Introduction
Colorectal cancer is one of the most common
malignancies in developing countries. Some patients exhibit a poor
prognosis due to resistance to chemotherapy and other treatments.
The underlying cause may be the enhanced viability of tumor cells.
In the life cycle of tumor cells, cells undergo a condition of
inadequate blood supply, and the ensuing absence of oxygen and
glucose deprivation may result in autophagy (1). In recent years, autophagy has been
attracting extensive attention as a common event caused by ambient
pressure or cellular stress. In detail, autophagy is a general term
for the degradation of cytoplasmic components within lysosomes,
producing amino acid, saccharides, and nucleotides to suffice the
physiological processes of cells which lack nutrients in turn
reducing the stress from apoptosis (2). However, it serves as only a temporary
relief for the survival of cells in poor condition, since
persistence of this process often results in cell death (3).
The expression of several specific proteins is often
accompanied by the process of autophagy, which can be recognized as
biomarkers related to the prognosis of colorectal cancer. These
proteins are specifically expressed in tumor cells but not in
normal cells. These include light chain 3 (LC3), Beclin-1, p62 and
GABA-A receptor-associated protein (GABARAP). LC3 accumulating
around the nucleus is closely related with the existence of
autophagy as well as indicative of a better prognosis of colon
cancer (4). In addition, in
advanced colorectal cancer, Beclin-1 is significantly highly
expressed in situ compared with metastatic sites and also
indicates a better prognosis (5).
In contrast, overexpression of GABARAP in colorectal cancer cells
results in poor differentiation and reduced patient survival
(6). Another study also indicated
that detection of the combination of the above biomarkers
facilitated prediction of prognosis in patients receiving 5-FU
treatment (7).
The prognosis of colorectal cancer depends on the
occurrence of tumor metastasis. Yet, the relationship between
autophagy and metastasis is still elusive (8). It is generally considered that
autophagy helps in reducing necrosis and inflammation, preventing
metastasis at the early stage of cancer. Yet, from another point of
view, it may also help in providing higher survival probability for
tumor cells circulated in blood and lymph nodes (9).
Vacuole membrane protein 1 (VMP1) is a transmembrane
protein which has been shown to be located mainly in the Golgi
apparatus and endoplasmic reticulum. It was first observed in
pancreatitis, participating in the process of autophagy (10). Subsequent study of tumor cells
demonstrated that it also plays a key role in the development of
tumors in processes such as proliferation (11), autophagy (12), metastasis (11) and drug sensitivity (13). A recent study on VMP1 showed that
colorectal cancer cells initiated the process of autophagy under
the stimulation of rapamycin or lack of nutrients with an increased
expression of VMP1 (14). However,
this phenomenon was diminished by siRNA interference of VMP1.
Further study indicated that colorectal cancer cells also became
more sensitive to etoposide and tended to undergo apoptosis under
starvation. In this previous study, VMP1 was considered as a key
regulator of autophagy. Namely active VMP1 promoted the process of
autophagy, maintained the survival of tumor cells as well as was
insensitive to apoptotic signaling. VMP1 could be regarded as a
suitable biomarker for colorectal cancer. However, this study
appeared to be limited owing to the lack of detection of VMP1 in
clinical samples as well as that etoposide is not the first-line
drug in treating patients with colorectal cancer.
Therefore, it is important to explore the effect of
VMP1 on the biological characteristics of colorectal cancer cells
and thus elucidate the underlying mechanisms, clarifying the
implications of VMP1 in the prognosis of patients suffering from
colorectal cancer. In the present study, expression of VMP1 was
detected in clinical samples as well as different colorectal cancer
cell lines to establish relationships between VMP1 and the
prognosis of colorectal cancer. Furthermore, an shRNA lentiviral
vector was constructed to silence the VMP1 gene to observe
heritable changes in the cell lines which originally highly express
VMP1. The present study focused on the proliferation, drug
sensitivity, metastasis as well as the signaling pathways related
to the processes mentioned above.
Materials and methods
Cell culture
The colorectal cell lines HT-29, SW620, RKO and LOVO
were obtained from Dr Zeng (State Key Laboratory of Oncology in
Southern China, Sun Yat-sen University Cancer Center, Guangzhou,
China) and were grown in RPMI-1640 medium (Invitrogen, Carlsbad,
CA, USA) supplemented with 10% fetal bovine serum (FBS). 293T and
SW480 cell lines were purchased from the Shanghai Institute of Cell
Biology (Shanghai, China). 293T and SW480 cells were separately
cultured in Dulbecco's modified Eagle's medium (DMEM) and RPMI-1640
medium with 10% FBS at 37°C in a humidified atmosphere of 5%
CO2.
Patient data
Paraffin-embedded, archived colorectal cancer
samples obtained from 94 patients were histologically and
clinically diagnosed at Fudan University Pudong Medical Center
between 2007 and 2009. Of the 94 colorectal cancer tissues, 52
matched adjacent non-cancerous tissues were used as controls. Prior
to the use of these clinical materials for investigation, informed
consent from patients and approval from the Institute Research
Ethics Committee were obtained. Primary cancers of the colorectum
were classified according to the pathological TNM classification
(15). Clinical information of the
samples is described in detail in Table
I. Patients included 47 males and 47 females, with ages ranging
from 24 to 85 years (mean, 65.4 years). The data for metastasis
pertain to its presence at any time during the follow-up. The
median follow-up time for overall survival was 59.0 months for
patients still alive at the time of analysis and ranged from 6 to
86 months. A total of 39 (41.5%) patients died during
follow-up.
 | Table ICorrelation between the
clinicopathological features and the expression of VMP1
protein. |
Table I
Correlation between the
clinicopathological features and the expression of VMP1
protein.
|
Characteristics | VMP1 expression
| P-value |
|---|
| Low | High |
|---|
| Age (years) |
| ≤60 | 26 (81.3) | 6 (18.8) | 0.069 |
| >60 | 39 (62.9) | 23 (37.1) | |
| Gender |
| Male | 32 (68.1) | 15 (31.9) | 0.826 |
| Female | 33 (70.2) | 14 (29.8) | |
| Stage |
| I | 11 (57.9) | 8 (42.1) | 0.008 |
| II | 19 (55.9) | 15 (44.1) | |
| III | 30 (85.7) | 5 (14.3) | |
| IV | 5 (83.3) | 1 (16.7) | |
| Histological
type |
| Mucinous
adenocarcinoma | 54 (72.0) | 21 (28.0) | 0.266 |
| Columnar
adenocarcinoma | 7 (53.8) | 6 (46.2) | |
| Others | 4 (66.7) | 2 (33.3) | |
| Histological
differentiation |
| Well | 4 (40.0) | 6 (60.0) | 0.058 |
| Moderate | 46 (70.8) | 19 (29.2) | |
| Poor | 15 (78.9) | 4 (21.1) | |
| Tumor diameter
(mm) |
| ≤50 | 46 (68.7) | 21 (31.3) | 0.872 |
| >50 | 19 (70.4) | 8 (29.6) | |
| pT
classification |
| T1-T2 | 20 (64.5) | 11 (35.5) | 0.500 |
| T3-T4 | 45 (71.4) | 18 (28.6) | |
| pN
classification |
| N0 | 30 (56.6) | 23 (43.4) | 0.002 |
| N1 | 25 (83.3) | 5 (16.7) | |
| N2 | 10 (90.9) | 1 (9.1) | |
| pMetastasis |
| Yes | 5 (83.3) | 1 (16.7) | 0.442 |
| No | 60 (68.2) | 28 (31.8) | |
VMP1 shRNA construction and lentiviral
production
Three target sequences for VMP1 mRNA were chosen
according to the RNAi Consortium (TRC) shRNA Library (Broad
Institute) (16). The VMP1 shRNA
single-strand oligonucleotides were: 1F,
5′-CCGGGTGCTTATAGCTACGTATTATCTCGAGATAATACGTAGCTATAAGCACTTTTTG-3′
and 1R,
5′-AATTCAAAAAGTGCTTATAGCTACGTATTATCTCGAGATAATACGTAGCTATAAGCAC-3′;
2F,
5′-CCGGCAACAGTATGTGCAACGTATACTCGAGTATACGTTGCACATACTGTTGTTTTTG-3′
and 2R,
5′-AATTCAAAAACAACAGTATGTGCAACGTATACTCGAGTATACGTTGCACATACTGTTG-3′;
3F,
5′-CCGGGCAATGAACAAGGAACATCATCTCGAGATGATGTTCCTTGTTCATTGCTTTTTG-3′
and 3R,
5′-AATTCAAAAAGCAATGAACAAGGAACATCATCTCGAGATGATGTTCCTTGTTCATTGC-3′.
