Introduction
Breast cancer is the leading cause of death from
cancer among women both in the developed and the developing world
(1). Approximately 1 in 8 women
(12%) in the United States will develop breast cancer during her
lifetime (2). In 2015, ~231,840 new
cases will be diagnosed and ~40,290 women will die from breast
cancer in the United States (3).
Radiotherapy is a highly targeted and effective treatment method
that uses high-energy rays or particles to inhibit the progression
of cancer. Radiation to the breast is often administered after
breast-conserving surgery to destroy cancer cells in the breast
that may not have been removed during surgery. Radiotherapy reduces
the risk of breast cancer recurrence by 70%. However, the
development of radioresistance is a significant issue after
prolonged exposure in the treatment of breast cancer patients.
Cancer stem cells (CSCs) have been identified as the important
factor for radioresistance by promoting repair of DNA damage,
redistribution of cells in the cell cycle, repopulation, and
reoxygenation of hypoxic tumor areas (4–6). In
addition, CSCs produce a high level of antioxidant proteins and
enhance their reactive oxygen species (ROS) defenses during
radiotherapy (7). Recently, Zhang
et al found that zinc finger E-box binding homeobox 1 (ZEB1)
plays an important role in radioresistance in breast cancer by
promoting homologous recombination-dependent DNA repair (8).
miRNAs are a class of small non-coding RNA molecules
that regulate the expression of multiple target mRNAs by binding to
the 3′-untranslated region (UTR) (9,10). The
pattern of miRNA expression is associated with cancer type, stage
and other clinical variables (11).
Recently, growing evidence suggests that miRNAs play important
roles in the tumorigenesis and progression of breast cancer
(12–14). Some miRNAs are found to be
upregulated, acting as oncogenes, while some miRNAs are found to be
downregulated, acting as tumor-suppressor genes (15). It has been reported that miRNA
dysregulation is responsible for the radioresistance in breast
tumors. For example, miR-95 is upregulated by ionizing radiation
and enhances the proliferation and invasive potential of breast
cancer cells by targeting the sphingolipid phosphatase SGPP1
(16). miR-34 may protect breast
cancer cells from non-apoptotic cell death (17). Studies have shown that miR-144 is
correlated with human disease (18)
and modulates metastasis (19).
Here, we examined the roles of miR-144 in breast
cancer. We found that overexpression of miR-144 inhibited
radiotherapy-induced apoptosis of breast cancer cells. Moreover,
miR-144 also enhanced epithelial-mesenchymal transition (EMT) of
breast cancer cells by activation of PTEN/Akt signaling. Our
findings indicate that miR-144 may serve as a potential biomarker
to predict the treatment reponse and prognosis of breast cancer and
may be utilized as a target for novel therapeutic strategies.
Materials and methods
Cell lines and culture
The human breast cancer cell lines MDA-MB-231 and
SKBR3 were obtained from the American Type Culture Collection
(ATCC). MDA-MB-231 and SKBR3 cells were grown in Dulbecco's
modified Eagle's medium (DMEM; Invitrogen, USA) supplemented with
10% heat-inactivated fetal bovine serum (FBS) and 100 U/ml of
penicillin and 100 µg/ml of streptomycin (Sigma-Aldrich,
USA). The cells were cultured at 37°C in a humidified incubator in
an atmosphere of 5% CO2−95% air.
Cell transfection
miR-144 mimics and anti-miR-144 inhibitor were
purchased from GenePharma (Shanghai, China). When breast cancer
cells reached 80% confluency, the miR-144 or anti-miR-144 inhibitor
was transfected into cells using Lipofectamine 2000 (Invitrogen)
according to the manufacturer's instructions. The scrambled
oligonucleotide was chosen as a negative control.
miRNA quantification by quantitative
real-time polymerase chain reaction (qRT-PCR)
To detect miR-144 expression, total RNA was
extracted with the mirVana miRNA isolation kit (Ambion, USA)
according to the manufacturer's instructions. cDNA was synthesized
from the isolated RNA with TaqMan microRNA reverse transcription
kit. The PCR conditions were 95°C for 5 min, followed by 35 cycles
of 95°C for 30 sec and 65°C for 30 sec, and a dissociation stage.
PCR was performed using the TaqMan Universal PCR Master Mix and
BioRad CFX384 machine. The endogenous reference gene GAPDH was used
for RNA quantification. The PCR primer sequences used were:
5′-GTCTCCTCTGACTTCAACAGCG-3′ and 5′-ACCACCCTGTTGCTGTAGCCAA-3′
(GAPDH).
Cell proliferation assay
Cell proliferation was assessed using the WST-1
assay (Roche, USA). Briefly, breast cancer cells, transfected with
the scrambled oligonucleotide or miR-144 mimic, were seeded in
triplicate in each well of 24-well plates at the density of
2×104 cells/well. After 12 h of culture, the cells were
irradiated with different doses of ionizing radiation. On every
other day, 10 µl of WST-1 was added to each well, and the
plates were incubated for 1.5 h at 37°C. Then 100 µl
WST-1/medium solution was transferred to a 96-well plate. Optical
density was measured at 440 (OD440) minus 600 nm using a microplate
spectrophotometer. All experiments were carried out in
triplicates.
