Introduction
Bladder cancer is the fourth most common malignancy
in men and the ninth most common in women, with 74,690 cases being
diagnosed in 2014 (1). Ten to 15%
of patients present with regional or distant metastatic disease at
diagnosis (2) and have 5-year
survival rates of 33 and 5%, respectively (3). Patients with muscle-invasive bladder
cancer have a poor prognosis, often with survival of <1 year
after metastasis to distant organs (4).
Metastatic bladder cancer is typically treated with
various combinations of systemic chemotherapy (5). However, the majority of patients with
metastatic bladder cancer succumb to the disease within 1–2 years
(5). Gemcitabine (Gem), has been
recognized for its activity against bladder cancer, and several
novel combination regimens including Gem have been reported
(6,7). The combination of Gem and cisplatin
has been shown to have similar efficacy to but lower toxicity than
the standard combination treatment (methotrexate, vinblastine,
doxorubicin, and cisplatin) (6).
However, there is no compelling evidence of improved antitumor
efficacy with the Gem plus cisplatin regimen. New agents that may
increase the efficacy of cytotoxic chemotherapy for bladder cancer
are needed.
Recent evidence suggests that bladder cancer has a
molecular subtype that resembles breast cancer (8). Estrogen receptors (ERs) play a major
role in breast cancer: ERα and ERβ belong to the same nuclear
receptor superfamily and act as transcriptional factors to mediate
important physiologic functions on regulating the growth of
estrogen-responsive tumors in breast cancer (9,10).
Strong correlations between ERβ expression and bladder tumor grade
and stage (11,12) and between ERβ expression and worse
progression-free survival rate have been identified in patients
with bladder cancer. Additionally, it has been shown that patients
with bladder cancer and high levels of ERβ have a worse
progression-free survival rate than patients without this molecular
subtype (11,13). As women have a disproportionate
incidence of bladder cancer and a worse prognosis than men,
estrogen may play a role in bladder cancer incidence and invasion
(14,15).
Tamoxifen (Tam), a non-steroidal selective ER
modulator that has strong efficacy against ER-positive breast
tumors, may be an ideal synergic agent to increase the cytotoxic
effects of Gem (16). We therefore
hypothesized that Tam would enhance the cytotoxicity of Gem in
human bladder cancer cell lines.
Materials and methods
Cells and maintenance
The TCC-Sup and 5637 cells were kindly provided by
Dr David J. McConkey (The University of Texas MD Anderson Cancer
Center, Houston, TX), and RT4 cells were purchased from the
American Type Culture Collection (ATCC; Manassas, VA, USA). The
three cell lines were fingerprinted by the Characterized Cell Line
Core of MD Anderson Cancer Center for the Specialized Program Of
Research Excellence in Bladder Cancer. These cell lines were
maintained as adherent cells in minimum essential medium (MEM)
supplemented with 10% fetal bovine serum, penicillin, streptomycin,
vitamins, glutamine, non-essential amino acids, and pyruvate at
37°C in a humidified atmosphere of 5% CO2.
Reagents and antibodies
Tam (T9262) was obtained from Sigma-Aldrich (St.
Louis, MO, USA). Gem was obtained from Sagent Pharmaceuticals
(Schaumburg, IL, USA). Antibodies used for western blot analysis
were purchased from the following manufacturers: poly(ADP-ribose)
polymerase (PARP) (sc-7150) and caspase-3 (sc7148) (Santa Cruz
Biotechnology, Inc., Dallas, TX, USA); ERα (2512) and p70 S6 kinase
(p70S6k, 2708) (Cell Signaling Technology, Beverly, MA, USA); ERβ
(ab3576; Abcam, Cambridge, MA, USA); and β-actin (A3853;
Sigma-Aldrich). For the in vitro studies, Tam and Gem were
reconstituted just before use in methanol and sterile water,
respectively, creating stock solutions that were then diluted in
medium to obtain the final concentrations indicated in the
figures.
Evaluation of cell viability
Cells were seeded in a 96-well plate
(3×103 cells/well) for 24 h and then treated with
various concentrations of Tam (1, 10, or 20 µM) or Gem (1
µM) alone or in combination for 24 to 72 h. Cell viability
was assessed by pulsing the cells for 2 h with
3-(4,5-dimeth-ylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
(from 5-mg/ml stock in phosphate-buffered saline solution).
Formazan crystals were solubilized in lysis buffer (100 µl)
containing 20% sodium dodecyl sulfate and 50% dimethylformamide.
Color development was quantified by measuring the optical densities
at 570 nm. The results are shown as means ± SEM. Each experimental
data point represents the mean value of four replicates, and each
experiment was performed ≥2 times.
Quantification of DNA fragmentation and
flow cytometry
Cells were grown in 6-well plates in MEM. At 24 h,
the cells were sequentially treated with Gem (1 µM) for 6 h
and then Tam (10 µM) (Gem→Tam) or Tam then Gem (Tam→Gem) for
an additional 24–72 h. For comparison, the cells were treated with
Tam or Gem alone, or were treated simultaneously (Tam+Gem). The
cells were harvested by trypsinization and pelleted by
centrifugation at 3,500 rpm for 5 min. The cells were then
resuspended in phosphate-buffered saline solution containing
propidium iodide (50 µg/ml), with 0.1% Triton X-100 and 0.1%
sodium citrate. DNA fragmentation was measured using propidium
iodide fluorescence-activated cell sorting (PI-FACS) (FC 500 flow
cytometer; Beckman Coulter, Brea, CA, USA). Cells exhibiting
hypodiploidy, indicative of DNA fragmentation, were scored as
apoptotic. PI-FACS analysis was performed in triplicate, and the
results are shown as the mean percentage of fragmentation ±
SEM.
