Introduction
Esophageal cancer is a common human gastrointestinal
cancer, accounting for 400,000 mortalities and 480,000 new cases in
2008 worldwide (1). Despite
advances in early detection and standardized treatment regimens,
relatively low 5-year survival rates for esophageal cancer have
been observed (2,3). In Eastern Asia and Africa, the
incidence rates of esophageal cancer are extremely high (4). Countries in this region are developing
countries and their patients may not be able to afford advanced
treatments. Therefore, low-cost treatments, such as hyperthermia
have been considered.
In the past decade, anticancer trials with
multimodal therapies, including hyperthermia have been reported
(5,6). These clinical trials demonstrated that
hyperthermia increased overall survival by making normally
inoperable tumors candidates for surgery and by improving the
efficacy of chemo- or radiotherapy with reduced toxicity (7–9).
Hyperthermia is considered the fifth pillar of cancer treatment
following surgery, chemotherapy, radiotherapy and biotherapy.
However, the mechanisms by which hyperthermia improves clinical
outcomes remain unclear. Recent findings suggest that hyperthermia
impairs protein synthesis, leading to apoptosis (10).
Apoptosis is a conserved and regulated cell suicide
process, which is activated and executed by cysteine
aspartate-specific proteases (caspases) (11). Survivin, a protein that is often
overexpressed in cancer, suppresses apoptosis by inhibiting caspase
activation (12). Due to its
upregulation in almost all human tumors and its key role in
apoptosis, proliferation and angiogenesis, Survivin is considered a
crucial target for anticancer therapies (13). Downregulation of Survivin in human
cancer cell lines and mouse models inhibits tumor growth and
sensitizes tumor cells to chemo- or radiotherapy (14,15).
In the present study, we investigated the function
of Survivin in hyperthermia-induced apoptosis in EC109 esophageal
cancer cells and examined Survivin expression in patients with
esophageal cancer, correlating its expression with clinico
pathological characteristics. The results showed that, hyperthermia
decreased the expression of Survivin, prevented its binding to
XIAP, activated caspase-3 and induced apoptosis. In addition,
Survivin expression was higher in esophageal cancer than in normal
tissues, and this higher level of expression was associated with
poor prognosis. Therefore, Survivin may be a crucial target for
hyperthermia in the esophageal cancer treatment.
Materials and methods
Construction of plasmids and
transfection
Full-length Survivin and XIAP were generated by PCR
from EC109 cell cDNA using the primers: Survivin sense,
5′-AGATCTGGATCCGGTGCCCCGACGTTGCCCCC-3′ and antisense,
5′-TCTAGAGCGGCCGCTCAATCCATGGCAGCCAGCTGCTCG-3′; XIAP sense,
5′-AGATCTGGATCCACTTTTAACAGTTTTGAAGG-3′ and antisense,
5′-TCTAGAGCGGCCGCTTAAGACATAAAAATTTTTTGCTTG-3′. The resulting
fragments were digested by BamHI and NotI (Thermo
Scientific, Rockford, IL, USA) and cloned into pcDNA3.0-Flag. The
resulting or control plasmids were transfected into the EC109 cells
with Lipofectamine 2000 (Invitrogen Life Technologies, Carlsbad,
CA, USA). Western blotting was used to identify protein
overexpression in EC109 cells. The siRNAs for XIAP and the control
siRNA were designed and produced by Shanghai GenePharma Co., Ltd.
(Shanghai, China).
Cell culture and heat treatment
The human EC109 cells were obtained from the
American Type Culture Collection; Manassas, VA, USA). The cells
were maintained at 37°C and 5% CO2 in RPMI-1640 medium,
supplemented with 10% fetal bovine serum (FBS) (both from GE
Healthcare Life Sciences, Logan, UT, USA), 100 U/ml penicillin and
100 g/ml streptomycin (both from Sigma, St. Louis, MO, USA). For
heat treatment, EC109 cells were collected into 1.5 ml Eppendorf
tubes and then incubated in a hot water bath at 37°, 39°, 41°, 42°,
43° or 45°C for 40 min. Following heat treatment, the cells were
recovered under standard culture conditions.
MTT assay
After heat treatment, 5×103 EC109 cells
were plated in each well of 96-well plates. The following day, 20
µl of MTT (Sigma) was added to each well, and the cells were
incubated for an additional 4 h at 37°C, prior to the addition of
150 µl dimethylsulfoxide (DMSO). After 20 min, absorbance at
490 nm (A490) was measured with a microplate reader (Bio-Rad
Laboratories, Richmond, CA, USA). Cell viability (%) was calculated
as: the average A490 in an experimental chemotherapy group/the
average A490 in the blank control group × 100%.
Flow cytometry
The cells were incubated in a water bath at
different temperatures for 40 min, and then plated into 24-well
plates at a concentration of 1×105 cells/well. After 24
h, the cells were trypsinized and incubated with 5 µl
propidium iodide (PI) and 5 µl Annexin V-FITC (both from
Invitrogen Life Technologies) for 15 min. The samples were then
analyzed for apoptosis using a FACScan flow cytometer (BD
Biosciences, Franklin Lakes, NJ, USA).
Western blot analysis
Following heat treatment, EC109 cells were collected
into a lysis buffer [50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM
EDTA (pH 8.0), 1% Triton X-100 and protease inhibitor cocktail
(Roche Molecular Biochemicals, Indianapolis, IN, USA)], and
incubated for 30 min on ice. Protein (30 µg) for each sample
were separated on 12% SDS-PAGE gels (Invitrogen Life Technologies)
and transferred to PVDF membranes (EMD Millipore, Billerica, MA,
USA). After blocking with 5% BSA (Shanghai Bioleaf Biotech Co.,
Ltd., Shanghai, China) at room temperature for 1 h, the membranes
were incubated with primary antibody overnight at 4°C, incubated
with horseradish peroxidase-labeled goat anti-rabbit IgG antibody
(1:5,000; sc-45101; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA) for 1 h at room temperature and developed using ECL detection
(Thermo Scientific, Waltham, MA, USA). β-actin was used as a
loading control. For immunodetection, the primary antibodies used
were: anti-Survivin rabbit polyclonal IgG antibody (1:1,000;
sc-10811; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA)
anti-Smac rabbit monoclonal antibody (1:1,000; 1012-1), anti-XIAP
rabbit polyclonal antibody (1:2,000; S1022) (both from Epitomics,
Burlingame, CA, USA), anti-bcl-2, anti-bax, anti-bcl-xl (rabbit
polyclonal antibody, 1:1,000, sc-783, sc-493, sc-7195; Santa Cruz
Biotechnology, Inc.), and anti-pro-caspase-3 rabbit monoclonal,
anti-active caspase-3 rabbit polyclonal antibodies (1:1,000;
ab32499; ab2302), and anti-β-actin rabbit polyclonal antibody
(1:5,000; ab75186) (all from Abcam, Cambridge, UK).
