Introduction
Esophageal squamous cell carcinoma (ESCC) is a
common malignancy worldwide (1). As
a result of lymph node metastases, deep tumor invasion and
difficulty in early diagnosis, the majority of esophageal cancer
patients have a relatively low survival rate (2,3).
Despite the many advances in surgery, chemotherapy and nutritional
aid therapies at present, the long-term ESCC survival rate has only
slightly improved in recent years (4). Therefore, it is a formidable task to
study and explore novel biomarkers or therapeutic targets for ESCC
patients. Recently some research findings have indicated that the
initiation and development of ESCC involved the mutation of
numerous oncogenes and anti-oncogenes, and thus were characterized
by the synergetic effect of multiple genes, factors and steps
(5,6), yet more effort is still needed to
study the genetic and molecular changes underlying the development
of ESCC.
MicroRNAs (miRNAs) are small non-coding RNAs with
19–23 nucleotides (7,8). Numerous studies have been performed on
the role and related mechanism of miRNAs in numerous different
kinds of diseases (9–12). miRNAs bind primarily to the
3′-untranslated region (3′UTR) of their target messenger RNAs
(mRNAs) to reduce their stability and decrease the expression of
target mRNAs at the post-transcriptional level (13), which play important roles in various
biological processes including cell growth, proliferation,
differentiation and death (14–19).
Concerning chemotherapy in various types of cancers such as breast
cancer, lung adenocarcinoma, glioblastoma, and ovarian cancer,
recent studies have shown that miRNAs also act in important roles
(20–24). In ESCC, altered expression of
miR-1290, miR-655, miR-375 and others, has been observed (25–27),
suggesting that miRNA deregulation plays an important role in ESCC
development.
Recently, miR-1291 was found to be significantly
downregulated in pancreatic and renal cell carcinomas, and
restoration of miR-1291 function repressed tumorigenesis. To the
best of our knowledge, there is no previous study concerning
miR-1291 in ESCC biology. To gain insight into the potential
mechanisms of miR-1291 in ESCC, we performed the relevant
bioinformatic analyses using TargetScan and miRanda (28), and found that mucin 1 (MUC1) is a
potential target of miR-1291. A previous study showed that the
alteration of MUC1 is correlated with regional lymph node
metastasis, and high-expression of MUC1 is associated with poor
prognosis for esophageal cancer patients (29). Based on the above information,
miR-1291 and MUC1 may play roles in the development of ESCC. The
present study investigated miR-1291 and MUC1 expression levels in
ESCC tissues from 54 patients, and evaluated the effect of miR-1291
upregulation on proliferation, invasion and apoptosis of ESCC
cells, which provides new insight into the potential mechanisms of
ESCC and identify a new possible target for early diagnosis or
therapy.
Materials and methods
Clinical sample collection
Paired tumorous and adjacent non-tumorous tissues
were obtained from 54 patients with ESCC who underwent radical
resection in the First Affiliated Hospital of Zhengzhou University
and the First Affiliated Hospital of Luohe Medical College between
2012 and 2014 (Table I). Tissues
were snap-frozen in liquid nitrogen and identified by pathological
examinations after resection. None had received chemotherapy or
radiotherapy before surgery. All patients consented to the use of
their tissue samples in the present study. Human Research Ethics
Committee of Zhengzhou University and Luohe Medical College
approved the present study.
 | Table IClinicopathological characteristics
and the expression of miRNA-1291 and MUC1 mRNA of ESCC
patients. |
Table I
Clinicopathological characteristics
and the expression of miRNA-1291 and MUC1 mRNA of ESCC
patients.
| Variables | n | miRNA-1291
expression (median ± SD) | P-value | Relative expression
of MUC1 mRNA (median ± SD) | P-value |
|---|
| Gender | | | 0.511 | | 0.066 |
| Male | 39 | 0.6159±0.32902 | | 1.3410±0.20273 | |
| Female | 15 | 0.5507±0.31224 | | 1.4527±0.17527 | |
| Age (years) | | | 0.080 | | 0.542 |
| ≥60 | 33 | 0.5364±0.25019 | | 1.3855±0.16354 | |
| <60 | 21 | 0.6943±0.40011 | | 1.3510±0.25058 | |
|
Differentiation | | | 0.072 | |
0.028a |
| Well | 15 | 0.5960±0.35603 | | 1.2693±0.22008 | |
| Moderate | 31 | 0.6584±0.32421 | | 1.3919±0.19580 | |
| Poor | 8 | 0.3663±0.08782 | | 1.4875±0.05548 | |
| Tumor location | | | 0.519 | |
0.001a |
| Low | 12 | 0.5442±0.35748 | | 1.2108±0.21660 | |
| Middle | 42 | 0.6131±0.31525 | | 1.4181±0.17178 | |
| TNM stage | | |
0.004a | | 0.232 |
| I | 14 | 0.7586±0.45578 | | 1.3207±0.26409 | |
| II | 29 | 0.6172±0.24326 | | 1.3645±0.19642 | |
| III | 11 | 0.3418±0.08577 | | 1.4573±0.04628 | |
| Nodal status | | |
<0.001a | |
0.002a |
| Positive | 37 | 0.7014±0.33548 | | 1.3159±0.21554 | |
| Negative | 17 | 0.3724±0.11519 | | 1.4941±0.07272 | |
Cell lines and cell culture
Human ESCC EC9706 and EC-1 cell lines were purchased
from the Type Culture Collection of the Chinese Academy of Sciences
(Shanghai, China), were maintained in a RPMI-1640 medium containing
100 U/ml penicillin, 100 µl/ml streptomycin and 10% fetal
bovine serum (FBS; Gibco-BRL, Gaithersburg, MD, USA) and incubated
at 37°C and 5% CO2.
RNA oligo-ribonucleotides and cell
transfection
miR-1291 mimic and a negative control (NC) were
chemically synthesized by Shanghai GenePharma Co. Ltd. The amount
of miR-1291 mimic and NC is 7.92 µg/4×106 cells,
respectively. Transfection was performed with a BTX ECM 2001 square
wave electroporator (Genetronics Inc., San Diego, CA, USA) with
electroporation settings adjusted according to the BTX ECM 2001
protocol. After transfection, EC9706 and EC-1 cells were seeded in
6-well plates (2×105 cells/well). Cells from each cell
line were subdivided into three groups: the non-transfected blank
(blank), a mimic NC-transfected (NC) and the miR-1291
mimic-transfection groups (miR-1291). Cells were harvested for
further experiments after 24–48 h post-transfection.
RNA extraction and quantitative real-time
PCR
Relative levels of miR-1291 and MUC1 mRNA in ESCC
tissue samples and adjacent non-tumorous tissue samples were
determined by quantitative real-time PCR (qRT-PCR) assays. Total
RNA was extracted with an RNA extraction kit (Qiagen, Venlo, The
Netherlands), and RNA quality was confirmed using a NanoDrop 1000
spectrophotometer (Thermo Fisher Scientific, Wilmington, DE, USA).
