Introduction
Gastric cancer is the fourth most common cancer and
the second leading cause of death from malignant disease worldwide,
with especially high mortality rates in East, South and Central
Asia; in Central and Eastern Europe; and in South America (1). In D1 dissection, the stomach (total or
distal) plus the perigastric lymph nodes are removed; and in D2
dissection, additional removal of the nodes along the left gastric
area, common hepatic splenic zone and left hepatoduodenal artery is
performed (2). The 3rd edition of
'The Gastric Cancer Treatment Guidelines' in Japan, defined D2
radical surgery as the standard operation. In recent years, D2
radical surgery has also been gradually accepted by European and
American doctors. The United States National Comprehensive Cancer
Network and the European Society for Medical Oncology have
recommended D2 radical surgery to improve the 5-year survival rate
of gastric cancer patients (3,4).
However, follow-up study conducted over 15 years suggested that D2
operation is associated with lower loco-regional recurrence and
gastric-cancer-related death rates, yet had significantly higher
postoperative mortality, morbidity and reoperation rates than D1
surgery (5). Obviously, a
standardized operation may lead to an expansion in the scope of
relative lymphadenectomy in some patients and increase unnecessary
damage and risk. Therefore, the accurate resection of tumors, even
accurate lymphadenectomy, is urgent and necessary.
Integrin is a major family of cell surface
receptors. It serves as the bridge for cell-cell and
cell-extracellular matrix (ECM). Integrin
α5β1, is one of the important members of the
integrin family and is a major receptor of fibronectin (FN).
Previous research has shown that integrin
α5β1 identifies and combines with the
Arg-Gly-Asp (RGD) sequence, an RGD sequence that is located in
III10 of FN and is the site of cell attachment via
α5β1 integrin on the cell surface (6–9).
Integrin α5β1 plays an important role in
tumor growth, invasion and metastasis (10–12).
In recent years, research suggests that expression of integrin is
associated with the differentiation and metastasis of gastric
cancer (13), and integrin
α5β1 may be an independent prognostic factor
for gastric cancer patients (14).
Therefore, artificial synthetic RGD, or an agent containing the RGD
sequence has been used as a ligand to target gastric cancer
(15,16).
Indocyanine green (ICG) was approved by the FDA for
use as a clinical near-infrared imaging agent, which has high
safety, less adverse reactions, and a high signal-to-noise ratio in
living tissue. It has been used for gastric cancer sentinel lymph
node (SLN) imaging (17,18). However, the intravenous
administration of ICG has a short half-life; for this reason, its
intravenous ICG study as a gastric cancer tracer has not been
carried out. Thus, it is necessary to search for new strategies for
prolong the half-live of ICG in vivo.
Liposomes were first described by the British
hematologist A.D. Bangham (19).
Liposomes are artificially prepared spherical vesicles composed of
a lamellar phase lipid bilayer. Liposomes have various advantages,
such as biocompatibility and no obvious toxicity or immunogenicity.
Liposomes have been used as an antitumor drug delivery carrier for
the treatment of tumors, as previously reported (20,21).
However, liposomes are easily utilized by the reticuloendothelial
system (RES) and gather in organs, such as the liver and spleen. In
order to avoid or reduce the uptake of RES, researchers have
prepared liposomes that are modified with polyethylene glycol
(PEG), which effectively prolongs their circulation time. In recent
years, researchers have used PEG-modified liposomes in combination
with targeted ligands (RGD, trastuzumab) to treat tumors in animal
models (22,23); and PEGylated liposome
(PLS)-encapsulated ICG has reportedly been used for the
identification of increased vascular permeability in arthritis
disease models (28) and lymphatic
function imaging (24,25). However, previous research has not
reported the use of liposome-encapsulated ICG to trace tumors.
In the present study, preparation of PLS-ICG was
carried out using the lipid thin-film hydration/extrusion method.
RGD was then added to the end of the PEG chain by the amidation
reaction. The prepared liposomes were used in a tracing study of
gastric cancer in a xenograft nude mouse model. The liposomes
effectively avoided uptake by the RES and exhibited prolonged
circulation time in vivo; furthermore, they are passive in
targeting tumor tissue by enhanced permeability and retention
effect (EPR) (26), are active in
targeting tumors through RGD, and increase the ICG accumulation in
tumors. The results of the present study may be able to help
surgeons toward greater accuracy in the resection of tumors under
the guidance of a tracer.
Materials and methods
Preparation of liposomes
Preparation of the liposomes was performed using the
lipid thin-film hydration/extrusion method (27). In brief, L-α-phosphatidylcholine
(PC; purity >98%; Aladdin Industrial Corporation, City of
Industry, CA, USA), cholesterol (CHOL; Sigma-Aldrich, St. Louis,
MO, USA) and
1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene
glycol)-2000] (DSPE-PEG2000),
1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[car
boxy(polyethylene glycol)-2000] (ammonium salt)
(DSPE-PEG2000-NH2; Avanti Polar Lipids, Inc.,
Alabaster, AL, USA) in a molar ratio of 2:1:0.08:0.02 were
dissolved in chloroform/methanol (2:1). The organic solvent was
evaporated under nitrogen, and the lipid film was placed under
vacuum overnight. The film was completely hydrated using a glucose
buffer solution (5%, w/v) with dissolved ICG (15 mmol). The
ICG-containing mixture was freeze-thawed 8 times and extruded 10
times through a polycarbonate film with a pore size of 100 nm using
the mini-lipid extruder (Morgan Machine Co. Inc., USA) to obtain
small unilamellar vesicles. Free-ICG was removed by size exclusion
chromatography on a PD Midi-Trap G-25 column (GE Healthcare,
Uppsala, Sweden) using glucose buffer as the eluent.
Coumarin-6-loaded liposomes were prepared using the
same procedures as previously mentioned, and the liposome
suspension was eluted using a Sephadex LH-20 column (GE Healthcare)
to remove free coumarin-6.
Attachment of RGD (Shanghai HD Biosciences Co.,
Shanghai, China) to the liposomes was based on free carboxyl group
of RGD and DSPE-PEG2000-NH2 from liposomes
with 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium
chloride (DMTMM; Sigma-Aldrich) as catalysts (28). Briefly, the liposomes of the above
preparation were incubated with RGD and DMTMM
(DSPE-PEG2000-NH2:RGD:DMTMM=1:1:1, molar
ratio), and then the mixture was slightly stirred for 1 h at room
temperature. The excess DMTMM and RGD were removed using a Sephadex
G-50 Mini-Column (Piscataway, NJ, USA).
Characteristics of the liposomes
The particle size and size distribution of the
liposomes were measured at 25°C by photon correlation spectroscopy
at a scattering angle of 90° using a Zetasizer Nano ZS90 (Malvern
Instruments, Worcestershire, UK). Each measurement was repeated 3
times for each sample. The morphology of the liposomes was observed
using an H-7650 transmission electron microscope (TEM; Hitachi
Ltd., Tokyo, Japan) with 2% phosphotungstic acid stain.
Stability of the liposomes
The UV absorbance spectra were obtained using an
Ultrospec 2100 pro UV/Visible Spectrophotometer (GE Healthcare Life
Sciences) at wavelengths from 600 to 900 nm. The stability of the
liposome-encapsulated ICG was monitored by a dialysis method
measuring the free-ICG concentration as an indicator of liposome
degradation. The dialysis system included solvent with 50% fetal
bovine serum (FBS) as a condition of blood, loaded ICG liposomes
and 50% FBS in the membrane (MWCO 500; Spectrum) inlet and only 50%
FBS in the membrane outlet as previously described (29).
Cell culture
Human gastric cancer cell lines SGC7901, BGC823 and
MGC803, and human mucosa endothelial cell line GES1 were purchased
from the Shanghai Institute of Cell Biology (Chinese Academy of
Sciences, Shanghai, China). Cells were cultured in Dulbecco's
modified Eagle's medium (DMEM) supplemented with 10% FBS, 100 U/ml
penicillin and 100 µg/ml streptomycin (all from Gibco,
Gaithersburg, MD, USA) at 37°C in a humidified atmosphere with 5%
CO2.
Quantitative real-time reverse
transcription-polymerase chain reaction (qRT-PCR)
Total RNA was isolated from each cell line with the
RNApure tissue kit following the manufacturer's instructions. The
RNA samples were treated with DNase I (both from ComWin Biotech
Co., Ltd., Beijing, China) to exclude contamination with traces of
genomic DNA. The amount of RNA isolated was quantified with a
NanoDrop 200 spectrophotometer (Thermo Scientific, Wilmington, DE,
USA) and 1 µg of purified total RNA was reversed
transcription using PrimeScript RT reagent kit with gDNA Eraser
(Takara, Dalian, China) in a Thermal Cycler Dice Real-Time System.
