Introduction
Oxygen is an essential nutrient for cellular
respiration and organisms must closely monitor fluctuations in
oxygen concentration to maintain homeostasis. Changes in oxygen
concentration can signal events in embryonic development (1), determine stem cell fate (2,3) and
contribute to pathological conditions (4,5).
Inadequate oxygen supply to a tissue, or hypoxia, triggers an
adaptive response mediated by hypoxia-inducible factors (HIFs)
(6). Hypoxia is a common feature of
malignant tumors and can be observed in central regions of solid
tumors (7,8). When tumor size reaches 1–2
mm3, the center of the tumor becomes hypoxic due to the
lack of adequate blood supply, hindering tumor growth. The growth
of rich, new vasculature or angiogenesis, is triggered by hypoxia
and supports the growing tumor by providing nutrients and oxygen
(9,10). Cellular responses to hypoxia are
diverse and include changes in metabolism, antioxidant gene
expression, cell proliferation, apoptosis and angiogenesis
(6,8,9).
Angiogenesis is essential for tumor growth and
progression (11), meaning that
tumor growth could be effectively inhibited when angiogenesis is
blocked (12). Angiogenesis is a
multistep process that begins when quiescent endothelial cells are
activated by signals from ischemic tissue or a hypoxic solid tumor.
Activated endothelial cells degrade the extracellular matrix,
proliferate and migrate toward the source of the stimuli, forming
an immature vascular network. The newly formed network undergoes a
process of maturation and stabilization that includes the
recruitment of supporting mural cells, association with mural cells
and placement of a new basement membrane (9,10). The
angiogenic process is tightly regulated through angiogenic and
anti-angiogenic factors (9).
Several factors containing heparin-binding domains,
including vascular endothelial growth factor (VEGF), basic
fibroblast growth factor (bFGF/FGF-2), acidic FGF (aFGF/FGF-1) and
heparin-binding EGF-like growth factor (HB-EGF) have angiogenic
functions (13–15). The hypoxia-inducible factor-1
(HIF-1) transcription factor is a key regulator of hypoxia-induced
angiogenesis (6,16). HIF-1 regulates genes affecting
vessel formation such as VEGF, placental growth factor and bFGF
(16). We are interested in
identifying and characterizing angiogenesis-related factors that
are sensitive to hypoxia; in the present study we focused on the
fibroblast growth factor (FGF) gene family containing
heparin-binding domains.
Twenty-two members of the FGF family (FGF1-FGF23)
have been reported in humans and rodents (17). Human FGF19 is the ortholog of rodent
FGF15. Fibroblast growth factors (FGFs) can be classified as
secretory (FGF1-FGF10 and FGF15-FGF23) or intracellular and
non-secretory (FGF11-FGF14) (17,18).
Most secretory FGFs and their surface FGF receptors have been well
characterized and carry out defined biological roles in cell
growth, differentiation and multiple developmental processes. The
functions of intracellular FGFs, also referred to as FGF homologous
factors (FHFs; FGF11-FGF14), remain to be explored.
In the present study, we described how FGF11 is
upregulated in endothelial cells in response to hypoxia. FGF11
overexpression stimulated the formation of capillary-like tube
structures in human endothelial cells and increased the levels of
tight junction (TJ) proteins. The promoter region of FGF11 contains
hypoxia response elements (HREs), which orchestrate FGF11
upregulation. Our results should facilitate the design of new
cancer therapeutics aimed at FGF11.
Materials and methods
Cell culture and hypoxic condition
Human umbilical vein endothelial cells (HUVECs)
(passages 5–8; Lonza) were cultured in M199 (Gibco) containing 20%
fetal bovine serum (FBS) (Lonza), bFGF (3 ng/ml; Invitrogen),
heparin (5 U/ml) and 1% penicillin/streptomycin (both from Gibco)
(5). HEK293a cells (ATCC CRL-1573)
were cultured in Dulbecco's modified Eagle's medium (DMEM) (Gibco)
containing 10% FBS. For hypoxic condition, cells were incubated in
a Forma hypoxia chamber (Forma Scientific), which is an anaerobic
system that strictly regulates oxygen levels; cells were maintained
at low oxygen tension (1% O2, 5% CO2 and
balanced with N2) to simulate hypoxia.
Real-time PCR and end-point PCR
Total RNA was isolated using the QIAshredder and
RNeasyPlus Mini kits (Qiagen Inc.). The PrimeScript™ First Strand
cDNA Synthesis kit (Takara) was used to synthesize cDNA from 1
µg of total RNA according to the manufacturer's
instructions. Real-time PCR was performed using the SYBR-Green PCR
Master Mix (Roche), using primers for human FGF11 as follows:
forward, 5′-TGTCGCTTTAAGGAGTGCGT-3′ and reverse,
5′-AGAGAAGGCTCCCGGTACAT-3′. Real-time PCR data were acquired using
an ABI PRISM-7500 sequence detection system (Applied Biosystems).