Analysis of apoptosis
Breast cancer cells, transfected with either the
scrambled oligonucleotide or miR-144 mimic, were seeded in 24-well
plates at a density of 1×105/well. After 18 h of
incubation in an atmosphere of 5% CO2−95% air, the
breast cancer cells were irradiated with different doses of
ionizing radiation. The apoptosis was measured using the
Caspase-Glo3/7 assay kit (Promega, Madison, WI, USA) according to
the manufacturer's instructions. Briefly, the breast cancer cells
were cultured for 36 h after ionizing radiation, then Caspase-Glo
reagent was added to each well and incubated at room temperature
for 8 h with gentle shaking. The luminescence value was measured
with a luminometer (Thermo Labsystems) using 1 min lag time and 0.5
sec/well read time. The experiments were performed in
triplicate.
Matrigel invasion assays
Cell invasion was examined by Matrigel invasion
assays according to the manufacturer's instructions. Briefly,
breast cancer cells, transfected with either miR-144 mimic or the
anti-miR-144 inhibitor, were placed on the upper BD BioCoat
Matrigel Invasion Transwell insert (BD Biosciences, USA) in 0.5 ml
DMEM with 0.1% BSA. DMEM containing 5% FBS was added to the lower
chamber. After 24 h, the non-invaded cells were removed with a
cotton swab, and the invaded cells were stained by Diff-Quik stain.
The invaded cells were counted under microscopy. The percentage of
invasion was expressed as the ratio of invading cells over cell
number normalized on day 2 of the growth curve.
Cell lysate and western blot
analysis
The transfected breast cancer cells were lysed with
ice-cold RIPA buffer (Beyotime, China). The cell debris and
insoluble material were removed by centrifugation at 4°C. The cell
lysates were mixed with loading buffer and boiled at 100°C, and
then the samples were separated by SDS-PAGE and transferred to
polyvinylidene fluoride (PVDF) membranes (Sigma-Aldrich). The
membranes were incubated in 5% non-fat dry milk in Tris-buffered
saline Tween-20 buffer (TBST: 10 mmol/l Tris-base, 150 mmol/l NaCl,
0.05% Tween-20; pH 7.4) for 1 h at room temperature to block
non-specific antibody binding sites. Then the membranes were
incubated overnight at 4°C with primary antibodies (N-cadherin,
Snail, Slug, vimentin, Twist, caspase-9, Bcl-2, Bax, AKT and PTEN;
Cell Signaling Technology, USA) in TBST with gentle agitation.
