Introduction
Nasopharyngeal carcinoma (NPC), a malignant tumor,
is highly prevalent in the Southeast Asian population (1). The pathological analysis of these
tumors mostly shows poorly differentiated squamous cells and
therefore, the preferred method of treatment is radiation therapy.
However, complications associated with radiation therapy are
common; the advantage offered by this treatment is only limited to
early in situ non-metastatic carcinoma and the 5-year
survival rate is quite low. Therefore, the development of new
chemotherapeutic alternatives with maximum efficacy and low
toxicity against NPC is necessary.
The development of malignant tumors such as NPC is
associated with tumor cell growth and the growth of new blood
vessels, which provide nutrients and oxygen capacity since the
existing vessels cannot meet the needs of the growing tumor. Tumor
cells require enhanced glycolysis to maintain strong autonomous and
physiological activities (2,3). The
enhanced glycolysis leads to reduced mitochondrial respiration in
tumor cells, which ultimately depends on aerobic glycolysis for
adenosine triphosphate (ATP) release as a priority source of energy
to meet their needs. Furthermore, even with an adequate oxygen
supply, enhanced glycolysis still weakens cellular aerobic
oxidation and enhances sugar fermentation (4). In order to elucidate the role of
glycolysis in ATP production in malignant and normal cells, we
first determined the intracellular levels of ATP after treatment
with a potent inhibitor of glycolysis in tumor cells.
It is well known that this cancer-specific metabolic
pathway has provided an exceptional opportunity for the development
of new chemotherapeutic agents (2).
These newly developed agents uniquely target this pathway and have
theoretical advantages, which include cancer specificity and low
toxicity to normal cells (5).
3-Bromopyruvate (3-BrPA), a member of this new class of anticancer
drugs, is a specific alkylating agent and a potent ATP inhibitor,
which blocks glycolytic tumor metabolism (6). Malignant cells generate a large amount
of ATP via glycolysis (7,8) and 3-BrPA has been shown to interrupt
the glycolytic metabolic pathway, leading to the death of the tumor
cells through apoptosis (9) with
almost no damage to the surrounding normal tissues. It has been
experimentally used to target hexokinase/glycolysis and possesses
chemical and structural properties that are very similar to the
acid, lactic acid and glucose metabolic products of glycolysis.
These similar properties enable 3-BrPA to be transported by the
same vector mechanisms that transport glycolytic products, and once
it gains access to the pathway, 3-BrPA penetrates tumor cells and
initiates its therapeutic effect (10).
Mitochondria are double membrane-enclosed organelles
that play an essential role in cellular metabolism, ATP generation,
ROS production and regulation of cell proliferation and death
(11). They are also the principal
source of ROS via electron leakiness in the electron transport
chain. Disruption of the mitochondrial membrane gradient leads to
increased electron leakage and potentially toxic ROS levels.
Mitochondrial ROS were previously studied to determine their role
in the tumor necrosis factor (TNF)α-induced necrotic death of L929
cells (12). ROS including singlet
oxygen, superoxide, hydroxyl free radical, hydrogen peroxides and
nitric oxide mainly generated from the mitochondria play a crucial
role in cell death (13). Necrosis
is considered a passive cell death process, which is disorderly,
unregulated and irreversible as opposed to the orderly programmed
manner of apoptosis. However, ongoing studies on the mechanisms of
cell death has put forth a new concept of type III programmed cell
necrosis, known as necroptosis, which is a basic cell death pathway
(14).
Z-VAD-fmk is a synthetic broad-spectrum caspase
inhibitor that inhibits apoptosis. The serine-threonine kinase
receptor-interacting protein (RIP) 1 is targeted by necrostatin-1
(Nec-1) a specific inhibitor of necroptosis, which depends on
RIP1/3 complex activation (15,16).
Necroptosis controls normal embryonic organic evolution, T-cell
propagation and chronic intestinal inflammation (17). In addition, the discovery of Nec-1,
a small molecule that inhibits non-apoptotic programmed cell
death-inducing substances, has provided a useful pharmacological
tool for the study of necroptosis. In the present study, we treated
NPC cells with 3-BrPA in the presence of Nec-1 and z-VAD-FMK to
observe the therapeutic effects. The association between
necroptosis and mitochondrial dysfunction remains to be examined in
NPC cells.
Materials and methods
Reagents and antibodies
3-BrPA,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT),
Nec-1, and NAC were purchased from Sigma (St. Louis, MO, USA).
Z-VAD-fmk was purchased from Calbiochem. Dihydroethidium (DHE),
JC-1, 4′,6-diamidino-2-phenylindole (DAPI) and propidium iodide
(PI) assay kits were purchased from Biotechnology (Beijing, China).
Dulbecco's modified Eagle's medium (DMEM) and fetal bovine serum
(FBS) were obtained from Gibco (Carlsbad, CA, USA). B-cell lymphoma
2 (Bcl-2), Bcl-2-associated protein X (Bax) and myeloid cell
leukemia 1 (Mcl-1) antibodies were purchased from Abcam (Cambridge,
UK). Mouse monoclonal antibodies were obtained from Cell Signaling
Technology (Beverly, MA, USA). Rabbit anti-β-actin antibodies were
obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Cell lines and cell culture
NPC HNE1 and CNE-2Z cells were obtained from the
Shanghai Cell Bank (Shanghai, China). The cells were grown in
Roswell Park Memorial Institute (RPMI)-1640 medium supplemented
with 10% fetal calf serum, streptomycin (100 U/ml), penicillin (10
U/ml), and HEPES (25 mM). They were maintained at 37°C in a 5%
CO2 humidified atmosphere. The NPC cells were seeded at
a density of 8,000 cells/well in a 96-well plate for 24 h and then
treated with different concentrations of 3-BrPA. At 24, 48 and 72
h, the cells were incubated with MTT [5 mg/ml in phosphate-buffered
saline, (PBS)] for 4 h at 37°C. The MTT solution was then replaced
with 100 µl of dimethyl sulfoxide (DMSO)/well and the
absorbance was measured using a plate reader at a wavelength of 490
nm.
PI staining
Prior to treatment with 3-BrPA, the cells
(2×105 cells/well) were plated in each well of a 12-well
plate and allowed to reach exponential growth (24 h). The cells
were then treated with increasing concentrations of 3-BrPA (40, 80
and 160 µM) for 24 h, stained with PI and then evaluated
using flow cytometry.
DAPI staining and quantification of
apoptotic cells
A DAPI nuclear staining assay was performed to
examine the effects of 3-BrPA on cancer cell apoptosis. The CNE-2Z
and HNE1 cells were plated in 6-well plates on glass slides
(2×105 cells/well). Following treatment with 3-BrPA for
24 h, the cells were fixed with immunostaining setting for 30 min
at 4°C, washed twice with PBS and then incubated with DAPI for 30
min at 4°C in the dark. The slides were then washed with PBS to
remove the excess DAPI and the cell nuclei were observed under a
laser confocal scanning microscope (LCSM, Olympus 1×71). The
apoptosis rate of the NPC cells treated with 3-BrPA (40, 80, and
160 µM) was determined by scoring the number of cells with
nuclear phenotypic changes in microscopic fields under the
LCSM.
