Introduction
Osteosarcoma is the most common primary bone cancer
in adolescents and young adults (1). Although treatment modalities have been
improved over the past decades, the survival rate of these patients
has limited improvement and the 3–5 years of survival rate after
amputation is only 5–20% (2,3).
Therefore, it is particularly important to understand the
pathogenesis of osteosarcoma in order to develop novel treatment
strategies.
Nephroblastoma overexpressed (NOV) gene maps
on chromosome 8q24.1 and was originally identified as aberrantly
expressed in avian nephroblastoma induced by
myeloblastosis-associated virus (4). NOV belongs to the CCN family of genes,
which comprises five additional members: cystein-rich protein 61
(Cyr61), connective tissue growth factor (CTGF), Wnt-1-induced
secreted protein (WISP)-1, WISP-2 and WISP-3 (5). The CCN genes encode secreted
proteins of 35–48 kDa associated with the extracellular matrix
(ECM) and cell membrane, which are involved in the regulation of
various cell functions such as proliferation, differentiation,
migration, attachment, angiogenesis and tumorigenesis (6). These genes are expressed in all
derivatives of the three embryonic sheets and are involved in the
development of kidney, nervous system, muscle, bone marrow,
cartilage and bone (7).
Unlike other CCN family members Cyr61 and CTGF, NOV
has been less studied and the functions of NOV protein among
different tissues are inconsistent and sometimes controversial. NOV
was originally described as antiproliferative (8) and its expression was associated with
differentiation and growth arrest in Wilm's tumor (9), rhabdomyosarcomas (10), cartilaginous tumors (11), renal cell carcinoma (12) and Ewing sarcoma (13), while more recent data associate NOV
expression with increased proliferative index of 3T3 fibroblast
(14,15) and tissue samples of prostate.
Furthermore, although NOV reduced the growth rate of renal cell
carcinoma and Ewing sarcoma transfectants in vitro, NOV
expression was associated with poor prognosis and shown to increase
cell motility, resulting in enhanced metastatic potential in these
tumors (12,13). It has been shown that in
osteosarcoma NOV is inversely associated with the expression of
liver/bone/kidney alkaline phosphatase isoform (16), an early marker of osteoblastic
differentiation, and has prognostic value of in osteosarcoma
(17). Nevertheless, the effect of
NOV on osteosarcoma cell biological behaviors and the underlying
molecular mechanisms remain unclear.
P38/MAPK and JNK/MAPK signaling pathways, which have
been confirmed to be tightly associated with tumor cell apoptosis
(18,19), including osteosarcoma (20,21),
are two important intracellular signaling pathways. Certain studies
have suggested that NOV was able to induce cell apoptosis or growth
inhibition in various types of cancer through the activation of
MAPKs signaling, such as choriocarcinoma, nephroblastoma and Ewing
sarcoma (13). Howerover, whether
p38/MPAK and JNK/MAPK signaling pathway activation is involved in
the NOV-induced effects of osteosarcoma remains to be
elucidated.
Based on the abovementioned studies, we aimed to
discuss the effects of recombinant adenovirus (22,23)-mediated NOV overexpression or
silencing on osteosarcoma cell lines, as well as to investigate the
probable molecular mechanisms underlying these effects. Our studies
found that NOV inhibited the proliferation while promoting the
apoptosis and migration of osteosarcoma cell lines in vitro
and the activation of p38/MAPK and JNK/MAPK signal may be involved
in these processes. These results offer a new approach for the
treatment of osteosarcoma.
Materials and methods
Cell culture and reagents
The 143B, U2OS, MG-63 and SaOS2 human osteosarcoma
cell lines were purchased from the American Type Culture Collection
(ATCC, Manassas, VA, USA). The cells were maintained in Dulbecco's
modified Eagle's medium (DMEM; Utah, HyClone, UT, USA) supplemented
with 10% fetal bovine serum (FBS; Gibco Life Technologies,
Carlsbad, CA, USA) and 100 U/ml streptomycin/penicillin at 37°C in
5% CO2. Recombinant adenovirus expressing green
fluorescent protein (AdGFP), red fluorescent protein (AdRFP), NOV
(AdNOV) with GFP and recombinant adenovirus expressing siRNA
targeted NOV (AdsiNOV) with RFP were donated by professor T.-C. He,
University of Chicago Medical Center. 3-(4,5)-Dimethylthiazol(-z-yl)-3,5-diphenyltetrazolium
bromide (MTT) and dimethyl sulphoxide (DMSO) were obtained from
Solarbio Biology (Beijing, China). TRIzol reagent was purchased
from Invitrogen-Life Technologies (Carlsbad, CA, USA). Reverse
transcription-polymerase chain reaction (RT-PCR) reagents were
purchased from Takara (Otsu, Japan). Reverse
transcription-quantitative PCR (RT-qPCR) reagents were purchased
from Thermo Fisher Scientific, (Waltham, MA, USA). Hoechst 33258
and western blot detection reagents were purchased from the
Beyotime Institute of Biotechnology (Jiangsu, China). Rabbit
anti-NOV monoclonal antibody was purchased from Abcam (Abcam,
Cambridge, MA, USA). Mouse anti-Bax, anti-Bcl-2 monoclonal
antibodies were purchased from Santa Cruz Biothechnology, Inc.
(Santa Cruz, CA, USA). Rabbit anti-p38, anti-phosphor-p38,
anti-JNK, anti-phosphor-JNK polyclonal antibodies were purchased
from Immunoway (Immunoway, Newark, DE, USA USA). Mouse anti-β-actin
monoclonal antibody, and secondary antibodies including
HRP-conjugated goat anti-mouse IgG antibody and anti-rabbit IgG
antibody were purchased from Zhongshan Goldenbridge Biotechnology
(Beijing, China). BeyoECL was purchased from Millipore (Billerica,
MA, USA).
Adenovirus infection
Οsteosarcoma cells (143B) were infected with
recombinant adenovirus AdNOV and negative control AdGFP,
respectively. MG63 osteosarcoma cells were infected with AdsiNOV
and negative control AdRFP, respectively. After being infected for
8–12 h, the medium was replaced with serum-free or low serum DMEM
followed by continued cell culturing for subsequent
experiments.
Cell viability assay
Cell proliferation was analyzed with the MTT assay.
Briefly, the cells infected with different adenovirus or blank
control were incubated in 96-well plates at a density of
1×104 cells/well with DMEM supplemented with 10% FBS. At
the indicated time-points (24, 48, and 72 h), 20 µl of the
MTT reagent (5 mg/ml) was added to each well and the mixture was
incubated for another 4 0068. The MTT solution was then removed and
formazan was dissolved in DMSO for 10 min at room temperature and
the color reaction was measured at 492 nm with enzyme immunoassay
analyzer (Bio-Rad, Hercules, CA, USA). The experiment was repeated
three times and the proliferative activities were calculated for
each well.
Colony-forming assay
Osteosarcoma cell lines during the log growth stage
were seeded in 6-well culture plates (4×102 cells/well)
and treated with AdNOV/AdsiNOV, respectively. After incubation for
2 weeks, the cells were stained with crystal violet and clones were
counted. The colony-forming rate was obtained using the formula:
(colony number/seeded cell number) × 100%. The experiment was
repeated three times.
Cell cycle and apoptosis analysis
Cell cycle and apoptosis analysis were assessed by
FCM. Briefly, the cells were seeded in 6-well plates at a density
of 2×105 cells/well and treated with relevant adenovirus
for 48 h. Log-phase cells from each group were harvested by
centrifugation. After being washed twice with ice-cold PBS and
resuspended, an aliquot of samples were fixed with pre-cold 75%
enthanol at 4°C overnight. The remaining samples were added into
apoptosis analysis solution and then analyzed by a FACSVantage SE
flow cytometer (Becton-Dickinson, San Jose, CA, USA). Each
experiment was performed three times.
