Introduction
Gastric cancer is one of the most common
malignancies worldwide (1,2). Recent advances in early diagnosis and
treatment have resulted in significant improvement in long-term
survival for gastric cancer patients. However, the prognosis for
advanced gastric cancer remains poor. A majority of patients with
advanced gastric cancer die due to complications caused by
metastases. Therefore, invasion and metastasis are critical
determinants of gastric cancer morbidity.
Ras-related C3 botulinum toxin substrate 1 (Rac1) is
an important member of the small molecule G-protein Rho family (Ras
homologue) and is an important class of intracellular signaling
molecules. It affects tumor growth, invasion and metastasis, and
tumor angiogenesis (3,4). p21-activated kinase 1 (Pakl) is a
conserved serine/threonine protein kinase that is an important
downstream target protein of Rho-GTPase Cdc42 and Rac1, which are
involved in numerous cellular activities and play an important role
in cytoskeletal reorganization, cell migration, apoptosis and
survival, cell cycle, gene transcription regulation and cell
transformation (5,6). Activation of Pak1 increases cell
motility in non-metastatic MCF-7 breast carcinoma cells (7), and overexpression of Pak1 was recently
found in NSCLC (6) and gastric
cancer (8). Many research groups
have shown that Rac1 and Pak1 may be important biomarkers of
gastric carcinoma invasion and metastasis (8,9).
p120-catenin (p120) belongs to the armadillo protein
superfamily and is originally identified as a substrate for
oncogenic Src family tyrosine kinase (10). It is best known for binding directly
to the cytoplasmic domain of cadherin or VE-cadherin and
contributing to regulation of cell-cell adhesion (11–13).
Due to its stabilizing function in the AJ, p120 has caught much
attention in the context of tumor development and progression. The
absence of membrane p120 or nuclear translocation of p120 in colon,
breast, bladder, lung, pancreas, prostate and stomach tumors is
well recognized, which has been associated with tumor malignancy
(14).
Research has focused on the relationship between
p120 and the expression of Rac1 and Pak1 in gastric carcinoma. On
one hand, several results indicated that p120-catenin also controls
the activity of small GTPases. For instance, overexpression of
p120-catenin represses RhoA activity (15,16)
and activates Rac1 (16,17). In contrast, there is a study
revealing that Pak5 and p120 co-localized in neuroblastoma cells
(18), Pak4, Pak5 and Pak6 were the
founding members of group B Paks, and Pak1, Pak2 and Pak3 compose
the group A Paks (19,20). For this reason, we hypothesize that
p120 participates in the development of gastric cancer through
regulating Rac1 and Pak1.
Materials and methods
Immunohistochemistry in gastric carcinoma
tissues
Gastric carcinoma tissue specimens both poorly
differentiated and well differentiated, were obtained from the
Institute of Pathology, Tongji Hospital, Tongji Medical College,
Wuhan, China. The specimens were fixed, dehydrated and embedded in
paraffin, then cut into 3-µm thin slices. After dewaxing and
rehydration, they were autoclaved for 2 min and were then incubated
with 3% hydrogen peroxide for 10 min at room temperature to remove
endogenous peroxidase activity. The slices were added with 5% BSA
for 30 min, followed by incubating with anti-p120, anti-Pak1,
anti-Rac1 (p120; Santa Cruz Biotechnology; Pak1 and Rac1; CST Co.)
antibodies at 4°C overnight, then washed in phosphate-buffered
saline (PBS) for 2 min three times and incubated with secondary
antibodies at 37°C for 1 h, then stained with DAB substrate
chromogen solution for 5 min at room temperature.
AGS and SGC7901 cell culture
Human gastric cancer SGC7901 and AGS cell lines were
cultured in RPMI-1640 with 10% fetal bovine serum (FBS), 200
µg/ml streptomycin, 200 IU/ml penicillin at 37°C under 5%
carbon dioxide.
Western blotting in AGS and SGC7901
cells
To investigate the level of the protein expression,
AGS and SGC7901 cells were cultured in 6-well inserts board, cells
were rinsed twice with ice-cold PBS, lysed in RARP buffer with 1%
protease inhibitor cocktail. Lysates were then cleared by
centrifugation, and protein concentration was determined by a BCA
kit. Equal amounts of proteins were fractionated by SDS-PAGE and
transferred to a nitrocellulose (NC) membrane or polyvinylidene
fluoride (PVDF) membrane. The membranes were blocked with 5%
fat-free milk in TBS and incubated with anti-p120 (1:300),
anti-RAC1 (1:500), anti-PAK1(1:500) and anti-β-actin (1:2,500)
overnight at 4°C. The signal was detected using a horseradish
peroxidase-conjugated secondary antibody and ECL and was then
exposed to X-ray film (Fuji, Japan).
Plasmids and transient transfection
Plasmid of RcCMV mp120-1A was generously provided by
Professor Enhua Wang (21).
Transient gene transfection was performed on cells in the
exponential phase of growth using Lipofectamine 2000 according to
the manufacturer's recommendations and the method described by
Tucker et al (22) with
minor modification. Forty-eight hours after transfection cells were
treated for further analysis.
RNA interference
The small interfering RNA (siRNA) oligonucleotides
of human p120 were purchased from Shanghai GenePharma Co. Ltd.
Cells were grown on a 6-well plate to 50% confluency with complete
medium and transfected with the siRNA using Lipofectamine 2000
according to the manufacturer's recommended procedure. Efficiency
of knockdown by siRNA was assessed by western blot analysis. The
non-silencing siRNA (scramble) was used as control. Forty-eight
hours after transfection cells were treated for further
analysis.
Wound healing assay
A wound healing assay was performed to examine the
capacity of cancer cell migration as previously described (23). Twenty-four hours after transfection
with p120 siRNA, the AGS and 7901 cells were resuspended with
serum-free RPMI-1640 in 6-well plates, when cancer cells were
90–95% confluent, a single scratch wound was generated with a 200
µl disposable pipette tip. The migration of the cells at the
edge of the scratch was analyzed at 0, 24 and 48 h. The images were
captured with a fluorescence microscope.
Transwell assay
Twenty-four hours after transfection with p120
siRNA, the AGS and 7901 cells were resuspended in serum-free
RPMI-1640 to adjust the density to 105/ml.
Twenty-four-well 8.0 µM Transwell inserts (3422; Corning)
were used for the experiments. We added 400 µl RPMI-1640
that containing 10% FBS to the lower chamber and 100 µl
medium that containing the cells to the upper chamber. After
incubation for 24 h, the cells that did not migrate to the upper
chamber were removed with a cotton swab. Then the migrated cells
were fixed with 4% paraformaldehyde for 20 min, stained with
crystal violet for 15 min, and were counted and photographed with a
fluorescence microscope at a magnification of ×200.
