Introduction
Cervical cancer is one of the most common
gynecological malignancies, resulting in a significant mortality
rate in women worldwide (1,2). In most cases, cervical cancer is
treated by hysterectomy or primary radiotherapy after diagnosis at
early stages (1,2). However, patients at an advanced stage
of cancer progression (regional and distant metastases) are
generally treated with the combination of radio and chemotherapy.
Despite the significant improvements in cancer treatment
strategies, the overall survival of cervical cancer patients has
yet to be determined. After treatment, >75% of patients have
recurrence within 1–2 years, resulting in tumor invasion and
metastasis (3).
Studies conducted on different solid tumors reported
that the persistence of cancer stem cells (CSCs) are the major
factor responsible for therapy failure and tumor recurrence
(4–7). A proposed cancer stem cell theory
suggested that CSCs are a small subpopulation within the
heterogenous tumor (7,8) and are highly potent with regard to
differentiation, infinite proliferation rate, self-renewal,
tumorigenesis and invasion (7–9).
Therefore, it is important to isolate and characterize CSCs to gain
a better understanding of CSC-meditated tumorigenesis and
metastasis. CSCs have been isolated based on their property of
Hoechst 33342 dye exclusion by using fluorescence-activated cell
sorting (FACS) analysis. During the FACS analysis, these CSCs were
reduced on the left side of the dot plot FACS analysis quadrant and
designated as side population (SP) cells (10). These SP cells share all the
significant features of CSCs and elevated ATP-binding cassette
(ABC) transporter protein, while ABCG2 in SP cells has been shown
to be involved in Hoechst 33342 efflux or multidrug resistance
(11–13). In the present study, we aimed to
characterize the cervical CSCs to gain a better understanding of
Nrf2-mediated drug and apoptosis resistance of CSCs.
Materials and methods
Cancer samples and cell culture
Cervical cancer samples were collected from patients
at the time of surgery in the Department of Obstetrics and
Gynecology in accordance with the ethical principles approved by
The Second Hospital of Jilin University, China. A total of 15
samples were collected by punch biopsies. The total number of
patients was 30, with an age range of 39–43. Three poorly
differentiated carcinomas (PDSCC), 15 high-grade SILs and 12
well-differentiated squamous cell carcinomas (WDSCC) were
identified. The corresponding control non-malignant cervical
epithelial tissues were obtained from healthy groups at the time of
hysterectomy. The cancer tissues were then washed and further
processed as previously described (14).
FACS analysis
By using a hemocytometer, ~106 cells were
taken and divided into two groups. In group I, the cells were
labeled with Hoechst 33342 dye alone (n=11) and in group II, the
cells were treated with the reserpine drug and Hoechst 33342 dye
(n=11). Subsequently, the cells were counterstained with propidium
iodide (PI) 2 µg/ml and analyzed by flow cytometer.
Assays
Cell resistance, Matrigel invasion, sphere formation
and TUNEL assays were performed exactly as previously described
(15,16).
Tumor cell implantation
The FACS sorted SP and non-SP cells
(4×103 cells) were administered into NOD/SCID mice by
subcutaneously injection. The density of the injected cells and
mice growth was monitored as previously described (17). After 3–4 weeks, the derived tumors
from the sacrificed mice were removed.
Reverse transcription (RT)-quantitative
polymerase chain reaction (qPCR)
Total RNA was extracted and complementary DNA was
prepared using Reverse Transcriptase kit (Fermentas). RT-qPCR
analysis was performed on an iCycler IQ real-time detection system,
using an IQ Supermix with SYBR-Green (both from Bio-Rad, Hercules,
CA, USA). The sequences of human specific primers used were as
follows (15,16): ABCG2 forward, TCA ATC AAA GTG CTT
CTT TTT TATG and reverse, TTG TGG AAG AAT CAC GTG GC); Nrf2
forward, ACA CGG TCC ACA GCT CAT C and reverse, TGC CTC CAA AGT ATG
TCA ATC A); GAPDH forward, ATG TCG TGG AGT CTA CTG GC and reverse,
TGA CCT TGC CCA CAG CCT TG); Oct-4 forward, TCG AGA ACC GAG TGA GAG
GC and reverse, CAC ACT CGG ACC ACA TCC TTC); Bmi-1 forward,
CTCCCAACTGGTTCGACCTT and reverse, CGGTTTCCATATTTCTCAGT); CD133
forward, TCT TGA CCG ACT GAG AC and reverse, ACT TGA TGG ATG CAC
CAA GCA C); EpCAM forward, CTG CCA AAT GTT TGG TGA TG and reverse,
ACG CGT TGT GAT CTC CTT CT); BCl-2 forward, ACA CTG TTA AGC ATG TGC
CG and reverse, CCA GCT CAT CTC ACC TCA CA); and Bax forward, GGA
TGC GTC CAC CAA GAA and reverse, ACT CCC GCC ACA AAG ATG) (17–20).
The PCR parameters used to set the PCR reactions were: Initial
denaturation at −95°C for 15 sec; annealing at −58°C for 45 sec;
and extension at −60°C for 30–45 sec, for 35 cycles. The amplified
products were visualized by ethidium bromide-stained 1.2% agarose
gels. Image J was used to measure the band intensity. The data
presented in the graph are the average values of three independent
experiments.
RNA interference
Small interfering RNA (siRNA) specific to the Nrf2
gene was purchased from Dharmacon (Lafayette, CO, USA). siRNA
transfection (final concentration of 200 nm) was performed as per
the Dharmacon protocol by using Oligofectamine 2000
(Invitrogen-Life Technologies, Carlsbad, CA, USA). The transfected
siRNA cells were analyzed after 48 h.
Immunofluorescent staining
The SP cells and main population cells were seeded
in cover slips on 12-well plates (1×105 cells/well). The
primary antibodies used were mouse anti-CD133 (1:100), anti-Oct-4
(1:200), anti-EpCAM (1:200), anti-ABCG2 (1:1,000), anti-bcl2
(1:100) and anti-Bax (1:200). After 24-h incubation in 1% BSA-TBS
the antibodies were incubated overnight at 40°C. After washing with
1X PBS, the cells were incubated with secondary antibody conjugated
with FITC (dilution: 1:1,000 in 1% BSA-TBS), at room temperature
for 1–2 h. For immunohistochemistry, the tissues were processed
with CD133 as previously described (21) and stained with mouse anti-CD133
(1:100) and rabbit anti-Nrf2. Tissues and cells were stained with
Hoechst 33342 (dilution: 1:100; Bio-Rad) dye to visualize the
nucleus.
The immunostaining of squamous spheres were
performed as previously described (12). Spheres were fixed onto glass slides
in ice-cold 4% paraformaldehyde (40°C for 10 min), blocked with
normal serum for 30 min, and incubated with mouse monoclonal
anti-Oct-4 and CD44 (1:200; Chemicon, Japan) overnight. After being
washed with PBS, the slides were incubated with FITC-conjugated
chicken anti-rat IgG overnight in the dark. Nuclei were
counterstained with 4,6-diamidino-2-phenylindole (DAPI). The cells
and tissues were subsequently viewed under a confocal laser
scanning microscope (Leica TCS, Mannheim, Germany). Image analysis
and figures were prepared using Adobe Photoshop CS6.