Two complementary single-strand oligonucleotides
containing the target sequences were synthesized (Shanghai, China)
chemically and annealed. The double-stranded oligonucleotides were
inserted between the AgeI and EcoRI restriction sites
in the pLKO-green fluorescent protein (GFP) small interfering RNA
(siRNA) vector that contains cytomegalovirus-driven enhanced green
fluorescent protein (EGFP) reporter gene. The ligated plasmid was
transformed into E. coli DH5α competent cells for plasmid
amplification. The plasmids from positive colonies were identified
by RT-PCR and DNA sequencing.
Recombinant lentiviruses were produced by
co-transfecting 293T cells with three combinant lentiviral vectors,
Δ8.91 and pVSV-G (10:10:1) using the cationic lipid complex method
(X-tremeGENE HP DNA Transfection Reagent; Roche). The culture
supernatants containing the produced viruses were harvested at 48 h
after transfection, and concentrated by centrifugation at 4,000 × g
at 4°C for 10–15 min. SW480 cells were subcultured at a density of
1×106 cells/well. Cells were divided into 4 groups: CON
(infected with negative control lentiviral vector) and VMP1 shRNA
1–3 (infected with the VMP1 lentiviral vectors). SW480 cells were
plated into 6-well dishes at 5×105 cells/well. The next
day, the cells were infected with the same viral titer with 2
μg/ml Polybrene. After 24 h post-infection, the media were
replaced with media containing 2 μg/ml puromycin. Cells were
maintained and allowed to grow for 7–9 days; media were replaced
without puromycin and then passaged for follow-up assays.
Western blot analysis
Equal numbers of cells were lysed in lysis buffer
composed of 0.6 M Tris-HCl (pH 6.8), 10% SDS and protease inhibitor
cocktail. Samples were incubated at 4°C for 10 min, and then
centrifuged at 10,000 × g for 15 min at 4°C. The supernatants were
transferred, mixed and boiled in sample buffer. The supernatants
were separated by polyacrylamide gel electrophoresis and
transferred to a PVDF membrane (Bio-Rad, Hercules, CA, USA). Then
incubation of the membrane was carried out at room temperature in
blocking buffer consisting of 5% fat-free milk dissolved in 1X TBST
(10 mM Tris-base, pH 7.5, 100 mM NaCl and 1% Tween-20) for 1 h
followed by incubation with the blocking buffer containing the
primary antibody, such as anti-VMP1, anti-AKT, anti-p-AKT,
anti-ZO-1 (Cell Signaling Technology, Danvers MA, USA),
anti-E-cadherin or anti-β-actin (BD, Shanghai, China) at 4°C
overnight. The membrane was washed with TBS-T, and incubated with
the secondary antibody for 1 h. The blot was exposed to ECL
(Amersham) after TBS washing.
Cell proliferation experiment by MTT
method
Cells of the SW480 CON group (infected with the
negative control lentiviral vector) and the SW480 VMP1 shRNA1 group
(infected with the VMP1 lentiviral vector) were seeded into a
96-well microplate at a density of 5×103 cells/well and
incubated overnight in 10% FBS medium. Each group had 3 wells.
After incubation for 12, 24, 48 and 72 h at 37°C, MTT reagent (5
mg/ml) in phosphate-buffered saline (PBS) was added to the cells
(10 μl/well), and the cells were incubated for 4 h at 37°C.
The supernatant was discarded and 200 μl of
dimethylsulfoxide (DMSO) was added to each well to solubilize the
MTT-formazan product. Finally, samples were measured on a
multi-well spectrophotometer (Thermo, USA) at a test wavelength of
560 nm with a reference wavelength of 650 nm.
Drug sensitivity experiment
Cells were seeded at 5,000 cells/well into 96-well
plates (200 μl/well) and allowed to grow for 24 h before
treatment with the complex. Different concentrations of cisplatin,
5-FU and oxaliplatin were added into the cells; each group had 3
wells. After a further 24 h, MTT reagent (5 mg/ml) in PBS was added
to the cells (10 μl/well) and incubated for 4 h at 37°C. Two
hundred microliters of DMSO was added to each well to solubilize
the MTT-formazan product after removal of the medium. Samples were
measured on a multi-well spectrophotometer (Thermo) at a test
wavelength of 595 nm and a reference of 650 nm.
Matrigel-based invasion assay
Assays were performed in Transwell chambers (Corning
Inc., USA) with an 8-μm pore size coated with Matrigel (BD,
USA). SW480 cells infected with the scrambled shRNA and VMP1 shRNA1
were trypsinized and suspended in 1% FBS. After counting, the cells
were plated into the upper chamber and 600 μl medium
supplemented with 10% FBS was placed into the lower chamber. After
incubation at 37°C for 6 h, cells on the upper surface of the
filters were removed and cells adhering to the undersurface of the
filter membrane were dyed with 0.5% crystal violet for 30 min. The
crystal violet was washed with PBS for 3 times. Cells on the lower
chamber were counted under a microscope in four fields randomly.
The mean cell numbers were recorded and analyzed. The experiment
was repeated 3 times.
Immunohistochemistry (IHC)
IHC was carried out to study altered protein
expression in 94 human colorectal cancer tissues and 52 matched
adjacent non-cancerous tissues. A commercially available antibody
against VMP1 (1:100 lot ab116006, rabbit polyclonal immunoglobulin
G; Abcam Biotechnology, USA) was used as the primary antibody. An
immunohistochemical kit (SP-9001 rabbit SP kit, lot 50581654) was
obtained from Zhongshan Golden Bridge Biotechnology Co., Ltd.
(Beijing, China). For each sample, one score was given according to
the percent of positive cells as: no positive cells, 0; <5%
positive cells, 1 point; 5–35% positive cells, 2 points; 36–70%
positive cells, 3 points; >70% positive cells, 4 points. To
achieve objectivity, the intensity of positive staining was also
used in a four-tier scoring system: 0 (negative staining), 1 (weak
staining exhibited as light yellow), 2 (moderate staining exhibited
as yellow brown), and 3 (strong staining exhibited as brown). A
final score was then calculated by multiplying the above two
scores. If the final score was ≥4, the tumor was considered to have
high expression; otherwise, the tumor was considered to have low
expression (17).
Reverse-transcriptase PCR (RT-PCR)
Cells were washed twice with cold PBS and were
collected by centrifugation at 1,000 × g for 5 min. Total RNA was
extracted using TRIzol® reagent (Invitrogen) following
the manufacturer's protocol. cDNA was generated with oligo(dT)
primers. Primers were designed online using the Primer3 designer
program (website, http://frodo.wi.mit.edu/) and were as follows: VMP1
forward, 5′-GAC CAG AGA CGT GTA GCA ATG-3′ and reverse, 5′-ACA ATG
CTT TGA CGA TGC CAT AA-3′; GAPDH (housekeeping gene) forward,
5′-GGA GAT TGT TGC CAT CAA CG-3′ and reverse, 5′-TTG GTG GTG CAG
GAT GCA TT-3′. The reaction systems consisted of 0.2 μl
cDNA, 2.5 μl 10X Taq enzyme buffer, 1 μl 10 mol/l
dNTP, the forward and reverse primers were 0.2 μl and 0.2
μl Taq enzymes. Water was then added to 25 μl. PCR
conditions were as follows: denaturation at 95°C for 2 min; each
cycle consisting of 1 min at 94°C, 1 min at 56°C; 1 min and 15 sec
at 72°C; and a final extension of 5 min at 75°C. After the
reaction, the PCR products were separated on 2% agarose gel
(BioWest) and visualized under UV light.