Statistical analysis
All data are expressed as the means ± standard
deviation (SD) of experiments repeated at least three times. The
statistical software SigmaStat was used to analyze the data. t-test
and one-way ANOVA were used for statistical analysis, and
statistical significance was assumed at p<0.05.
Results
Expression of p120, Pak1 and Rac1 in
different types of gastric cancer tissues and the AGS and SGC7901
cells
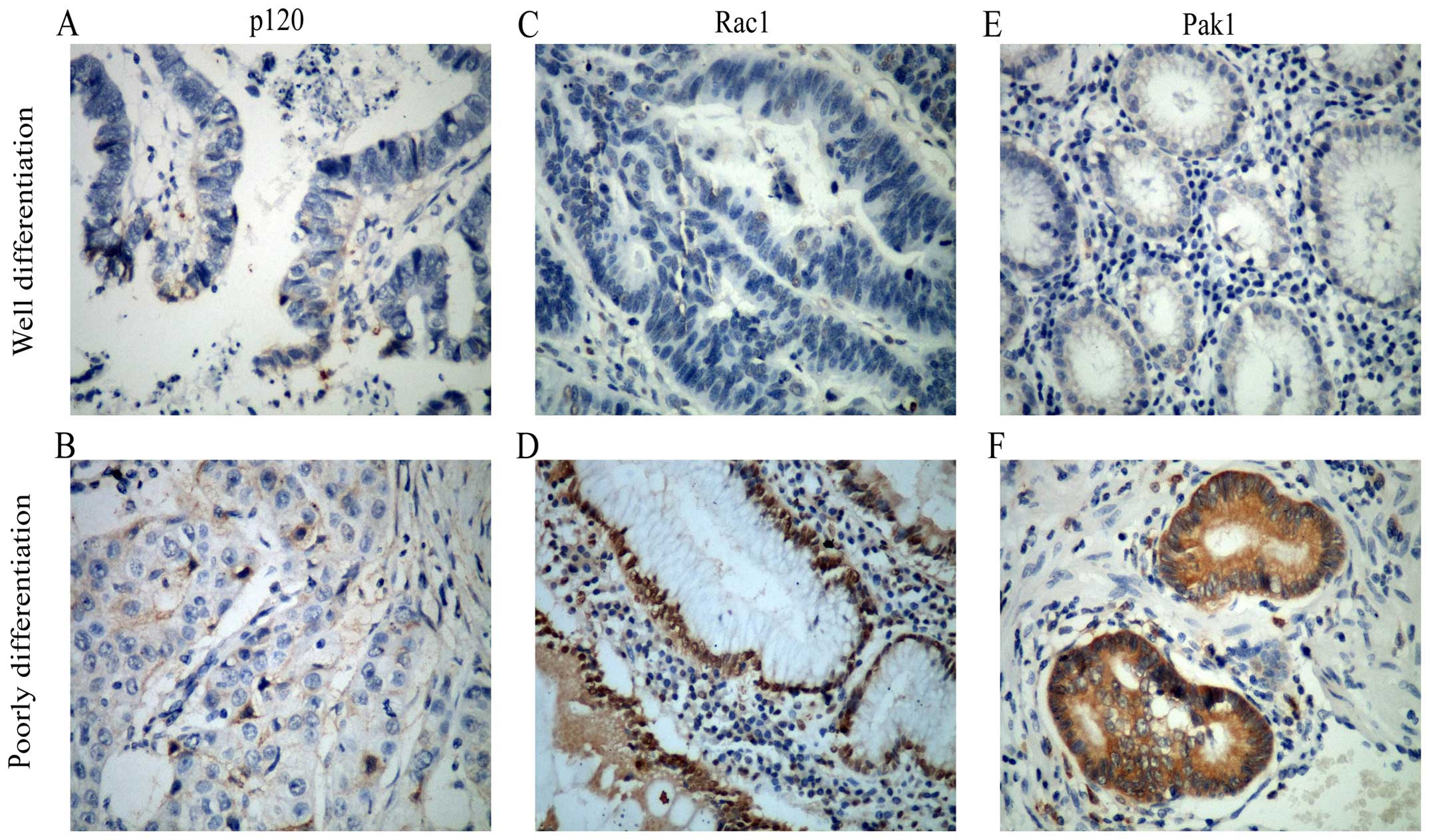
In order to determine the protein expression of
different stages of gastric cancer patients, immunohistochemistry
was used to detect the expression level of p120, Pak1 and Rac1. As
shown in Fig. 1,
immunohistochemistry revealed that expression level of Rac1 and
Pak1 proteins were low in well differentiated gastric cancer
tissues, yet were high in poorly differentiated tissues.
Differently, the expression level of p120 proteins were low both in
poorly differentiated group and well differentiated ones.
Nevertheless, there was a significant nuclear location of p120 in
poorly differentiated tissues.
