Introduction
Insulin-like growth factor (IGF)-binding protein-3
(IGFBP-3) is one of the six characterized binding partners of
IGF-I, and is the most abundant IGFBP found in circulation
(1). However, growing evidence
points to IGF-independent effects of IGFBP-3 on cell growth
(2). IGFBP-3 has been shown to act
as a primary growth inhibitor and can be induced by other
growth-inhibitory (and apoptosis-inducing) agents such as
transforming growth factor-β1 (TGF-β1), retinoic acid, tumor
necrosis factor-α (TNF-α) and the tumor-suppressor gene p53
(3). Growing evidence also suggests
that IGFBP-3 is involved in induction of apoptosis (4). Notably, IGFBP-3 promotes
ceramide-induced apoptosis in a breast cancer cell line (5) and enhances both p53-dependent
(6,7) and p53-independent (8) apoptosis. However, IGFBP-3 alone shows
little effect on cell apoptosis, indicating that other proteins are
necessary in this process.
Interleukin (IL)-24 was originally identified as
melanoma differentiation-associated gene-7 (mda-7), which is a
cytokine tumor suppressor located in a cluster on chromosome 1q32
encoding IL-10, -19 and -20 (9).
Interleukin-24 (IL-24) protein expression is lost during the
progression of melanoma tumors; virtually all metastatic melanomas
lack IL-24 expression (10). IL-24
functions as a pro-Th1 cytokine in human peripheral blood
mononuclear cells and induces secretion of IL-6, interferon (IFN)-γ
and TNF-α (11). Numerous studies
demonstrate potent antitumor functions of IL-24 in a broad spectrum
of human cancers, with no effects seen on normal cells (12).
This tumor cell-specific growth-inhibitory effect
has also been observed in multiple animal models in vivo and
in human clinical trials. IL-24 exerts its antitumor function
mainly through inhibition of the epidermal growth factor receptor
(EGFR) and phosphatidylinositol 3-kinase (PI3K) signaling pathways,
and the induction of expression of double-stranded RNA-activated
protein kinase (PKR) in human non-small cell lung cancer (NSCLC)
and breast cancer (13,14). The tumor suppressor activity of
IL-24 is independent of the status of other tumor suppressor genes,
such as p53, Rb, p16 or Ras (15,16).
IL-24 regulates many proliferative control mechanisms in tumor
cells and downregulates anti-apoptotic proteins (Bcl-2/Bcl-xL) and
upregulate pro-apoptotic proteins (Bax and Bak); this effect was
not seen in normal cells (17,18).
IL-24 has been established as a promising therapeutic candidate
with potent antitumor, antiangiogenic and cytokine activities.
However, the precise molecular mechanisms and signaling pathways of
IL-24 in melanoma suppression remain largely unknown.
The mammalian target of rapamycin (mTOR) is a highly
conserved serine/threonine kinase that regulates cell growth, cell
cycle progression and metabolism. The PI3K/AKT signaling pathway
activates mTOR, which in turn directly phosphorylates ribosome
protein S6 kinase 1 (S6K) and eIF4E-binding protein 1, both of
which are important in control of protein translation initiation
(19,20). S6K phosphorylates S6, which
regulates the translation of 50 terminal oligopyrimi-dine mRNAs
that encode ribosomal proteins and translational factors. 4EBP1
binds to and inhibits eIF4E, initiating cap-dependent translation.
mTOR is constitutively activated in the development of various
types of human cancers, including ovarian, pancreatic and lung
carcinomas (21). Thus, mTOR
signaling networks have emerged as attractive targets for novel
anticancer therapies.
IGFBP-3 is differentially expressed across normal
prostate tissue types (6) and may
be important in the regulation of prostate cell survival. However,
though IGFBP-3 is both antiproliferative and pro-apoptotic, the
molecular mechanisms behind its actions have not been elucidated.
In the present study, we found that IGFBP-3 selectively enhances
IL-24-induced cytotoxicity in prostate cancer (PC) cells, yet has
no effect on the survival of adenoma-derived cells, which are
resistant to IL-24-induced cell death. This result combined with
previous findings leads us to hypothesize that IGFBP-3 promotes
apoptosis through regulation of survival pathways activated in
response to the induction of apoptosis. This hypothesis potentially
explains why IGFBP-3 does not cause apoptosis when added directly
to cell cultures (6,8). Our present results show, for the first
time, that IGFBP-3 inhibits mTOR activation in response to
IL-24-induced apoptosis. Inhibition of mTOR activation is important
in improving efficacy of both chemotherapy and radiotherapy
(22). Therefore, we propose that
through inhibition of the mTOR pro-survival pathway, IGFBP-3
coupled with IL-24 may be a potent adjuvant in a number of cancer
treatment regimens.
Materials and methods
Reagents, cell lines and cell
culture
LNCap, PC-3, P69 and HEK293 cell lines were obtained
from the American Type Culture Collection (ATCC; Manassas, VA, USA)
and cultured in the recommended growth medium (Invitrogen,
Carlsbad, CA, USA) at 5% CO2, 37°C. HEK293 cells
transfected with, and stably expressing the pcDNA3 expression
vector containing IGFBP-3 (IGFBP-3 cDNA was constructed by our
laboratory). HEK293/IGFBP-3, and a vector control
(HEK293/pcDNA3-control) were grown in 10% fetal bovine serum (FBS)
Dulbecco's modified Eagle's medium (DMEM) supplemented with 200
mg/ml G418 for selection. Soluble recombinant human IGFBP-3 and
IL-24 were purchased from PeproTech, USA.
Treatment with IL-24
Cells were seeded in triplicate flasks and grown
under standard conditions until ~70% confluency. Cells were grown
for 24 h in a serum-free medium (SFM) to remove IGFBP-3 from the
serum and then grown for up to 24 h in SFM supplemented with or
without IL-24 (0.2, 0.4 and 0.8 µM) to induce apoptosis.
Measurement of cell viability
Cell viability was measured by mitochondrial
conversion of 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium
bromide (MTT) to a colored product. Following treatment with drugs,
MTT was added. Cells were then solubilized in dimethylsulphoxide
(DMSO). The amount of converted MTT was determined by measuring
absorbance at 570 nm.
Plasmids and siRNAs
Full-length Mcl-1 or IGFBP-3 cDNA was cloned into
the BamHI/XbaI sites of pcDNA3.1(+) (Invitrogen). S6K
cDNA containing an N-terminal Myc (plasmid 26610) was purchased
from Addgene (Cambridge, MA, USA). All constructs were verified by
DNA sequencing. Mcl-1 (sc-35877), adenosine monophosphate-activated
protein kinase (AMPK)α1/2 (sc-45312) and control (no. 1, sc-37007)
siRNAs were purchased from Santa Cruz Biotechnology (Santa Cruz CA,
USA). S6K (Hs_RPS6KB1_5) and control (1022076) siRNAs were
purchased from Qiagen (Valencia, CA, USA), and mTOR (6381) and
control (6568) siRNAs were purchased from Cell Signaling Technology
(Beverly, MA, USA). Transfection experiments with plasmids and
siRNAs were performed using Lipofectamine Plus and Lipofectamine
2000 (Invitrogen, UK) according to the manufacturer's
instructions.
Colony formation assay
LNCap cells were transfected with an IGFBP-3
overexpression vector or Mcl-1 siRNAs for 24 h. The cells were
reseeded in 60-mm plates and colony formation was monitored over
the subsequent 10 days.