Revere transcription reaction was performed at 37°C for 15 min,
followed by 85°C for 15 sec and cooled at 4°C.
qPCR analysis was performed in accordance with the
manufacturer's instructions using SYBR® Premix Ex Taq™
II (Tli RNaseH Plus) (Takara). Two microliters of reverse
transcription reaction was used for a total 20 µl
quantitative PCR reactions in Applied Biosystems 7500 Real-Time PCR
System (Life Technologies, Carlsbad, CA, USA). The parameters were
as follows: stage 1, pre-degeneration, at 95°C for 30 sec; stage 2,
counting for 40 cycles, at 95°C for 5 sec, and 60°C for 34 sec;
stage 3, dissociation, at 95°C for 15 sec, 60°C for 1 min, at 95°C
for 15 sec. In comparison to the relative gene expression between
the experimental and control groups, the 2−ΔΔCt values
were calculated (30), where ΔΔCt =
(ΔCt value of the experimental group - ΔCt value of the control
group) and ΔCt = (Ct value of a selected gene - Ct value of
β-actin). The following sets of primers were used in the PCR
amplification: β-actin (forward, 5′-GCGGGAAATCGTGCGTGAC-3′ and
reverse, 5′-CAGGAAGGAAGGCTGGAAGAGTG-3′); α5 (forward,
5′-GGATACTCTGTGGCTGTTGGTGAA-3′ and reverse,
5′-GGATGGTGACATAGCCGTAAGTGA-3′); β1 (forward,
5′-ACTATCCCATTGACCTCTACTACCT-3′ and reverse,
5′-GTAATCCTCCTCATTTCATTCATCA-3′).
Western blot analysis
Whole cell lysates were prepared as previously
described (31). Total protein
concentrations were determined by a Bicinchoninic acid protein
assay kit (KeyGen, Jiangsu, China). Equal amounts (30 µg) of
protein extracted from the cultured cells were run on 10% SDS-PAGE,
followed by electro-transferring to polyvinylidene fluoride
membranes (Millipore, Bedford, MA, USA). The membranes were blocked
with 5% skim milk powder for 2 h. Then, the membranes were
incubated overnight at 4°C with the primary antibodies
anti-integrin α5 (1:400), anti-integrin β1
(1:400) (both from Abcam, USA), then with horseradish
peroxidase-conjugated secondary antibody goat anti-rabbit IgG
(1:2,000; ZSGB-Bio, Beijing, China) for 2 h at room temperature.
The membranes were visualized by chemiluminescence (Millipore) and
exposed with an automatic exposure machine (Bio-Rad, USA) in a dark
room, and then Quantity One software was used for gray-scale
analysis.
Flow cytometric analysis
The cell binding and internalization of the various
coumarin-6-loaded liposomes were analyzed by flow cytometry
(32). GES1, SGC7901, BGC823 and
MGC803 cells were seeded onto 6-well plates with about
6×105 cells/well and cultured for 24 h. Then, the cells
were incubated for 1 h at 37°C with coumarin-6-loaded PLS and
RGD-PLS in serum-free medium, in which the final concentration of
coumarin-6 was 2 µg/ml. The free culture medium was applied
as the blank control. After incubation, the cells were washed 3
times with cold phosphate-buffered saline (PBS; pH 7.4) and the
cells underwent digestion with trypsin. Next, the cells were
collected by centrifugation; resuspended in 500 µl of PBS,
followed by centrifugation and resuspension was repeated 3 times.
The cellular uptake of the fluorescence was measured on a BD
FACSCanto II flow cytometer (BD Biosciences, USA) equipped with an
argon ion air cooled laser (488 nm). Approximately 10,000 events
were counted for each sample.
Confocal microscopic analysis
The binding and internalization of the liposomes
were also examined by laser confocal fluorescence microscopy
(32). Aliquots of 5×104
SGC7901 and GES1 cells were seeded in 35-mm dishes with a glass
coverslip at the bottom. After a 24-h proliferation, the cells were
incubated with coumarin-6-loaded PLS and RGD-PLS in serum-free
medium for 1 h at 37°C. The final concentration of coumarin-6 was 2
µg/ml. After incubation, the medium was removed and the
cells were washed 5 times with cold PBS. Next, the cells were fixed
with 4% of p-formaldehyde (PFA) for 15 min, followed by cell
nuclear staining with Hoechst 33342 (Molecular Probes, Inc.,
Eugene, OR, USA) for 10 min, and then washed 5 times with PBS for
confocal microscopy analysis under oil mirror in magnification of
63 folds. The fluorescence images were analyzed with a laser
confocal fluorescence microscope (LSCM; Zeiss LSM 710,
Germany).
In vivo imaging in the gastric cancer
animal model
Male BALB/C nude mice (18–20 g) were obtained from
SLAC Laboratory Animal Co., Ltd. (Shanghai, China). All animals
were treated according to the guidelines for the use of
experimental animals and approved by the Institutional Animal Care
and Research Advisory Committee at Nanjing University (Nanjing,
China). ICG-loaded liposomes were used to investigate the tumor
targeting efficacy in a heterotopic transplantation tumor model.
Briefly, SGC7901 cells (5×106 cells/mouse) were
subcutaneously transplanted into the armpit of nude mice. Tumor
volume was measured by a Vernier caliper and calculated as [length
× (width)2]/2. When the tumor volume reached a size of
~150–200 mm3, the mice were injected with PLS, ICG
loaded PLS and ICG loaded RGD-PLS at a dose of 40 mg ICG/kg body
weight via tail vein, respectively. Animals were anesthetized with
oxygen/air mixture containing 2% isoflurane. Fluorescence of the
injected ICG was visualized using the Maestro EX in vivo
fluorescence imaging system (CRi, Woburn, MA, USA). The imaging
parameters were as follows: λex = 735 nm, λem = 840 nm, exposure
time = 1–5 sec, f/stop = 2, medium binning, field of view = 6.6×6.6
cm2. The mouse scans were carried out at 1, 2, 4, 8, 16,
24, 48, 72, 96 and 120 h post-injection. Thereafter, the
tumor-bearing mice were sacrificed and the tumors, liver, spleen,
kidney and stomach were harvested for isolated organ imaging.
Statistical analysis
Data are presented as mean ± SD. For statistical
analysis between two groups, the Student's t-test for independent
means was used. A value of P<0.05 was considered as
statistically significant, and a P-value <0.01 was considered as
very significant. Statistical analysis was performed using SPSS
19.0 software.
Results
Synthesis and characterization of the
liposomes
The physical properties of the liposomes are shown
in Table I. The mean size of the
coumarin-6 encapsulated PLS was ~112 nm with a particle size
distribution index (PDI) of 0.056 as measured by dynamic light
scattering (DLS) method. The ICG-loaded liposomes were ~106 nm,
with a PDI of 0.093. Both of the liposomes were slightly increased
following conjugation with RGD, yet there was no statistical
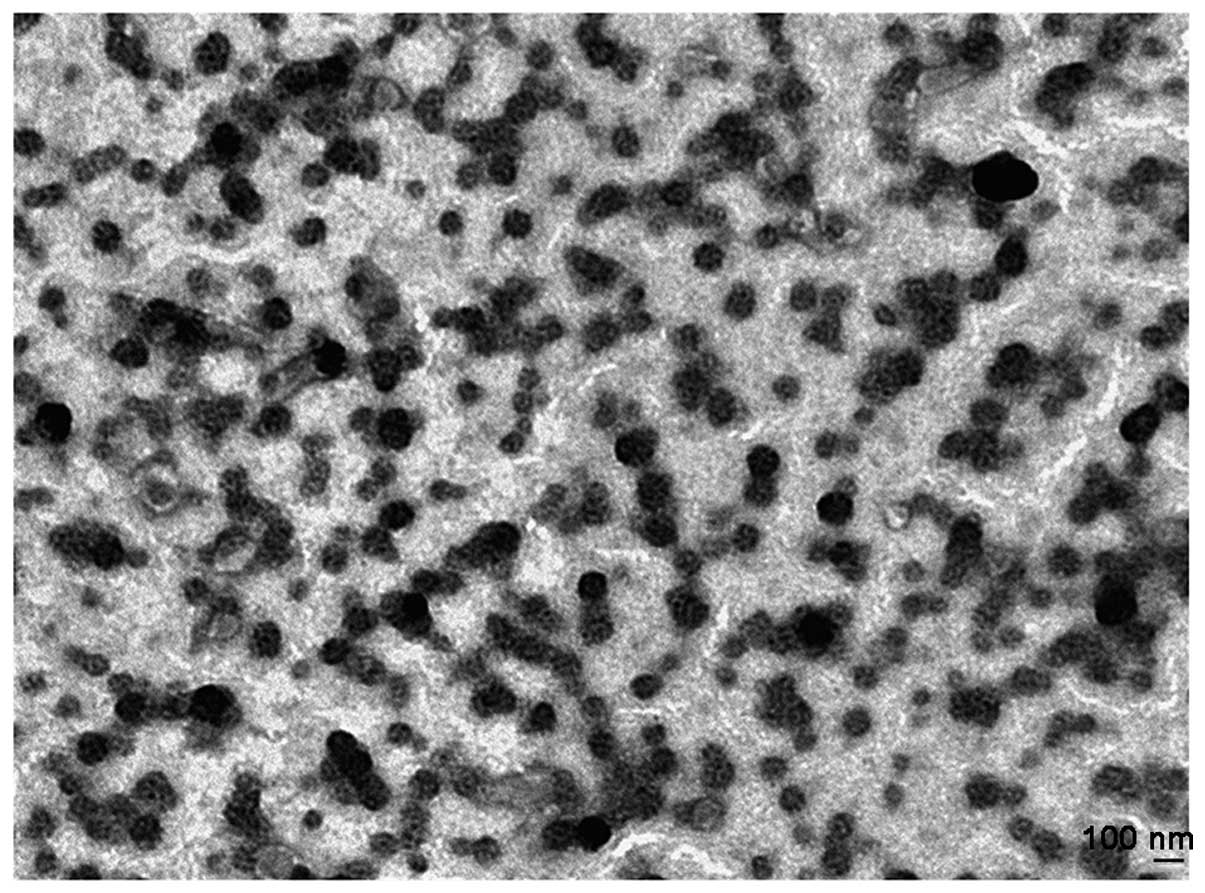
significance and the PDI also did not show significant change. TEM
(Fig. 1) indicated that the
morphology of the liposomes were approximate circular and the
particle size was similar. The results were consistent with the
DLS.