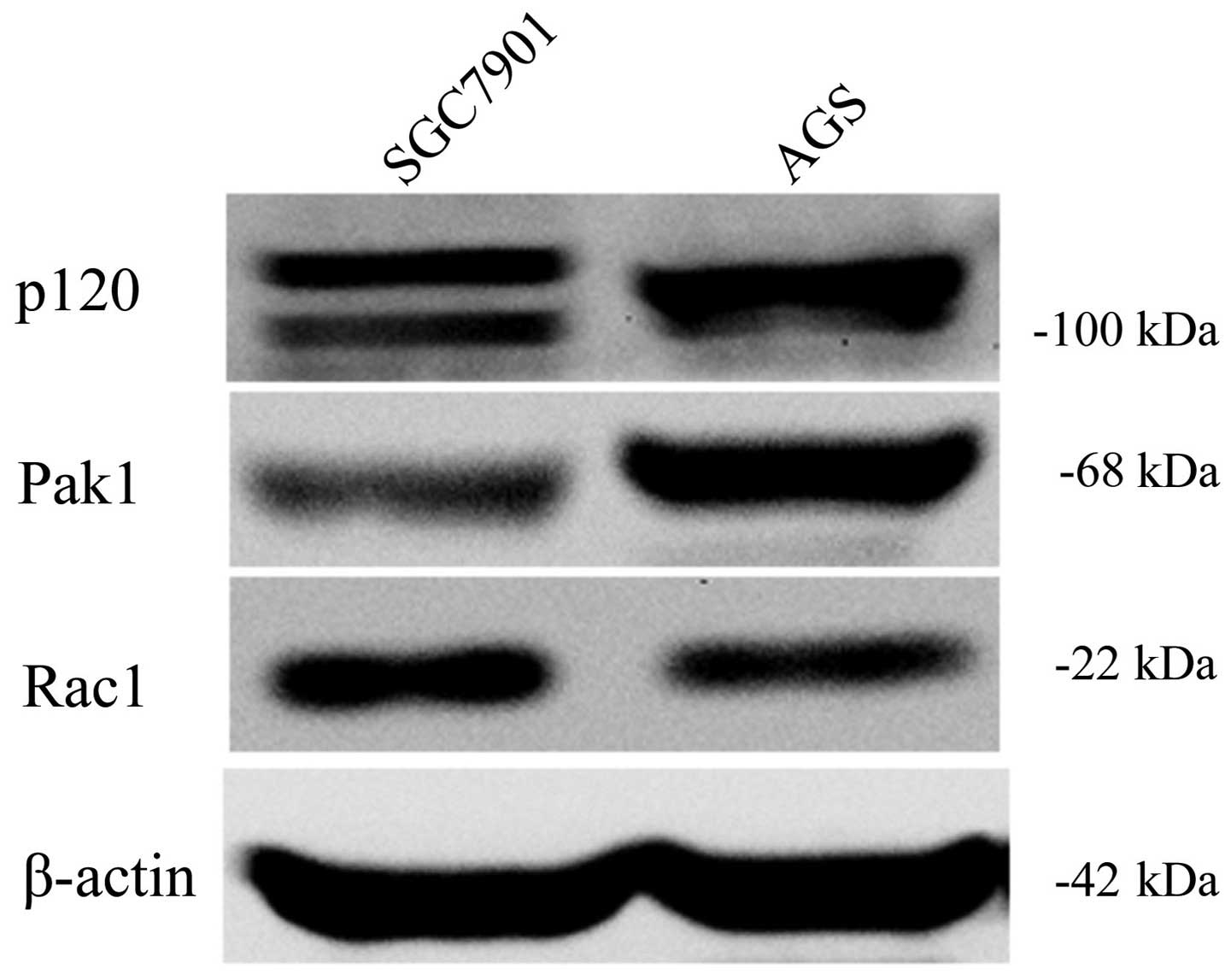
Next, we explored the expression of p120, Rac1 and
Pak1 in two types of GC cells. Western blotting showed that
expression of Pak1 and Rac1 in SGC7901 cells were both higher than
that in the AGS cells. p120 isoform 1 and 3 was detected in SGC7901
cells, while only p120 isoform 1 was detected in AGS cells, which
was identical with a previous study (24) (Fig.
2).
Overexpression of p120 1A inhibits the
expression of Pak1 and Rac1 in GC cells
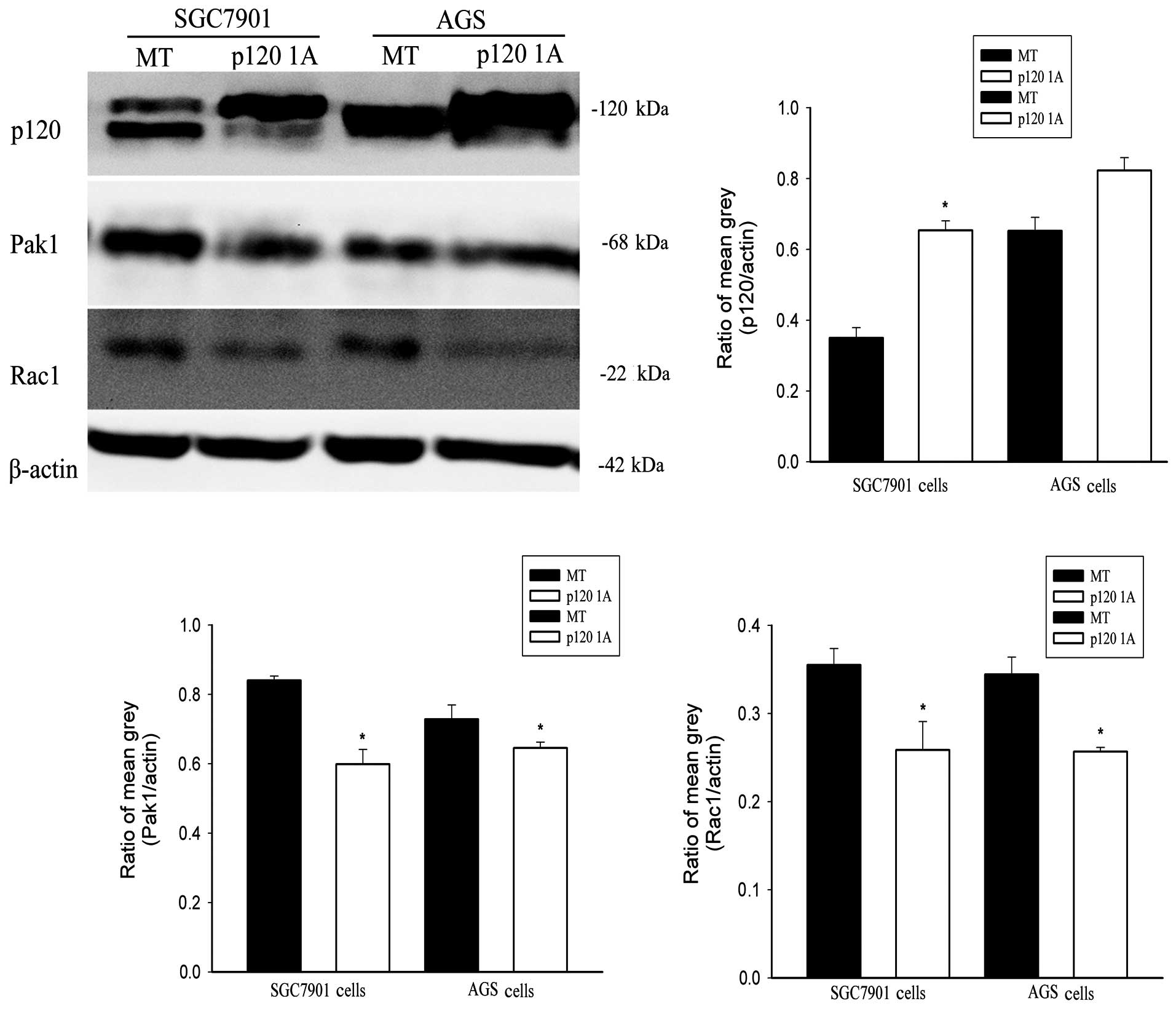
To investigate the relationship between
downregulated p120 and upregulated Rac1 and Pak1, we transfected
plasmid of p120 1A into the two cell types to detect the expression
of Rac1 and Pak1. The transfection efficiency was detected by
western blotting (Fig. 3). Compared
with MT groups, the expression of Rac1 and Pak1 were both
downregulated when p120 1A was overexpressed in GC cells (Fig. 3).
