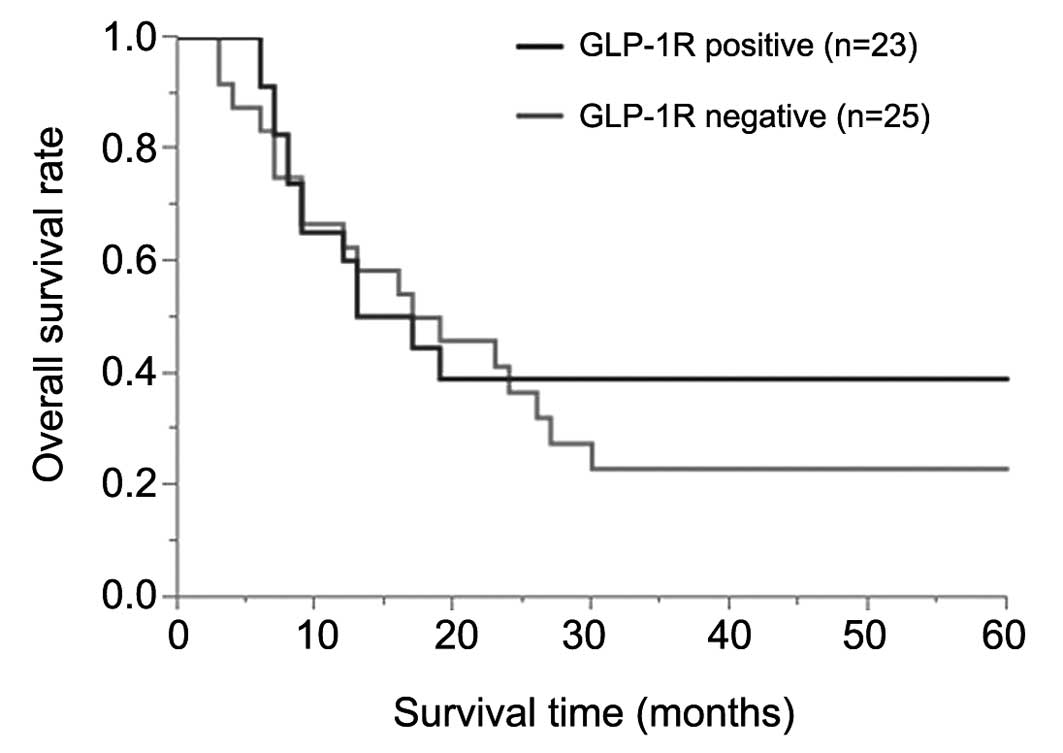
GLP-1R expression and its correlations
with clinicopathological features and the survival analysis
Table II shows the
associations between GLP-1R expression and the clinicopathological
features of the 48 patients. There were no significant associations
between the receptor expression status and the clinicopathological
features. Similarly, the overall survival rates showed no
significant difference between the positive and negative groups for
GLP-1R expression (P=0.74; Fig.
2).
 | Table IIRelationships between GLP-1R
expression and clinicopathological features (n=48). |
Table II
Relationships between GLP-1R
expression and clinicopathological features (n=48).