Reverse transcription was performed using MMLV RTase cDNA synthesis
kit (Takara, Dalian, China). A cDNA library of miRNAs was
constructed by QuantiMir cDNA kit (Takara). All protocols were
performed according to the manufacturers' instructions, and qPCR
was performed using the ABI Power SYBR-Green PCR Master Mix
(Applied Biosystems, Foster City, CA, USA). The relative expression
of miR-1291 was calculated using the comparative cycle threshold
(CT; 2−ΔCt) method with U6 snRNA as an endogenous
control to normalize the data. β-actin was used as normalization
for the relative levels of MUC1 mRNA. The calculation method was
the same as presented.
Western blotting
Proteins were precipitated and determined by western
blot analysis following the previously described procedures
(26). Proteins were subjected to
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE) and transferred onto polyvinylidene difluoride (PVDF)
membranes. After blocking, membranes were incubated overnight at
4°C with the diluted (1:500) primary antibody (polyclonal rabbit
anti-MUC1; Santa Cruz). Following extensive washing, the membranes
were incubated with the diluted (1:3,000) horseradish
peroxidase-conjugated goat anti-rabbit IgG (Santa Cruz). Signals
were detected using a chemiluminescence detection kit (Amersham
Pharmacia Biotech, Piscataway, NJ, USA). An antibody against
β-actin (Santa Cruz) served as an endogenous reference. Relative
protein levels were calculated using β-actin as a loading
control.
Cell Counting Kit-8 (CCK-8) assay
We used the CCK-8 assay (Dojindo Laboratories,
Japan) according to the manufacturer's instructions to determine
cell viability. Briefly, cells were seeded at a density of
2×103 cells/well in 96-well plates (in three replicate
wells) and treated daily for four consecutive days with 10
µl/well of CCK-8 solution. Optical density was measured at
450 nm to estimate the number of viable cells.
Colony formation assay
Twenty-four hours after RNA transfection, cells were
suspended in 0.3% agarose with RPMI-1640 containing 10% FBS and
plated into four 6-cm cell culture dishes on top of an existing
layer of 0.6% agarose prepared with the same medium. The plates
were incubated at 37°C in a 5% CO2 incubator for 12
days. Colonies were stained with crystal violet, and those with
>50 cells were scored as surviving colonies. The cloning
efficiency was calculated by dividing the average number of
colonies/dish by the number of plated cells.
Cell apoptosis detection
Flow cytometric assays were conducted using the
Annexin V-FITC apoptosis detection kit I (BestBio, Shanghai,
China), according to the manufacturer's instructions. Cells from
the two groups (miR-1291 and NC) were harvested by trypsinization
and resuspended at a density of 1×106 cells/ml in 1X
binding buffer. After double staining with propidium iodide (PI)
and Annexin V-FITC, the samples were analyzed by flow cytometry and
the data were analyzed using CellQuest software.
Transwell invasion assay
We assayed the invasion ability of cells using 6.5
mm diameter Transwell chambers with 8-µm membranes (Corning,
USA). Twenty-four hours after post-transfection, EC9706 or EC-1
cells were added to the upper chambers, and the bottom wells were
coated with 1 mg/ml Matrigel for the invasion assays, while a
medium containing 10% FBS was added to the lower chamber. After 24
h at 37°C in a 5% CO2 humidified atmosphere, cells in
the upper chamber were carefully scraped off using a cotton swab,
and cells that had migrated to the basal side of the membrane were
fixed in methanol, stained with hematoxylin, mounted and dried at
80°C for 30 min. The number of cells invading the Matrigel was
counted in three randomly selected fields using an inverted
microscope (magnification, ×200). Each test was performed in
triplicate.
Luciferase reporter assays
The wild-type MUC1 3′UTR fragments containing
putative seed region for miR-1291 were amplified from the human
genomic DNA by PCR. The mutagenesis of the seed region was achieved
by overlap PCR. The wild-type (wt) MUC1 3′UTR and mutant (mut) MUC1
3′UTR fragments were inserted into the pmirGLO promoter vector
(Promega) downstream of the luciferase gene to generate the
recombinant luciferase reporter vectors pmirGLO-MUC1-wt and
pmirGLO-MUC1-mut, respectively. For the luciferase reporter assay,
the recombinant plasmids were co-transfected with miR-1291 mimic or
NC into EC9706 cells. After 24 h, luciferase activity was measured
using the Dual-Luciferase Reporter assay system (Promega) according
to the manufacturer's instructions.
Statistical analysis
Statistical analyses were performed using SPSS 17.0
software. Data are expressed as mean ± standard deviation (SD). The
Student's t-test was performed to compare sample means. One-way
analysis of variance (ANOVA) was used to analyze the significance
between different samples. P<0.05 was considered to indicate a
statistically significant result. Pearson's correlation analysis
was used to analyze the correlation between different variable
elements.
Results
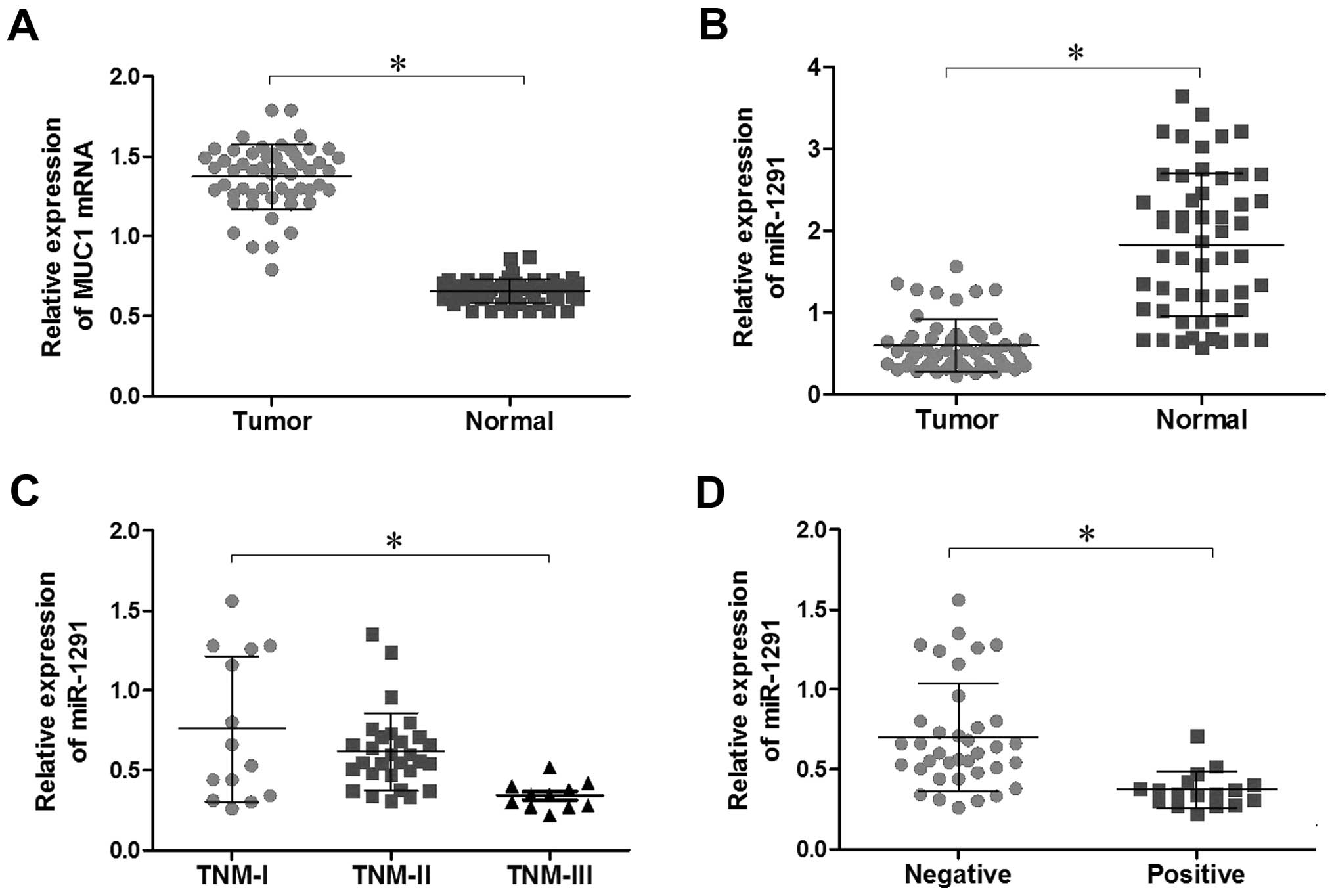
Expression of miR-1291 and MUC1 mRNA in
ESCC samples
We investigated the expression of miR-1291 and MUC1
in ESCC samples by qRT-PCR array. Compared with adjacent
non-tumorous tissues, miR-1291 expression was significantly lower
in ESCC tissues (P<0.05; Fig.