Western blot analysis
Proteins were extracted from the SP and non-SP
cells, and protein concentration was determined using the Bradford
assay (15). Primary antibodies
(rabbit anti-human ABCG2, Nrf2 and GAPDH) were incubated overnight,
followed by a secondary antibody (goat anti-rabbit IgG with
alkaline phosphatase markers) and a chemiluminescence reagent.
Blots were detected using gel documentation and scanned using a
densitometer (GS-710; Bio-Rad).
Statistical analysis
A one-way analysis of variance (ANOVA) and Student's
t-test were performed to determine the significant difference
between the treatment and control groups. A probability level of
P<0.05 or 0.01 was considered statistically significant.
Results
Flow cytometry-based purification of
cancer stem-like SP cells
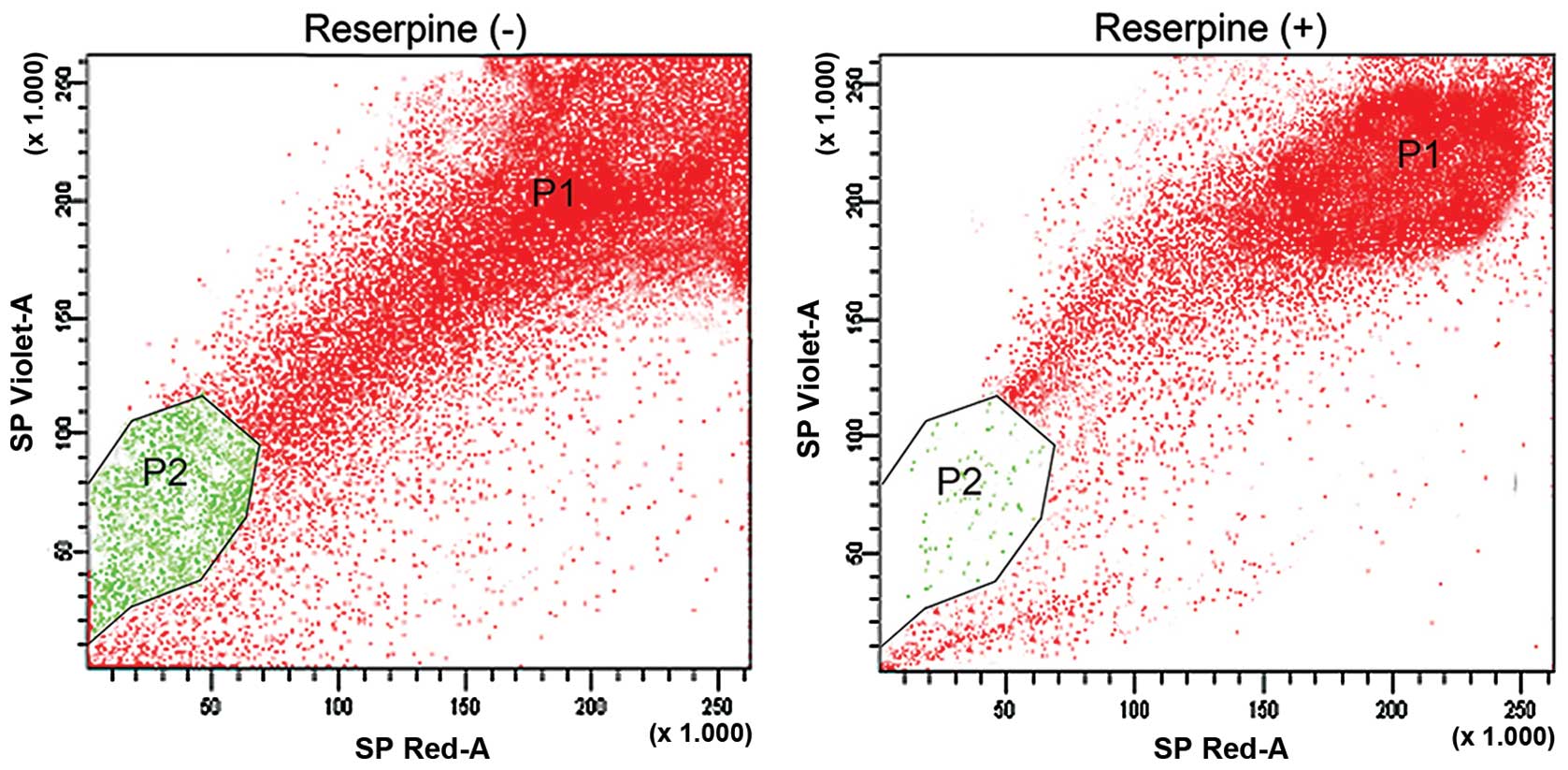
In the FACS analysis, cells passing through the
laser beam were considered forward and side scatter cells
correlating with cell size and population density, respectively.
Using PI staining, dead cells were excluded and the main population
was gated as a P1 region. The cancer cells that efficiently
effluxed the DNA binding dye Hoechst 33342 and were reduced in the
left lower quadrant of the FACS profile were termed as 'side
population (SP)' cells. We found ~3.1% of SP cells (P2-gated
population) from cervical cancer samples, which actively pumped out
the Hoechst 33342 dye (Fig. 1A).
The process of drug expulsion by SP cells occurred due to the
overexpression of the ABC transporter protein, ABCG2. As a
confirmatory test we treated the cervical cancer samples with the
ABC inhibitor reserpine and analyzed using FACS. Following
treatment with reserpine, the SP cell population was dramatically
reduced to 0.9% (Fig. 1B). Thus,
the results suggested that the presence of ABC proteins in SP cells
is significant for the expulsion of DNA binding dyes.