Intracellular ATP measurements
The cells were plated in duplicate in 96-well
culture plates and the cellular ATP levels were determined using
the Cell Titer-Glo luminescent cell viability assay (Promega,
Madison, WI, USA) according to the manufacturer's instructions. The
luminescent levels were measured using a microplate reader
(Varioskan Flash spectral scanning multimode reader, Thermo Fisher,
Waltham MA, USA).
Mitochondrial membrane potential (MMP)
analysis
MMP detection was performed using the JC-1 staining
assay kit according to the manufacturer's instructions. Briefly,
following treatment with 3-BrPA, the cells were incubated with 10
µM JC-1 for 30 min at 37°C in the dark. Then, the
fluorescence in the cells was detected using a fluorescence
microscope (Olympus 1×71).
Detection of superoxide anion levels
The HNE1 and CNE-2Z cells were seeded at a density
of 2×105 cells/well in a 6-well plate and labeled with 5
µM DHE. This was immediately followed by treatment with
3-BrPA (160 or 320 µM) for the indicated times and then the
cells were collected, washed and analyzed using
fluorescence-activated cell sorting (FACS).
Western blot analysis
The cells were rinsed with ice-cold PBS and lysed in
radioimmunoprecipitation assay (RIPA) buffer for 30 min on ice,
after which the lysates were centrifuged at 12,000 × g for 30 min
at 4°C. The proteins were then separated on a 15% sodium dodecyl
sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) gel and
subsequently transferred to a nitrocellulose membrane (Bio-Rad,
Berkeley, CA, USA). The membranes were incubated with the
appropriate primary antibodies overnight at 4°C and then further
incubated with the corresponding secondary antibodies. β-actin was
used as the loading control.
In vivo tumor experiment
The nude mice (3- to 4-weeks old) weighed 18–21 g at
the time of tumor implantation. The mice were kept under a 12/12-h
light/dark cycle at 24±2°C and were given standard food and clean
water. The mice were inoculated with the human CNE-2Z cells
(8×106 cells/ml) subcutaneously to induce tumor
formation. Fifteen mice that developed tumors (100–200
mm3) were randomly assigned to three groups (5
mice/group). On day 7 after inoculation, the groups began to be
treated intraperitoneally with vehicle [0.9% normal saline, (NS)],
3-BrPA (8 mg·kg−1) or cisplatin (DDP, 3
mg·kg−1) treatment every 4 days for 28 days. Tumor
growth was monitored every 4 days by obtaining two-dimensional
measurements of the individual tumors of each mouse. The tumor
volume was calculated using the formula: length x
width2/2. After the treatment ended, all the mice were
euthanized and the tumors were excised. The tumor growth inhibition
rate was calculated using the following formula: tumor inhibition
rate = (1 - treatment group tumor weight/control group tumor
weight) × 100%. The tumors were then fixed in 4% formalin solution,
embedded in paraffin and stained with hematoxylin and eosin
(H&E).
Statistical analysis
Data are expressed as the mean ± SEM of three
experiments. SPSS v.16.0 software (SPSS Inc., Chicago, IL, USA) was
used for data analysis. The statistical analyses were carried out
using one-way analysis of variance (ANOVA). P<0.05 and P<0.01
indicated values that were significantly different from the control
values.
Results
Inhibition of NPC cell proliferation by
varying concentrations of 3-BrPA
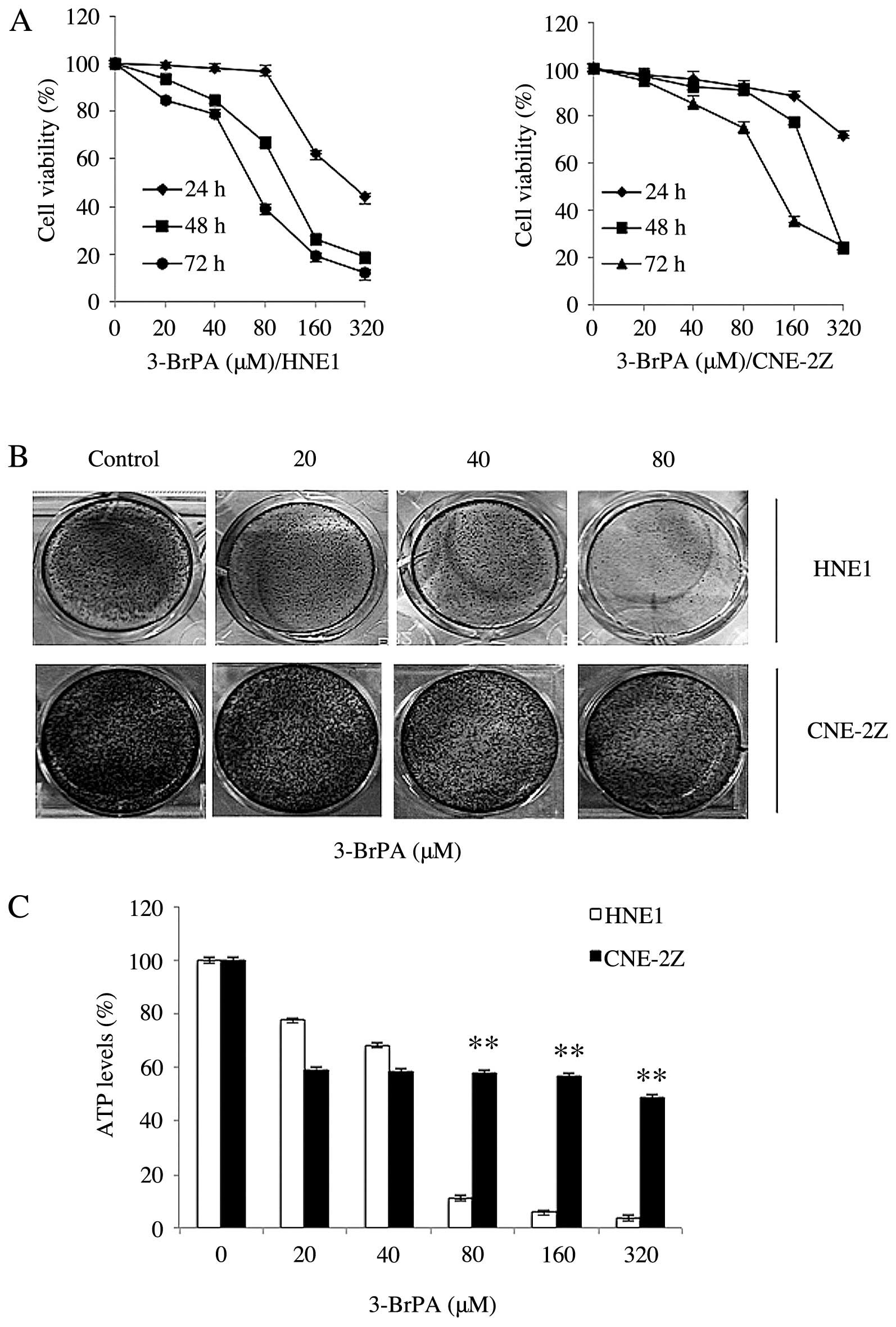
The effects of different concentrations of 3-BrPA
were evaluated on the cell proliferation of two NPC cell lines
treated for 24, 48 and 72 h. We found that 3-BrPA inhibited
proliferation and induced the apoptosis of HNE1 and CNE-2Z cells
(Fig. 1A and B). The results
demonstrated that the rate of apoptosis had a direct ratio
relationship with the dose-response curve. Next, we determined the
effects of 3-BrPA on the colony formation of the HNE1 and CNE-2Z
cells by evaluating their 5-day survival rate in different
concentrations of 3-BrPA. The results showed that the
colony-forming ability of the cells was clearly reduced by 3-BrPA
(Fig. 1B). The results show that
the NPC cells with clonogenic capacity overcame the effects of
glycolysis inhibition.
