Introduction
Hepatocellular carcinoma (HCC) is the most common
form of liver cancer in men and women over 50 years of age. In the
past decade, despite the great advances in HCC diagnosis and
treatment, the incidence rates, as well as the mortality rates of
HCC patients worldwide are increasing (1). Particularly in the Asian or Chinese
population, the numbers of HCC patients and HCC-related cancer
deaths are almost twice these numbers in Caucasian patients, due to
common infection with Helicobacter pylori or hepatitis B
virus (2). Thus, it is critical to
elucidate the underlying mechanisms of HCC proliferation and
metastasis in order to provide more accurate diagnosis and advanced
optimal treatment strategies for HCC patients.
MicroRNAs (miRNAs) are families of 18–22 nucleotide
non-coding RNAs that bind to the 3′-untranslated regions (3′-UTR)
of target mRNAs to negatively modulate gene and protein expression
by DNA or protein degradation in both animals and humans (3). In the past few decades, mounting
evidence demonstrates that miRNAs play a critical role in various
stages of carcinogenesis, cancer proliferation and cancer
metastasis of human cancers, including HCC (4–6). In
HCC, numerous cancer-associated miRNAs act as either oncogenes,
such as miR-494, miR-93 and miR-184 (7–9), or
tumor-suppressors, such as miR-31, miR-29c and miR-148a (10–12). A
recent study found that miR-137 is a tumor-suppressor miRNA in HCC,
as miR-137 was downregulated in HCC and its subsequent upregulation
inhibited HCC proliferation and migration in in vivo
xenografts (13). However, it is
known that miR-137 may exert its cancer regulatory effects through
multiple genes (14,15). Therefore, it is important to explore
the full scope of the downstream genes associated with miR-137 to
better understand its regulation in HCC.
One of the common target genes of miR-137 is cell
division cycle 42 (CDC42), a GTPase of the Rho family (16–18).
CDC42 itself is also highly associated with cancer regulation in
many types of human carcinomas (19,20). A
mouse model of CDC42 deficiency showed that it induced the
development of HCC (21). In
humans, CDC42 was found to be weakly expressed in clinical tumor
samples (22). While this body of
evidence points to a tumor-suppressing role of CDC42 in HCC, it is
notable that CDC42 also acts as an oncogenic factor as knockdown of
CDC42 inhibited HCC migration in vitro (23). Therefore, this conflicting body of
evidence suggests that complex signaling pathways may be associated
with CDC42 regulation in HCC.
In the present study, we explored the possible
molecular association between CDC42 and miR-137 in HCC regulation.
We examined whether miR-137 directly targets CDC42 in HCC, and
whether CDC42 exerts any regulatory effects on miR-137-induced
inhibition of HCC proliferation and metastasis, the two key
properties of human HCC. Furthermore, we examined whether there is
crosstalk between CDC42 and other miR-137 target genes in HCC. The
results of our study may help elucidate the molecular profile of
miRNA regulation in human HCC.
Materials and methods
HCC cell lines
Human HCC cell lines, HuH7, BEL-7402, HEPG2 and
HEP3B and a normal human hepatocyte cell line (THLE-2) were
obtained from the Cell Bank of the Chinese Academy of Sciences
(Shanghai, China). MHCC97L was kindly provided by the Liver Cancer
Institute of Zhongshan Hospital at Fudan University (Shanghai,
China). All cell lines were maintained in Dulbecco's modified
Eagle's medium (DMEM) supplemented with 10% fetal bovine serum
(FBS) (both from Sigma-Aldrich, USA), 100 IU/ml penicillin G and
100 µg/ml streptomycin at 37°C in 5% CO2. Once
confluency was achieved, the cells were passaged with replenished
medium every 3 or 4 days.
RNA isolation and quantitative real-time
PCR (qRT-PCR)
To extract RNA, the cell or tumor samples were
homogenized in TRIzol reagent (Invitrogen, USA).
Reverse-transcription was carried out by regular PCR using a
RT-PreMix kit (SBS Genetech, China) according to the manufacturer's
protocol. To quantitatively measure the gene expression level of
miR-137, miRNA qRT-PCR was carried out using a Hairpin-it™ miRNAs
Real-Time PCR Quantitation kit (GenePharma, China) with internal
control of U6 snRNA, according to the manufacturer's protocol. To
quantify mRNA expression of CDC42 and AKT2, qRT-PCR was carried out
using a SYBR Green PCR Master Mix (Applied Biosystems, USA) with
internal control of the 18s gene, according to the manufacturer's
protocol. All primer sets were purchased from SBS Genetech. Gene
expression levels were quantified by 2−ΔΔct methods
against internal control genes and are reported as relative
fold-changes.
MicroRNA-137 upregulation
Lentiviruses carrying the mature hsa-miR-137 mimic
(miR-137) or its negative control miRNA (miR-C) were obtained from
RiboBio (China). Lentiviral transfection of the HuH7 and MHCC97L
cells was carried out using Lipofectamine 2000 reagent (Invitrogen)
according to the manufacturer's protocol for 24 h, followed by
replenishment with fresh medium.