Co-immunoprecipitation
The EC109 cells were transfected with Flag-Survivin,
Flag-XIAP or Flag control vector for 48 h, collected in lysis
buffer [50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM EDTA (pH 8.0),
1% Triton X-100 and protease inhibitor cocktail (Roche Molecular
Biochemicals, Indianapolis, IN, USA)], and then incubated for 30
min on ice. Protein (500 µg) from the cell lysates was
incubated with 20 µl Anti-FLAG M2 Affinity Gel (Sigma) for 2
h at 4°C. The collected beads were washed three times with lysis
buffer and then eluted with SDS-PAGE sample buffer. Immunoblotting
was detected using western blot analysis.
Clinical samples
Primary tumor specimens were obtained from 86
patients diagnosed with esophageal squamous cell carcinomas, who
underwent complete resection at the First Affiliated Hospital of
Xi'an Jiaotong University from February, 2009 to February, 2011.
Normal esophageal tissues were collected at >5 cm from the edge
of the tumor. The tissues were fixed in 10% formalin immediately
and embedded in paraffin within 24 h after surgical resection.
Follow-up information was obtained from a review of the patient
medical records. There were 64 males and 22 females and their mean
age was 59.8 years (42–77 years). The patients had a single tumor,
without distant metastasis and none of them had been previously
treated with chemo- or radiotherapy. After surgical resection, the
patients underwent standard therapeutic procedures according to the
Clinical Oncology Information Network guidelines. Carcinomas were
classified in accordance with the tumor-node-metastasis (TNM)
classification system of the International Union Against Cancer
(UICC) (16). The study design and
procedure were approved by the Ethics Committee of the hospital and
each participant signed an informed consent document prior to
enrollment.
Immunohistochemistry
Resected specimens were fixed with 10% formaldehyde
and embedded in paraffin blocks. Sections (5 µm) were
deparaffinized with xylene and rehydrated in a series of ethanol
concentrations. Endogenous peroxidase activity was blocked by
immersion in 0.3% methanolic peroxide for 15 min. Immunoreactivity
of the target antigens was enhanced by microwaving the sections for
10 min in 0.1 M citrate buffer, pH 6.0. Test sections were
incubated with anti-Survivin rabbit polyclonal IgG antibody
(sc-10811; Santa Cruz Biotechnology, Inc.) at a dilution of 1:100
in the blocking solution at 4°C overnight and goat anti-rabbit
IgG/HRP secondary antibody (bs-0295G-HRP; Beijing Biosynthesis
Inc., Beijing, China) was used at a dilution of 1:250 for 1 h at
room temperature. After washing, the signal was detected using a
DAB kit (Beijing Biosynthesis, Inc.). The section was
counterstained with hematoxylin and photographed under a light
microscope (Nikon, Tokyo, Japan). For the negative controls, the
anti-Survivin antibody was replaced with 1% bovine serum albumin
(Shanghai Bioleaf Biotech Co., Ltd.) in phosphate-buffered saline
(PBS). No staining was detected in any control section.
Immunohistochemical scoring
The stained slides were assessed by two independent
pathologists, without knowledge of the patient clinical data. Ten
randomly-selected fields for each slide were scored for the area
and intensity of positively stained (brown) cytoplasm and/or cell
membrane under light microscopy. The intensity of Survivin staining
was scored as: 0, no signal; 1, weak; 2, moderate; and 3, marked.
Percentage scores were assigned as: 1, 1–25%; 2, 26–50%; 3, 51–75%;
and 4, 76–100%. The scores of each tumor sample were multiplied to
give a final score of 0–12, and the tumors were designated as
negative expression (−), score 0–4; weak expression (+), score 5–8;
high expression (++), score 9–12. Tumor samples scored (+) to (++)
were considered positive.
Statistical analysis
The χ2 and Fisher's exact tests were used
to compare frequencies. The Kaplan-Meier method was used to assess
prognosis after surgery, and the log-rank test was used to compare
survival curves. Univariate analysis was performed with the Cox
regression model. Most pathological variables were used as
dichotomized variables: Age (<60 vs. ≥60 years), location
(upper+middle vs. lower thoracic), tumor differentiation
(high+middle vs. low), tumor size (<5 vs. ≥5 cm), lymph node
metastasis (N0 vs. N1+N2+N3) and stage (I+II vs. III). Statistical
analyses were performed using SPSS version 16.0 (SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant result.
Results
Hyperthermia induces apoptosis and
necrosis in EC109 cells
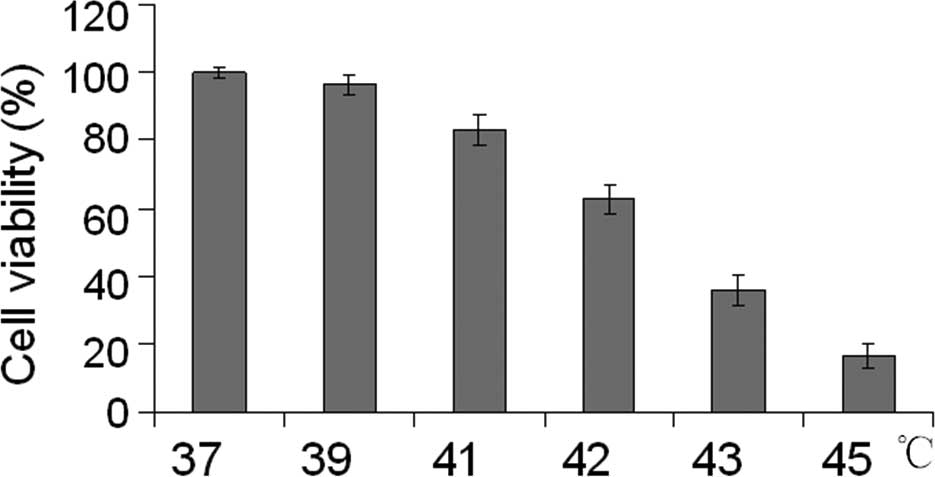
To analyze the effect of hyperthermia in esophageal
cancer cells, we first treated EC109 cells at different
temperatures (37°C, 39°C, 41°C, 42°C, 43°C and 45°C) for 40 min.