After being washed with TBST, the membranes were incubated with the
horseradish peroxidase-conjugated secondary antibody for 1 h at
room temperature. The immune blot signals were visualized using the
EasySee Western Blot kit (Transgen, China). The protein bands were
detected by densitometric scanning (Tanon-1600 Gel Image System;
Tanon, Shanghai, China).
Statistical analysis
Statistical analyses of the above results were
performed using the Student's t-test using the SPSS program
(version 11.0; SPSS Inc., USA). Differences were considered
statistically significant at p<0.05.
Results
miR-144 promotes the survival of breast
cancer cells following irradiation
To examine the effect of miR-144 on radiation
resistance in breast cancer cells, miR-144 was transiently
transfected into MDA-MB-231 and SKBR3 cells with Lipofectamine
2000, and the cell survival rate was measured by WST-1 assay. As
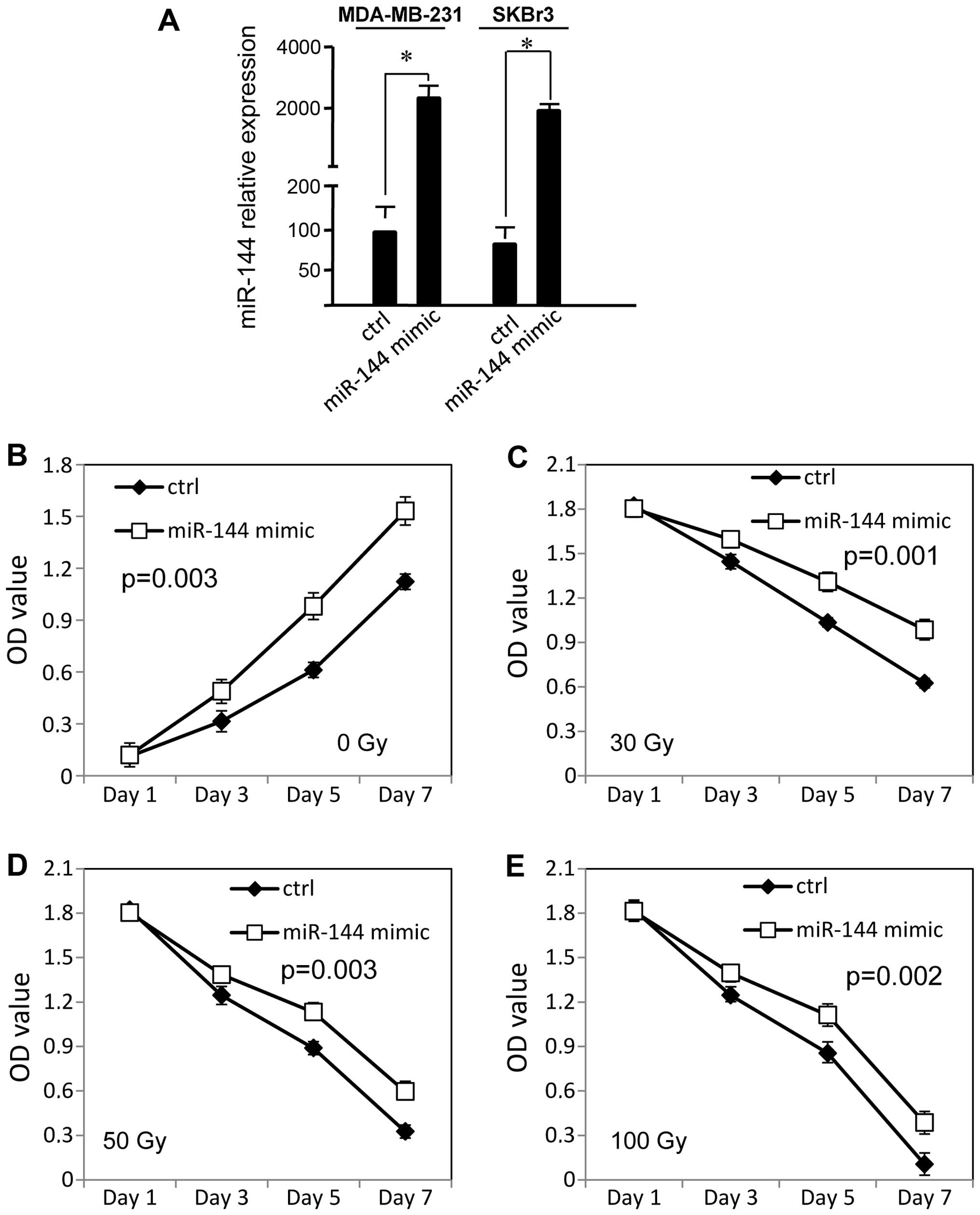
shown in Fig. 1A, we confirmed that
the miR-144 expression level was significantly increased in the
breast cancer cells using qRT-PCR (p<0.01). Without radiation,
overexpression of miR-144 increased the rate of proliferation of
the MDA-MB-231 cells (p<0.05, Fig.
1B) and SKBR3 cells (p<0.05, Fig. 1F), as compared with the negative
control. The MDA-MB-231 cells displayed significantly increased
radiation resistance after overexpression of miR-144, as compared
with the control cells (p<0.05, Fig.
1C–E). The miR-144 mimic-transfected SKBR3 cells also exhibited
increased radiation resistance (p<0.05, Fig. 1G–I).
miR-144 inhibits radiation-induced
apoptosis in breast cancer cells
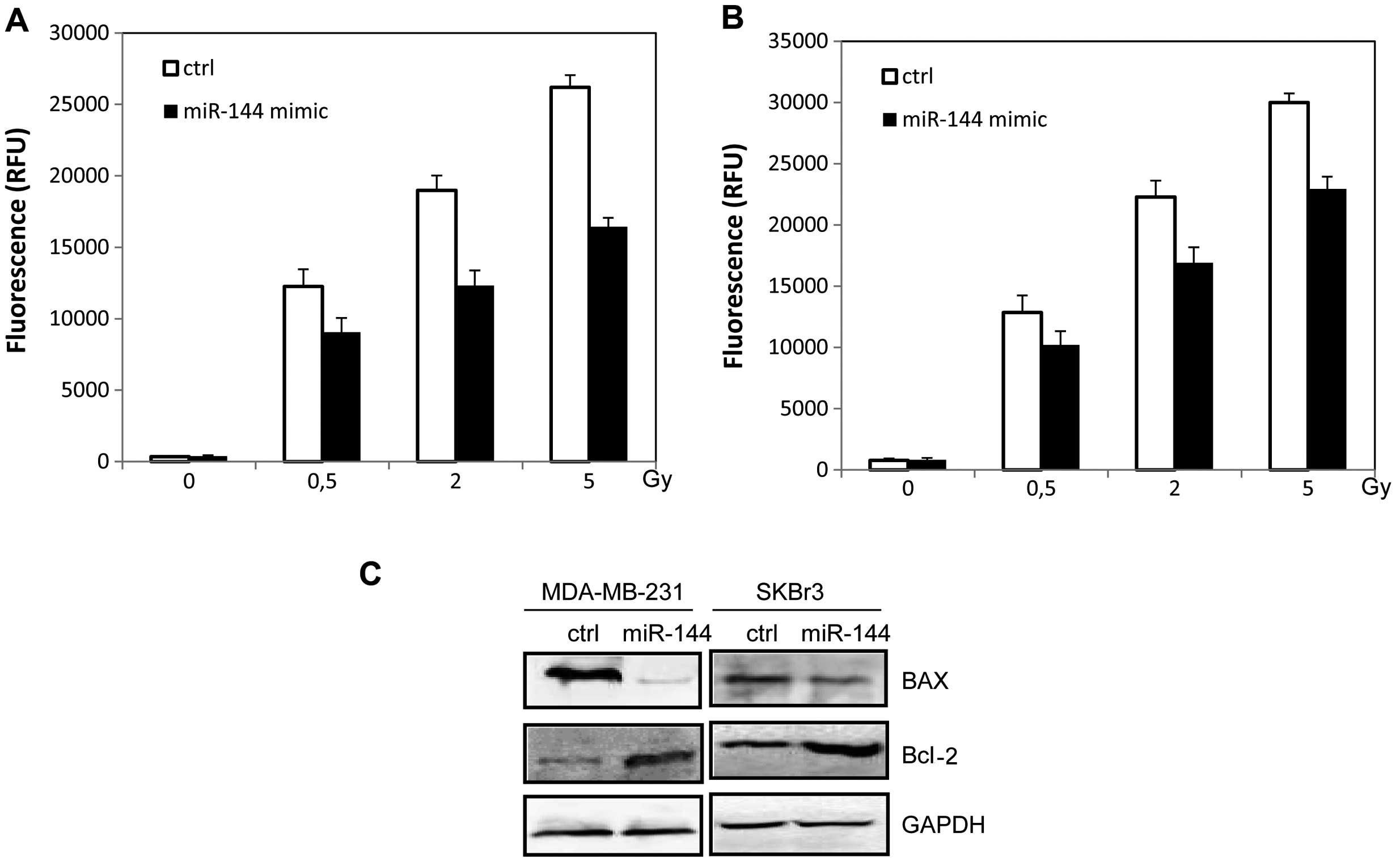
We further examined the effect of miR-144 on
radiation resistance by apoptosis analysis. The miR-144 mimic was
transiently transfected into MDA-MB-231 and SKBR3 cells with
Lipofectamine 2000. Then the cells were irradiated followed by
analysis of caspase-3/-7 activity. The expression of apoptotic
proteins was evaluated by western blot analysis. We found that
overexpression of miR-144 reduced the sensitivity of breast cancer
cells to the effects of irradiation by inhibiting caspase-3/-7
activities (p<0.05, Fig. 2A and
B). As shown in Fig. 2C, the
ability of irradiation to increase BAX and caspase-9 expression was
inhibited as a result of miR-144 over-expression (p<0.05). The
Bcl-2 expression was also increased in the miR-144-overexpressing
cells (p<0.05, Fig. 2C).