1B), while MUC1 mRNA showed the opposite trend (P<0.05;
Fig. 1A). These observations
demonstrate that the expression of miR-1291 and MUC1 was negatively
correlated in ESCC tissues. In addition, we further analyzed our
data and found that miR-1291 expression level in ESCC tissues was
associated with lymph node metastases and TNM stage (P<0.05;
Table I; Fig. 1C and D). No significant differences
were observed between miR-1291 expression and gender, age,
differentiation or tumor location (P>0.05; Table I). Distinctly, the MUC1 mRNA
expression level in ESCC tissues was associated with lymph node
metastases, differentiation and tumor location (P<0.05; Table I). There were no significant
differences between MUC1 mRNA expression and TNM stage, gender or
age (P>0.05; Table I).
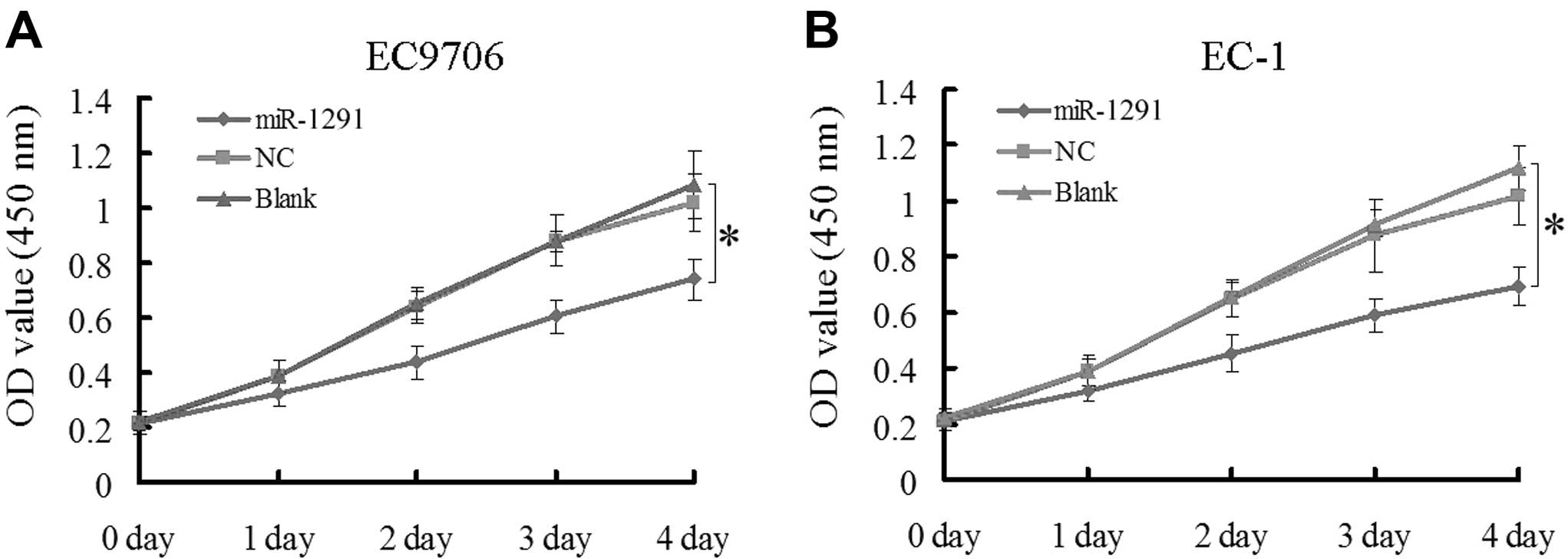
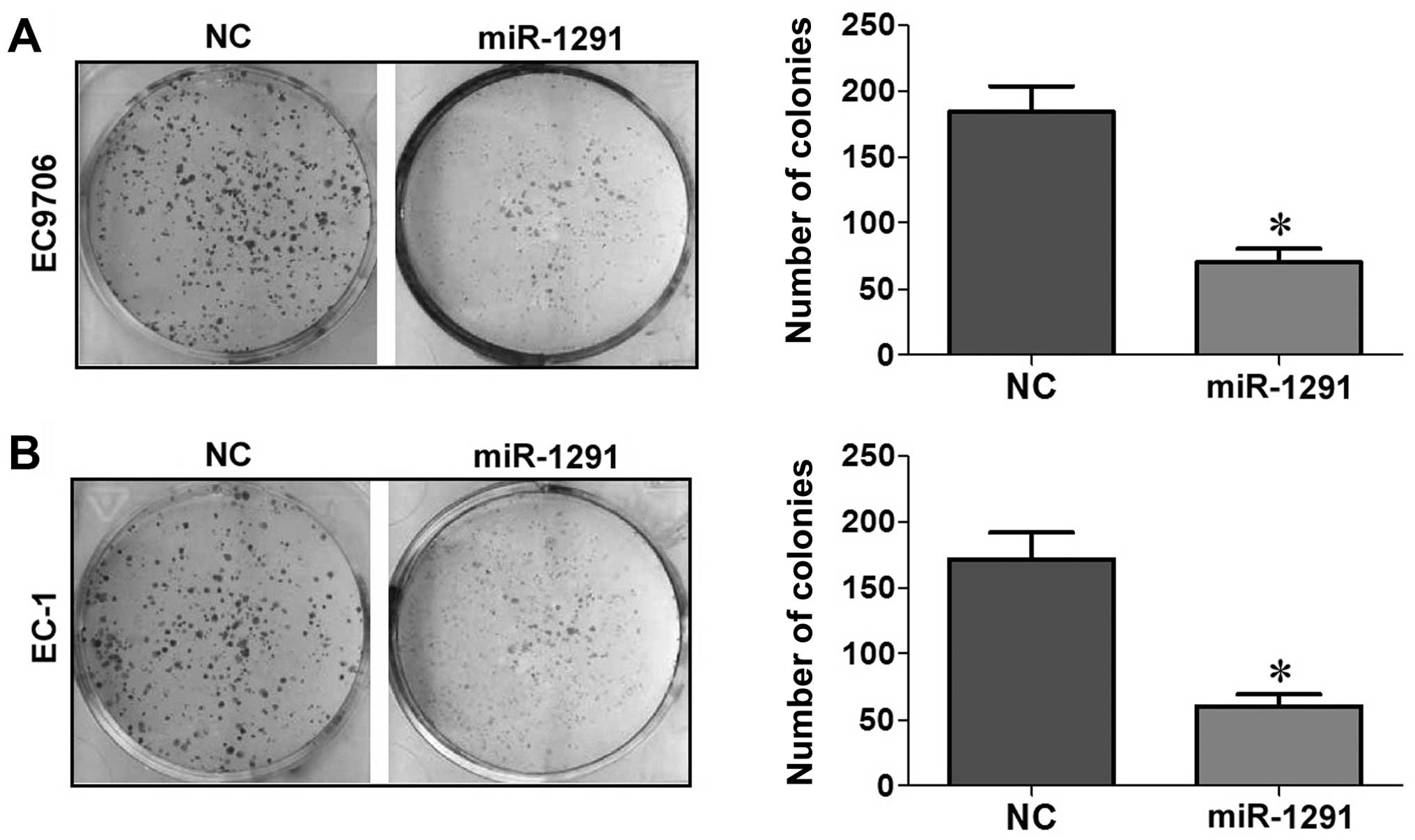
Upregulation of miR-1291 inhibits cell
proliferation in EC9706 and EC-1 cells
The CCK-8 and colony formation assays in EC9706 and
EC-1 cell lines were performed to evaluate the effect of miR-1291