Hoechst 33258 staining assay
The Hoechst staining assay was performed according
to the manufacturer's instructions using the Hoechst 33258. The
cells seeded in 24-well plates were treated with adenovirus for 48
h and then washed twice with cold PBS. The cells were fixed with 4%
paraformaldehyde for 10 min and continued to be washed twice with
PBS. Two hundred mililiters of 1:1,000 Hoechst staining solution
was added to each well and the cells were incubated for 30 min at
room temperature in the dark. The cells were then washed twice with
PBS and viewed under a UV microscope. Each experiment was performed
three times.
Transwell chamber migration assay
Cell migration assay were performed by 24-well
Transwell chambers (8 µm pore size; Millipore). Briefly, the
cells (5×104/400 µl) treated with different
adenovirus in serum-free DMEM were seeded onto the upper chambers
and fresh DMEM (600 µl/well) containing 20% FBS was added to
the bottom chambers. After 24 h, cells that migrated to the
underside of the filter were fixed with methanol and stained with
crystal violet, and counted under brightfield microscopy. The
experiments were repeated three times.
RNA extraction, RT-PCR and RT-qPCR
analysis
Total RNA was extracted from osteosarcoma cells in
different groups using TRIzol reagent, according to the
manufacturer's instructions. First-strand DNA was synthesized using
the Reverse Transcriptase M-MLV (RNase H) kit with random hexamer
primers. Touchdown PCR analysis determining the gene expression
level was performed under the following conditions: 95°C × 5 min
for one cycle, 12 cycles at 95°C × 30 sec, 68°C × 30 sec (with a
decrease of 1 degree/cycle) and 72°C x 30 sec and 25 cycles at 95°C
x 30 sec, 55°C x 30 sec, and 72°C x 30 sec, 72°C x 10 min for 1
cycle. PCR products were separated by electrophoresis on a 2%
agarose gel. The expression level of mRNA were normalized to GAPDH.
RT-qPCR was run in the Rotor-Gene 6000 Real-Time PCR machine
(Corbett Research, Sydney, Australia) using SYBR Premix Ex Taq
(Thermo) with the following protocol: initial activation of HotStar
Taq DNA polymerase at 95°C for 10 min, then 45 cycles of 95°C for 5
sec and 60°C for 20 sec. GAPDH was used as an internal control. The
primer efficiencies were confirmed to be high (>90%) and
comparable (Table I). Data were
analyzed according to the 2−ΔΔCt method. The expression
level of mRNA were normalized to GAPDH. Three separate experiments
were performed for each group.
 | Table IThe primer sequences and their
product lengths in RT-PCR. |
Table I
The primer sequences and their
product lengths in RT-PCR.
| Gene | Primer
sequences | Length of product
(bp) |
|---|
| GAPDH | F: 5′-CAG CGA CAC
CCA CTC CTC-3′ | 120 |
| R: 5′-TGA GGT CCA
CCA CCC TGT-3′ | |
| NOV | F: 5′-ACC TTC CTG
CTT CTC CAT C-3′ | 490 |
| R: 5′-CTC CCA GTG
AAT CCT CCT C-3′ | |
| BAX | F: 5′-CCC TTT TGC
TTC AGG GTT TC-3′ | 150 |
| R: 5′-TGT TAC TGT
CCA GTT CGT CC-3′ | |
| BCL-2 | F: 5′-GAG ACA GCC
AGG AGA AAT CA-3′ | |
| R:
5′-CCTGTGGATGACTGAGTACC-3′ | 128 |
Western blot assay
Briefly, osteosarcoma cells with different
treatments were lysed with RIPA buffer, then centrifuged for 30 min
at 4°C and supernatants were collected. The protein concentration
was determined by the BCA assay. Cell extracts were boiled for 10
min in loading buffer and then an equal amount of cell extracts was
separated on 10% SDS-PAGE gels and transferred subsequently onto
PVDF membranes. After blocking with 5% BSA (Bovine Serum Albumin,
Solarbio) in TBST, the membranes were probed with the primary
antibody and incubated at 4°C overnight. Horseradish
peroxidase-conjugated secondary antibodies were added at a dilution
ratio of 1:5,000 and incubated at 37°C for 1 h. Protein levels were
quantified using the Supersignal West Pico Chemiluminescent
Substrate kit. Three separate experiments were performed for each
group.
Inhibition of p38 and JNK with specific
inhibitors and its effects on the NOV-induced antiproliferative
effect and apoptosis of 143B cells
The cells were treated with 10 µM SB203580
(p38 inhibitor) or 20 µM SP600125 (JNK inhibitor) for 30
min, followed by treatment with AdGFP or AdNOV for the indicated
time points. Following treatment for 48 h, the phosphorylation of
p38 and JNK was detected by western blot analysis. At the same
time, the expression of Bax and Bcl-2 was detected by western blot
analysis. The experiments were repeated three times.
Statistical analysis
Data are presented as the means ± standard deviation
(SD) from at least three independent experiments. One-way analysis
of variance (ANOVA) was used to analyze the differences between
groups and the LSD method of multiple comparisons was used when the
probability for ANOVA was statistically significant using GraphPad
Prism 5 and SPSS 17.0. Statistical significance was set at
P<0.05.
Results
The endogenous expression of NOV in
osteosarcoma cell lines
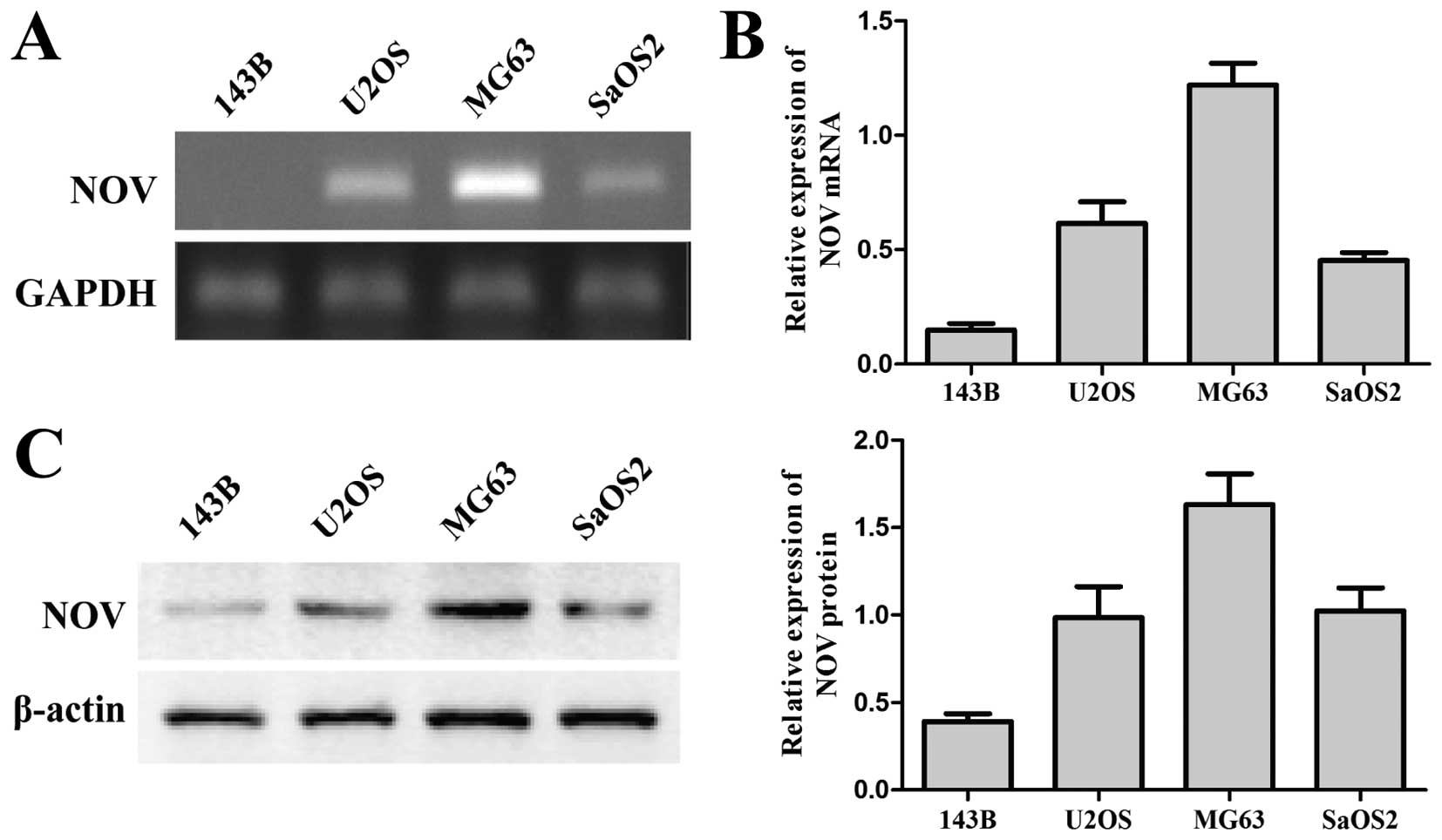
In the present study, the endogenous expression of
NOV in human 143B, U2OS, MG-63 and SaOS2 osteosarcoma cell lines
was evaluated using RT-PCR analysis (Fig. 1A). As shown in Fig. 1A, the mRNA expression level of NOV
in these osteosarcoma cell lines was different. The expression
level was significantly higher in MG63 cells although obviously
lower in 143B cells as compared to the remaining cells. The result
was confirmed by RT-qPCR (Fig. 1B)
and western blot assays (Fig. 1C).