The role of glycolysis in ATP production in the HNE1
and CNE-2Z cells was evaluated by measuring the intracellular
levels of ATP following treatment with the potent glycolysis
inhibitor 3-BrPA for 5 h. We found that the concentration of ATP
decreased with increasing concentrations of 3-BrPA in both cell
lines. However, the downward trend was more pronounced in the HNE1
cells. The results showed that 3-BrPA inhibits ATP production in
the NPC cells (Fig. 1C).
3-BrPA induces apoptosis in NPC
cells
In the MTT assay, we treated HNE1 and CNE-2Z cells
with three different concentrations of 3-BrPA for 24 h and then
used PI staining and flow cytometry to distinguish the apoptotic
cells. The results showed that 3-BrPA induced apoptosis in the
human HNE1 and CNE-2Z cells and the rate of apoptosis induction
increased in a concentration-dependent manner (Fig. 2A). Many alkylating agents used to
treat tumors act by inducing apoptosis (18) and therefore, we sought to determine
whether 3-BrPA inhibited NPC cell proliferation by inducing
apoptosis. The CNE-2Z and HNE1 cells were treated with different
concentrations of 3-BrPA for 24 h and DAPI staining was used to
detect apoptotic cells. The results showed that the 3-BrPA-treated
HNE1 and CNE-2Z cells exhibited apoptotic characteristics of
chromatin condensation with typical apoptotic bodies and the number
of apoptotic nuclei was significantly increased in these groups
(Fig. 2B).
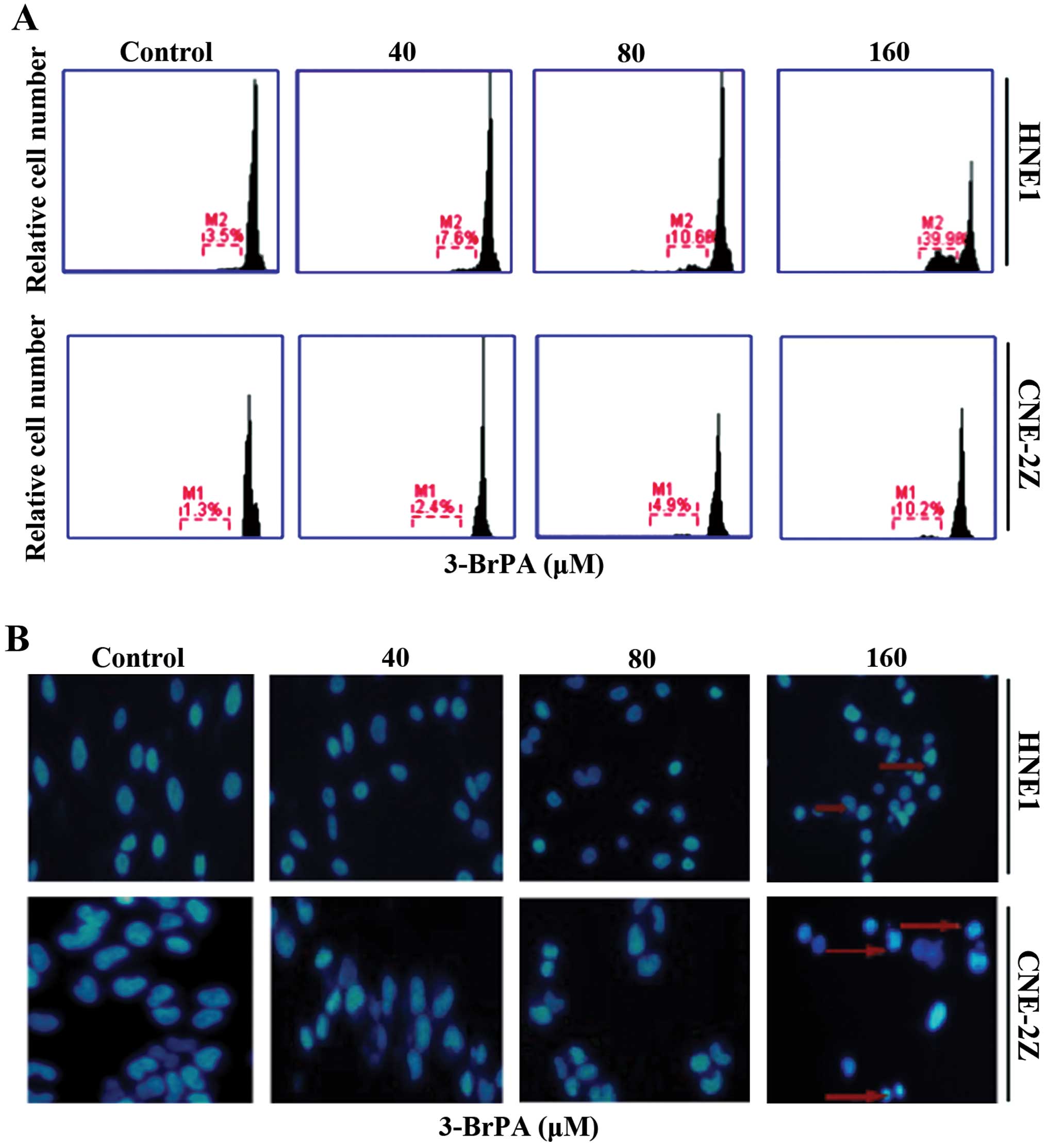 | Figure 23-BrPA induces cell death in HNE1
cells. (A) HNE1 cells were treated with medium (Ctrl) and 40, 80
and 160 µM 3-BrPA for 24 h. Cell death was measured by the
PI staining method using flow cytometry. (B) NPC cells were treated
with 0, 40, 80, and 160 µM 3-BrPA for 24 h, subjected to
DAPI staining (showing nucleus) and then visualized under a
fluorescence microscope. 3-BrPA, 3-bromopyruvate; PI, propidium
iodide; NPC, nasopharyngeal carcinoma; DAPI,
4′,6-diamidino-2-phenylindole. |
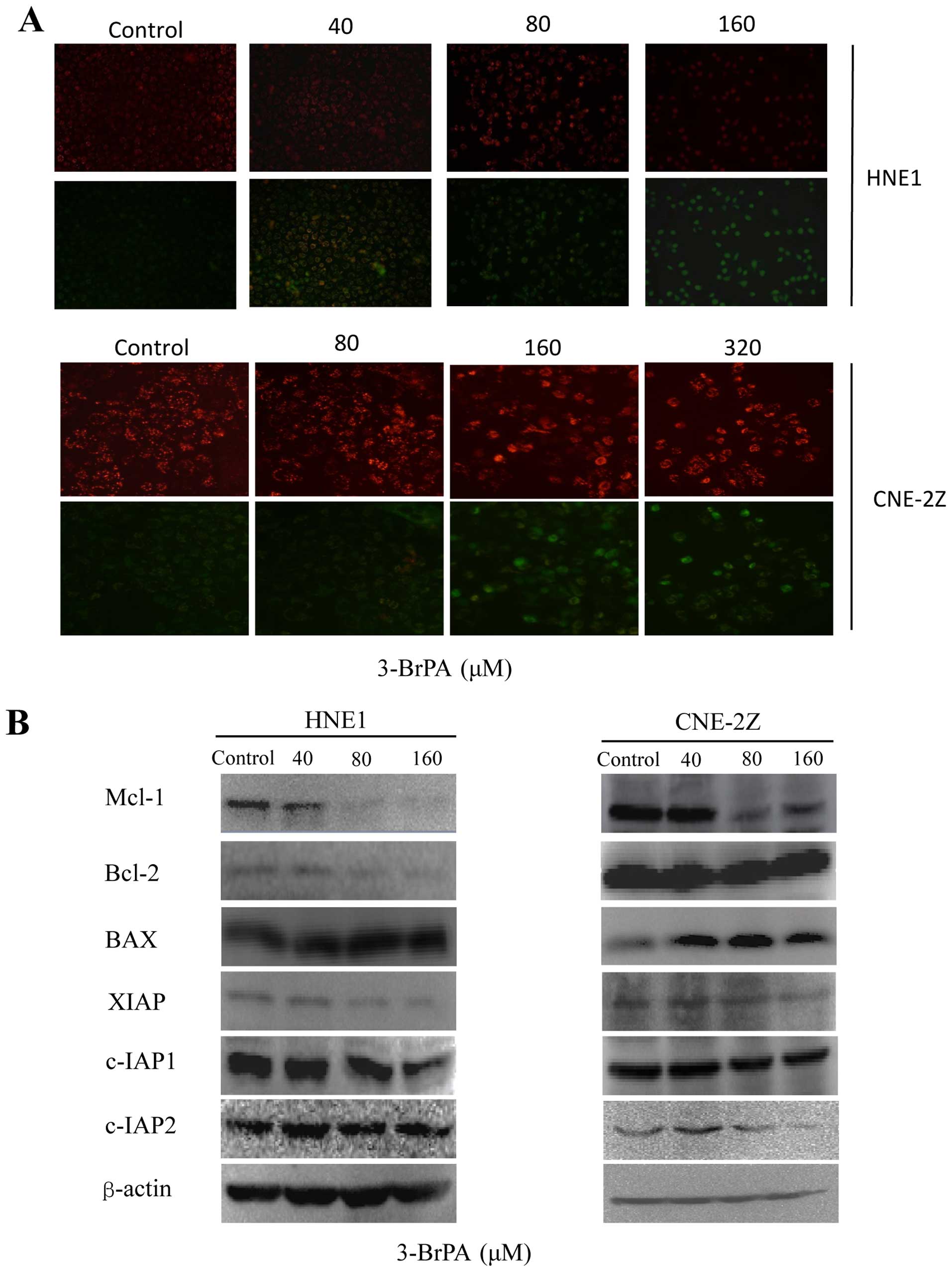
3-BrPA reduces MMP and protein expression
in NPC cells
JC-1 is a carbocyanine fluorescent probe widely used
to detect MMP fluorescence, which we used to explore the mechanisms
by which 3-BrPA induced apoptotic cell death in HNE1 and CNE-2Z
cells. The normal mitochondria of the untreated control cells were