|
Characteristics | GLP-1R positive
(n=23) | GLP-1R negative
(n=25) | P-value |
|---|
| Age, years | | | 0.77 |
| ≥65 | 11 (46) | 13 (54) | |
| <65 | 12 (50) | 12 (50) | |
| Gender | | | 0.15 |
| Female | 13 (59) | 9 (41) | |
| Male | 10 (38) | 16 (62) | |
| pT category | | | 0.95 |
| pT1/pT2 | 1 (50) | 1 (50) | |
| pT3/pT4 | 22 (48) | 24 (52) | |
| pN category | | | 0.44 |
| pN0 | 2 (33) | 4 (67) | |
| pN1 | 21 (50) | 21 (50) | |
| UICC stage | | | 0.45 |
| I | 1 | 0 | |
| II | 20 (48) | 22 (52) | |
| III/IV | 2 (40) | 3 (60) | |
| Histological
grade | | | 0.77 |
| G1/G2 | 12 (50) | 12 (50) | |
| G3 | 11 (46) | 13 (54) | |
| Lymphatic
invasion | | | 0.36 |
| Negative | 5 (63) | 3 (37) | |
| Positive | 18 (45) | 22 (55) | |
| Vascular
invasion | | | 0.52 |
| Negative | 3 (37) | 5 (63) | |
| Positive | 20 (50) | 20 (50) | |
| Neural
invasion | | | 0.71 |
| Negative | 2 (40) | 3 (60) | |
| Positive | 21 (49) | 22 (51) | |
| Surgical
margin | | | 0.51 |
| Negative | 15 (52) | 14 (48) | |
| Positive | 8 (42) | 11 (58) | |
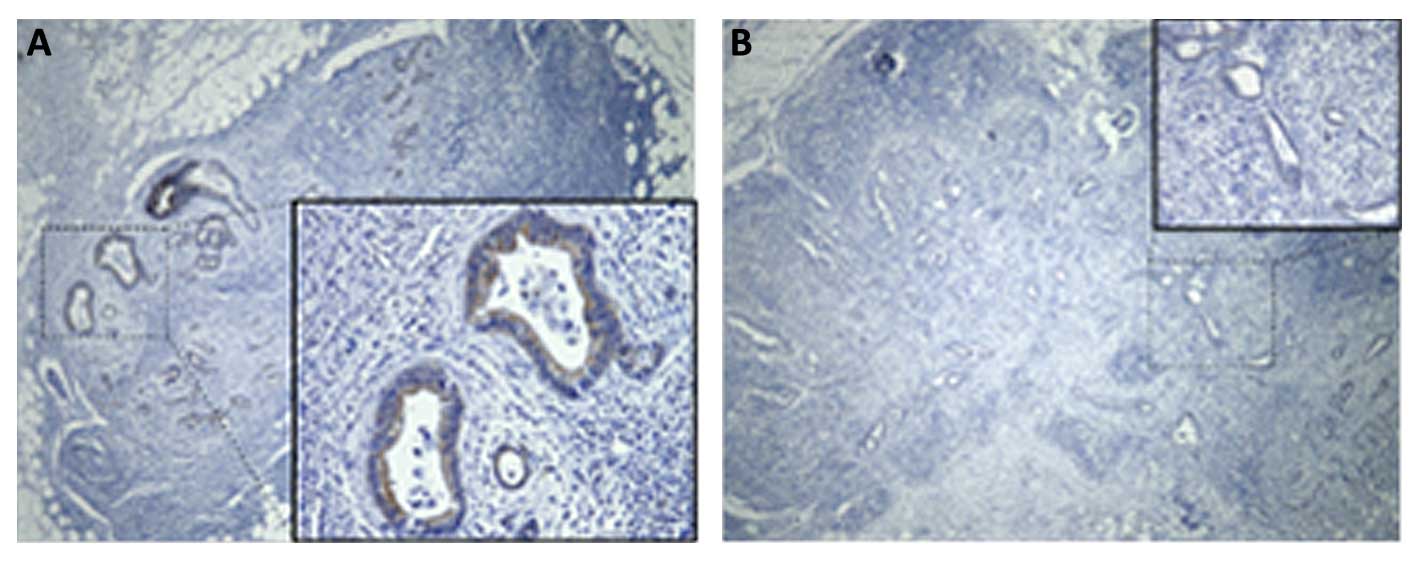
GLP-1R analysis in metastatic sites
From the total of 42 patients with lymph node
metastasis (Table I), 15 metastatic
lymph node samples were randomly selected and immunohistochemically
evaluated for the expression of GLP-1R. Most of the samples (11 of
15; 73%) showed positive staining for GLP-1R (Table III, Fig. 3A), while the remaining four samples
(27%) were classified as GLP-1R-negative (Table III, Fig. 3B). Although there was a correlation
between GLP-1R expression in the primary site and the metastatic
site in 13 samples (i.e. positive-positive or negative-negative),
two samples with negative expression in the primary sites showed
positive-GLP-1R expression in the metastatic sites.
 | Table IIIImmunohistochemical evaluation of
GLP-1R in metastatic sites: analysis of 15 metastatic lymph node
samples. |
Table III
Immunohistochemical evaluation of
GLP-1R in metastatic sites: analysis of 15 metastatic lymph node
samples.