Thus, we selected 143B and MG63 osteosarcoma cell lines for
infection with the AdNOV and AdsiNOV adenovirus, respectively.
NOV is successfully overexpressed in 143B
cells and interfered in MG63 cells
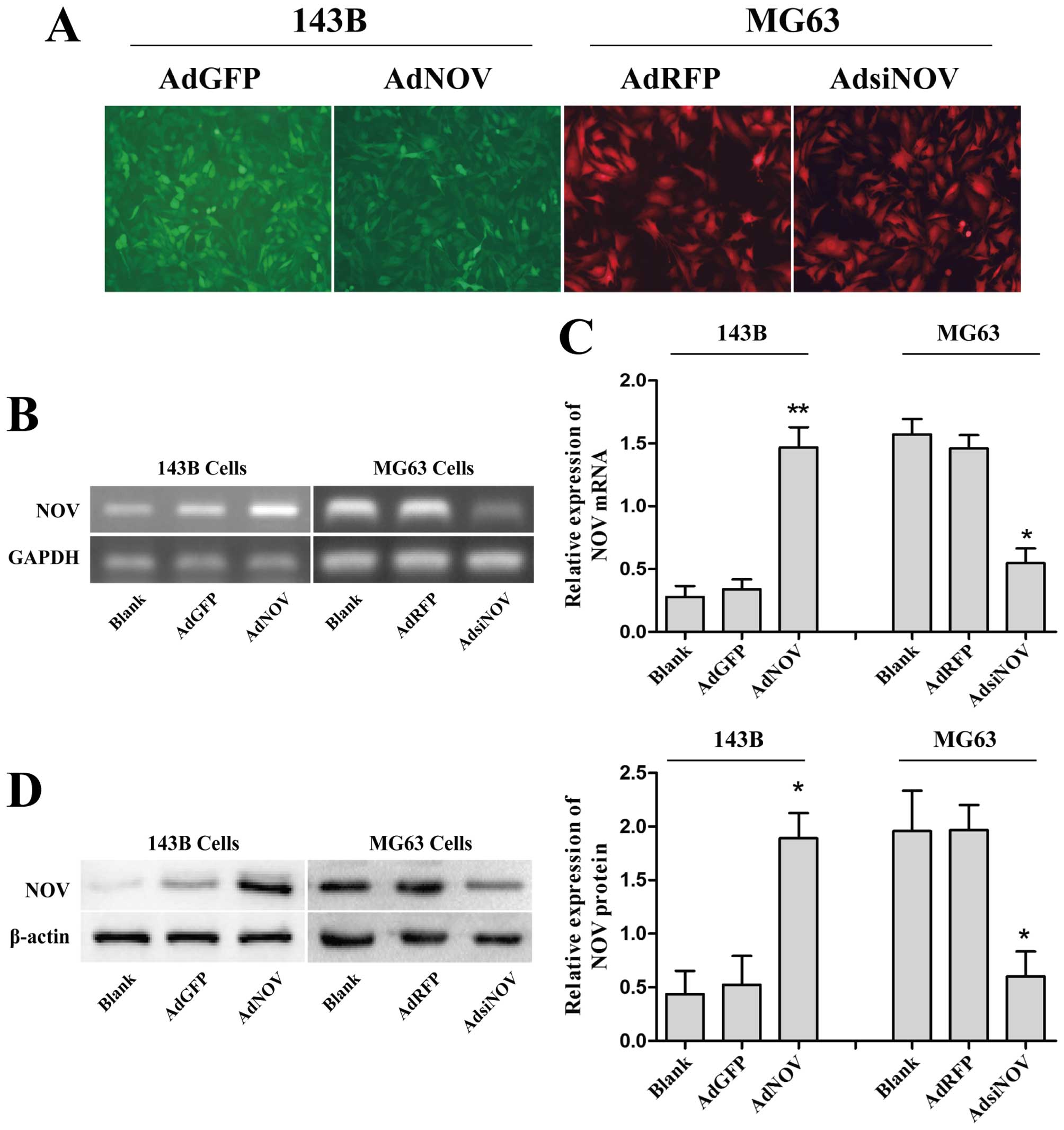
To identify the role of NOV in osteosarcoma cell
lines, recombinant adenovirus AdNOV and AdsiNOV were used to infect
143B and MG63 cell lines, respectively. In the present study, the
infection efficiency of AdNOV and AdsiNOV in 143B and MG63 cell
lines was >60% under fluorescence microscopy (Fig. 2A). In addition, RT-PCR (Fig. 2B), RT-qPCR (Fig. 2C) and western blot assays (Fig. 2D) were performed at 48 h recovery to
measure the expression level of NOV after adenovirus infection. An
obvious increase of NOV expression was observed in the AdNOV group
compared with the AdGFP and control groups (P<0.01 and
P<0.05) in 143B cells, while a marked decrease of NOV expression
was found in the AdsiNOV group compared with the AdRFP and control
groups in MG-63 cells (P<0.05) (Fig.
2B and C). These data indicated that NOV was successfully
overexpressed in 143B cells and interfered in MG63 cells.