Western blot analysis
After plating TCC-Sup and 5637 cells, the cells were
treated with the same combinations agents used in the flow
cytometry experiments. The cells were scraped in medium at 24 h,
pelleted by centrifugation at 3,500 rpm for 5 min, washed once with
ice-cold phosphate-buffered saline solution, and re-pelleted by
centrifugation at 3,500 rpm for 5 min. The pellets were subjected
to lysis buffer [50 mM of Tris-HCl, pH 7.4; 150 mM of sodium
chloride; 5 mM of ethylenediaminetetraacetic acid; 25 mM of sodium
fluoride; 1% Triton X-100; 1% NP-40; 0.1 mM of sodium
orthovanadate; 12.5 mM of β-glycerophosphate; 1 mM of
phenylmethylsulfonyl fluoride; and complete protease inhibitor
cocktail (Roche, Basel, Switzerland)] and were clarified at 13,000
rpm for 10 min at 4°C. The supernatants were measured and subjected
to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and
western blotting on a nitrocellulose membrane. The blots were
developed using ECL reagent (GE Healthcare, Pittsburgh, PA, USA).
Equal protein loading was confirmed using β-actin blots.
Soft agar colony formation/transformation
assay
TCC-Sup cells were grown to semi-confluence in 10-cm
plates and were treated with MEM, methanol, and Tam (10 µM)
or Gem (1 µM) alone or with sequential Gem→Tam for 72 h. The
cells were then subjected to trypsinization and were counted.
TCC-Sup cells (5×103 cells/ml) were then seeded in
6-well plates in MEM with 0.5% agarose or were layered on top of
0.4% agar in MEM in 6-well plates (for the soft agar assay). The
plates were incubated at 37°C in a humidified atmosphere of 5%
CO2. After 2 weeks, the cells were stained with MTT (5
mg/ml), and cell colonies were counted and photographed on the
stage of Desk Top Light Box (Logan Electric, Texarkana, AR, USA)
using Canon IXY120 (Tokyo, Japan).
Statistical analysis
Statistical analysis was performed using
Ekuseru-Toukei 2010 software (Social Survey Research Information
Co., Ltd., Tokyo, Japan). Analysis of variance and the Student's
t-test were used to evaluate statistical significance of
differences between cells treated at each drug concentration and
untreated control cells. These data were normal distributed.
P<0.05 was considered statistically significant.
Results
ER expression in bladder cancer cell
lines
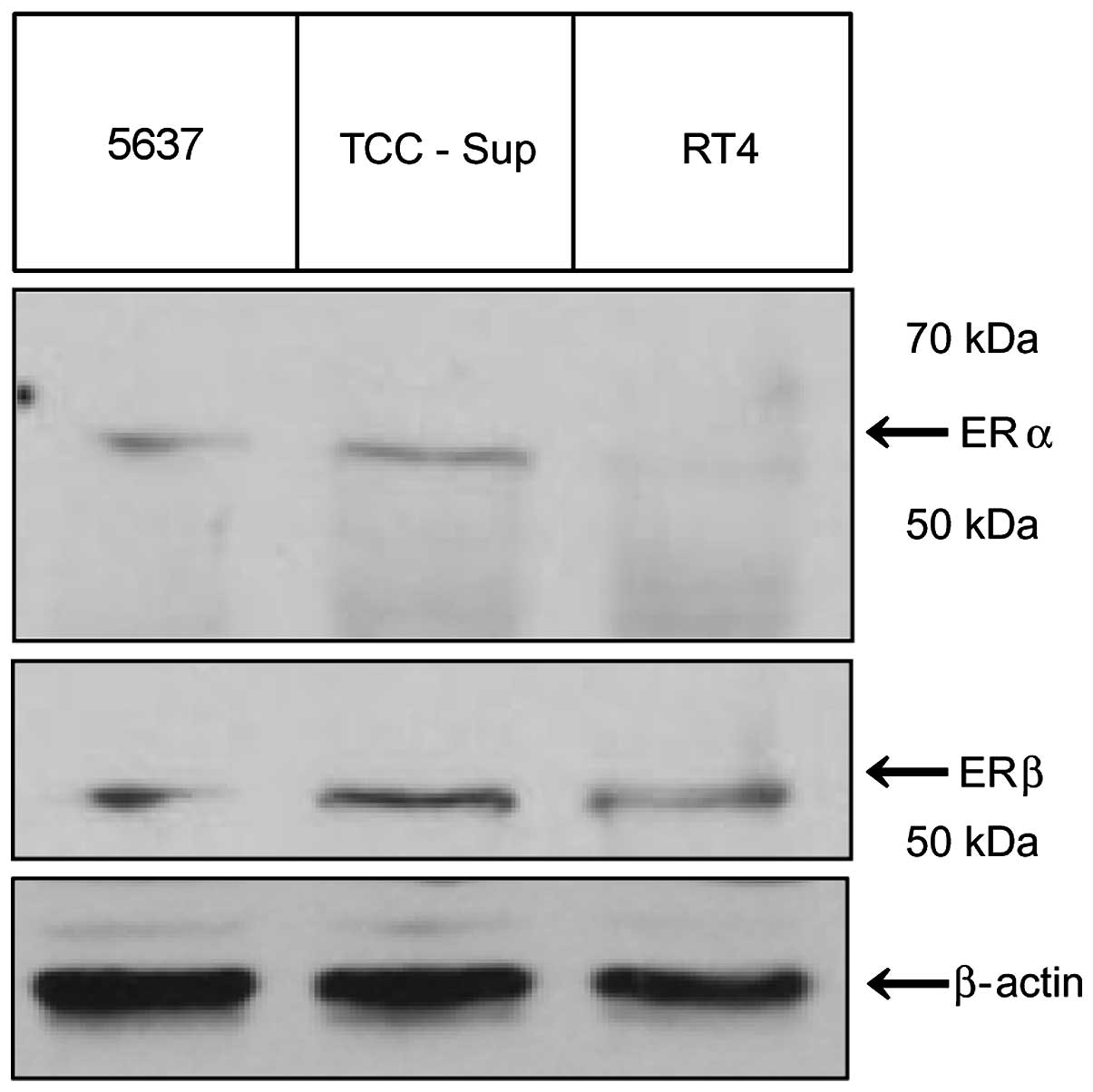
Immunoblotting results for RT4, 5637, and TCC-Sup
cells revealed that RT4 cells had no ERα expression than TCC-Sup
and 5637 cells. However, the three cell lines expressed ERβ, with
the highest levels in TCC-Sup cells (Fig. 1).