Xenograft model
Mice were treated in accordance with the NIH Guide
for the Care and Use of Laboratory Animals (18), and as approved by the Ethics
Committee of Ningbo University. Mice were housed in a
temperature-controlled room with a proper dark-light cycle, fed a
regular diet, and maintained under the care of the Laboratory
Animal Unit, Ningbo University, China. The mice were acclimated for
1 week before the experiment. VMP1KD and
VMP1scramble cells growth in logarithmic phase were
trypsinized, and the cell suspension was harvested. After staining
with trypan blue, the cells were counted under a hemocytometer to
test the viability. Cell concentration was adjusted with culture
medium to 1×107 cells/ml. The nude mice were randomly
divided into 2 groups. In the VMP1 knockout group, 10 mice were
intraperitoneal injected with VMP1KD cells, while in the
scramble shRNA group, 10 mice were injected with the
VMP1scramble cells. Each nude mouse was inoculated with
0.5 ml of the cell suspension. The needle was stopped internally
for 5 sec, rotated and pulled out to avoid leakage of the cell
suspension. The activity, diet and mental state of the nude mice
were observed daily. On day 20 of the experiment, all of the mice
were sacrificed by cervical vertebra disjointing, the abdominal
cavity was opened and the nodules within the abdominal were
stripped, counted and confirmed by fluorescence microscopy.
Statistical analysis
All statistical analyses were carried out using the
SPSS 13.0 statistical software package. The χ2 test was
employed to evaluate differences in expression of VMP1 between the
two categories of tissues. The Mann-Whitney U test was used to
analyze the relationship between VMP1 expression and
clinicopathological characteristics. Survival curves were plotted
by the Kaplan-Meier method and compared by the log-rank test. The
significance of various variables for survival was analyzed by the
Cox proportional hazards model in the multivariate analysis.
P<0.05 in all cases was considered to indicate a statistically
significant result.
Results
Expression of VMP1 in colorectal cancer
and analysis of prognosis
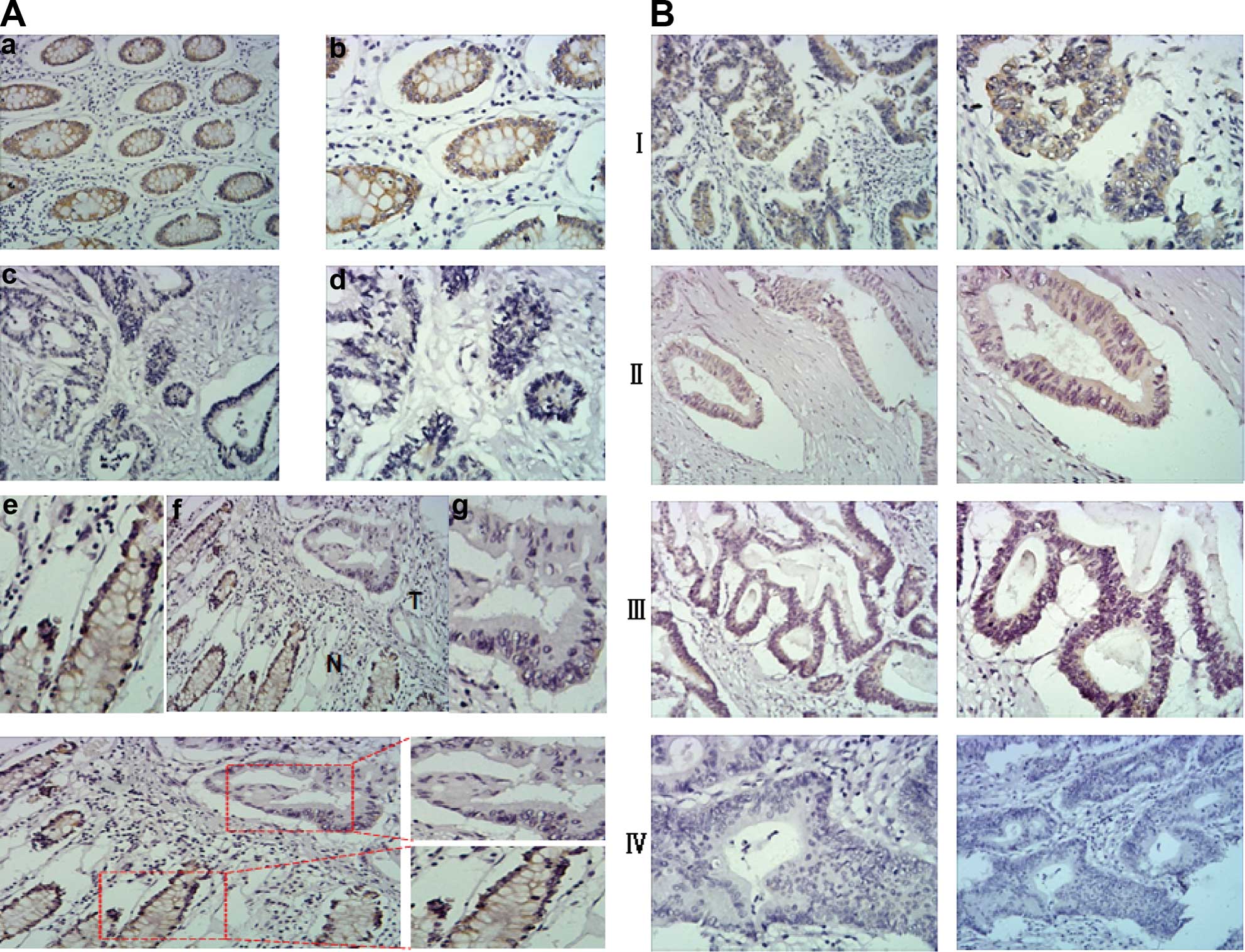
Immunohistochemical assay showed that the expression
of VMP1 in the same patients was significantly decreased in primary
cancer tissues when compared with that in the matched adjacent
non-cancerous tissues (χ2=12.962, P=0.008; Fig. 1). Among the matched adjacent
non-cancerous tissues, 61.5% (32 of 52) of the individual tissues
had either high or strong VMP1 expression (Fig. 1Aa, b, e and g). However among the
colorectal cancer cases only 30.9% (29 of 94) of the cancer tissues
were defined as having high expression of VMP1 (Fig. 1Ac–f). The subcellular location of
VMP1 was mainly cytoplasmic.
Correlation between VMP1 protein
expression and clinicopathological features
Table I shows the
relationship between the expression of VMP1 protein and clinical
characteristics. There was no significant correlation between the
expression level of VMP1 protein and age, histological
classification, histological differentiation, tumor diameter, pT
classification or distant metastasis of the colorectal cancer
patients. However, the expression of VMP1 was closely associated
with stage of the colorectal cancer patients (P=0.008) and pN
classification (P=0.002). The expression of VMP1 protein was
negatively correlated with staging and pN classification (Table II). As shown in Fig. 1B, higher staging was correlated with
lower VMP1 expression.
 | Table IIUnivariate and multivariate analyses
of different prognostic parameters in patients with colorectal
cancer by Cox-regression analysis. |
Table II
Univariate and multivariate analyses
of different prognostic parameters in patients with colorectal
cancer by Cox-regression analysis.