Cervical cancer SP cells possess
properties of embryonic stem cells
The FACS sorted SP and non-SP cells were further
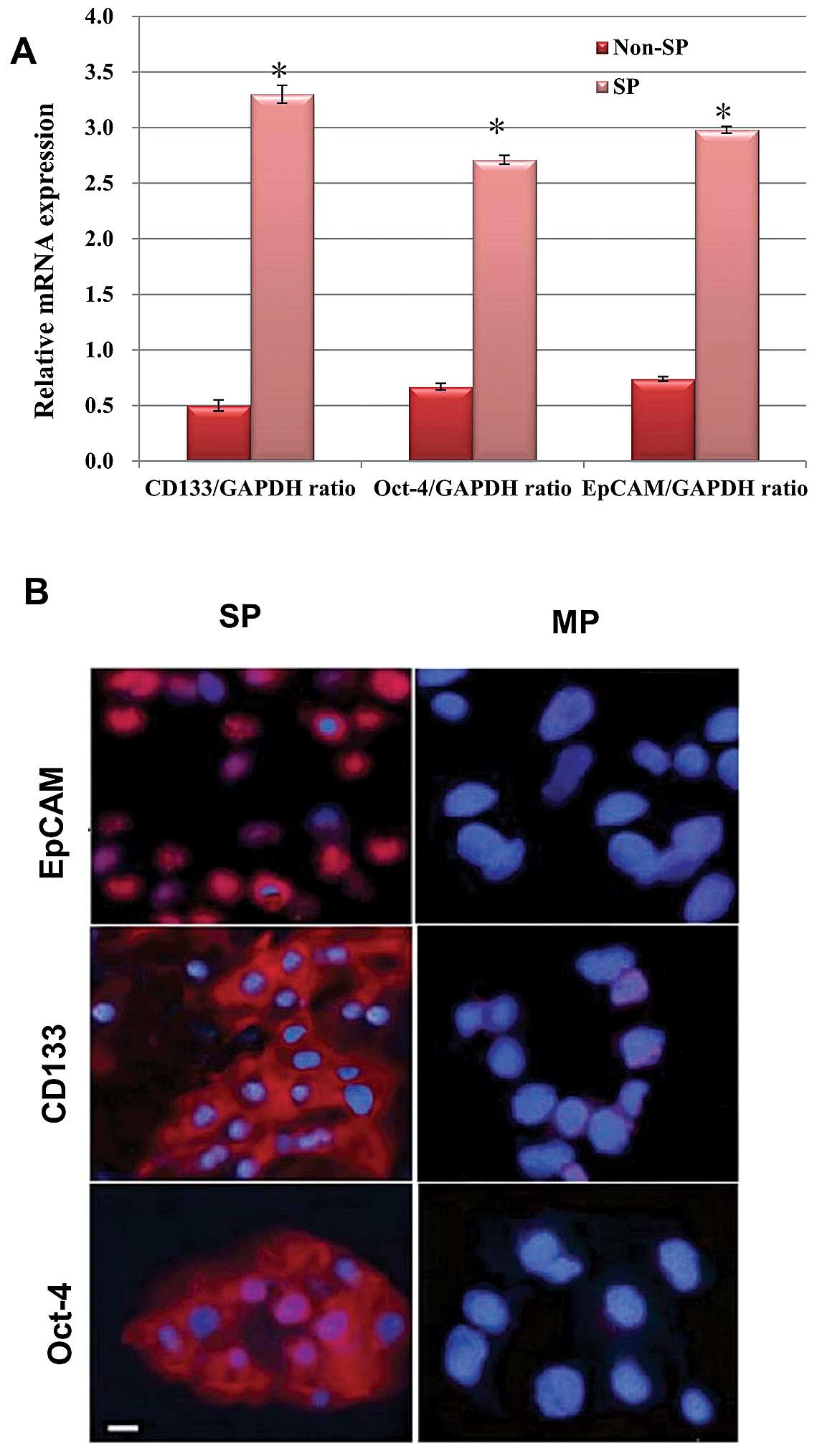
analyzed for the association of stem cell features. First, we
evaluated the expression of stem cell surface genes, such as Oct-4,
CD133 and EpCAM, between the SP and non-SP cells using RT-PCR
analysis. As shown in the graph (Fig.
2A), the transcriptional regulation of the abovementioned genes
are significantly upregulated in the SP cells than non-SP cells. In
addition, our immunofluorescence assay revealed that the SP cells
exhibited increased positivity towards these proteins, whereas the
non-SP cells exhibited null or less positivity towards these
proteins (Fig. 2B). Thus, the
over-expression of these stem cell proteins may be involved in the
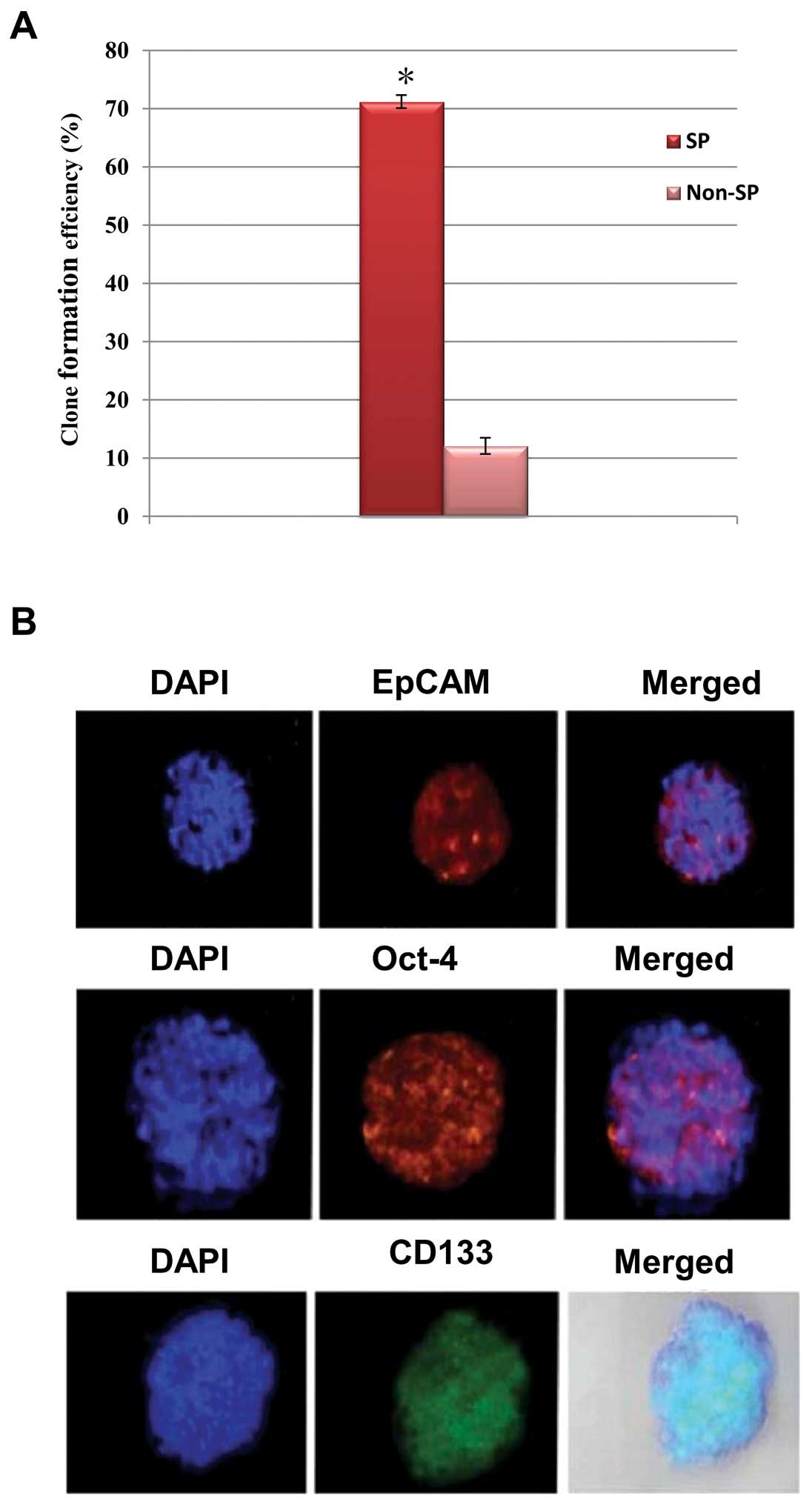
self-renewal process. Therefore, we performed a sphere formation
assay to determine the ability of self-renewal of SP cells. As
expected, the SP cells produced significantly more tumor spheres
and generally the tumor size was much larger than that of the SP
cells (Fig. 3A). Of note, the
spheres generated by the SP cells were highly positive towards stem
cell surface proteins such as CD133, EpCAM and Oct-4 (Fig. 3B). Therefore, an increased
expression of stemness genes in SP is crucial for the maintenance
of self-renewal of the cancer stem-like SP cells.