After the cells were incubated at 37°C in 5% CO2 for
another 24 h, cell viability was assessed using an MTT assay. With
higher temperatures, EC109 cells showed significantly decreased
viability (Fig. 1). To determine
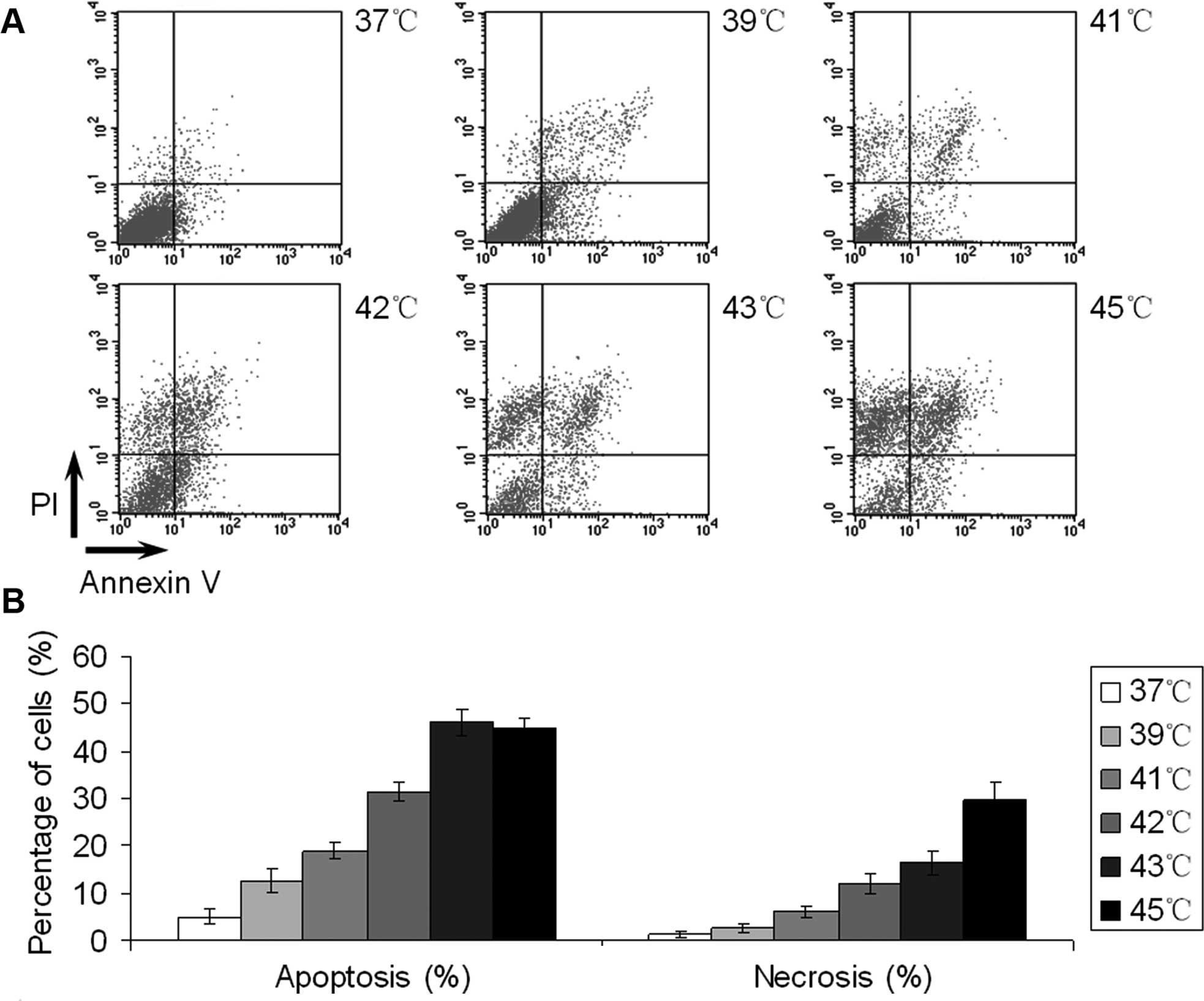
whether EC109 cells treated with hyperthermia underwent apoptosis,
we treated cells as before, and after 24 h the cells were stained
with Annexin V-FITC and PI to assess necrosis and apoptosis
induction by flow cytometry. As shown in Fig. 2A, necrosis was calculated as Annexin
V (−) and PI (+) while apoptosis was
indicated by Annexin V (+) and PI (±).
Necrotic EC109 cells increased with higher temperatures, whereas
apoptotic cells reached their peak at 43°C. The levels of apoptosis
were similar between the last two groups, but at 45°C the number of
necrotic EC109 cells was significantly higher than at 43°C
(Fig. 2B).
Hyperthermia inhibits Survivin and
activates caspase-3 in EC109 cells
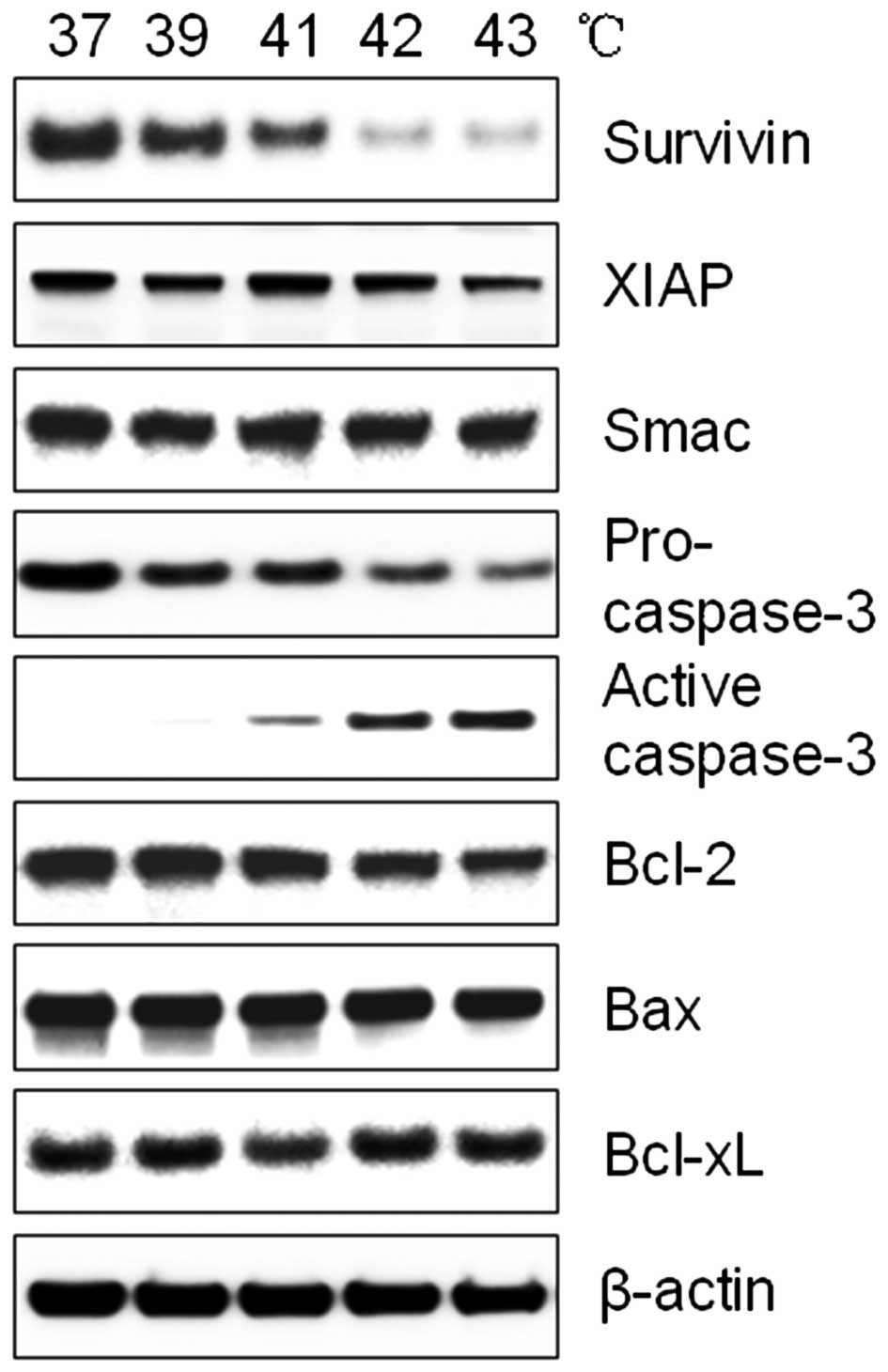
To investigate the increased induction of apoptosis
in EC109 cells following heat treatment, western blotting was
performed for components of the apoptotic pathway. Following
treatment with increased temperature, the expression of XIAP and
Smac did not change, whereas active caspase-3 and Survivin were
significantly altered. Hyperthermia reduced Bcl-2, while Bax and
Bcl-xL were unaffected. Notably, the activation of caspase-3
coincided with the decrease of Survivin expression (Fig. 3). Survivin is a member of the IAP
familiy, which inhibits caspase activation. It is possible that a
reduced Survivin expression leads to caspase-3 activation during
hyperthermia.