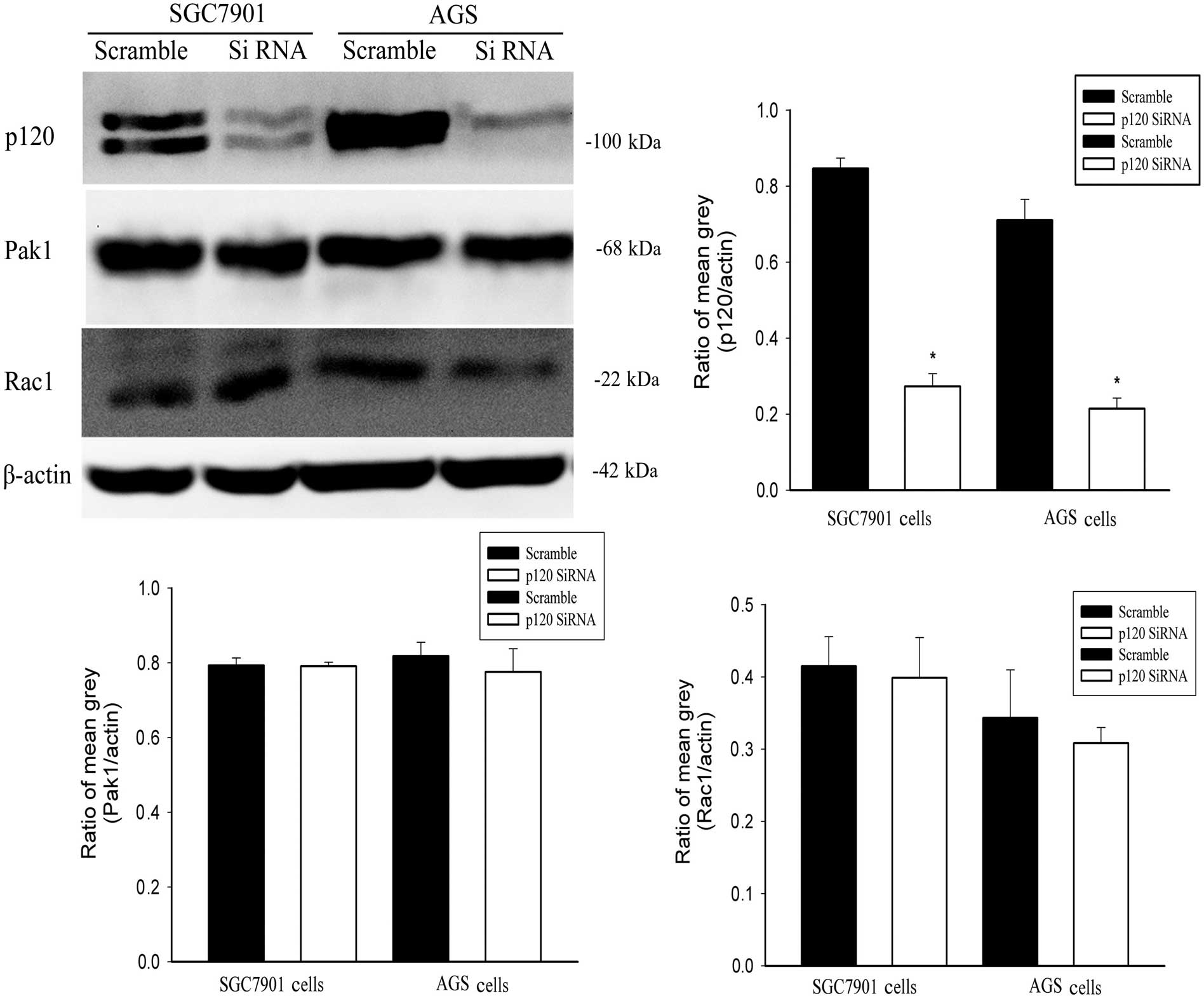
In contrast, we used p120 siRNA to silence p120,
then detected the changes of Rac1 and Pak1. Compared with the
scrambled groups, the silencing efficiency of p120 was detected by
western blotting (Fig. 4). Notably,
the expression of Rac1 and Pak1 remained unchanged (Fig. 4).
Overexpression of p120 1A decreases the
proliferation and invasion of AGS and SGC7901 cells
Cell migration and invasion were considered to have
important value in progress of cancer (25), were one of the crucial events in
metastasis of cancer cells. Therefore, in order to find whether
p120 impacts the progress of gastric cancer, we used wound healing
and Transwell assays to explore the biological behavior changes of
SGC7901 and AGS cells in overexpression of p120 1A.