miR-144 promotes migration and invasion
in breast cancer cells
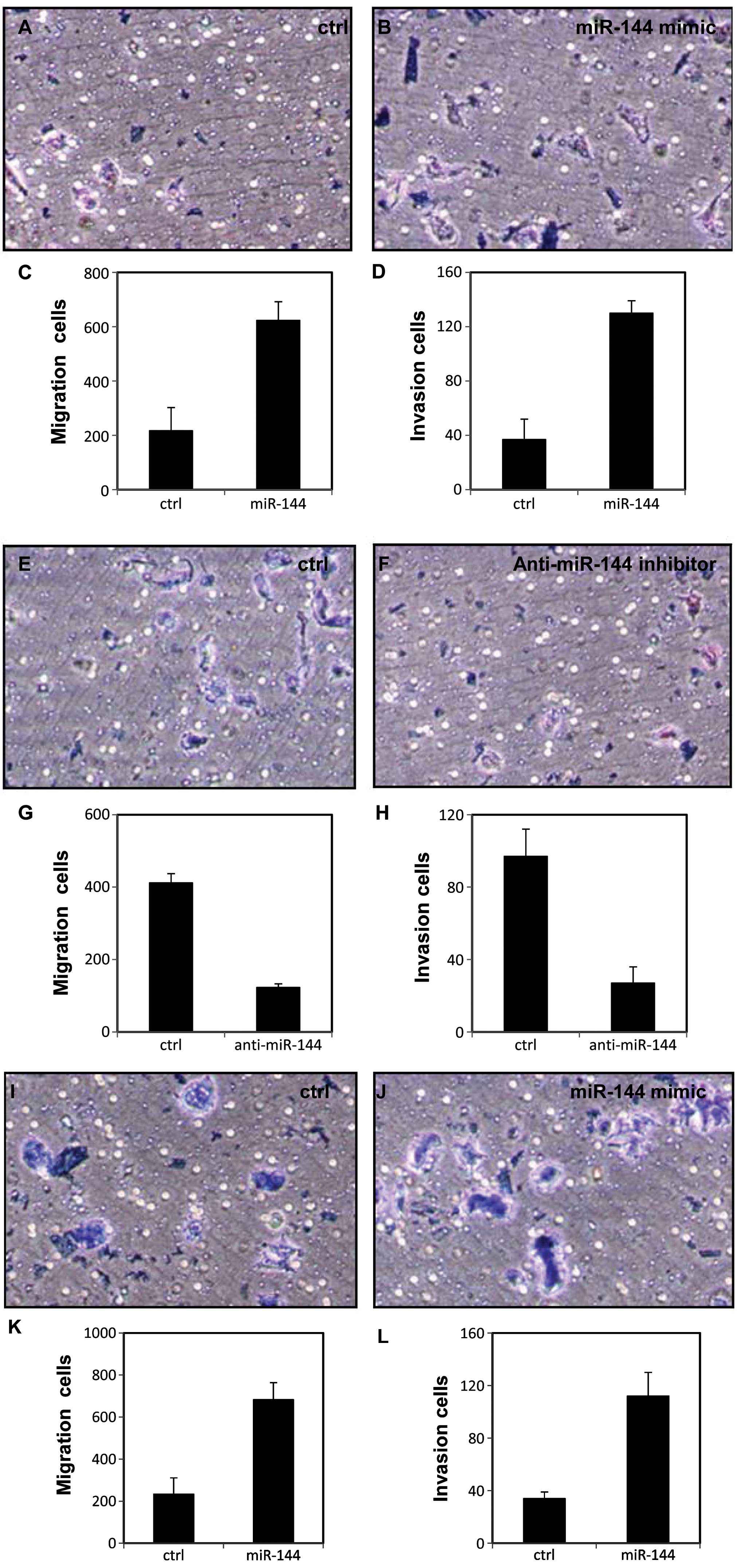
To further investigate the effect of miR-144 on cell
aggression, we examined the effect of miR-144 on migration and
invasion of breast cancer cells with Transwell migration and
Matrigel invasion assays. As shown in Fig. 3A–D, the migration and invasion of
the MDA-231 cells were enhanced after transfection with the miR-144
mimic. The same effects were observed in another breast cancer cell
line SKBR3 (p<0.05, Fig. 3I–L).