Treatment with IL-24 or IGFBP-3
Cells were seeded in duplicate flasks and grown
under standard conditions until ~70% confluency, and then grown for
24 h in SFM to remove IGFBP-3 from the serum. The cells were then
grown for up to 24 h in SFM supplemented with IL-24 (0.2–0.8
µM). The secreted proteins were harvested from human
embryonic kidney HEK-(293) cells transfected with the IGFBP-3
pcDNA3 expression vector or from mock-transfected HEK293 cells.
Upon reaching 70% confluency, cells were transfected using
Lipofectamine 2000. After 6 h, the medium was replaced by SFM and
the cells were harvested 48 h later. The IGFBP-3 content was
adjusted to ~100 ng/ml by western blot analysis compared to IGFBP-3
protein standards (Upstate Biotechnology, USA).
Treatment with IL-24/IGF-IR antibody or
anti-IGFBP-3 antibodies
Cells were first incubated in SFM for 24 h, and then
in SFM plus an anti-IGF-IR antibody (Santa Cruz Biotechnology) or
an anti-IGFBP-3 antiserum at a content level of 5 µg/ml for
24 h, followed by 0.8 µM IL-24 in the presence or absence of
IGFBP-3 (GroPep, AUS). The cell yield and floating cell population
were counted using a Neubauer counting chamber (VWR, UK).
Assessment of IGFBP-3
Subconfluent monolayers were grown in SFM for 24 h.
The cells were then grown in SFM for 24 h, with or without IL-24.
The media were then harvested, centrifuged to remove floating cells
and stored at −70°C. The number of attached cells was quantified
using a haemocytometer. Proteins from conditioned medium were
concentrated using 'Microsep' 10 K centrifuge columns (Gelman
Laboratory). The levels of IGFBP-3 were assessed by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE)
immunoblotting and the IGFBP-3 protein was detected by anti-IGFBP-3
antiserum (polyclonal from Diagnostic System Laboratory, USA) using
an enhanced chemiluminescence (ECL) system (KPL, USA).
Western blotting
Cell lysates and immunoprecipitates were separated
by SDS-PAGE and transferred to nitrocellulose membranes, followed
by immunoblotting with the specified primary antibodies and
horseradish peroxidase-conjugated secondary antibodies.
Immunoreactive bands were visualized with SuperSignal West Pico
Chemiluminescent Substrates (Thermo Scientific Pierce, Rockford,
IL, USA).
Generation of recombinant
adenoviruses
Recombinant adenoviruses (pAd/CMV/IGFBP-3 or
pAd/CMV/IL-24) contained IGFBP-3 or IL-24 between the
cytomegalovirus (CMV) promoter and the polyadenylation signal
(SV40pA). These adenoviral vectors were propagated in HEK293 cells
(Invitrogen) using a Stratagene MBS mammalian transfection kit (La
Jolla, CA, USA) with a modified calcium phosphate transfection
protocol. The transfected cells were incubated at 37°C for 7 days,
harvested and subjected to 4 cycles of freezing (dry ice and
alcohol)/thawing in a 37°C water bath. The cell lysates were
centrifuged at 12,000 × g and 4°C for 10 min; then the supernatant
(primary virus stock) was transferred to a fresh screw-cap
mini-centrifuge tube and stored at −80°C. Recombinant adenoviruses
were further amplified using the same procedure; the cell lysates
were centrifuged with cesium chloride step gradients at 60,000 × g
and 4°C for 2 h, allowing the viruses to separate from the
defective particles and empty capsids. The recovered virus bands
were dialyzed 3 times against phosphate-buffered saline (PBS). The
viruses Ad5.IGFBP-3 or Ad5.IL-24 were aliquoted in a buffer
containing 10 mmol/l Tris-HCl (pH 8.1), 10 mmol/l MgCl2
and 10% v/v glycerol and then stored at −80°C.
Studies in vivo
Four-week-old athymic nude mice were obtained from
the Animal Research Center, Shanghai Laboratory Animal Research
Center, China. All procedures were performed in accordance with an
animal protocol approved by the First Affiliated Hospital of
Medical School, Xi'an Jiaotong University, and by the local
Experimental Ethics Committee. All efforts were made to minimize
animal suffering and reduce the number of animals used. Tumors were
established by subcutaneous injection of 5×106 LNcap
cells into the right flank of the mice. When a tumor grew to the
size of ~50 mm3, the host mouse was randomly assigned to
one of the 3 groups (each n=6): control (DMSO dissolved in 50
µl PBS and administered i.p. every other day), Ad5.IGFBP-3
(5×108 PFU in 50 µl of PBS, administered i.p.
every other day) and Ad5.IGFBP-3+Ad5.IL-24 (5×108 PFU in
50 µl of PBS, administered i.p. once daily). Tumor size was
measured every other day using calipers, and tumor volume
(mm3) was estimated as length × width2/2. The
mice were closely monitored for 24 days prior to euthanization and
removal of tumors. Each tumor was stored at −80°C for further
analysis.
Statistical analysis
All data represent the mean of 3 separate
experiments. Each experiment was carried out in duplicate or
triplicate parallel flasks. Each experiment was repeated 3 times,
and results are presented as the mean of the 3 separate
experiments. Statistical analysis was carried out using SPSS for
Windows statistical software (release 10.0.5; SPSS, Inc., Chicago,
IL, USA). Analysis of variance (ANOVA) was used to determine
differences among means. Pair-wise comparisons were made using
Tukey's post hoc test for multiple comparisons.
Results
LNCap and PC-3 lines are sensitive to
IL-24-induced proliferation inhibition, yet not the P69 cell
line
Differential sensitivity to IL-24 was observed in PC
cell lines. Whereas the LNCap and PC-3 cell lines were sensitive to
the inhibition of cell proliferation, the P69 cell line was not
sensitive to the drug treatment. However, as experiments on the
effects of IGFBP-3 were carried out in SFM, we initially sought to
verify that the differential sensitivity to IL-24-induced cell
proliferation inhibition was retained under serum-free conditions.