on cell growth. According to the CCK-8 assay results, the
absorbance of EC9706 or EC-1 cells transfected with miR-1291 mimic
was markedly decreased compared to the control groups (NC and blank
control) from the second day onwards (P<0.05; Fig. 2A and B). In the colony formation
assay, the colony-forming activity of the miR-1291 mimic group was
lower than that of the NC group in both EC9706 (Fig. 3A) and EC-1 (Fig. 3B) cells (P<0.05). Based on these
results, we concluded that upregulation of miR-1291 inhibited the
proliferation of EC9706 and EC-1 cells.
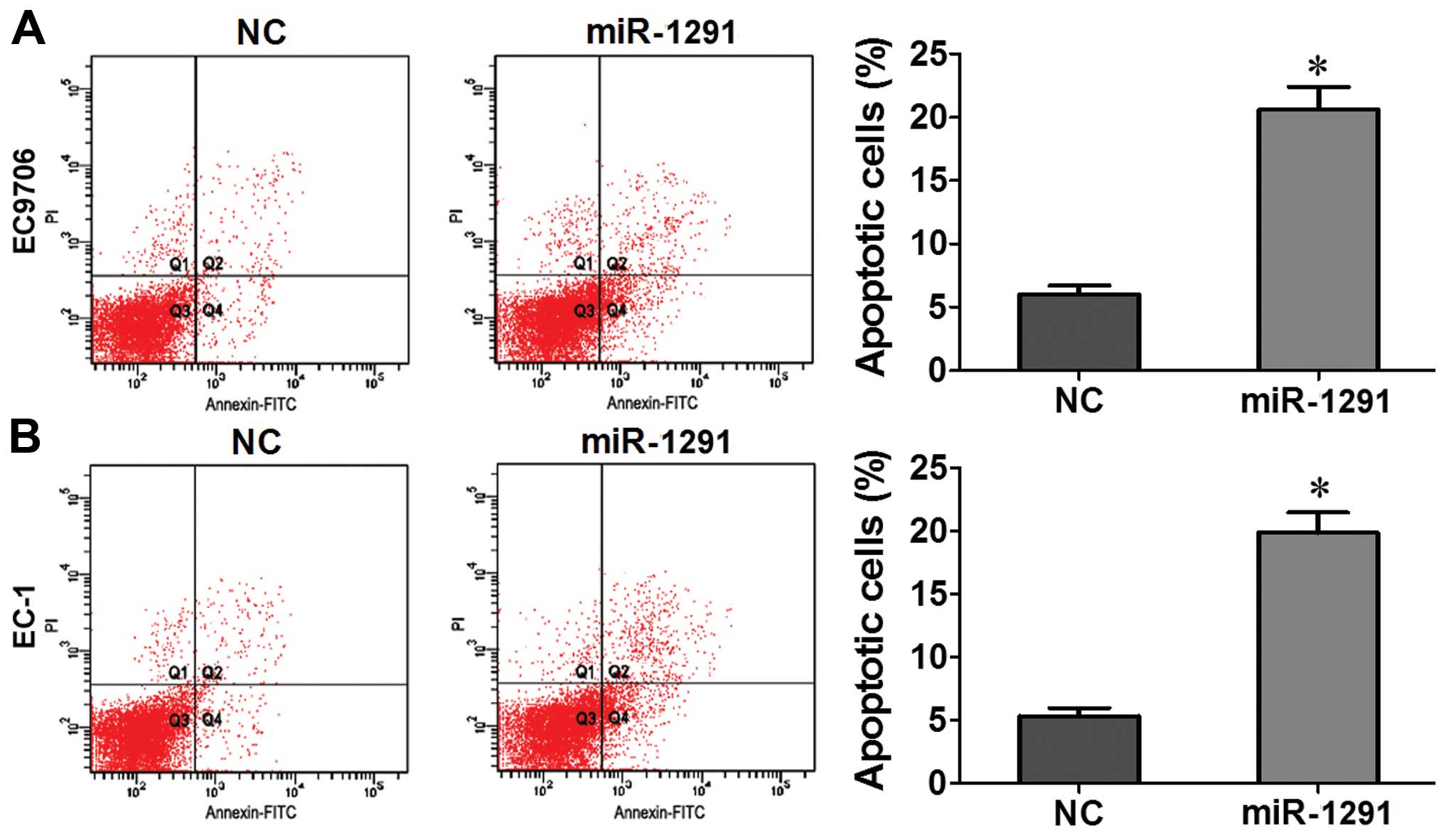
Upregulation of miR-1291 promotes
apoptosis in EC9706 and EC-1 cells
In order to determine whether apoptosis resulted in
proliferation inhibition, we performed a flow cytometric apoptosis
assay. Our data showed that in contrast to NC groups the number of
apoptotic EC9706 or EC-1 cells transfected with miR-1291 mimic
increased significantly after 48 h (P<0.05; Fig. 4A and B). Based on these results, we
concluded that upregulation of miR-1291 promotes apoptosis of
EC9706 and EC-1 cells.