| Patient no. | Age (years) | Gender | Degree of
differentiation | GLP-1R expression
in primary site | GLP-1R expression
in lymph node sample |
|---|
| 1 | 61 | F | G3 | Positive | Positive |
| 2 | 79 | F | G3 | Positive | Positive |
| 3 | 66 | M | G3 | Positive | Positive |
| 4 | 63 | F | G1 | Negative | Positive |
| 5 | 55 | M | G3 | Positive | Positive |
| 6 | 67 | F | G3 | Positive | Positive |
| 7 | 61 | M | G3 | Negative | Negative |
| 8 | 65 | M | G3 | Negative | Positive |
| 9 | 59 | M | G3 | Positive | Positive |
| 10 | 73 | M | G2 | Positive | Positive |
| 11 | 60 | F | G3 | Positive | Positive |
| 12 | 50 | F | G2 | Negative | Negative |
| 13 | 73 | M | G2 | Negative | Negative |
| 14 | 55 | F | G3 | Positive | Positive |
| 15 | 60 | M | G3 | Negative | Negative |
GLP-1R knockdown has a positive impact on
the malignant behavior of CFPAC-1 pancreatic cancer cells
First, we investigated the presence of GLP-1R
expression in several pancreatic cancer cell lines as well as in
normal pancreatic cells by qRT-PCR and western blot analysis
(Fig. 4A). Next, for knockdown
experiments, the cell line CFPAC-1 was selected due to its high
receptor expression at both the mRNA and protein levels. Knockdown
efficacy was verified by qRT-PCR and western blot analysis at 48 h
post-transfection (Fig. 4B). We
found that GLP-1R knockdown significantly decreased the
proliferation of CFPAC-1 cells after 4 days of culture when using
siGLP-1R 4, and after 5 days of culture when using both siGLP-1R 4
and siGLP-1R 5, as compared with siControl (Fig. 4C). Similarly, the receptor knockdown
significantly decreased the cell migration and invasion compared
with siControl (Fig. 4D).
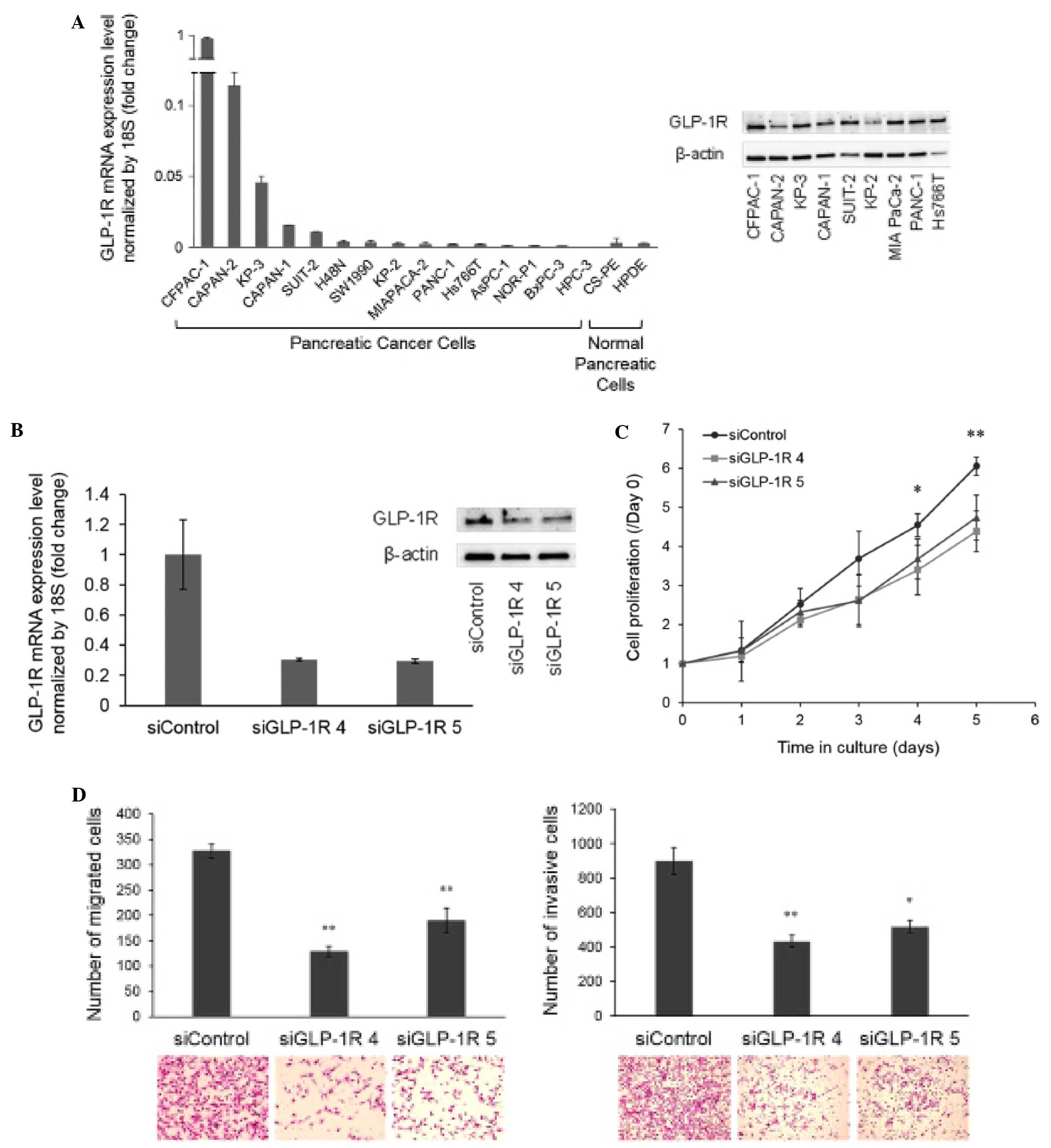 | Figure 4Presence of GLP-1R in cell lines and
effects of its knockdown. (A) qRT-PCR was carried out to examine
the presence of GLP-1R in several pancreatic cancer cell lines and
normal pancreatic cells. (B) For additional western blot analysis,
we selected cells with high expression at the mRNA level (CFPAC-1,
CAPAN-2 and KP-3), and some cells with low expression (CAPAN-1,
SUIT-2, KP-2, MIA PaCa-2, PANC-1 and Hs766T). We then chose the
CFPAC-1 cell line for further experiments due to its high
expression of GLP-1R at both the mRNA and protein levels. GLP-1R
knockdown was achieved in CFPAC-1 cells using two different siRNAs,
siGLP-1R 4 and siGLP-1R 5. Both siGLP-1R 4 and siGLP-1R 5
effectively reduced the receptor expression at the mRNA and protein
levels in CFPAC-1 cells, as compared with siControl. (C) The cells