Overexpression of Survivin inhibits
hyperthermia-induced apoptosis through caspase-3 inactivation
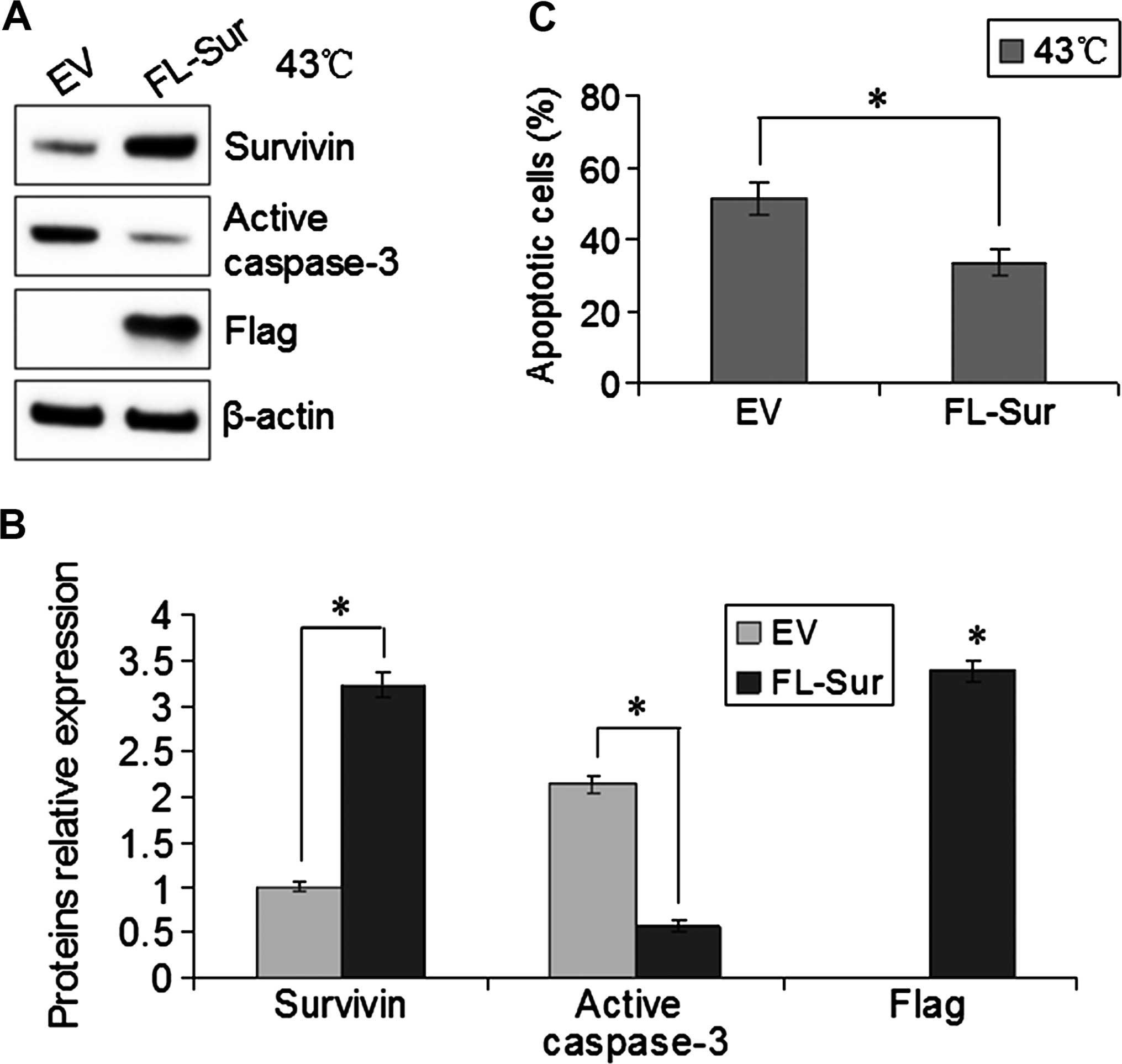
To investigate the function of Survivin in EC109
cells with heat treatment, we transfected pcDNA3.0-Flag-Survivin
(FL-Sur) or empty vector (EV) into EC109 cells for 48 h and then
incubated the cells at 43°C for 40 min. The following day, western
blotting confirmed that the expression of Survivin in the
Survivin-transfected cells was higher than that in the control
cells, and heat-induced active caspase-3 was inhibited by the
overexpression of Survivin (Fig. 4A and
B). Furthermore, Survivin-overexpressing and control cells were
stained with PI and Annexin V-FITC to assess apoptosis induction by
flow cytometry. At 43°C cultures, the number of apoptotic EC109
cells with a higher Survivin expression was significantly lower
than that in the control cells (P<0.05, Fig. 4C). These results indicated that the
overexpression of Survivin inhibited the activation of caspase-3,
resulting in decreased hyperthermia-induced apoptosis.
