Introduction
Vasculogenic mimicry (VM) is the conversion of
aggressive cancer cells to an endothelial cell-like phenotype and
subsequent formation of tumor cell-lined vasculature. Thus, tumors
can form their own vasculature for nourishment through VM,
independent of host blood vessels. The presence of VM correlates
with an increased risk of metastasis and therefore poor clinical
outcome (1). VM has been reported
in many malignant tumor types, including melanoma, liver, prostate
and breast cancer, glioma and renal cancer (2–7); yet
what fascinates us most is the discovery of VM in the most lethal
gynecologic malignancy, ovarian cancer (8).
The etiology of VM remains unclear. A dynamic,
complex relationship exists between VM and the microenvironment,
which plays a pivotal role in cancer progression and has been shown
to affect VM formation in melanoma (9). Hypoxia, a feature of the tumor
microenvironment, promotes VM formation in three-dimensional
cultures (10–12). Morphological analysis of
choriocarcinoma demonstrated that central blood channels were
surrounded by neoplastic trophoblastic cells rather than
endothelial cells. In the periphery of choriocarcinoma, tumor cells
invaded uterine stroma-derived blood vessels, where trophoblastic
cells replaced endothelial cells, forming anastomoses between
endothelium-lined vessels and trophoblast-lined pseudovascular
channels. These findings indicated that choriocarcinoma, noted for
its high level of human chorionic gonadotropin (HCG) secretion, is
among the few tumor types that utilize VM (13). However, little is known about the
role of HCG in VM.
HCG is a cytokine that is ectopically expressed in a
variety of malignant tumor microenvironments, including ovarian
cancer, endometrial carcinoma, cervical, seminomatous testicular,
bladder and breast cancer (14–20).
HCG expression in ovarian cancer tissue varies relative to grade
and stage. HCG acts on the luteinizing hormone (LH)/HCG receptor
(LH-R), and HCG expression correlates with LH-R expression in
ovarian cancer tissue which has prognostic value (21). Ziecik et al (22) demonstrated ubiquitous HCG protein
expression in ovarian cancer samples, and at least 40% of
epithelial ovarian cancer expressed LH-R at high levels.
Co-expression of the HCG protein and LH-R in cancer cells may
indicate an autocrine or paracrine mechanism of tumor-derived HCG
activity in the tumor microenvironment. HCG has now been proposed
as a novel angiogenic factor that could mediate angiogenic pathways
(23,24).
Our recent study showed that treatment of ovarian
cancer cell line OVCAR-3 with recombinant HCG resulted in formation
of tumor cell-lined vasculature and significantly increased
vascular marker expression (25).
Herein, we extend our previous study to determine the influence of
the trophoblast cell microenvironment on VM in ovarian cancer. To
further explore a possible effect of HCG secreted by trophoblast
cells on VM in ovarian cancer cells, we developed an
endothelial-trophoblast cell co-culture system as a preconditioned,
HCG-enriched microenvironment. After the co-cultured cells were
removed (26), HCG
receptor-positive OVCAR-3 cells or HCG receptor-negative SKOV3
ovarian cancer cells were implanted into the preconditioned matrix.
VM was identified morphologically and by detection of vascular cell
markers expressed by cancer cells.
Hypoxia mediates tumor VM through hypoxia inducible
factor-1α (HIF-1α) (27), and
ovarian cancer cells form vasculogenic-like networks in a
three-dimensional culture under hypoxic conditions (10). We further used the dual cell system
under hypoxic conditions to gain insight into the interaction
between HCG and hypoxia in promoting VM in ovarian cancer.
Materials and methods
Cell culture
Human umbilical vein endothelial cells (HUVECs) were
purchased from Cambrex (East Rutherford, NJ, USA) and maintained in
Dulbecco's modified Eagle's medium (DMEM) growth media supplemented
with 8% fetal bovine serum. The first trimester human extravillous
trophoblast cell line HTR-8 and the ovarian cancer OVCAR-3 and
SKOV3 cell lines were purchased from the American Type Culture
Collection (ATCC; Manassas, VA, USA) and cultured in 1640 growth
medium. OVCAR-3 cells were incubated under normoxic (21%
O2) or hypoxic (1% O2) conditions. Culture
dishes in the hypoxia group were placed in a manually controlled
airtight chamber and hypoxic conditions were created by flushing 5%
CO2 and 94% nitrogen through the GENbox jar 2.5L chamber
(bioMérieux SA, Japan) until the O2 concentration was
reduced to 1%, as measured by a mini oxygen meter.
Establishment of an
endothelial-trophoblast cell co-culture system and preconditioned
microenvironment
Undiluted Matrigel was plated into 24-well tissue
culture plates at 300 µl/well and polymerized for 30 min at
37°C. HUVECs (1.0×105) and HTR-8 (1.0×105)
cells were stained with the green fluorescent linker dye PKH67 and
the red fluorescent linker dye PKH26, respectively, and then
co-cultured in the Matrigel. The formation of tube-like structures
was monitored by fluorescence microscopy. After establishment of
the endothelial-trophoblast cell co-culture system for three days,
co-cultured cells were removed with 20 mM NH4OH to
establish the preconditioned microenvironment. Furthermore, ovarian
cancer OVCAR-3 and SKOV3 cells were planted in the preconditioned
Matrigel.
ELISA
After establishing the co-culture system, the
expression of HCG in the supernatant was investigated at 0, 24, 48,
72 and 96 h by ELISA using an HCG ELISA kit (Beijing North
Institute of Biological Technology, Beijing, China). Assay diluent
RD1N (50 µl) and 50 µl of each sample or standard
were mixed in the wells of a microplate coated with a polyclonal
antibody specific for β-HCG, and then 100 µl of substrate
solution was added. The enzyme reaction was terminated with the
addition of a diluted hydrochloric acid solution. The intensity of
the color was measured at a wavelength of 450 nm. The specificity
of the effects of exogenous HCG in the microenvironment was further
assessed by complete inhibition with a 2.4 µg/ml
neutralizing anti-HCG antibody.