characterized by red fluorescence while the depolarized
mitochondria of the 3-BrPA-treated cells showed
concentration-dependent, characteristic diffused green
fluorescence. This result indicates that 3-BrPA increases
mitochondrial membrane permeability (Fig. 3A). Although apoptosis may be induced
by death receptors, the mitochondrial pathway is the major mediator
of apoptosis and the induction of major outer-membrane proteins is
essential for the initiation of this process. We sought to
determine the mechanisms by which 3-BrPA induced apoptosis by
measuring its effects on the expression of major outer-membrane
proteins by using western blot analysis. The results showed that
3-BrPA inhibited the expression of the antiapoptotic proteins Mcl-1
and Bcl-2 in a dose-dependent manner and also the expression of the
pro-apoptotic protein Bax. These results suggest that 3-BrPA may
regulate the expression of apoptosis-related proteins. The
expression of the inhibitors of apoptosis proteins (IAPs) including
c-IAP1, c-IAP2, and XIAP was decreased by 3-BrPA in a
dose-dependent manner, which also promoted the apoptosis of NPC
cells (Fig. 3B).
Reactive oxygen species (ROS) production
is involved in 3-BrPA-induced cell death
Next, we investigated the role of mitochondrial ROS
activity in 3-BrPA-induced necroptosis. We examined the effects of
3-BrPA on intracellular ROS production by using DHE, a fluorescent
probe. As shown in Fig. 4A, cells
treated with 3-BrPA showed a higher increase in the fluorescence
intensity than did the control group (P<0.05). In addition, the
fluorescence intensity of the cells treated with the antioxidant
N-acetyl-L-cysteine (NAC, 5 mM) alone was reduced more than that of
the cells treated with 3-BrPA alone. Furthermore, the DHE assay
revealed high levels of green fluorescence in cells co-treated with
3-BrPA and NAC, while the addition of more NAC darkened the cells
again (Fig. 4A). In addition, we
discovered that 3-BrPA increased the ROS levels in a time-dependent
manner within 6 h (Fig. 4B). These
results showed that NAC (5 mM) completely blocked the effect of
3-BrPA and reduced the intracellular ROS levels (Fig. 4B). Finally, the viability of the NPC
cells treated with 160 or 320 µM 3-BrPA was decreased
significantly, and this effect was blocked by 5 mM NAC (Fig. 4C).
