Introduction
Gastric cancer (GC) is one of the most common and
fatal malignancies worldwide. According to Globocan 2012, GC is
considered the fifth most common malignancy after lung, breast,
colorectum and prostate malignancies (1). It is estimated that >70% of GC
cases occur in developing countries such as China, Japan and Korea
(1,2). Additionally, GC is associated with a
high mortality case and is the third leading cause of
cancer-related death; the fatality rate is ~75% of total GC cases
worldwide, representing an important public health burden (1,3).
Chronic inflammation-related carcinomas are closely
associated with chronic infections, chemical and physical factors,
and autoimmune and inflammatory responses of uncertain etiology
(4–8). The development, progression and
metastasis of GC are closely associated with chronic inflammation
associated with Helicobacter pylori infection, the host
immune system and other agents (9–12).
Inflammation was first linked to cancer by Rudolf Virchow in 1863
(4). Persistent inflammation can
induce the formation of cancer cells, and inflammatory cells,
cytokines and chemokines in the tumor microenvironment can
contribute to cancer growth, invasion and metastasis and to the
transformation to an immunosuppressive environment in malignant
disease (7,8,13).
Recently, CD4+ T helper (Th17) cells, a
new subtype of T cells and its associated inflammatory cytokines,
such as interleukin-17A (IL-17A), IL-23 and IL-1β, play critical
roles in the development and metastasis of various tumors including
GC, colorectal cancer (CRC), hepatocellular carcinoma (HCC) and
ovarian cancer (14–19). IL-17A production has been found not
only in Th17 cells but also in other immune cells, including mast
cells, IL-17A-secreting γδT (γδT17) cells, IL-17A-secreting
CD8+ T (Tc17) cells and NKT cells (20–24).
These immune cells have also been suggested to predominately
produce IL-17A in other tumor types or in the same tumor type in
different species (25–28). Mast cells but not T cells or
macrophages mainly secrete IL-17A in esophageal squamous cell
carcinoma tissues and in GC tissue (21,25).
Additionally, intracellular IL-17A is produced predominantly by
Tc17 cells in human HCC tissue but is mainly produced by Th17 cells
in the peripheral blood of human HCC patients (26,27).
Although γδT17 cells are the main cellular source of IL-17A in
human CRC, it has been suggested that IL-17A is mainly secreted by
Th17 cells in murine colon cancer models (16,28).
Similarly, mast cells are the main source of IL-17A in human GC
tissue (21); however, the source
of IL-17A-producing cells in peripheral blood from human GC
patients remains poorly defined. Recent studies have shown that
increased percentages of Th17 cells, Tc17 cells and their
associated cytokines (such as IL-17A, IL-23, IL-6 and IL-22)
promote GC development and progression (15,29,30).
However, the roles of γδT17 cells have not been elucidated in human
and mouse GC.
Here, we found that the frequencies of Th17 and
γδT17 cells were significantly higher in peripheral blood from
patients with GC than in peripheral blood from healthy donors
(HDs); however, Tc17 cell numbers were lower in patients with GC.
Moreover, Th17 cells were found to be the main source of
intracellular IL-17A-secreting cells, and the second most important
source was Tc17 cells; γδT17 cells represented a weak source of
these cells in peripheral blood obtained from patients with GC and
HDs. In addition, the expression levels of IL-17A and its
associated cytokines (such as IL-23 and IL-1β) in serum and the
relative mRNA levels in human peripheral blood mononuclear cells
(PBMCs) obtained from patients with GC were markedly higher than in
those obtained from HDs, and these IL-17A-producing T cells and
associated cytokines were significantly associated with partial
clinical pathological characteristics of the patients with GC.
Finally, we speculated that Th17 cells, Tc17 cells, γδT17 and
IL-17A-associated cytokines may be involved in the progression and
metastasis of GC in patients.
Materials and methods
Patients and specimens
The present study included 47 patients (32 males and
15 females) with GC who underwent surgical operations at the Second
Affiliated Hospital, Soochow University from March to October,
2014. The patients did not receive anticancer treatment (such as
chemotherapy and radiotherapy) before surgery. Patients with
autoimmune diseases or viral infections were excluded from the
study. The clinical characteristics of the studied 47 patients with
GC and 35 HDs are summarized in Table
I. Tumor stage was identified according to the International
Union Against Cancer TNM Classification for GC. Tumor tissues were
obtained from resected GCs and embedded in paraffin. Serum and
peripheral blood specimens were collected from these patients
before surgery and from HDs. In addition, written informed consent
was obtained from all individuals in accordance with the
Declaration of Helsinki (1964); the local Medical Ethics Committee
of the Second Affiliated Hospital of Soochow University approved
the study.
 | Table IClinical features of the patients
with GC and healthy donors. |
Table I
Clinical features of the patients
with GC and healthy donors.
| Features | GC patients | Healthy donors |
|---|
| Gender | | |
| Male | 32 | 21 |
| Female | 15 | 12 |
| Age (years) | | |
| Median | 59 | 57 |
| Range | 32–76 | 30–75 |
| Histologic
grade | | |
| Well/moderate | 29 | |
| Poor | 18 | |
| Tumor stage | | |
| T1 | 6 | |
| T2 | 24 | |
| T3 | 13 | |
| T4 | 4 | |
| Lymphoid nodal
status | | |
| N0 | 27 | |
| N1 | 13 | |
| N2 | 5 | |
| N3 | 2 | |
| Distant metastasis
status | | |
| M0 | 44 | |
| M1 | 3 | |
| TNM stage | | |
| I | 22 | |
| II | 6 | |
| III | 13 | |
| IV | 6 | |
Cell isolation and flow cytometric
analysis
Human PBMCs were isolated from the fresh peripheral
blood of all individuals using Ficoll-Hypaque density gradient
centrifugation and transferred to sterile tubes containing
RPMI-1640 medium supplemented with 10% fetal bovine serum, 2 mM
glutamine, 100 U/ml penicillin and 100 µg/ml streptomycin
(Invitrogen, Carlsbad, CA, USA). The PBMCs (1×106/cells)
were stimulated for 5 h with 50 ng/ml phorbol 12-myristate
13-acetate (PMA), 1 µg/ml ionomycin and 500 ng/ml monensin
(eBscience, San Diego, CA, USA) in 24-well plates. Subsequently,
the cells were harvested and washed twice in phosphate-buffered
saline (PBS). When analyzing Th17, Tc17 and γδT17 cells, the cells
were stained with phycoerythrin (PE)-conjugated anti-human CD3,
fluorescein isothiocyanate (FITC)-conjugated anti-human γδTCR,
allophycocyanin (APC)-conjugated anti-human CD8 and Pacific
Blue-conjugated anti-human CD4 antibodies at 4°C for 30 min. After
surface staining, the cells were incubated with
PerCP-Cy5.5-conjugated anti-human IL-17 antibody after fixation and
permeabilization according to the manufacturer's protocols.