Effects of Tam and Gem on cell
viability
We examined the effects of Gem or Tam alone and
simultaneous treatment on the viability of the three cell lines. On
the MTT assay, cell viability was affected by the two agents in a
concentration-dependent manner (Fig.
2). Tam (10 µM) alone treatment for 72 h reduced the
viability of TCC-Sup, 5637, and RT4 cells to 36.4, 40.6, and 45.5%,
respectively, of the viability of untreated control cells (Fig. 2). The three cell lines treated with
the highest dose of Tam (20 µM) alone had a significantly
lower cell viability than the controls, regardless of treatment
time 24 h: [TCC-Sup cells, 1.9%; 5637 cells, 11.9%; RT4 cells,
6.2%; (P<0.05) 48 h: TCC-Sup cells, 5.2%; 5637 cells, 12.4%; RT4
cells, 17.9%; (P<0.05); 72 h: TCC-Sup cells, 1.2%; 5637 cells,
4.1%; RT4 cells, 7.4%; (P<0.05)] (Fig. 2). At the 72-h time-point, Gem (1
µM) reduced the viability of TCC-Sup, 5637, and RT4 cells to
30.5, 21.0, and 21.1%, respectively (P<0.05).
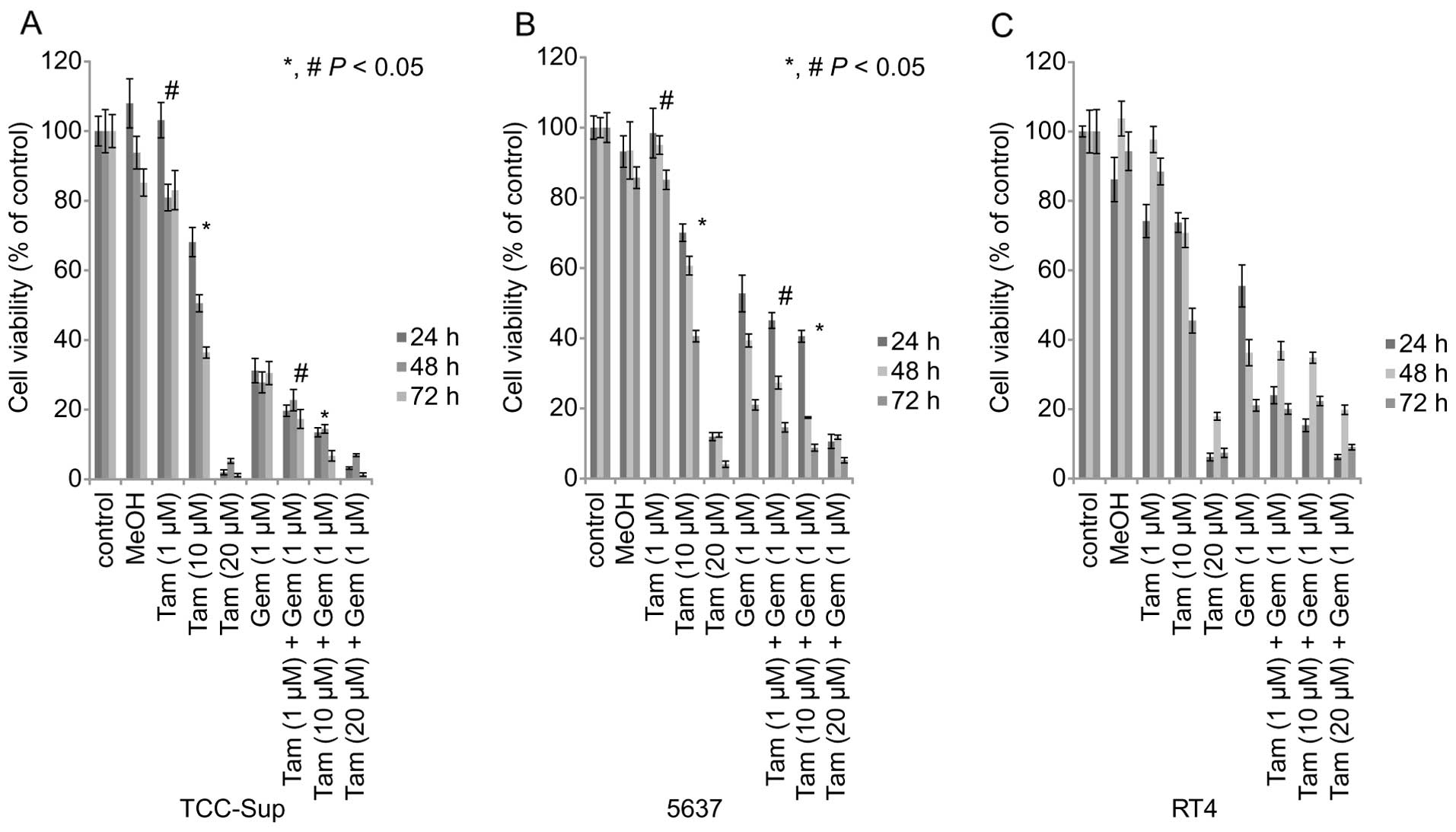 | Figure 2Cell viability of three bladder cancer
cell lines after treatment with Tam or Gem alone or in combination.