Elevated Nrf2 attenuates apoptosis and
multidrug intake
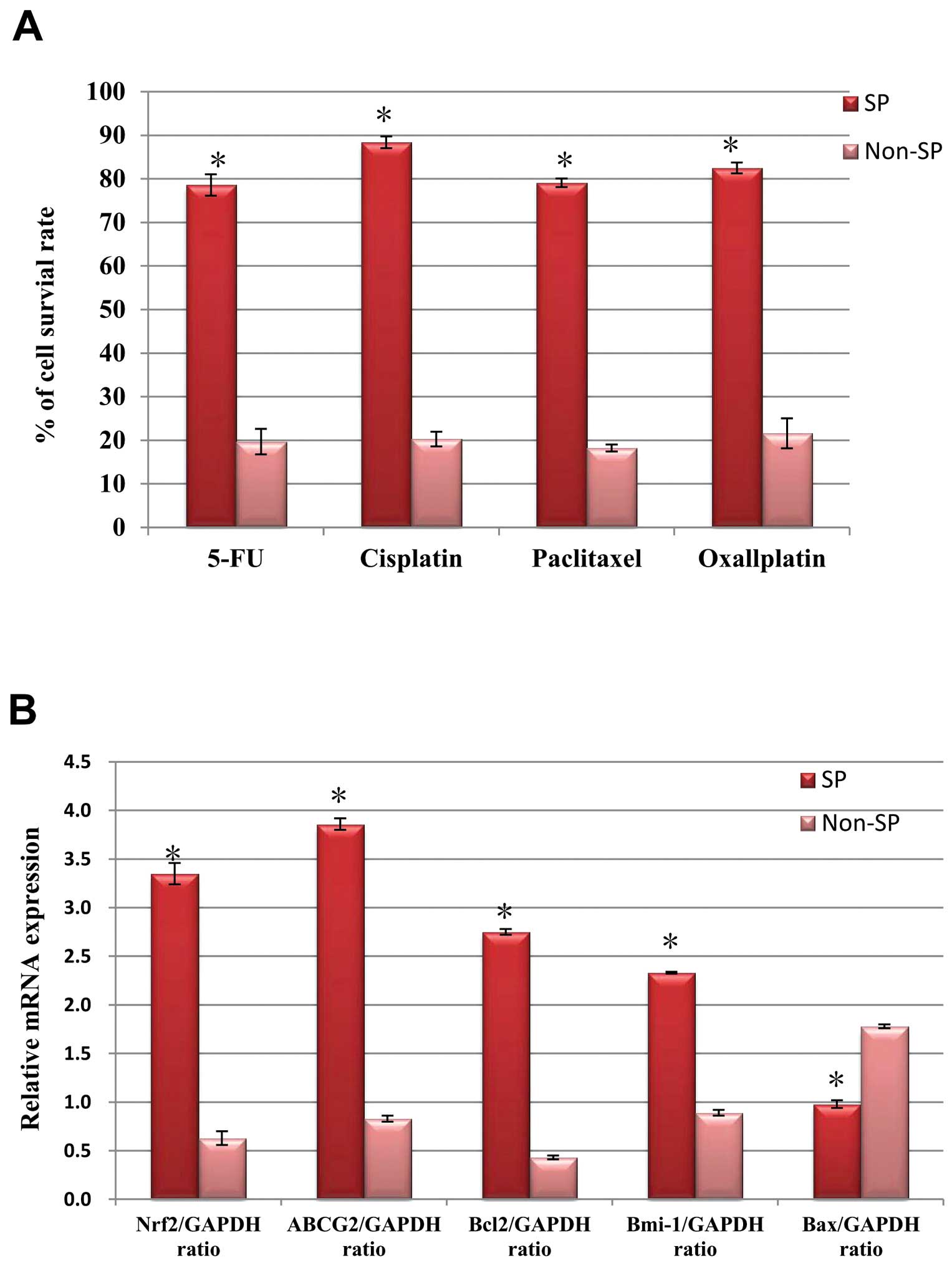
We performed a drug resistance assay to compare the
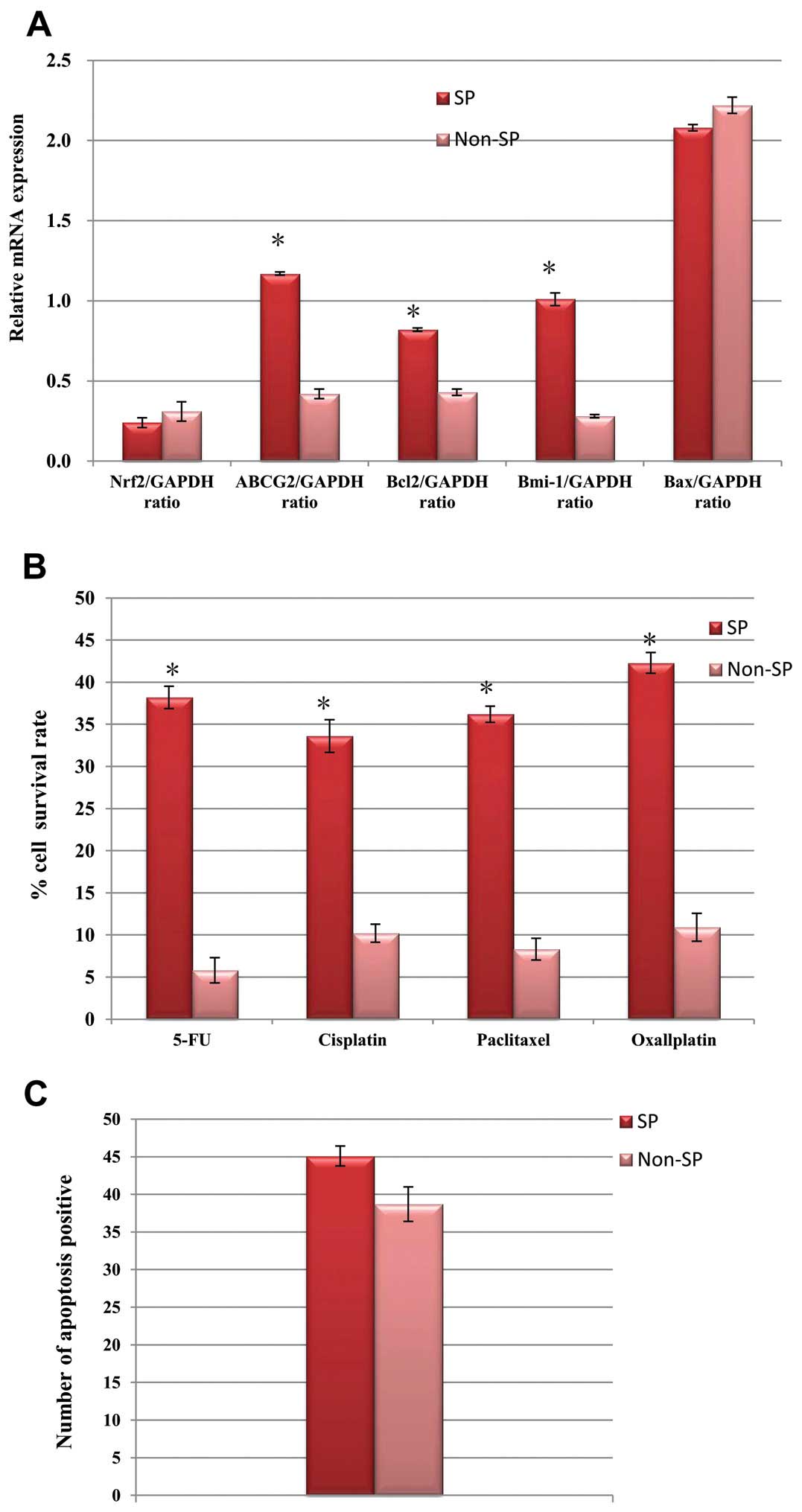
cell survival rate between the SP and non-SP cells. As shown in
Fig. 4A, the SP cells were more
resistant to 5-fluorouracil (5-FU), cisplatin, paclitaxel and
oxaliplatin. The survival rates of the SP cells were significantly
higher (>75%) following treatment with the multidrugs than the
non-SP cells (<20%). It has been shown that the aberrant
upregulation of Nrf2 was involved in the regulation of ABC genes,
thus ultimately resulting in chemotherapy resistance of the SP
cells (22). Therefore, we
evaluated the transcriptional expression of the Nrf2 gene in the SP
and non-SP cells. The relative mRNA expression of the Nrf2 gene was
significantly enhanced in the SP cells in conjunction with the
elevated mRNA expression of the ABC transporter ABCG2 and
anti-apoptotic genes such as Bcl-2 and Bmi-1 than in the non-SP
cells (Fig. 4B). However, Bax gene
expression was significantly reduced in the SP cells as compared to
the non-SP cells. In addition, our immunofluorescence results
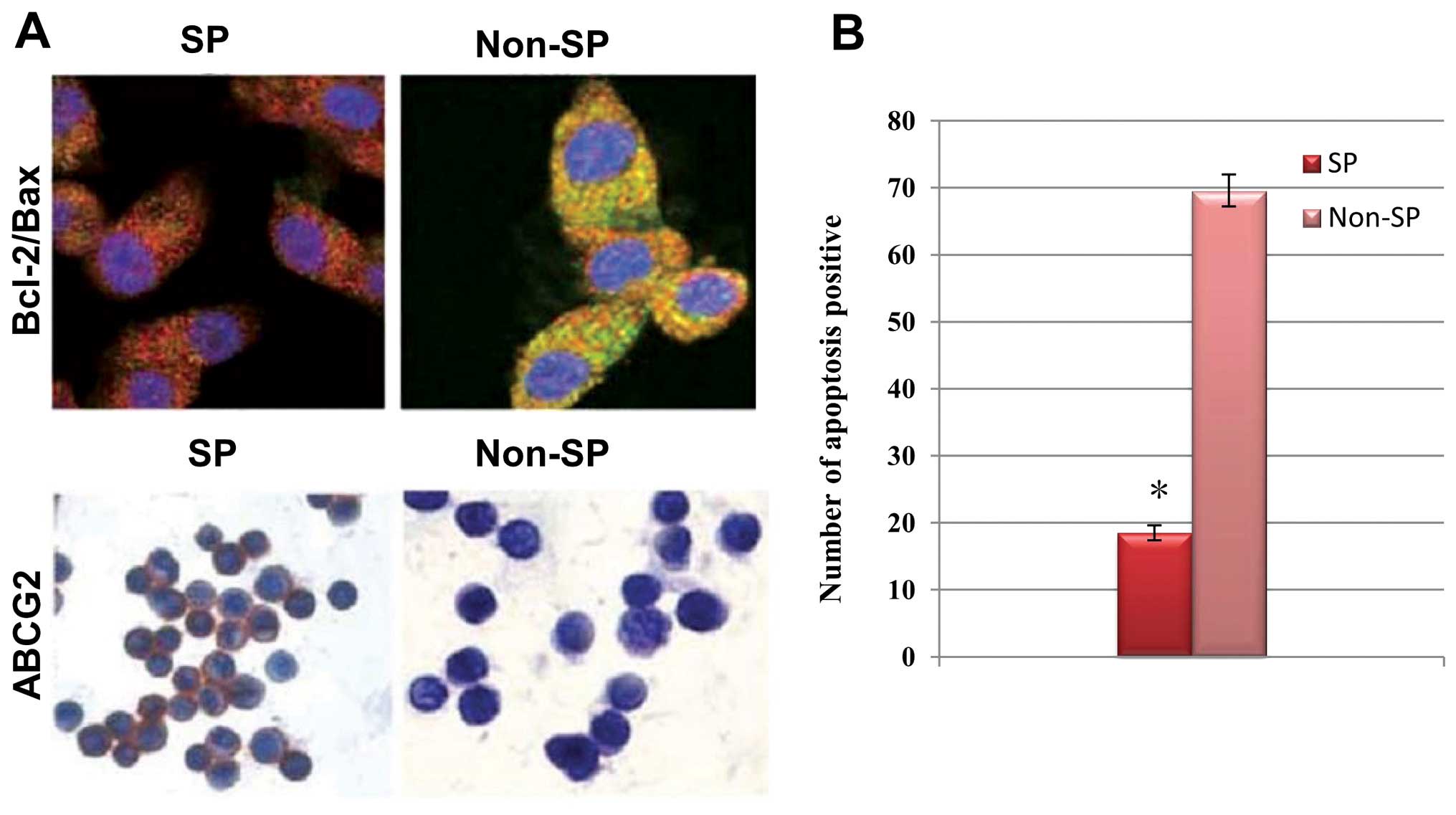
revealed that the cervical cancer SP cells exhibited enhanced
positivity towards ABCG2 and Bcl-2, but decreased positivity