were seeded at 48 h post-transfection, and cell proliferation was
measured at the indicated time points using a propidium iodide
assay. GLP-1R knockdown significantly decreased the cell
proliferation. *P<0.05, siGLP-1R 4 vs. siControl;
**P<0.05, siGLP-1R 4 and siGLP-1R 5 vs. siControl.
(D) Knockdown of GLP-1R expression significantly reduced the
migration and invasion abilities of CFPAC-1 cells.
*P=0.0001; **P<0.0001. Representative
photomicrographs of migrated cells (H&E, original
magnification, ×200) and invasive cells (H&E, original
magnification, ×100) are shown. GLP-1R, glucagon-like peptide 1
(GLP-1) receptor; H&E, hematoxylin and eosin. |
Discussion
We analyzed the expression of GLP-1R in 48
surgically resected PDAC specimens, and examined its relationships
with several clinicopathological factors. The immunohistochemical
analysis procedure was the same as that used in our previous study
(16), in which we examined GLP-1R
expression in PNETs and found no significant associations between
the receptor expression status and several clinicopathological
characteristics. Similar to our previous study, GLP-1R expression
was not found to be a predictive factor for survival in PDAC
patients. The receptor expression showed dissimilar levels (at both
the mRNA and protein levels) even among pancreatic cancer cells,
indicating that the receptor expression was markedly variable from
one cell to another (heterogeneity), and that even if there was a
relationship between GLP-1R and tumor behavior, this was lost when
the samples were evaluated together.
We evaluated samples of metastatic lymph nodes, and
found that most of these samples were GLP-1R-positive (11 of 15
samples; 73%). It was interesting that 13 samples showed a direct
correlation for GLP-1R expression between the primary site and its
metastatic counterpart, while the remaining two samples with
negative GLP-1R expression in the primary site showed positive
GLP-1R expression in the metastatic site, suggesting that cells
expressing GLP-1R may have an increased metastatic ability. In
other words, even though the expression status in the primary site
was judged to be negative, there could be a minor compartment of
GLP-1R-positive cells arising through tumor heterogeneity, and
these cells may be able to metastasize to lymph nodes. To our
knowledge, this is the first study to reveal the expression of
GLP-1R in metastatic samples of PDAC, a finding to consider given
the negative impact that metastases have on patient survival.