Therefore, both LNCap, PC-3 and P69 cells were treated with IL-24
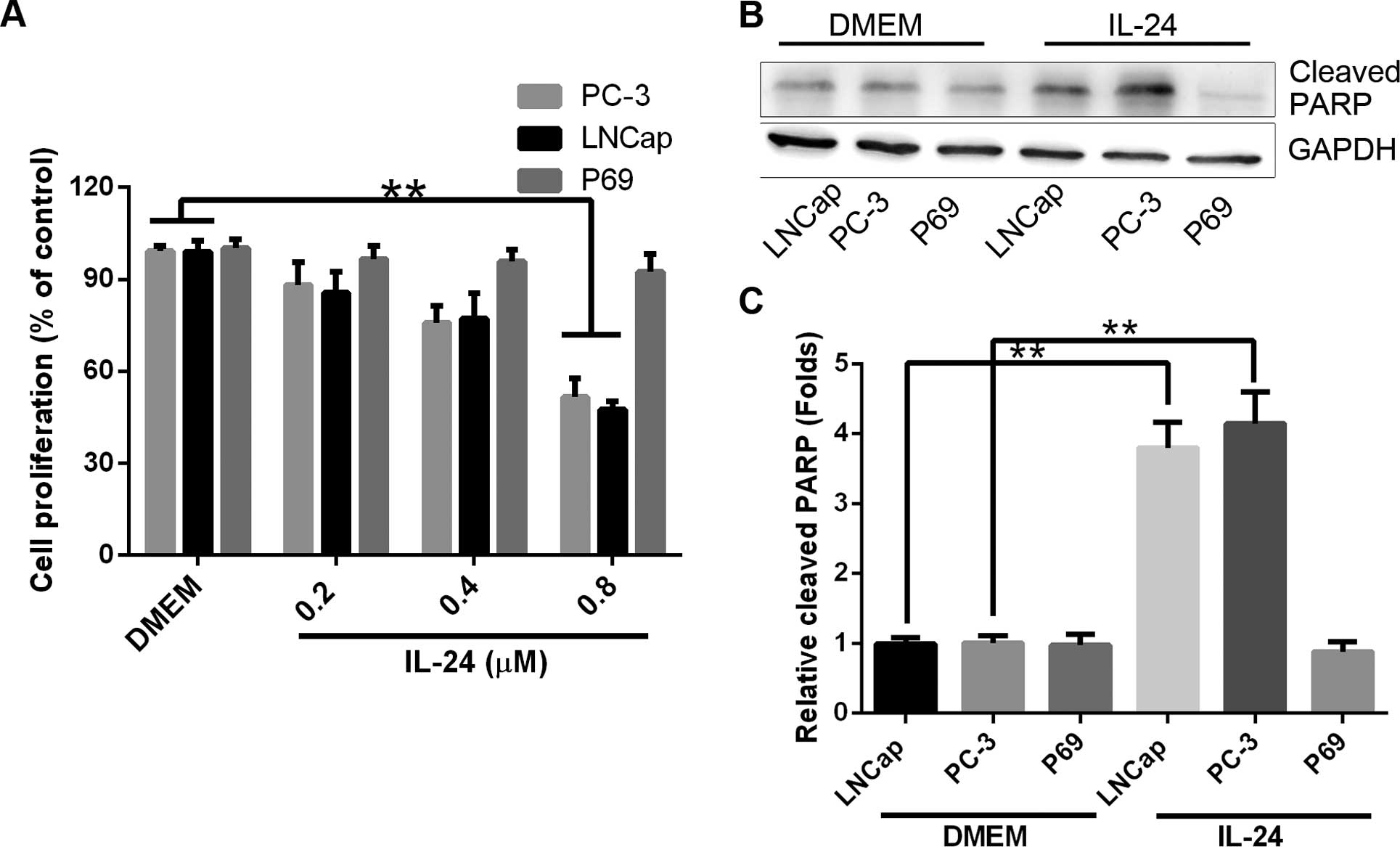
(0.2, 0.4 and 0.8 µM) for 24 h in SFM (Fig. 1A). In the 3 investigated tumorigenic
cell lines, there was a significant decrease in attached cell yield
and an associated increase in cell death [confirmed as apoptotic by
poly(ADP-ribose) polymerase (PARP) cleavage, Fig. 1B and C] in the LNCap and PC-3 cell
lines. In contrast, P69 cell line remained relatively resistant to
IL-24-induced cell proliferation inhibition.
IGFBP-3 potentiates IL-24-induced cell
proliferation inhibition in LNCap and PC-3 cells whereas not in the
P69 cell line
As the pro-apoptotic IGFBP-3 protein improves the
sensitivity of cancer cell lines to the induction of apoptosis
(6,8), we investigated whether IGFBP-3
affected the response of cells to IL-24-induced cell proliferation
inhibition. Cells were treated with 100 ng/ml IGFBP-3, which is a
physiologically relevant dose (6)
and equal to ~50 ng/106 cells. We investigated the
exogenous IGFBP-3 protein to control in vitro. Protein was
collected from the conditioned medium of HEK293 cells, which were
transiently transfected to express IGFBP-3 protein. The secreted
protein was harvested from the culture medium (referred to as
IGFBP-3 medium, IGFBP3-M). The resulting concentrated conditioned
medium contained ~100 ng/ml IGFBP-3 protein assessed by ELISA.
These experiments were controlled by treatment of parallel cultures
with concentrated media from mock-transfected HEK293 cells
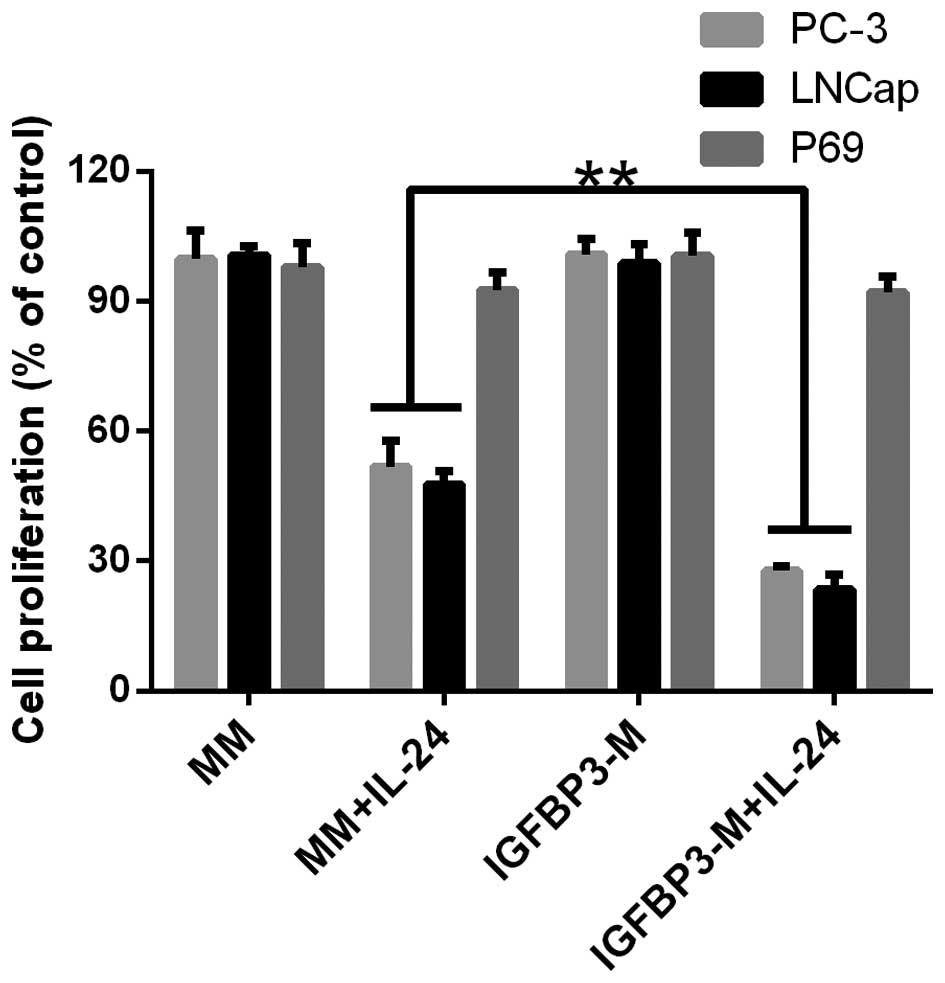
(referred to as mock media; MM). The results are shown in Fig. 2. Cancer cells were treated with
IL-24 at a dose of 0.8 µM to induce moderate cell
proliferation inhibition. For the PC cell lines, increasing the
amount of IGFBP-3 in the media significantly enhanced cellular
sensitivity to IL-24-induced cell proliferation inhibition
(Fig. 2). However, the addition of
IGFBP3-M to P69 had no effect on cell proliferation.