Isotype-matched antibody controls were used in all procedures. The
stained cells were analyzed using a BD FACSVerse flow cytometer
(FCM) and FlowJo software, version 7.6.5 (TreeStar, San Carlos, CA,
USA). All antibodies were obtained from Biolegend (San Diego, CA,
USA).
Analysis of serum cytokines
Serum cytokines were tested in all individuals using
the Bio-Plex Pro Human Th17 Cytokine Panel kit (Bio-Rad
Laboratories, Shanghai, China). The cytokines comprised IL-1β,
IL-17A and IL-23. Multiplex cytokine analysis was performed
according to the manufacturer's protocols. Briefly, 50 µl of
diluted (1:1) beads that were coated with anti-human antibodies
raised against the tested cytokine antigen were mixed with 150
µl of each diluted sample (50 µl of serum, standards
or controls were diluted with 100 µl of dilution buffer) and
then incubated for 1 h at room temperature with vigorous shaking.
After washing the plate three times, 25 µl of biotinylated
detection antibodies was added and incubated for 30 min. After a
wash cycle, 50 µl streptavidin (SA)-PE was added to the
beads, and the mixture was incubated for 10 min. After washing the
plate three times, the bead mixtures were resuspended in 125
µl assay buffer and measured using a Bio-Plex 200 system
(Bio-Rad Laboratories, Inc., Munich, Germany). All samples were
detected in triplicate. The data were analyzed using BioPlex
Manager 6.1 software (Bio-Rad Laboratories, Inc.)
RNA isolation and quantitative real-time
PCR (qRT-PCR)
To analyze the mRNA expression of IL-1β, IL-17A and
IL-23, total RNA was extracted from human PBMCs using TRIzol
(Invitrogen) after stimulating the cells for 5 h with 50 ng/ml
phorbol 12-myristate 13-acetate (PMA) and 1 µg/ml ionomycin.
cDNA was synthesized using reverse transcription reagent kits
(Takara, Dalian, China) according to the manufacturer's
instructions. Real-time PCR was performed in triplicate using the
QuantiFast™ SYBR-Green PCR kit (Qiagen, Hilden, Germany) and an ABI
7500 analysis system (Applied Biosystems, Foster, CA, USA). The
amplification conditions were as follows: 5 min at 95°C
(denaturation); then, 40 cycles of 95°C for 10 sec and 60°C for 30
sec; the fluorescence was recorded at 60°C. The primer sequences
used were as follows: IL-1β forward, 5′-CCACAGACCTTCCAGGAGA ATG-3′
and IL-1β reverse, 5′-GTGCAGTTCAGTGATCGTA CAGG-3′; IL-17A forward,
5′-CGGACTGTGATGGTCAAC CTGA-3′ and IL-17A reverse,
5′-GCACTTTGCCTCCCAGA TCACA-3′; IL-23p19 forward,
5′-GAGCCTTCTCTGCTCCCTGATA-3′ and IL-23p19 reverse,
5′-GACTGAGGCTTGGAATCTGCTG-3′; β-actin forward,
5′-TGGAATCCTGTGGCATCCATGAAAC-3′ and β-actin reverse,
5′-TAAAACGCAGCTCAGTAACAGTCCG-3′. The data were analyzed using ABI
7500 software (Applied Biosystems).
Statistical analysis
One-way ANOVA analysis was performed to confirm the
statistical significance of differences among the groups. Data are
presented as the means ± standard deviation (SD). Between-group
comparisons were performed using Student's t-test. For
non-parametric data, the Mann-Whitney U test was performed between
the two studied groups. Correlations between variables were
determined using Spearman's correlation coefficient. P<0.05 was
considered to indicate a statistically significant difference. The