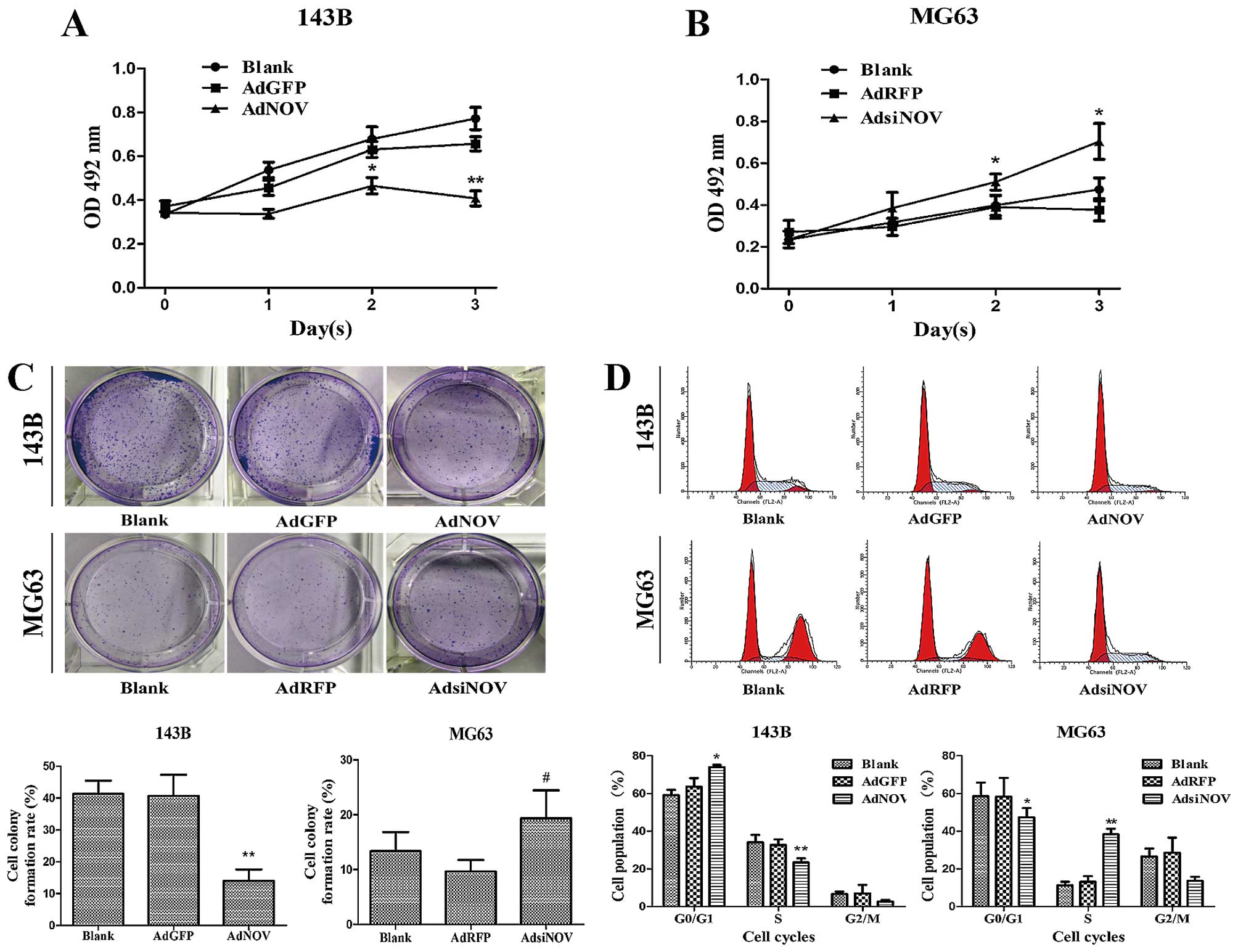
NOV inhibits cell viability of
osteosarcoma cells
To investigate the effects of NOV on cell growth,
osteosarcoma cells were treated with AdNOV and AdsiNOV,
respectively, for 24, 48 and 72 h, and cell viability was examined
by MTT assay (Fig. 3A and B). as
shown in Fig. 3A and B, the cell
viability of 143B cells treated with AdNOV was significantly
inhibited at 48 h (P<0.05) and was more significant at 72 h
(P<0.01), whereas the cell viability of MG63 cells treated with
AdsiNOV was significantly promoted at 48 h (P<0.05) and 72 h
(P<0.05). Thus, we selected 48 h as the time-point for the
remaining experiments. Furthermore, after treatment with AdNOV for
2 weeks, we found a significant decrease in colony formation in the
AdNOV group and the colony-formation rate decreased by 23% in 143B
cells compared with that of the AdGFP and control groups
(P<0.01), while a slight increase in colony formation in the
AdsiNOV group in MG63 cells, compared with that of the AdRFP and
control groups (P>0.05, Fig.
3C).
NOV induces cell cycle arrest in
osteosarcoma cell lines
Cell cycle arrest plays a crucial role in the
process of cell proliferation. Flow cytometric assay was used to
detect the change of the cell cycle induced by NOV expression
(Fig. 3D). The data showed that NOV
overexpression decreased the percentage of 143B cells in the S
phase from (35.14±3.29) to (23.83±4.66)% (P<0.01) compared to
the AdGFP group, and increased the percentage of G1 phase from
(63.61±4.55) to (73.92±1.22)% (P<0.05) compared to the AdGFP
group, respectively. Conversely, knockdown of NOV expression
through RNA interference in MG63 cells, led to the percentage of
MG63 cells in the S phase being increased from (19.32±5.16) to
(39.07±4.16)% (P<0.01) and the percentage of G1 phase was
decreased from (58.15±2.33) to (43.29±5.37)% (P<0.05) compared
to the AdRFP group, respectively (Fig.
3D).
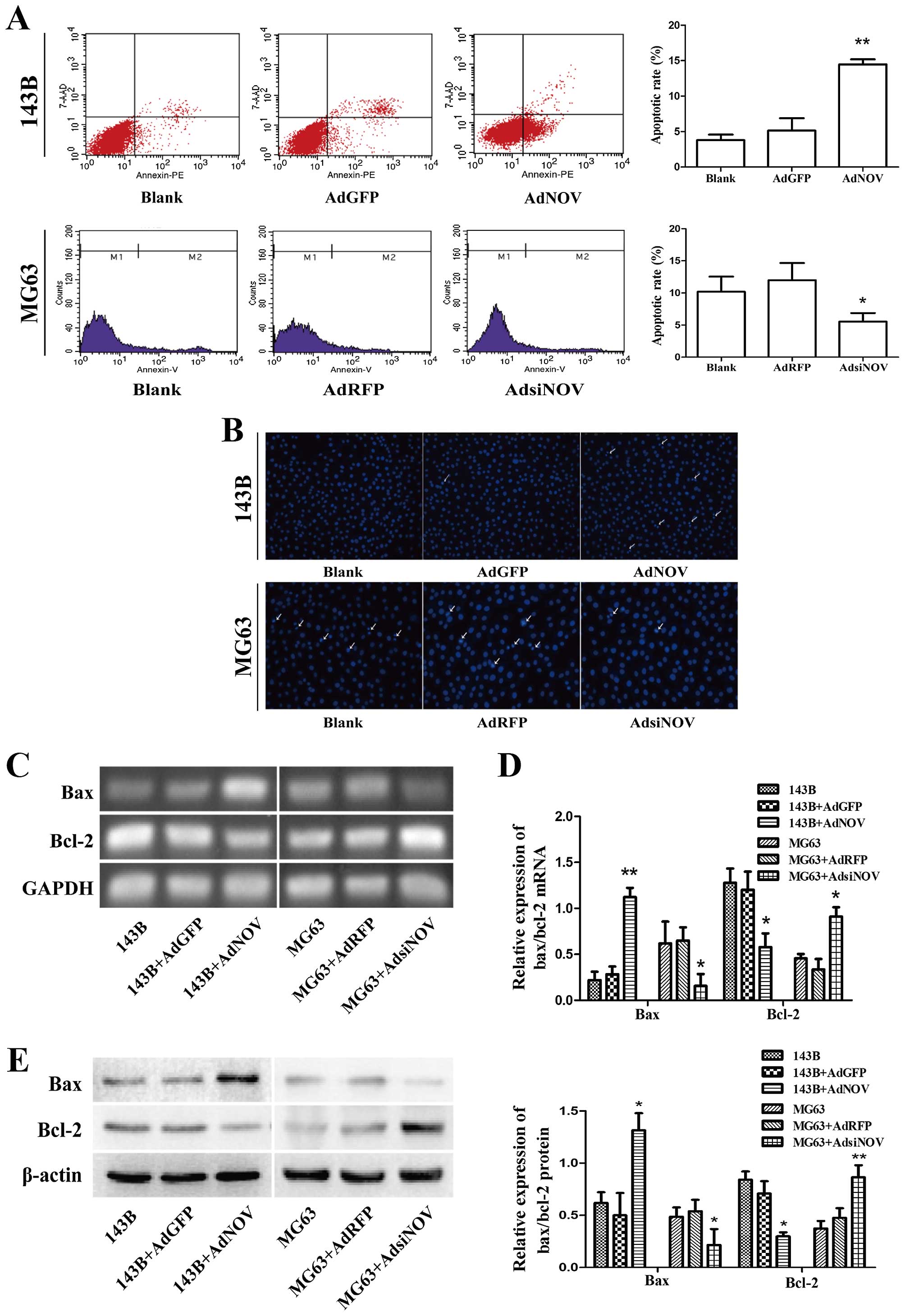
NOV stimulates apoptosis of osteosarcoma
cell lines in vitro
To evaluate apoptotic cell death in osteosarcoma
cells after AdNOV/AdsiNOV treatment, flow cytometric assay
(Fig. 4A), Hoechst staining
(Fig. 4B), RT-qPCR (Fig. 4C and D) and western blot analysis
(Fig. 4E) were performed. In
Fig. 4A it is shown that the
apoptotic rate of 143B cells was significantly increased from
(5.23±2.61) to (11.91±2.29)% (P<0.01) compared to the AdGFP
groups following treatment with AdNOV for 48 h, whereas the
apoptotic rate of MG63 cells was significantly reduced as compared
to that of the AdRFP groups after AdsiNOV treatment for 48 h
(P<0.05). Staining with Hoechst 33258 (Fig. 4B) was used to visualise the
apoptosis induced by NOV expression, and extensive nuclear
condensation and cell fragmentation were observed. Moreover, a
significant increase in Bax expression and a parallel Bcl-2
decrease was observed in 143B cells after NOV overexpression, while
the contrary result was evident in MG63 cells after AdsiNOV
infection (P<0.05 and P<0.01, Fig. 4C–E).