Upregulation of miR-1291 restricts cell
invasion ability of EC9706 and EC-1 cells
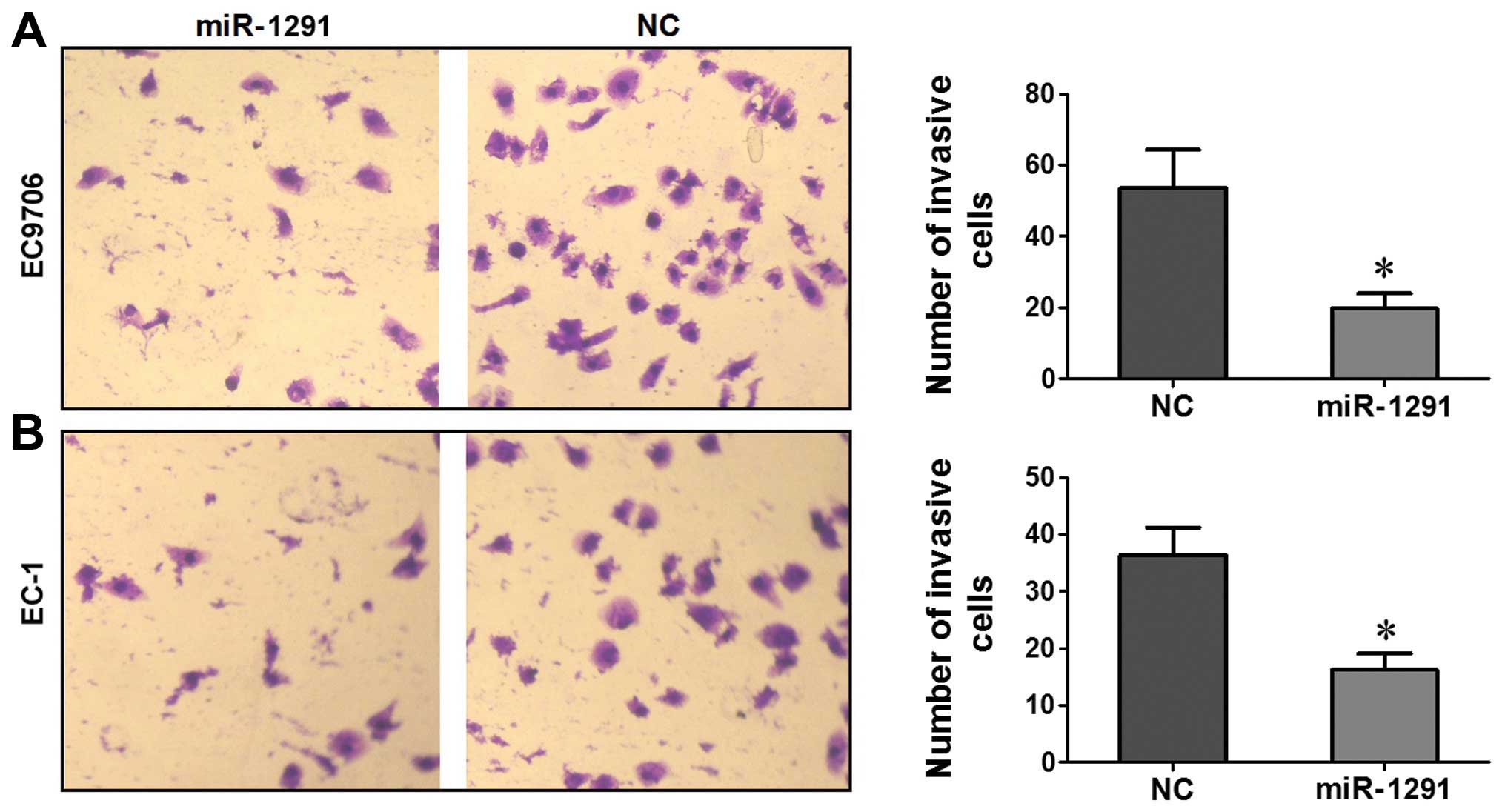
A Transwell assay was performed to evaluate the role
of miR-1291 in regulating the invasion activity of EC9706 and EC-1
cells. Compared to the NC group, the number of EC9706 or EC-1 cells
transfected with miR-1291 mimic that penetrated the Transwell
membrane was significantly lower (P<0.05; Fig. 5A and B). These results indicated
that upregulation of miR-1291 restricted the invasion capacity of
EC9706 or EC-1 cells.
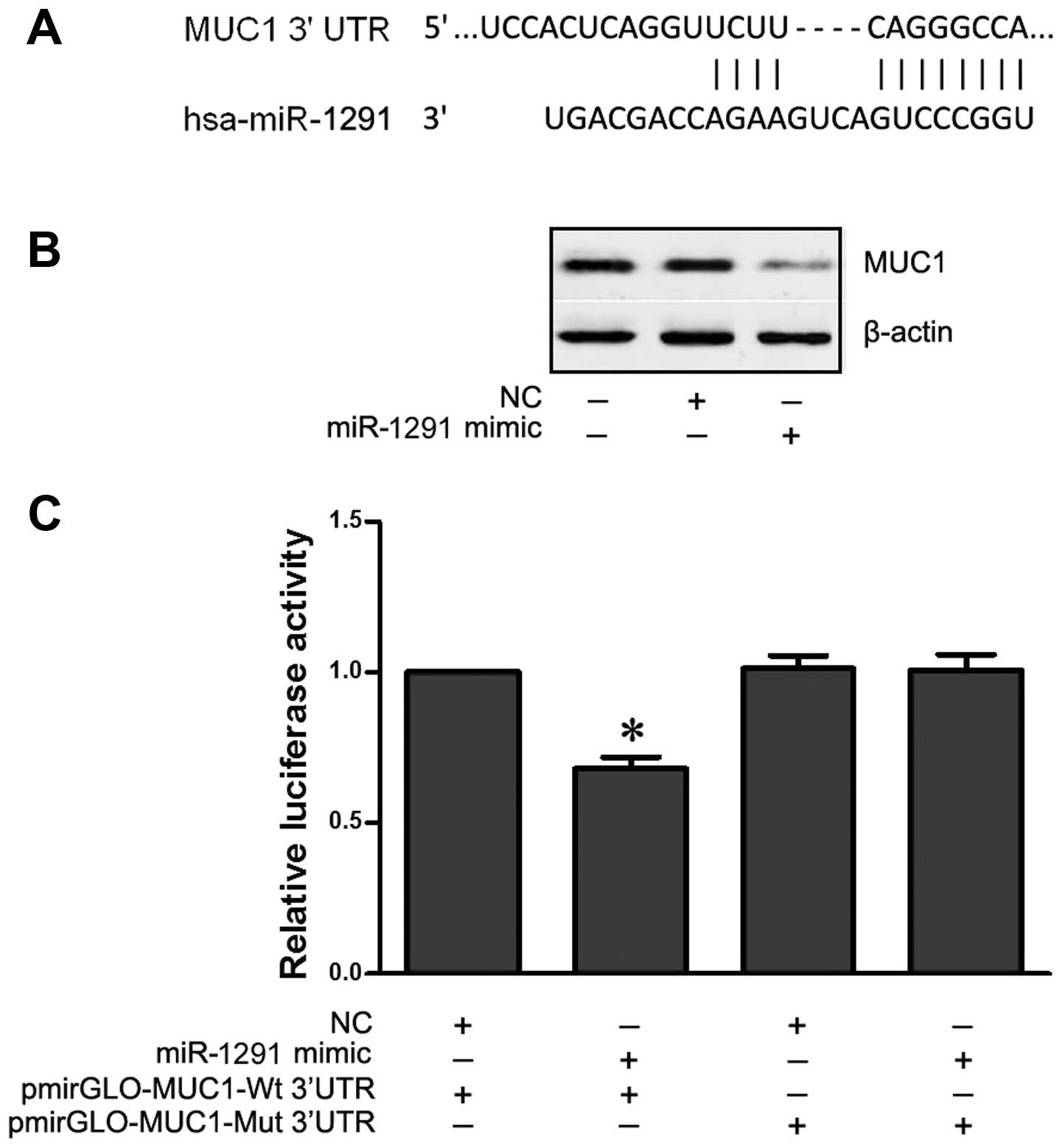
miR-1291 downregulates MUC1 expression by
binding to its 3′UTR
The TargetScan and miRanda prediction algorithms
showed that the MUC1 3′UTR may be directly targeted by miR-1291
(Fig. 6A). In order to verify this
targeting relationship, the wild-type and mutant human MUC1 3′UTR
fragments were cloned downstream of the firefly luciferase reporter
gene in pmirGLO vector (pmirGLO-MUC1-wt and pmirGLO-MUC1-mut) and
then co-transfected with a miR-1291 mimic (or NC) into EC9706
cells. The relative luciferase activity of the reporter gene in
EC9706 cells co-transfected with pmirGLO-MUC1-wt and miR-1291 mimic
was significantly lower (P<0.05) compared with the control group
(co-transfected pmirGLO-MUC1-wt and NC) (Fig. 6C). Among cells co-transfected with
pmirGLO-MUC1-mut, no difference was found between the relative
luciferase activity of cells co-transfected with miR-1291 mimic and
cells co-transfected with NC. This result was confirmed by western
blot analysis showing that MUC1 expression was downregulated in the
EC9706 cells following transfection with miR-1291 mimic (Fig. 6B). These results indicated that
miR-1291 negatively regulated MUC1 expression by directly binding
to the 3′UTR seed region in ESCC.