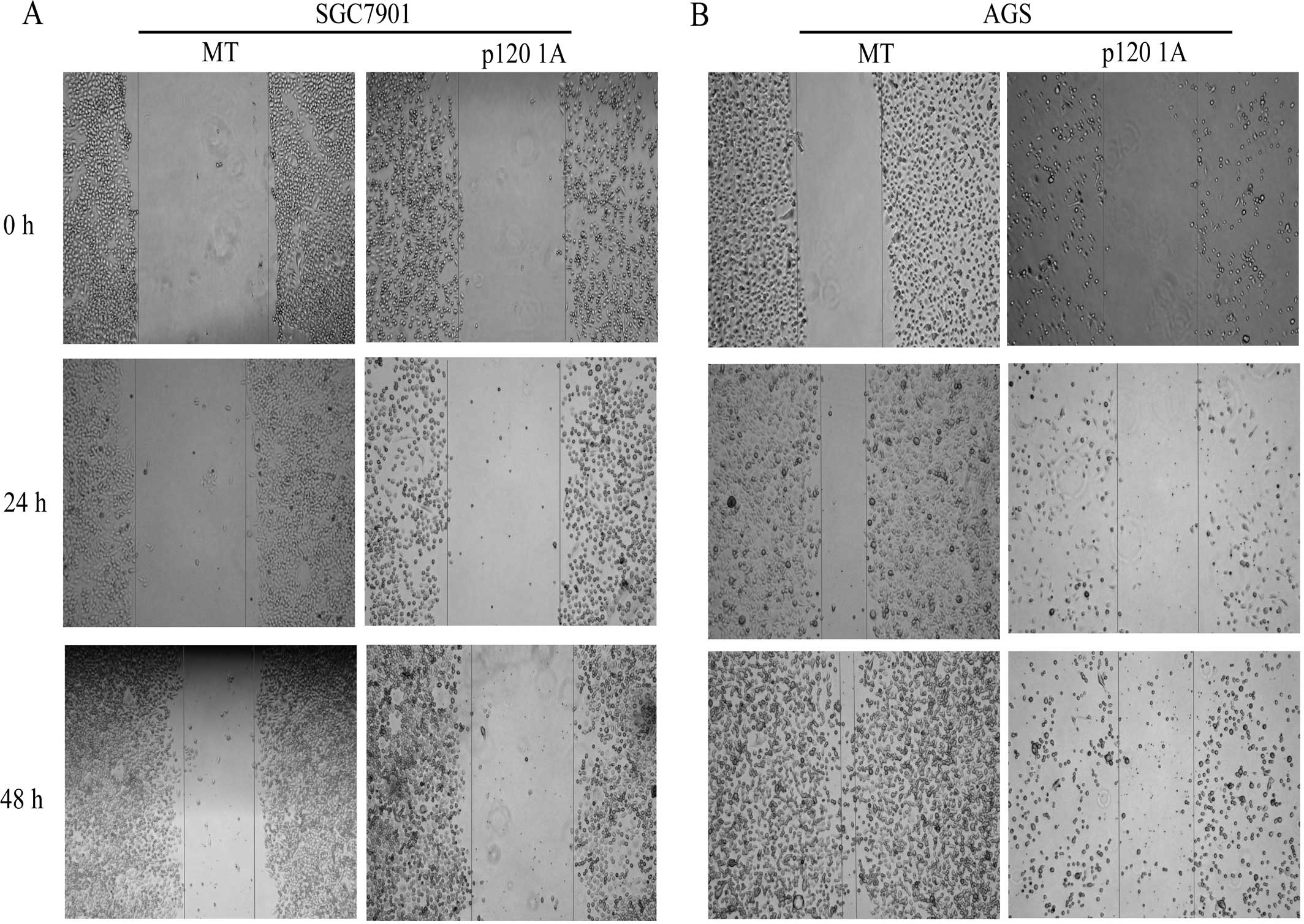
Overexpression of p120 inhibited the migration
capacity of the two GC cell types at 24 and 48 h, while the GC
cells covered the wound at 48 h in MT groups (Fig. 5). Moreover, the serum-stimulated
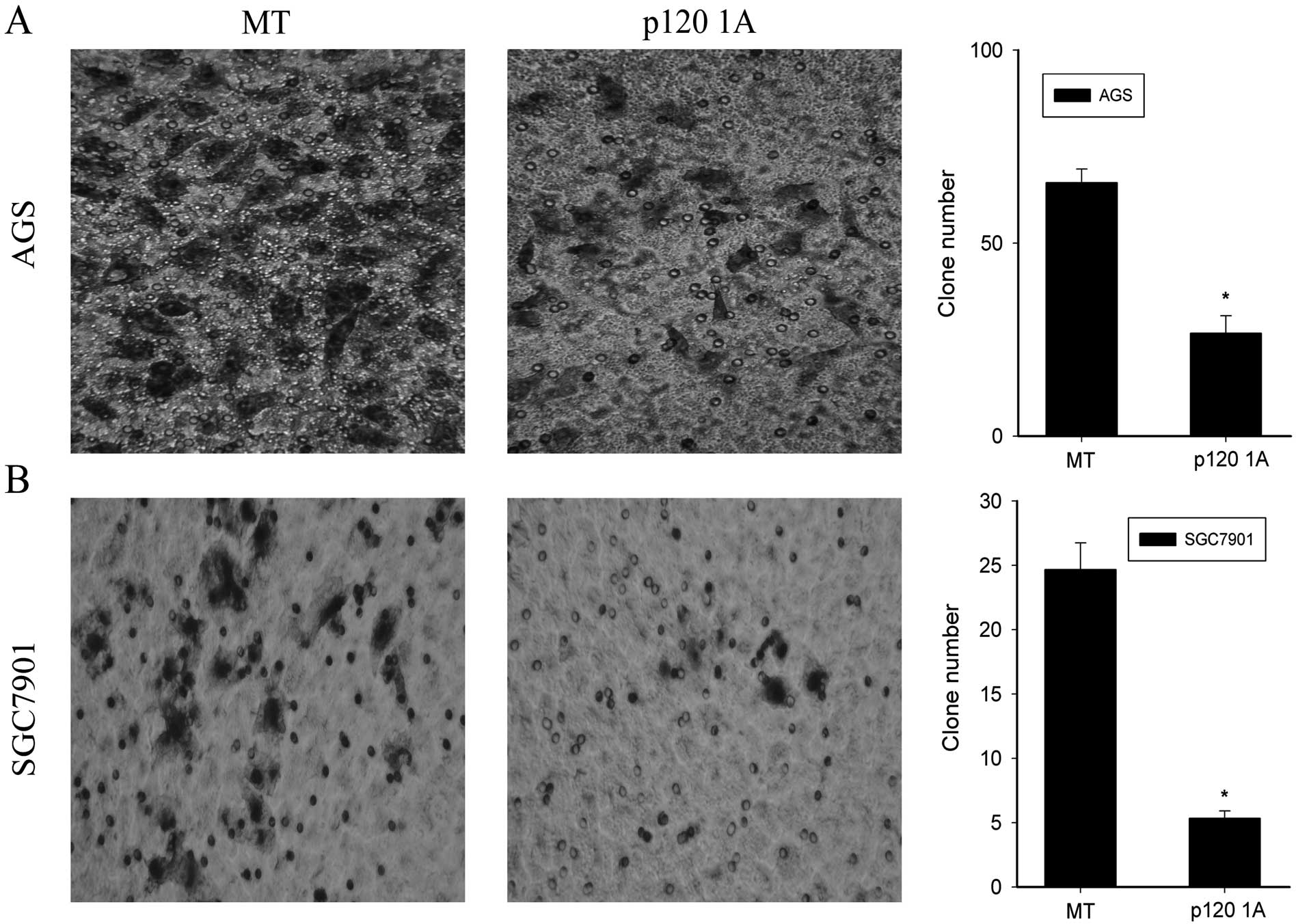
Matrigel invasion assay demonstrated that overexpression of p120 1A
significantly decreased the invasiveness of AGS and SGC7901 cells
at 24 and 48 h, compared to the MT groups (P<0.05, Fig. 6).