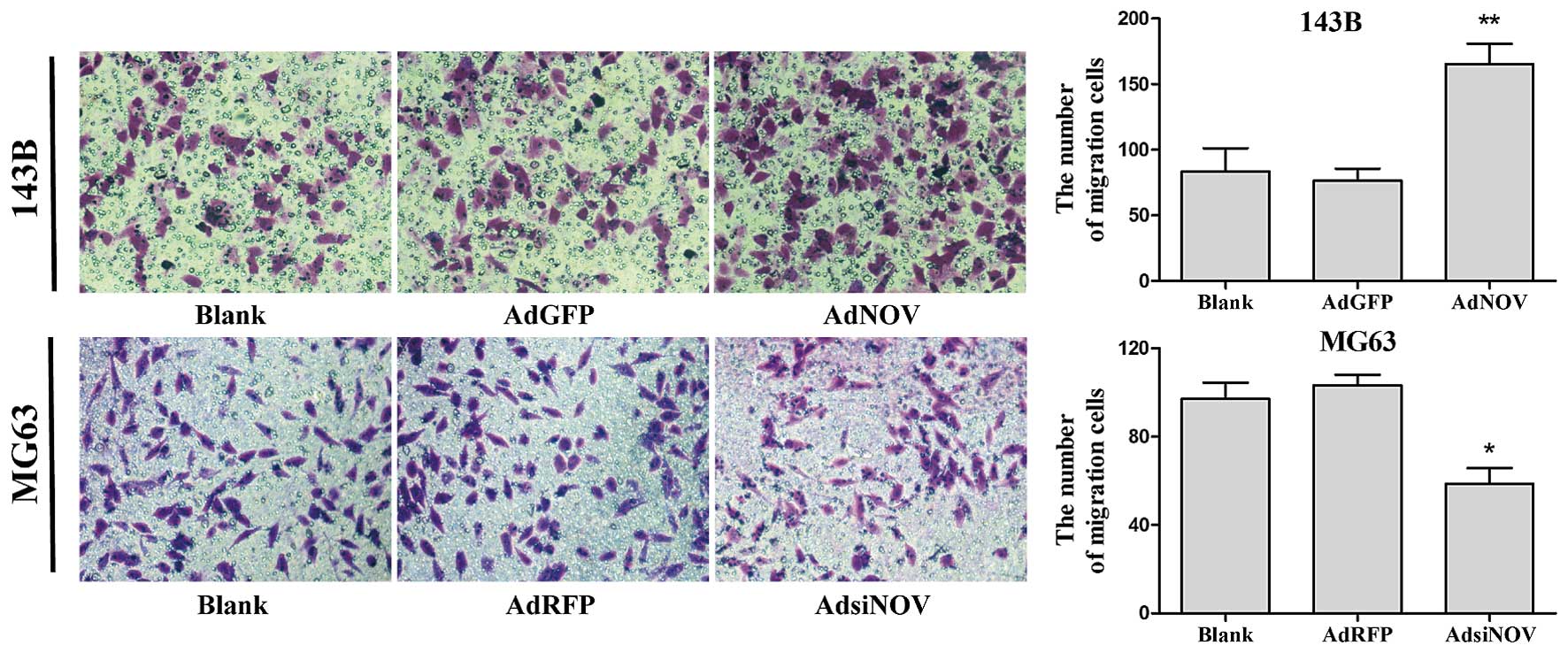
NOV promotes osteosarcoma cell migration
in vitro
Cell migration plays a crucial role in the process
of tumor metastasis. In the present study, Transwell migration
assay was used to detect the change in cell migration induced by
NOV. After treatment with adenovirus for 24 h, the number of
trans-membrane cells in the NOV upregulation group increased
significantly compared with the control group in 143B and MG63
cells (P<0.05 and P<0.01, Fig.
5).