| Univariate analysis
| Multivariate
analysis
|
|---|
| No. of pts. | P-value | Regression
coefficient (SE) | P-value | RR | 95% CI |
|---|
| Stage | | <0.001 | 1.316 (0.292) | <0.001 | 3.729 | 2.103–6.612 |
| I | 19 | | | | | |
| II | 34 | | | | | |
| III | 35 | | | | | |
| IV | 6 | | | | | |
| pT
classification | | 0.026 | 0.502 (0.424) | 0.236 | 1.653 | 0.720–3.791 |
| T1-T2 | 31 | | | | | |
| T3-T4 | 63 | | | | | |
| pN metastasis | | <0.001 | 0.275 (0.443) | 0.535 | 1.317 | 0.552–3.140 |
| N0 | 53 | | | | | |
| N1 | 30 | | | | | |
| N2 | 11 | | | | | |
| VMP1
expression | | 0.021 | −0.496 (0.462) | 0.283 | 0.609 | 0.246–1.507 |
| Low | 65 | | | | | |
| High | 29 | | | | | |
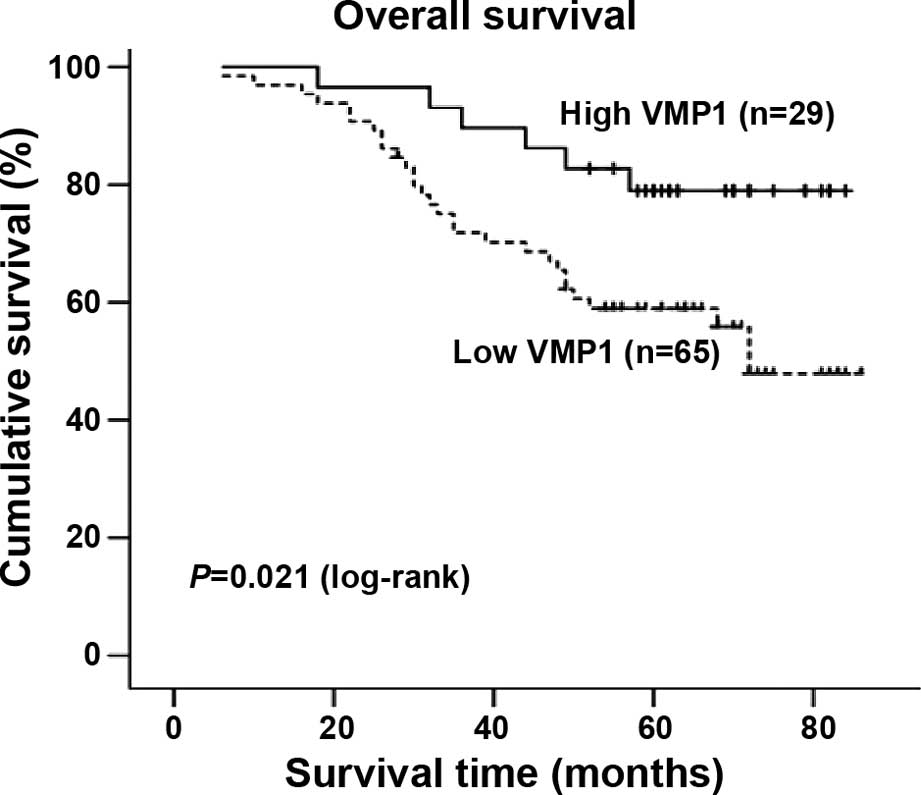
Survival analysis
Kaplan-Meier analysis and the log-rank test were
used to calculate the effect of classic clinicopathological
characteristics (including stage, T classification, N
classification) and VMP1 expression on survival. The expression
level of VMP1 protein in the colorectal cancer cases was
significantly correlated with the patient survival time (P=0.021),
indicating that lower levels of VMP1 expression were correlated
with shorter survival time. The high VMP1 expression group had
better survival, whereas the low VMP1 expression group had shorter
survival (Fig. 2). The median
survival of patients with high VMP1 expression was much longer (62
months) than the median survival of patients with low VMP1
expression (55 months) (P=0.021, log-rank).
In addition, T classification, N classification and
stage were also significantly correlated with survival in the
Kaplan-Meier analysis and log-rank test (for T classification,
P=0.026; for N classification, P<0.001; for stage, P<0.001).
We did multivariate survival analysis, which included VMP1
expression level, stage, T classification and N classification, to
determine whether VMP1 expression is an independent prognostic
factor of patient outcome. In this analysis, stage was recognized
as an independent prognostic factor, but VMP1 expression was not an
independent prognostic factor of outcome (Table II).
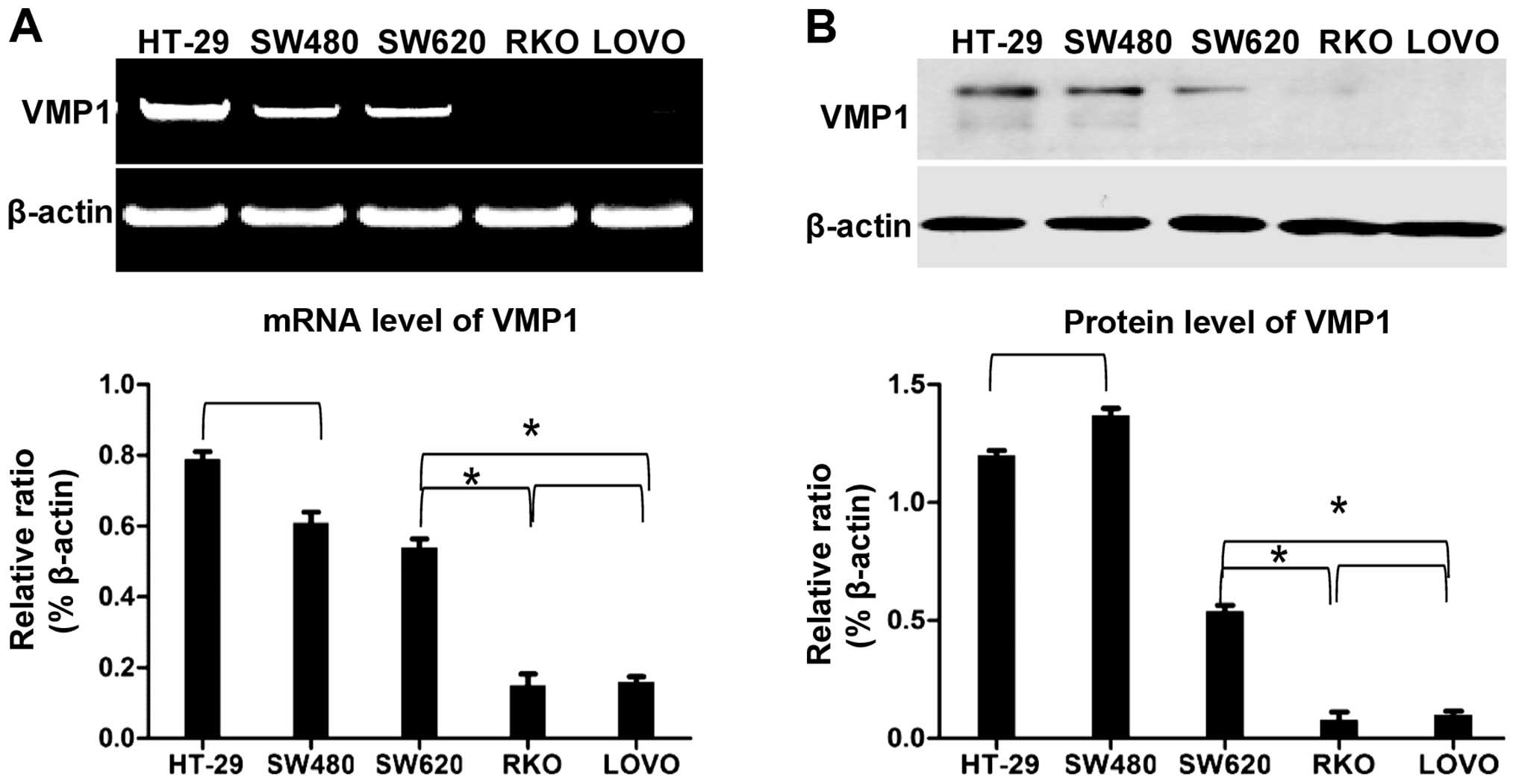
Expression of VMP1 in different
colorectal cell lines
The expression of VMP1 at the protein and mRNA
levels in different colorectal cancer cell lines was detected. A
negative correlation between VMP1 expression and metastasis was
found. Namely, the cell lines expressing VMP1 had a significantly
lower ability for metastasis and vice versa. These results suggest
that VMP1 may act as a key factor for preventing the progression of
colorectal cancer as well as in balancing the level between
autophagy and apoptosis (Fig.
3).