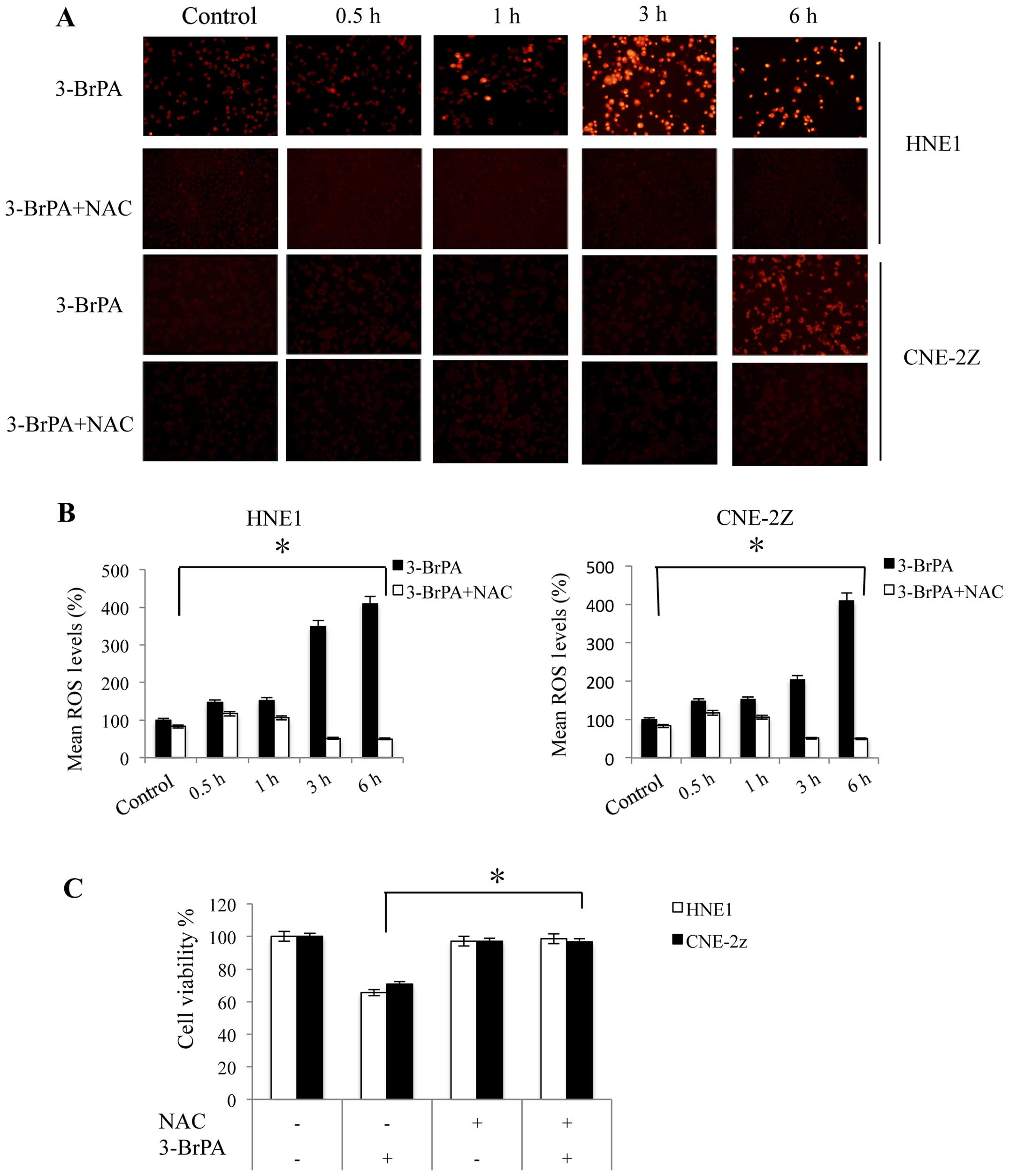 | Figure 4ROS production contributes to
3-BrPA-induced cell death in NPC cells. (A) HNE1 and CNE-2Z cells
were treated with 160 and 320 µM 3-BrPA, respectively, with
or without 5 mM NAC for 0.5, 1, 3 and 6 h. Cells were photographed
using a fluorescence microscope camera. (B) After pre-incubation
with 5 mM NAC for 1 h, cells were treated as above. ROS production
was analyzed using DHE staining and flow cytometry. Mean ROS (100%)
values are shown. *P<0.05 vs. the control at a given
time-point. (C) After pre-incubation with 5 mM NAC for 1 h, HNE1
and CNE-2Z cells were treated with 160 and 320 µM 3-BrPA,
respectively, for 24 h and the relative cell viability was assessed
using the MTT assay. *P<0.05. Experiments were
performed in triplicate. ROS, reactive oxygen species; 3-BrPA,
3-bromopyruvate; NPC, nasopharyngeal carcinoma; NAC,
N-acetyl-L-cysteine; DHE, dihydroethidium. |
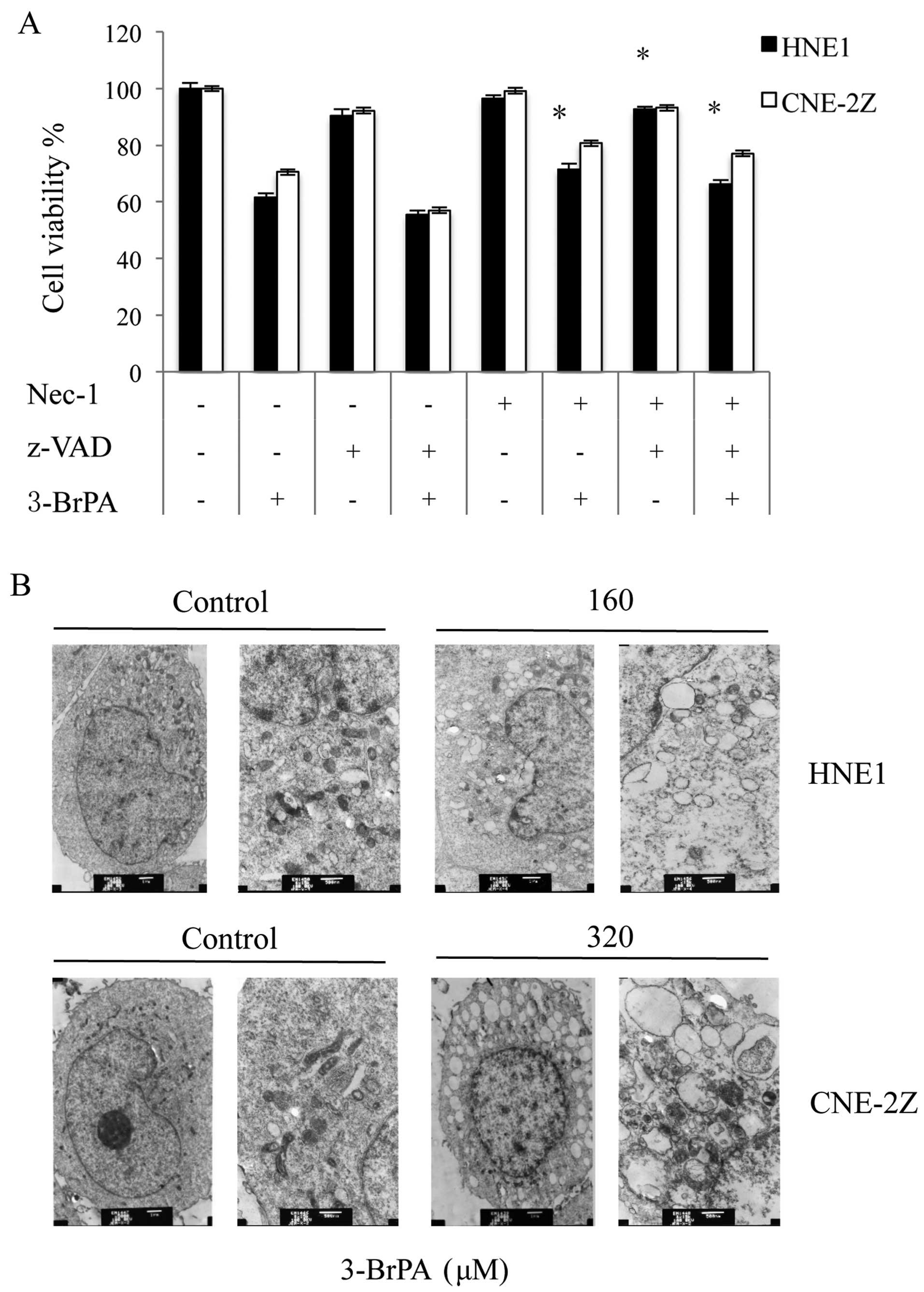
3-BrPA induces cell death in NPC cells by
necroptosis
To confirm the type of cell death induced in HNE1
and CNE-2Z cells by 3-BrPA, we evaluated the inhibitory effects of
z-VAD-fmk, a broad-spectrum caspase inhibitor, on 3-BrPA. The MTT