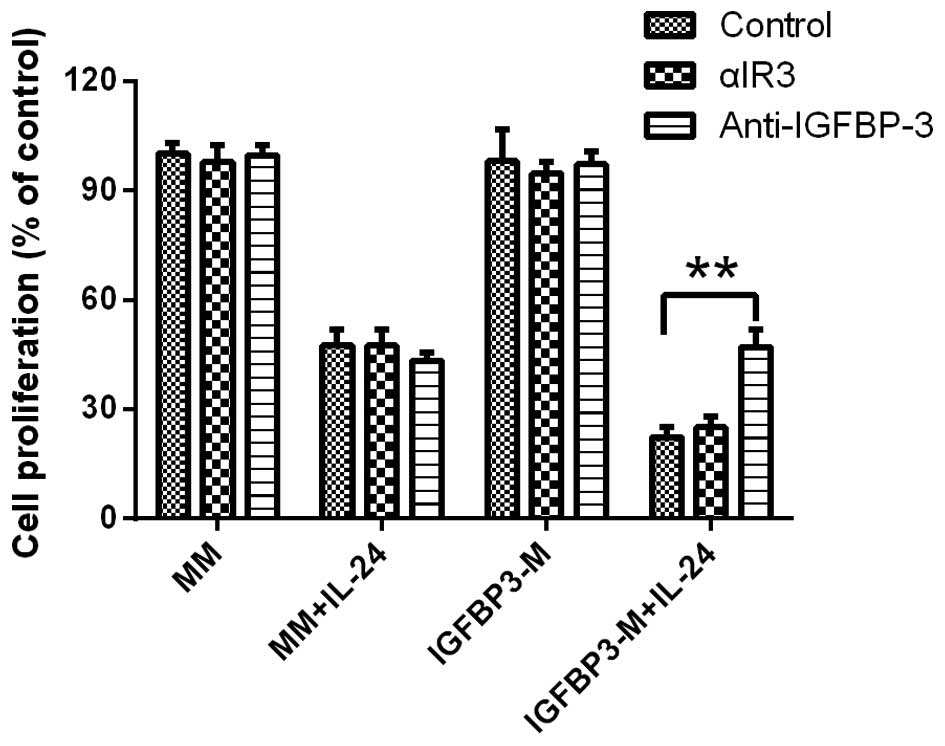
The IGF-1R blocking antibody αIR3 (5 µg/ml)
failed to block IGFBP-3 potentiation of IL-24-induced cell
proliferation inhibition in LNCap cell line, indicating that the
cell proliferation decreasing observed was not due to the
inhibition of IGF-dependent signaling pathways (Fig. 3). Furthermore, the potentiation of
IGFBP-3 in IL-24-induced cell proliferation inhibition was
completely blocked by the addition of a neutralizing IGFBP-3
antibody, confirming that IGFBP-3 promotes IL-24-induced cell
proliferation inhibition.
IGFBP-3 overexpression leads to
inactivity of mTOR, which plays an important role in Mcl-1
downregulation
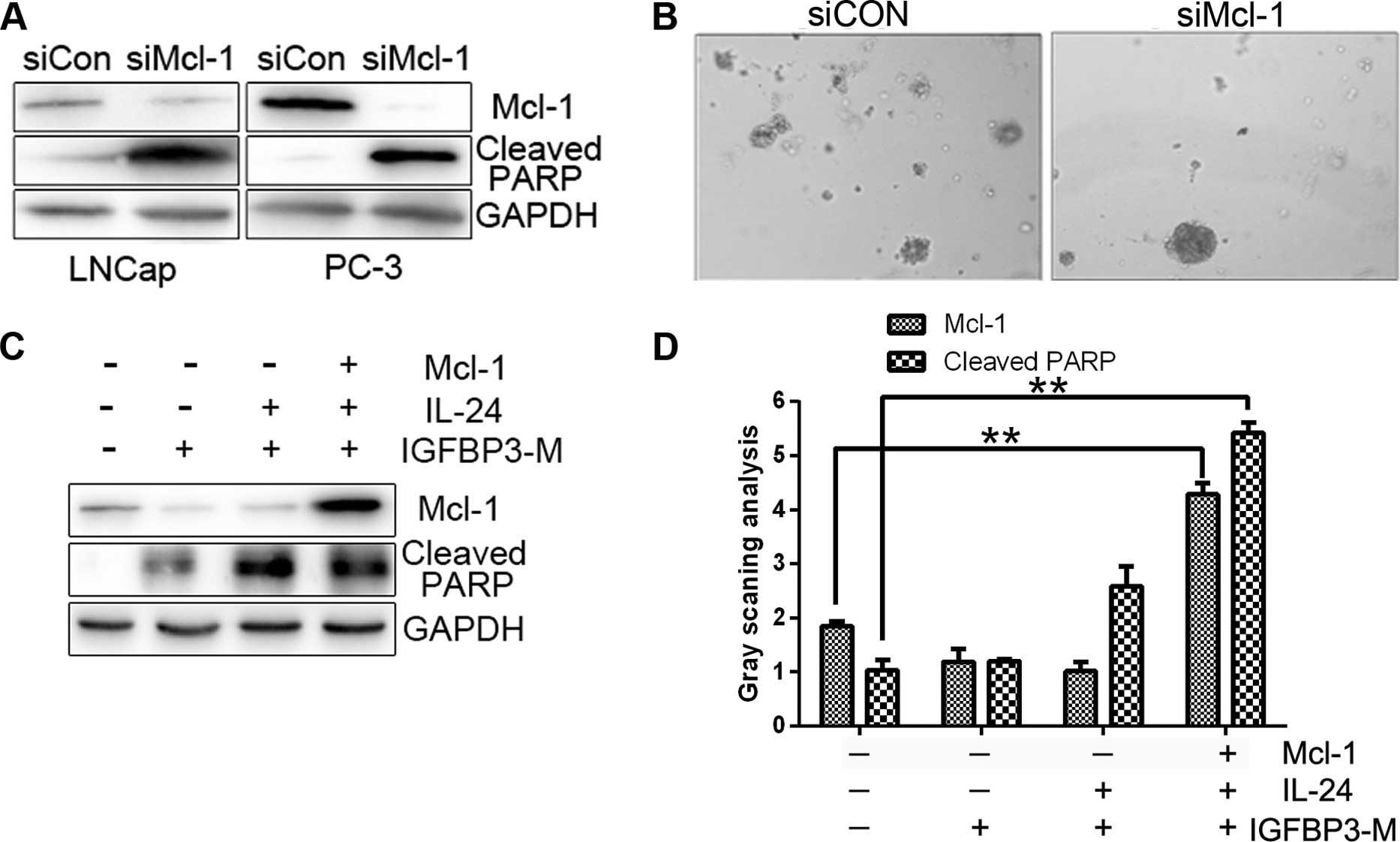
A recent study shows that PC cell lines express high
levels of Mcl-1, and that suppression of Mcl-1 induces significant
cell death (23). As expected, the
downregulation of Mcl-1 with siRNA led to increased cell death and
PARP protein cleavage in our experiments (Fig. 4A). Colony formation was also
diminished in the presence of Mcl-1 siRNA compared with the control
siRNA (Fig. 4B). To further clarify
whether Mcl-1 plays an essential role in the survival of PC cells,
we transfected Mcl-1 plasmids into cells treated with IGFBP3-M and
IL-24 protein. The level of IGFBP3-M and IL-24-induced PARP
cleavage were reduced upon overexpression of Mcl-1 (Fig. 4C and D). Our results indicate that
downregulation of Mcl-1 is critical for the sensitizing effect of
IGFBP-3 on IL-24-induced cell death.