The 18S rRNA gene was used as a positive control and for
normalization. End-point PCR for FGF11 was also performed. GAPDH
was used for normalization.
Oligonucleotide primers for PCR were designed as
follows: FGF11 forward, 5′-GTCACCATCCAGAGTGCCAA-3′ and FGF11
reverse, 5′-CACTGTGGAGAGAAGGCTCC-3′; GAPDH forward,
5′-CATGACAACTTTGGCATTGTG-3′ and GAPDH reverse,
5′-GTTGAAGTCGCAGGAGACAAC-3′. The PCR products were analyzed using a
1.2 % agarose gel.
Plasmid cloning, transfection and western
blot analysis
Full-length human FGF11 was synthesized by PCR and
cloned into the pcDNA3.1/HA vector (Invitrogen). Transfection was
carried out using Metafectene Pro (Biontex). For western blotting,
cells were harvested and lysed with lysis buffer containing
protease inhibitors (Roche). Total protein (20–30 µg) was
immunoblotted with antibodies specific to FGF11 (R&D Systems),
zonula occludens-1 (ZO-1) (Invitrogen), occludin (Invitrogen) or
claudin-5 (Abcam). α-tubulin (Calbiochem) was used as an internal
control. Quantification of band intensity was analyzed using ImageJ
(NIH).
Tube formation assay
The tube formation assay was performed as previously
described (19). Briefly, 200
µl of growth factor-reduced Matrigel (BD Biosciences) was
pipetted into a well of a 24-well culture plate and polymerized for
30 min at 37°C. After transfection, HUVECs (1×104
cells/well) were seeded onto polymerized Matrigel and incubated in
M199 containing 2% FBS and heparin (10 U/ml). Every hour up to 16
h, the cultures were photographed with an Olympus TH4-200
microscope. Capillary-like tube networks were observed and the
branch point number was counted.
Endothelial cell migration assay
HUVECs were transfected with an FGF11-overexpression
plasmid or control mock plasmid. After one day, cells were plated
on 60-mm culture dishes and the migration assay was performed as
previously described (20).
Briefly, confluent HUVECs were wounded and incubated in M199 media
with 2% FBS and 1 mM thymidine. After 16 h, HUVECs were fixed with
absolute methanol for 2 min and stained with Giemsa solution for 3
min. Migration activity was quantitated by counting the number of
cells that moved beyond the reference line (20).
Promoter luciferase assay
A partial genomic DNA sequence encompassing the
human FGF11 promoter region bearing putative HREs was amplified by
PCR and cloned into the luciferase pGL3 promoter vector (Promega).
Primer information was as follows: forward,
5′-CTGCTAGCCCAACCTCTCCTTCCTACC-3′ (pGL3-FGF11-HREs); forward,
5′-GTGCTAGCGGGGCTGGTTAGATTGGAG-3′ (pGL3-FGF11-ΔHREs); and reverse,
5′-ATAGATCTACTAGGGCATGCTCTTGACG-3′. HEK293a cells were plated at a
density of 2×105 cells/well of a 6-well plate and
transfected with various combinations of effector plasmids.
Luciferase assays were performed using the luciferase assay system
kit with a GloMax luminometer (both from Promega), according to the
manufacturer's instructions. Relative luciferase activity was
normalized to relative light units and β-galactosidase
activity.
Statistical analysis
The data are expressed as means ± standard
deviations (SD). The statistical differences between the groups
were compared using the unpaired t-test or the one-way analysis of
variance (ANOVA). P-values ≤0.05 were considered to indicate
statistically significant results.
Results
Hypoxia-induced FGF11 expression in
endothelial cells
Solid tumor angiogenesis is initiated by hypoxic
conditions that serve as a strong stimulus for new vessel formation
(9,10). Since we are interested in
identifying hypoxia-induced genes, we first investigated the mRNA
expression of FGF homologous factors (FHFs; FGF11-FGF14) in HUVECs
after exposure to hypoxia (1% O2). FGF14 mRNA was not
detected in HUVECs by real-time PCR. FGF12 and FGF13 expression
increased slightly under hypoxia, yet their expression level was
very low in HUVECs. Whereas FGF11 mRNA expression was relatively
high in comparison to FGF12 and FGF13 expression (data not shown).