towards Bax proteins as compared to the non-SP cells (Fig. 5A). As a consequence of the aberrant
regulation of the ABCG2 and anti-apoptotic gene expression
profiles, the number of SP cells that underwent apoptosis was
markedly decreased compared to that of the non-SP cells (Fig. 5B). These results suggested that the
elevated expression of Nrf2 is crucial in the long-term survival of
cancer stem cells by upregulating and downregulating the drug
efflux and apoptosis mechanism, respectively.
siRNA interference of Nrf2 enhances drug
and apoptosis sensitivity of SP cells
We examined whether the rapid inactivation of Nrf2
downregulated the ABC transporter and anti-apoptotic genes. Thus,
we used a silencing RNA approach to inactivate the Nrf2 gene
rapidly and temporarily. After silencing the Nrf2 gene in the SP
cells, the transcriptional regulation of the ABCG2, Bcl-2 and Bmi-1
genes was significantly downregulated (Fig. 6A). Consequently, the SP cells were
more sensitive towards multidrug treatment, as the survival rate of
SP cells was significantly reduced (Fig. 6B). Similarly, the number of SP cells
that underwent apoptosis was significantly elevated after Nrf2
inactivation (Fig. 6C). These
results suggested that an elevated Nrf2 is a major causative factor
for chemotherapy failure and poor survival rate of cancer
patients.