In the present study, we have shown that IGFBP-3
potentiates IL-24-induced apoptosis in PC cell lines. Adding to the
former findings that IGFBP-3 enhanced apoptosis by suppressing
certain survival signalling pathway. We then investigated the
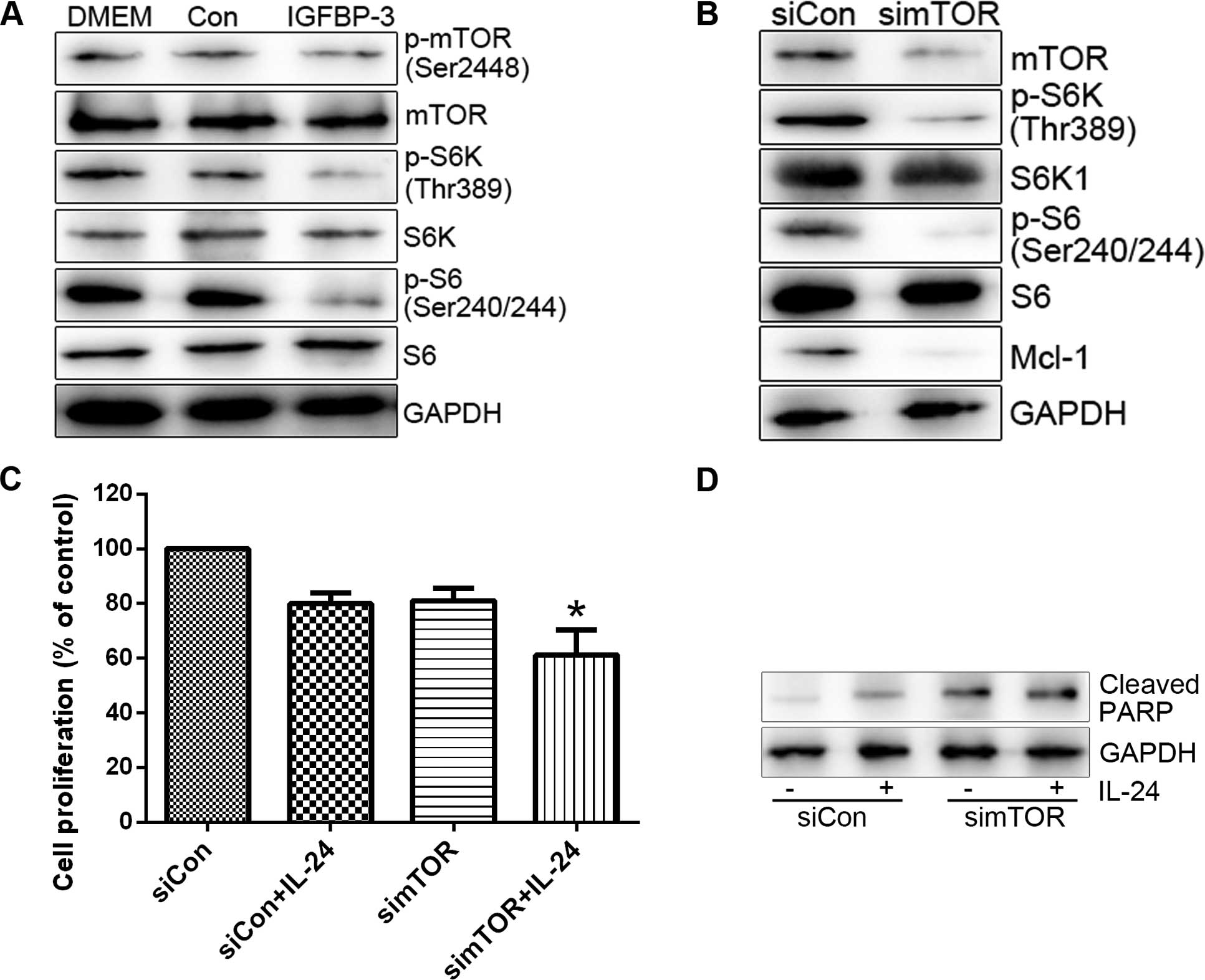
mechanism by which IGFBP-3 overexpression suppresses Mcl-1 protein.
Initially, mTOR activity was assessed in cells treated with
IGFBP3-M. IGFBP3-M suppressed mTOR activity, assessed on basis of
weakened phosphorylation of mTOR and its effectors (S6K and S6) in
LNCap cells (Fig. 5A). Since mTOR
promotes cell survival through translational control of Mcl-1
(24), we determined the level of
Mcl-1 protein in cells treated with mTOR siRNA. As expected,
suppression of mTOR resulted in decreased expression of Mcl-1
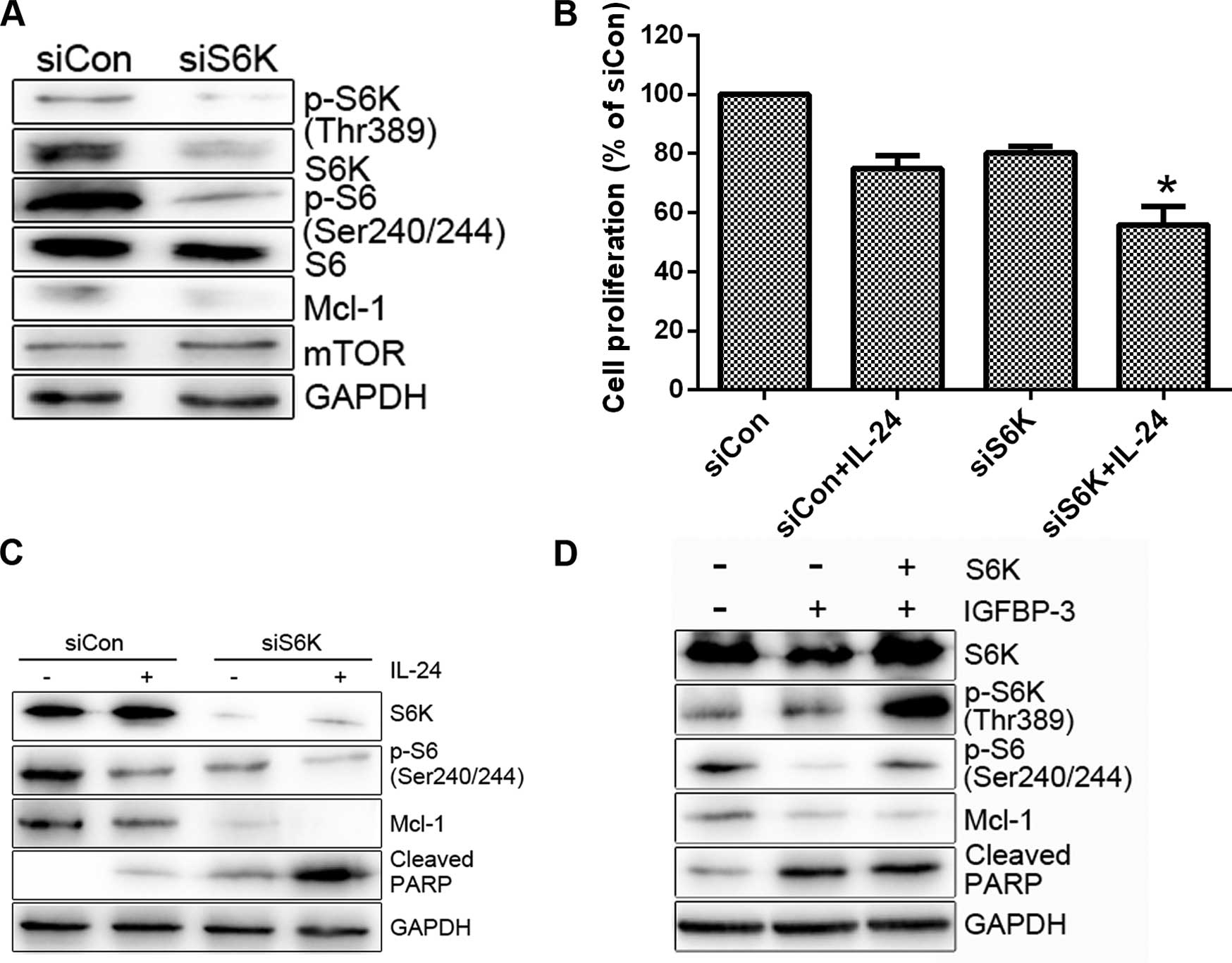
protein and enhanced LNCap cell sensitivity to IL-24 (Fig. 5B and C). S6K as a downstream target
of mTOR plays important roles in cell proliferation, protein
translation and cell survival and is thus an effective target in
cancer therapy (25). To ascertain
whether S6K is involved in the regulation of Mcl-1 expression and
consequent cell survival, we transfected the cells with S6K siRNA
and then treated them with IL-24. S6K siRNA induced a decrease in
Mcl-1 protein levels compared to the control siRNA (Fig. 6A). Moreover, knockdown of S6K
enhanced IL-24-induced cell death (Fig.