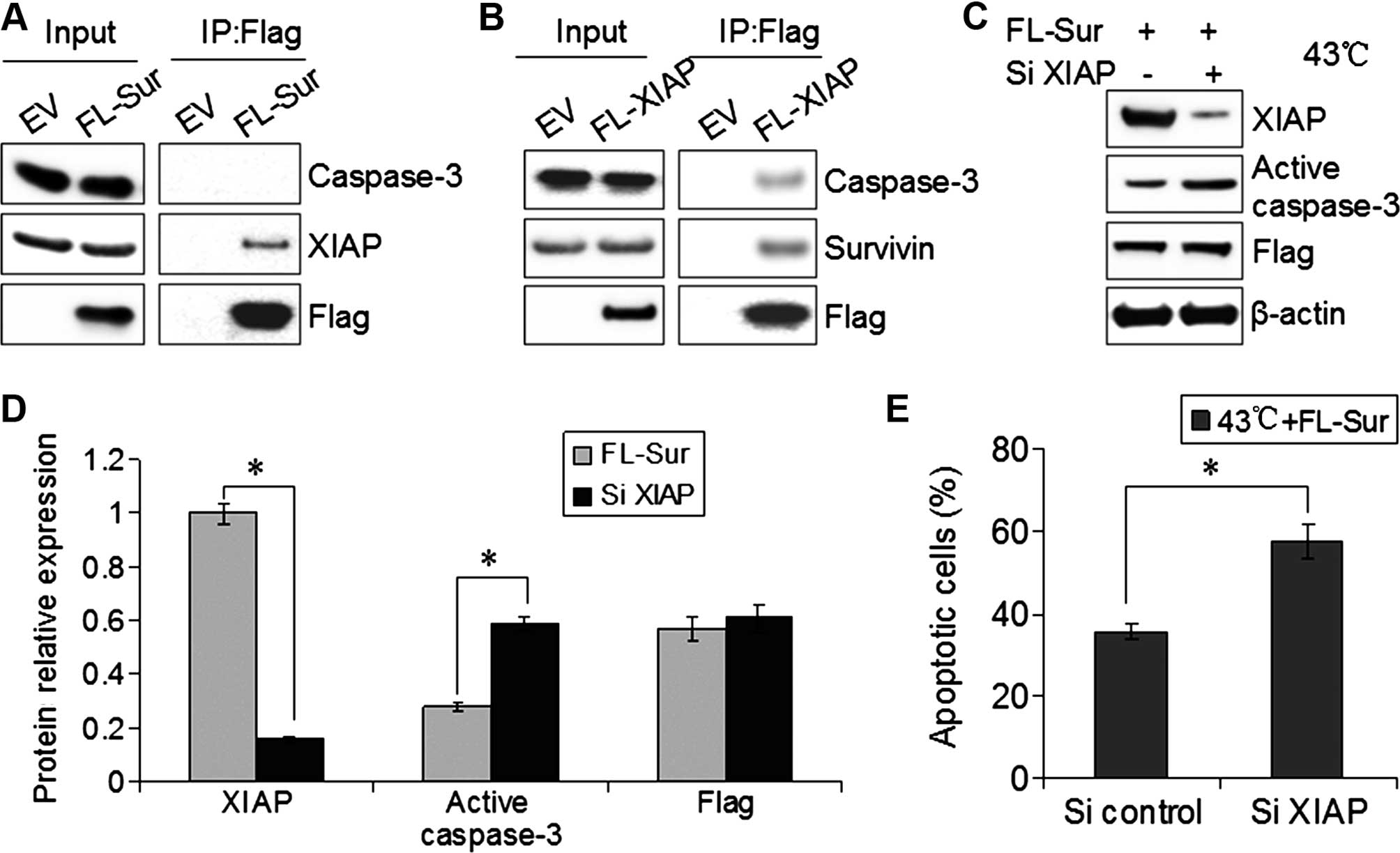
Survivin inactivates caspase-3 by binding
to XIAP
We determined whether Survivin and caspase-3
physically interacted in EC109 cells. EC109 cells were transfected
with Flag-Survivin, although immunoprecipitates of Flag from
transfected cells rarely contained caspase-3 by western blotting.
By contrast, XIAP was detected in Flag-Survivin immunoprecipitates
from cells (Fig. 5A). When we
overexpressed Flag-XIAP in EC109 cells and performed Flag
immunoprecipitations, there was an interaction between XIAP and
Survivin or caspase-3 (Fig. 5B). To
demonstrate the interaction of XIAP with Survivin and caspase-3, we
co-transfected Flag-Survivin and SiXIAP into EC109 cells, and then
treated cells with hyperthermia (43°C for 40 min). The inhibitions
of active caspase-3 and apoptosis by Survivin were reversed in the
absence of XIAP (Fig. 5C–E). These
results supported the model that Survivin inactivated caspase-3 by
binding XIAP.
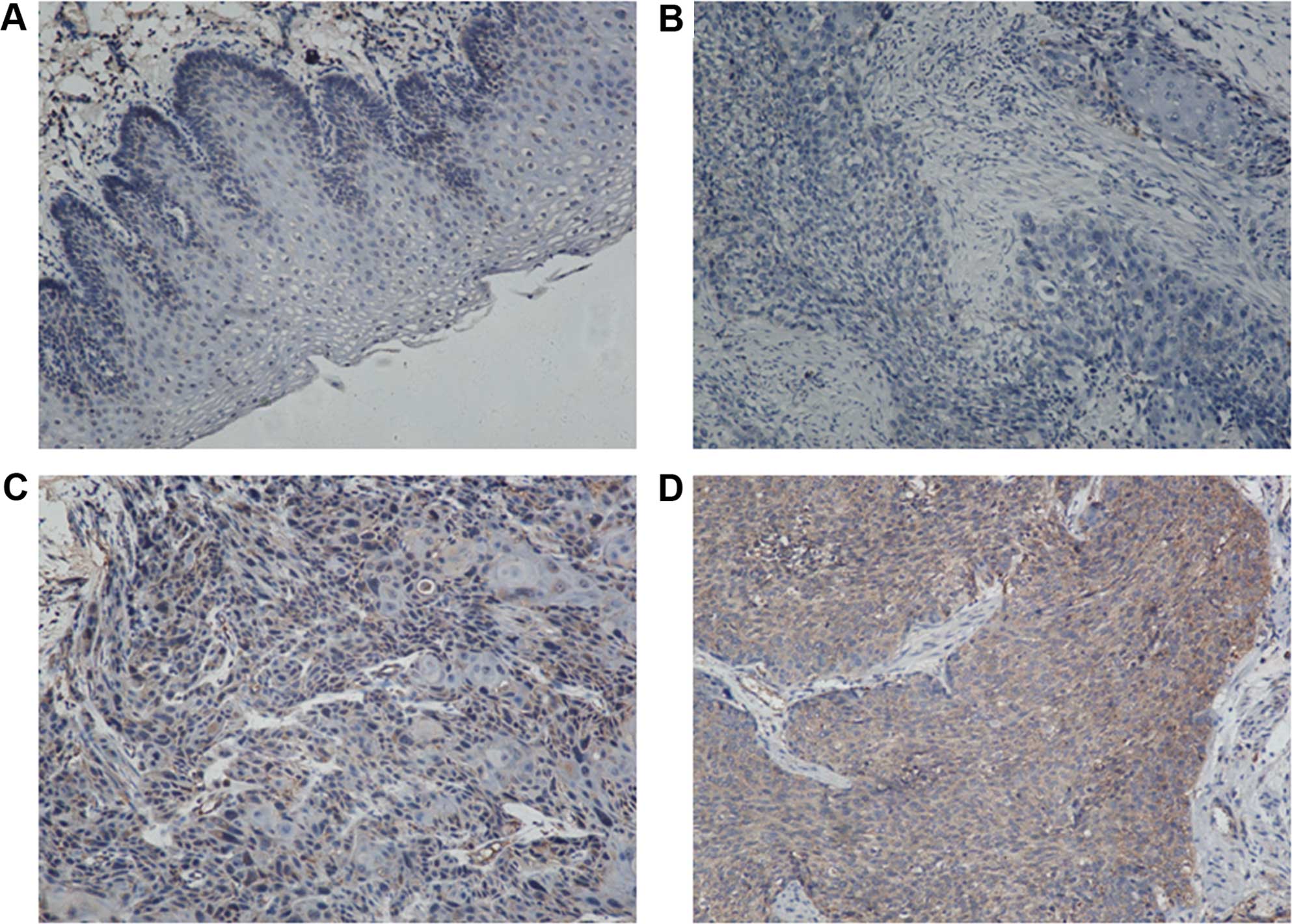
Expression of Survivin in esophageal
cancer and para-tumor tissues
Using immunohistochemistry and patient samples, we
found that the expression rate of Survivin in the esophageal cancer
tissues was 57.0% (49 of 86 patients), and positive staining was
identified in the cytoplasm. Of the 62 para-tumor tissues, 16
samples exhibited positive staining, and the remaining stained
negative. The positive staining rate was 25.8% (16 of 62 patients)
(Fig. 6). The expression of
Survivin in esophageal cancer was significantly higher than that in
the para-tumor tissues (P<0.01) (Table I).