MTT assay shows inhibition of viability in TCC-Sup, 5637, and RT4
bladder cancer cells treated with Gem or Tam alone or in
combination. Cell viability analysis of (A) TCC-Sup, (B) 5637, and
(C) RT4 bladder cancer cell lines treated with various
concentrations of Tam (1, 10, or 20 µM) or Gem (1 µM)
alone or simultaneous Gem and Tam for 24–72 h.
*,#P<0.05, statistically significant. Tam, tamoxifen;
Gem, gemcitabine. |
TCC-Sup and 5637 cells treated with simultaneous Gem
(1 µM) + Tam (1 or 10 µM) had a significantly lower
cell viability than those treated with Gem or Tam alone for 72 h
[TCC-Sup cells: Tam (1 µM) + Gem (1 µM), 17.3%; Tam
(10 µM) + Gem (1 µM), 6.7% (P<0.05); 5637 cells:
Tam (1 µM) + Gem (1 µM), 14.6%; Tam (10 µM) +
Gem (1 µM), 8.8% (P<0.05)] (Fig. 2).
However, simultaneous treatment did not
significantly affect cell viability in comparison with Tam or Gem
alone in RT4 cells after 72 h of treatment [RT4 cells: Tam (1
µM) + Gem (1 µM), 20.1%; Tam (10 µM) + Gem (1
µM), 22.4%].
These results demonstrated that high concentrations
of Tam produced substantial cytotoxic effects in the three cell
lines. Treating cells with simultaneous Tam+Gem increased
cytotoxicity with lower concentrations of Tam.
Effect of sequential treatment on DNA
fragmentation
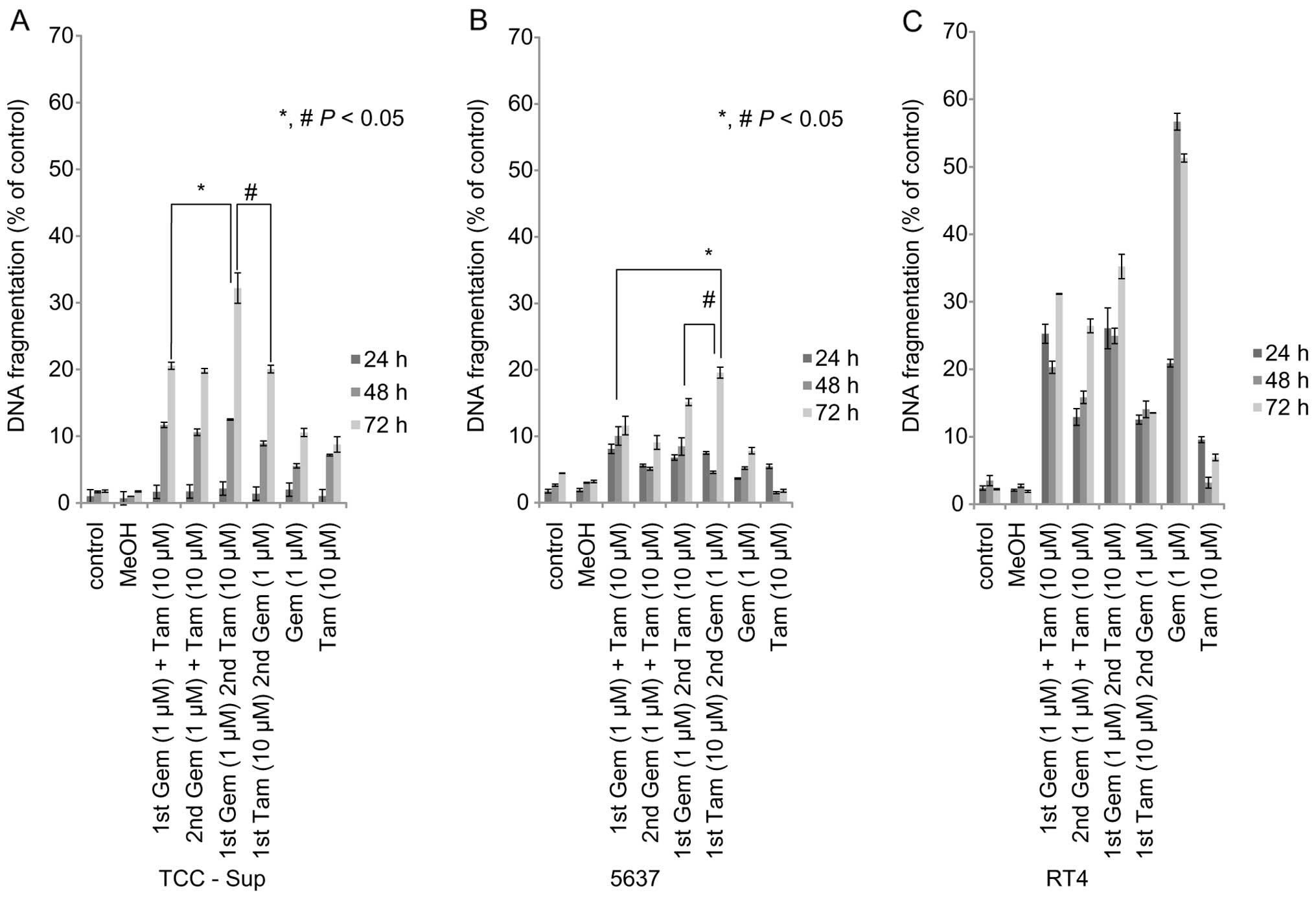
We examined the apoptosis-inducing effects of
sequential of Gem and Tam treatment in the three cell lines. In
TCC-Sup and 5637 cells in response to sequential Gem and Tam
treatment, an increase in DNA fragmentation was consistently
observed with treatment periods >24 h (Fig. 3A and B). TCC-Sup and 5637 cells
treated with sequential Tam (10 µM)→Gem (1 µM) or Gem
(1 µM)→Tam (10 µM) had greater DNA fragmentation than
those treated with Gem or Tam alone at the 72-h time-point
(TCC-Sup, 10.6%; 5637, 7.8%; P<0.05) (Fig. 3A and B).