Light and electron microscopy
Ovarian cancer cells were examined by an inverted
phase contrast microscope (Carl Zeiss, Inc., Thornwood, NY, USA).
For scanning electron microscopy, cells were fixed in 2.5% cold
glutaraldehyde in 0.1 M sodium cacodylate buffer and post-fixed in
1% osmium tetroxide. Specimens were dehydrated in a graded series
of ethanol, displaced, critically point-dried and sputter-coated
with gold before scanning electron microscopy analysis (S-520;
Hitachi, Tokyo, Japan).
Real-time polymerase chain reaction
(PCR)
Reverse transcription (RT) of RNA was performed
using the TaqMan Gold RT-PCR kit as described by the manufacturer
(Applied Biosystems, Foster City, CA, USA). Real-time quantitative
PCR was performed with the Applied Biosystems 9700HT sequence
detection system. Each target was amplified in triplicate with an
HCG-specific primer probe set. We devised primers as follows:
HIF-1α, 5′-CTGCCACCACTGATGA ATTA-3′ and 5′-GTATGTGGGTAGGAGATGGA-3′;
LH-R, 5′-TGGCTGCTGTAAACGTCGGG-3′ and 5′-GGAGAGC
TGTACCTTGACAGTGC-3′; CD31, 5′-ATTGCAGTGGTTA TCATCGGAGTG-3′ and
5′-CTCGTTGTTGGAGTTCAGA AGTGG-3′; factor VIII (VWF),
5′-ATCGAGGGTCTCG GGGATGCG-3′ and 5′-TGCGAAGAGTGCTGCGAAT GCT-3′;
VEGFA, 5′-GACATCTTCCAGGAGTACC-3′ and 5′-TGCTGTAGGAAGCTCATCTC-3′;
β-HCG, 5′-TGCCAC CCTGGCTGTGGA-3′ and 5′-GCGGTAGTTGCACACCAC CTG-3′.
A 5-fold titration of control template was included along with
RT-negative and no-template controls. Relative mRNA levels were
calculated using the standard curve method (ABI User Bulletin no.
2) with 18S as the endogenous control and 0 h as the
calibrator.
Western blot analysis
The mouse monoclonal antibodies raised against VEGF,
VWF, HIF-1α, β-HCG and LH-R were obtained from Santa Cruz
Biotechnology (Santa Cruz, CA, USA). The rabbit monoclonal antibody
raised against CD31 was purchased from Bioworld (Dublin, OH, USA).
Cellular proteins were isolated, separated on an 8% SDS-tris
polyacrylamide gel and were transferred to a PVDF membrane. The
membranes were blocked before incubation with primary antibodies
(1:100) and horseradish peroxidase-conjugated secondary antibody
(1:1,000). Immunocomplexes were visualized by
electrochemiluminescence. Protein expression was semi-quantified
using a Tiannen imager and analysis system (Shanghai, China).
Small interfering RNA (siRNA)
HCG-β siRNAs were synthesized and ligated into a
pcDNA™6.2-GW/EmGFPmiR vector (Invitrogen, Carlsbad, CA, USA). The
sequences of pSilencer/HCG-β were: AGCAGCAACAGCAGCAGCCTC.
Transfections were performed with 3 µg silencing plasmid
pcDNA™6.2-GW/EmGFP-CGB5 miR, 9 µg ViraPower Packaging Mix
and 2 µl Lipofectamine 2000 (Invitrogen). Control cells were
mock-transfected. Stably transfected cells were selected by 0.4
mg/ml G418 (Merck, Darmstadt, Germany) after two weeks.
Indirect immunofluorescence
Cultured cells were fixed in 4% paraformaldehyde
then incubated with 2% BSA. After blocking, the sections were
incubated with mouse anti-human LH-R/HCG antibody (Santa Cruz
Biotechnology). Sections were stained with 1:1,000 FITC-conjugated
goat anti-mouse IgG1 secondary antibody (Caltag). Nuclei were
visualized by staining with 4′,6-diamidino-2-phenylindole (DAPI).
Sections were imaged by immunofluorescence microscopy. Negative
controls were produced by omitting the primary antibody.
Statistical analysis
The results are presented as the mean ± SD (standard
deviation). Data were analyzed using SPSS 16.0 for Windows (SPSS,
Inc., Chicago, IL, USA). One-way ANOVA analysis was performed to
identify differences.