assay (Fig. 5A) showed that
z-VAD-fmk aggravated cell death in the HNE1 and CNE-2Z cells. The
cell death observed was possibly mediated by necroptosis, the
programmed form of necrosis, which involves RIP1 and RIP3
triggering the death receptors (19). Nec-1 alone had no effect on both
HNE1 and CNE-2Z cells, but radically restored cell survival of
3-BrPA-treated cells (Fig. 5A).
Electron microscopy (EM) showed that the 3-BrPA-treated HNE1 and
CNE-2Z cells had ruptured plasma membranes, which is indicative of
necrosis (Fig. 5B).
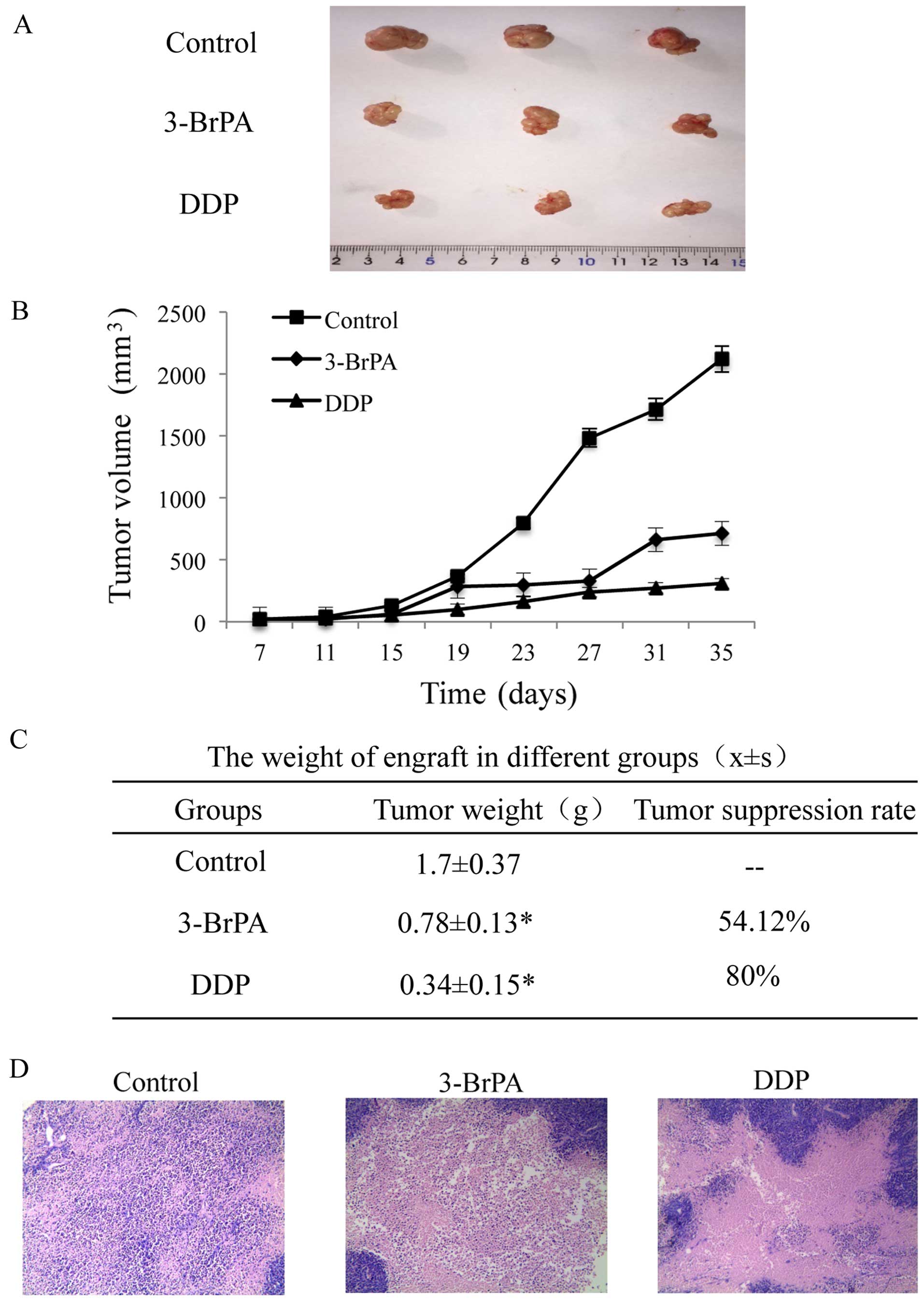
In vivo antitumor efficacy of 3-BrPA
We investigated the in vivo antitumor
activity of 3-BrPA using tumor-bearing nude mice. On day 7 after
inoculation, mice bearing CNE-2Z tumors were intraperitoneally
administered 3-BrPA (8 mg·kg−1) and DDP (3
mg·kg−1) treatment every 4 for 28 days. The tumor
volumes of the treated mice increased at a much slower rate than
those of the control group (Fig. 6A and
B). The tumors were completely excised 35 days after the cancer
cell inoculation. The tumor weight and growth inhibition rate for
the three groups are shown in Fig.