 | Table IComparison of Survivin expression in
esophgeal cancer and para-tumor tissues. |
Table I
Comparison of Survivin expression in
esophgeal cancer and para-tumor tissues.
| Tissues | Total | Survivin
| Positive rate
(%) |
|---|
| + | − |
|---|
| Esophageal
cancer | 86 | 49 | 37 | 57.0 |
| Para-tumor | 62 | 16 | 46 | 25.8 |
Correlation between Survivin expression
and clinicopatho-logical characteristics
To examine the association between Survivin
expression and the clinicopathological characteristics of patients,
we compared Survivin expression with common parameters, such as
age, gender, tumor size, tumor location, pathological type, tumor
differentiation, tumor size, depth of tumor invasion, lymph node
metastasis and clinical stage (Table
II). In the 86 esophageal squamous carcinoma samples, the
expression of Survivin was directly correlated with clinical stage
and lymph node metastasis (P<0.05), but not age, gender, tumor
location, pathological type, tumor differentiation, tumor size or
depth of tumor invasion (P>0.05).
 | Table IIRelationship between the expression of
Survivin and the characteristics of patients. |
Table II
Relationship between the expression of
Survivin and the characteristics of patients.
| Characteristics | Total | The expression of
Survivin
| χ2 | P-value |
|---|
| Positive | Negative | Positive rate
(%) |
|---|
| Age (years) | | | | | | |
| <60 | 45 | 27 | 18 | 60.0 | 0.352 | 0.553 |
| ≥60 | 41 | 22 | 19 | 53.7 | | |
| Gender | | | | | | |
| Male | 64 | 36 | 28 | 56.3 | 0.054 | 0.861 |
| Female | 22 | 13 | 9 | 59.1 | | |
| Location | | | | | | |
| Upper+middle
thoracic | 43 | 25 | 18 | 58.1 | 0.047 | 0.828 |
| Lower
thoracic | 43 | 24 | 19 | 55.8 | | |
| Pathological
type | | | | | | |
| Polypoid | 9 | 5 | 4 | 55.6 | 0.051a | 0.829a |
| Medullary | 41 | 24 | 17 | 58.5 | | |
| Ulceration | 29 | 18 | 11 | 62.1 | | |
| Erosive | 7 | 2 | 5 | 28.6 | | |
| Tumor
differentiation | | | | | | |
| High+middle | 60 | 33 | 27 | 55.0 | 0.316 | 0.574 |
| Low | 26 | 16 | 10 | 61.5 | | |
| Tumor size
(cm) | | | | | | |
| <5 | 59 | 35 | 24 | 59.3 | 0.422 | 0.516 |
| ≥5 | 27 | 14 | 13 | 51.9 | | |
| Depth of
invasion | | | | | | |
| T1
(submucosa) | 12 | 5 | 7 | 41.7 | 1.251 | 0.263 |
| T2 (muscle) | 15 | 8 | 7 | 53.3 | | |
| T3 (serosa) | 41 | 26 | 15 | 63.4 | | |
| T4 (adjacent
organs) | 18 | 10 | 8 | 55.6 | | |
| Lymph node
metastasis | | | | | | |
| Yes | 37 | 28 | 9 | 75.7 | 9.263 | 0.002 |
| No | 49 | 21 | 28 | 42.9 | | |
| Stage | | | | | | |
| I+II | 46 | 20 | 26 | 43.5 | 7.351 | 0.007 |
| III | 40 | 29 | 11 | 72.5 | | |
| Total | 86 | 49 | 37 | 57.0 | | |
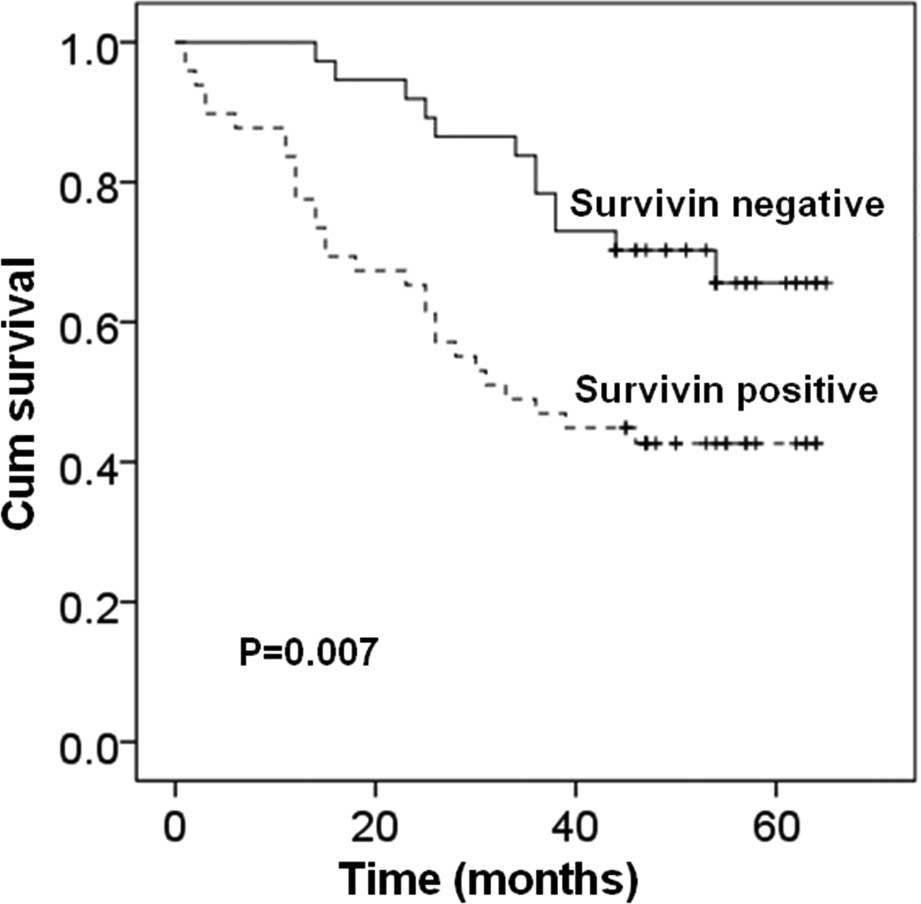
Prognostic value of Survivin and other
clinical characteristics
We used the Kaplan-Meier survival curves and the
log-rank test to examine the prognostic value of Survivin and other
clinical characteristics (Fig. 7).