Additional investigations of GLP-1R expression in other metastatic
sites, such as the liver and lung are warranted.
Although a previous study described an
immunohistochemical analysis of GLP-1R in PDAC and its
relationships with several clinicopathological factors (5), the present study involved a larger
number of patients for immunohistochemical evaluation of the
receptor, and included samples from metastatic sites. In the
previous study, Zhao et al (5) found that negative GLP-1R expression in
PDAC samples was associated with a larger tumor diameter (this
information was additionally presented by the same authors in a
second study) (6), conjointly with
lymphatic metastasis and a poorer overall survival compared with
patients with GLP-1R-positive tumors. We think that the differences
compared with our findings exceed the mere facts of using
antibodies from different commercial brands or different sample
numbers and could possibly arise through methodological differences
in the immunohistochemical evaluations.
As we observed GLP-1R-positive tumor cells in
invasive areas as well as in metastatic lymph nodes, we questioned
whether the receptor influences the metastatic ability of
pancreatic cancer cells. To address this question, we performed
siRNA experiments to achieve receptor knockdown, and found that
GLP-1R knockdown decreased the proliferation of CFPAC-1 cells, and
also induced noticeable decreases in the cell migration and
invasion abilities. To the best of our knowledge, this is the first
study to disclose that GLP-1R silencing decreases the malignant
behavior of PDAC cells, and these results may support our
immunohistochemical findings, wherein metastatic lymph nodes were
frequently GLP-1R-positive. Taken together, the data obtained in
the present study suggest that GLP-1R plays a role in the
metastatic potential of PDAC cells.
In the absence of any treatment, we hypothesized
that GLP-1R may be functioning without a ligand to mediate its
activation; for example, through autophosphorylation with the
consequent triggering of cellular signals. Alterations in GPCRs
have been described and linked to carcinogenesis (3,24), and
thus GLP-1R may not be an exception. Notably, at the beginning of
the experiments using CFPAC-1 cells with GLP-1R knockdown, a dose
of 10% FBS in the medium was used, yet the results were not
significant. Subsequently, we decided to reduce the FBS
concentration to 1%, resulting in significant differences under
this condition. In view of this situation, and since the sequences
of the GLP-1 peptide are quite similar among mammals (25), our hypothesis was that traces of
bovine GLP-1 in the FBS could be stimulating some GLP-1Rs still
present in the cell membrane, thereby interfering with the results.
Using western blot analysis and ELISA, we searched for the presence
of bovine GLP-1 in the FBS, but the results were negative (data not
shown). Nevertheless, we cannot totally confirm the absence of
GLP-1 in the FBS, or the possible presence of another metabolite
that functions as a receptor agonist. In our previous study
(16), we reported that GLP-1
treatment increased the proliferation as well as the migration of
the insulinoma cell line MIN6, and numerous studies have associated
GLP-1-based therapies with pancreatic disease in recent years
(7–14,17).
Moreover, using mutant GLP-1Rs, Ge et al (26) described that even with a mutation of
the putative signal sequence, ligand binding to the mutant receptor
was observed with conserved cAMP production. In summary, although
an aberrant GLP-1R presented an agonist-independent functionality
as the siRNA data suggest, an agonist-dependent functionality
cannot be discarded. One limitation of the present study is that
the downstream signals related to GLP-1R knockdown were not
investigated. However, the in vitro data firmly demonstrated
that GLP-1R silencing decreased the malignant ability of PDAC
cells. Further experiments will be necessary to clarify the cell
signals involved in this hypothetical ligand-independent receptor
process.
In our previous study (16), we discussed the potential use of
GLP-1R for the diagnosis and treatment of metastatic PNETs. In a
similar way, we can also think of GLP-1R as a molecular target not
only for the treatment of PDAC, but also as a diagnostic molecular
marker for metastatic PDAC, e.g., its utilization in scintigraphy
techniques. However, given the results described in the present
study, wherein GLP-1R promoted the metastatic potential of PDAC
cells, it is useful to mention that GLP-1R showed an excellent
response to radiolabeled antagonist binding (19). The latter observation posits that
the hypothetical use of a radiolabeled antagonist for the diagnosis
of advanced PDAC would not involve undesired side-effects, unlike
the case for many agonists. Although GLP-1R showed minimal
antagonist-induced internalization compared with agonists (27), receptor internalization is
considered to be a very attractive feature in terms of retention of
radioactivity for radionuclide therapy application (28).