Silencing of p120 increases the
proliferation and invasion of the GC7901 and AGS cells
To further confirm the changes of p120 effects in
the biological behavior of SGC7901 and AGS cells, we silenced p120
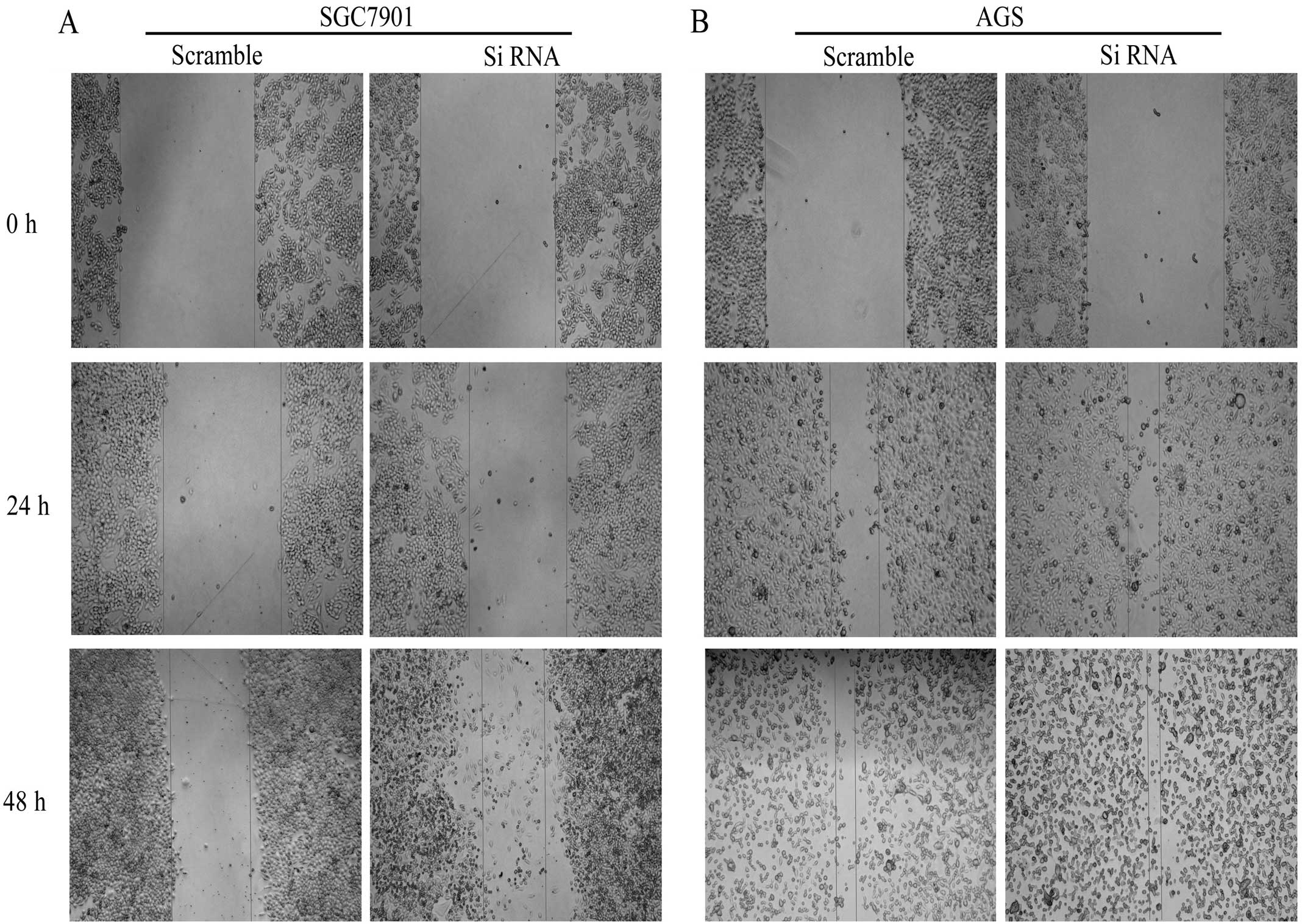
to observe the proliferation and invasion of the cells. The wound
assay showed that when p120 was silenced, the migration of SGC7901
and AGS cells were increased in 24 h and 48 h, particularly AGS
cells were more prominent (Fig. 7).