6B and C). Overexpression of S6K alleviated IGFBP3-M-induced
PARP cleavage (Fig. 6D). Based on
these results, we propose that mTOR activity is suppressed upon
IGFBP-3 overexpression and is critical for Mcl-1
downregulation.
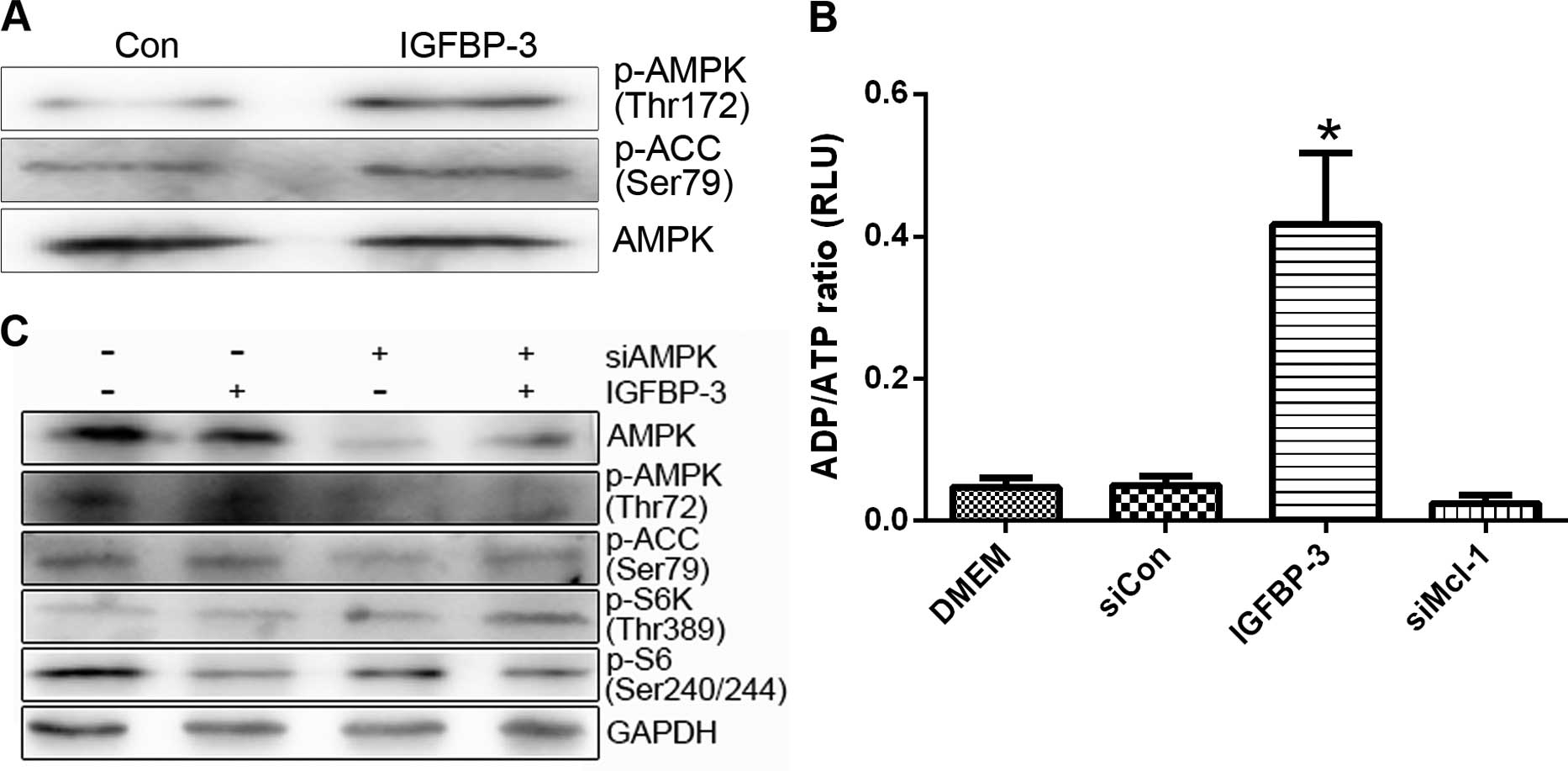
Overexpression of IGFBP-3 leads to
inhibition of mTOR through activation of AMPK
mTOR activity can be suppressed upon energy
deprivation, as depleted ATP levels trigger AMPK activation
(26). We further investigated
whether AMPK is involved in the mTOR inhibition induced by IGFBP-3
overexpression. Treatment with IGFBP3-M stimulated the
phosphorylation of AMPK (p-AMPK) and its substrate (acetyl-CoA
carboxylase, p-ACC) in LNCap and PC-3 cells (Fig. 7A). Moreover, overexpression of
IGFBP-3 led to a marked increase in the cellular ADP/ATP ratio,
indicating a decrease in ATP content in both cell lines (Fig. 7B). To clarify the involvement of
AMPK in IGFBP3-M-induced mTOR inactivity, cells overexpressing
IGFBP-3 were transfected with AMPKα 1/2 siRNA. Downregulation of
AMPK led to recovery of mTOR activity (Fig. 7C). These results suggest that
IGFBP-3 overexpression is associated with ATP depletion, leading to
AMPK-induced mTOR inhibition.