In contrast, migration and invasion were decreased with
anti-miR-144 inhibitor treatment in the MDA-231 cells (p<0.05,
Fig. 3E–H) and SKBR3 cells
(p<0.05, Fig. 3M–P).
miR-144 affects the expression of EMT
proteins and activates AKT in breast cancer cells
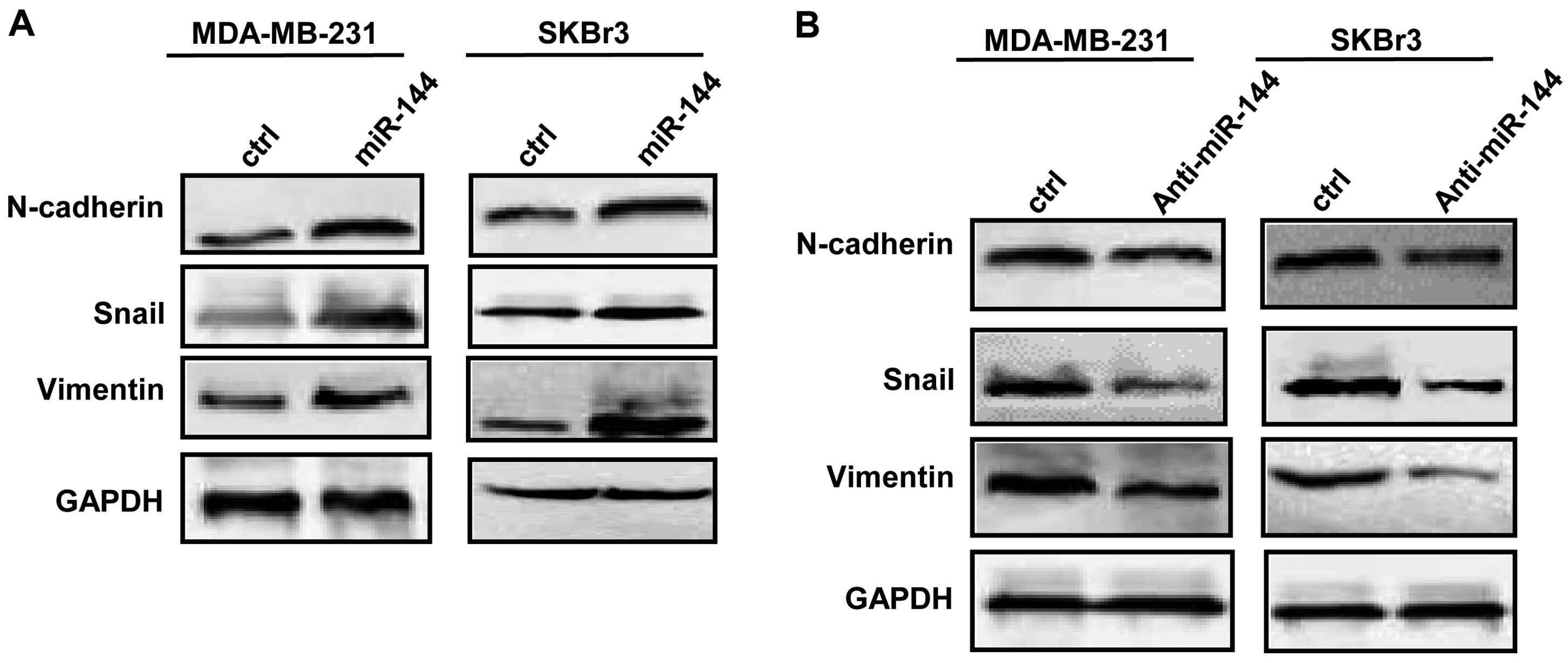
We next determined expression of EMT markers by
western blot analysis after breast cancer cells were transfected
with either the miR-144 mimic or the anti-miR-144 inhibitor.