Results
Establishment of an
endothelial-trophoblast cell co-culture system and preconditioned
microenvironment
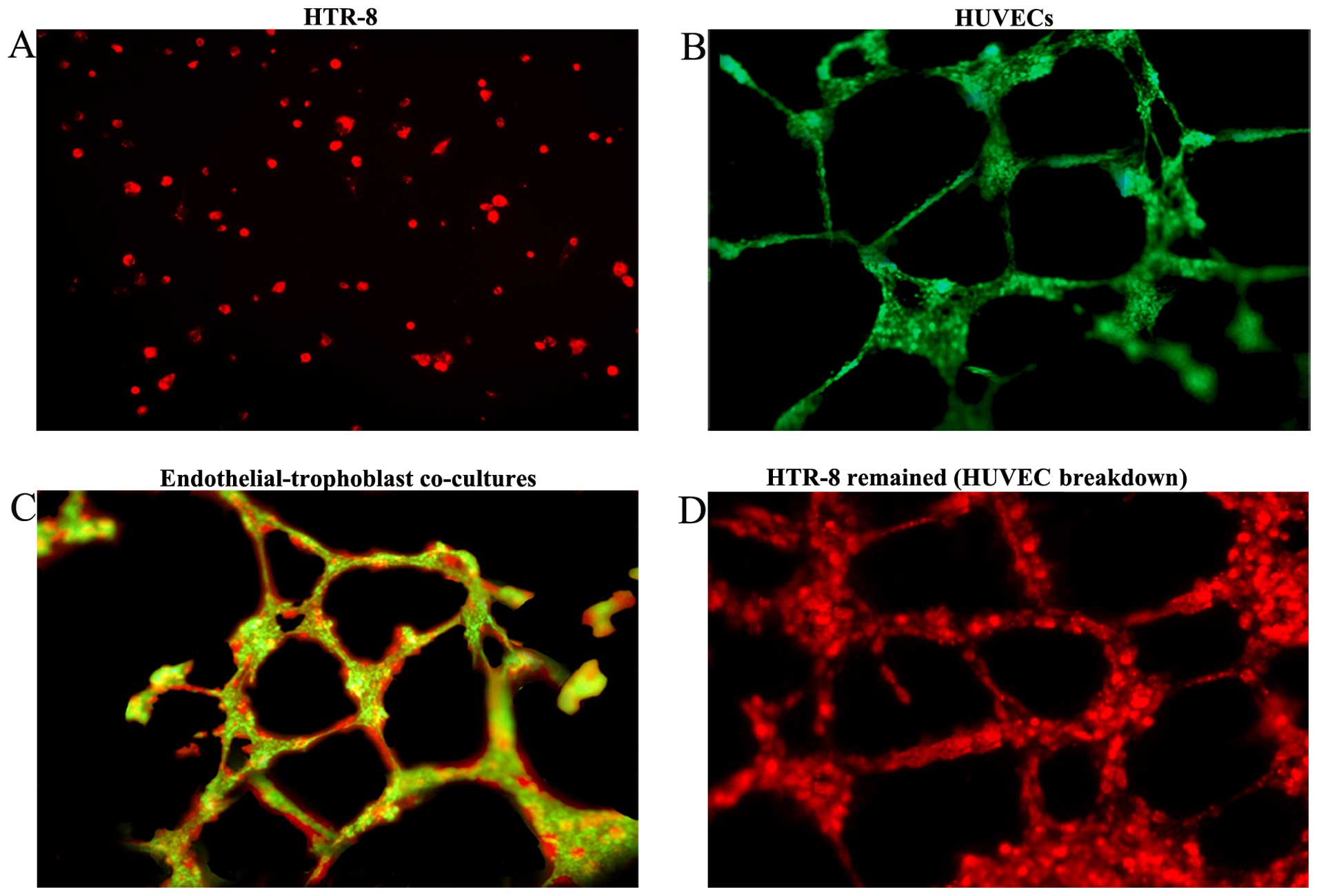
We established an endothelial-trophoblast cell
co-culture system on Matrigel. First trimester trophoblast HTR-8
cells failed to form tube-like structures even after 72 h of
incubation and remained aggregated as lumps or acinar structures
(Fig. 1A). Under identical culture
conditions, HUVECs migrated, polarized, underwent cytoskeleton
remodeling, branched and formed the classical capillary-like tube
(Fig. 1B). However, when the two
cell types were co-cultured, HTR-8 cells spontaneously
'fingerprinted' the capillary tube-like structures of the HUVECs
(Fig. 1C). After 72 h of
co-culturing, HUVECs began to breakdown, and only the tube
structures formed by HTR-8 cells remained. HTR-8 cells distributed
along the walls of the tubes and achieved complete replacement of
the HUVECs (Fig. 1D). After
establishing the endothelial-trophoblast cell co-culture system for
three days, co-cultured cells implanted in the matrix were removed
with NH4OH to establish the preconditioned
microenvironment.
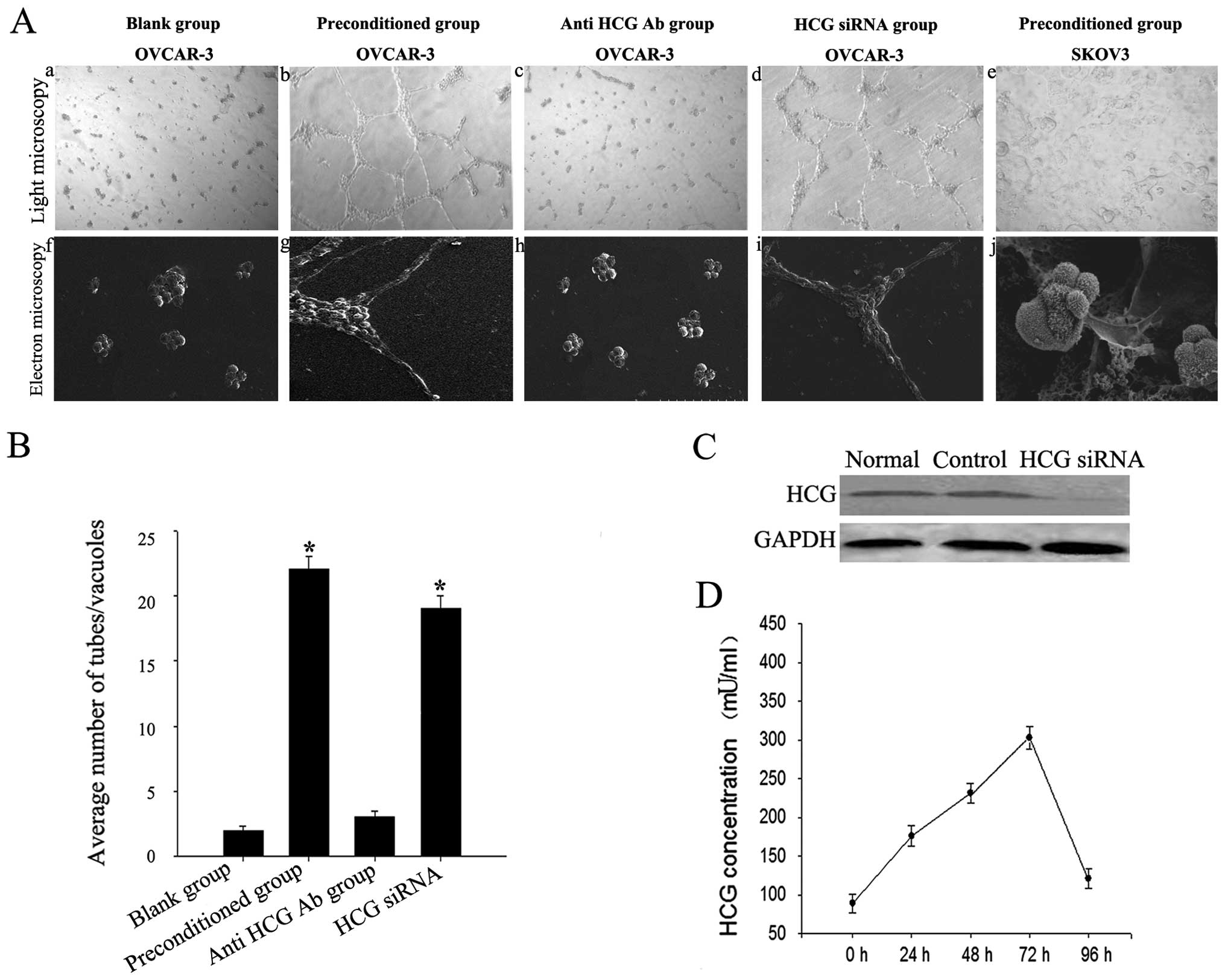
Morphological flexibility of OVCAR-3
cells induced by the HCG-rich microenvironment
OVCAR-3 cells aggregated as lumps on normal Matrigel
(Fig. 2A-a and -f) and migrated,
polarized, underwent cytoskeleton remodeling, branched and formed
classical capillary-like tubes on the preconditioned Matrigel