Sequential Gem→Tam treatment resulted in greater DNA
fragmentation (32.2%) than simultaneous Gem+Tam (20.1%) or
sequential Tam→Gem (20.1%) in TCC-Sup cells (Fig. 3A). DNA fragmentation was slightly
higher in 5637 cells treated with sequential Tam→Gem (19.6%) than
in those treated with simultaneous Gem+Tam (11.6%) or sequential
Gem→Tam (15.1%) (Fig. 3B).
However, RT4 cells treated with sequential Tam→Gem
or Gem→Tam had lower DNA fragmentation than those treated with Gem
alone at the 48- and 72-h time-points (Fig. 3C). In RT4 cells, Gem alone induced a
high amount of DNA fragmentation (48 h, 56.7%), which did not
differ from the DNA fragmentation in RT4 cells treated with either
sequential Tam→Gem or Gem→Tam.
These results demonstrate that sequential Gem→Tam or
Tam→Gem induced apoptosis to a greater extent than Gem or Tam alone
or simultaneous treatment in TCC-Sup and 5637 cells, and these
effects of sequential treatment in TCC-Sup were higher than in 5637
(Fig. 3A and B). These findings
also indicate that TCC-Sup and 5637 cells are resistant to Gem
therapy whereas RT4 cells are not resistant to Gem therapy.
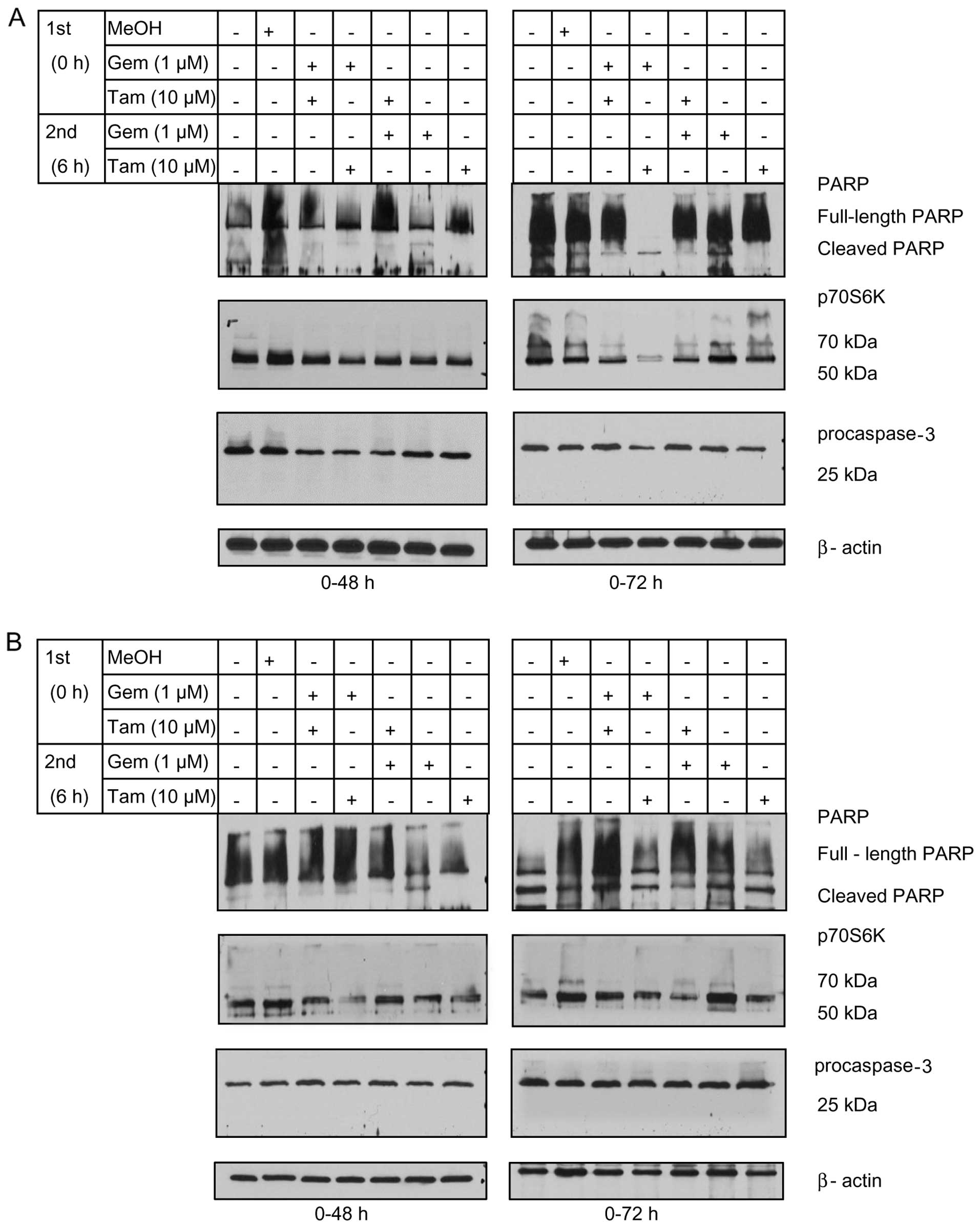
Expression of markers of apoptosis and
cell transformation
To confirm that the cytotoxic effects of sequential
therapy in TCC-Sup and 5637 cells were associated with apoptosis
and reduced cell transformation, we performed western blot analysis
to assess the expression of cleaved caspase-3 as an apoptosis
markers and the cell transformation of cleaved PARP and p70S6k as
markers in treated cells. Caspase and PARP cleavage marked
apoptosis whereas the expression of p70S6k marked the ability of
cells to form spheres (transformation) after apoptosis (17). TCC-Sup cells treated with sequential
Gem→Tam for 72 h had lower levels of full-length PARP, p70S6k, and
procaspase-3 and had higher levels of cleaved PARP than the
controls (Fig. 4A). The expression
levels of the apoptotic markers were lower in 5637 cells treated
with the regimen, including the Tam→Gem sequence (Fig. 4B). Notably, p70S6k was downregulated
by sequential Gem→Tam treatment in TCC-Sup cells (Fig. 4A) at the 72-h time-point, indicating
that sequential treatment may inhibit cell transformation. Taken
together, these data demonstrated that sequential Gem→Tam treatment
enhanced PARP cleavage and downregulated p70S6k in TCC-Sup
cells.