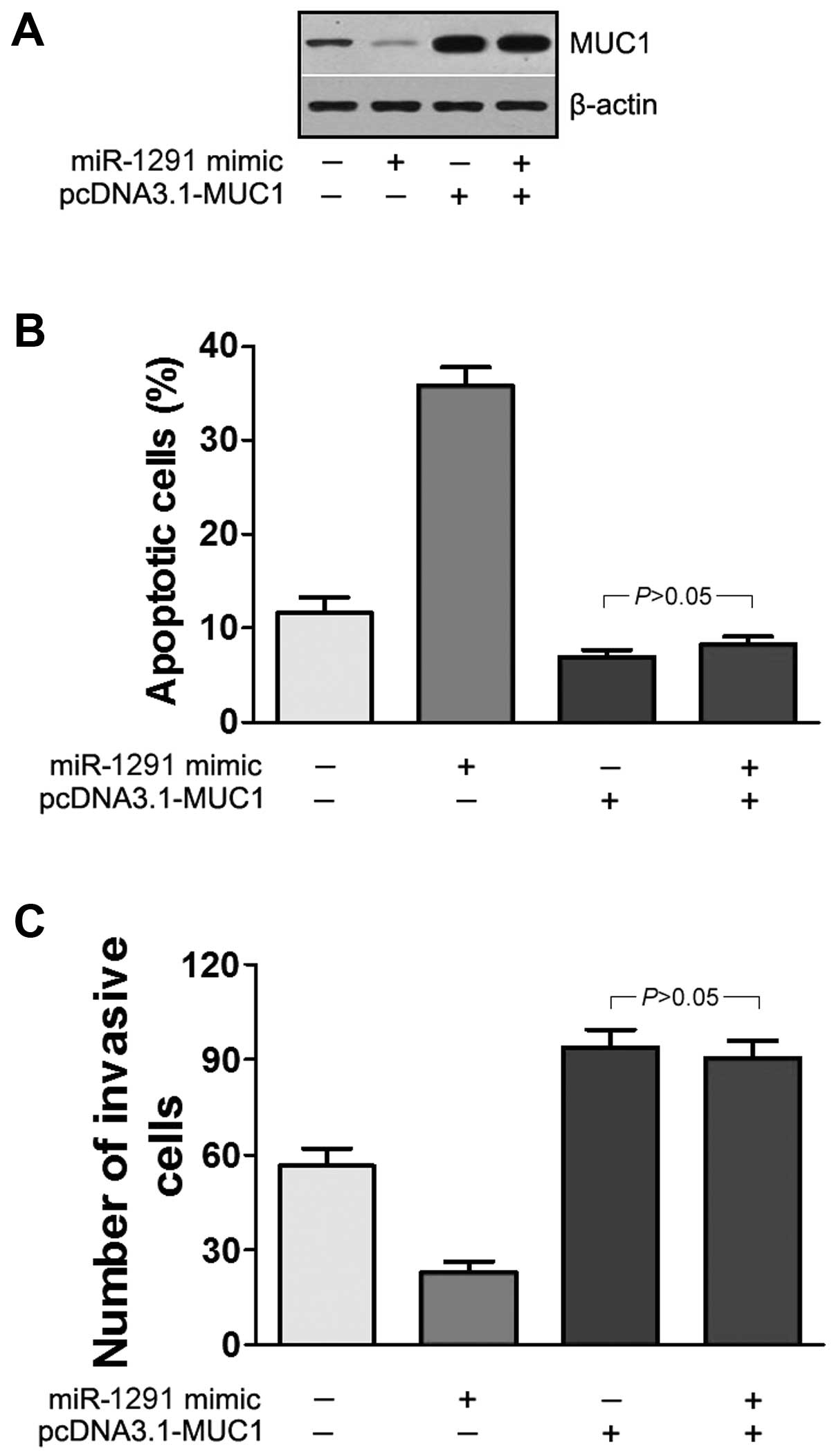
Expression of MUC1 abrogates the
anti-invasion and pro-apoptosis function of miR-1291
To further clarify MUC1 as a direct target of
miR-1291, the function of MUC1 in miR-1291-mediated invasion and
apoptosis was investigated. We constructed a recombinant expressing
vector pcDNA3.1-MUC1, which contains MUC1 lacking the 3′UTR. The
EC9706 cells were transfected with miR-1291 mimic or pcDNA3.1-MUC1
or co-transfected with both. Western blotting showed that
co-transfection of miR-1291 mimic and pcDNA3.1-MUC1 led to an
increase in MUC1 expression, and abrogated the effects of miR-1291
mimic (Fig. 7A). Furthermore, the
apoptosis assay demonstrated that compared to cells only subjected
to serum deprivation, the percentage of apoptotic cells transfected
with miR-1291 mimic was significantly increased (P<0.05;
Fig. 7B). However, the percentage
of apoptotic cells was significantly decreased in transfection with
pcDNA3.1-MUC1 or co-transfection with pcDNA3.1-MUC1 and miR-1291
mimic. These data indicated that co-transfection with pcDNA3.1-MUC1
and miR-1291 mimic abrogated the effects of miR-1291 on cell
apoptosis (P<0.05; Fig. 7B). In
the Transwell assays, we found that co-transfection of
pcDNA3.1-MUC1 and miR-1291 mimic increased the average number of
invading cells (P<0.05; Fig.
7C). Based on these results we further confirmed that 3′UTR of
MUC1 is the action site of miR-1291.