(Fig. 2A-b and -g). The number of
vessel-like network structures formed by OVCAR-3 cells was
significantly increased in the preconditioned microenvironment
(Fig. 2B). However, SKOV3 cells
failed to form capillary-like tubes (Fig. 2A-e and -j).
We detected expression of HCG in the liquid
supernatant collected after co-culturing for 0, 24, 48, 72 and 96
h. HCG expression increased gradually, peaked at 72 h, then began
to decrease but remained higher than normal levels (Fig. 2D). Tube formation was significantly
inhibited in the OVCAR-3 cells treated with the neutralizing
anti-HCG antibody added to the endothelial-trophoblast cell
co-culture in Matrigel (p<0.05). This result indicated that HCG
in the microenvironment was critical for VM formation in OVCAR-3
cells (Fig. 2A-c and -h).
OVCAR-3 ovarian cancer cells were previously
reported to secrete HCG in an autocrine manner (15). To clarify the source of HCG, we used
HCG siRNA to knockdown endogenous expression of HCG in OVCAR-3
cells (Fig. 2C). We found that HCG
siRNA treatment in OVCAR-3 cells failed to block VM formation in
the preconditioned microenvironment (Fig. 2A-d and -i). This result indicated
that exogenous HCG secreted by trophoblasts may promote VM
formation in OVCAR-3 cells.
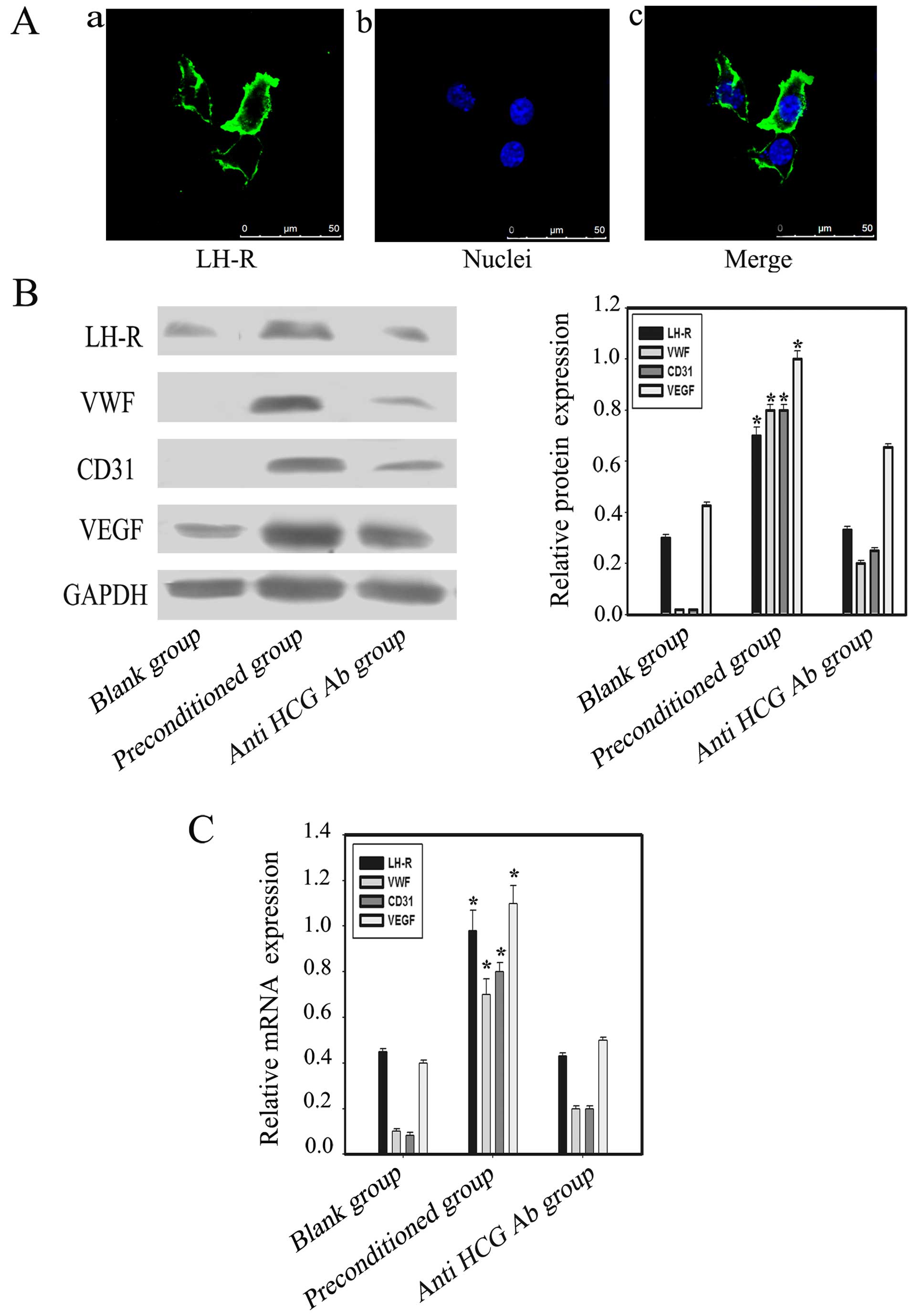
HCG-induced expression of LH-R and
vascular cell markers in OVCAR-3 cells
OVCAR-3 ovarian cancer cell line expresses LH-R
(28), which is consistent with our
immunofluorescence results (Fig.