 | Table ICharacteristics of coumarin-6 and
ICG-loaded liposomes. |
Table I
Characteristics of coumarin-6 and
ICG-loaded liposomes.
| Formulation | Fluorescence
probe | Particle size
(nm) | PDI |
|---|
| PLS | Coumarin-6 | 112.7±1.1 | 0.056±0.014 |
| RGD-PLS | Coumarin-6 | 119.6±2.4 | 0.077±0.022 |
| PLS | ICG | 106.4±2.2 | 0.093±0.041 |
| RGD-PLS | ICG | 117.5±2.1 | 0.070±0.014 |
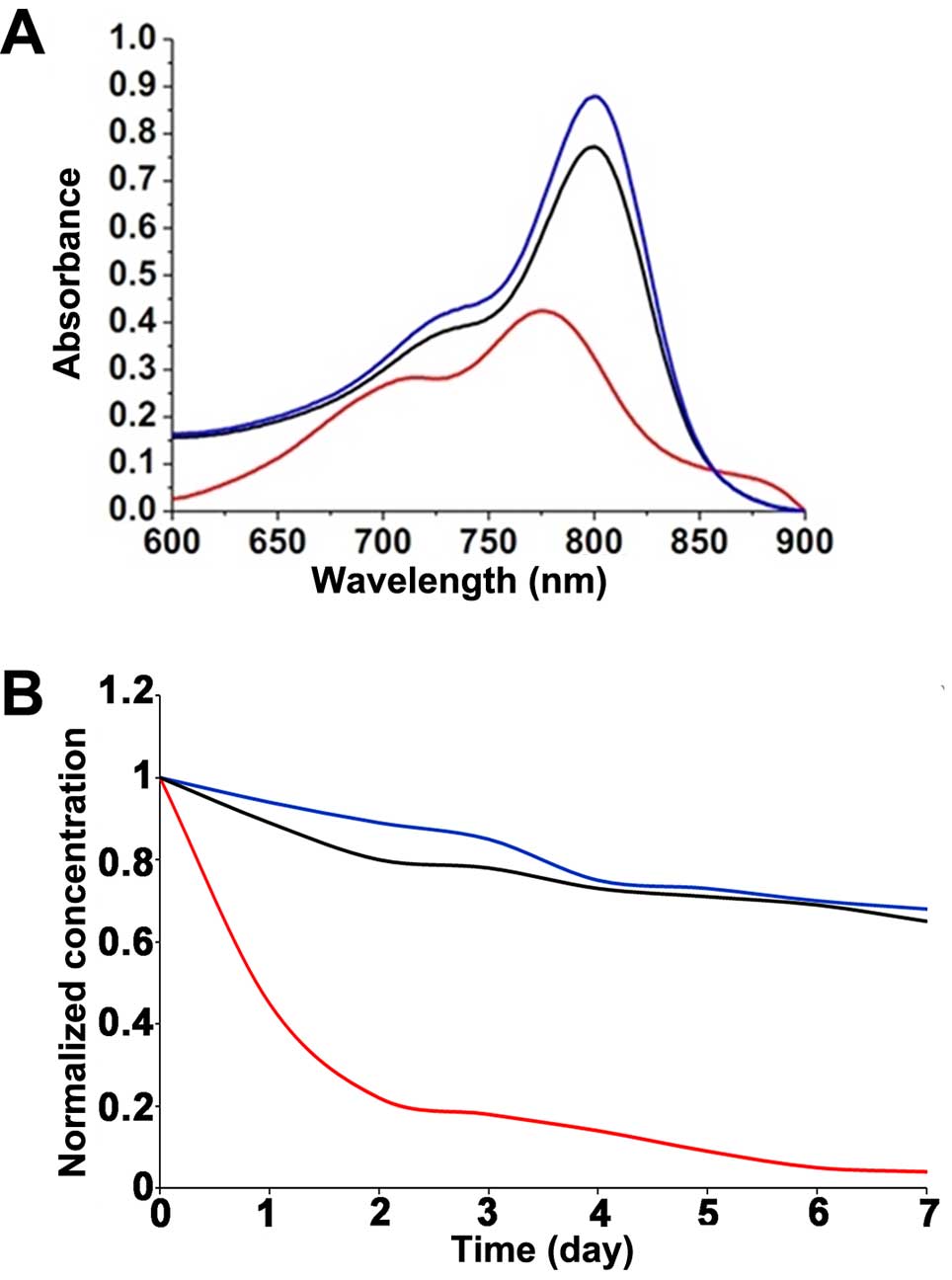
Spectral properties and stability of the
liposomes
Fig. 2 shows the
absorption (Fig. 2A) spectra of
free-ICG dissolved in a 50% FBS solution compared with the spectra
of LP-ICG and RGD-LP-ICG. The marked red shift (23 nm) in the
absorption spectrum of the ICG-loaded liposomes confirmed the
affinity of ICG for the lipid bilayers (33,34).
The seemingly minor shifts toward longer wavelengths resulted in a
marked decrease in the in vivo background signal during
detection, leading to an improved signal-to-noise ratio in
vivo (25).
The stability of PLS-ICG and RGD-PLS-ICG was
determined using the dialysis method to measure the free-ICG
concentration as an indicator of liposome degradation. As
illustrated in Fig. 2, the UV
absorbance and ICG concentrations of PLS-ICG and RGD-PLS-ICG were
higher than the free-ICG, and the ICG concentrations of PLS-ICG and
RGD-PLS-ICG were slightly decreased over time, while the free-ICG
was obviously decreased.