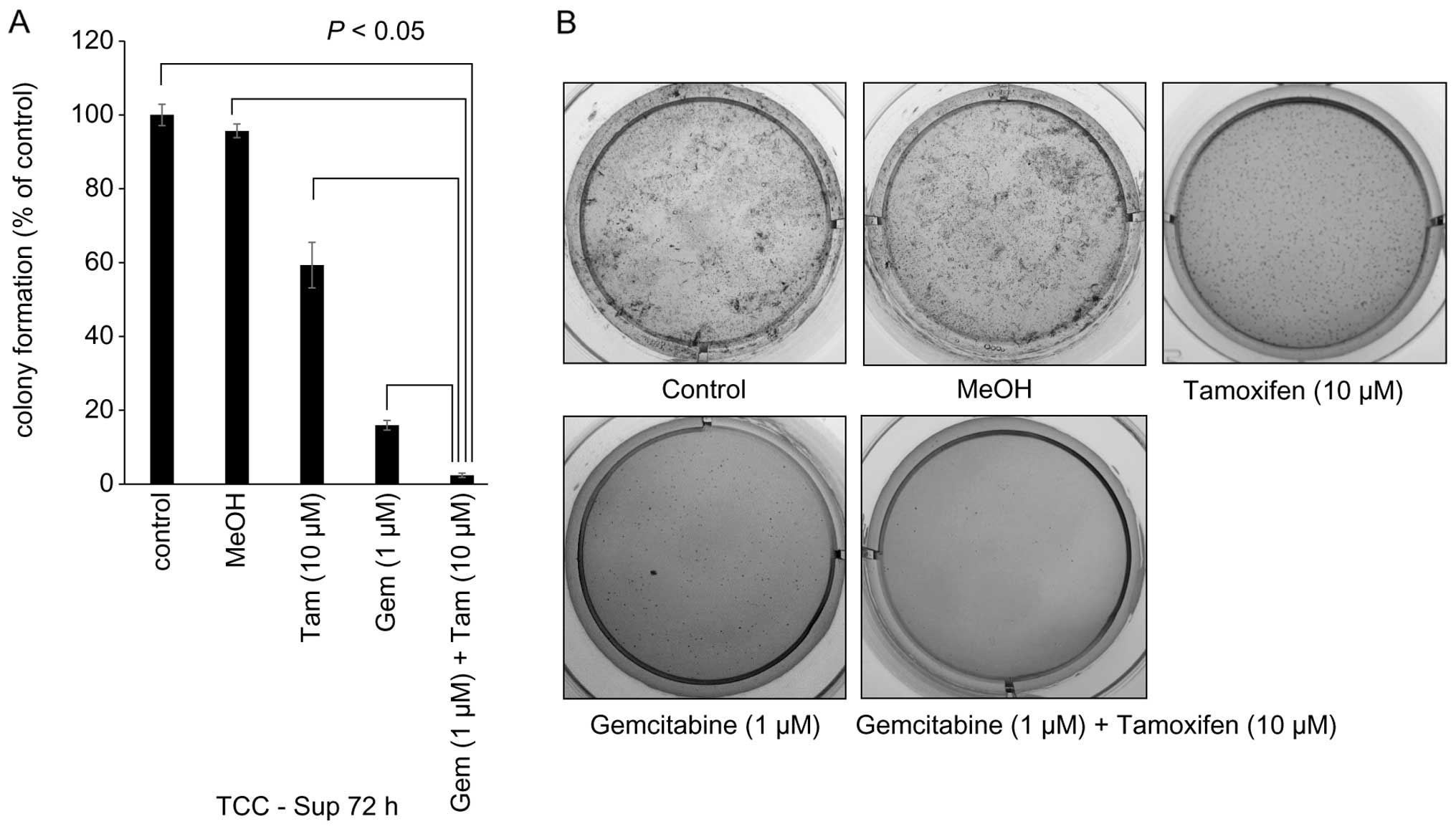
Inhibition of cell transformation by
sequential Gem→Tam treatment in TCC-Sup cells
Since p70S6k was downregulated by sequential Gem→Tam
treatment in TCC-Sup cells, we performed soft agar colony formation
assays to determine the effects of these agents on cellular
transformation in TCC-Sup cells. Cells treated with sequential
Gem→Tam had significantly less transformation (2.4%) than those
treated with Tam (59.3%) or Gem (15.9%) alone (P<0.05, Fig. 5A). These results demonstrated that
sequential Gem→Tam treatment effectively inhibited cell
transformation.