data were analyzed using GraphPad Prism 5 software (GraphPad
Software, Inc., San Diego, CA, USA).
Results
Clinical characteristics of the patients
with GC
The clinical characteristics of the patients with GC
are presented in Table I.
Forty-seven patients, including 32 males and 15 females, were
recruited in this study. The age of the patients ranged from 32 to
76 years. Twenty-nine patients presented with well/moderate
histology, whereas 18 presented with severe histology. Twenty-seven
patients had no lymphoid nodal metastasis, although 13 were
classified as N1, 5 were classified as N2 and 2 were classified as
N3. Moreover, most patients (93.6%, 44/47) had no distant
metastasis, but 3 were classified as M1. Additionally, there were
63.8% (30) cases of tumor stage
T1+T2 and 17 cases of stage T3+T4 according to the 7th edition of
the American Joint of Committee on Cancer. Of the cases with GC,
22, 6, 13 and 6 were classified as stages I, II, III and IV,
respectively according to the TNM classification for GC.
Frequency of circulating Th17, Tc17 and
γδT17 cells in peripheral blood from patients with GC
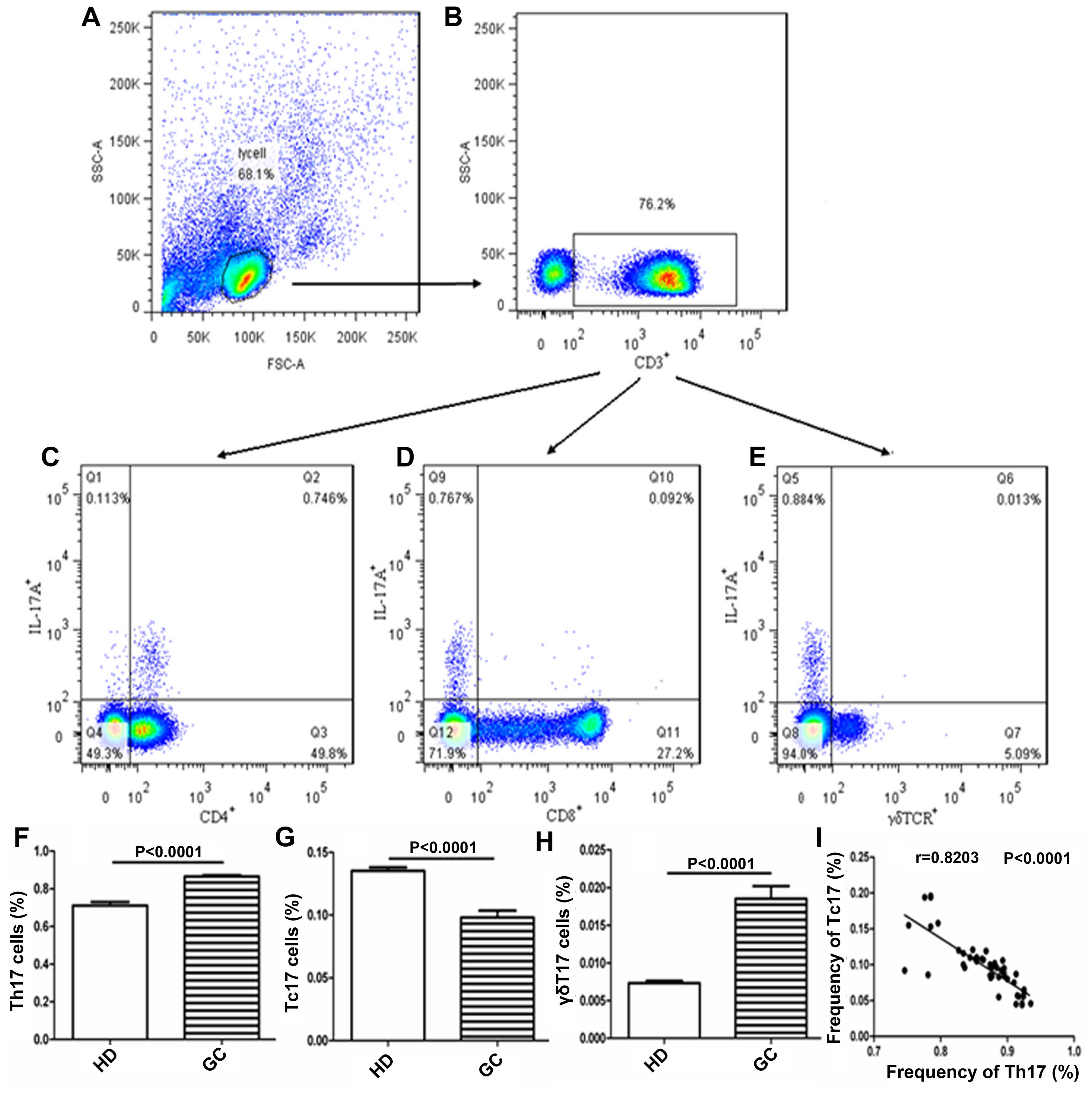
The frequencies of circulating Th17, Tc17 and γδT17
cells in gated CD3+ T cells in the PBMCs obtained from
patients with GC were detected to explore their roles in the
patients with GC (Fig. 1A–I). The
frequencies of circulating Th17 and γδT17 cells were significantly
higher in patients with GC than in the HDs (Fig. 1F and H), although the frequency of
circulating Tc17 cells was notably lower in patients with GC than
in the HDs (Fig. 1G). Additionally,
the frequency of Th17 cells was negatively associated with that of
Tc17 cells in the patients with GC (Fig. 1I). Further analysis indicated that
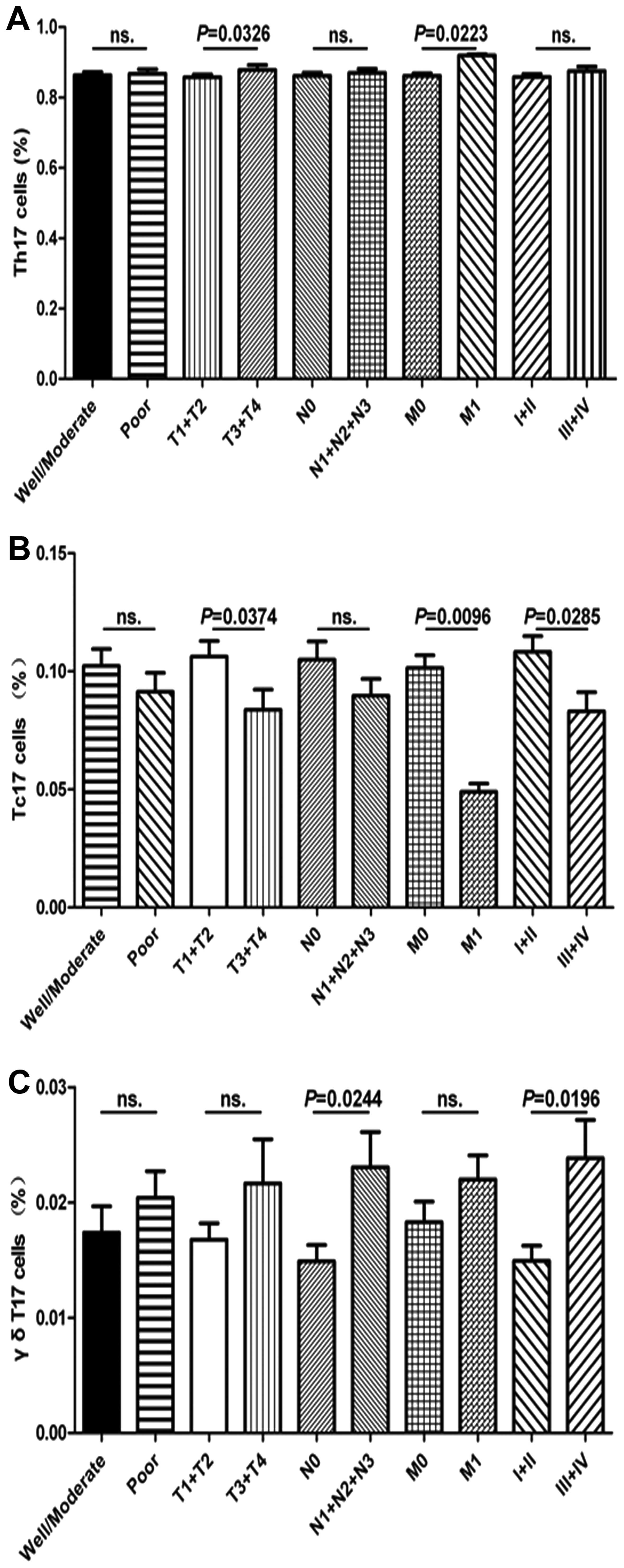
there was no significant difference in regards to the frequency of
Th17 cells in regards to clinicopathologic features such as tumor
differentiation (well/moderate vs. poor), TNM stage (I+II and
III+IV) and lymphoid nodal metastasis (N0 vs. N1+N2+N3); however, a
significant association with tumor invasion (T1+T2 vs. T3+T4) and
distant metastasis (M0 vs. M1) was observed in patients with GC
(Fig. 2A). The percentage of γδT17
cells in patients with GC with lymphoid nodal metastasis (N1+N2+N3)
was significantly higher than that in patients with GC with no
lymphoid nodal metastasis (N0), similar to TNM stage (I+II and
III+IV); however, no significant difference was found between other
clinical characteristics and the percentage of γδT17 cells,
respectively (Fig. 2C). The
percentage of Tc17 cells in patients with III+IV stage GC was
significantly lower than that in patients with I+II stage GC;
similar findings were observed for some clinical features, such as
distant metastasis (M0 vs. M1) and depth of tumor invasion (T1+T2
vs. T3+T4), but not for lymphoid nodal metastasis (N1+N2+N3) or
tumor differentiation (good/moderate vs. poor) (Fig. 2B), respectively. These findings
indicate that the patients with GC generally had higher populations
of circulating Th17 and γδT17 cells and a decreased percentage of
circulating Tc17 cells in peripheral blood, which were involved in
the progression of GC in the patients.