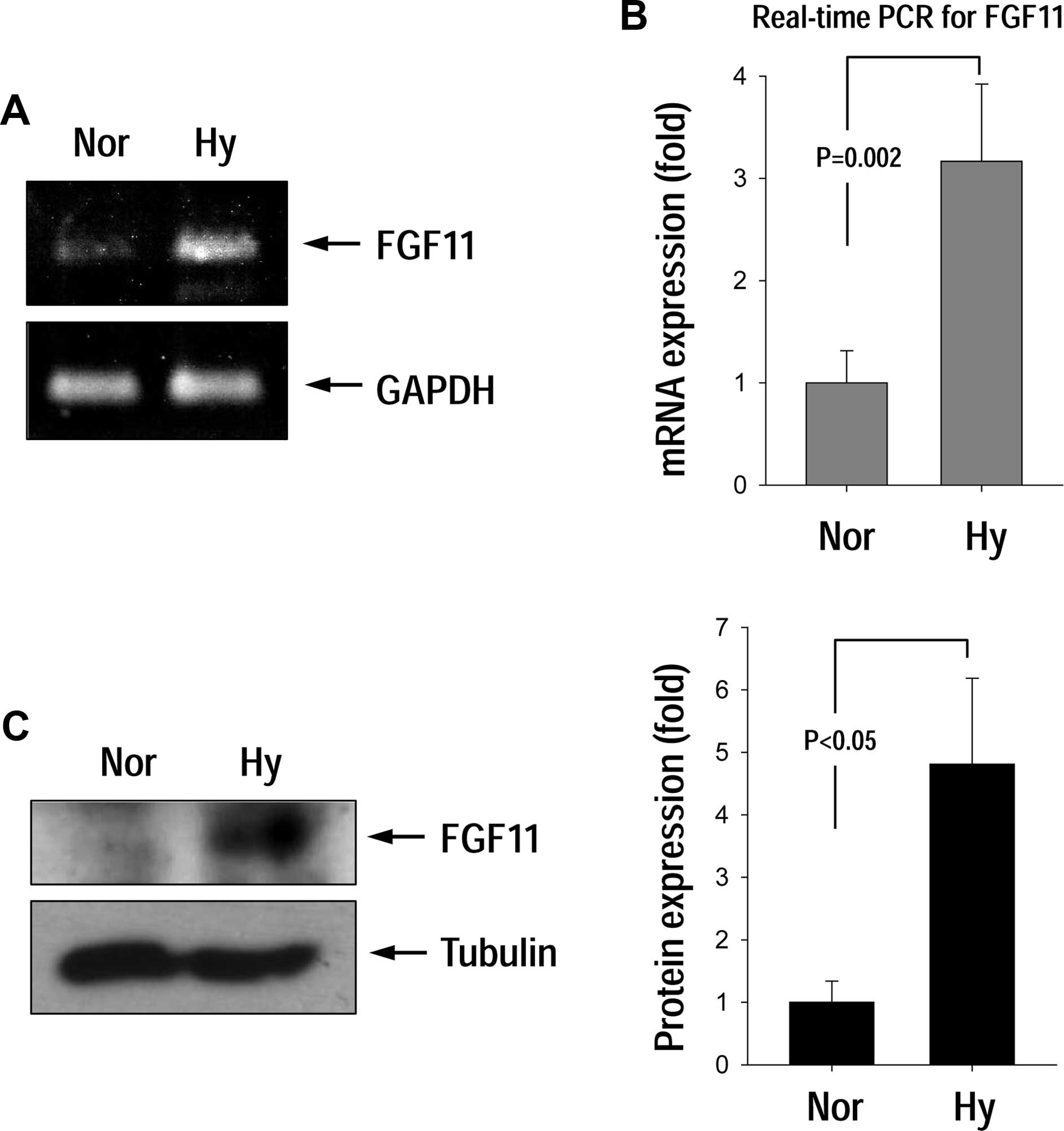
FGF11 mRNA expression was significantly increased in response to
hypoxic conditions (Fig. 1A and B).
Western blotting results for the FGF11 protein suggests that the
protein level was significantly increased under hypoxia (Fig. 1C).
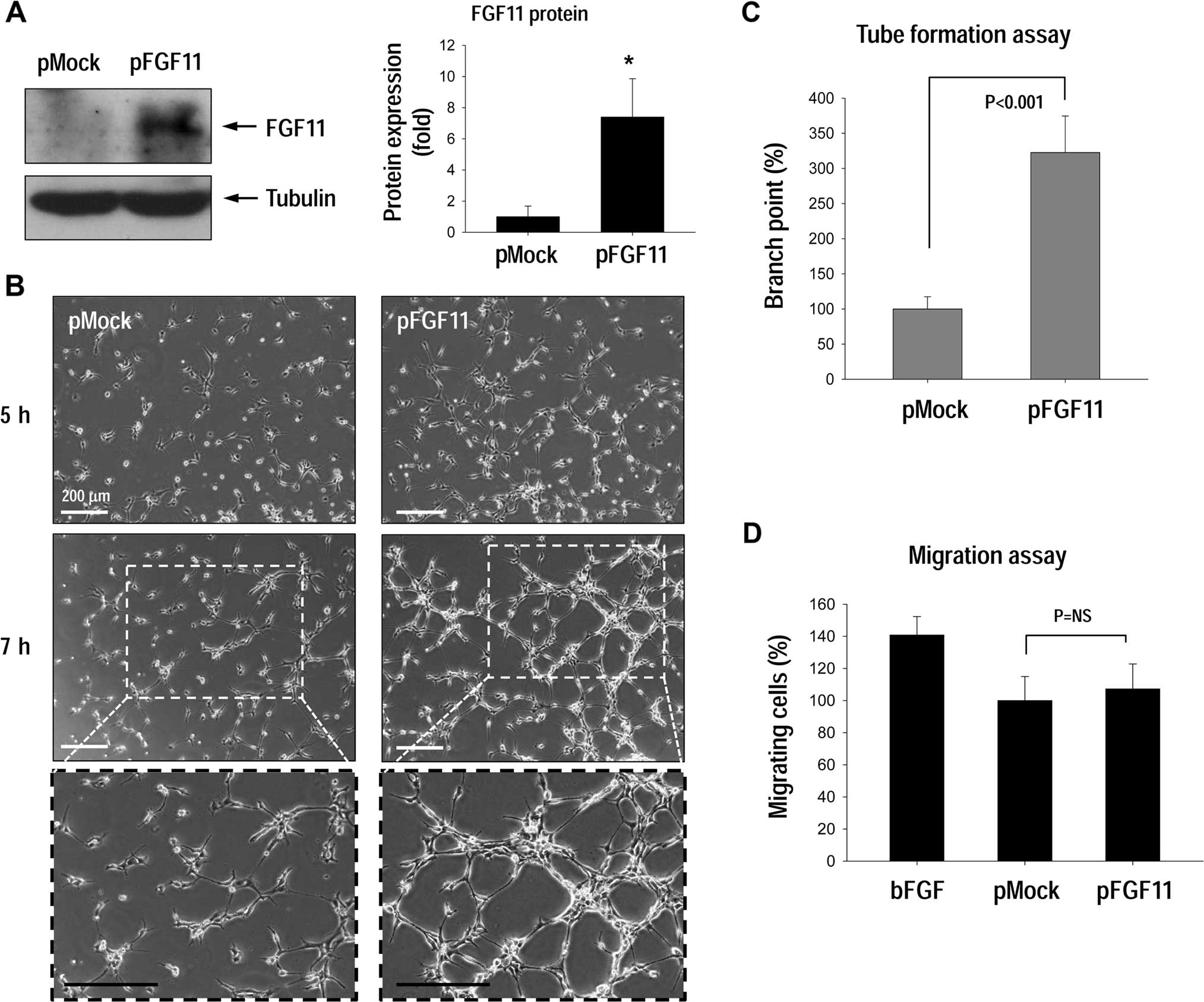
FGF11 overexpression in HUVECs increases
capillary-like tube formation
To investigate the effect of FGF11 expression on
angiogenesis, tube formation and cell migration were examined for
HUVECs transfected with pFGF11 (Fig.
2A). FGF11 overexpression significantly stimulated tube
formation compared to the control cells (Fig. 2B and C), yet did not stimulate
migration activity (Fig. 2D).
HUVECs treated with basic FGF (bFGF) as a positive migration
control (14) migrated normally,
thus we concluded that FGF11-overexpression did not affect
endothelial migration activity. Instead, FGF11 may be involved in
stabilizing capillary-like tube structures.
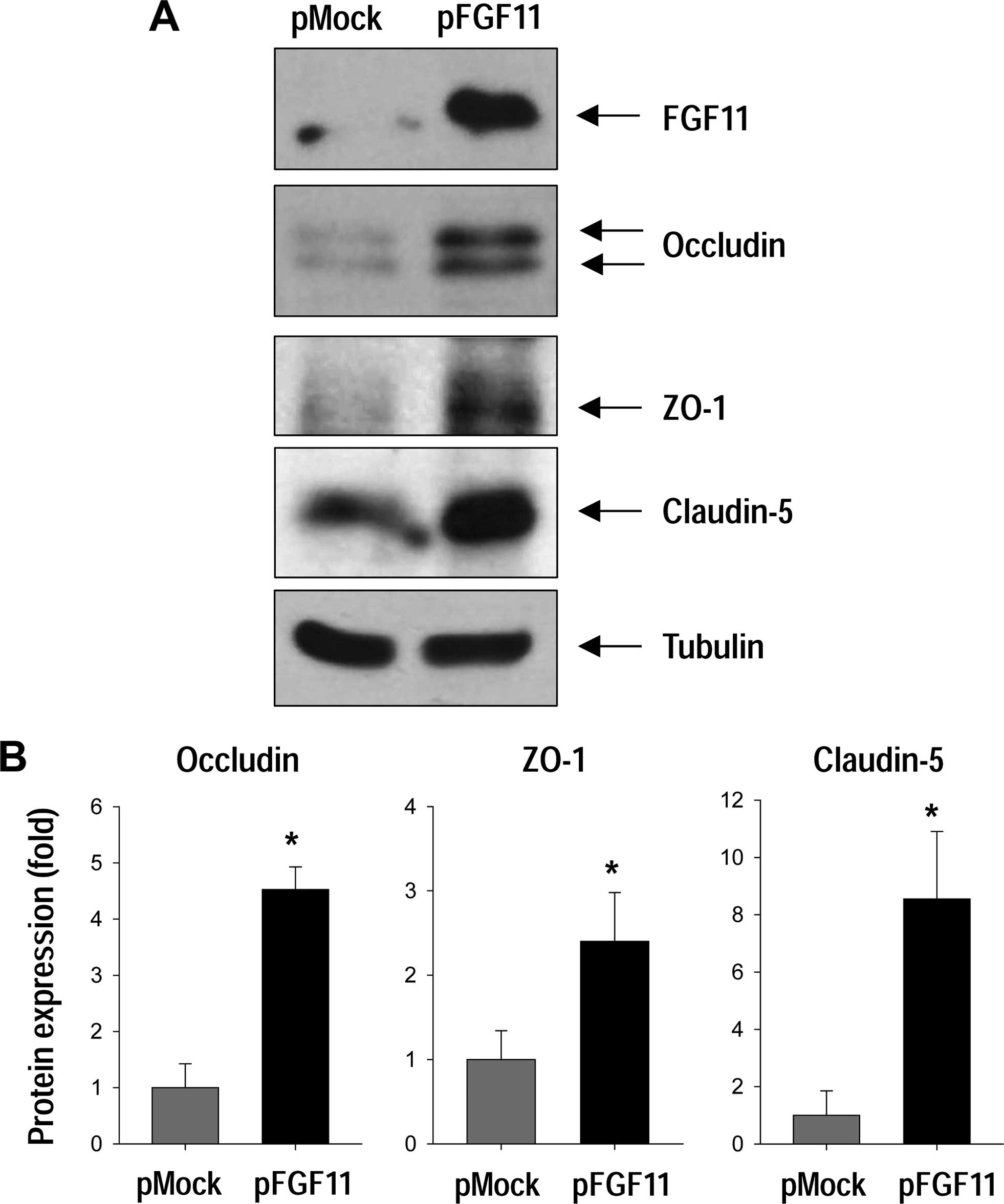
FGF11 overexpression increases the
expression of TJ proteins
Since capillary tube formation in HUVECs was
increased with FGF11 overexpression, we examined whether FGF11
overexpression in endothelial cells affects the expression of TJ
proteins by western blotting (Fig.
3). TJ proteins play a role in stabilizing capillary structure