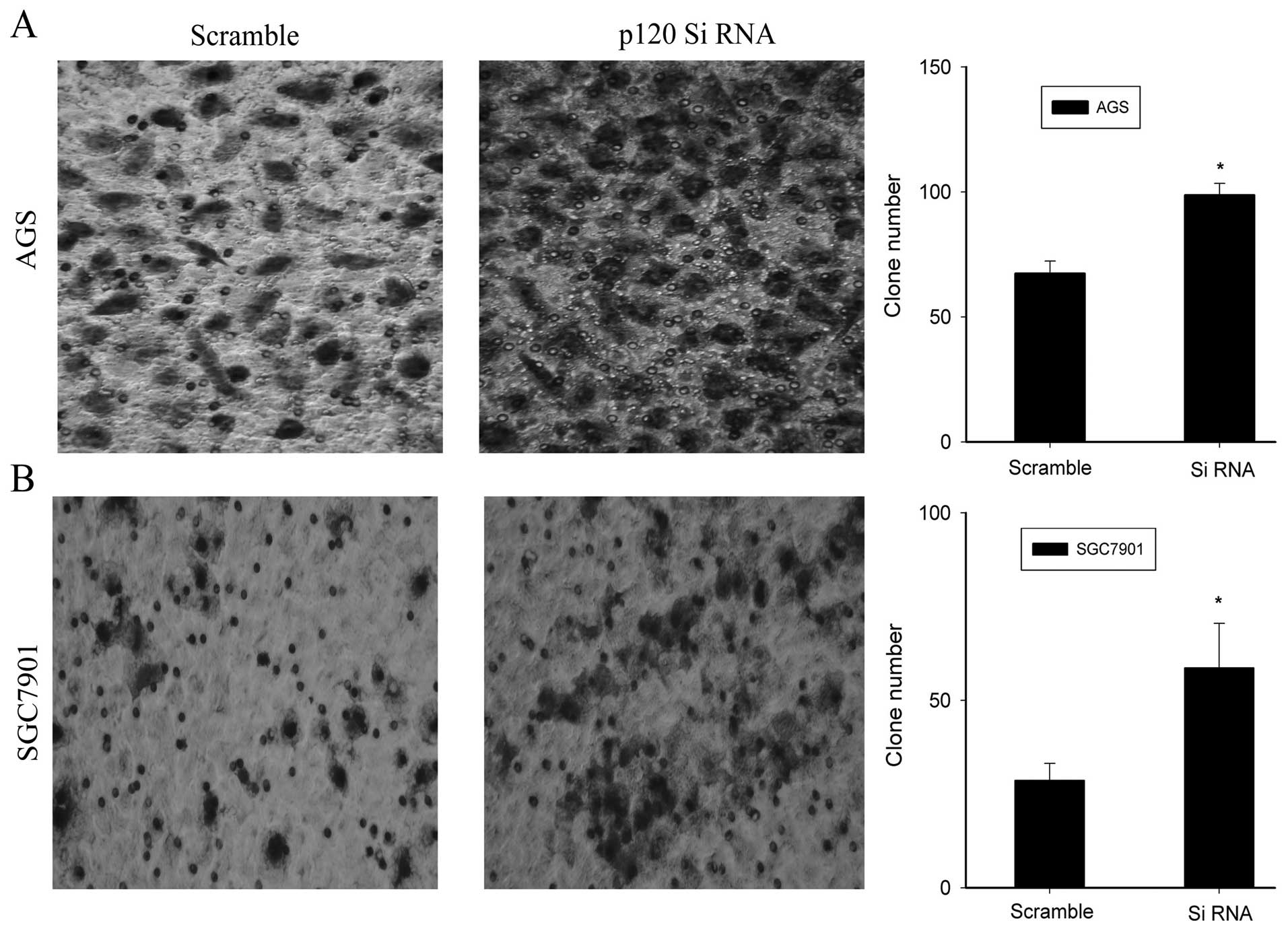
Moreover, knockdown of p120 significantly reduced the invasiveness
of the two GC cell types at 48 h when compared to the scrambled
group (P<0.05, Fig. 8).
Discussion
In the present study, p120 was downregulated and
Rac1 and Pak1 were upregulated in the different tissues of human
gastric cancer by immunohistochemistry. Then western blotting
showed that expression of Pak1 and Rac1 in SGC7901 cells were
higher than in the AGS cells. p120 isoform 1 and 3 was detected in
SGC7901 cells, while only p120 isoform 1 was detected in AGS cells.
Next, overexpression of p120 1A downregulates the expression of
Pak1 and Rac1 in SGC7901 and AGS cells. Notably, the expression of
Rac1 and Pak1 remained unchanged when silencing the p120 by p120
siRNA. Furthermore, overexpression of p120 1A decreased the
migration and invasion of the GC cells, while silencing of p120
increased the migration and invasion of the two GC cell types. In
conclusion, we speculated that in addition to Rac1 and Pak1, p120
also participates in the progress of gastric cancer and this may be
through regulating Rac1 and Pak1, which provides a potential
prevention and a promising therapeutical approach for patients with
gastric cancer.
In previous studies, Pak1 and Rac1 signaling pathway
was shown to play a crucial role in malignant tumors (26,27).
Positive rates of Rac1 and Pak1 expression in normal tissue,
dysplasia and gastric carcinoma showed an increasing trend and were
correlated with tumor lymph node metastasis and TNM stage (9). We found that the expression of Rac1
and Pak1 was higher in poorly differentiated than well
differentiated gastric cancer tissues. p120 has been shown to be
crucial in contributing to the cell-cell adhesion and strengthen
the stability of cadherin-catenin complex (28). A number of studies have shown that
an absence of p120 expression is common in colon, bladder, stomach,
breast and prostate cancer (15),
and in many cases the absence of p120 expression is associated with
poor prognosis, indicating that reduced expression of p120
correlates closely with the progression of cancer. For the first
time, we found that p120 was also absent in gastric cancer. Four
different subtypes of p120 exist as a result of differential
splicing. Our results indicated that p120 isoform 1 and 3 were
mainly expressed in SGC7901 cells and p120 isoform 1 was expressed
in AGS cells. The present study further confirmed that p120 isoform
1 was involved in promoting cell invasiveness (29).
It has been found that Rac1 and Pak1 were downstream
factors of p120 (30). Thus, we
overexpressed or silenced p120 to explore the relationship among
p120, Rac1 and Pak1 at the cellular level. Notably, overexpression
of p120 1A decreased the expression of Rac1 and Pak1 in both GC
cell types. Silencing p120 did not change the expression of Rac1
and Pak1, but possibly the activity of Rac1 and Pak1 changed.
Rac1 and Pak1 may be important biomarkers of gastric
carcinoma invasion and metastasis (10). p120 can partially regulate the
migration and invasiveness of GC cells via Rac1 and Pak1.
Overexpression of p120 1A decreases the migration and invasion of
the GC cells, while silencing p120 increases the migration and
invasion of two GC cell types. These results indicated that p120
may be an important biomarker of gastric carcinoma invasion and
metastasis.
In conclusion, our studies demonstrated that not
only Rac1 and Pak1, but also p120 participates in the progress of
gastric cancer and this may be through regulating Rac1 and Pak1.
Most importantly, p120 as an upstream protein of Rac1 and Pak1 may
be a new target for the treatment of gastric cancer, which provides
a potential prevention and a promising therapeutical approach for
patients with gastric cancer.
Acknowledgments
This study was supported by grants from the Initial
Project for Post-Graduates of Hubei University of Medicine
(2013QDJZR09), and the Scientific and Technological Project of
Shiyan City of Hubei Province.