3A). HCG exerts its effects by binding to LH-R. Western
blotting and PCR results indicated upregulation of LH-R expression
in OVCAR-3 cells in the co-culture environment. Furthermore, the
expression of the vascular markers CD31, VEGF and VWF in the
preconditioned group was significantly increased when compared with
the blank and anti-HCG antibody groups (p<0.05) (Fig. 3B and C).
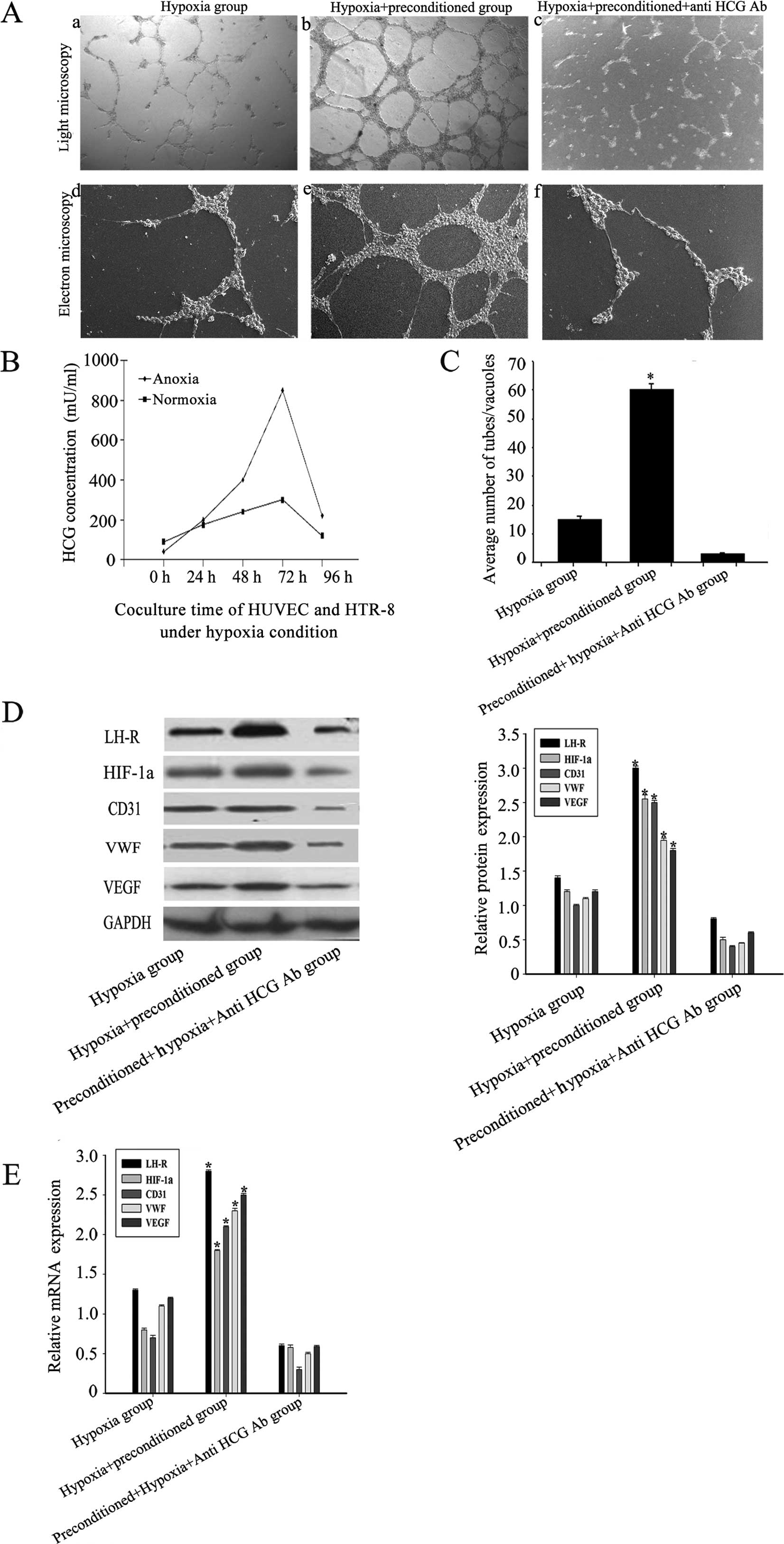
The role of the HCG-rich microenvironment
in regulating VM formation and HIF-1α expression under hypoxic
conditions
Under hypoxic conditions, OVCAR-3 cells were
implanted in normal or preconditioned Matrigel. Our data showed
that the morphological flexibility, i.e., the appearance of network
structures and channels, of OVCAR-3 cells on the preconditioned
Matrigel was significantly greater than that of untreated cells on
normal Matrigel. This plasticity was largely inhibited by a
neutralizing anti-HCG antibody. The expression of the vascular
markers in OVCAR-3 cells implanted in preconditioned Matrigel was
also significantly higher than in the blank and anti-HCG antibody
groups (Fig. 4A and C). The peak
level of HCG was significantly higher under hypoxic conditions than
normoxia (Fig. 4B).