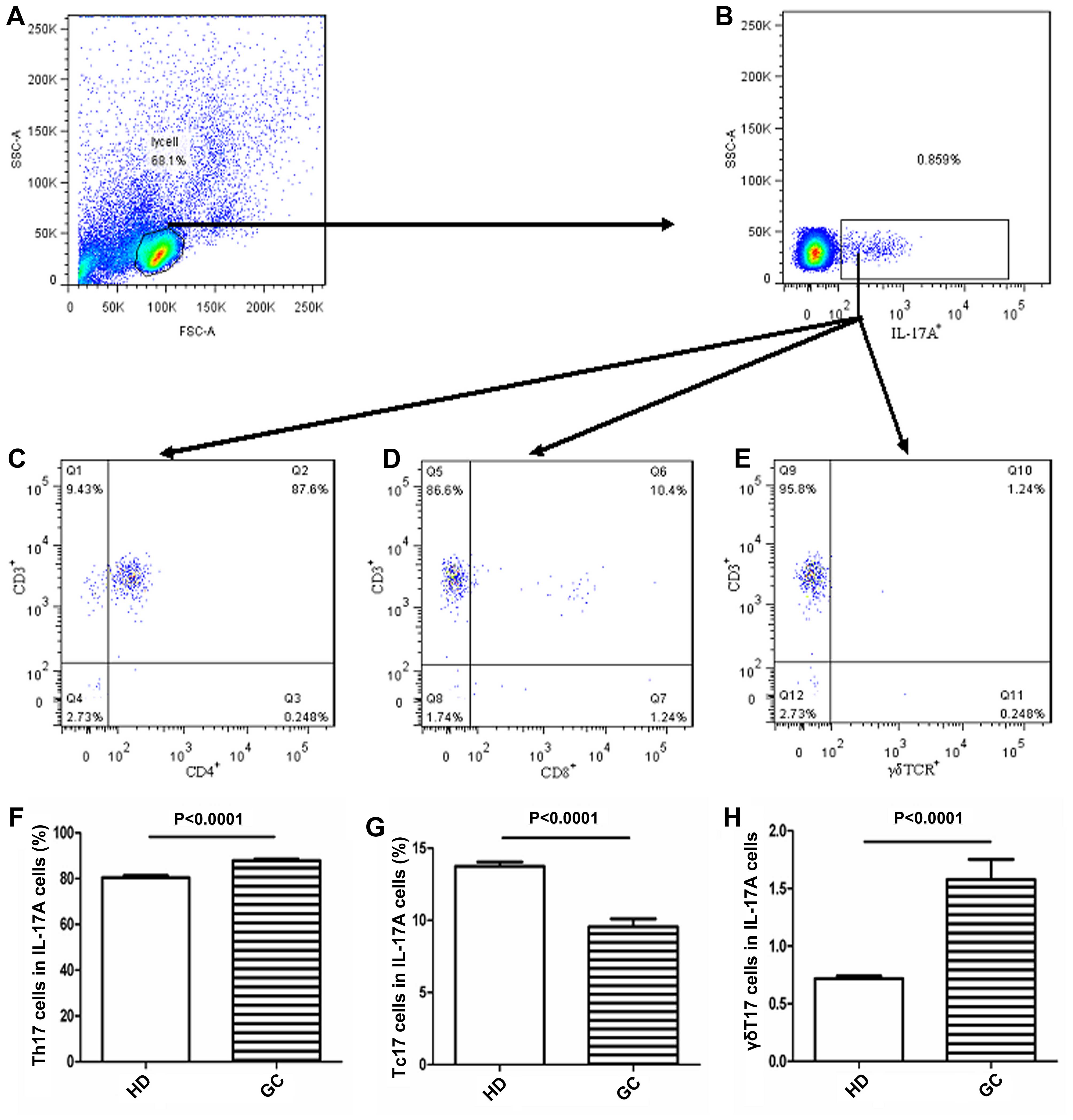
The principal source of intracellular
IL-17A cells in peripheral blood
Since circulating Th17, Tc17 and γδT17 cells play
important roles in patients with GC, we determined the principal
source of intracellular IL-17A cytokines in peripheral blood
obtained from the patients with GC by measuring the frequencies of
circulating Th17, Tc17 and γδT17 cells in gated intracellular
IL-17A cells obtained from patients with GC using FCM (Fig. 3A–E). The proportion of circulating
Th17 cells was highest in intracellular IL-17A cells of peripheral
blood cells from patients with GC or the HDs (Fig. 3C). The second-largest population of
intracellular IL-17A cells were circulating Tc17 cells (Fig. 3D) and the lowest population of
circulating Tc17 cells was γδT17 cells (Fig. 3E). Moreover, the percentages of
intracellular IL-17A cells represented by circulating Th17 and
γδT17 cells in peripheral blood were significantly higher in
patients with GC than in HDs (Fig. 3F
and H). In contrast, the percentage of intracellular IL-17A
cells represented by circulating Tc17 obtained from patients with
GC was obviously lower than that in the HDs (Fig. 3G). These data imply that circulating
Th17 cells represent the main source of intracellular IL-17A
cytokine in peripheral blood and that the percentages of
circulating Th17 and γδT17 cells were notably increased in patients
with GC than in the HDs.
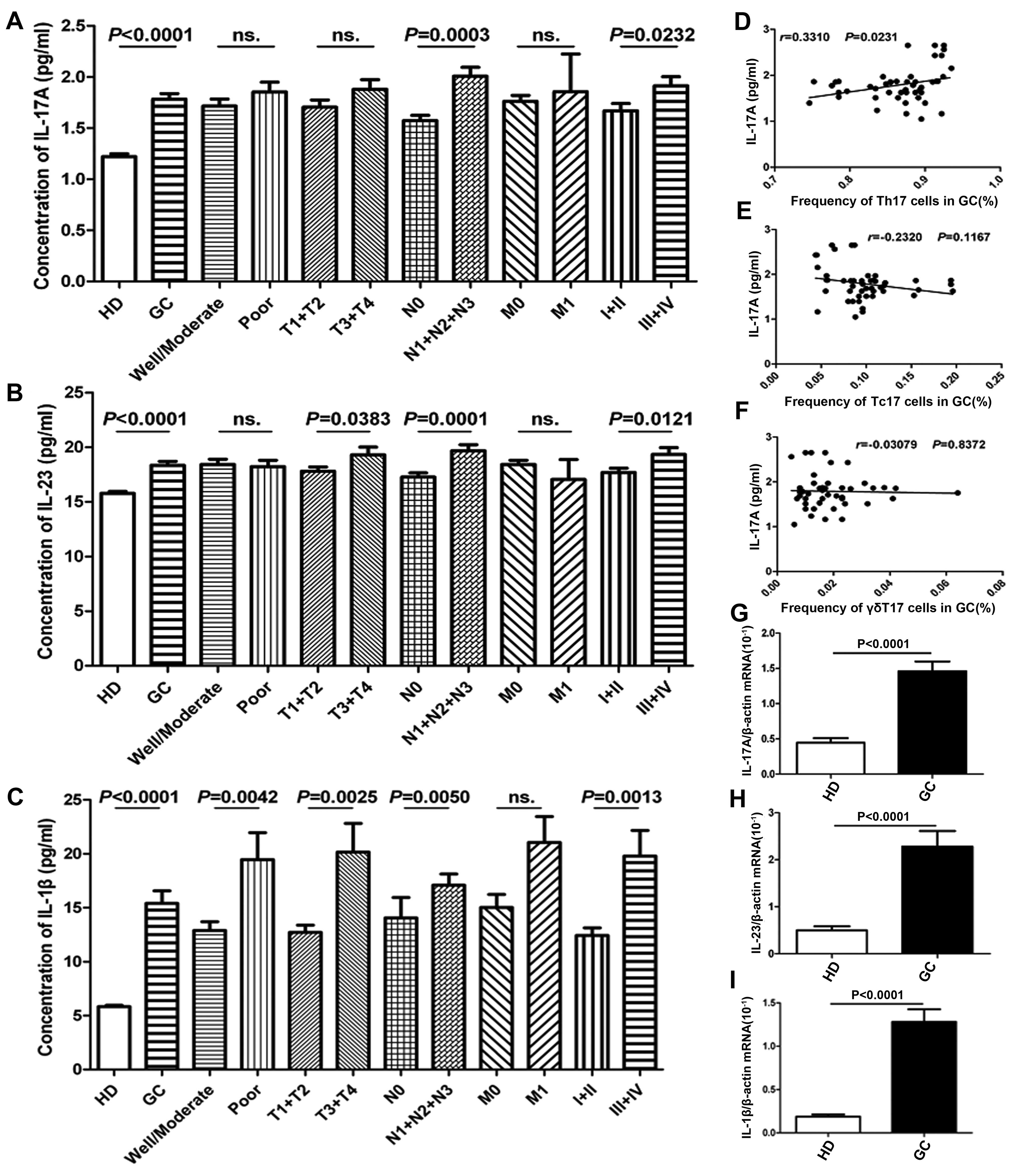
Expression of IL-17A and associated
cytokines in patients with GC
Since circulating Th17, Tc17 and γδT17 cells were
found to be an important source of intracellular IL-17A cytokines
in peripheral blood, we further detected the expression levels of
IL-17A and its associated cytokines (IL-23 and IL-1β) in serum and
PBMCs obtained from patients with GC and from HDs (Fig. 4A–G). The concentrations of the
cytokines IL-17A, IL-23 and IL-1β were significantly higher in sera
obtained from patients with GC than in sera obtained from the HDs
(Fig. 4A–C). Moreover, significant
associations were found between IL-17A levels and various clinical
pathological characteristics including lymphoid nodal metastasis
(N0 vs. N1+N2+N3) and TNM stage (I+II and III+IV), respectively.
However, no significant association between IL-17A concentration
and tumor histopathology (well/moderate vs. poor), depth of tumor
invasion (T1+T2 vs. T3+T4) or distant metastasis (M0 vs. M1) was
observed in the patients with GC (Fig.
4A). Similar findings regarding IL-23 and IL-1β concentrations
were observed in patients with GC (Fig.
4B and C). Additionally, serum IL-23 and IL-1β levels were
markedly different between patients with GC at stages T1+T2 and
T3+T4 and IL-1β levels were also significantly different between
good/moderate and poor tumor histopathology (Fig. 4B and C). Moreover, serum IL-17A
concentrations were markedly associated with Th17 cell frequency in
patients with GC but not with Tc17 and γδT17 cell frequencies
(Fig. 4D–F). In addition, the mRNA
expression levels of IL-1β, IL-17A and IL-23 were notably higher in
PBMCs in patients with GC than in those of the HDs (Fig. 4G–I).
Discussion
In the present study, we found that the frequencies
of circulating Th17 and γδT17 cells were significantly higher in
peripheral blood obtained from the patients with GC than in the
HDs; however, the percentage of Tc17 cells was lower. Moreover, the
expression levels of the associated cytokines, such as IL-17A,
IL-23 and IL-1β in serum and PBMCs obtained from patients with GC
were also significantly higher than those of the HDs, and Th17,
Tc17 and γδT17 cells and cytokines were partly associated with