6C. The transplanted tumor tissue sections from all three
groups showed poorly differentiated squamous cancer cells with
varying degrees of pathological mitosis and eosinophilic necrosis.
The DDP and 3-BrPA-treated CNE-2Z tumors showed a consistent size
and shape, and the cell size was smaller than that of the untreated
control cells. These tumors also showed a decreased
nuclear-cytoplasmic ratio. In addition, the number of nucleoli,
pathological mitosis and number of vessels were lower in the
treated tumors than these in the tumors in the control group, while
fibrous tissue increased owing to eosinophilic necrosis (Fig. 6D).
Discussion
There are currently several treatment options for
NPC, including radiotherapy, chemotherapy, surgery and
chemotherapy. However, none of them provides an ideal or optimal
therapeutic effect. The commonly used chemotherapeutic drugs are
highly toxic and susceptible to drug resistance. Thus, strategies
for reducing the toxic side effects of the drugs while
simultaneously improving drug efficacy are an important aspect of
anticancer drug development (20).
3-BrPA has recently become a popular drug among
research scholars worldwide (21).
This drug is a glycolytic inhibitor that acts via hexokinase II and
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and plays a
critical role in apoptosis induced by intracellular ATP depletion.
At the same time, it also inhibits succinate dehydrogenase (SDH)
activity, which causes an imbalance in the cellular redox state.
The additional inhibition of the hexokinase II pentose phosphate
pathway leads to a decrease in the activity of the NADPH-generating
pentose phosphate pathway (PPP), ultimately reducing the equivalent
intracellular NADPH levels and further decreasing the peroxide
state. Therefore, 3-BrPA lowers the levels of intracellular ATP and
induces an imbalance in the intracellular oxidative metabolism,
with good antitumor effects (22,23).
Our results showed that 3-BrPA inhibited NPC HNE1 and CNE-2Z cell
proliferation, triggered apoptosis, reduced ATP production and
increased intracellular ROS levels.
Bcl-2 and Bax exist as either homologous dimers or
heterodimers and form an apoptosis regulatory system in combination
with the B-cell lymphoma-extra-large (Bcl-xL) protein. The
formation of Bax homologous dimers induces apoptosis while Bcl-2
protein expression causes the separation of these dimers, thereby
increasing the Bcl-2 to Bax-Bax ratio. This increase in Bcl-2
facilitates the creation of the more stable Bax-Bcl-2 heterodimer,
thereby 'neutralizing' the Bax-Bax dimer-induced apoptosis. Thus,
the Bcl-2 and Bax proportion controls apoptosis (24,25).
Our results revealed a decrease in the expression of Bcl-2 in HNE-1
cells with an increase in Bax expression. However, in the CNE-2Z
cells, the Bcl-2 protein expression remained essentially unchanged
while BAX expression increased. Members of the Bcl-2 protein family
can induce imbalances in the endogenous pathway regulating
apoptosis (26). The IAP-induced
direct inhibition of caspases or pro-caspases mainly suppresses key
members of the caspase family. Thus, IAPs such as c-IAP1, c-IAP2,
XIAP, livin and survivin proteins directly inhibit caspases 3, 7
and 9. The IAPs possess a highly conserved BIR and RING domain
structure, which interacts with the protease caspases and plays an
antiapoptotic role by inhibiting caspase activity (25).
Many studies have shown that ROS such as peroxide
induce apoptosis in many different cell types including neutrophils
(gas apoptosis) and this process is mediated by catalase inhibition
(27). The mercapto group donor in
NAC confers antioxidant properties by clearing the generated free
groups, thereby regulating cellular metabolic activity (28,29).
In contrast, 3-BrPA facilitates an increase in intracellular ROS
levels and has been widely used in clinical studies because of this
activity. The actions of NAC and 3-BrPA render them highly useful
joint pharmacological tools. While NAC protects the cells, 3-BrPA
enhances the intracellular ROS content, which induces apoptosis by
regulating cell death. The resultant apoptotic cell death model is
well known and widely used.
The pathway of essential cell death is invoked by
various cellular stresses, including DNA damage, oxidative stress,
growth factor deprivation, heat shock and endoplasmic reticulum
stress (30). With the passage of
time, experimental advances and discoveries, researchers are now
more interested in the concept of necrotic cell death. Necrotic
death generally occurs as a result of high-dose cytotoxic abuse and
does not require any specific molecular programming. However, it is
now recognized that mechanisms other than those mediated by caspase
induce cellular suppression through a caspase-independent pathway
of cell death that exhibits the morphological characteristics of
necrosis. This form of regulated cell death was recently termed
'necrotizing apoptosis' or 'necroptosis' (14), because it shares features of both
apoptosis and necrosis. As in the case of apoptosis, necroptosis
involves an explicit molecular cascade. However, both necroptosis
and necrosis are characterized by swelling of the cell and its
organelles and cell rupture, which leads to the release of cellular
contents (31). One example of
necroptosis can be observed in HT-29 cells, wherein z-VAD-fmk
accelerates Smac analog-induced cell death, which is inhibited by
Nec-1, the necrosis-specific chemical inhibitor (32). We also discovered that Nec-1
protects HNE1 and CNE-2Z cells against the acceleration of
3-BrPA-induced cell death via z-VAD-fmk. However, additional
experiments are required to confirm how 3-BrPA induces necroptosis
in HNE1 and CNE-2Z cells treated with caspase inhibitors.
Necroptosis and apoptosis are two different forms of death
receptor-mediated cell death. These processes are
caspase-independent and do not require mitochondrial release of
cytochrome c, but are controlled by a signal-regulated
process mediated by RIP1/RIP3. Inhibition of caspase activity in
cells will inhibit apoptosis but not necrosis. Nec-1 acts as a
specific inhibitor of programmed necrosis by inhibiting the
formation of the RIP1-RIP3 complex; therefore, the inhibition of
RIP1 interferes with necroptosis (33).
In conclusion, the results of the present study
showed that 3-BrPA significantly inhibits the proliferation of
human NPC HNE1 and CNE-2Z cells and induces apoptosis by increasing
ROS levels. In addition, the mechanism of action of 3-BrPA may be
mediated via the mitochondrial pathway in cells and the
death-receptor pathway. These results suggest that 3-BrPA may be a
potential candidate for development as a new anticancer drug.
However, the instability of 3-BrPA in solution poses a serious
challenge to developing a safe and stable potent inhibitor of
hexokinase, which is certain to become an important research
topic.
Acknowledgments
The present study was supported by grants from the
National Natural Science Foundation of China (81000992 and
81372899), the Natural Science Foundation of the Anhui Province
(1508085MH166), the Graduate Scientific Research and Innovation
Projects of Bengbu Medical College of Anhui Province (Byycx1421)
and the Science Foundation of Bengbu Medical College
(Byky1447).