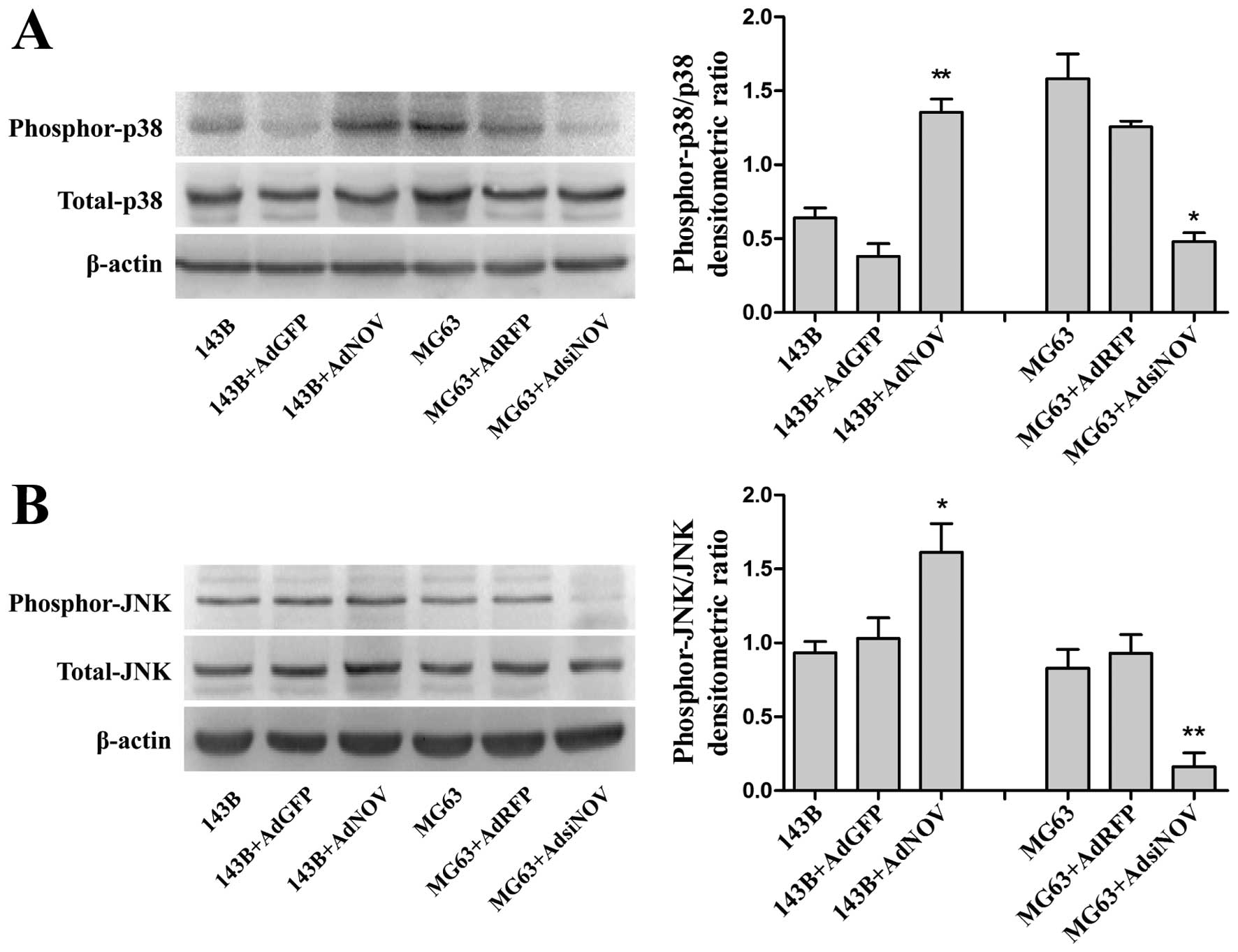
NOV-induced activation of the MAPK
signaling pathway in osteosarcoma cell lines
It has been previously reported that activation of
p38/MAPK and JNK/MAPK signaling pathway was associated with cell
apoptosis. To determine whether NOV expression is involved in
activation of the MAPK signaling pathway in osteosarcoma cell
lines, we detected and analyzed the phosphorylation of MAPKs in
cell lysates of osteosarcoma cells in different groups by western
blot analysis (Fig. 6A and B). The
results showed that NOV overexpression enhanced the phosphorylation
of p38 and JNK/MAPKs in 143B cells, whereas downregulated NOV had
an obvious suppressant effect on the phosphorylation of MAPKs in
MG63 cells (P<0.05 and P<0.01, Fig. 6A and B). These results demonstrated
that NOV enhances the activity of the MAPK signaling pathway (p38
and JNK) in osteosarcoma cell lines.
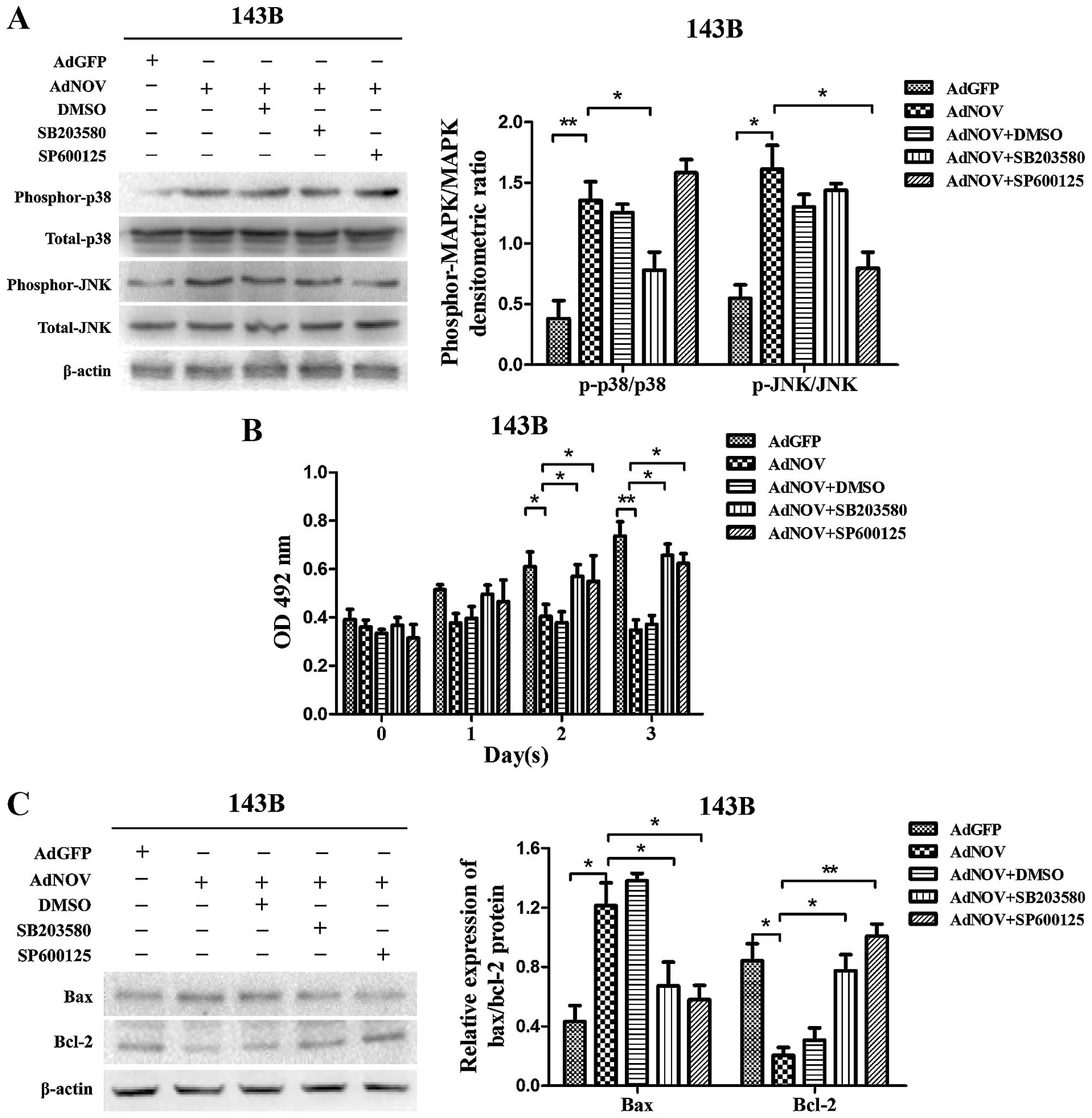
Impact of the inhibition of MAPK
signaling on NOV-induced apoptosis of osteosarcoma cells
To investigate whether activation of the MAPK
signaling pathway is involved in the NOV-induced apoptosis of
osteosarcoma cells, the specific inhibitors of p38 (SB203580) and
JNK (SP600125) were used to pre-treat the 143B cells. We detected
the phosphorylation of MAPKs by western blot analysis (Fig. 7A) and found that the NOV-induced
phosphorylation of p38 and JNK was reversed by SB203580 (P<0.05)
and SP600125 (P<0.05), respectively. Cell viability was measured
by MTT assay (Fig. 7B). We found
that both SB203580 and SP600125 significantly suppressed the
NOV-induced antiproliferative effect at 48 h (P<0.05) and 72 h
(P<0.05). At the same time, we detected changes in the
expression levels of Bax and Bcl-2 in the presence and absence of
SB203580 or SP600125 (Fig. 7C). We
found that the NOV-induced upregulated expression of Bax was
reversed by SB203580 (P<0.05) and SP600125 (P<0.05), while
the down-regulated expression of Bcl-2 was also reversed by
SB203580 (P<0.05) and SP600125 (P<0.01) in 143B cells. These
results suggested that the promotive role of NOV in the apoptosis
of osteosarcoma cells may be mediated by the phosphorylation of p38
and JNK.