clinicopathologic characteristics such as cancer invasion,
metastasis and TNM stage. Furthermore, the main source of
intracellular IL-17A cytokine was Th17 cells, the second-most
important source was Tc17 cells and γδT17 cells also acted as a
source of intracellular IL-17A cytokine in peripheral blood
obtained from patients with GC. Taken together, these findings
suggest that Th17, Tc17 and γδT17 cells might play crucial roles in
human GC development and metastasis.
Th17 cells play pivotal roles in inflammation and in
autoimmune diseases of humans and mice, thus exacerbating the
severity of diseases; they also act in host defense against
pathogens (31–33). Recently, accumulating evidence has
suggested that Th17 cells exhibit complex and controversial roles
in the development, progression and metastasis of tumors (30,34,35).
An increased prevalence of Th17 cells in peripheral blood, tumor
tissue or tumor-draining lymph nodes contributes to the
development, progression and/or metastasis of human cancers, such
as gastric carcinoma, CRC, HCC and pancreatic cancer (16,26,30).
In contrast, a high frequency of tumor-infiltrating Th17 cells is
associated with improved survival in ovarian and lung carcinoma
patients and with slower progression in patients with prostate
cancer (34–36). Similarly, increased numbers of Th17
cells in the tumor environment improve survival in mouse models of
pancreatic and melanoma cancer (37,38).
Although the frequency of Th17 cells was obviously higher in
peripheral blood obtained from patients with GC, it was not
directly confirmed that these cells were the main source of IL-17
in human peripheral blood.
Our results demonstrated that the frequency of
circulating Th17 cells was significantly higher in PBMCs obtained
from patients with GC than in that obtained from HDs, consistent
with previous studies (15,21,30).
Moreover, it was clear that intracellular IL-17A cytokines were
mainly produced by Th17 cells obtained from the PBMCs of patients
with GC and HDs. Additionally, the high percentage of circulating
Th17 cells was individually associated with depth of tumor invasion
(T1+T2 vs. T3+T4) and distant metastasis (M0 vs. M1) but not with
other factors such as TNM stage (I+II and III+IV) and lymphoid
nodal metastasis (N0 vs. N1+N2+N3). These findings suggest that
Th17 cells partly participate in the progression and metastasis of
human GC, a finding that is partially consistent with previous
reports (15,30). The discrepancies found might be due
to the numbers of recruited patients, age, lifestyle and other
causative agents, such as infections, chemical and physical factors
and other factors of uncertain etiology.
Similar to Th17 cells, Tc17 cells that are defined
by a signature of IL-17A-secreting CD8+ T cells play a
critical role in various diseases, such as those related to
infection and autoimmunity (39–42).
For example, in rhesus macaques with pathogenic simian
immunodeficiency virus (SIV) infection, Tc17 cells regulate the
disease progression and contribute to the disease outcome (39). Additionally, Tc17 cells can protect
mice against lethal influenza infection, which is accompanied by an
early enhanced influx of neutrophils into the lung (40). Conversely, Tc17 cells, but not Th17
cells, notably caused severe autoimmune colitis in a mouse model
(41). In an animal model of
multiple sclerosis (MS), the numbers of
IL-17+CD8+ T (Tc17) cells were found to be
significantly higher in active regions of MS lesions than in
inactive areas and controls, suggesting that Tc17 cells are
associated with active disease in MS (42). However, the clinical prevalence and
role of Tc17 cells remain elusive in human and mouse tumors
(43).