Furthermore, we detected the expression of HIF-1α in
the three groups. Our data showed a significantly increased amount
of HIF-1α expression in the preconditioned group compared with the
other two groups, indicating possible synergistic effects of HCG
and hypoxia (Fig. 4D and E).
Discussion
Vasculogenic mimicry (VM) is found in many highly
invasive tumors and may greatly influence tumor metastasis and
recurrence (29). VM provides a new
perspective on antitumor therapy and has become a hot topic and
difficult problem in gynecological cancer research. The interaction
between tumors and other cell types, i.e., the tumor
microenvironment, is thought to play an important role in VM.
However, the molecular details governing the microenvironment
remain poorly understood. Seftor et al (26) investigated the unique epigenetic
effects of the microenvironment of aggressive melanoma cells on the
behavior of non-aggressive melanoma cells. Collagen matrices
preconditioned with aggressive melanoma cells capable of forming
VM-induced poorly aggressive melanoma cells, which are initially
unable to form VM, to express vasculogenic genes and form VM in
vitro. In this process, the factors secreted by tumor cells or
other niche components in the microenvironment play a critical
role.
HCG, a recently identified angiogenic factor, is
secreted ectopically in the microenvironment of some
non-trophoblastic tumors. It not only stimulates growth and
inhibits apoptosis of cancer cells (30), yet also has a role in angiogenesis
by increasing capillary formation and endothelial cell migration
in vivo and in vitro (31,32).
HCG has been regarded as a stage- and grade-independent prognostic
factor that identifies a subgroup of patients with increased risk
of aggressive disease (33).
Elevated serum or tissue levels of β-HCG are frequently associated
with higher cancer aggressiveness and resistance to therapy
(34), while the reduction of β-HCG
expression by modified U1 snRNA caused apoptosis in cervical cancer
cells (19).
Notably, the sinus in choriocarcinoma is similar to
VM formation in ovarian cancer. Trophoblast cells, which produce
high levels of HCG, invade the endovascular tube and replace the
endothelial cells lining the vessels, a process known as vascular
remodeling. In the present study, we designed a three-dimensional
endothelial-trophoblast cell co-culture system as a preconditioned
β-HCG-rich microenvironment mimicking vascular remodeling to
further investigate the role of β-HCG on ovarian cancer cell
differentiation into endothelioid cells.
We found that OVCAR-3 cells formed VM in the
micro-environment preconditioned by the endothelial-trophoblast
cell co-culture system. The fundamental events in the process were
not clearly defined, and any contribution of HCG to VM formation in
the microenvironment was unknown. To clarify the role of HCG from
the microenvironment in VM formation, we measured the concentration
of HCG in the preconditioned microenvironment. After 72 h of
co-culture, we measured up to 4,000 mU/ml β-HCG in the
microenvironment, and the network structures formed by OVCAR-3 were
inhibited by a neutralizing anti-HCG antibody. Moreover, in
preconditioned Matrigel, CD31, VEGF and VWF expression levels were
upregulated in the OVCAR-3 cells, and these inductive effects were
mostly neutralized by treatment with an anti-HCG antibody, which
was verified by western blotting and real-time PCR. The relative
protein expressions of vascular markers were still slightly higher
in anti-HCG antibody group than in blank group from our data,
possibly since that the neutralizing anti-hCG antibody failed to
recognize all the dimeric forms of the hormone. However,
LH-R-negative SKOV3 ovarian cancer cells could not form VM under
identical conditions. These results indicated that HCG secreted in
the microenvironment contributes to OVCAR-3 differentiation into
endothelioid cells.
Hypoxia induces HIF-1α expression and formation of
VM (35). Under hypoxic conditions,
HIF-1α is the main regulator of cancer cell transcriptional
responses to hypoxia and VM channels (35,36).
We verified this finding herein by analyzing HIF-1α expression and
further investigated the possible relationship between hypoxia and
an HCG-rich preconditioned microenvironment. When the
preconditioned Matrigel was placed under hypoxic conditions, the
number and length of VM were significantly increased compared with
the normoxic condition. Moreover, the expression levels of HCG,
LH-R and HIF-1α, and the vascular markers were also significantly
increased compared with the hypoxia group. These results indicated
that HCG and hypoxia may have synergistic effects on ovarian cancer
cell differentiation into endothelioid cells.
Nearly 90% of advanced-stage ovarian cancer patients
develop recurrence and eventually die from the development of
resistance to cytotoxic chemotherapy. Therefore, there is an urgent
need to develop novel therapeutics that selectively kill ovarian
cancer cells (37). Since there is
abundant LH-R expression in numerous epithelial ovarian cancers,
LH-R may become an attractive therapeutic target. Vaccines against
β-HCG have been successfully demonstrated, suggesting a potential
adjuvant therapy in cancer treatment (38). Our results demonstrate the powerful
influence of the HCG-rich endothelial-trophoblast microenvironment
on endothelial differentiation and VM formation in OVCAR-3 ovarian
cancer cells. We anticipate that these findings may provide new
perspectives on tumor cell plasticity and offer new targets for
novel therapeutic strategies. In addition, further investigation is
required to clarify the signal transduction pathways involved in
HCG-induced OVCAR-3 ovarian cancer cell differentiation.