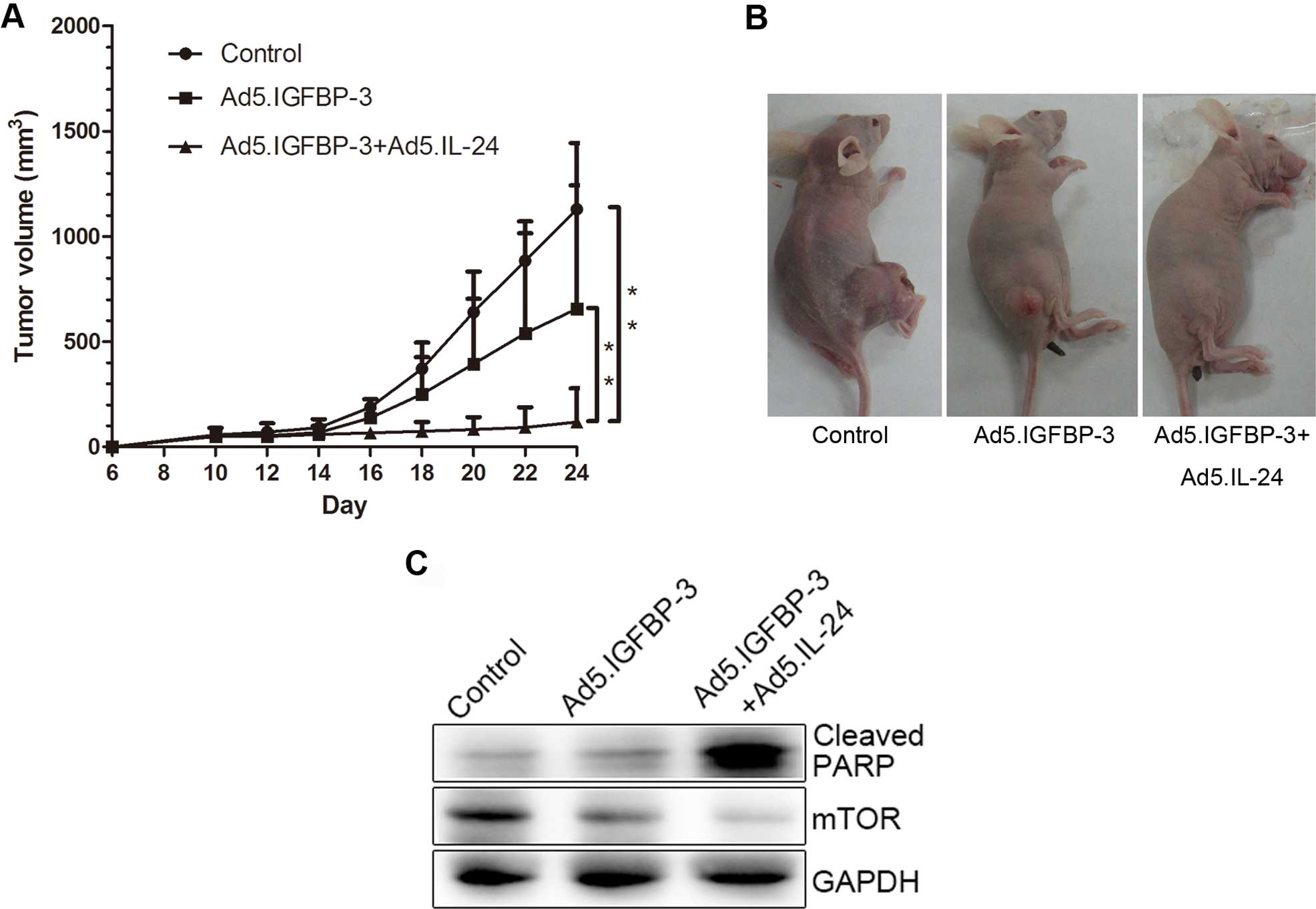
IGFBP-3 and IL-24 significantly inhibit
growth of human PC cells in vivo
To investigate the effects of IGFBP-3 and IL-24 on
cell growth in vivo, LNcap cells were subcutaneously
xenografted in nude mice. Tumor volume was reduced in the
Ad5.IGFBP-3 group compared with the control group. After treatment
with a combination of Ad5.IGFBP-3 and Ad5.IL-24, tumor size was
significantly reduced compared with the control group (P<0.01)
or the Ad5.IGFBP-3 group (P<0.01), indicating a higher
suppression of tumor growth in vivo (Fig. 8A and B). Western blotting of tumor
tissue lysates also revealed that the expression of PARP was
significantly upregulated and mTOR was downregulated in the
Ad5.IGFBP-3+Ad5.IL-24 group to a higher extent than in the
Ad5.IGFBP-3 group (Fig. 8C). These
results are consistent with our studies in vitro, providing
further evidence that IL-24 potentiates the antitumor activity of
IGBP-3 in vivo.
Discussion
IGF-binding protein-3 (IGFBP-3) is a pro-apoptotic
protein previously shown to potentiate apoptotic cell death in a
number of systems. In the present study, we showed that in human
prostate cancer (PC) cells IGFBP-3 promotes IL-24-induced
apoptosis. This important finding suggests that IGFBP-3 may be used
to improve IL-24-induced apoptosis in cancer cells without
affecting the survival of non-tumorigenic cells.
Apoptosis is induced by both extrinsic and intrinsic
pathways initiated by the activation of death receptors and
stress-inducing stimuli (27).
Results from the present study contribute to the current literature
demonstrating that IGFBP-3 enhances both extrinsic and intrinsic
apoptotic pathways (4).
Furthermore, IGFBP-3 is pro-apoptotic in several cell systems
(breast, esophageal, colonic cancer cells), although its direct
addition to cells will not induce apoptosis (7,8). These
observations led us to hypothesize that instead of being an
integral part of cell death signaling, IGFBP-3 instead modify the
activity of pathways that modulate the cellular response to
apoptosis. This hypothesis represents a more general mechanism by
which IGFBP-3 is able to potentiate many death-inducing
pathways.
The mTOR pathway plays multiple roles in cell
growth, proliferation and survival. In the present study, we
determined whether mTOR and its downstream factors are involved in
IL-24 sensitization induced by IGFBP-3 overexpression.
Unexpectedly, IGFBP-3 overexpression led to reduced levels of
phosphorylated mTOR, as well as its downstream effector S6K.
Downregulation of mTOR and S6K using siRNA made cells more
sensitive to IL-24. Importantly, we showed that knockdown of either
mTOR or S6K downregulates Mcl-1 protein levels. These results
indicated that Mcl-1 is a downstream target of mTOR and S6K.
Furthermore, Mcl-1 may be regulated at the translational level, as
the main function of mTOR is to control translation via direct
regulation of S6K and 4E-BP. This assumption is supported by
several studies in the literature that Mcl-1 is a potential
downstream target of eIF4E, which contains a unique G+C-rich 5′
untranslated region among the anti-apoptotic Bcl-2 members
(24,28). In addition, inhibition of mTOR with
rapamycin blocked the synthesis of Mcl-1 protein, but not that of
Bcl-2 or Bcl-xL.
Surprisingly, overexpression of IGFBP-3 led to a
marked increase in the ADP/ATP ratio within cells, indicating a
decrease in ATP content. AMPK is a central cellular energy-sensing
system that actively participates in the metabolism-cancer
interaction via regulation of the mTOR pathway. AMPK activation
directly phosphorylates and activates TSC2 by enhancing its GAP
activity, leading to inhibition of mTOR signaling (29). Our findings confirm that
overexpression of IGFBP-3 leads to AMPK activation and consequently
mTOR inhibition. Downregulation of AMPK with AMPKα1/2 siRNA
counteracted the inhibition of mTOR activity by IGFBP3-M. The
present study, shows that overexpression of IGFBP-3 is associated
with ATP depletion, leading to AMPK-induced mTOR inhibition. This
finding highlights a novel underlying mechanism linking the IGFBP-3
and mTOR pathways. However, further research is necessary to
clarify the exact mechanisms that link the overexpression of
IGFBP-3 to ATP-depletion and subsequent activation of AMPK.
In summary, through inhibiting mTOR activation,
IGFBP-3 increases the sensitivity of cancer cells to IL-24-induced
apoptosis and may also be important in both the prevention of PC,
and as a potential adjuvant for a number of other therapeutic
regimens used in the treatment of metastatic prostate cancer.
Acknowledgments
The present study was supported by the National
Science Foundation of China (grant nos. 30901500, 81072107 and
81372736), and the Science and Technology Program of Shaanxi
Province (grant no. 2009JQ4002).