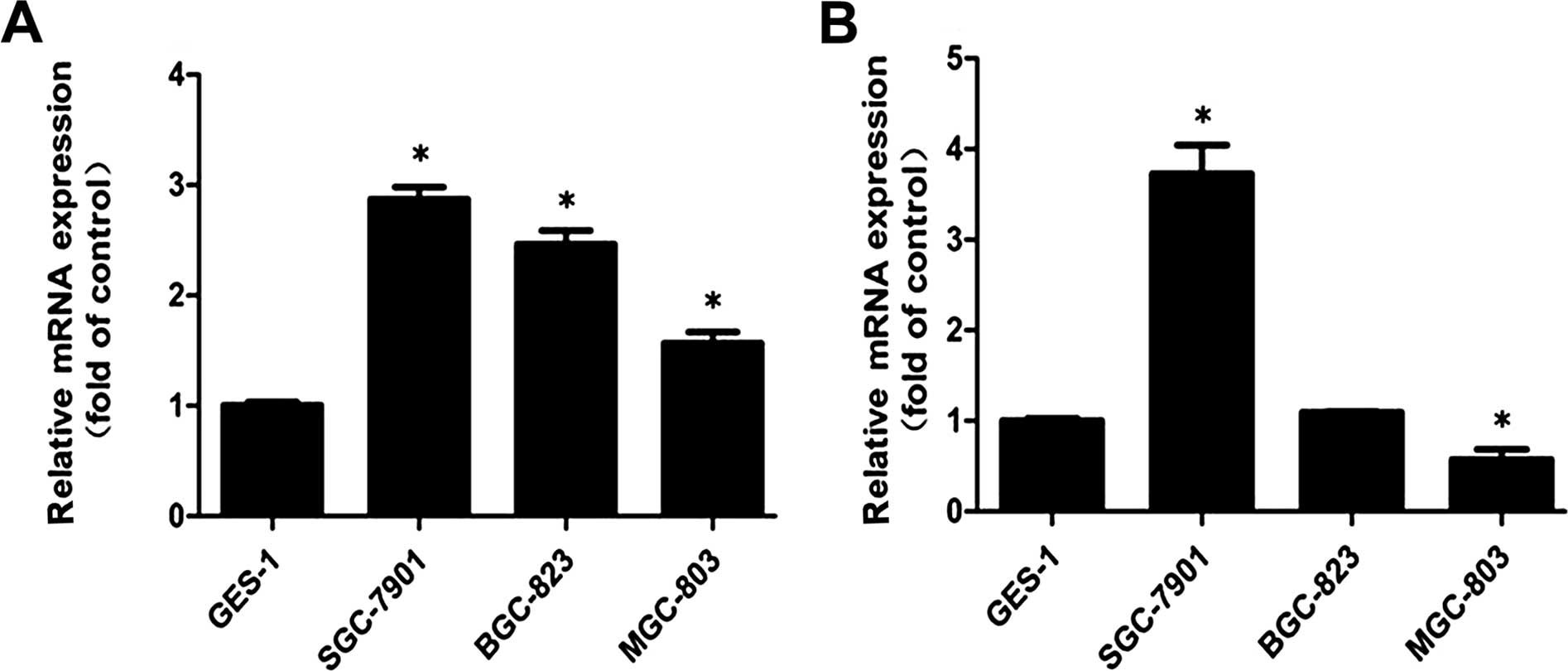
Integrin expression in the cell
lines
Integrin α5β1 was previously
reported in gastric cancer (13,35)
and prostate cancer (36), and
glioma (37), and those that have
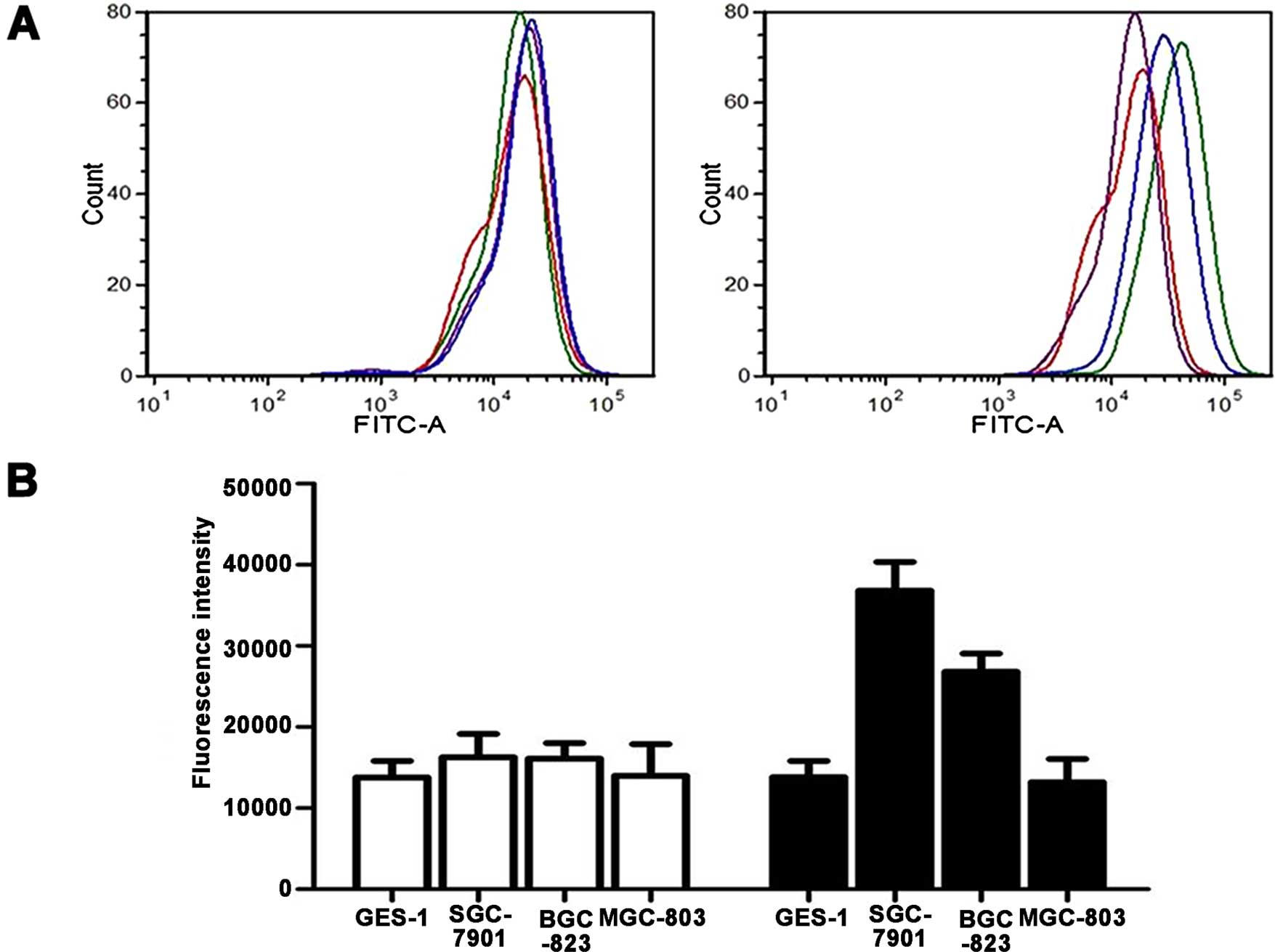
been revealed as FN receptors were explored. Fig. 3 shows the mRNA relative expression
levels of integrin α5 and β1 in the SGC7901,
BGC823, MGC803 and GES1 cell lines. We found that the expression of
α5 was higher in the SGC7901, BGC823 and MGC803 cells
than that in the GES1 cells (Fig.
3A). α5 showed the highest level in the SGC7901
cells, while the expression level of α5 was higher in
the BGC823 than that in the MGC803 cells. For β1, the
results revealed that β1 had the highest expression
level in the SGC7901 cells when compared with that in the GES1
cells, whereas MGC803 exhibited low expression. The expression
level of β1 exhibited no significant difference between
the GES1 and BGC823 cells (Fig.
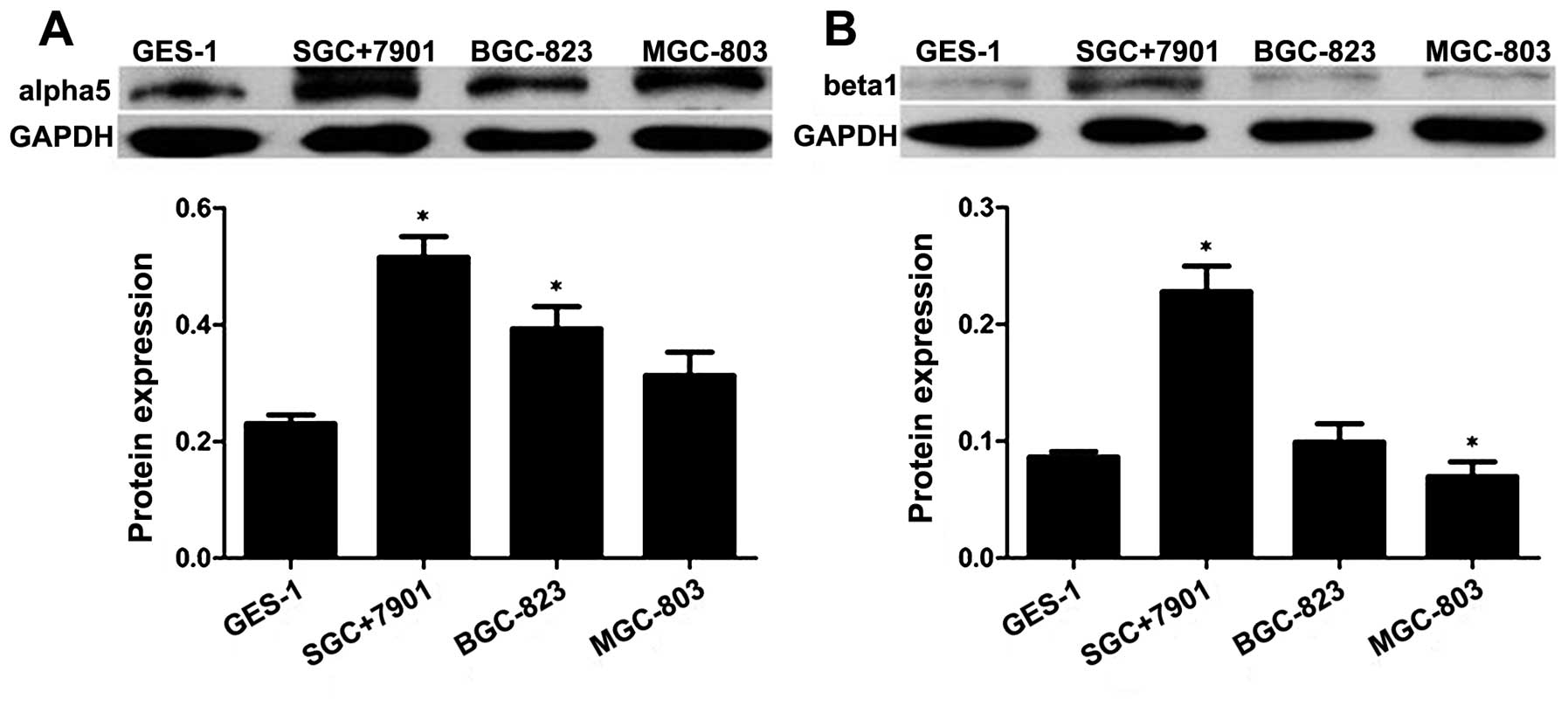
3B). As shown in Fig. 4, we
also confirmed that the protein expression of integrin
α5 and β1 was higher in the SGC7901 cells
than that in the BGC823, MGC803 and GES1 cell lines by western
blotting. These results were consistent with PCR.