Discussion
In the present study, we found TCC-Sup and 5637
cells were resistant to Gem therapy whereas RT4 cells were not
resistant to Gem therapy. However, the sequential treatment of Gem
and Tam enhanced apoptosis in TCC-Sup and 5637 cells. Sequential
Gem followed by Tam (Gem→Tam) treatment caused the largest increase
in DNA fragmentation at 72 h in TCC-Sup cells compared to the other
treatments in the remaining two cell lines, and this regimen
enhanced PARP cleavage and downregulated p70S6k and blocked
transformation in bladder cancer cells.
Our results demonstrate that Tam induced significant
apoptosis when treated in sequence after gemcitabine with prolonged
incubation in a cell type-dependent manner. Bladder cancer is shown
to have similarities to breast cancer in terms of gene expression
data profiling (8). Breast cancer
is known to have therapeutic efficiency based on ER expression.
Thus, we examined whether bladder cancer cells express ERs or not.
This cell-type dependency likely reflects the expression of of ERs
for the cell lines. TCC-Sup and 5637 cells, which had the higher
ERβ levels, had more resistance to apoptosis when treated with Gem
alone than RT4 cells, which had lower levels of ERβ than TCC-Sup
and 5637 cells. Our findings support those of Shen et al
(12) who reported that ERβ
expression occurred more frequently in high-grade bladder cancers
and significantly more often in muscle-invasive and metastatic
bladder cancers. Shen et al (12) suggested that ERβ possibly plays a
role in tumor progression and metastasis, possibly by conferring a
growth advantage.
TCC-Sup cells is a bladder cancer cell line that
exhibits metastasis and cell transformation and 5637 cells exhibit
invasiveness, whereas RT4 bladder cell lines represent papillary
tumor. Our findings showing that TCC-Sup and 5637 cells are
resistant to Gem reflect the clinical outcomes of patients with
metastatic or invasive bladder cancer. These patients are typically
treated with systemic chemotherapy regimens that include Gem,
although the median progression-free and overall survival durations
are only 7.7 and 14.0 months, respectively (18). Regimens that increase the efficiency
of Gem are needed to improve patient outcomes.
Gem is a nucleoside analogue that interferes with
DNA synthesis to induce apoptosis (19). Tam is a standard endocrine therapy
for the treatment of steroid receptor-positive breast cancer
(20,21). Our results support those of another
study which found that Tam treatment inhibited bladder cancer cell
proliferation (12). In the present
study, Tam alone showed significant cytotoxic effects in the three
cell lines, but only at high concentrations. Combining Tam with Gem
resulted in increased cytotoxicity at lower concentrations.
Our results show that the sequential treatment
exerted significant apoptotic effects in TCC-Sup and 5637 cells,
which showed resistance to Gem alone. Previous findings have
suggested that combining Gem and Tam is a valid and effective
therapy for advanced breast cancer, (22). Additionally, the efficacy of this
combination treatment in breast cancer was previously demonstrated
(23). Sequential Gem→Tam treatment
resulted in greater DNA fragmentation in TCC-Sup cells, whereas
Tam→Gem treatment resulted in greater DNA fragmentation in 5637
cells. However, the effects level of sequential treatment itself
resulted in greater DNA fragmentation in TCC-Sup cells than in 5637
cells. Therefore, the results of the present study have effectively
shown the significance of utilizing this drug schedule (Gem→Tam) in
TCC-Sup. Our results demonstrate that sequential treatment for 72 h
effectively induced TCC-Sup cell death through apoptosis. Several
studies have investigated ERα and ERβ protein levels in bladder
cancer development and have found correlations between ER
expression and bladder cancer stage (11–13,24),
which underscores the importance of targeting ERs in bladder cancer
therapy.
There is also a possibility of sequential treatment
using Gem and Tam in metastastic bladder cancer. However, we were
not able to determine the usefulness of this treatment, including
clinical background. Our results have demonstrated that sequential
Gem→Tam treatment effectively inhibited cell transformation in
TCC-Sup cells. Previous findings showed that apoptotic cells
expressing p70S6k are capable of undergoing cell transformation
(sphere formation) (17). We found
that the sequential Gem→Tam treatment reduced p70S6k expression,
which efficiently inhibited cell transformation in TCC-Sup cells.
Thus, our study identified a viable sequential treatment strategy
in vitro that remains to be confirmed via in vivo
studies.
In conclusion, prolonged sequential Gem→Tam
treatment induced significant apoptosis in a bladder cancer cell
type-dependent manner and inhibited cell transformation. This
sequential treatment may be useful in the increase of the
efficiency of Gem against bladder cancer in a subset of patients
and should be investigated.
Acknowledgments
The authors would like to thank the Department of
Scientific Publications, The University of Texas MD Anderson Cancer
Center for the English Language Review. This study was supported in
part by the National Institutes of Health through MD Anderson
Cancer Center's Support Grant, CA016672.
References
|
1
|
DeSantis CE, Lin CC, Mariotto AB, Siegel
RL, Stein KD, Kramer JL, Alteri R, Robbins AS and Jemal A: Cancer
treatment and survivorship statistics, 2014. CA Cancer J Clin.
64:252–271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rosenberg JE, Carroll PR and Small EJ:
Update on chemotherapy for advanced bladder cancer. J Urol.
174:14–20. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stein JP, Lieskovsky G, Cote R, Groshen S,
Feng AC, Boyd S, Skinner E, Bochner B, Thangathurai D, Mikhail M,
et al: Radical cystectomy in the treatment of invasive bladder
cancer: Long-term results in 1,054 patients. J Clin Oncol.