References
|
1
|
Gill ZP, Perks CM, Newcomb PV and Holly
JM: Insulin-like growth factor-binding protein (IGFBP-3)
predisposes breast cancer cells to programmed cell death in a
non-IGF-dependent manner. J Biol Chem. 272:25602–25607. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nakamura M, Takakura M, Fujii R, Maida Y,
Bono Y, Mizumoto Y, Zhang X, Kiyono T and Kyo S: The PRB-dependent
FOXO1/IGFBP-1 axis is essential for progestin to inhibit
endometrial epithelial growth. Cancer Lett. 336:68–75. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hanafusa T, Shinji T, Shiraha H, Nouso K,
Iwasaki Y, Yumoto E, Ono T and Koide N: Functional promoter
upstream p53 regulatory sequence of IGFBP3 that is silenced by
tumor specific methylation. BMC Cancer. 5:92005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Baxter RC, Butt AJ, Schedlich LJ and
Martin JL: Antiproliferative and pro-apoptotic activities of
insulin-like growth factor-binding protein-3. Growth Horm IGF Res.
10(10 Suppl A): S10–S11. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ho L, Stojanovski A, Whetstone H, Wei QX,
Mau E, Wunder JS and Alman B: Gli2 and p53 cooperate to regulate
IGFBP-3-mediated chondrocyte apoptosis in the progression from
benign to malignant cartilage tumors. Cancer Cell. 16:126–136.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Butt AJ and Williams AC: IGFBP-3 and
apoptosis - a license to kill? Apoptosis. 6:199–205. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Williams AC, Collard TJ, Perks CM, Newcomb
P, Moorghen M, Holly JM and Paraskeva C: Increased p53-dependent
apoptosis by the insulin-like growth factor binding protein IGFBP-3
in human colonic adenoma-derived cells. Cancer Res. 60:22–27.
2000.PubMed/NCBI
|
|
8
|
Hollowood AD, Lai T, Perks CM, Newcomb PV,
Alderson D and Holly JM: IGFBP-3 prolongs the p53 response and
enhances apoptosis following UV irradiation. Int J Cancer.
88:336–341. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Collard TJ, Guy M, Butt AJ, Perks CM,
Holly JM, Paraskeva C and Williams AC: Transcriptional upregulation
of the insulin-like growth factor binding protein IGFBP-3 by sodium
butyrate increases IGF-independent apoptosis in human colonic
adenoma-derived epithelial cells. Carcinogenesis. 24:393–401. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang EY, Madireddi MT, Gopalkrishnan RV,
Leszczyniecka M, Su Z, Lebedeva IV, Kang D, Jiang H, Lin JJ,
Alexandre D, et al: Genomic structure, chromosomal localization and
expression profile of a novel melanoma differentiation associated
(mda-7) gene with cancer specific growth suppressing and apoptosis
inducing properties. Oncogene. 20:7051–7063. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ellerhorst JA, Prieto VG, Ekmekcioglu S,
Broemeling L, Yekell S, Chada S and Grimm EA: Loss of MDA-7
expression with progression of melanoma. J Clin Oncol.
20:1069–1074. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chada S, Sutton RB, Ekmekcioglu S,
Ellerhorst J, Mumm JB, Leitner WW, Yang HY, Sahin AA, Hunt KK,
Fuson KL, et al: MDA-7/IL-24 is a unique cytokine - tumor
suppressor in the IL-10 family. Int Immunopharmacol. 4:649–667.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dent P, Yacoub A, Hamed HA, Park MA, Dash
R, Bhutia SK, Sarkar D, Wang XY, Gupta P, Emdad L, et al: The
development of MDA-7/IL-24 as a cancer therapeutic. Pharmacol Ther.
128:375–384. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu S, Oshima T, Imada T, Masuda M, Debnath
B, Grande F, Garofalo A and Neamati N: Stabilization of MDA-7/IL-24
for colon cancer therapy. Cancer Lett. 335:421–430. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gupta P, Emdad L, Lebedeva IV, Sarkar D,
Dent P, Curiel DT, Settleman J and Fisher PB: Targeted
combinatorial therapy of non-small cell lung carcinoma using a
GST-fusion protein of full-length or truncated MDA-7/IL-24 with
Tarceva. J Cell Physiol. 215:827–836. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Deng WG, Kwon J, Ekmekcioglu S, Poindexter
NJ and Grimm EA: IL-24 gene transfer sensitizes melanoma cells to
erlotinib through modulation of the Apaf-1 and Akt signaling
pathways. Melanoma Res. 21:44–56. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sauane M, Gopalkrishnan RV, Sarkar D, Su
ZZ, Lebedeva IV, Dent P, Pestka S and Fisher PB: MDA-7/IL-24: Novel
cancer growth suppressing and apoptosis inducing cytokine. Cytokine
Growth Factor Rev. 14:35–51. 2003. View Article : Google Scholar
|
|
18
|
Mhashilkar AM, Schrock RD, Hindi M, Liao
J, Sieger K, Kourouma F, Zou-Yang XH, Onishi E, Takh O, Vedvick TS,
et al: Melanoma differentiation associated gene-7 (mda-7): A novel
anti-tumor gene for cancer gene therapy. Mol Med. 7:271–282.
2001.PubMed/NCBI
|
|
19
|
Chada S, Mhashilkar AM, Liu Y, Nishikawa
T, Bocangel D, Zheng M, Vorburger SA, Pataer A, Swisher SG, Ramesh
R, et al: mda-7 gene transfer sensitizes breast carcinoma cells to
chemotherapy, biologic therapies and radiotherapy: Correlation with
expression of bcl-2 family members. Cancer Gene Ther. 13:490–502.
2006. View Article : Google Scholar
|
|
20
|
Lebedeva IV, Sarkar D, Su ZZ, Kitada S,
Dent P, Stein CA, Reed JC and Fisher PB: Bcl-2 and
Bcl-xL differentially protect human prostate cancer
cells from induction of apoptosis by melanoma differentiation
associated gene-7, mda-7/IL-24. Oncogene. 22:8758–8773. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ma XM and Blenis J: Molecular mechanisms
of mTOR-mediated translational control. Nat Rev Mol Cell Biol.
10:307–318. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
She QB, Halilovic E, Ye Q, Zhen W,
Shirasawa S, Sasazuki T, Solit DB and Rosen N: 4E-BP1 is a key
effector of the oncogenic activation of the AKT and ERK signaling
pathways that integrates their function in tumors. Cancer Cell.
18:39–51. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shaw RJ and Cantley LC: Ras, PI(3)K and
mTOR signalling controls tumour cell growth. Nature. 441:424–430.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cusack JC Jr: Overcoming antiapoptotic
responses to promote chemosensitivity in metastatic colorectal
cancer to the liver. Ann Surg Oncol. 10:852–862. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fabbri F, Brigliadori G, Carloni S, Ulivi
P, Vannini I, Tesei A, Silvestrini R, Amadori D and Zoli W:
Zoledronic acid increases docetaxel cytotoxicity through pMEK and
Mcl-1 inhibition in a hormone-sensitive prostate carcinoma cell
line. J Transl Med. 6:432008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mills JR, Hippo Y, Robert F, Chen SM,
Malina A, Lin CJ, Trojahn U, Wendel HG, Charest A, Bronson RT, et
al: mTORC1 promotes survival through translational control of
Mcl-1. Proc Natl Acad Sci USA. 105:10853–10858. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chan S: Targeting the mammalian target of
rapamycin (mTOR): A new approach to treating cancer. Br J Cancer.
91:1420–1424. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Petroulakis E, Mamane Y, Le Bacquer O,
Shahbazian D and Sonenberg N: mTOR signaling: Implications for
cancer and anticancer therapy. Br J Cancer. 96(Suppl): R11–R15.
2007.PubMed/NCBI
|
|
29
|
Adams JM: Ways of dying: Multiple pathways
to apoptosis. Genes Dev. 17:2481–2495. 2003. View Article : Google Scholar : PubMed/NCBI
|