A certain type of α-subunit integrin can be paired
with a specific type of β-subunit; and it has been shown that
integrin α5 binds only to β1 (38). For integrin
α5β1, the expression of α5
determines the maximal levels of integrin
α5β1. Hence, we speculated that the
expression of integrin α5β1 was found in all
cell lines, and SGC7901 cells showed the highest expression level
when compared with that in the other cell lines.
Flow cytometric analysis of cell binding
and cellular uptake
Flow cytometric analysis was performed to quantify
the cell binding and cellular uptake. First, the uptake of RGD-PLS
by SGC7901, BGC823, MGC803 and GES1 cells was compared to PLS,
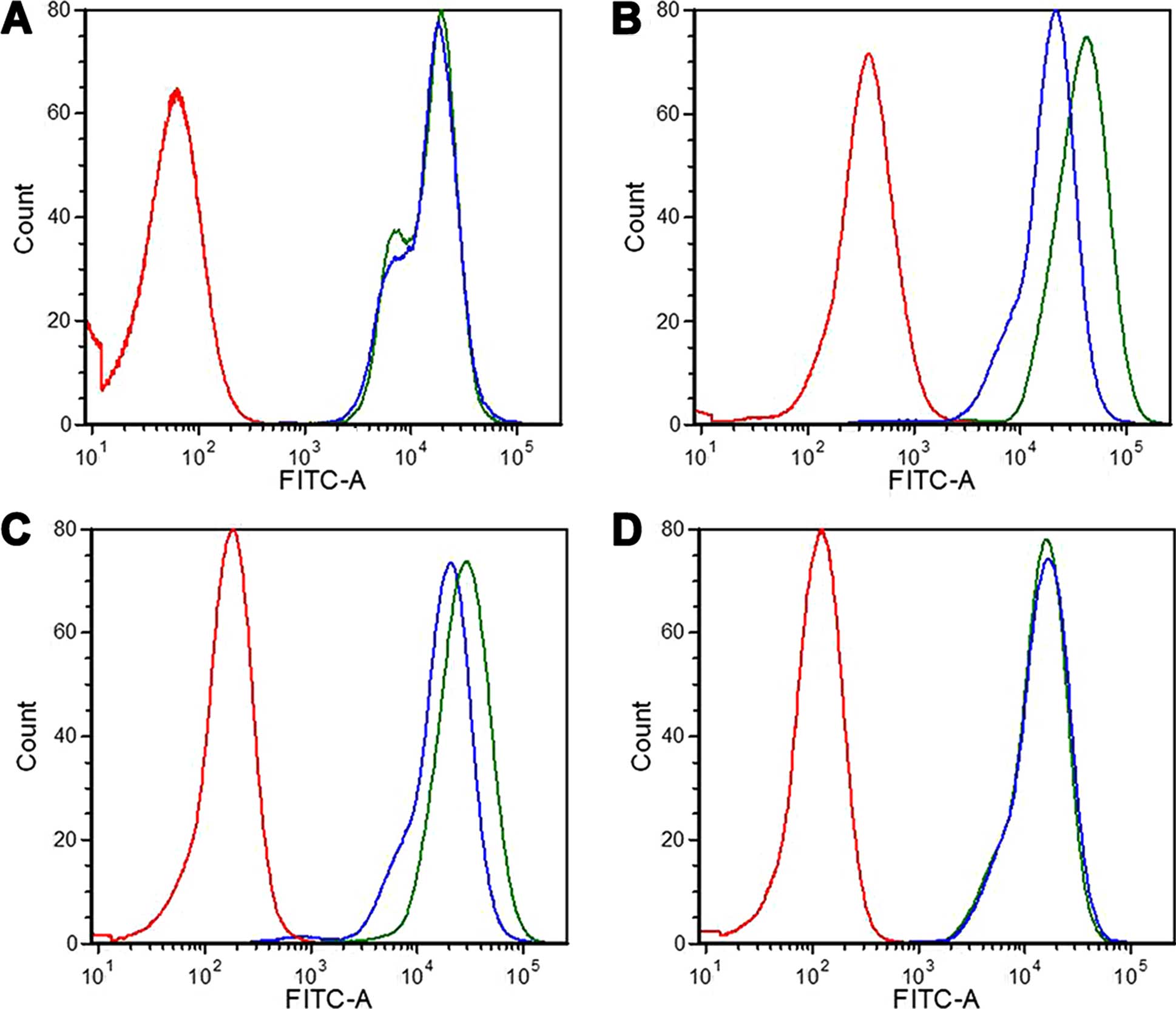
which chosen as the negative control. As shown in Figs. 5 and 6A, the cell-associated fluorescence of
RGD-PLS by overexpression of integrin α5β1 in
the SGC7901 and BGC823 cells was higher than PLS at 37°C; however,
GES1 and MGC803 cells treated with RGD-PLS and PLS had no obvious
difference in binding fluorescence. For the SGC7901 and BGC823
cells, the cellular fluorescence intensities of the RGD-PLS cells
were as much as 2.26-fold (P<0.05) and 1.67-fold (P<0.05)
that of PLS at 37°C (Fig. 6B),
respectively.
To confirm the specificity of RGD-PLS towards
SGC7901 and BGC823 cells, GES1 cells were chosen as the negative
control. As presented in Figs. 5
and 6B, there was no significant
difference in binding to GES1 cells among the two liposome
formulations as expected. The different cell association of RGD-PLS
in the different cells indicated that specific cell binding and
cellular uptake were dependent on the cell surface expression
levels of integrin α5β1.
Confocal microscopic analysis of cell
binding and cellular uptake
In order to investigate the interactions between
RGD-PLS and target cells, confocal microscopy was employed to
evaluate the cell uptake of coumarin-6-loaded liposomes by SGC7901
and GES1 cells, and non-active targeted liposomes PLS were chosen
as the negative controls.