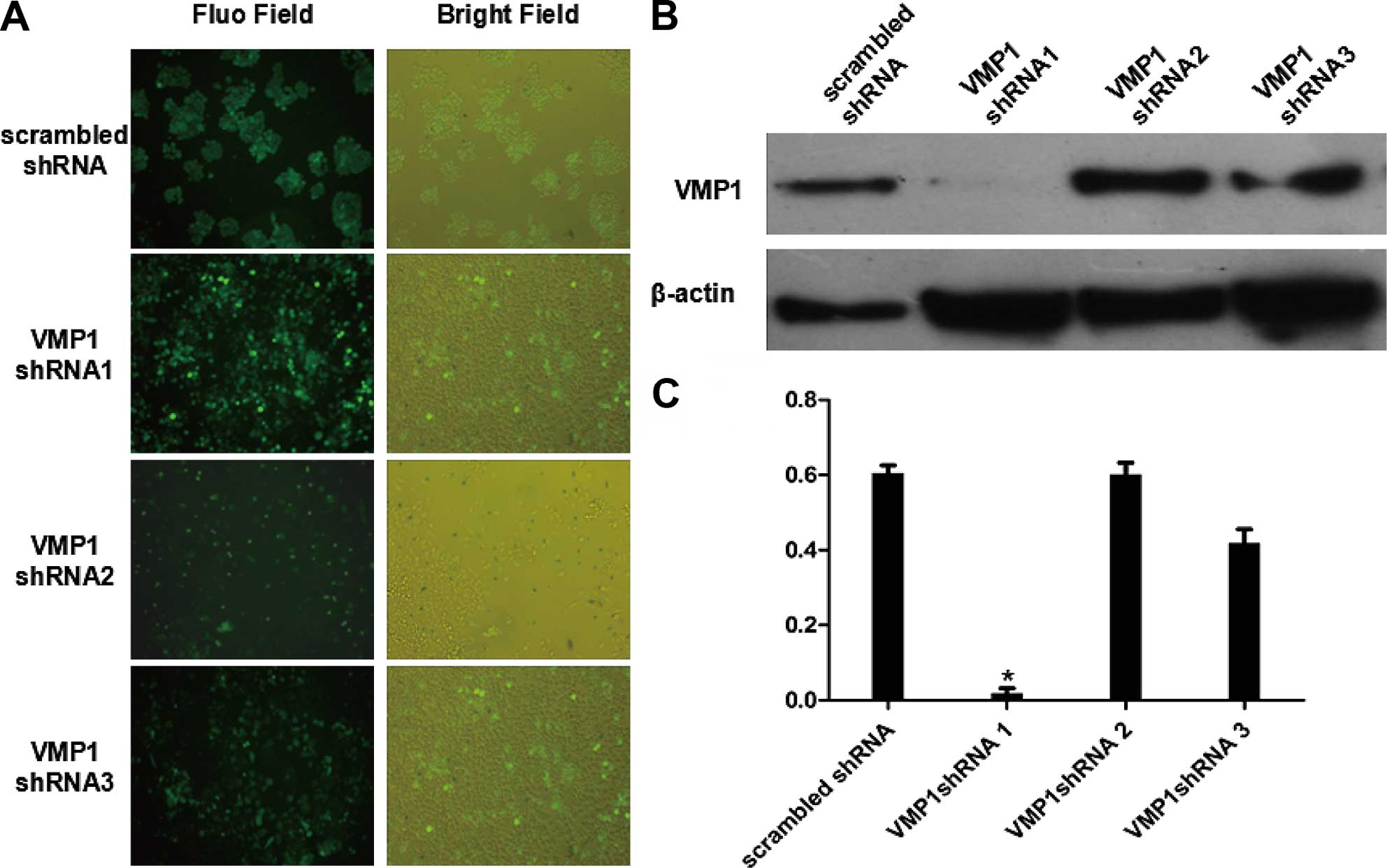
Construction and identification of VMP1
stable gene silencing
In order to clarify the role of VMP1 in the
progression of colorectal cancer, a shRNA lentiviral vector was
constructed to silence the VMP1 gene for heritable changes. We
designed 3 pairs of shRNAs. After sequencing was confirmed and
amplification in the plasmid, we packaged the 293T cells with
liposome and plasmid for a transfection of 48 h. After that, the
lentivirus in the culture supernatant was harvested to infect the
target cells. Results of fluorescence (Fig. 4A) showed that the cell infection
rate reached ~98%. In contrast, the western blotting indicated that
shRNA1 was the best sequence for silencing VMP1 at nearly 95%
(Fig. 4B and C). Thus, the
colorectal cancer cells were used for subsequent experiments.
Effect of VMP1 gene silencing on cell
proliferation and drug sensitivity
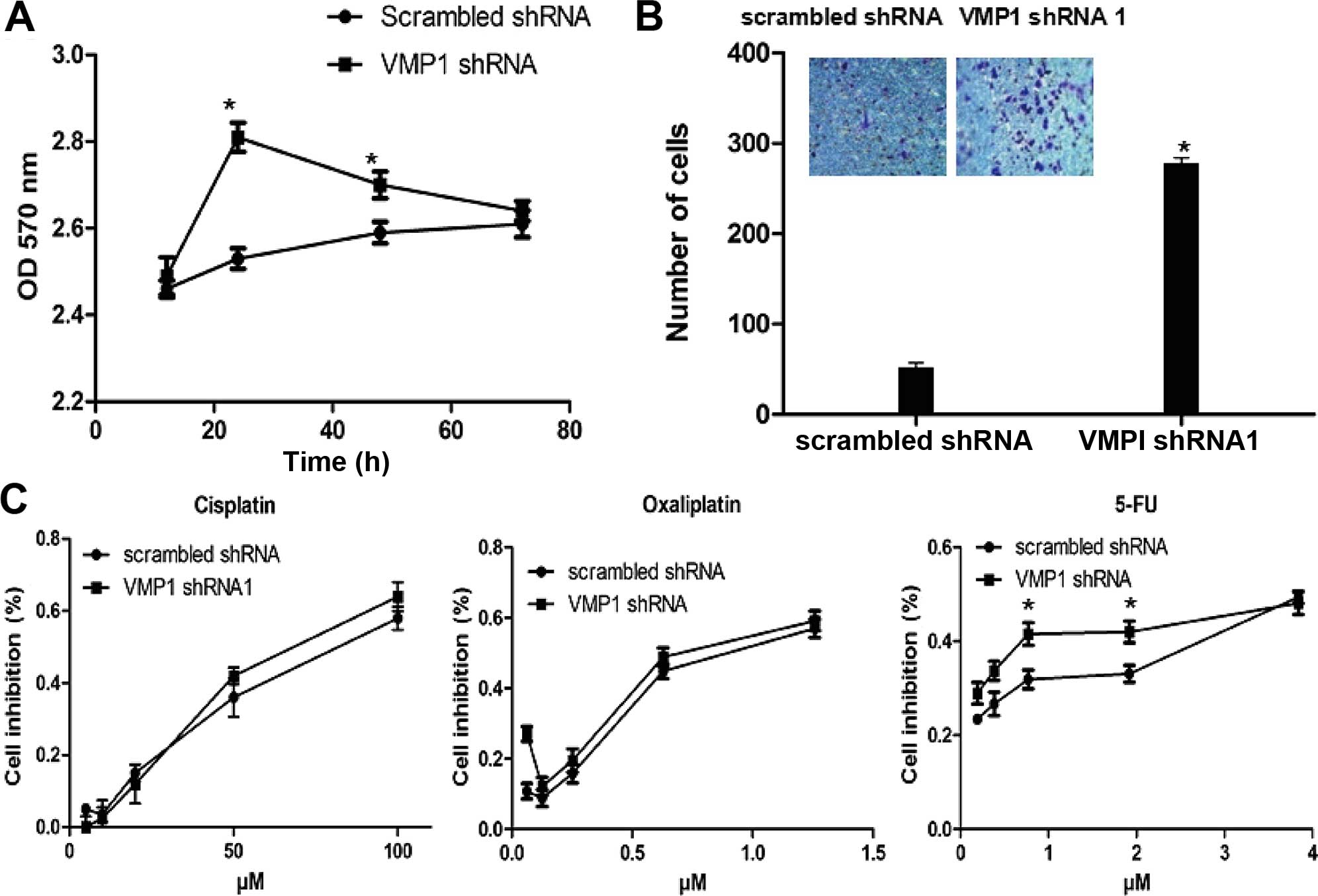
First, we evaluated the cell proliferation after
VMP1 silencing, which indicated a significantly higher
proliferation rate at 48 h and a balanced level at 72 h compared
with the scrambled shRNA group (Fig.