Recent studies indicate that Tc17 cells have
important roles in the development and metastasis of tumors
(44–48). Elevated proportions of Tc17 cells in
patients with cervical cancers are associated with cancer
progression that is accompanied by increased infiltrations of Th17
cells as well as enhanced tumor vasculogenesis and metastasis
(44). Similar results were also
observed in patients with endometrial carcinoma (45). In contrast, adoptive transfer of
Tc17 cells was found to mediate effective antitumor immunity
against an established murine melanoma model, and this depended on
the effects of IFN-γ in the tumor environment (46,47).
A high proportion of Th17 and a low proportion of
Tc17 cells are observed in peripheral blood obtained from patients
with thyroid tumors, and these proportions are negatively
correlated with tumor size, suggesting that Tc17 cells might have
an antitumor role in thyroid tumors and that its effective function
may not be involved with IL-17 production (48). Recently, Tc17 cell numbers were
found to be elevated in peripheral CD8+ T cells and in
tumor tissues obtained from patients with GC (29). However, increased Tc17 cell
frequency was not directly shown in PBMCs obtained from patients
with GC, and it was unclear whether Tc17 cells were the main source
of intracellular IL-17A in patients with GC and in HDs.
Here, a significantly decreased frequency of Tc17
cells was observed in patients with GC and was notably associated
with depth of tumor invasion and TNM stage but not with other
factors, such as lymphoid nodal metastasis and tumor
differentiation. Moreover, Tc17 cells were the second most
important source of intracellular IL17A cytokine in the patients
with GC and in HDs. Interestingly, a negative association was found
between the frequency of Th17 and Tc17 cells in patients with GC,
indicating that increased percentage of Th17 cells might negatively
regulate Tc17 cells in patients with GC and that Tc17 cells might
exert antitumor activity in patients with GC; however, this notion
requires further investigation by studying the role of Tc17 cells
in human GC. Taken together, these findings indicate that a low
percentage of Tc17 cells might affect the development and
progression of GC. The different age, population and causative
factors in our patients with GC, together with differences in the
study aim, might account for the discrepancies between our results
and those described in a previous study (29).
γδT17 cells, as innate cells that secrete the IL-17
cytokine, are involved in the pathogenesis of various
inflammation-associated diseases in humans and mice (49,50).
γδT17 cells play an effective and pivotal role in psoriasiform
plaque formation in mice and are the primary source of IL-17A
cytokine but not Th17 cells (51).
Additionally, a high frequency of γδT17 cells is found in the
brains of mice with experimental autoimmune encephalomyelitis
(EAE), and the activation of γδT17 cells can promote the expansion
of Th17 cells and increase susceptibility to EAE (50). Elevated γδT17 cells exacerbate
arthritis and are the main source of IL-17 cytokine in mice with
collagen-induced arthritis (CIA) (52). However, the role of γδT17 cells is
poorly understood in tumors.
Tumor-infiltrating IL-17 promotes the progression of
skin carcinoma in mice by inducing angiogenesis and is mainly
produced by γδT17 cells in T cell tumor-infiltrating lymphocytes
(TILs) but not in Th17 or Tc17 cells (53). Additionally, the frequencies of
Th17, Tc17 and γδT17 cells are all clearly elevated in human PBMCs
and in the tumor tissues of patients with CRC (16). Moreover, γδT17 cells are the main
producers of IL-17A and promote the progression of human CRC
(16). Dissimilarly, Th17 cells,
not γδT17 cells, are considered the main source of IL-17 and were
found to improve the progression of a murine colon cancer model
(28). Although the frequency of
Th17 and/or Tc17 cells was obviously elevated in patients with GC
(15,16,30),
it has not been clearly proven that γδT17 cells are the main source
of IL-17, and the role of γδT17 cells has not yet been elucidated
in human GC.
Our results demonstrated that a significantly
increased frequency of γδT17 cells was observed in patients with
GC, and this increase was notably associated with lymphoid nodal
metastasis and TNM stage but not with other factors, such as depth
of tumor invasion, distant metastasis and tumor differentiation. In
addition, γδT17 cells were not an abundant source of intracellular
IL-17A cytokine; rather, they were a poorer source than Th17 and
Tc17 cells in patients with GC and in HDs. Taken together, these
findings indicate that an increased percentage of γδT17 cells might
contribute to the development and progression of GC. Moreover,
human GC represents a possible cause for the low production of
intracellular IL-17A from γδT17 cells, implying that various tumors
might affect the phenotype and distribution of IL-17A-producing T
cells differently and act as the main source of intracellular
IL-17A cytokine. We will explore the role of γδT17 cells in human
GC in future studies.
IL-23 and/or IL-1β cytokines, which are secreted via
innate immune cells, such as dendritic cells and macrophages, can
contribute to the proliferation and differentiation of Th17, Tc17
or γδT17 cells, which promote the production of the IL-17 cytokine
(54–56). Increased IL-17, IL-23 and/or IL-1β
cytokines, which are accompanied by elevated percentages of Th17,
Tc17 or γδT17 cells, play important roles in
inflammation-associated diseases, including those related to
infection, autoimmunity and tumors (14,49,50,54).
Recently, serum concentrations and mRNA expression levels of
IL-17A, IL-23 and/or IL-1β, which are associated with Th17 and Tc17
cells, have been found to be significantly increased in patients
with GC compared to those in healthy individuals and are closely
associated with the development of gastric carcinoma (15,21,29,30).
IL-23 and IL-1β cytokines, which are produced by tumor-activated
monocytes, promote the differentiation of Tc17 cells that
contribute to cancer progression in patients with GC (29).
Our results showed that serum IL-17, IL-23 and IL-1β
levels were obviously increased in patients with GC, and this
increase was associated with stages of GC development, such as
lymphoid nodal metastasis and the TNM stage and was partly
associated with depth of tumor invasion and distant metastasis; in
addition, Th17 cell percentage was positively associated with serum
IL-17A concentrations in patients with GC. Additionally, the
relative mRNA expression of these cytokines was significantly
higher in patients with GC than in HDs, consistent with previous
studies (15,21,30).
These findings indicate that these increases in the inflammatory
cytokines IL-17, IL-23 and IL-1β contribute to the progression and
metastasis of human GC and to the proliferation of inflammatory
cells, such as Th17 cells. These inflammatory cytokines and
associated inflammation-related cells play important roles in the
development, progression and metastasis of human GC.