We found that the patients with a negative expression of Survivin
had a significantly longer overall survival than those with a
positive expression of Survivin (P=0.007). Lymph node metastasis
and clinical stage also showed a significant relationship with
overall survival (P<0.001). Other clinical characteristics, such
as age, gender, tumor location, had no correlation with overall
survival of patients (P>0.05). Univariate Cox regression
analysis results revealed that, Survivin expression (P=0.009),
tumor differentiation (P=0.028), lymph node metastasis (P<0.001)
and clinical stage (P<0.001) were the best predictors of
survival of esophageal cancer patients.
Discussion
Survivin is an essential regulator of cell death,
apoptosis, cell division and proliferation, which is rarely
expressed in normal tissues. However, it is upregulated in the
majority of cancers (17,18). As a tumor-specific molecule,
Survivin promoted angiogenesis, antagonized apoptosis and
facilitated resistance to chemotherapy/radiotherapy (19). Survivin is a good diagnostic
biomarker for estimating the prognosis of cancer patients (20). Therefore, it is a potentially
valuable target for drug identification. In our experiments, we
found that Survivin is expressed at higher levels in esophageal
cancer tissues than in normal tissues, and its high expression
correlated with poor prognosis, suggesting that Survivin is also a
target for esophageal cancer treatment.
Hyperthermia, the heating of tumors to 42–43°C, is
considered the fifth pillar of cancer treatment and is strongly
supported by evidence-based medicine (21,22).
However, the mechanisms by which hyperthermia improves clinical
outcomes remain unclear. There are possible mechanisms that are to
be considered. First, hyperthermia induces apoptosis via the cell
death pathway. Second, hyperthermia alters the cell cycle by
disrupting S phase and blocking of mitosis. Third, hyperthermia
causes chronic ischemia inside the tumor and reduces vessel
regulation (23,24). In the present study, hyperthermia
was found to induce esophageal cancer apoptosis by inhibiting
Survivin. Due to the high expression of Survivin in esophageal
cancer tissues, hyperthermia is a valuable therapy for targeting
Survivin in esophageal cancer.
Survivin plays a crucial role in inhibiting
caspase-dependent apoptosis in different tumors, such as lung and
thyroid tumors. As an IAP family member, Survivin contains a BIR
domain that may bind to caspases and interfere with their functions
(12). There is a debate concerning
the Survivin ability to directly bind to caspases (such as other
IAP members) with its single BIR domain. Previous findings have
demonstrated that Survivin binds to caspase-3 and -7 under specific
conditions (25). By contrast,
other studies have shown that Survivin inhibited the activation of
caspases by binding and stabilizing XIAP rather than directly
binding to caspases (26,27). In the present study, we found that
hyperthermia inhibits Survivin, activates caspase-3 and induces
apoptosis. Overexpression of Survivin reduced hyperthermia-induced
apoptosis by inhibiting the activation of caspase-3. However,
Survivin did not interact with caspase-3 directly. Caspase-3 and
Survivin bind to XIAP and the inhibition of activated caspase-3 by
Survivin was reversed in the absence of XIAP. Therefore, Survivin
assisted XIAP in inactivating caspase-3 and inhibiting
apoptosis.
It has been shown that inhibiting Survivin increases
the sensitivity of cancer cells to chemo- or radiotherapy, in
vitro and in vivo. For example, YM155, a small molecule
inhibitor of Survivin, is potent in targeting various renal cancer
and lymphoma cell lines. It inhibits the transcription of Survivin
and participates in numerous antitumor activities (28–30).
LY2181308, a Survivin antisense oligonucleotide, has been proven to
be effective by itself or in combination with chemotherapy in
pre-clinical and clinical studies. However, these small molecules
have many adverse effects in patients, including fatigue, nausea,
pyrexia or even renal failure (31,32).
In the present study, we reported that hyperthermia is a new method
to treat cancer by inhibiting Survivin. Due to its low adverse
effects, hyperthermia behaved as a better Survivin inhibitor and
improved the function of chemotherapy or radiotherapy for
esophageal cancer patients. However, more studies are required to
precisely identify the effects of hyperthermia in esophageal cancer
models and to determine whether there is synergy in combination
with chemotherapy or radiotherapy.
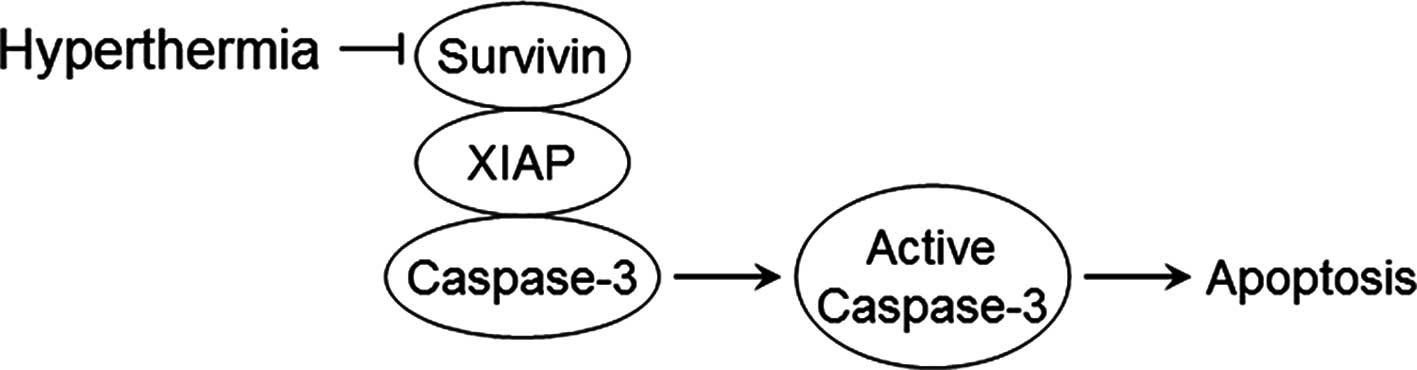
In conclusion, hyperthermia decreases the expression
of Survivin, prevents its binding to XIAP, activates caspase-3 and
induces apoptosis (Fig. 8).
Additionally, the expression of Survivin is higher in esophageal
cancer than in the normal tissues, and its higher level in cancer
is associated with poor prognosis. Therefore, Survivin may be an
essential target for hyperthermia in the treatment of esophageal
cancer.
Acknowledgments
We would like to thank J.R. Skaar for critically
reading the manuscript. The present study was supported by the
National Natural Science Foundation of China, approved ID nos.:
81402506 and 81272418.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kato H and Nakajima M: Treatments for
esophageal cancer: A review. Gen Thorac Cardiovasc Surg.