Acknowledgments
We thank the Laboratory of Immunology, Nantong
University, China for technical assistance. The present study was
supported by grants from the National Natural Science Foundation of
China (30801226). The manuscript was edited by the professional
language editing service Elsevier Webshop.
References
|
1
|
Vartanian AA, Stepanova EV, Gutorov SL,
Solomko ESH, Grigorieva IN, Sokolova IN, Baryshnikov AY and
Lichinitser MR: Prognostic significance of periodic
acid-Schiff-positive patterns in clear cell renal cell carcinoma.
Can J Urol. 16:4726–4732. 2009.PubMed/NCBI
|
|
2
|
Maniotis AJ, Folberg R, Hess A, Seftor EA,
Gardner LM, Pe'er J, Trent JM, Meltzer PS and Hendrix MJ: Vascular
channel formation by human melanoma cells in vivo and in vitro:
Vasculogenic mimicry. Am J Pathol. 155:739–752. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sun D, Sun B, Liu T, Zhao X, Che N, Gu Q,
Dong X, Yao Z, Li R, Li J, et al: Slug promoted vasculogenic
mimicry in hepatocellular carcinoma. J Cell Mol Med. 17:1038–1047.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Qin L, Ren Y, Chen AM, Guo FJ, Xu F, Gong
C, Cheng P, Du Y and Liao H: Peroxisome proliferator-activated
receptor γ ligands inhibit VEGF-mediated vasculogenic mimicry of
prostate cancer through the AKT signaling pathway. Mol Med Rep.
10:276–282. 2014.PubMed/NCBI
|
|
5
|
El Hallani S, Boisselier B, Peglion F,
Rousseau A, Colin C, Idbaih A, Marie Y, Mokhtari K, Thomas JL,
Eichmann A, et al: A new alternative mechanism in glioblastoma
vascularization: Tubular vasculogenic mimicry. Brain. 133:973–982.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Francescone RA III, Faibish M and Shao R:
A Matrigel-based tube formation assay to assess the vasculogenic
activity of tumor cells. J Vis Exp. 7:30402011.
|
|
7
|
Zhang Y, Sun B, Zhao X, Liu Z, Wang X, Yao
X, Dong X and Chi J: Clinical significances and prognostic value of
cancer stem-like cells markers and vasculogenic mimicry in renal
cell carcinoma. J Surg Oncol. 108:414–419. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sood AK, Fletcher MS, Zahn CM, Gruman LM,
Coffin JE, Seftor EA and Hendrix MJ: The clinical significance of
tumor cell-lined vasculature in ovarian carcinoma: Implications for
anti-vasculogenic therapy. Cancer Biol Ther. 1:661–664. 2002.
View Article : Google Scholar
|
|
9
|
Chen LX, Sun BC, Zhang SW, He YJ, Li XR
and He ZJ: The mechanisms of microenvironments influence on
vasculogenic mimicry between intraocular and subcutaneous melanoma.
Zhonghua Yan Ke Za Zhi. 45:641–646. 2009.In Chinese. PubMed/NCBI
|
|
10
|
Yao LQ, Feng YJ, Ding JX, Jing HM, Xu CJ,
Chen SF, Su M and Yin LH: Characteristics and differentiated
mechanism of vascular endothelial cells-like derived from
epithelial ovarian cancer cells induced by hypoxia. Int J Oncol.
30:1069–1075. 2007.PubMed/NCBI
|
|
11
|
Du J, Sun B, Zhao X, Gu Q, Dong X, Mo J,
Sun T, Wang J, Sun R and Liu Y: Hypoxia promotes vasculogenic
mimicry formation by inducing epithelial-mesenchymal transition in
ovarian carcinoma. Gynecol Oncol. 133:575–583. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Su M, Feng YJ, Yao LQ, Cheng MJ, Xu CJ,
Huang Y, Zhao YQ and Jiang H: Plasticity of ovarian cancer cell
SKOV3ip and vasculogenic mimicry in vivo. Int J Gynecol Cancer.
18:476–486. 2008. View Article : Google Scholar
|
|
13
|
Shih IeM: Trophoblastic vasculogenic
mimicry in gestational choriocarcinoma. Mod Pathol. 24:646–652.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Muller CY and Cole LA: The quagmire of hCG
and hCG testing in gynecologic oncology. Gynecol Oncol.
112:663–672. 2009. View Article : Google Scholar
|
|
15
|
Szajnik M, Nowak-Markwitz E, Szczepański
MJ and Spaczyński M: Assessment of expression of luteinizing
hormone (LH)/human chorionic gonadotropin (hCG) receptor (LH/hCGR)
and hCG protein in ovarian cancer tissues. Ginekol Pol. 78:939–943.
2007.In Polish.
|
|
16
|
Lempiäinen A, Stenman UH, Blomqvist C and
Hotakainen K: Free beta-subunit of human chorionic gonadotropin in
serum is a diagnostically sensitive marker of seminomatous
testicular cancer. Clin Chem. 54:1840–1843. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Iles RK, Delves PJ and Butler SA: Does hCG
or hCGβ play a role in cancer cell biology? Mol Cell Endocrinol.
329:62–70. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Burczynska B, Booth MJ, Iles RK, Shah A,
Shiled A and Butler SA: Stable knockdown of hCGβ mRNA expression in
bladder cancer cells results in significant growth inhibition.