Nrf2-upregulated SP cells are highly
tumorigenic and invasive
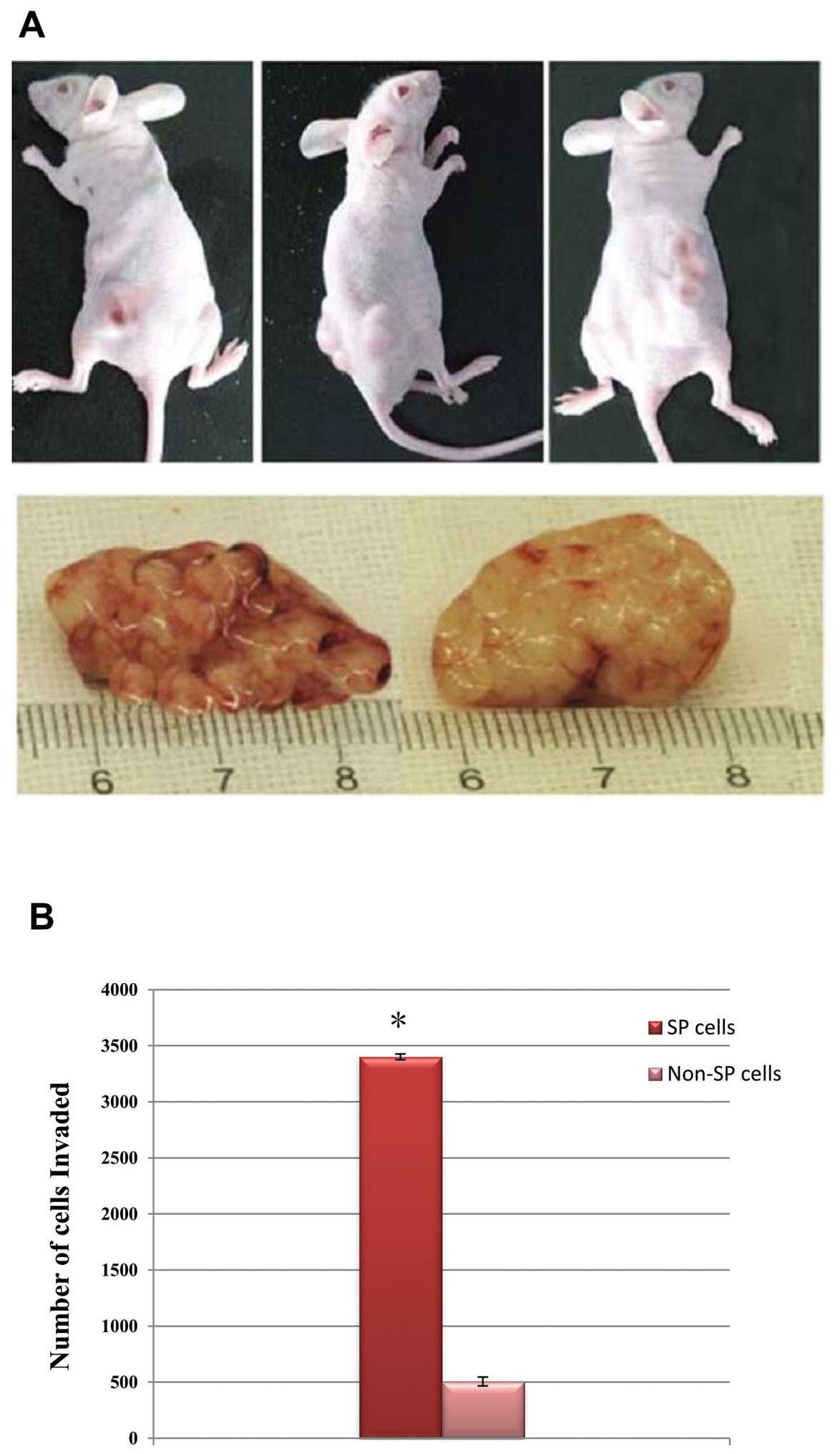
To determine the tumorigenicity of FACS-sorted
Nrf2-overexpressed SP cells, we implanted the lowest density
(4×103 cells) of the SP and non-SP cells separately into
the NOD/SCID mice. The SP cells were highly potent for initiating
rapid tumor growth in the NOD/SCID mice and the tumor size was
larger (Fig. 7A), whereas the
non-SP cells failed to generate tumors at this cell concentration.
The Matrigel invasive assay revealed that the SP cells were highly
invasive (Fig. 7B). Furthermore,
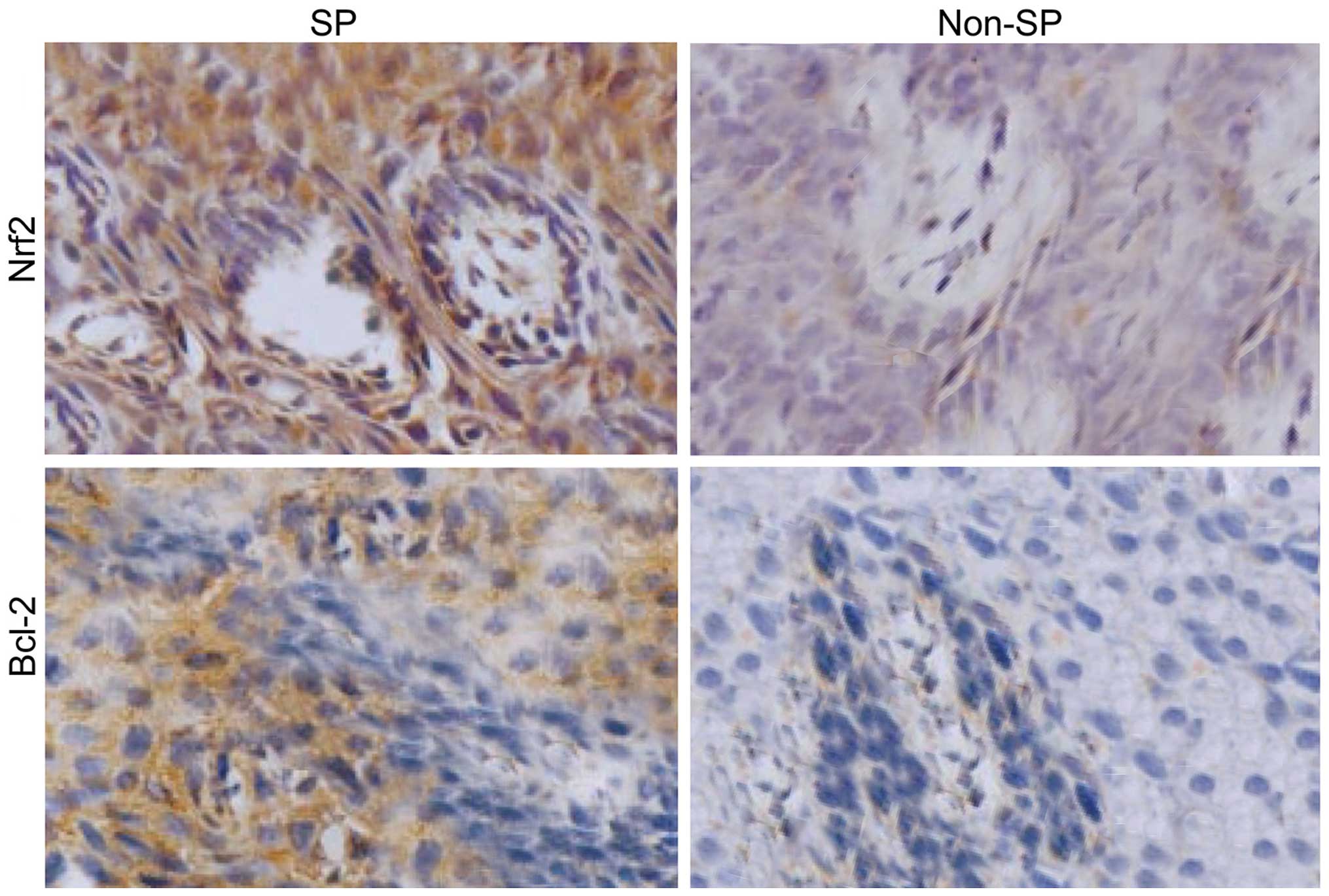
the tumor tissues derived from SP-induced cells showed an enhanced
Nrf2 and Bcl-2 staining compared to the non-SP tissues (Fig. 8). These results clearly suggested
that the presence of SP cells are the major factor involved in
tumor recurrence, invasion and metastasis after chemotherapy
failure.