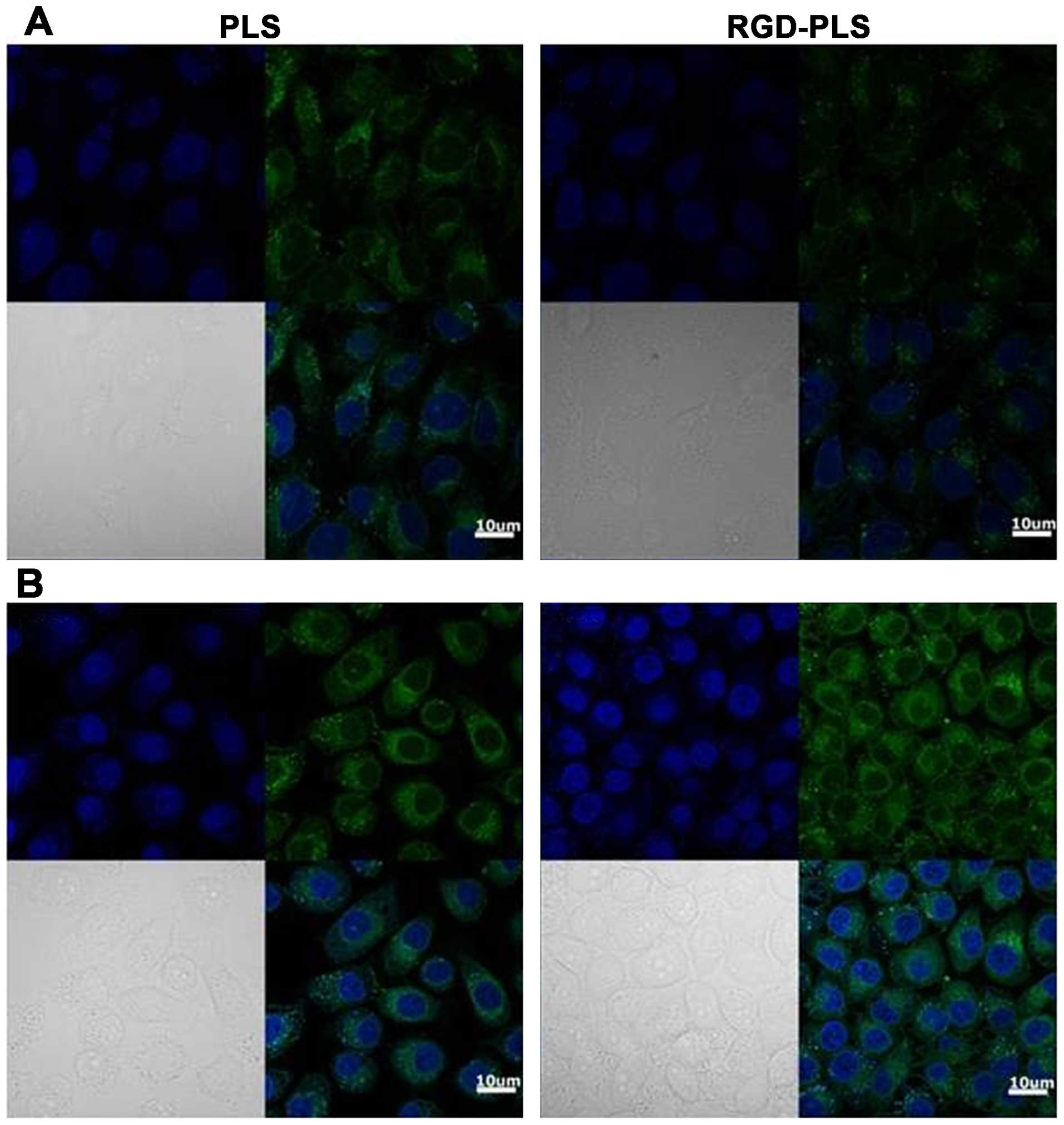
Fig. 7 shows the
fluorescence images of SGC7901 and GES1 cells treated with
liposomes after a 1-h incubation at 37°C. For the target SGC7901
cells, some non-specific cell binding was observed with PLS. The
fluorescence intensity of the RGD-PLS binding to SGC7901 cells was
significantly greater than the intensity of the PLS. For the GES1
cells, we also observed non-specific cell binding treated with PLS,
and the fluorescence signal of PLS and RGD-PLS were as weak as the
SGC7901 cells with PLS. This suggests that cells had non-specific
uptake of the liposomes and RGD markedly improved the specific cell
binding and cellular uptake of liposomes by SGC7901 cells.
In vivo imaging in the tumor model
In order to test the active targeting efficiency of
the liposomes toward gastric cancer, we established a nude mouse
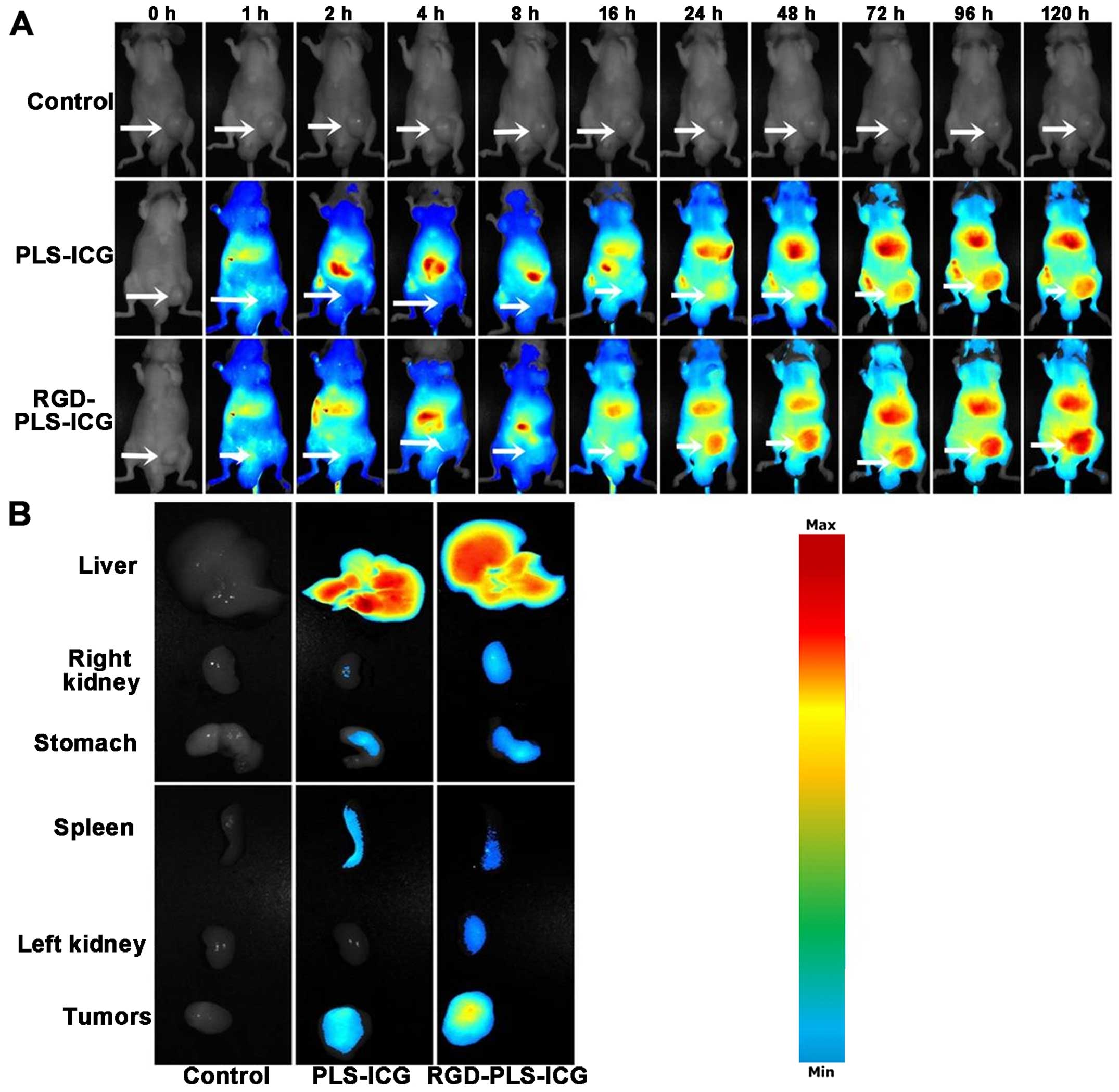
tumor model. Fig. 8A represents the
real-time distribution and tumor accumulation ability of the
fluorescence ICG-loaded liposomes in the tumor model by intravenous
injection into the tail. The fluorescence accumulation was found in
tumors of the RGD-PLS groups starting at 16 h post injection, while
limited fluorescence was observed in tumors of the PLS group. With
the extension of time, the ICG accumulation in the tumors of the
RGD-PLS groups gradually increased and the degree of increase was
higher than the ICG accumulation in tumors of the PLS group at the
same time point. After 120 h, the ICG accumulation in tumors was
the largest and still higher than those of PLS. The ex vivo
image of excised organs (Fig. 8B)
confirmed the higher fluorescence accumulation in the tumors of the
RGD-PLS group compare with those of the PLS group. The ICG
accumulation in tumors of the PLS group showed that the liposomes
can be trapped and retained for longer time periods through the
process called enhanced permeation and retention (EPR) effect.
However, ICG accumulation of RGD-PLS in the tumors did not only
depend on the EPR effect, yet also relied on active targeting of
RGD. These results are further proof of the selective accumulation
of RGD-PLS in gastric cancer. An additional explanation for the
high fluorescence intensity observed in the liver and spleen is the
naturally high uptake of liposomes by the macrophages in these
organs (Fig. 8B).
Discussion
In the present study, a novel NIR contrast system
containing ICG was developed and its targeting efficiency to
gastric cancer in a tumor model was investigated. RGD-PLS-ICG had
several advantages over ICG or PLS-ICG, including: i) increased
fluorescence signal with a shift toward the longer wavelength
absorption; ii) vastly improved stability in 50% FBS solution; iii)
specificity combined with the cells; iv) prolonged circulation time
in vivo; v) effective reduction RES uptake; and vi) improved
visualization of tumor tissues in vivo. These
characteristics of RGD-LP-ICG have enabled quantitative development
of tumor tissues in the tumor model.
Fibronectin (FN) is a high-molecule glycoprotein of
the ECM that binds to membrane-spanning receptor proteins called
integrins, and play an important role in cell adhesion, growth,
migration and differentiation. It was previously reported that the
majority of integrin-mediated interactions with FN occur through
the arg-gly-asp (RGD) cell-binding sequence in repeat
III10, such as integrin α5β1,
αvβ3 and αIIbβ3
(6,7). In recent years, integrin
α5β1 was found to be overexpressed in several
types of cancers (35,36). Furthermore, substantial evidence
suggests that RGD binds to gastric cancer (15,16).