5A). As shown in Fig. 5C,
determination of drug sensitivity indicated an increasing
sensitivity to 5-FU after VMP1 silencing but an unconspicuous
function on platinum, which resulted from the increased sensitivity
to apoptosis signaling.
The effect and mechanisms of cell
migration after VMP1 gene silencing
We used a Transwell chamber model to study the
ability of cell migration. As expected, a significant increase in
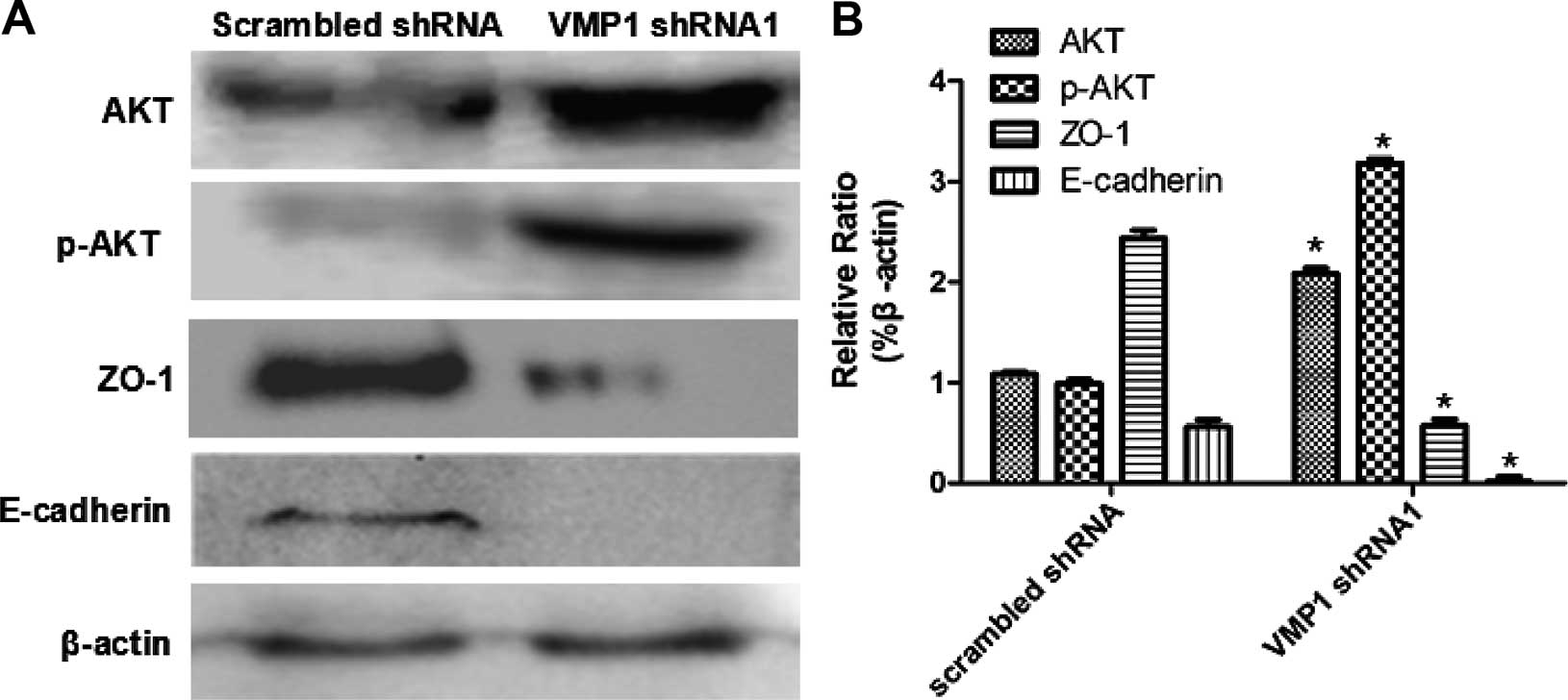
migration was found after VMP1 silencing (Fig. 5B). Western blotting showed an
increase in AKT/p-AKT as well as a downregulation of ZO-1, which
was related to the activation of the PI3K/AKT signaling pathway and
the phosphorylation of ZO-1. In addition, expression of E-cadherin
was also downregulated at the same time that acts as a key factor
inducing the EMT of tumor cells and promoting migration (Fig. 6).
Biological characteristics of the VMP1
silenced cell lines in vivo
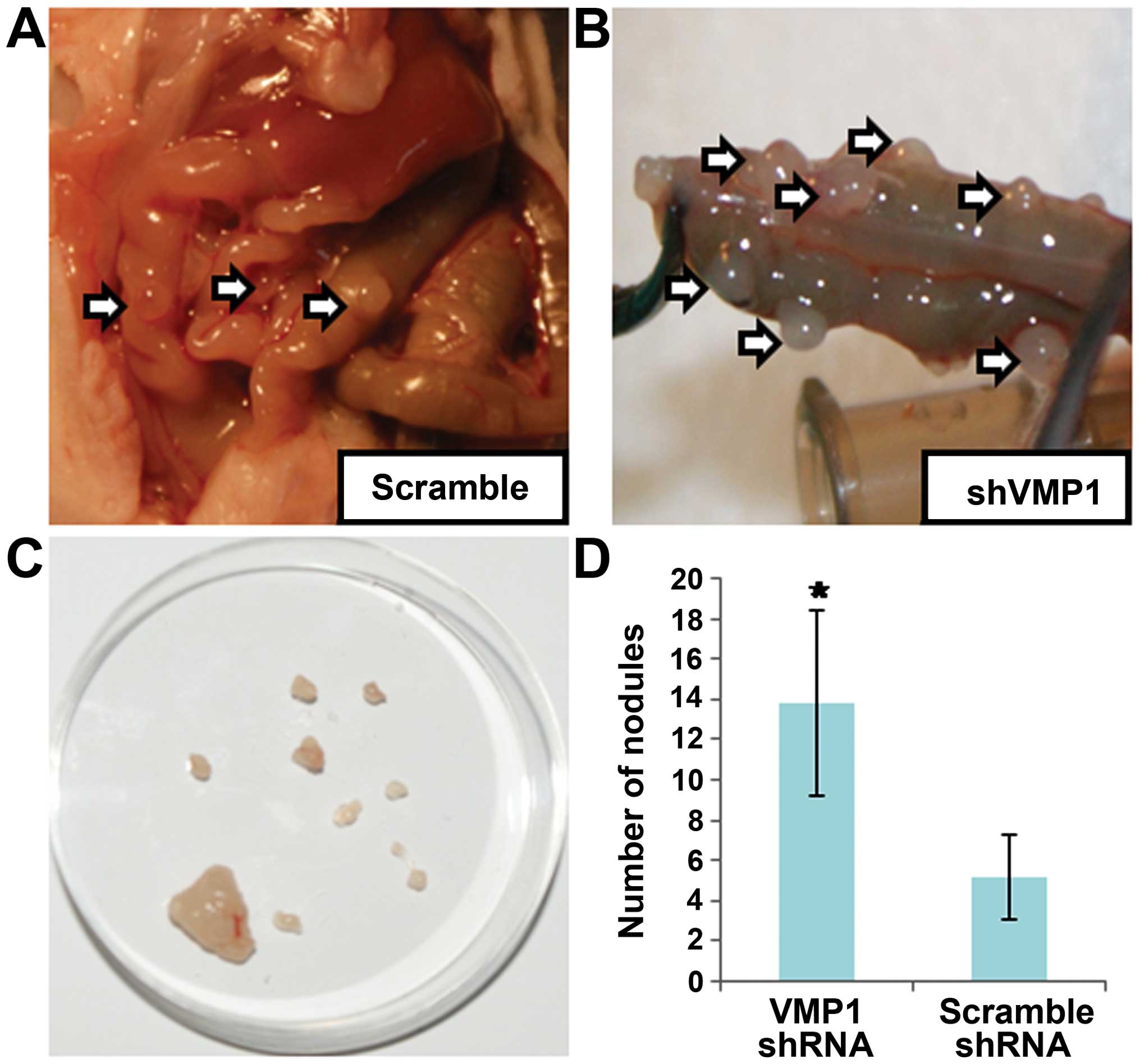
Finally, we compared the tumor formation in a nude
mouse model. The results indicated that the tumor formation rate
was 100%. There were no significant differences among the two
groups of mice at day 7 after inoculation of the normal SW480 cell
and the silenced ones. Furthermore, listlessness, poor appetite and
slow action were found in the VMP1 shRNA group but no significant
changes were noted in the scrambled shRNA group 7 days later. In
addition, 20 days after inoculation, separation of nodules
surrounded by intestinal tissue was carried out, to evaluate the
biological characteristics by counting the nodules with fluorescent
signaling. An increasing number of nodules was formed in the VMP1
silenced group compared with the GFP groups (Fig. 7).