Expression of N-cadherin, vimentin and Snail was increased after
breast cancer cells were transfected with the miR-144 mimic
(Fig. 4A). In contrast, decreased
N-cadherin, vimentin and Snail was observed in the breast cancer
cells transfected with the anti-miR-144 inhibitor (Fig. 4B). We further found that the
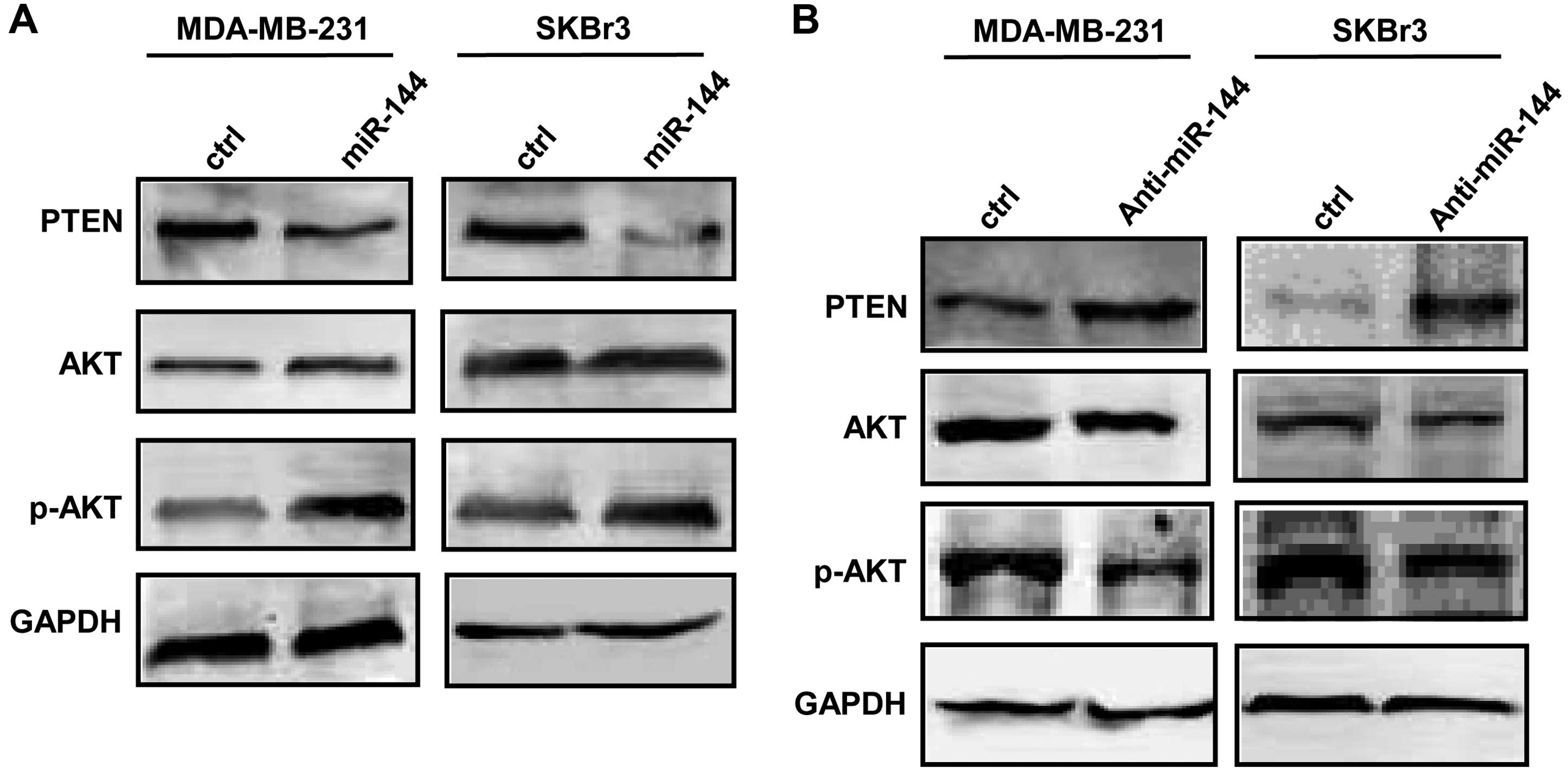
expression of AKT was increased, and the PTEN expression level was
decreased in the breast cancer cells after transfection with the
miR-144 mimic (Fig. 5A). As shown
in Fig. 5B, the transfection with
anti-miR-144 inhibitor decreased the AKT protein level and
increased PTEN expression in the breast cancer cells.
Discussion
Although the 5-year survival rate of breast cancer
patients has been markedly improved due to early diagnosis and
effect of treatment, the development of radiotherapy resistance is
a significant issue in the treatment of breast cancer patients
(20,21). Studies have shown that the
development of radiotherapy resistance correlates with various
signaling pathways that regulate cell survival, proliferation and
apoptosis. For example, Tessner et al found that
prostaglandin E2 inhibited radiation-induced apoptosis by
activation of AKT and BAX translocation (22). Zhang et al found that protein
ZEB1 promotes radioresistance in breast cancer by regulating
homologous recombination-dependent DNA repair (8). Cellular response to ionizing radiation
activates signaling pathways mediating the DNA damage response.
Growing evidence has demonstrated that miRNAs are involved in the
regulation of the DNA damage response (23). Aberrant expression of miR-144 has
been found in many different human cancers. Iwaya et al
found that downregulation of miR-144 could predict the poor
prognosis of colorectal cancer patients by activation of the mTOR
signaling pathway (24). Guo et
al reported that miR-144 could regulate proliferation in
bladder cancer cells by targeting enhancer of zeste homolog 2
(EZH2) (25).
Buffa et al found that a high level of
miR-144 is associated with poor prognosis in breast cancer
(26). In our study, we found that
miR-144 increased the rate of proliferation of MDA-MB-231 and SKBR3
cells. Moreover, both MDA-MB-231 and SKBR3 cells displayed
significantly increased radiation resistance after overexpression
of miR-144. Our results indicate that miR-144 may play an important
role in the radiation resistance of breast cancer cells.