Discussion
The heterogenous population of cancer cells contains
a small proportion of tumor-initiating cells termed cancer stem
cells (CSCs), responsible for multidrug therapy failure and tumor
relapse as they are capable of self-renewal (23–25).
CSCs have been isolated and characterized in different solid tumors
such as pancreatic carcinoma, ovarian and prostate cancer (4–7).
Additionally, CSCs are highly aggressive and resistant to multidrug
treatment and apoptosis (4–7). The increased multidrug resistance
properties of CSCs are mainly due to the higher expression levels
of drug efflux pumps such as ABC transporters ABCG2 and Bcrp1 which
are encoded by multi-drug-resistant (MDR) gene 1 (12). However, investigations into CSCs to
identify novel signaling and aberrantly upregulated proteins in
CSCs may be useful to developing novel and effective anticancer
drugs.
In the present study, by using the Hoechst 33342 dye
exclusion assay we have isolated and characterized the cancer
stem-like SP cells from cervical cancer samples. We have found a
significant amount (3.1%) of cervical cancer SP cells. The SP cell
population possesses overexpression of ABC transporter proteins
such as ABCG2. Similarly, it has been previously showed in cervical
cancer that HeLa stem cells possess the highly expressed MDR
protein Bcrp1 (3). Furthermore, we
have demonstrated that FACs-sorted cervical cancer SP cells are
capable of self-renewal as they express higher levels of stem cell
surface proteins such as CD133, CD44, EpCAM and Oct-4, which are
involved in the maintenance of self-renewal. These proteins are
also responsible for the higher cell proliferation rate and rapid
generation of tumor spheres because the FACs-purified SP cells are
able to initiate tumor spheres. These tumor spheres are highly
positive for CD133, CD44 and Oct-4. With regard to these findings,
it has been previously shown that Bcrp1+ cells underwent
rapid proliferation and they are capable of self-renewal (3,26).
Another interesting phenotype of the SP cells we identified is that
they are highly resistant to multidrugs and apoptosis. Furthermore,
these SP cells can initiate tumor growth in NOD/SCID mice even at
the lowest cell concentration, confirming that SP cells are capable
of causing tumor recurrence and invasion following therapy failure.
Similarly, the Bcrp1+/CD44+ cervical cancer
SP cells are highly tumorigenic and induce rapid tumor growth in
NOD/SCID mice (9). Overexpression
of the stem cell surface protein CD133 in different types of cancer
has been shown to be involved chemoresistance, self-renewal and
tumorigenesis in vitro and in vivo (27). However, the signaling mechanism and
downstream pathways involved in drug resistance, apoptosis
resistance and tumor recurrence remain to be determined.
The major role of Nrf2 is providing protection to
the cells from oxidative stress and other environmental cues, such
as xenobiotics. Generally, the downstream signaling of Nrf2 leads
to activation of several antioxidant enzymes and an elevated
expression of various drug efflux transporter proteins on the cell
surface (28–30). On the other hand, negative
regulation of Nrf2 leads to ubiquitin-mediated proteosomal
degradation by KEAP1 (31,32). loss of Nrf2-KEAP1 interaction or
point mutations in KEAP1 has been found to lead to the upregulation
of the Nrf2 gene, which is often present in primary tumors
(33,34). Studies in lung cancer stem cells
reported that upregulated Nrf2 leads to the overexpression of ABC
transporter proteins, contributing to chemotherapy failure
(2,12,17).
Furthermore, depletion of Nrf2 leads to the suppression of ABC
transporter gene expression and its function (22). Similarly, we have demonstrated that
cervical cancer SP cells possess an aberrantly elevated level of
the Nrf2 transcript and consequently the transcriptional regulation
of ABCG2 and Bcl2/Bmi-1 are highly upregulated. Furthermore, we
have shown that silencing of the Nrf2 gene expression attenuated
the expression of ABCG2, Bcl-2 and Bmi-1, rendering the SP cells
more sensitive to drug treatment and efficiently subjected to
apoptosis. There is a high risk of alteration in programmed cell
death during carcinogenesis associated with mutation in the p53
gene and modulation of apoptotic regulatory Bcl-2, Bmi-1 and Bax
proteins in cervical CSCs (18).
Inactivation of the p53 gene induces the production of other
pro-apoptotic proteins such as Bax and represses the anti-apoptotic
factors such as Bcl-2 and Bmi-1 (18). Therefore, we hypothesized that an
elevated level of Nrf2 may be involved in the inactivation of the
p53 gene and its function.
Taken together, our results suggest that elevated
Nrf2 signaling in cervical cancer SP cells are a major root cause
for DNA targeting drug and cell death resistance. Future studies
concerning elucidation of the precise molecular mechanism behind
the regulatory pathways and additional factors involved in
Nrf2-mediated ABCG2/Bcl-2 overexpression may provide insight into
the development of novel anticancer drugs that effectively suppress
Nrf2 signaling and the function of drug efflux pumps.
Acknowledgments
The present study is supported by the Youth Fund of
Science and Technology Department, Jilin (no. 201201034). We would
like to thank Dr Wanshan Li (Department of Oral and Maxillofacial
Surgery, Children's Hospital, Chongqing Medical University); Dr
Yang Liu (Department of Thoracic Surgery, Chinese PLA General
Hospital); Dr Nan Jiang (Department of Hepatic Surgery, The Third
Affiliated Hospital of Sun Yat-Sen University); Dr Yang Liu,
(Department of Thoracic Surgery, Chinese PLA General Hospital); and
Dr Xue-Hui Wang (Department of Gynecology, The Affiliated Zhongshan
Hospital of Dalian University) for their helpful guidance,
collaboration and sharing of all the important protocols and
primers.
References
|
1
|
Bansal N, Herzog TJ, Shaw RE, Burke WM,
Deutsch I and Wright JD: Primary therapy for early-stage cervical
cancer: Radical hysterectomy vs radiation. Am J Obstet Gynecol.
201:485.e1–485.e9. 2009. View Article : Google Scholar
|
|
2
|
Kosmas C, Mylonakis N, Tsakonas G, Vorgias
G, Karvounis N, Tsavaris N, Daladimos T, Kalinoglou N, Malamos N,
Akrivos T, et al: Evaluation of the paclitaxel-ifosfamide-cisplatin
(TIP) combination in relapsed and/or metastatic cervical cancer. Br
J Cancer. 101:1059–1065. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang SL, Wang YS, Zhou T, Yu XW, Wei ZT
and Li YL: Isolation and characterization of cancer stem cells from
cervical cancer HeLa cells. Cytotechnology. 64:477–484. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bapat SA, Mali AM, Koppikar CB and Kurrey
NK: Stem and progenitor-like cells contribute to the aggressive
behavior of human epithelial ovarian cancer. Cancer Res.