This research suggests that RGD may be a promising target ligand
for gastric cancer. However, previous studies have mainly focused
on RGD as a targeting ligand for the treatment of tumors and
limited research has reported its use for tracing tumors. In the
present study, we explored expression of integrin
α5β1 in 3 gastric cancer cell lines and
normal gastric mucosa epithelial cells. The results showed that
integrin α5β1 had higher expression in the
SGC7901 cells than that in the other cell lines. Therefore, we
prepared RGD-PLS-ICG to confirm that RGD enhanced the gastric
cancer targeting effect by increasing specific binding to gastric
cancer cells.
ICG is considered one of the most attractive
exogenous contrast agents for in vivo NIR fluorescent (NIRF)
imaging, due to its spectral properties, minimal toxicity, low cost
and FDA-approved status as a medical diagnostic compound (39). Injected ICG is rapidly cleared
through the liver and bile duct (40) and it is unstable, aggregates in
solution, self-quenches and has low quantum properties (41). These disadvantages are overcome by
combining ICG with colloidal particles, such as micelles and
liposomes (42). The design of an
efficient ICG delivery system preserves its optical properties and
prolongs the half-life. For the current application, the optimized
formulation exhibited an increased absorbance and a bathochromic
shift in the absorption spectrum. The spectral properties of
RGD-PLS-ICG and PLS-ICG were preserved in 50% FBS solution for one
week.
In the present experiment, the conjugation of RGD
onto the surface of PEGylated liposomes was carried out by DMT-MM
as a catalyst, which created stable amide bonds between the amine
groups of the PEG chains and the carboxyl groups of RGD in the
water phase (28). The particle
size of the liposomes plays an important role for the
pharmacokinetics. Liposomes with particle size <200 nm increase
drug accumulation in the tumor via the EPR effect (43). Meanwhile, the particle size of
liposomes also has an effect on their interactions with target
cells. Particles up to 100–200 nm can be internalized by
receptor-mediated endocytosis (44). In the present study, the sizes of
PLS-ICG and RGD-PLS-ICG were mainly 100–130 nm, which would be
favorable for passive and active targeting and for cellular
uptake.
To demonstrate the specific cell binding and
internalization of RGD-PLS, SGC7901, BGC803 and MGC803 cells were
chosen as experimental groups, while GES1 cells were applied as a
control group. Non-active targeted liposomes, PLS, was also
prepared and used as the negative controls. The flow cytometric
data showed that the cell associated fluorescence of RGD-PLS in
SGC7901 and BGC823 cells was higher than that of PLS, while MGC803
cells treated with RGD-PLS and PLS had no obvious differences in
the GES1 cells. Receptor-mediated binding was proceeded at both 4°C
and 37°C, yet endocytosis and internalization occurred only at 37°C
(45). Therefore, we explored how
SGC7901, BGC823, MGC803 and GES1 cells may incubate with RGD-PLS
and PLS at 37°C. In the SGC7901 and BGC823 cells, except the
non-specific binding event, an additional specific cell binding
named the receptor-mediated binding also occurs for RGD-PLS, which
was different from PLS (non-specific binding only) (45). In addition, the markedly enhanced
uptake of RGD-PLS compared with PLS by SGC7901 and BGC823 cells
suggested an easier endocytosis may results from the presence of
specific binding mediated by RGD.
Meanwhile, confocal microscopy images demonstrated
that RGD-PLS resulted in a significant higher cell association by
SGC7901 cells compared to PLS. However, similar cellular behavior
was found in the two liposomal formulations when they were
incubated in the GES1 cells. All of these phenomena suggested that
RGD-PLS enhanced the specific cell binding and cellular uptake in
the SGC7901 cells due to RGD, and also depending on the integrin
α5β1 expression level at the cell
surface.
One of the most important concerns of a new active
targeting delivery system is its tolerability and toxicity upon the
interaction with the target and normal cells. PEGylated liposomes,
which have been approved by the FDA, are regarded as promising drug
carriers with good biocompatibility, biodegradability and low
cytotoxicity for cancer therapeutics (46). In addition, RGD has been used in the
clinic (15,16). In the present study, we did not
explore RGD for cell toxicity in vitro, yet no obvious
side-effect was noted during the period of the animal experiments.
However, long-term in vivo toxicity and immunogenicity of
RGD-PLS in animals should be investigated further.
A biodistribution study was also conducted to
investigate the tumor targeting effect of RGD-PLS in vivo.
The biodistribution of PLS and RGD-PLS indicated that both passive
and active tumor targeting mechanisms were involved in the ICG
accumulation within the tumor, in which passive targeting was
attributed to the EPR effect of PLS and active targeting may be
achieved by RGD conjugation to PLS. As shown in Fig. 8, the accumulation of ICG in tumors
was significantly increased after the attachment of RGD to PLS.
These results suggest that RGD modification of PEGylated liposomes
facilitates the accumulation of PLS in gastric cancer, and the
gastric cancer targeting ability of RGD was proven. However, a
higher fluorescence intensity was observed in the liver and spleen
due to the RES uptake nature for liposomes; how to reduce or to
avoid RES uptake is a topic for future research. Based on the
present study, RGD-PLS may be a promising delivery system to target
gastric cancer with favorable integrin α5β1
targeting ability. However, future study will be aimed at reducing
the uptake of liposomes in the liver and spleen and at finding more
specific targeting ligands to gastric cancer.
In conclusion, a gastric cancer targeting
fluorescent dye delivery system was successfully constructed by the
DMTMM-mediated conjugation of PEGylated liposomes with RGD, where
RGD was used for active targeting to gastric cancer and the
PEG-lipid was applied for prolonging circulation time in
vivo. The coumarin-6 and ICG-loaded RGD-PLS had a particle size
of <200 nm, which would be favorable for passive and active
tumor targeting. RGD-PLS was demonstrated to be a promising active
targeting tracer delivery system for targeting gastric cancer cells
overexpressing integrin, as it possessed marked binding affinity
and specificity towards gastric cancer cells, and improved
accumulation in stomach tumors. This targeting delivery carrier may
be applied in the clinical and may facilitate accurate resection of
gastric cancers in the future.
Acknowledgments
The present study was supported by the National
Nature Science Foundation of China (81372364), the Digestive System
Disease Clinical Medical Center of Jiangsu Province (BK2012001),
and the Nature Science Foundation of Nanjing (ZKX11015).
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar
|
|
2
|
Dikken JL, van de Velde CJ, Coit DG, Shah
MA, Verheij M and Cats A: Treatment of resectable gastric cancer.
Therap Adv Gastroenterol. 5:49–69. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ajani JA, Barthel JS, Bekaii-Saab T,
Bentrem DJ, D'Amico TA, Das P, Denlinger C, Fuchs CS, Gerdes H,
Hayman JA, et al: NCCN Gastric Cancer Panel: Gastric cancer. J Natl
Compr Canc Netw. 8:378–409. 2010.PubMed/NCBI
|
|
4
|
Okines A, Verheij M, Allum W, Cunningham D
and Cervantes A; ESMO Guidelines Working Group: Gastric cancer:
ESMO Clinical Practice Guidelines for diagnosis, treatment and
followup. Ann Oncol. 21(Suppl 5): v50–v54. 2010. View Article : Google Scholar
|
|
5
|
Songun I, Putter H, Kranenbarg EMK, Sasako
M and van de Velde CJH: Surgical treatment of gastric cancer:
15-year follow-up results of the randomised nationwide Dutch D1D2
trial. Lancet Oncol. 11:439–449. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ruoslahti E: Integrins. J Clin Invest.
87:1–5. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hynes RO: Integrins: Versatility,
modulation, and signaling in cell adhesion. Cell. 69:11–25. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Aota S, Nomizu M and Yamada KM: The short
amino acid sequence Pro-His-Ser-Arg-Asn in human fibronectin
enhances cell-adhesive function. J Biol Chem. 269:24756–24761.
1994.PubMed/NCBI
|
|
9
|
Rajeswari J and Pande G: The significance
of alpha 5 beta 1 integrin dependent and independent actin
cytoskelton organization in cell transformation and survival. Cell
Biol Int. 26:1043–1055. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Toquet C, Colson A, Jarry A, Bezieau S,
Volteau C, Boisseau P, Merlin D, Laboisse CL and Mosnier JF: ADAM15
to α5β1 integrin switch in colon carcinoma cells: A late event in
cancer progression associated with tumor dedifferentiation and poor
prognosis. Int J Cancer. 130:278–287. 2012. View Article : Google Scholar
|
|
11
|
Nam JM, Onodera Y, Bissell MJ and Park CC:
Breast cancer cells in three-dimensional culture display an
enhanced radio-response after coordinate targeting of integrin
alpha5beta1 and fibronectin. Cancer Res. 70:5238–5248. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mitra AK, Sawada K, Tiwari P, Mui K, Gwin
K and Lengyel E: Ligand-independent activation of c-Met by
fibronectin and α5 β1-integrin regulates
ovarian cancer invasion and metastasis. Oncogene. 30:1566–1576.