In summary, our results revealed that the
frequencies of circulating Th17 and γδT17 cells were significantly
higher in patients with GC than in HDs; however, the frequency of
Tc17 cells, which were involved in the progression and metastasis
of patients with GC, was decreased. Moreover, the intracellular
IL-17A cytokine was predominantly produced by Th17 cells in human
peripheral blood. Additionally, the expression levels of Th17
cell-associated cytokines, such as IL-17A, IL-23 and IL-1β, were
significantly elevated in the serum and PBMCs of patients with GC
and played important roles in the progression and metastasis in the
patients with GC. In future studies, a large number of patients
with GC will be studied to determine the roles of Th17, Tc17 and
γδT17 cells in the pathogenesis of GC.
Acknowledgments
This study was supported by the Scientific Research
Innovation Projects for Ordinary University Graduate Students,
Jiangsu, China (grant no. CXLX12_0842), the Pre-Research Fund of
Soochow University (grant no. SDY2012B28) and the Pre-Research Fund
of the Second Affiliated Hospital of Soochow University (grant no.
SDFEYGJ1301). The manuscript had been edited for English language
by American Journal Experts (http://www.aje.com).
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar
|
|
2
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar
|
|
3
|
McLean MH and El-Omar EM: Genetics of
gastric cancer. Nat Rev Gastroenterol Hepatol. 11:664–674. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Balkwill F and Mantovani A: Inflammation
and cancer: Back to Virchow? Lancet. 357:539–545. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Okada F: Inflammation-related
carcinogenesis: Current findings in epidemiological trends, causes
and mechanisms. Yonago Acta Med. 57:65–72. 2014.PubMed/NCBI
|
|
6
|
De Flora S and Bonanni P: The prevention
of infection-associated cancers. Carcinogenesis. 32:787–795. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Trinchieri G: Cancer and inflammation: An
old intuition with rapidly evolving new concepts. Annu Rev Immunol.
30:677–706. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Grivennikov SI, Greten FR and Karin M:
Immunity, inflammation and cancer. Cell. 140:883–899. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Correa P: Helicobacter pylori and gastric
carcinogenesis. Am J Surg Pathol. 19(Suppl 1): S37–S43.
1995.PubMed/NCBI
|
|
10
|
Yakirevich E and Resnick MB: Pathology of
gastric cancer and its precursor lesions. Gastroenterol Clin North
Am. 42:261–284. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Piazuelo MB and Correa P: Gastric cáncer:
Overview. Colomb Med (Cali). 44:192–201. 2013.
|
|
12
|
Grabsch HI and Tan P: Gastric cancer
pathology and underlying molecular mechanisms. Dig Surg.
30:150–158. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang B, Kang H, Fung A, Zhao H, Wang T and
Ma D: The role of interleukin 17 in tumour proliferation,
angiogenesis, and metastasis. Mediators Inflamm. 2014:6237592014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang B, Rong G, Wei H, Zhang M, Bi J, Ma
L, Xue X, Wei G, Liu X and Fang G: The prevalence of Th17 cells in
patients with gastric cancer. Biochem Biophys Res Commun.
374:533–537. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu P, Wu D, Ni C, Ye J, Chen W, Hu G, Wang
Z, Wang C, Zhang Z, Xia W, et al: γδT17 cells promote the
accumulation and expansion of myeloid-derived suppressor cells in
human colorectal cancer. Immunity. 40:785–800. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
He D, Li H, Yusuf N, Elmets CA, Li J,
Mountz JD and Xu H: IL-17 promotes tumor development through the
induction of tumor promoting microenvironments at tumor sites and
myeloid-derived suppressor cells. J Immunol. 184:2281–2288. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Miyahara Y, Odunsi K, Chen W, Peng G,
Matsuzaki J and Wang RF: Generation and regulation of human
CD4+ IL-17-producing T cells in ovarian cancer. Proc
Natl Acad Sci USA. 105:15505–15510. 2008. View Article : Google Scholar
|
|
19
|
Langowski JL, Zhang X, Wu L, Mattson JD,
Chen T, Smith K, Basham B, McClanahan T, Kastelein RA and Oft M:
IL-23 promotes tumour incidence and growth. Nature. 442:461–465.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Alizadeh D, Katsanis E and Larmonier N:
The multifaceted role of Th17 lymphocytes and their associated
cytokines in cancer. Clin Dev Immunol. 2013:9578782013. View Article : Google Scholar
|
|
21
|
Liu X, Jin H, Zhang G, Lin X, Chen C, Sun
J, Zhang Y, Zhang Q and Yu J: Intratumor IL-17-positive mast cells
are the major source of the IL-17 that is predictive of survival in
gastric cancer patients. PLoS One. 9:e1068342014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lockhart E, Green AM and Flynn JL: IL-17
production is dominated by gammadelta T cells rather than CD4 T
cells during Mycobacterium tuberculosis infection. J Immunol.
177:4662–4669. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu SJ, Tsai JP, Shen CR, Sher YP, Hsieh
CL, Yeh YC, Chou AH, Chang SR, Hsiao KN, Yu FW, et al: Induction of
a distinct CD8 Tnc17 subset by transforming growth factor-beta and
interleukin-6. J Leukoc Biol. 82:354–360. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yoshiga Y, Goto D, Segawa S, Ohnishi Y,
Matsumoto I, Ito S, Tsutsumi A, Taniguchi M and Sumida T: Invariant
NKT cells produce IL-17 through IL-23-dependent and -independent
pathways with potential modulation of Th17 response in
collagen-induced arthritis. Int J Mol Med. 22:369–374.
2008.PubMed/NCBI
|
|
25
|
Wang B, Li L, Liao Y, Li J, Yu X, Zhang Y,
Xu J, Rao H, Chen S, Zhang L, et al: Mast cells expressing
interleukin 17 in the muscularis propria predict a favorable
prognosis in esophageal squamous cell carcinoma. Cancer Immunol
Immunother. 62:1575–1585. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang JP, Yan J, Xu J, Pang XH, Chen MS,
Li L, Wu C, Li SP and Zheng L: Increased intratumoral
IL-17-producing cells correlate with poor survival in
hepatocellular carcinoma patients. J Hepatol. 50:980–989. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kuang DM, Peng C, Zhao Q, Wu Y, Zhu LY,
Wang J, Yin XY, Li L and Zheng L: Tumor-activated monocytes promote
expansion of IL-17-producing CD8+ T cells in
hepatocellular carcinoma patients. J Immunol. 185:1544–1549. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Grivennikov SI, Wang K, Mucida D, Stewart
CA, Schnabl B, Jauch D, Taniguchi K, Yu GY, Osterreicher CH, Hung
KE, et al: Adenoma-linked barrier defects and microbial products
drive IL-23/IL-17-mediated tumour growth. Nature. 491:254–258.
2012.PubMed/NCBI
|
|
29
|
Zhuang Y, Peng LS, Zhao YL, Shi Y, Mao XH,
Chen W, Pang KC, Liu XF, Liu T, Zhang JY, et al: CD8(+) T cells
that produce interleukin-17 regulate myeloid-derived suppressor
cells and are associated with survival time of patients with
gastric cancer. Gastroenterology. 143:951–962. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Iida T, Iwahashi M, Katsuda M, Ishida K,
Nakamori M, Nakamura M, Naka T, Ojima T, Ueda K, Hayata K, et al:
Tumor-infiltrating CD4+ Th17 cells produce IL-17 in
tumor microenvironment and promote tumor progression in human
gastric cancer. Oncol Rep. 25:1271–1277. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fouser LA, Wright JF, Dunussi-Joannopoulos
K and Collins M: Th17 cytokines and their emerging roles in
inflammation and autoimmunity. Immunol Rev. 226:87–102. 2008.