Discussion
Vacuole membrane protein 1 (VMP1) is associated with
cancer patient prognosis which was first observed in studies of
pancreatic cancer. VMP1 was found to be overexpressed in pancreatic
cancer, and was found to be closely linked with poorly
differentiated human pancreatic cancer (13). In addition, zymophagy, a new pathway
through VMP1-USP9x-p62 preventing cell death of the pancreas, was
reported (12). Furthermore, a
study of lung cancer patients found that expression of VMP1 was
positively related to poor prognosis (19). However, some studies have shown that
VMP1 has various antitumor properties, increasing the degree of
malignancy if absent. VMP1 is an important component of cell
connections between cells and the formation of tight junctions, and
enhances cell junctions and cell adhesion function. In research on
breast cancer and renal cell carcinoma, researchers found that VMP1
expression was significantly reduced in cancer metastatic when
compared to primary tumors. When VMP1 expression was downregulated,
cell adhesion was lost and the invasive capacity of cells increased
(20). Another expression study in
breast cancer found that patients with VMP1-positive expression had
favorable prognosis, and patients with negative expression had poor
prognosis (21). Liver cancer
studies found similar results. Patients with low VMP1 expression
had lower overall survival and disease-free survival than those
with high expression of VMP1. Upregulation of VMP1 not only
inhibited the proliferation of cancer cells, but also inhibited
liver cancer cell metastasis in nude mice (11). The results of the present study and
the results in breast and liver cancer are in accordance. Prognosis
of patients with VMP1 high expression was significantly prolonged
when compared to patients with low expression and the difference
was statistically significant. Yet, our results also found that
patients with different stages had VMP1 expression level
differences. In addition, these results were consistent with
previous findings.
VMP1 had a high positive rate in normal tissues and
a low positive rate in cancer tissues. In the same patient, VMP1
was positive in the tissue adjacent to the carcinoma tumor tissue,
but was not positive in the tumor tissue. Furthermore, a recent
study showed that VMP1 was located in the plasma membrane playing a
critical role in cell-cell contact (20). It is well known that reduced levels
of cell-cell adhesion proteins often correlate with tumor invasion
and metastasis (22). Guo et
al and other studies have shown that upregulation of VMP1
inhibits metastasis of tumor cells (11). However, to date, no studies have
shown the impact of VMP1 in colorectal metastases. Therefore, our
results indicated that the development of colorectal cancer may
have a negative relationship with the expression of VMP1, which may
initiate autophagy and limit the transformation of cancer cells.
The results from our study also strengthen the connection between
autophagy and cancer metastasis.
In order to further illustrate the concepts
mentioned above, an shRNA lentiviral vector was constructed to
silence the VMP1 gene to observe heritable changes in cell lines
which originally highly express VMP1. After that, the proliferation
and migration of shVMP1 cells increased significantly. The benefit
of lentiviral vector transfection lies in inducing the cells to
gain heritable changes, which benefit subsequent study (23). The traditional method of siRNA
interference only functions as a temporary transfection, which
affects the proliferation of cells in turn as well as instable
function in gene silencing (24).
Of course, lentiviral technique also has some defects. It would be
time-consuming in the process of puromycin screening in requiring
the target cells.
As a tumor-suppressor gene involved in tumor
progression, it has been demonstrated that upregulation of VMP1
inhibited the proliferation of liver cancer cells (11). In the present study, after VMP1
knockdown, a maximum proliferation was observed at 24 h and an
ensuing increase which was finally equivalent to the control group.
All the mentioned above indicate that VMP1 plays a crucial role in
controlling the proliferation of cells. Such a role for VMP1 was
further supported by our in vivo study in which we showed
that the proliferation of cells was lower than that of the VMP1
gene silenced group.
VMP1 also plays a crucial role in the regulation of
cell migration. On the one hand, recent studies indicate that VMP1
is essential for cell-cell contacts and tight junction formation,
and it co-localizes with the tight junction protein ZO-1 in spots
between neighboring HEK293 cells (20). ZO-1 is important for the clustering
of claudins and occludin, resulting in the formation of tight
junctions. Downregulation of VMP1 by RNAi results in loss of cell
adherence and increased ability for migration. In this process,
ZO-1 is closely associated with E-cadherin (25), both of which participate in the
course of epithelial-mesenchymal transition (EMT). It was also
demonstrated by downregulation of E-cadherin in advanced metastatic
colon cancer. In contrast, it was reported that VMP1 may be a new
player in the regulation of autophagy-specific phosphatidylinositol
3-kinase complex activation (26).
However, the underlying mechanisms involved in the migration of
colon cancer remain elusive. To further confirm the subcellular
localization of VMP1 and its regulatory mechanism, in our Transwell
study, a significant increase in migration was noted in colon
cancer cells after VMP1 gene silencing. At the protein levels, a
decrease in expression of phosphorylated ZO-1 as well as
downregulation of E-cadherin were noted by western blotting,
indicating a weak function of ZO-1 after VMP1 gene silencing. As
ZO-1 and E-cadherin play important roles in cancer-related cell
biological systems (27), the
direct interaction of VMP1 with ZO-1 and E-cadherin may provide
some evidence on the phenomenon that VMP1 controls colorectal
cancer behavior thorough regulation of cell-cell contacts.
Moreover, this phenomenon may be associated with activation of the
PI3K/Akt signaling pathway, which was embodied by the increasing
level of p-Akt/Akt. The PI3K/Akt signaling pathway was confirmed to
play a significant role in tumor cell survival, angiogenesis,
differentiation, growth and metastasis (28). Similarly, other researches also
pointed out the relationship between VMP1 and the PI3K/Akt
signaling pathway (29) and the
significant role of the PI3K/Akt signaling pathway in colon cancer
(30). In the present study, all
the findings mentioned above demonstrate the occurrence of EMT and
the ensuing cellular invasion may function through the PI3K/Akt
signaling pathway.
VMP1 may be a bridge between autophagy and
apoptosis. Under conditions of stress, cells may tend to upregulate
VMP1 to promote autophagy. In contrast, when VMP1 is downregulated,
the cell may turn to apoptosis. In addition to previous studies in
colon cancer (14), in our study,
it was demonstrated that selective apoptosis occurred, which was
embodied by an increasing sensitivity to 5-FU but an invariable
sensitivity to platinum drugs such as cisplatin and
oxaliplatin.
Integrated with the above mentioned clinical data,
the downregulation of VMP1 caused sensitivity to apoptotic
signaling pathways. It will be imperative to discuss the role of
VMP1 in in vivo studies, namely to verify the sensitivity to
5-FU of cancer cells with VMP1 gene silencing implanted in animal
models. Furthermore, for clinical studies, the effectiveness of
5-FU should be noted in patients with low expression of VMP1, which
may be a potential target for individual treatment in colorectal
cancer.
In conclusion, the present study firstly indicated
that the expression of VMP1 was negatively related to the prognosis
of colorectal cancer by analyzing clinical samples and different
cell lines. After gene silencing with a lentivirus, a significantly
increased ability of invasion was noted and an increased
sensitivity to 5-FU was also verified. To some extent, VMP1 could
be regarded as a new prognostic biomarker of colorectal cancer and
a potential target concerned with migration of cancer cells. In
conclusion, VMP1 is not only a bridge in the balance of autophagy
and metastasis, but is also a biomarker to predict which patients
may benefit from 5-FU in regards to different expression levels of
VMP1.