61:330–335. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim T, Grobmyer SR, Smith R, Ben-David K,
Ang D, Vogel SB and Hochwald SN: Esophageal cancer - the five year
survivors. J Surg Oncol. 103:179–183. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang Y: Epidemiology of esophageal
cancer. World J Gastroenterol. 19:5598–5606. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lordick F, Hölscher AH, Haustermans K and
Wittekind C: Multimodal treatment of esophageal cancer. Langenbecks
Arch Surg. 398:177–187. 2013. View Article : Google Scholar
|
|
6
|
van Hagen P, Hulshof MC, van Lanschot JJB,
Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, Richel DJ,
Nieuwenhuijzen GA, Hospers GA, Bonenkamp JJ, et al CROSS Group:
Preoperative chemoradiotherapy for esophageal or junctional cancer.
N Engl J Med. 366:2074–2084. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Heijkoop ST, van Doorn HC, Stalpers LJA,
Boere IA, van der Velden J, Franckena M and Westermann AM: Results
of concurrent chemotherapy and hyperthermia in patients with
recurrent cervical cancer after previous chemoradiation. Int J
Hyperthermia. 30:6–10. 2014. View Article : Google Scholar
|
|
8
|
Gadaleta-Caldarola G, Infusino S, Galise
I, Ranieri G, Vinciarelli G, Fazio V, Divella R, Daniele A,
Filippelli G and Gadaleta CD: Sorafenib and locoregional deep
electro-hyperthermia in advanced hepatocellular carcinoma: A phase
II study. Oncol Lett. 8:1783–1787. 2014.PubMed/NCBI
|
|
9
|
Petryk AA, Giustini AJ, Gottesman RE,
Kaufman PA and Hoopes PJ: Magnetic nanoparticle hyperthermia
enhancement of cisplatin chemotherapy cancer treatment. Int J
Hyperthermia. 29:845–851. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Januszewski A and Stebbing J: Hyperthermia
in cancer: Is it coming of age? Lancet Oncol. 15:565–566. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qin S, Yang C, Li S, Xu C, Zhao Y and Ren
H: Smac: Its role in apoptosis induction and use in lung cancer
diagnosis and treatment. Cancer Lett. 318:9–13. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Altieri DC: Survivin, cancer networks and
pathway-directed drug discovery. Nat Rev Cancer. 8:61–70. 2008.
View Article : Google Scholar
|
|
13
|
Pennati M, Folini M and Zaffaroni N:
Targeting survivin in cancer therapy. Expert Opin Ther Targets.
12:463–476. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ryan BM, O'Donovan N and Duffy MJ:
Survivin: A new target for anti-cancer therapy. Cancer Treat Rev.
35:553–562. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Or YY, Chow AK, Ng L, Fan ST, Yau TC, Poon
RT and Pang RW: Survivin depletion inhibits tumor growth and
enhances chemosensitivity in hepatocellular carcinoma. Mol Med Rep.
10:2025–2030. 2014.
|
|
16
|
Rice TW: Staging of esophageal cancer: TNM
and beyond. Esophagus. 7:189–195. 2010. View Article : Google Scholar
|
|
17
|
Kanwar JR, Kamalapuram SK and Kanwar RK:
Survivin signaling in clinical oncology: A multifaceted dragon. Med
Res Rev. 33:765–789. 2013. View Article : Google Scholar
|
|
18
|
Coumar MS, Tsai FY, Kanwar JR, Sarvagalla
S and Cheung CH: Treat cancers by targeting Survivin: Just a dream
or future reality? Cancer Treat Rev. 39:802–811. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mobahat M, Narendran A and Riabowol K:
Survivin as a preferential target for cancer therapy. Int J Mol
Sci. 15:2494–2516. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Waligórska-Stachura J, Jankowska A, Waśko
R, Liebert W, Biczysko M, Czarnywojtek A, Baszko-Błaszyk D, Shimek
V and Ruchała M: Survivin - prognostic tumor biomarker in human
neoplasms - review. Ginekol Pol. 83:537–540. 2012.
|
|
21
|
Zhao P, Jiang H, Su D, Feng J, Ma S and
Zhu X: Inhibition of cell proliferation by mild hyperthermia at
43°C with Paris Saponin I in the lung adenocarcinoma cell line
PC-9. Mol Med Rep. 11:327–332. 2015.
|
|
22
|
Soares PI, Ferreira IM, Igreja RA, Novo CM
and Borges JP: Application of hyperthermia for cancer treatment:
Recent patents review. Recent Patents Anticancer Drug Discov.
7:64–73. 2012. View Article : Google Scholar
|
|
23
|
Palazzi M, Maluta S, Dall'Oglio S and
Romano M: The role of hyperthermia in the battle against cancer.
Tumori. 96:902–910. 2010.
|
|
24
|
Ahmed K and Zaidi SF: Treating cancer with
heat: Hyperthermia as promising strategy to enhance apoptosis. J
Pak Med Assoc. 63:504–508. 2013.PubMed/NCBI
|
|
25
|
Shin S, Sung BJ, Cho YS, Kim HJ, Ha NC,
Hwang JI, Chung CW, Jung YK and Oh BH: An anti-apoptotic protein
human survivin is a direct inhibitor of caspase-3 and -7.
Biochemistry. 40:1117–1123. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dohi T, Okada K, Xia F, Wilford CE, Samuel
T, Welsh K, Marusawa H, Zou H, Armstrong R, Matsuzawa S, et al: An
IAP-IAP complex inhibits apoptosis. J Biol Chem. 279:34087–34090.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hu D, Liu S, Shi L, Li C, Wu L and Fan Z:
Cleavage of survivin by granzyme M triggers degradation of the
survivin-X-linked inhibitor of apoptosis protein (XIAP) complex to
free caspase activity leading to cytolysis of target tumor cells. J
Biol Chem. 285:18326–18335. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Koike H, Nitta T, Sekine Y, Arai S, Furuya
Y, Nomura M, Matsui H, Shibata Y, Ito K, Oyama T, et al: YM155
reverses rapamycin resistance in renal cancer by decreasing
Survivin. J Cancer Res Clin Oncol. 140:1705–1713. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Winter GE, Radic B, Mayor-Ruiz C, Blomen
VA, Trefzer C, Kandasamy RK, Huber KV, Gridling M, Chen D, Klampfl
T, et al: The solute carrier SLC35F2 enables YM155-mediated DNA
damage toxicity. Nat Chem Biol. 10:768–773. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kaneko N, Mitsuoka K, Amino N, Yamanaka K,
Kita A, Mori M, Miyoshi S and Kuromitsu S: Combination of YM155, a
survivin suppressant, with bendamustine and rituximab: A new
combination therapy to treat relapsed/refractory diffuse large
B-cell lymphoma. Clin Cancer Res. 20:1814–1822. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Talbot DC, Ranson M, Davies J, Lahn M,
Callies S, André V, Kadam S, Burgess M, Slapak C, Olsen AL, et al:
Tumor survivin is downregulated by the antisense oligonucleotide
LY2181308: A proof-of-concept, first-in-human dose study. Clin
Cancer Res. 16:6150–6158. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tanioka M, Nokihara H, Yamamoto N, Yamada
Y, Yamada K, Goto Y, Fujimoto T, Sekiguchi R, Uenaka K, Callies S,
et al: Phase I study of LY2181308, an antisense oligonucleotide
against survivin, in patients with advanced solid tumors. Cancer
Chemother Pharmacol. 68:505–511. 2011. View Article : Google Scholar
|