65:3025–3029. 2005.PubMed/NCBI
|
|
5
|
Collins AT, Berry PA, Hyde C, Stower MJ
and Maitland NJ: Prospective identification of tumorigenic prostate
cancer stem cells. Cancer Res. 65:10946–10951. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Olempska M, Eisenach PA, Ammerpohl O,
Ungefroren H, Fandrich F and Kalthoff H: Detection of tumor stem
cell markers in pancreatic carcinoma cell lines. Hepatobiliary
Pancreat Dis Int. 6:92–97. 2007.PubMed/NCBI
|
|
7
|
Tu SM, Lin SH and Logothetis CJ: Stem-cell
origin of metastasis and heterogeneity in solid tumours. Lancet
Oncol. 3:508–513. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Szotek PP, Pieretti-Vanmarcke R, Masiakos
PT, Dinulescu DM, Connolly D, Foster R, Dombkowski D, Preffer F,
Maclaughlin DT and Donahoe PK: Ovarian cancer side population
defines cells with stem cell-like characteristics and Mullerian
inhibiting substance responsiveness. Proc Natl Acad Sci USA.
103:11154–11159. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Goodell MA, Brose K, Paradis G, Conner AS
and Mulligan RC: Isolation and functional properties of murine
hematopoietic stem cells that are replicating in vivo. J Exp Med.
183:1797–1806. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou S, Schuetz JD, Bunting KD, Colapietro
AM, Sampath J, Morris JJ, Lagutina I, Grosveld GC, Osawa M,
Nakauchi H, et al: The ABC transporter Bcrp1/ABCG2 is expressed in
a wide variety of stem cells and is a molecular determinant of the
side-population phenotype. Nat Med. 7:1028–1034. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hirschmann-Jax C, Foster AE, Wulf GG,
Nuchtern JG, Jax TW, Gobel U, Goodell MA and Brenner MK: A distinct
'side population' of cells with high drug efflux capacity in human
tumor cells. Proc Natl Acad Sci USA. 101:14228–14233. 2004.
View Article : Google Scholar
|
|
13
|
Wulf GG, Wang RY, Kuehnle I, Weidner D,
Marini F, Brenner MK, Andreeff M and Goodell MA: A leukemic stem
cell with intrinsic drug efflux capacity in acute myeloid leukemia.
Blood. 98:1166–1173. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qin Zl, Zheng XX and Xu YH: Increased
angiogenesis and decreased programmed cell death increases the risk
of uterine cervical cancer. Drug Res. Oct 21–2014.Epub ahead of
print.
|
|
15
|
Yang B, Ma YF and Liu Y: Elevated
expression of Nrf-2 and ABCG2 involved in multi-drug resistance of
lung cancer SP cells. Drug Res. Nov 4–2014.Epub ahead of print.
|
|
16
|
Zhang QH, Dou HT, Xu P, Zhuang SC and Liu
PS: Tumor recurrence and drug resistance properties of side
population cells in high grade ovary cancer. Drug Res. 65:153–157.
2015.
|
|
17
|
Shi Y, Fu X, Hua Y, Han Y, Lu Y and Wang
J: The side population in human lung cancer cell line NCI-H460 is
enriched in stem-like cancer cells. PLoS One. 7:e333582012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fan J, Li R, Zhang R, Liu HL, Zhang N,
Zhang FQ and Dou KF: effect of Bcl-2 and Bax on survival of side
population cells from hepatocellular carcinoma cells. World J
Gastroenterol. 13:6053–6059. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Park JR, Kim RJ, Lee YK, Kim SR, Roh KJ,
Oh SH, Kong G, Kang KS and Nam JS: Dysadherin can enhance
tumorigenesis by conferring properties of stem-like cells to
hepatocellular carcinoma cells. J Hepatol. 54:122–131. 2011.
View Article : Google Scholar
|
|
20
|
Shimamura T, Yasuda J, Ino Y, Gotoh M,
Tsuchiya A, Nakajima A, Sakamoto M, Kanai Y and Hirohashi S:
Dysadherin expression facilitates cell motility and metastatic
potential of human pancreatic cancer cells. Cancer Res.
64:6989–6995. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim RJ, Kim SR, Roh KJ, Park SB, Park JR,
Kang KS, Kong G, Tang B, Yang YA, Kohn EA, et al: Ras activation
contributes to the maintenance and expansion of Sca-1pos
cells in a mouse model of breast cancer. Cancer Lett. 287:172–181.
2010. View Article : Google Scholar
|
|
22
|
Singh A, Wu H, Zhang P, Happel C, Ma J and
Biswal S: expression of ABCG2 (BCRP) is regulated by Nrf2 in cancer
cells that confers side population and chemoresistance phenotype.
Mol Cancer Ther. 9:2365–2376. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bonnet D and Dick JE: Human acute myeloid
leukemia is organized as a hierarchy that originates from a
primitive hematopoietic cell. Nat Med. 3:730–737. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lapidot T, Sirard C, Vormoor J, Murdoch B,
Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA and
Dick JE: A cell initiating human acute myeloid leukaemia after
transplantation into SCID mice. Nature. 367:645–648. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer, and cancer stem cells. Nature.
414:105–111. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hass R, Giese G, Meyer G, Hartmann A, Dörk
T, Köhler L, Resch K, Traub P and Goppelt-Strübe M: Differentiation
and retrodifferentiation of U937 cells: Reversible induction and
suppression of intermediate filament protein synthesis. Eur J Cell
Biol. 51:265–271. 1990.PubMed/NCBI
|
|
27
|
Salnikov AV, Gladkich J, Moldenhauer G,
Volm M, Mattern J and Herr I: CD133 is indicative for a resistance
phenotype but does not represent a prognostic marker for survival
of non-small cell lung cancer patients. Int J Cancer. 126:950–958.
2010.
|
|
28
|
Itoh K, Chiba T, Takahashi S, Ishii T,
Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, et
al: An Nrf2/small Maf heterodimer mediates the induction of phase
II detoxifying enzyme genes through antioxidant response elements.
Biochem Biophys Res Commun. 236:313–322. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ramos-Gomez M, Kwak MK, Dolan PM, Itoh K,
Yamamoto M, Talalay P and Kensler TW: Sensitivity to carcinogenesis
is increased and chemoprotective efficacy of enzyme inducers is
lost in nrf2 transcription factor-deficient mice. Proc Natl Acad
Sci USA. 98:3410–3415. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kwak MK, Kensler TW and Casero RA Jr:
Induction of phase 2 enzymes by serum oxidized polyamines through
activation of Nrf2: effect of the polyamine metabolite acrolein.
Biochem Biophys Res Commun. 305:662–670. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kobayashi A, Kang MI, Okawa H, Ohtsuji M,
Zenke Y, Chiba T, Igarashi K and Yamamoto M: Oxidative stress
sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to
regulate proteasomal degradation of Nrf2. Mol Cell Biol.
24:7130–7139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang DD, Lo SC, Cross JV, Templeton DJ
and Hannink M: Keap1 is a redox-regulated substrate adaptor protein
for a Cul3-dependent ubiquitin ligase complex. Mol Cell Biol.
24:10941–10953. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Singh A, Misra V, Thimmulappa RK, Lee H,
Ames S, Hoque MO, Herman JG, Baylin SB, Sidransky D, Gabrielson E,
et al: Dysfunctional KEAP1-NRF2 interaction in non-small-cell lung
cancer. PLoS Med. 3:e4202006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shibata T, Ohta T, Tong KI, Kokubu A,
Odogawa R, Tsuta K, Asamura H, Yamamoto M and Hirohashi S: Cancer
related mutations in NRF2 impair its recognition by Keap1-Cul3 E3
ligase and promote malignancy. Proc Natl Acad Sci USA.
105:13568–13573. 2008. View Article : Google Scholar : PubMed/NCBI
|