Discussion
Increasing studies have shown that miRNAs play
important roles in carcinogenesis and progression of esophagus
cancer (EC). For instance, miR-1290 was significantly upregulated
in ESCC tissue samples, and ectopic miR-1290 expression potently
promoted ESCC cell growth, migration and invasion in vitro
(25). miR-140 expression was
decreased in the EC tissues and regulated the cell invasion of EC
via controlling Slug expression (30). It has also been shown that miR-625
expression is lower in EC tissues than in the corresponding
adjacent tissues and may regulate the proliferation and invasion of
EC cells by targeting Sox2 (31).
miR-103/107 expression has been associated with poor survival rates
for ESCC patients (32).
In the present study, miR-1291 expression was
explored in ESCC. We analyzed the relationship between the miR-1291
expression levels and clinicopathological characteristics of ESCC.
The results showed that miR-1291 was significantly downregulated in
ESCC tissues and was correlated with lymph node metastases and TNM
stage. These results indicated that miR-1291 may be a new diagnosis
molecule or therapeutic target for ESCC. Moreover, cell biology
experiments in EC9706 and EC-1 cells showed overexpression of
miR-1291 inhibited proliferation, restricted cell invasion and
resulted in an increased rate of cell apoptosis. These observations
suggested that miR-1291 functioned as a tumor-suppressor in
ESCC.
Bioinformatics analysis using TargetScan and miRanda
revealed that MUC1 is one of the targets of miR-1291. MUC1 is a
transmembrane protein that has been identified by its marked
overexpression in human carcinomas (33,34).
Studies have shown that epithelial Muc1/MUC1 facilitates mucosal
wound healing by enhancing cell migration and proliferation,
protecting against apoptosis and mediating expression of mucosal
modulators (35). In most
epithelial-derived cancer cells, MUC1 is overexpressed and loses
its apical polarity (36,37). In various adenocarcinoma including
ESCC, MUC1 overexpression is correlated with tumor growth, lymph
node metastasis and resistance to apoptosis (38), involved in multiple
cancer-associated pathways. MUC1 suppresses activation of the
ARF-MDM2-p53 pathway (39). In
MUC1-expressing cells, a MUC1 co-operating NF-κB signaling pathway
plays a critical role in cancer cell invasion (40). MUC1 deficiency impairs NFKB p65, Akt
and MAPK pathways, which indicated that MUC1 appears to be a good
therapeutic target to slow down esophageal tumor progression
(41). In the present study, we
detected MUC1 mRNA of tumorous and adjacent non-tumorous human
esophagus tissues from 54 patients with ESCC. Our data showed that
MUC1 mRNA levels were significantly increased in ESCC tissues and
related to lymph node metastasis. In addition, we found that
altered MUC1 expression levels were associated with ESCC
differentiation status and tumor location. Our results clarified
the relationship between the MUC1 expression levels and
clinico-pathological characteristics of ESCC.
To confirm that MUC1 was one of the direct
functional targets of miR-1291, the 3′UTR region of MUC1 was
amplified from human genomic DNA and inserted into the pmirGLO
vector to construct a luciferase reporter plasmid, and qRT-PCR,
western blotting, luciferase reporter and knockdown assays were
performed. Further, restoration assays on invasion and apoptosis
were performed and the results showed expression of MUC1 abrogates
the anti-invasion and pro-apoptosis function of miR-1291, which
confirmed that miR-1291 bound to the 3′UTR of MUC1 mRNA reduced
stability and/or inhibited translation involved in proliferation,
invasion and apoptosis of ESCC cells.
Collectively, our investigation identified
significantly lower expression of miR-1291 in ESCC tissues and that
over-expression of miR-1291 suppressed cell growth, invasion and
promoted apoptosis in ESCC cells. Our results indicated that
miR-1291 acts as a tumor suppressor by targeting MUC1 in ESCC.
These new findings suggest that miR-1291 plays an important role in
regulating carcinogenesis and development of ESCC and is of value
to the diagnosis and therapy of ESCC.
Acknowledgments
The present study was supported by the Luohe Medical
College (no. 2014-S-LMC23). We would also like to thank Alison
Beamish at the University of British Columbia for her assistance
with English language and grammatical editing of the
manuscript.
References
|
1
|
Napier KJ, Scheerer M and Misra S:
Esophageal cancer: A Review of epidemiology, pathogenesis, staging
workup and treatment modalities. World J Gastrointest Oncol.
6:112–120. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kunisaki C, Makino H, Kimura J, Oshima T,
Fujii S, Takagawa R, Kosaka T, Ono HA and Akiyama H: Impact of
lymph-node metastasis site in patients with thoracic esophageal
cancer. J Surg Oncol. 101:36–42. 2010. View Article : Google Scholar
|
|
3
|
Rice TW, Rusch VW, Apperson-Hansen C,
Allen MS, Chen LQ, Hunter JG, Kesler KA, Law S, Lerut TE, Reed CE,
et al: Worldwide esophageal cancer collaboration. Dis Esophagus.
22:1–8. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mizoguchi K, Ishiguro H, Kimura M,
Takahashi H, Sakamoto N, Tanaka T and Takeyama H: Induction of
apoptosis by eicosapentaenoic acid in esophageal squamous cell
carcinoma. Anticancer Res. 34:7145–7149. 2014.PubMed/NCBI
|
|
5
|
Zhu X, Ding M, Yu ML, Feng MX, Tan LJ and
Zhao FK: Identification of galectin-7 as a potential biomarker for
esophageal squamous cell carcinoma by proteomic analysis. BMC
Cancer. 10:2902010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shigeoka M, Urakawa N, Nishio M, Takase N,
Utsunomiya S, Akiyama H, Kakeji Y, Komori T, Koma Y and Yokozaki H:
Cyr61 promotes CD204 expression and the migration of macrophages
via MEK/ERK pathway in esophageal squamous cell carcinoma. Cancer
Med. 4:437–446. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hobert O: Gene regulation by transcription
factors and microRNAs. Science. 319:1785–1786. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schaefer JS, Attumi T, Opekun AR, Abraham
B, Hou J, Shelby H, Graham DY, Streckfus C and Klein JR: MicroRNA
signatures differentiate Crohn's disease from ulcerative colitis.
BMC Immunol. 16:52015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hoss AG, Labadorf A, Latourelle JC, Kartha
VK, Hadzi TC, Gusella JF, MacDonald ME, Chen JF, Akbarian S, Weng
Z, et al: miR-10b-5p expression in Huntington's disease brain
relates to age of onset and the extent of striatal involvement. BMC
Med Genomics. 8:102015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Trionfini P, Benigni A and Remuzzi G:
MicroRNAs in kidney physiology and disease. Nat Rev Nephrol.
11:23–33. 2015. View Article : Google Scholar
|
|
12
|
Gupta S and Li L: Modulation of miRNAs in
pulmonary hypertension. Int J Hypertens. 2015:1690692015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kong W, He L, Richards EJ, Challa S, Xu
CX, Permuth-Wey J, Lancaster JM, Coppola D, Sellers TA, Djeu JY, et
al: Upregulation of miRNA-155 promotes tumour angiogenesis by
targeting VHL and is associated with poor prognosis and
triple-negative breast cancer. Oncogene. 33:679–689. 2014.