by maintaining adhesive cell-cell interactions (21). We found that FGF11 overexpression
markedly increased the levels of the TJ proteins, such as occludin,
ZO-1, and claudin-5 (Fig. 3).
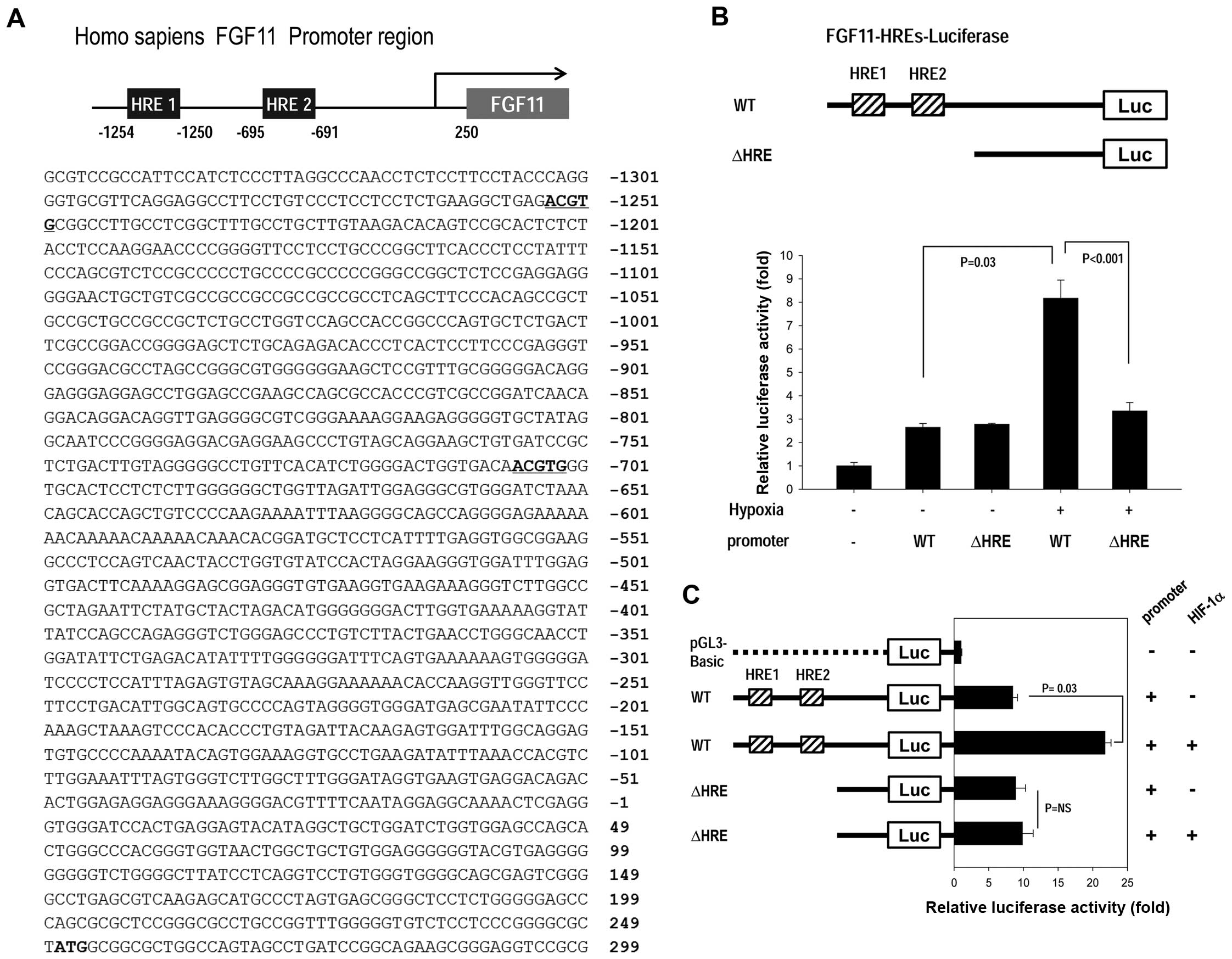
Hypoxia increases the FGF11 promoter
activity through HIF-1α
We further examined the novel finding that FGF11
expression was upregulated in response to hypoxic conditions by
determining the mechanism through which hypoxia stimulates FGF11
expression, focusing on the FGF11 promoter. HIF-1α is a key
transcription factor that activates genes involved in the hypoxic
response by binding to HREs in the gene promoter region (2,16).
Notably, the FGF11 promoter contains two HREs (5′-ACGTG-3′)
(Fig. 4A).
We determined the effects of HIF-1 on the FGF11
promoter containing two HREs (FGF11-HREs; Fig. 4B and C) using a promoter luciferase
assay. Reporter gene activity was significantly increased in
response to hypoxia for the cells transfected with FGF11-HREs (WT).
In contrast, reporter gene activity was not changed by hypoxia for
cells transfected with the HRE-deletion-fragment (ΔHRE) (Fig. 4B), suggesting that the HREs in the
FGF11 promoter region are sensitive to hypoxia. To determine
whether FGF11-HREs are responsive to hypoxia via the HIF-1α
transcription factor, we co-transfected cells with both WT