Discussion
Osteosarcoma is a high-mortality cancerous tumor
localized at the end of metaphysis, which is most prevalent in
children and young adults. Although early diagnosis and timely
treatment have greatly enhanced the survival rates, the patients
have a poor prognosis because of the high rate of lung metastasis
and drug resistance (24). Tumor
microenvironment, which contains large amounts of ECM generated by
tumor cells including growth factors, chemotactic factors and
matrix-degrading enzymes, is greatly associated with tumorigenesis,
development and metastasis by influencing the proliferation,
apoptosis, migration and invasion of tumor cells (25). As mentioned earlier, it is necessary
to elucidate a novel strategy that efficiently inhibits the
progression of osteosarcoma.
NOV, also known as CCN3, is a matricellular protein
encoded by the NOV gene, that can interact with numerous
cell-surface receptors and participate in various pathological
physiological activities including tumor (26–28).
NOV interacts with the integrin receptor of cell surface to trigger
intracellular signal trans duction and activate downstream
signaling pathways, thus regulate the occurrence and development of
tumor. NOV has been shown to play different roles in various types
of tumors. A previous study demonstrated that NOV expression was
associated with the prognosis judgment of osteosarcoma (17). Nonetheless, the biological
foundation of this effect and the exact role of NOV expression in
the progression of osteosarcoma has not been extensively clarified.
Therefore, in the present study, we investigated the effects of NOV
on the proliferation, apoptosis and migration of osteosarcoma cells
and the possible underlying mechanisms.
In the present study, we firstly evaluated the
endogenous expression of NOV in human osteosarcoma cell lines
through PCR and western blot analysis and found it was different
among the cell lines. Thus, we selected adenovirus-mediated NOV
overexpression/downregulation to investigate the effects of NOV on
different osteosarcoma cell lines. The present results showed that
NOV overexpression had a significant inhibitory effect on
osteosarcoma 143B cell viability in a time-dependent manner,
whereas NOV silencing induced opposite effects in MG63 cells. These
results have shown an inhibitory effect of NOV overexpression on
cancer cell growth including renal cell carcinoma and Ewing sarcoma
(12,13), which is consistent with previous
studies. A possible explanation for the growth inhibition is cell
cycle arrest or apoptosis increase. Our results derived from FCM
indicated that NOV overexpression may induce cell cycle arrest in
the G1 phase in 143B cells, while its downregulation reversed the
effect in MG63 cells, leading to increased cell proliferation
ability. Since the G1 phase is necessary for the material and
energy preparation for DNA replication into the subsequent S
period, the abnormity of this phase may inevitably lead to the
obstacles of DNA replication and eventually cause the inhibition of
cell proliferation (29). At the
same time, we found that NOV expression induced cell apoptosis of
osteosarcoma cell lines. Bax and Bcl-2 are two primary proteins
that are often used as markers for cell apoptosis (30,31).
Our results showed a marked increase of Bax and a decrease of Bcl-2
in the AdNOV group compared with the AdGFP and CON groups in 143B
cells, but a decrease of Bax and an increase of Bcl-2 in AdsiNOV
group compared with the AdRFP and CON groups in MG63 cells, further
comfirming that NOV may promote the apoptosis of osteosarcoma
cells.
P38/MAPK and JNK/MAPK are two major pathways for
malignant progression in various tumors. It has been confirmed that
they are involved in mediating apoptosis signals that cause cell
death (32). Integrin
receptor-dependent signaling pathways may activate P38/MAPK and
JNK/MAPK, thereby promoting the phosphorylation of Bcl-2, the
expression of Bax and induce the apoptosis of tumor cells (33,34).
Moreover, previous findings have shown that NOV induced cell
apoptosis or growth inhibition in many types of cancer through
activation of the MAPK signaling pathway (35,36).
Therefore, we detected the effects of NOV on the MAPK signaling
pathway in osteosarcoma cell lines. Our results show that NOV
expression enhanced the phosphorylation of p38 and JNK in
osteosarcoma cells in vitro. Furthermore, the
phosphorylation of p38 and JNK was inhibited by the specific
inhibitors, SB203580 and SP600125, respectively. The results from
the present study also demonstrate that NOV-induced cell
proliferation inhibition and apoptosis was reversed by SB203580 and
SP600125, suggesting that NOV is involved in osteosarcoma cell
apoptosis by activating the MAPK signaling pathway. These results
are consistent with those of previous findings for Ewing sarcoma
(13).
Besides participating in the growth and prognosis,
NOV also exerted effects on the adhesion, migration and invasion of
tumor cells (7). Previous studies
indicated that NOV activated PI3K/AKT and NF-κB signaling pathways
by combining with αvβ3 integrin receptor, thus promoting the
transcription and expression of transfer relevant factors (37). Nonetheless, with regard to Ewing
sarcoma, NOV overexpression reduced the expression of integrin α2β1
at the transcription level, thus reducing tumor cell anchor
activity and promoting migration, and caused the abnormal
distribution of MMP-9, leading to MMP-9 overexpression on the cell
surface and promoting invasion (13). Our Transwell migration results
demonstrated that the migration ability of osteosarcoma was greatly
enhanced in 143B cells following NOV overexpression and markedly
inhibited in MG63 cells after NOV silencing, which was consistent
with previous findings for Ewing sarcoma. We hypothesized that the
integrin receptor may also be involved in this progression, but
whether it was consistent with Ewing sarcoma needs to be further
examined. In combination with other clinical reports concerning NOV
upregulation in primary osteosarcoma, it was identified that NOV
overexpression has a close relationship with the high metastatic
rate of osteosarcoma. Findings of previous clinical studies
investigating the prognostic value of NOV including osteosarcoma
patients receiving chemotherapy (17), showed that primary osteosarcoma
cells with a low expression of NOV were easily eliminated using
chemotherapeutic drugs due to their high proliferative capacity. By
contrast, cells with NOV upregulation show hyposensitivity to drugs
because of its lower proliferation and higher migration ability,
thus making it possible for distant metastasis to occur. Therefore,
NOV overexpression has a tendency to promote osteosarcoma
progression in the clinic.
To the best of our knowledge, in the present study,
we first confirmed that NOV expression can inhibit cell
proliferation, while promoting the apoptosis and migration of
osteosarcoma cell lines in vitro. We also demonstrated that
NOV expression-induced phosphorylation of p38/MAPK and JNK/MAPK may
be involved in these progressions. These results offer an
experimental basis for further clarification of the effects of NOV
on osteosarcoma and its probable mechanism. Furthermore, our
results indicate that NOV overexpression may prove to be a valuable
tool for inhibiting cancer growth. Future studies to elucidate the
relationship between NOV and osteosarcoma are required.
Acknowledgments
We would like to thank Dr T.C. He of The University
of Chicago Medical Center for providing the adenoviruses. This
study was supported by the National Natural Science Foundation of
China (NSFC 81102035) and Natural Science Foundation Project of
Chongqing Science and Technology Commission (no. 2011BB5126).
References
|
1
|
Damron TA, Ward WG and Stewart A:
Osteosarcoma, chon-drosarcoma, and Ewing's sarcoma: National Cancer
Data Base Report. Clin Orthop Relat Res. 459:40–47. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mirabello L, Troisi RJ and Savage SA:
International osteosarcoma incidence patterns in children and
adolescents, middle ages and elderly persons. Int J Cancer.