19:666–675. 2001.PubMed/NCBI
|
|
4
|
Galsky MD, Moshier E, Krege S, Lin CC,
Hahn N, Ecke T, Sonpavde G, Godbold J, Oh WK and Bamias A: Nomogram
for predicting survival in patients with unresectable and/or
metastatic urothelial cancer who are treated with cisplatin-based
chemotherapy. Cancer. 119:3012–3019. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sternberg CN, Yagoda A, Scher HI, Watson
RC, Geller N, Herr HW, Morse MJ, Sogani PC, Vaughan ED, Bander N,
et al: Methotrexate, vinblastine, doxorubicin, and cisplatin for
advanced transitional cell carcinoma of the urothelium. Efficacy
and patterns of response and relapse. Cancer. 64:2448–2458. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
von der Maase H, Hansen SW, Roberts JT,
Dogliotti L, Oliver T, Moore MJ, Bodrogi I, Albers P, Knuth A,
Lippert CM, et al: Gemcitabine and cisplatin versus methotrexate,
vinblastine, doxorubicin, and cisplatin in advanced or metastatic
bladder cancer: Results of a large, randomized, multinational,
multi-center, phase III study. J Clin Oncol. 18:3068–3077.
2000.PubMed/NCBI
|
|
7
|
Hussain M, Vaishampayan U, Du W, Redman B
and Smith DC: Combination paclitaxel, carboplatin, and gemcitabine
is an active treatment for advanced urothelial cancer. J Clin
Oncol. 19:2527–2533. 2001.PubMed/NCBI
|
|
8
|
Choi W, Porten S, Kim S, Willis D, Plimack
ER, Hoffman-Censits J, Roth B, Cheng T, Tran M, Lee IL, et al:
Identification of distinct basal and luminal subtypes of
muscle-invasive bladder cancer with different sensitivities to
frontline chemotherapy. Cancer Cell. 25:152–165. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nilsson S, Mäkelä S, Treuter E, Tujague M,
Thomsen J, Andersson G, Enmark E, Pettersson K, Warner M and
Gustafsson JA: Mechanisms of estrogen action. Physiol Rev.
81:1535–1565. 2001.PubMed/NCBI
|
|
10
|
Harris HA: Estrogen receptor-beta: Recent
lessons from in vivo studies. Mol Endocrinol. 21:1–13. 2007.
View Article : Google Scholar
|
|
11
|
Miyamoto H, Yao JL, Chaux A, Zheng Y, Hsu
I, Izumi K, Chang C, Messing EM, Netto GJ and Yeh S: Expression of
androgen and oestrogen receptors and its prognostic significance in
urothelial neoplasm of the urinary bladder. BJU Int. 109:1716–1726.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shen SS, Smith CL, Hsieh JT, Yu J, Kim IY,
Jian W, Sonpavde G, Ayala GE, Younes M and Lerner SP: Expression of
estrogen receptors-alpha and -beta in bladder cancer cell lines and
human bladder tumor tissue. Cancer. 106:2610–2616. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tuygun C, Kankaya D, Imamoglu A, Sertcelik
A, Zengin K, Oktay M and Sertcelik N: Sex-specific hormone
receptors in urothelial carcinomas of the human urinary bladder: A
comparative analysis of clinicopathological features and survival
outcomes according to receptor expression. Urol Oncol. 29:43–51.
2011. View Article : Google Scholar
|
|
14
|
Pfister C: New trends for optimal
management of bladder tumors. J Urol. 165:65–66. 2001. View Article : Google Scholar
|
|
15
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zheng A, Kallio A and Härkönen P:
Tamoxifen-induced rapid death of MCF-7 breast cancer cells is
mediated via extracellularly signal-regulated kinase signaling and
can be abrogated by estrogen. Endocrinology. 148:2764–2777. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jinesh GG, Choi W, Shah JB, Lee EK, Willis
DL and Kamat AM: Blebbishields, the emergency program for cancer
stem cells: Sphere formation and tumorigenesis after apoptosis.
Cell Death Differ. 20:382–395. 2013. View Article : Google Scholar :
|
|
18
|
von der Maase H, Sengelov L, Roberts JT,
Ricci S, Dogliotti L, Oliver T, Moore MJ, Zimmermann A and Arning
M: Long-term survival results of a randomized trial comparing
gemcitabine plus cisplatin, with methotrexate, vinblastine,
doxorubicin, plus cisplatin in patients with bladder cancer. J Clin
Oncol. 23:4602–4608. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang P, Chubb S, Hertel LW, Grindey GB
and Plunkett W: Action of 2′,2′-difluorodeoxycytidine on DNA
synthesis. Cancer Res. 51:6110–6117. 1991.PubMed/NCBI
|
|
20
|
Brauch H and Jordan VC: Targeting of
tamoxifen to enhance anti-tumour action for the treatment and
prevention of breast cancer: The 'personalised' approach? Eur J
Cancer. 45:2274–2283. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Marshall E: Tamoxifen. 'A big deal,' but a
complex hand to play. Science. 280:1961998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Silvestris N, Cinieri S, La Torre I,
Pezzella G, Numico G, Orlando L and Lorusso V: Role of gemcitabine
in metastatic breast cancer patients: A short review. Breast.
17:220–226. 2008. View Article : Google Scholar
|
|
23
|
Cosco D, Paolino D, Cilurzo F, Casale F
and Fresta M: Gemcitabine and tamoxifen-loaded liposomes as
multidrug carriers for the treatment of breast cancer diseases. Int
J Pharm. 422:229–237. 2012. View Article : Google Scholar
|
|
24
|
Han B, Cui D, Jing Y, Hong Y and Xia S:
Estrogen receptor β (ERβ) is a novel prognostic marker of
recurrence survival in non-muscle-invasive bladder cancer
potentially by inhibiting cadherin switch. World J Urol.
30:861–867. 2012. View Article : Google Scholar : PubMed/NCBI
|