2011. View Article : Google Scholar :
|
|
13
|
Chi F, Fu D, Zhang X, Lv Z and Wang Z:
Expression of the c-Met proto-oncogene and Integrin α5β1 in human
gastric cardia adenocarcinoma. Biosci Biotechnol Biochem.
76:1471–1476. 2012. View Article : Google Scholar
|
|
14
|
Ren J, Xu S, Guo D, Zhang J and Liu S:
Increased expression of α5β1-integrin is a prognostic marker for
patients with gastric cancer. Clin Transl Oncol. 16:668–674. 2014.
View Article : Google Scholar
|
|
15
|
Chen CH, Liu DZ, Fang HW, Liang HJ, Yang
TS and Lin SY: Evaluation of multi-target and single-target
liposomal drugs for the treatment of gastric cancer. Biosci
Biotechnol Biochem. 72:1586–1594. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang C, Bao C, Liang S, Fu H, Wang K, Deng
M, Liao Q and Cui D: RGD-conjugated silica-coated gold nanorods on
the surface of carbon nanotubes for targeted photoacoustic imaging
of gastric cancer. Nanoscale Res Lett. 9:2642014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kusano M, Tajima Y, Yamazaki K, Kato M,
Watanabe M and Miwa M: Sentinel node mapping guided by indocyanine
green fluorescence imaging: A new method for sentinel node
navigation surgery in gastrointestinal cancer. Dig Surg.
25:103–108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tajima Y, Murakami M, Yamazaki K, Masuda
Y, Kato M, Sato A, Goto S, Otsuka K, Kato T and Kusano M: Sentinel
node mapping guided by indocyanine green fluorescence imaging
during laparoscopic surgery in gastric cancer. Ann Surg Oncol.
17:1787–1793. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bangham AD and Horne RW: Negative staining
of phospholipids and their structural modification by
surface-active agents as observed in the electron microscope. J Mol
Biol. 8:660–668. 1964. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Anders CK, Adamo B, Karginova O, Deal AM,
Rawal S, Darr D, Schorzman A, Santos C, Bash R, Kafri T, et al:
Pharmacokinetics and efficacy of PEGylated liposomal doxorubicin in
an intracranial model of breast cancer. PLoS One. 8:e613592013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lin YY, Kao HW, Li JJ, Hwang JJ, Tseng YL,
Lin WJ, Lin MH, Ting G and Wang HE: Tumor burden talks in cancer
treatment with PEGylated liposomal drugs. PLoS One. 8:e630782013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jiang J, Yang SJ, Wang JC, Yang LJ, Xu ZZ,
Yang T, Liu XY and Zhang Q: Sequential treatment of drug-resistant
tumors with RGD-modified liposomes containing siRNA or doxorubicin.
Eur J Pharm Biopharm. 76:170–178. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yamamoto Y, Yoshida M, Sato M, Sato K,
Kikuchi S, Sugishita H, Kuwabara J, Matsuno Y, Kojima Y, Morimoto
M, et al: Feasibility of tailored, selective and effective
anticancer chemotherapy by direct injection of docetaxel-loaded
immunoliposomes into Her2/neu positive gastric tumor xenografts.
Int J Oncol. 38:33–39. 2011.
|
|
24
|
Sandanaraj BS, Gremlich HU, Kneuer R,
Dawson J and Wacha S: Fluorescent nanoprobes as a biomarker for
increased vascular permeability: Implications in diagnosis and
treatment of cancer and inflammation. Bioconjug Chem. 21:93–101.
2010. View Article : Google Scholar
|
|
25
|
Proulx ST, Luciani P, Derzsi S,
Rinderknecht M, Mumprecht V, Leroux JC and Detmar M: Quantitative
imaging of lymphatic function with liposomal indocyanine green.
Cancer Res. 70:7053–7062. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Duncan R and Sat YN: Tumour targeting by
enhanced permeability and retention (EPR) effect. Ann Oncol.
9(Suppl 2): 39, abs. 149. 1998. View Article : Google Scholar
|
|
27
|
Hope MJ, Bally MB, Webb G and Cullis PR:
Production of large unilamellar vesicles by a rapid extrusion
procedure: Characterization of size distribution, trapped volume
and ability to maintain a membrane potential. Biochim Biophys Acta.
812:55–65. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kunishima M, Kawachi C, Hioki K, Terao R
and Tani S: Formation of carboxamides by direct condensation of
carboxylic acids and amines in alcohols using a new alcohol-and
water-soluble condensing agent: DMT-MM. Tetrahedron. 57:1551–1558.
2001. View Article : Google Scholar
|
|
29
|
Jeong HS, Lee CM, Cheong S-J, Kim EM,
Hwang H, Na KS, Lim ST, Sohn MH and Jeong HJ: The effect of
mannosylation of liposome-encapsulated indocyanine green on imaging
of sentinel lymph node. J Liposome Res. 23:291–297. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
31
|
Kao E, Shinohara M, Feng M, Lau MY and Ji
C: Human immunodeficiency virus protease inhibitors modulate
Ca2+ homeostasis and potentiate alcoholic stress and
injury in mice and primary mouse and human hepatocytes. Hepatology.
56:594–604. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Simard P and Leroux JC: pH-sensitive
immunoliposomes specific to the CD33 cell surface antigen of
leukemic cells. Int J Pharm. 381:86–96. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mordon S, Devoisselle JM, Soulie-Begu S
and Desmettre T: Indocyanine green: Physicochemical factors
affecting its fluorescence in vivo. Microvasc Res. 55:146–152.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Desmettre T, Devoisselle JM, Soulie-Begu S
and Mordon S: Fluorescence properties and metabolic features of
indocyanine green (ICG). J Fr Ophtalmol. 22:1003–1016. 1999.In
French. PubMed/NCBI
|
|
35
|
Gulubova M and Vlaykova T:
Immunohistochemical assessment of fibronectin and tenascin and
their integrin receptors alpha-5beta1 and alpha9beta1 in gastric
and colorectal cancers with lymph node and liver metastases. Acta
Histochem. 108:25–35. 2006. View Article : Google Scholar
|
|
36
|
Albrecht M, Renneberg H, Wennemuth G,
Möschler O, Janssen M, Aumüller G and Konrad L: Fibronectin in
human prostatic cells in vivo and in vitro: Expression,
distribution, and pathological significance. Histochem Cell Biol.
112:51–61. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kesanakurti D, Chetty C, Dinh DH, Gujrati
M and Rao JS: Role of MMP-2 in the regulation of IL-6/Stat3
survival signaling via interaction with α5β1 integrin in glioma.
Oncogene. 32:327–340. 2013. View Article : Google Scholar
|
|
38
|
Gahmberg CG, Fagerholm SC, Nurmi SM,
Chavakis T, Marchesan S and Grönholm M: Regulation of integrin
activity and signalling. Biochim Biophys Acta. 1790:431–444. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Frangioni JV: In vivo near-infrared
fluorescence imaging. Curr Opin Chem Biol. 7:626–634. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Unno N, Nishiyama M, Suzuki M, Yamamoto N,
Inuzuka K, Sagara D, Tanaka H and Konno H: Quantitative lymph
imaging for assessment of lymph function using indocyanine green
fluorescence lymphography. Eur J Vasc Endovasc Surg. 36:230–236.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Saxena V, Sadoqi M and Shao J: Indocyanine
green-loaded biodegradable nanoparticles: Preparation,
physicochemical characterization and in vitro release. Int J Pharm.
278:293–301. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ohnishi S, Lomnes SJ, Laurence RG,
Gogbashian A, Mariani G and Frangioni JV: Organic alternatives to
quantum dots for intraoperative near-infrared fluorescent sentinel
lymph node mapping. Mol Imaging. 4:172–181. 2005.PubMed/NCBI
|
|
43
|
Gao J, Zhong W, He J, Li H, Zhang H, Zhou
G, Li B, Lu Y, Zou H, Kou G, et al: Tumor-targeted PE38KDEL
delivery via PEGylated anti-HER2 immunoliposomes. Int J Pharm.
374:145–152. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Couvreur P and Puisieux F: Nanoparticles
and microparticles for the delivery of polypeptides and proteins.
Adv Drug Deliv Rev. 10:141–162. 1993. View Article : Google Scholar
|
|
45
|
Xie H, Zhu Y, Jiang W, Zhou Q, Yang H, Gu
N, Zhang Y, Xu H, Xu H and Yang X: Lactoferrin-conjugated
superparamagnetic iron oxide nanoparticles as a specific MRI
contrast agent for detection of brain glioma in vivo. Biomaterials.
32:495–502. 2011. View Article : Google Scholar
|
|
46
|
Allen TM and Cullis PR: Drug delivery
systems: Entering the mainstream. Science. 303:1818–1822. 2004.
View Article : Google Scholar : PubMed/NCBI
|