References
|
1
|
Jemal A, Siegel R, Ward E, Murray T, Xu J
and Thun MJ: Cancer statistics, 2007. CA Cancer J Clin. 57:43–66.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gómez del Pulgar T, Bandrés E, Espina C,
Valdés-Mora F, Pérez-Palacios R, García-Amigot F, García-Foncillas
J and Lacal JC: Differential expression of Rac1 identifies its
target genes and its contribution to progression of colorectal
cancer. Int J Biochem Cell Biol. 39:2289–2302. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rathinam R, Berrier A and Alahari SK: Role
of Rho GTPases and their regulators in cancer progression. Front
Biosci. 16:2561–2571. 2011. View
Article : Google Scholar
|
|
5
|
Kumar R and Vadlamudi RK: Emerging
functions of p21-activated kinases in human cancer cells. J Cell
Physiol. 193:133–144. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ong CC, Jubb AM, Haverty PM, Zhou W, Tran
V, Truong T, Turley H, O'Brien T, Vucic D, Harris AL, et al:
Targeting p21-activated kinase 1 (PAK1) to induce apoptosis of
tumor cells. Proc Natl Acad Sci USA. 108:7177–7182. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vadlamudi RK, Adam L, Wang RA, Mandal M,
Nguyen D, Sahin A, Chernoff J, Hung MC and Kumar R: Regulatable
expression of p21-activated kinase-1 promotes anchorage-independent
growth and abnormal organization of mitotic spindles in human
epithelial breast cancer cells. J Biol Chem. 275:36238–36244. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang J-X, Zhou Y-N, Zou SJ, Ren TW and
Zhang ZY: Correlations of p21-activated kinase 1 expression to
clinicopathological features of gastric carcinoma and patients'
prognosis. Chin J Cancer. 29:649–654. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu YJ, Tang Y, Li ZF, Li Z, Zhao Y, Wu ZJ
and Su Q: Expression and significance of Rac1, Pak1 and Rock1 in
gastric carcinoma. Asia Pac J Clin Oncol. 10:e33–e39. 2014.
View Article : Google Scholar :
|
|
10
|
Reynolds AB, Roesel DJ, Kanner SB and
Parsons JT: Transformation-specific tyrosine phosphorylation of a
novel cellular protein in chicken cells expressing oncogenic
variants of the avian cellular src gene. Mol Cell Biol. 9:629–638.
1989.PubMed/NCBI
|
|
11
|
Shibamoto S, Hayakawa M, Takeuchi K, Hori
T, Miyazawa K, Kitamura N, Johnson KR, Wheelock MJ, Matsuyoshi N,
Takeichi M, et al: Association of p120, a tyrosine kinase
substrate, with E-cadherin/catenin complexes. J Cell Biol.
128:949–957. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ferber A, Yaen C, Sarmiento E and Martinez
J: An octapeptide in the juxtamembrane domain of VE-cadherin is
important for p120ctn binding and cell proliferation. Exp Cell Res.
274:35–44. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ishiyama N, Lee SH, Liu S, Li GY, Smith
MJ, Reichardt LF and Ikura M: Dynamic and static interactions
between p120 catenin and E-cadherin regulate the stability of
cell-cell adhesion. Cell. 141:117–128. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Thoreson MA and Reynolds AB: Altered
expression of the catenin p120 in human cancer: Implications for
tumor progression. Differentiation. 70:583–589. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Anastasiadis PZ, Moon SY, Thoreson MA,
Mariner DJ, Crawford HC, Zheng Y and Reynolds AB: Inhibition of
RhoA by p120 catenin. Nat Cell Biol. 2:637–644. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Noren NK, Liu BP, Burridge K and Kreft B:
p120 catenin regulates the actin cytoskeleton via Rho family
GTPases. J Cell Biol. 150:567–580. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Grosheva I, Shtutman M, Elbaum M and
Bershadsky AD: p120 catenin affects cell motility via modulation of
activity of Rho-family GTPases: A link between cell-cell contact
formation and regulation of cell locomotion. J Cell Sci.
114:695–707. 2001.PubMed/NCBI
|
|
18
|
Wong LE, Reynolds AB, Dissanayaka NT and
Minden A: p120-catenin is a binding partner and substrate for group
B Pak kinases. J Cell Biochem. 110:1244–1254. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jaffer ZM and Chernoff J: p21-activated
kinases: Three more join the Pak. Int J Biochem Cell Biol.
34:713–717. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Parrini MC, Lei M, Harrison SC and Mayer
BJ: Pak1 kinase homodimers are autoinhibited in trans and
dissociated upon activation by Cdc42 and Rac1. Mol Cell. 9:73–83.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang X and Wang E: Impact of p120-catenin
isforms 1A and 3A on epithelial mesenchymal transition of lung
cancer cells expression e-cadherin in different subcellular
locations. PLoS One. 9:880642014. View Article : Google Scholar
|
|
22
|
Tucker TA, Varga K, Bebok Z, Zsembery A,
McCarty NA, Collawn JF, Schwiebert EM and Schwiebert LM: Transient
transfection of polarized epithelial monolayers with CFTR and
reporter genes using efficacious lipids. Am J Physiol Cell Physiol.
284:C791–C804. 2003. View Article : Google Scholar
|
|
23
|
Sun T, Tian H, Feng YG, Zhu YQ and Zhang
WQ: Egr-1 promotes cell proliferation and invasion by increasing
β-catenin expression in gastric cancer. Dig Dis Sci. 58:423–430.
2013.
|
|
24
|
Jiang G, Wang Y, Dai S, Liu Y, Stoecker M
and Wang E and Wang E: P120-catenin isoforms 1 and 3 regulate
proliferation and cell cycle of lung cancer cells via β-catenin and
Kaiso respectively. PLoS One. 7:e303032012. View Article : Google Scholar
|
|
25
|
Zhang ZY and Ge HY: Micrometastasis in
gastric cancer. Cancer Lett. 336:34–45. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tang Y, Chen Z, Ambrose D, Liu J, Gibbs
JB, Chernoff J and Field J: Kinase-deficient Pak1 mutants inhibit
Ras transformation of Rat-1 fibroblasts. Mol Cell Biol.
17:4454–4464. 1997.PubMed/NCBI
|
|
27
|
Qu J, Cammarano MS, Shi Q, Ha KC, de
Lanerolle P and Minden A: Activated PAK4 regulates cell adhesion
and anchorage-independent growth. Mol Cell Biol. 21:3523–3533.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Thoreson MA, Anastasiadis PZ, Daniel JM,
Ireton RC, Wheelock MJ, Johnson KR, Hummingbird DK and Reynolds AB:
Selective uncoupling of p120ctn from E-cadherin disrupts
strong adhesion. J Cell Biol. 148:189–202. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang Y, Zhao Y, Jiang G, Zhang X, Zhao H,
Wu J, Xu K and Wang E: Impact of p120-catenin isoforms 1A and 3A on
epithelial mesenchymal transition of lung cancer cells expressing
E-cadherin in different subcellular locations. PLoS One.
9:e880642014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu Y, Chen N, Cui X, Zheng X, Deng L,
Price S, Karantza V and Minden A: The protein kinase Pak4 disrupts
mammary acina architecture and promotes mammary tumorigenesis.
Oncogene. 29:883–894. 2010. View Article : Google Scholar
|