References
|
1
|
Hildesheim A and Levine PH: Etiology of
nasopharyngeal carcinoma: A review. Epidemiol Rev. 15:466–485.
1993.PubMed/NCBI
|
|
2
|
Geschwind JF, Ko YH, Torbenson MS, Magee C
and Pedersen PL: Novel therapy for liver cancer: Direct
intraarterial injection of a potent inhibitor of ATP production.
Cancer Res. 62:3909–3913. 2002.PubMed/NCBI
|
|
3
|
Warburg O: On the origin of cancer cells.
Science. 123:309–314. 1956. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nelson K: 3-Bromopyruvate kills cancer
cells in animals. Lancet Oncol. 3:5242002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Finley RS: Overview of targeted therapies
for cancer. Am J Health Syst Pharm. 60:S4–S10. 2003.
|
|
6
|
Mathupala SP, Ko YH and Pedersen PL:
Hexokinase-2 bound to mitochondria: Cancer's stygian link to the
'Warburg Effect̓ and a pivotal target for effective therapy. Semin
Cancer Biol. 19:17–24. 2009. View Article : Google Scholar :
|
|
7
|
Buchakjian MR and Kornbluth S: The engine
driving the ship: Metabolic steering of cell proliferation and
death. Nat Rev Mol Cell Biol. 11:715–727. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kondoh H: Cellular life span and the
Warburg effect. Exp Cell Res. 314:1923–1928. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nakano A, Tsuji D, Miki H, Cui Q, El Sayed
SM, Ikegame A, Oda A, Amou H, Nakamura S, Harada T, et al:
Glycolysis inhibition inactivates ABC transporters to restore drug
sensitivity in malignant cells. PLoS One. 6:e272222011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dolmans DE, Kadambi A, Hill JS, Flores KR,
Gerber JN, Walker JP, Borel Rinkes IH, Jain RK and Fukumura D:
Targeting tumor vasculature and cancer cells in orthotopic breast
tumor by fractionated photosensitizer dosing photodynamic therapy.
Cancer Res. 62:4289–4294. 2002.PubMed/NCBI
|
|
11
|
Peter ME: Programmed cell death: Apoptosis
meets necrosis. Nature. 471:310–312. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Goossens V, Stange G, Moens K, Pipeleers D
and Grooten J: Regulation of tumor necrosis factor-induced,
mitochondria- and reactive oxygen species-dependent cell death by
the electron flux through the electron transport chain complex I.
Antioxid Redox Signal. 1:285–295. 1999. View Article : Google Scholar
|
|
13
|
Trachootham D, Lu W, Ogasawara MA, Nilsa
RD and Huang P: Redox regulation of cell survival. Antioxid Redox
Signal. 10:1343–1374. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Degterev A, Huang Z, Boyce M, Li Y, Jagtap
P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA and Yuan J:
Chemical inhibitor of nonapoptotic cell death with therapeutic
potential for ischemic brain injury. Nat Chem Biol. 1:112–119.
2005. View Article : Google Scholar
|
|
15
|
Declercq W, Vanden Berghe T and
Vandenabeele P: RIP kinases at the crossroads of cell death and
survival. Cell. 138:229–232. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Degterev A, Zhou W, Maki JL and Yuan J:
Assays for necroptosis and activity of RIP kinases. Methods
Enzymol. 545:1–33. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Humphries F, Yang S, Wang B and Moynagh
PN: RIP kinases: Key decision makers in cell death and innate
immunity. Cell Death Differ. 22:225–236. 2015. View Article : Google Scholar
|
|
18
|
Parlato M, Souza-Fonseca-Guimaraes F,
Philippart F, Misset B, Adib-Conquy M and Cavaillon JM; Captain
Study Group: CD24-triggered caspase-dependent apoptosis via
mitochondrial membrane depolarization and reactive oxygen species
production of human neutrophils is impaired in sepsis. J Immunol.
192:2449–2459. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Galluzzi L and Kroemer G: Necroptosis: A
specialized pathway of programmed necrosis. Cell. 135:1161–1163.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ahmad S and Ansari AA: Therapeutic roles
of heparin anticoagulants in cancer and related disorders. Med
Chem. 7:504–517. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shoshan MC: 3-Bromopyruvate: Targets and
outcomes. J Bioenerg Biomembr. 44:7–15. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cardaci S, Desideri E and Ciriolo MR:
Targeting aerobic glycolysis: 3-bromopyruvate as a promising
anticancer drug. J Bioenerg Biomembr. 44:17–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dell'Antone P: Targets of 3-bromopyruvate,
a new, energy depleting, anticancer agent. Med Chem. 5:491–496.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Scarfò L and Ghia P: Reprogramming cell
death: BCL2 family inhibition in hematological malignancies.
Immunol Lett. 155:36–39. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ndubaku C, Varfolomeev E, Wang L, Zobel K,
Lau K, Elliott LO, Maurer B, Fedorova AV, Dynek JN, Koehler M, et
al: Antagonism of c-IAP and XIAP proteins is required for efficient
induction of cell death by small-molecule IAP antagonists. ACS Chem
Biol. 4:557–566. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rossé T, Olivier R, Monney L, Rager M,
Conus S, Fellay I, Jansen B and Borner C: Bcl-2 prolongs cell
survival after Bax-induced release of cytochrome c. Nature.
391:496–499. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kasahara Y, Iwai K, Yachie A, Ohta K,
Konno A, Seki H, Miyawaki T and Taniguchi N: Involvement of
reactive oxygen intermediates in spontaneous and CD95
(Fas/APO-1)-mediated apoptosis of neutrophils. Blood. 89:1748–1753.
1997.PubMed/NCBI
|
|
28
|
De Flora S, Izzotti A, D'Agostini F and
Balansky RM: Mechanisms of N-acetylcysteine in the prevention of
DNA damage and cancer, with special reference to smoking-related
end-points. Carcinogenesis. 22:999–1013. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang CC, Liu TY, Cheng CH and Jan TR:
Involvement of the mitochondrion-dependent pathway and oxidative
stress in the apoptosis of murine splenocytes induced by areca nut
extract. Toxicol In Vitro. 23:840–847. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
de Almagro MC and Vucic D: Necroptosis:
Pathway diversity and characteristics. Semin Cell Dev Biol.
39:56–62. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kaczmarek A, Vandenabeele P and Krysko DV:
Necroptosis: The release of damage-associated molecular patterns
and its physiological relevance. Immunity. 38:209–223. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
He S, Wang L, Miao L, Wang T, Du F, Zhao L
and Wang X: Receptor interacting protein kinase-3 determines
cellular necrotic response to TNF-alpha. Cell. 137:1100–1111. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xu X, Chua CC, Kong J, Kostrzewa RM,
Kumaraguru U, Hamdy RC and Chua BH: Necrostatin-1 protects against
glutamate-induced glutathione depletion and caspase-independent
cell death in HT-22 cells. J Neurochem. 103:2004–2014. 2007.
View Article : Google Scholar : PubMed/NCBI
|