In conclusion, although GLP-1R expression was not
found to be an independent prognostic factor in PDAC patients by
immunohistochemical analysis, it appears to have some implications
for PDAC metastatic ability. It is necessary to monitor the
long-term safety of GLP-1 mimetic therapies, as well as to examine
GLP-1R as a possible molecular target for the diagnosis and
treatment of advanced PDACs.
Acknowledgments
The authors thank Ms. Emiko Manabe and Ms. Miyuki
Omori (Pancreatic Cancer Laboratory, Department of Surgery and
Oncology, Kyushu University) for their technical assistance. The
present study was supported by JSPS KAKENHI grant nos. 24390318,
24390319, 25293285, 25670582 and 26293305. A.I.C. is supported by a
doctoral fellowship provided by the Ministry of Education, Culture,
Sports, Science and Technology of Japan. B.Z. is supported by a
doctoral fellowship provided by the China Scholarship Council. K.S.
is supported by a postdoctoral fellowship provided by the Japan
Society for the Promotion of Science.
References
|
1
|
Vincent A, Herman J, Schulick R, Hruban RH
and Goggins M: Pancreatic cancer. Lancet. 378:607–620. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pierce KL, Premont RT and Lefkowitz RJ:
Seven-transmembrane receptors. Nat Rev Mol Cell Biol. 3:639–650.
2002. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dorsam RT and Gutkind JS:
G-protein-coupled receptors and cancer. Nat Rev Cancer. 7:79–94.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Koehler JA and Drucker DJ: Activation of
glucagon-like peptide-1 receptor signaling does not modify the
growth or apoptosis of human pancreatic cancer cells. Diabetes.
55:1369–1379. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhao H, Wang L, Wei R, Xiu D, Tao M, Ke J,
Liu Y, Yang J and Hong T: Activation of glucagon-like peptide-1
receptor inhibits tumourigenicity and metastasis of human
pancreatic cancer cells via PI3K/Akt pathway. Diabetes Obes Metab.
16:850–860. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao H, Wei R, Wang L, Tian Q, Tao M, Ke
J, Liu Y, Hou W, Zhang L, Yang J, et al: Activation of
glucagon-like peptide-1 receptor inhibits growth and promotes
apoptosis of human pancreatic cancer cells in a cAMP-dependent
manner. Am J Physiol Endocrinol Metab. 306:E1431–E1441. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ayoub WA, Kumar AA, Naguib HS and Taylor
HC: Exenatide-induced acute pancreatitis. Endocr Pract. 16:80–83.
2010. View Article : Google Scholar
|
|
8
|
Lee PH, Stockton MD and Franks AS: Acute
pancreatitis associated with liraglutide. Ann Pharmacother.
45:e222011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Iyer SN, Drake AJ III, West RL, Mendez CE
and Tanenberg RJ: Case report of acute necrotizing pancreatitis
associated with combination treatment of sitagliptin and exenatide.
Endocr Pract. 18:e10–e13. 2012. View Article : Google Scholar
|
|
10
|
Elashoff M, Matveyenko AV, Gier B,
Elashoff R and Butler PC: Pancreatitis, pancreatic, and thyroid
cancer with glucagon-like peptide-1-based therapies.
Gastroenterology. 141:150–156. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Singh S, Chang HY, Richards TM, Weiner JP,
Clark JM and Segal JB: Glucagonlike peptide 1-based therapies and
risk of hospitalization for acute pancreatitis in type 2 diabetes
mellitus: A population-based matched case-control study. JAMA
Intern Med. 173:534–539. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Matveyenko AV, Dry S, Cox HI, Moshtaghian
A, Gurlo T, Galasso R, Butler AE and Butler PC: Beneficial
endocrine but adverse exocrine effects of sitagliptin in the human
islet amyloid polypeptide transgenic rat model of type 2 diabetes:
Interactions with metformin. Diabetes. 58:1604–1615. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gier B, Matveyenko AV, Kirakossian D,
Dawson D, Dry SM and Butler PC: Chronic GLP-1 receptor activation
by exendin-4 induces expansion of pancreatic duct glands in rats
and accelerates formation of dysplastic lesions and chronic
pancreatitis in the KrasG12D mouse model. Diabetes.