Invasion and metastasis of aggressive breast cancer
cells are the final and fatal step during cancer progression
(27). Metastasis is an extremely
complicated multistep pathophysiological process involving
intravasation, survival in circulation, extravasation, and
colonization and growth at a distant site (28). EMT is an important process during
the metastasis of cancer cells. EMT is a biological process that
allows a polarized epithelial cell to acquire a mesenchymal cell
phenotype. These changes enhance the migratory capacity,
invasiveness, and elevated resistance to apoptosis and are
associated with the high aggressiveness or recurrence of tumors
(29). Commonly used molecular
markers for EMT include E-cadherin, N-cadherin, Snail and vimentin
(30). Here, we showed that miR-144
enhanced the migration and invasion of MDA-MB-231 and SKBR3 cells
after overexpression of miR-144. In contrast, the migration and
invasion of MDA-MB-231 and SKBR3 cells were decreased when breast
cancer cells were treated with the anti-miR-144 inhibitor.
Moreover, we found that the expression level of EMT biomarkers was
altered in breast cancer cells with aberrant expression of miR-144.
These results indicate that miR-144 plays important roles in breast
cancer progression.
PTEN, a tumor-suppressor gene, plays important roles
in the regulation of tumor cell growth, cell cycle, apoptosis,
invasion and metastasis (31). The
loss or mutation of PTEN in many primary and metastatic human
cancers leads to activation of the phosphoinositide-3-kinase
(PI3K)-PKB/Akt signaling pathway (32). Studies have demonstrated that PTEN
is important for breast cancer development and prognosis (33,34).
Recent studies have shown that PTEN signaling plays roles in EMT
and metastasis (35). Growing
evidence has shown that miRNAs play critical roles in the
regulation of tumor development, progression and prognosis by
directly targeting the PTEN/Akt signaling pathway (36,37).
In the present study, we found that miR-144 decreased the
expression of PTEN and increased the expression of pAKT in the
MDA-MB-231 and SKBR3 cells. These results indicate that the
PTEN/Akt signaling pathway may be targeted by miR-144 in breast
cancer. miR-144 plays important roles in breast cancer and
radiotherapy resistance through the PTEN/Akt signaling pathway.
In summary, miR-144 plays important roles in the
regulation of tumorigenesis and malignant progression in breast
cancer. miR-144 may be a potential molecular target in breast
cancer treatment in the future.
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Donepudi MS, Kondapalli K, Amos SJ and
Venkanteshan P: Breast cancer statistics and markers. J Cancer Res
Ther. 10:506–511. 2014.PubMed/NCBI
|
|
3
|
Abels JC, Gorham AT, Pack GT and Rhoads
CP: Metabolic studies in patients with cancer of the
gastro-intestinal tract. I. Plasma vitamin A levels in patients
with malignant neoplastic disease, particularly of the
gastro-intestinal tract. J Clin Invest. 20:749–764. 1941.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Peitzsch C, Kurth I, Kunz-Schughart L,
Baumann M and Dubrovska A: Discovery of the cancer stem cell
related determinants of radioresistance. Radiother Oncol.
108:378–387. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rich JN: Cancer stem cells in radiation
resistance. Cancer Res. 67:8980–8984. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pajonk F, Vlashi E and McBride WH:
Radiation resistance of cancer stem cells: The 4 R's of
radiobiology revisited. Stem Cells. 28:639–648. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie
MJ, Kulp AN, Qian D, Lam JS, Ailles LE, Wong M, et al: Association
of reactive oxygen species levels and radioresistance in cancer
stem cells. Nature. 458:780–783. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang P, Wei Y, Wang L, Debeb BG, Yuan Y,
Zhang J, Yuan J, Wang M, Chen D, Sun Y, et al: ATM-mediated
stabilization of ZEB1 promotes DNA damage response and
radioresistance through CHK1. Nat Cell Biol. 16:864–875. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Manikandan J, Aarthi JJ, Kumar SD and
Pushparaj PN: OncomiRs: The potential role of non-coding microRNAs
in understanding cancer. Bioinformation. 2:330–334. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Esquela-Kerscher A and Slack FJ: OncomiRs
- microRNAs with a role in cancer. Nat Rev Cancer. 6:259–269. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee YS and Dutta A: MicroRNAs in cancer.
Annu Rev Pathol. 4:199–227. 2009. View Article : Google Scholar :
|
|
12
|
Nana-Sinkam SP and Croce CM: MicroRNA
regulation of tumorigenesis, cancer progression and interpatient
heterogeneity: Towards clinical use. Genome Biol. 15:4452014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou W, Shi G, Zhang Q, Wu Q, Li B and
Zhang Z: MicroRNA-20b promotes cell growth of breast cancer cells
partly via targeting phosphatase and tensin homologue (PTEN). Cell
Biosci. 4:622014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li L, Xiao B, Tong H, Xie F, Zhang Z and
Xiao GG: Regulation of breast cancer tumorigenesis and metastasis
by miRNAs. Expert Rev Proteomics. 9:615–625. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang B, Pan X, Cobb GP and Anderson TA:
microRNAs as oncogenes and tumor suppressors. Dev Biol. 302:1–12.
2007. View Article : Google Scholar
|
|
16
|
Huang X, Taeb S, Jahangiri S, Emmenegger
U, Tran E, Bruce J, Mesci A, Korpela E, Vesprini D, Wong CS, et al:
miRNA-95 mediates radioresistance in tumors by targeting the
sphingolipid phosphatase SGPP1. Cancer Res. 73:6972–6986. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kato M, Paranjape T, Müller RU, Nallur S,
Gillespie E, Keane K, Esquela-Kerscher A, Weidhaas JB and Slack FJ:
The miR-34 microRNA is required for the DNA damage response in vivo
in C. elegans and in vitro in human breast cancer cells. Oncogene.