FGF11-HREs and HIF-1α under normoxic conditions (Fig. 4C). To promote assembly of the
functional HIF1 complex, cells were also co-transfected with the
partner of HIF-1α, HIF-1β. With HIF-1α overexpression, reporter
gene activity was high even under normoxic conditions, whereas the
HRE-deletion-fragment (ΔHRE) was unaffected by HIF-1α
overexpression (Fig. 4C), which
indicates that FGF11 promoter induction occurs via HIF-1.
Discussion
Tumor growth is strongly limited by oxygen
availability; tumorigenesis is dependent on angiogenesis for the
formation of rich vasculature around the tumor that delivers oxygen
and nutrients (11). Under hypoxic
conditions, the transcription factor HIF-1 binds to HREs in the
promoter regions of hypoxia-induced genes, which then orchestrate
hypoxia adaptations and promote angiogenesis (6). We identified a member of the
fibroblast growth factor family, intracellular FGF11 whose
expression was upregulated in response to hypoxic conditions
(Fig. 1). Furthermore, FGF11 has
been reported to play a role in tumorigenesis, particularly in
mitogenic and cell-survival activities that are related to tumor
invasion and growth (22).
Infiltrating T cells enhanced prostate cancer growth through
regulation of FGF11-mediated MMP9 signaling (23). Microarray-based expression profiles
for oral cancer cells indicated that increased FGF11 expression is
associated with increased cell proliferation, resistance to
apoptosis and enhanced capillary-like structures (24).
Since FGF11 is associated with tumorigenesis
(22–24) and its expression was upregulated
under hypoxic conditions in endothelial cells (Fig. 1), we examined whether FGF11 is
involved in angiogenesis which is essential for tumorigenesis
(9–11). FGF11 overexpression in HUVECs
stimulated capillary tube formation (Fig. 2); however, FGF11 overexpression did
not affect endothelial migration. Notably, the expression of tight
junction (TJ) proteins including occludin, ZO-1 and claudin-5
increased by FGF11 overexpression (Fig.
3). TJ complexes are composed of occludins, claudins and
junctional adhesion molecules (JAMs), which are stabilized by ZO
scaffold proteins (21). TJs in
epithelial and endothelial cells establish a barrier to diffusion
through the paracellular pathway and block diffusion of membrane
proteins between the apical and basal regions of the cell (19,21,25).
Numerous studies have reported TJ protein
downregulation in multiple types of cancer. However, upregulation
of TJ proteins has been observed in various types of cancers,
indicating that there is an emerging role for TJ proteins in cancer
cell proliferation, transformation and metastasis (21,26).