Acknowledgments
The present study was supported by the Young Medical
Talents Training Program of Pudong Health Bureau of Shanghai (no.
PWRq2012-31), the Academic Leaders Training Program of Pudong
Health Bureau of Shanghai (no. PWRd2012-15), and the Natural
Science Foundation of Ningbo (no. 2011A610048).
References
|
1
|
Song J, Guo X, Xie X, Zhao X, Li D, Deng
W, Song Y, Shen F, Wu M and Wei L: Autophagy in hypoxia protects
cancer cells against apoptosis induced by nutrient deprivation
through a Beclin1-dependent way in hepatocellular carcinoma. J Cell
Biochem. 112:3406–3420. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mizushima N: Autophagy: Process and
function. Genes Dev. 21:2861–2873. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Debnath J, Baehrecke EH and Kroemer G:
Does autophagy contribute to cell death? Autophagy. 1:66–74. 2005.
View Article : Google Scholar
|
|
4
|
Giatromanolaki A, Koukourakis MI, Harris
AL, Polychronidis A, Gatter KC and Sivridis E: Prognostic relevance
of light chain 3 (LC3A) autophagy patterns in colorectal
adenocarcinomas. J Clin Pathol. 63:867–872. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Koukourakis MI, Giatromanolaki A, Sivridis
E, Pitiakoudis M, Gatter KC and Harris AL: Beclin 1 over-and
underexpression in colorectal cancer: Distinct patterns relate to
prognosis and tumour hypoxia. Br J Cancer. 103:1209–1214. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Miao Y, Zhang Y, Chen Y, Chen L and Wang
F: GABARAP is overexpressed in colorectal carcinoma and correlates
with shortened patient survival. Hepatogastroenterology.
57:257–261. 2010.PubMed/NCBI
|
|
7
|
Park JM, Huang S, Wu TT, Foster NR and
Sinicrope FA: Prognostic impact of Beclin 1, p62/sequestosome 1 and
LC3 protein expression in colon carcinomas from patients receiving
5-fluorouracil as adjuvant chemotherapy. Cancer Biol Ther.
14:100–107. 2013. View Article : Google Scholar :
|
|
8
|
Yang SY and Winslet MC: Dual role of
autophagy in colon cancer cell survival. Ann Surg Oncol. 18(Suppl
3): S2392011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kenific CM, Thorburn A and Debnath J:
Autophagy and metastasis: Another double-edged sword. Curr Opin
Cell Biol. 22:241–245. 2010. View Article : Google Scholar :
|
|
10
|
Dusetti NJ, Jiang Y, Vaccaro MI, Tomasini
R, Azizi Samir A, Calvo EL, Ropolo A, Fiedler F, Mallo GV, Dagorn
JC, et al: Cloning and expression of the rat vacuole membrane
protein 1 (VMP1), a new gene activated in pancreas with acute
pancreatitis, which promotes vacuole formation. Biochem Biophys Res
Commun. 290:641–649. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guo L, Yang LY, Fan C, Chen GD and Wu F:
Novel roles of Vmp1: Inhibition metastasis and proliferation of
hepatocellular carcinoma. Cancer Sci. 103:2110–2119. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Grasso D, Ropolo A, Lo Ré A, Boggio V,
Molejón MI, Iovanna JL, Gonzalez CD, Urrutia R and Vaccaro MI:
Zymophagy, a novel selective autophagy pathway mediated by
VMP1-USP9x-p62, prevents pancreatic cell death. J Biol Chem.
286:8308–8324. 2011. View Article : Google Scholar :
|
|
13
|
Gilabert M, Vaccaro MI, Fernandez-Zapico
ME, Calvo EL, Turrini O, Secq V, Garcia S, Moutardier V, Lomberk G,
Dusetti N, et al: Novel role of VMP1 as modifier of the pancreatic
tumor cell response to chemotherapeutic drugs. J Cell Physiol.
228:1834–1843. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qian Q, Zhou H, Chen Y, Shen C, He S, Zhao
H, Wang L, Wan D and Gu W: VMP1 related autophagy and apoptosis in
colorectal cancer cells: VMP1 regulates cell death. Biochem Biophys
Res Commun. 443:1041–1047. 2014. View Article : Google Scholar
|
|
15
|
Hu H, Krasinskas A and Willis J:
Perspectives on current tumor-node-metastasis (TNM) staging of
cancers of the colon and rectum. Semin Oncol. 38:500–510. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Root DE, Hacohen N, Hahn WC, Lander ES and
Sabatini DM: Genome-scale loss-of-function screening with a
lentiviral RNAi library. Nat Methods. 3:715–719. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Reed J, Ouban A, Schickor FK, Muraca P,
Yeatman T and Coppola D: Immunohistochemical staining for c-Kit
(CD117) is a rare event in human colorectal carcinoma. Clin
Colorectal Cancer. 2:119–122. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
No authors listed. NIH requests
information on care of laboratory animals. Am J Vet Res.
67:204–205. 2006.PubMed/NCBI
|
|
19
|
Zhou SQ, Zhou JH, Deng T, Zeng F, Wang YK
and Liu XG: Expression and prognostic value of vacuole membrane
protein 1 in non-small-cell lung cancer. Zhonghua Yi Xue Za Zhi.
91:2828–2831. 2011.In Chinese.
|
|
20
|
Sauermann M, Sahin O, Sültmann H, Hahne F,
Blaszkiewicz S, Majety M, Zatloukal K, Füzesi L, Poustka A, Wiemann
S, et al: Reduced expression of vacuole membrane protein 1 affects
the invasion capacity of tumor cells. Oncogene. 27:1320–1326. 2008.
View Article : Google Scholar
|
|
21
|
Liu F, Niu Y, Liu N, Zhang W, Wang SL, Liu
H and Zhang TX: Expression of vacuole membrane protein 1 and its
prognostic value in invasive ductal carcinoma of the breast.
Zhonghua Bing Li Xue Za Zhi. 42:86–89. 2013.In Chinese. PubMed/NCBI
|
|
22
|
Okegawa T, Li Y, Pong RC and Hsieh JT:
Cell adhesion proteins as tumor suppressors. J Urol. 167:1836–1843.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lattanzi A, Salvagno C, Maderna C,
Benedicenti F, Morena F, Kulik W, Naldini L, Montini E, Martino S
and Gritti A: Therapeutic benefit of lentiviral-mediated neonatal
intracerebral gene therapy in a mouse model of globoid cell
leukodystrophy. Hum Mol Genet. 23:3250–3268. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Subramanya S, Kim SS, Manjunath N and
Shankar P: RNA interference-based therapeutics for human
immunodeficiency virus HIV-1 treatment: Synthetic siRNA or
vector-based shRNA? Expert Opin Biol Ther. 10:201–213. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ando-Akatsuka Y, Yonemura S, Itoh M,
Furuse M and Tsukita S: Differential behavior of E-cadherin and
occludin in their colocalization with ZO-1 during the establishment
of epithelial cell polarity. J Cell Physiol. 179:115–125. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Molejon MI, Ropolo A and Vaccaro MI: VMP1
is a new player in the regulation of the autophagy-specific
phosphatidylinositol 3-kinase complex activation. Autophagy.
9:933–935. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wodarz A and Näthke I: Cell polarity in
development and cancer. Nat Cell Biol. 9:1016–1024. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Acosta KB, Tibolla MM, Tiscornia MM,
Lorenzati MA and Zapata PD: Recent patents related to
phosphorylation signaling pathway on cancer. Recent Pat DNA Gene
Seq. 5:175–184. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Molejon MI, Ropolo A, Re AL, Boggio V and
Vaccaro MI: The VMP1-Beclin 1 interaction regulates autophagy
induction. Sci Rep. 3:10552013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huang XF and Chen JZ: Obesity, the
PI3K/Akt signal pathway and colon cancer. Obes Rev. 10:610–616.
2009. View Article : Google Scholar : PubMed/NCBI
|