28:2419–2424. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Keller A, Leidinger P, Vogel B, Backes C,
ElSharawy A, Galata V, Mueller SC, Marquart S, Schrauder MG, Strick
R, et al: miRNAs can be generally associated with human pathologies
as exemplified for miR-144. BMC Med. 12:2242014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang J, Qin X, Sun Q, Guo H, Wu X, Xie F,
Xu Q, Yan M, Liu J, Han Z, et al: Transcriptional control of
PAX4-regulated miR-144/451 modulates metastasis by suppressing
ADAMs expression. Oncogene. 34:3283–3295. 2015. View Article : Google Scholar
|
|
20
|
DeSantis CE, Lin CC, Mariotto AB, Siegel
RL, Stein KD, Kramer JL, Alteri R, Robbins AS and Jemal A: Cancer
treatment and survivorship statistics, 2014. CA Cancer J Clin.
64:252–271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nakshatri H: Radiation resistance in
breast cancer: Are CD44+/CD24−/proteosome
low/PKH26+ cells to blame? Breast Cancer Res.
12:1052010. View
Article : Google Scholar
|
|
22
|
Tessner TG, Muhale F, Riehl TE, Anant S
and Stenson WF: Prostaglandin E2 reduces radiation-induced
epithelial apoptosis through a mechanism involving AKT activation
and bax translocation. J Clin Invest. 114:1676–1685. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cellini F, Morganti AG, Genovesi D,
Silvestris N and Valentini V: Role of microRNA in response to
ionizing radiations: Evidences and potential impact on clinical
practice for radiotherapy. Molecules. 19:5379–5401. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Iwaya T, Yokobori T, Nishida N, Kogo R,
Sudo T, Tanaka F, Shibata K, Sawada G, Takahashi Y, Ishibashi M, et
al: Downregulation of miR-144 is associated with colorectal cancer
progression via activation of mTOR signaling pathway.
Carcinogenesis. 33:2391–2397. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Guo Y, Ying L, Tian Y, Yang P, Zhu Y, Wang
Z, Qiu F and Lin J: miR-144 downregulation increases bladder cancer
cell proliferation by targeting EZH2 and regulating Wnt signaling.
FEBS J. 280:4531–4538. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Buffa FM, Camps C, Winchester L, Snell CE,
Gee HE, Sheldon H, Taylor M, Harris AL and Ragoussis J:
microRNA-associated progression pathways and potential therapeutic
targets identified by integrated mRNA and microRNA expression
profiling in breast cancer. Cancer Res. 71:5635–5645. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
McAllister SD, Murase R, Christian RT, Lau
D, Zielinski AJ, Allison J, Almanza C, Pakdel A, Lee J, Limbad C,
et al: Pathways mediating the effects of cannabidiol on the
reduction of breast cancer cell proliferation, invasion, and
metastasis. Breast Cancer Res Treat. 129:37–47. 2011. View Article : Google Scholar
|
|
28
|
Webb CP and Vande Woude GF: Genes that
regulate metastasis and angiogenesis. J Neurooncol. 50:71–87. 2000.
View Article : Google Scholar
|
|
29
|
Kalluri R and Neilson EG:
Epithelial-mesenchymal transition and its implications for
fibrosis. J Clin Invest. 112:1776–1784. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee JM, Dedhar S, Kalluri R and Thompson
EW: The epithelial-mesenchymal transition: New insights in
signaling, development, and disease. J Cell Biol. 172:973–981.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liang WT, Cheng ZY, Jia ZQ and Wang BY:
PTEN: A new target in inhibiting of tumor invasion and metastasis.
Sheng Li Ke Xue Jin Zhan. 42:201–205. 2011.In Chinese. PubMed/NCBI
|
|
32
|
Carracedo A and Pandolfi PP: The PTEN-PI3K
pathway: Of feedbacks and cross-talks. Oncogene. 27:5527–5541.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hühns M, Salem T, Schneider B, Krohn M,
Linnebacher M and Prall F: PTEN mutation, loss of heterozygosity,
promoter methylation and expression in colorectal carcinoma: Two
hits on the gene? Oncol Rep. 31:2236–2244. 2014.PubMed/NCBI
|
|
34
|
Kechagioglou P, Papi RM, Provatopoulou X,
Kalogera E, Papadimitriou E, Grigoropoulos P, Nonni A, Zografos G,
Kyriakidis DA and Gounaris A: Tumor suppressor PTEN in breast
cancer: Heterozygosity, mutations and protein expression.
Anticancer Res. 34:1387–1400. 2014.PubMed/NCBI
|
|
35
|
Mulholland DJ, Kobayashi N, Ruscetti M,
Zhi A, Tran LM, Huang J, Gleave M and Wu H: Pten loss and RAS/MAPK
activation cooperate to promote EMT and metastasis initiated from
prostate cancer stem/progenitor cells. Cancer Res. 72:1878–1889.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang ZX, Lu BB, Wang H, Cheng ZX and Yin
YM: MicroRNA-21 modulates chemosensitivity of breast cancer cells
to doxorubicin by targeting PTEN. Arch Med Res. 42:281–290. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ma F, Zhang J, Zhong L, Wang L, Liu Y,
Wang Y, Peng L and Guo B: Upregulated microRNA-301a in breast
cancer promotes tumor metastasis by targeting PTEN and activating
Wnt/β-catenin signaling. Gene. 535:191–197. 2014. View Article : Google Scholar
|