Occludin and claudin-5 expression were upregulated in human
hepatocellular carcinoma tissue in comparison to non-neoplastic
liver or normal control tissues (27). Claudin-5 expression was elevated in
borderline ovarian tumors and was implicated in malignant
transformation (28). Strong
claudin-5 expression is a biomarker for elevated risk of pancreatic
adenocarcinoma and breast cancer (29,30).
Increased ZO-1 expression and altered localization were observed in
primary and metastatic pancreatic cancers (31). Clearly, the role of TJ proteins in
tumor initiation and development is more complicated than
originally understood and a more systematic examination is
warranted for clarification. Since FGF11 overexpression is
associated with increased occludin, ZO-1 and claudin-5 expression,
it is reasonable to expect that FGF11 modulates tumorigenesis.
HIF-1 is a master transcription factor that
regulates genes involved in the adaptive response to hypoxia
through binding to cis-acting HREs (2,16,30)
and it regulates genes affecting cell survival, metabolism and
tumor vessel formation such as VEGF, erythropoietin, placental
growth factor and bFGF (6,16,32).
In the present study, we found that FGF11 expression can be induced
through HIF-1 binding sites in its promoter region (Fig. 4). Based on our results, we suggest
that FGF11 acts as a novel modulator of hypoxia-induced
pathological processes such as tumor progression. Future studies
focusing on the role of FGF11 in human tumors, as well as a more
systematic examination of FGF11 biology, may facilitate the
development of new cancer therapeutics.
Acknowledgments
This study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF) funded by the Ministry of Science, ICT and Future Planning
(2013R1A1A3012024, awarded to S.-W.L.), the Basic Science Research
Program through the NRF grant funded by the Ministry of Education
(NRF-2011-0025506, awarded to W.-J.K.), and the KIOST in-house
program (PE99314, awarded to S.-W.L.).
References
|
1
|
Semenza GL: Regulation of mammalian
O2 homeostasis by hypoxia-inducible factor 1. Annu Rev
Cell Dev Biol. 15:551–578. 1999. View Article : Google Scholar
|
|
2
|
Lee SW, Jeong HK, Lee JY, Yang J, Lee EJ,
Kim SY, Youn SW, Lee J, Kim WJ, Kim KW, et al: Hypoxic priming of
mESCs accelerates vascular-lineage differentiation through
HIF1-mediated inverse regulation of Oct4 and VEGF. EMBO Mol Med.
4:924–938. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee SW, Yang J, Kim SY, Jeong HK, Lee J,
Kim WJ, Lee EJ and Kim HS: MicroRNA-26a induced by hypoxia targets
HDAC6 in myogenic differentiation of embryonic stem cells. Nucleic
Acids Res. 43:2057–2073. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee SW, Lee YM, Bae SK, Murakami S, Yun Y
and Kim KW: Human hepatitis B virus X protein is a possible
mediator of hypoxia-induced angiogenesis in hepatocarcinogenesis.
Biochem Biophys Res Commun. 268:456–461. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee SW, Won JY, Kim WJ, Lee J, Kim KH,
Youn SW, Kim JY, Lee EJ, Kim YJ, Kim KW, et al: Snail as a
potential target molecule in cardiac fibrosis: Paracrine action of
endothelial cells on fibroblasts through snail and CTGF axis. Mol
Ther. 21:1767–1777. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Carmeliet P, Dor Y, Herbert JM, Fukumura
D, Brusselmans K, Dewerchin M, Neeman M, Bono F, Abramovitch R,
Maxwell P, et al: Role of HIF-1alpha in hypoxia-mediated apoptosis,
cell proliferation and tumour angiogenesis. Nature. 394:485–490.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Moulder JE and Rockwell S: Hypoxic
fractions of solid tumors: Experimental techniques, methods of
analysis, and a survey of existing data. Int J Radiat Oncol Biol
Phys. 10:695–712. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Folkman J: What is the evidence that
tumors are angiogenesis dependent? J Natl Cancer Inst. 82:4–6.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Holash J, Wiegand SJ and Yancopoulos GD:
New model of tumor angiogenesis: Dynamic balance between vessel
regression and growth mediated by angiopoietins and VEGF. Oncogene.
18:5356–5362. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dimmeler S and Zeiher AM: Endothelial cell
apoptosis in angiogenesis and vessel regression. Circ Res.
87:434–439. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hanahan D and Folkman J: Patterns and
emerging mechanisms of the angiogenic switch during tumorigenesis.