View Article : Google Scholar
|
|
32
|
Wilke CM, Bishop K, Fox D and Zou W:
Deciphering the role of Th17 cells in human disease. Trends
Immunol. 32:603–611. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Muranski P and Restifo NP: Essentials of
Th17 cell commitment and plasticity. Blood. 121:2402–2414. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kryczek I, Banerjee M, Cheng P, Vatan L,
Szeliga W, Wei S, Huang E, Finlayson E, Simeone D, Welling TH, et
al: Phenotype, distribution, generation, and functional and
clinical relevance of Th17 cells in the human tumor environments.
Blood. 114:1141–1149. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sfanos KS, Bruno TC, Maris CH, Xu L,
Thoburn CJ, DeMarzo AM, Meeker AK, Isaacs WB and Drake CG:
Phenotypic analysis of prostate-infiltrating lymphocytes reveals
TH17 and Treg skewing. Clin Cancer Res. 14:3254–3261. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ye ZJ, Zhou Q, Gu YY, Qin SM, Ma WL, Xin
JB, Tao XN and Shi HZ: Generation and differentiation of
IL-17-producing CD4+ T cells in malignant pleural
effusion. J Immunol. 185:6348–6354. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gnerlich JL, Mitchem JB, Weir JS, Sankpal
NV, Kashiwagi H, Belt BA, Porembka MR, Herndon JM, Eberlein TJ,
Goedegebuure P, et al: Induction of Th17 cells in the tumor
microenvironment improves survival in a murine model of pancreatic
cancer. J Immunol. 185:4063–4071. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Muranski P, Boni A, Antony PA, Cassard L,
Irvine KR, Kaiser A, Paulos CM, Palmer DC, Touloukian CE, Ptak K,
et al: Tumor-specific Th17-polarized cells eradicate large
established melanoma. Blood. 112:362–373. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nigam P, Kwa S, Velu V and Amara RR: Loss
of IL-17-producing CD8 T cells during late chronic stage of
pathogenic simian immunodeficiency virus infection. J Immunol.
186:745–753. 2011. View Article : Google Scholar
|
|
40
|
Hamada H, Garcia-Hernandez ML, Reome JB,
Misra SK, Strutt TM, McKinstry KK, Cooper AM, Swain SL and Dutton
RW: Tc17, a unique subset of CD8 T cells that can protect against
lethal influenza challenge. J Immunol. 182:3469–3481. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tajima M, Wakita D, Noguchi D, Chamoto K,
Yue Z, Fugo K, Ishigame H, Iwakura Y, Kitamura H and Nishimura T:
IL-6-dependent spontaneous proliferation is required for the
induction of colitogenic IL-17-producing CD8+ T cells. J
Exp Med. 205:1019–1027. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tzartos JS, Friese MA, Craner MJ, Palace
J, Newcombe J, Esiri MM and Fugger L: Interleukin-17 production in
central nervous system-infiltrating T cells and glial cells is
associated with active disease in multiple sclerosis. Am J Pathol.
172:146–155. 2008. View Article : Google Scholar :
|
|
43
|
Hinrichs CS, Kaiser A, Paulos CM, Cassard
L, Sanchez-Perez L, Heemskerk B, Wrzesinski C, Borman ZA, Muranski
P and Restifo NP: Type 17 CD8+ T cells display enhanced
antitumor immunity. Blood. 114:596–599. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang Y, Hou F, Liu X, Ma D, Zhang Y, Kong
B and Cui B: Tc17 cells in patients with uterine cervical cancer.
PLoS One. 9:e868122014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang W, Hou F, Zhang Y, Tian Y, Jiao J,
Ma D, Kong B and Cui B: Changes of Th17/Tc17 and Th17/Treg cells in
endometrial carcinoma. Gynecol Oncol. 132:599–605. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yu Y, Cho HI, Wang D, Kaosaard K, Anasetti
C, Celis E and Yu XZ: Adoptive transfer of Tc1 or Tc17 cells
elicits antitumor immunity against established melanoma through
distinct mechanisms. J Immunol. 190:1873–1881. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Garcia-Hernandez ML, Hamada H, Reome JB,
Misra SK, Tighe MP and Dutton RW: Adoptive transfer of
tumor-specific Tc17 effector T cells controls the growth of B16
melanoma in mice. J Immunol. 184:4215–4227. 2010. View Article : Google Scholar
|
|
48
|
Jiang G, Ma S, Wei Y, Wu Y, Yu X and Liu
H: The prevalence and distribution of Th17 and Tc17 cells in
patients with thyroid tumor. Immunol Lett. 162:68–73. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Petermann F, Rothhammer V, Claussen MC,
Haas JD, Blanco LR, Heink S, Prinz I, Hemmer B, Kuchroo VK, Oukka
M, et al: γδ T cells enhance autoimmunity by restraining regulatory
T cell responses via an interleukin-23-dependent mechanism.
Immunity. 33:351–363. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Sutton CE, Lalor SJ, Sweeney CM, Brereton
CF, Lavelle EC and Mills KH: Interleukin-1 and IL-23 induce innate
IL-17 production from gammadelta T cells, amplifying Th17 responses
and autoimmunity. Immunity. 31:331–341. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Cai Y, Shen X, Ding C, Qi C, Li K, Li X,
Jala VR, Zhang HG, Wang T, Zheng J, et al: Pivotal role of dermal
IL-17-producing γδ T cells in skin inflammation. Immunity.
35:596–610. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ito Y, Usui T, Kobayashi S,
Iguchi-Hashimoto M, Ito H, Yoshitomi H, Nakamura T, Shimizu M,
Kawabata D, Yukawa N, et al: Gamma/delta T cells are the
predominant source of interleukin-17 in affected joints in
collagen-induced arthritis, but not in rheumatoid arthritis.
Arthritis Rheum. 60:2294–2303. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wakita D, Sumida K, Iwakura Y, Nishikawa
H, Ohkuri T, Chamoto K, Kitamura H and Nishimura T:
Tumor-infiltrating IL-17-producing gammadelta T cells support the
progression of tumor by promoting angiogenesis. Eur J Immunol.
40:1927–1937. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Miossec P: IL-17 and Th17 cells in human
inflammatory diseases. Microbes Infect. 11:625–630. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Tesmer LA, Lundy SK, Sarkar S and Fox DA:
Th17 cells in human disease. Immunol Rev. 223:87–113. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Korn T, Bettelli E, Oukka M and Kuchroo
VK: IL-17 and Th17 cells. Annu Rev Immunol. 27:485–517. 2009.
View Article : Google Scholar : PubMed/NCBI
|