61:1250–1262. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Butler AE, Campbell-Thompson M, Gurlo T,
Dawson DW, Atkinson M and Butler PC: Marked expansion of exocrine
and endocrine pancreas with incretin therapy in humans with
increased exocrine pancreas dysplasia and the potential for
glucagon-producing neuroendocrine tumors. Diabetes. 62:2595–2604.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Reubi JC: Peptide receptors as molecular
targets for cancer diagnosis and therapy. Endocr Rev. 24:389–427.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cases AI, Ohtsuka T, Fujino M, Ideno N,
Kozono S, Zhao M, Ohuchida K, Aishima S, Nomura M, Oda Y, et al:
Expression of glucagon-like peptide 1 receptor and its effects on
biologic behavior in pancreatic neuroendocrine tumors. Pancreas.
43:1–6. 2014. View Article : Google Scholar
|
|
17
|
Nakamura T, Ito T, Uchida M, Hijioka M,
Igarashi H, Oono T, Kato M, Nakamura K, Suzuki K, Jensen RT, et al:
PSCs and GLP-1R: Occurrence in normal pancreas, acute/chronic
pancreatitis and effect of their activation by a GLP-1R agonist.
Lab Invest. 94:63–78. 2014. View Article : Google Scholar
|
|
18
|
Hörsch D, Göke R, Eissele R, Michel B and
Göke B: Reciprocal cellular distribution of glucagon-like peptide-1
(GLP-1) immunoreactivity and GLP-1 receptor mRNA in pancreatic
islets of rat. Pancreas. 14:290–294. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Waser B and Reubi JC: Radiolabelled GLP-1
receptor antagonist binds to GLP-1 receptor-expressing human
tissues. Eur J Nucl Med Mol Imaging. 41:1166–1171. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Furukawa T, Kuboki Y, Tanji E, Yoshida S,
Hatori T, Yamamoto M, Shibata N, Shimizu K, Kamatani N and
Shiratori K: Whole-exome sequencing uncovers frequent GNAS
mutations in intraductal papillary mucinous neoplasms of the
pancreas. Sci Rep. 1:1612011. View Article : Google Scholar :
|
|
21
|
Sato N, Mizumoto K, Beppu K, Maehara N,
Kusumoto M, Nabae T, Morisaki T, Katano M and Tanaka M:
Establishment of a new human pancreatic cancer cell line, NOR-P1,
with high angiogenic activity and metastatic potential. Cancer
Lett. 155:153–161. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Furukawa T, Duguid WP, Rosenberg L,
Viallet J, Galloway DA and Tsao MS: Long-term culture and
immortalization of epithelial cells from normal adult human
pancreatic ducts transfected by the E6E7 gene of human papilloma
virus 16. Am J Pathol. 148:1763–1770. 1996.PubMed/NCBI
|
|
23
|
Zhang L, Mizumoto K, Sato N, Ogawa T,
Kusumoto M, Niiyama H and Tanaka M: Quantitative determination of
apoptotic death in cultured human pancreatic cancer cells by
propidium iodide and digitonin. Cancer Lett. 142:129–137. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Allen LF, Lefkowitz RJ, Caron MG and
Cotecchia S: G-protein-coupled receptor genes as protooncogenes:
Constitutively activating mutation of the alpha 1B-adrenergic
receptor enhances mitogenesis and tumorigenicity. Proc Natl Acad
Sci USA. 88:11354–11358. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Knudsen LB, Hastrup S, Underwood CR, Wulff
BS and Fleckner J: Functional importance of GLP-1 receptor species
and expression levels in cell lines. Regul Pept. 175:21–29. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ge Y, Yang D, Dai A, Zhou C, Zhu Y and
Wang MW: The putative signal peptide of glucagon-like peptide-1
receptor is not required for receptor synthesis but promotes
receptor expression. Biosci Rep. 34:e001522014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Brom M, Joosten L, Oyen WJ, Gotthardt M
and Boerman OC: Radiolabelled GLP-1 analogues for in vivo targeting
of insulinomas. Contrast Media Mol Imaging. 7:160–166. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bodei L, Paganelli G and Mariani G:
Receptor radionuclide therapy of tumors: A road from basic research
to clinical applications. J Nucl Med. 47:375–377. 2006.PubMed/NCBI
|