Cell. 86:353–364. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Folkman J: Seminars in Medicine of the
Beth Israel Hospital, Boston. Clinical applications of research on
angiogenesis. N Engl J Med. 333:1757–1763. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Neufeld G, Cohen T, Gengrinovitch S and
Poltorak Z: Vascular endothelial growth factor (VEGF) and its
receptors. FASEB J. 13:9–22. 1999.PubMed/NCBI
|
|
14
|
Friesel RE and Maciag T: Molecular
mechanisms of angiogenesis: Fibroblast growth factor signal
transduction. FASEB J. 9:919–925. 1995.PubMed/NCBI
|
|
15
|
Yotsumoto F, Tokunaga E, Oki E, Maehara Y,
Yamada H, Nakajima K, Nam SO, Miyata K, Koyanagi M, Doi K, et al:
Molecular hierarchy of heparin-binding EGF-like growth
factor-regulated angiogenesis in triple-negative breast cancer. Mol
Cancer Res. 11:506–517. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Semenza GL: Targeting HIF-1 for cancer
therapy. Nat Rev Cancer. 3:721–732. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Itoh N and Ornitz DM: Functional
evolutionary history of the mouse Fgf gene family. Dev Dyn.
237:18–27. 2008. View Article : Google Scholar
|
|
18
|
Itoh N and Ornitz DM: Fibroblast growth
factors: From molecular evolution to roles in development,
metabolism and disease. J Biochem. 149:121–130. 2011. View Article : Google Scholar :
|
|
19
|
Lee SW, Kim WJ, Choi YK, Song HS, Son MJ,
Gelman IH, Kim YJ and Kim KW: SSeCKS regulates angiogenesis and
tight junction formation in blood-brain barrier. Nat Med.
9:900–906. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee SW, Jung KH, Jeong CH, Seo JH, Yoon
DK, Suh JK, Kim KW and Kim WJ: Inhibition of endothelial cell
migration through the down-regulation of MMP-9 by A-kinase
anchoring protein 12. Mol Med Rep. 4:145–149. 2011.PubMed/NCBI
|
|
21
|
Runkle EA and Mu D: Tight junction
proteins: From barrier to tumorigenesis. Cancer Lett. 337:41–48.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ding I, Liu W, Sun J, Fenton B and
Okunieff P: Comparison and modulation of angiogenic responses by
FGFs, VEGF and SCF in murine and human fibrosarcomas. Comp Biochem
Physiol A Mol Integr Physiol. 132:17–25. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hu S, Li L, Yeh S, Cui Y, Li X, Chang HC,
Jin J and Chang C: Infiltrating T cells promote prostate cancer
metastasis via modulation of FGF11→miRNA-541→androgen receptor
(AR)→MMP9 signaling. Mol Oncol. 9:44–57. 2015. View Article : Google Scholar
|
|
24
|
Zhuang Z, Jian P, Longjiang L, Bo H and
Wenlin X: Oral cancer cells with different potential of lymphatic
metastasis displayed distinct biologic behaviors and gene
expression profiles. J Oral Pathol Med. 39:168–175. 2010.
View Article : Google Scholar
|
|
25
|
Lee SW, Kim WJ, Jun HO, Choi YK and Kim
KW: Angiopoietin-1 reduces vascular endothelial growth
factor-induced brain endothelial permeability via upregulation of
ZO-2. Int J Mol Med. 23:279–284. 2009.PubMed/NCBI
|
|
26
|
Brennan K, Offiah G, McSherry EA and
Hopkins AM: Tight junctions: A barrier to the initiation and
progression of breast cancer? J Biomed Biotechnol. 2010:4606072010.
View Article : Google Scholar
|
|
27
|
Bouchagier KA, Assimakopoulos SF, Karavias
DD, Maroulis I, Tzelepi V, Kalofonos H, Karavias DD, Kardamakis D,
Scopa CD and Tsamandas AC: Expression of claudins-1, -4, -5, -7 and
occludin in hepatocellular carcinoma and their relation with
classic clinicopathological features and patients' survival. In
Vivo. 28:315–326. 2014.PubMed/NCBI
|
|
28
|
Nissi R, Talvensaari-Mattila A, Kuvaja P,
Pääkkö P, Soini Y and Santala M: Claudin-5 is associated with
elevated TATI and CA125 levels in mucinous ovarian borderline
tumors. Anticancer Res. 35:973–976. 2015.PubMed/NCBI
|
|
29
|
Soini Y, Eskelinen M, Juvonen P, Kärjä V,
Haapasaari KM, Saarela A and Karihtala P: Strong claudin 5
expression is a poor prognostic sign in pancreatic adenocarcinoma.
Tumour Biol. 35:3803–3808. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sugimoto H, Nagahara M, Bae Y, Nakagawa T,
Ishikawa T, Sato T, Uetake H, Eishi Y and Sugihara K:
Clinicopathologic relevance of claudin 5 expression in breast
cancer. Am J Clin Pathol. 143:540–546. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kleeff J, Shi X, Bode HP, Hoover K,
Shrikhande S, Bryant PJ, Korc M, Büchler MW and Friess H: Altered
expression and localization of the tight junction protein ZO-1 in
primary and metastatic pancreatic cancer. Pancreas. 23:259–265.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang Y, Sun M, Wang L and Jiao B: HIFs,
angiogenesis, and cancer. J Cell Biochem. 114:967–974. 2013.
View Article : Google Scholar
|