Anticancer Res. 33:3611–3614. 2013.PubMed/NCBI
|
|
19
|
Jankowska A, Gunderson SI, Andrusiewicz M,
Burczynska B, Szczerba A, Jarmolowski A, Nowak-Markwitz E and
Warchol JB: Reduction of human chorionic gonadotropin beta subunit
expression by modified U1 snRNA caused apoptosis in cervical cancer
cells. Mol Cancer. 7:262008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jankowska AG, Andrusiewicz M, Fischer N
and Warchol PJ: Expression of hCG and GnRHs and their receptors in
endometrial carcinoma and hyperplasia. Int J Gynecol Cancer.
20:92–101. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lenhard M, Tsvilina A, Schumacher L, Kupka
M, Ditsch N, Mayr D, Friese K and Jeschke U: Human chorionic
gonadotropin and its relation to grade, stage and patient survival
in ovarian cancer. BMC Cancer. 12:22012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ziecik AJ, Kaczmarek MM, Blitek A,
Kowalczyk AE, Li X and Rahman NA: Novel biological and possible
applicable roles of LH/hCG receptor. Mol Cell Endocrinol.
269:51–60. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pietrowski D, Wiehle P, Sator M, Just A
and Keck C: Regulation of the angiopoietin-2 gene by hCG in ovarian
cancer cell line OVCAR-3. Horm Metab Res. 42:328–333. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Brouillet S, Hoffmann P, Chauvet S,
Salomon A, Chamboredon S, Sergent F, Benharouga M, Feige JJ and
Alfaidy N: Revisiting the role of hCG: New regulation of the
angiogenic factor EG-VEGF and its receptors. Cell Mol Life Sci.
69:1537–1550. 2012. View Article : Google Scholar
|
|
25
|
Su M, Wei W, Xu X, Wang X, Chen C, Su L
and Zhang Y: Role of hCG in vasculogenic mimicry in OVCAR-3 ovarian
cancer cell line. Int J Gynecol Cancer. 21:1366–1374. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Seftor EA, Meltzer PS, Kirschmann DA,
Margaryan NV, Seftor RE and Hendrix MJ: The epigenetic
reprogramming of poorly aggressive melanoma cells by a metastatic
microenvironment. J Cell Mol Med. 10:174–196. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao N, Sun BC, Sun T, Ma YM, Zhao XL, Liu
ZY, Dong XY, Che N, Mo J and Gu Q: Hypoxia-induced vasculogenic
mimicry formation via VE-cadherin regulation by Bcl-2. Med Oncol.
29:3599–3607. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gebauer G, Mueller N, Fehm T, Berkholz A,
Beck EP, Jaeger W and Licht P: Expression and regulation of
luteinizing hormone/human chorionic gonadotropin receptors in
ovarian cancer and its correlation to human chorionic
gonadotropin-doxorubicin sensitivity. Am J Obstet Gynecol.
190:1621–1628. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sun Q, Zou X, Zhang T, Shen J, Yin Y and
Xiang J: The role of miR-200a in vasculogenic mimicry and its
clinical significance in ovarian cancer. Gynecol Oncol.
132:730–738. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Elzarrad K, Haroon A, Reed D and Al-Mehdi
AB: Early incorporated endothelial cells as origin of metastatic
tumor vasculogenesis. Clin Exp Metastasis. 26:589–598. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Michel RM, Aguilar JL and Arrieta O: Human
chorionic gonadotropin as an angiogenic factor in breast cancer
during pregnancy. Med Hypotheses. 68:1035–1040. 2007. View Article : Google Scholar
|
|
32
|
Phan B, Rakenius A, Pietrowski D,
Bettendorf H, Keck C and Herr D: hCG-dependent regulation of
angiogenic factors in human granulosa lutein cells. Mol Reprod Dev.
73:878–884. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lempiäinen A, Hotakainen K, Blomqvist C,
Alfthan H and Stenman UH: Hyperglycosylated human chorionic
gonadotropin in serum of testicular cancer patients. Clin Chem.
58:1123–1129. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Crabb SJ, Sharp A, Head J, Chau C, Wheater
M, Geldart TR, et al: Investigating serum β-HCG as an independent
prognostic factor in patients (pts) receiving chemotherapy (Ct) for
transitional cell carcinoma (TCC) of the urothelial tract. J Clin
Oncol. 31:45492013.
|
|
35
|
Sun W, Shen ZY, Zhang H, Fan YZ, Zhang WZ,
Zhang JT, Lu XS and Ye C: Overexpression of HIF-1α in primary
gallbladder carcinoma and its relation to vasculogenic mimicry and
unfavourable prognosis. Oncol Rep. 27:1990–2002. 2012.PubMed/NCBI
|
|
36
|
Comito G, Calvani M, Giannoni E, Bianchini
F, Calorini L, Torre E, Migliore C, Giordano S and Chiarugi P:
HIF-1α stabilization by mitochondrial ROS promotes Met-dependent
invasive growth and vasculogenic mimicry in melanoma cells. Free
Radic Biol Med. 51:893–904. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
McCann GA, Rath KS, Naidu S, Lata P,
Hemant B, Sudhakar M, Hideg K, Houghton P, Kuppusamy P, Cohn DE, et
al: HO-3867, is selectively cytotoxic to ovarian cancer cells
through a dual mechanism of action involving the STAT3 and AKT
pathways. Cancer Res. 73(Suppl 8): 10392013. View Article : Google Scholar
|
|
38
|
Yang J, Zhang Y, Wang H, Gao Z, Wang Z,
Liu B, Zhang X, Du M, Huang X, Xu M, et al: Vaccination with the
repeat β-hCG C-terminal peptide carried by heat shock protein-65
(HSP65) for inducing antitumor effects. Tumour Biol. 33:1777–1784.
2012. View Article : Google Scholar : PubMed/NCBI
|