View Article : Google Scholar :
|
|
15
|
Yang YK, Xi WY, Xi RX, Li JY, Li Q and Gao
YE: MicroRNA-494 promotes cervical cancer proliferation through the
regulation of PTEN. Oncol Rep. 33:2393–2401. 2015.PubMed/NCBI
|
|
16
|
Zhang Y, Han T, Wei G and Wang Y:
Inhibition of microRNA-17/20a suppresses cell proliferation in
gastric cancer by modulating UBE2C expression. Oncol Rep.
33:2529–2536. 2015.PubMed/NCBI
|
|
17
|
Cao Q, Dong P and Wang Y, Zhang J, Shi X
and Wang Y: miR-218 suppresses cardiac myxoma proliferation by
targeting myocyte enhancer factor 2D. Oncol Rep. 33:2606–2612.
2015.PubMed/NCBI
|
|
18
|
Hwang HW and Mendell JT: MicroRNAs in cell
proliferation, cell death, and tumorigenesis. Br J Cancer.
96(Suppl): R40–R44. 2007.PubMed/NCBI
|
|
19
|
Wang Z, Ma X, Cai Q, Wang X, Yu B, Cai Q,
Liu B, Zhu Z and Li C: MiR-199a-3p promotes gastric cancer
progression by targeting ZHX1. FEBS Lett. 588:4504–4512. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yao YS, Qiu WS, Yao RY, Zhang Q, Zhuang
LK, Zhou F, Sun LB and Yue L: miR-141 confers docetaxel
chemoresistance of breast cancer cells via regulation of EIF4E
expression. Oncol Rep. 33:2504–2512. 2015.PubMed/NCBI
|
|
21
|
Hu H, Li S, Cui X, Lv X, Jiao Y, Yu F, Yao
H, Song E, Chen Y, Wang M, et al: The overexpression of
hypomethylated miR-663 induces chemotherapy resistance in human
breast cancer cells by targeting heparin sulfate proteoglycan 2
(HSPG2). J Biol Chem. 288:10973–10985. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Feng B, Wang R, Song HZ and Chen LB:
MicroRNA-200b reverses chemoresistance of docetaxel-resistant human
lung adenocarcinoma cells by targeting E2F3. Cancer. 118:3365–3376.
2012. View Article : Google Scholar
|
|
23
|
Lee D, Sun S, Zhang XQ, Zhang PD, Ho AS,
Kiang KM, Fung CF, Lui WM and Leung GK: MicroRNA-210 and
endoplasmic reticulum chaperones in the regulation of
chemoresistance in glioblastoma. J Cancer. 6:227–232. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhou Y, Wang M, Wu J, Jie Z, Chang S and
Shuang T: The clinicopathological significance of miR-1307 in
chemotherapy resistant epithelial ovarian cancer. J Ovarian Res.
8:232015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li M, He XY, Zhang ZM, Li S, Ren LH, Cao
RS, Feng YD, Ji YL, Zhao Y and Shi RH: MicroRNA-1290 promotes
esophageal squamous cell carcinoma cell proliferation and
metastasis. World J Gastroenterol. 21:3245–3255. 2015.PubMed/NCBI
|
|
26
|
Wang Y, Zang W, Du Y, Ma Y, Li M, Li P,
Chen X, Wang T, Dong Z and Zhao G: Mir-655 upregulation suppresses
cell invasion by targeting pituitary tumor-transforming gene-1 in
esophageal squamous cell carcinoma. J Transl Med. 11:3012013.
View Article : Google Scholar
|
|
27
|
Isozaki Y, Hoshino I, Akutsu Y, Hanari N,
Mori M, Nishimori T, Murakami K, Akanuma N, Takeshita N, Maruyama
T, et al: Usefulness of microRNA 375 as a prognostic and
therapeutic tool in esophageal squamous cell carcinoma. Int J
Oncol. 46:1059–1066. 2015.
|
|
28
|
John B, Enright AJ, Aravin A, Tuschl T,
Sander C and Marks DS: Human microRNA targets. PLoS Biol.
2:e3632004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Song ZB, Gao SS, Yi XN, Li YJ, Wang QM,
Zhuang ZH and Wang LD: Expression of MUC1 in esophageal
squamous-cell carcinoma and its relationship with prognosis of
patients from Linzhou city, a high incidence area of northern
China. World J Gastroenterol. 9:404–407. 2003.PubMed/NCBI
|
|
30
|
Li W, Jiang G, Zhou J, Wang H, Gong Z,
Zhang Z, Min K, Zhu H and Tan Y: Downregulation of miR-140 induces
EMT and promotes invasion by targeting Slug in esophageal cancer.
Cell Physiol Biochem. 34:1466–1476. 2014. View Article : Google Scholar
|
|
31
|
Wang Z, Qiao Q, Chen M, Li X, Wang Z, Liu
C and Xie Z: miR-625 down-regulation promotes proliferation and
invasion in esophageal cancer by targeting Sox2. FEBS Lett.
588:915–921. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Guo Y, Chen Z, Zhang L, Zhou F, Shi S,
Feng X, Li B, Meng X, Ma X, Luo M, et al: Distinctive microRNA
profiles relating to patient survival in esophageal squamous cell
carcinoma. Cancer Res. 68:26–33. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kufe DW: Functional targeting of the MUC1
oncogene in human cancers. Cancer Biol Ther. 8:1197–1203. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kufe DW: Mucins in cancer: Function,
prognosis and therapy. Nat Rev Cancer. 9:874–885. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Banerjee D, Fernandez HR, Patil PB,
Premaratne P, Quiding-Järbrink M and Lindén SK: Epithelial MUC1
promotes cell migration, reduces apoptosis and affects levels of
mucosal modulators during acetylsalicylic acid (aspirin)-induced
gastropathy. Biochem J. 465:423–431. 2015. View Article : Google Scholar
|
|
36
|
Singh PK and Hollingsworth MA: Cell
surface-associated mucins in signal transduction. Trends Cell Biol.
16:467–476. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mather IH, Jack LJ, Madara PJ and Johnson
VG: The distribution of MUC1, an apical membrane glycoprotein, in
mammary epithelial cells at the resolution of the electron
microscope: Implications for the mechanism of milk secretion. Cell
Tissue Res. 304:91–101. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nath S and Mukherjee P: MUC1: A
multifaceted oncoprotein with a key role in cancer progression.
Trends Mol Med. 20:332–342. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Raina D, Ahmad R, Chen D, Kumar S,
Kharbanda S and Kufe D: MUC1 oncoprotein suppresses activation of
the ARF-MDM2-p53 pathway. Cancer Biol Ther. 7:1959–1967. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mori Y, Akita K, Tanida S, Ishida A, Toda
M, Inoue M, Yashiro M, Sawada T, Hirakawa K and Nakada H: MUC1
protein induces urokinase-type plasminogen activator (uPA) by
forming a complex with NF-κB p65 transcription factor and binding
to the uPA promoter, leading to enhanced invasiveness of cancer
cells. J Biol Chem. 289:35193–35204. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gronnier C, Bruyère E, Lahdaoui F,
Jonckheere N, Perrais M, Leteurtre E, Piessen G, Mariette C and Van
Seuningen I: The MUC1 mucin regulates the tumorigenic properties of
human esophageal adenocarcinomatous cells. Biochim Biophys Acta.
1843:2432–2437. 2014. View Article : Google Scholar : PubMed/NCBI
|