125:229–234. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Eyre R, Feltbower RG, Mubwandarikwa E,
Eden TO and McNally RJ: Epidemiology of bone tumours in children
and young adults. Pediatr Blood Cancer. 53:941–952. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Joliot V, Martinerie C, Dambrine G,
Plassiart G, Brisac M, Crochet J and Perbal B: Proviral
rearrangements and overexpression of a new cellular gene (nov) in
myeloblastosis-associated virus type 1-induced nephroblastomas. Mol
Cell Biol. 12:10–21. 1992.PubMed/NCBI
|
|
5
|
Perbal B: NOV (nephroblastoma
overexpressed) and the CCN family of genes: Structural and
functional issues. Mol Pathol. 54:57–79. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen CC and Lau LF: Functions and
mechanisms of action of CCN matricellular proteins. Int J Biochem
Cell Biol. 41:771–783. 2009. View Article : Google Scholar :
|
|
7
|
Ouellet V, Tiedemann K, Mourskaia A, Fong
JE, Tran-Thanh D, Amir E, Clemons M, Perbal B, Komarova SV and
Siegel PM: CCN3 impairs osteoblast and stimulates osteoclast
differentiation to favor breast cancer metastasis to bone. Am J
Pathol. 178:2377–2388. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Scholz G, Martinerie C, Perbal B and
Hanafusa H: Transcriptional down regulation of the nov
proto-oncogene in fibroblasts transformed by p60v-src. Mol Cell
Biol. 16:481–486. 1996.PubMed/NCBI
|
|
9
|
Chevalier G, Yeger H, Martinerie C,
Laurent M, Alami J, Schofield PN and Perbal B: novH: Differential
expression in developing kidney and Wilm's tumors. Am J Pathol.
152:1563–1575. 1998.PubMed/NCBI
|
|
10
|
Manara MC, Perbal B, Benini S, Strammiello
R, Cerisano V, Perdichizzi S, Serra M, Astolfi A, Bertoni F, Alami
J, et al: The expression of ccn3(nov) gene in musculoskeletal
tumors. Am J Pathol. 160:849–859. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu C, Le AT, Yeger H, Perbal B and Alman
BA: NOV (CCN3) regulation in the growth plate and CCN family member
expression in cartilage neoplasia. J Pathol. 201:609–615. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu S, Liu Z, Bi D, Yuan X, Liu X, Ding S,
Lu J and Niu Z: CCN3 (NOV) regulates proliferation, adhesion,
migration and invasion in clear cell renal cell carcinoma. Oncol
Lett. 3:1099–1104. 2012.PubMed/NCBI
|
|
13
|
Benini S, Perbal B, Zambelli D, Colombo
MP, Manara MC, Serra M, Parenza M, Martinez V, Picci P and
Scotlandi K: In Ewing's sarcoma CCN3(NOV) inhibits proliferation
while promoting migration and invasion of the same cell type.
Oncogene. 24:4349–4361. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu C, Liu X-J, Crowe PD, Kelner GS, Fan
J, Barry G, Manu F, Ling N, De Souza EB and Maki RA: Nephroblastoma
overexpressed gene (NOV) codes for a growth factor that induces
protein tyrosine phosphorylation. Gene. 238:471–478. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ren Z, Hou Y, Ma S, Tao Y, Li J, Cao H and
Ji L: Effects of CCN3 on fibroblast proliferation, apoptosis and
extracellular matrix production. Int J Mol Med. 33:1607–1612.
2014.PubMed/NCBI
|
|
16
|
Owen TA, Aronow M, Shalhoub V, Barone LM,
Wilming L, Tassinari MS, Kennedy MB, Pockwinse S, Lian JB and Stein
GS: Progressive development of the rat osteoblast phenotype in
vitro: Reciprocal relationships in expression of genes associated
with osteoblast proliferation and differentiation during formation
of the bone extracellular matrix. J Cell Physiol. 143:420–430.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Perbal B, Zuntini M, Zambelli D, Serra M,
Sciandra M, Cantiani L, Lucarelli E, Picci P and Scotlandi K:
Prognostic value of CCN3 in osteosarcoma. Clin Cancer Res.
14:701–709. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kuo WH, Chen JH, Lin HH, Chen BC, Hsu JD
and Wang CJ: Induction of apoptosis in the lung tissue from rats
exposed to cigarette smoke involves p38/JNK MAPK pathway. Chem Biol
Interact. 155:31–42. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mansouri A, Ridgway LD, Korapati AL, Zhang
Q, Tian L, Wang Y, Siddik ZH, Mills GB and Claret FX: Sustained
activation of JNK/p38 MAPK pathways in response to cisplatin leads
to Fas ligand induction and cell death in ovarian carcinoma cells.
J Biol Chem. 278:19245–19256. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen HJ, Lin CM, Lee CY, Shih NC, Peng SF,
Tsuzuki M, Amagaya S, Huang WW and Yang JS: Kaempferol suppresses
cell metastasis via inhibition of the ERK-p38-JNK and AP-1
signaling pathways in U-2 OS human osteosarcoma cells. Oncol Rep.
30:925–932. 2013.PubMed/NCBI
|
|
21
|
Tsagaraki I, Tsilibary EC and Tzinia AK:
TIMP-1 interaction with αvβ3 integrin confers resistance to human
osteosarcoma cell line MG-63 against TNF-α-induced apoptosis. Cell
Tissue Res. 342:87–96. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Luo J, Deng ZL, Luo X, Tang N, Song WX,
Chen J, Sharff KA, Luu HH, Haydon RC, Kinzler KW, et al: A protocol
for rapid generation of recombinant adenoviruses using the AdEasy
system. Nat Protoc. 2:1236–1247. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
He TC, Zhou S, da Costa LT, Yu J, Kinzler
KW and Vogelstein B: A simplified system for generating recombinant
adenoviruses. Proc Natl Acad Sci USA. 95:2509–2514. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fidler IJ: The pathogenesis of cancer
metastasis: The 'seed and soil' hypothesis revisited. Nat Rev
Cancer. 3:453–458. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Perbal B: The CCN3 protein and cancer. New
Trends in Cancer for the 21st Century; Springer; pp. 23–40.
2006
|
|
27
|
Denduluri SK, Idowu O, Wang Z, Liao Z, Yan
Z, Mohammed MK, Ye J, Wei Q, Wang J, Zhao L, et al: Insulin-like
growth factor (IGF) signaling in tumorigenesis and the development
of cancer drug resistance. Genes Dis. 2:13–25. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Perbal B: CCN proteins: A centralized
communication network. J Cell Commun Signal. 7:169–177. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bartek J and Lukas J: Mammalian G1- and
S-phase checkpoints in response to DNA damage. Curr Opin Cell Biol.
13:738–747. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yin X-M, Oltvai ZN and Korsmeyer SJ: BH1
and BH2 domains of Bcl-2 are required for inhibition of apoptosis
and heterodimerization with Bax. Nature. 369:321–323. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kroemer G: The proto-oncogene Bcl-2 and
its role in regulating apoptosis. Nat Med. 3:614–620. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wagner EF and Nebreda ÁR: Signal
integration by JNK and p38 MAPK pathways in cancer development. Nat
Rev Cancer. 9:537–549. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
De Chiara G, Marcocci ME, Torcia M,
Lucibello M, Rosini P, Bonini P, Higashimoto Y, Damonte G,
Armirotti A, Amodei S, et al: Bcl-2 phosphorylation by p38 MAPK:
Identification of target sites and biologic consequences. J Biol
Chem. 281:21353–21361. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nicholson DW and Thornberry NA: Apoptosis.
Life and death decisions. Science. 299:214–215. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Doghman M, Arhatte M, Thibout H, Rodrigues
G, De Moura J, Grosso S, West AN, Laurent M, Mas JC, Bongain A, et
al: Nephroblastoma overexpressed/cysteine-rich protein
61/connective tissue growth factor/nephroblastoma overexpressed
gene-3 (NOV/CCN3), a selective adrenocortical cell proapoptotic
factor, is downregulated in childhood adrenocortical tumors. J Clin
Endocrinol Metab. 92:3253–3260. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gupta N, Wang H, McLeod TL, Naus CC,
Kyurkchiev S, Advani S, Yu J, Perbal B and Weichselbaum RR:
Inhibition of glioma cell growth and tumorigenic potential by CCN3
(NOV). Mol Pathol. 54:293–299. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen PC, Cheng HC and Tang CH: CCN3
promotes prostate cancer bone metastasis by modulating the
tumor-bone microenvironment through RANKL-dependent pathway.
Carcinogenesis. 34:1669–1679. 2013. View Article : Google Scholar : PubMed/NCBI
|