Dual-luciferase reporter assay
The 3′-UTR of human CDC42 including the putative
miR-137 binding site was amplified from a human liver cDNA library
and then cloned into the SpeI/HindIII site of a
pMIR-REPORT luciferase vector to generate the wild-type (WT) CDC42
luciferase reporter (CDC42-WT; Ambion, USA). A mutant CDC42 3′UTR
with a modified miR-137 binding site was generated using a
Site-Directed Mutagenesis kit (SBS Genetech). It was also cloned
into the pMIR-REPORT vector to generate the mutant CDC42 luciferase
reporter (CDC42-MT). In both HuH7 and MHCC97L cells, miR-137 was
co-transfected with CDC42-WT, CDC42-MT or a Renilla
luciferase control vector (Luc-C) for 48 h. The luciferase
activities were measured by a dual-luciferase reporter assay
(Promega, USA), and normalized to the activity of the
Renilla control.
Human tumor specimens
Thirteen human HCC specimens were collected by
surgery between June 2013 and April 2015 at the Department of
Oncology of The First Affiliated Hospital of Zhengzhou University,
and the Department of Oncology of The First People's Hospital of
Zhengzhou in Zhengzhou, China. Consent forms were signed by all
patients. The clinical and laboratory protocols were approved by
the Ethic Committees at the participating institutes.
Western blot analysis
HuH7 and MHCC97L cells were collected and lysed in a
lysis buffer containing 50 mM Tris (pH 7.6), 150 mM NaCl, 1 mM
EDTA, 10% glycerol, and 0.5% NP-40 and protease inhibitor cocktail
(Millipore, USA). The extracted cell proteins were dissolved on 10%
SDS-PAGE gel, transferred to nitrocellulose membranes, and
incubated with a primary rabbit antibody against human CDC42
(1:200; Sigma-Aldrich) at 4°C overnight. On the second day, after
washing with Tris-buffered saline (3 × 10 min), the membranes were
incubated with a horseradish peroxidase-conjugated secondary
antibody (Millipore). Each blot was visualized with enhanced
chemiluminescence (Pierce, USA) according to the manufacturer's
protocol.
Overexpression assay
Whole DNA sequences of CDC42 and AKT2 were amplified
from a human liver cDNA library and confirmed with sequencing. The
DNA sequences were then cloned into a recombinant plasmid
eukaryotic expression vector pcDNA3.1 (Invitrogen) to construct
overexpressing vectors of CDC42 (pcDNA3.1/CDC42), and AKT2
(pcDNA3.1/AKT2). An empty vector (pcDNA3.1/+) was used as control.
The transfection was carried out using Lipofectamine 2000 reagent
according to the manufacturer's protocol for 24 h, followed by
replenishment with fresh medium.
In vitro proliferation assay
HuH7 and MHCC97L cells were initially transfected
with the lentiviruses and/or the over-expressing vectors for 24 h.
After 24 h, the culture medium was replenished and the
proliferation of HCC cells was characterized using a
3-(4,5-dimethylthazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay for 5 consecutive days. Briefly, after washing the cells with
PBS, 1 ml of 0.5 mg/ml MTT was added into the culture for 4 h. MTT
was aspirated and 300 µl isopropanol was immediately added.
The optical density (OD) was measured at a wavelength of 570
nm.
In vitro migration assay
Metastasis in the HuH7 and MHCC97L cells was
measured using a QCM chemotaxis 96-well migration assay (Chemicon,
USA). On the first day, HuH7 and MHCC97L cells were transfected
with the lenti-viruses and/or the overexpressing vectors. During
this time, the upper chamber of the QCM chemotaxis plate was coated
with 0.1% gelatin (in PBS) overnight. On the second day, HuH7 and
MHCC97L cells were resuspended and re-plated in the upper chamber
with RPMI-1640 medium. The lower chamber was filled with RPMI-1640
medium with the addition of 10% FBS as a chemoattractant. After 24
h, the HCC cells that migrated into the lower chamber were fixed
with 4% PFA and stained with hematoxylin and eosin (H&E). The
relative migration rates were measured by averaging the cell
numbers in 5 random 0.1 × 0.1 mm regions and normalizing to the
cell number under control conditions.
In vivo transplantation assay
HuH7 cells were transfected with the lentiviruses
and/or the overexpressing vectors. After 24 h, the cells were
collected and resuspended. Approximately 1 million cells were
subcutaneously inoculated into the left flank of 2-month-old female
nude mice. The in vivo tumor growth assay was carried out by
measuring the in vivo tumor volume based on the equation:
length × width2/2. At the end of the 5-week
transplantation, the tumors were extracted and Ki-67 immunostaining
(BD Biosciences) was carried out on paraffin sections.
Statistical analysis
In the present study, data are presented as the mean
± standard deviation. Statistical analysis with the two-tailed
Student's t-test (SPSS, version 11.0) was carried out to evaluate
the difference between results. Differences were considered
significant at P<0.05. All experiments were repeated in
triplicates.
Results
MicroRNA-137 is downregulated in HCC cell
lines and targets CDC42
A previous study demonstrated that miR-137 is
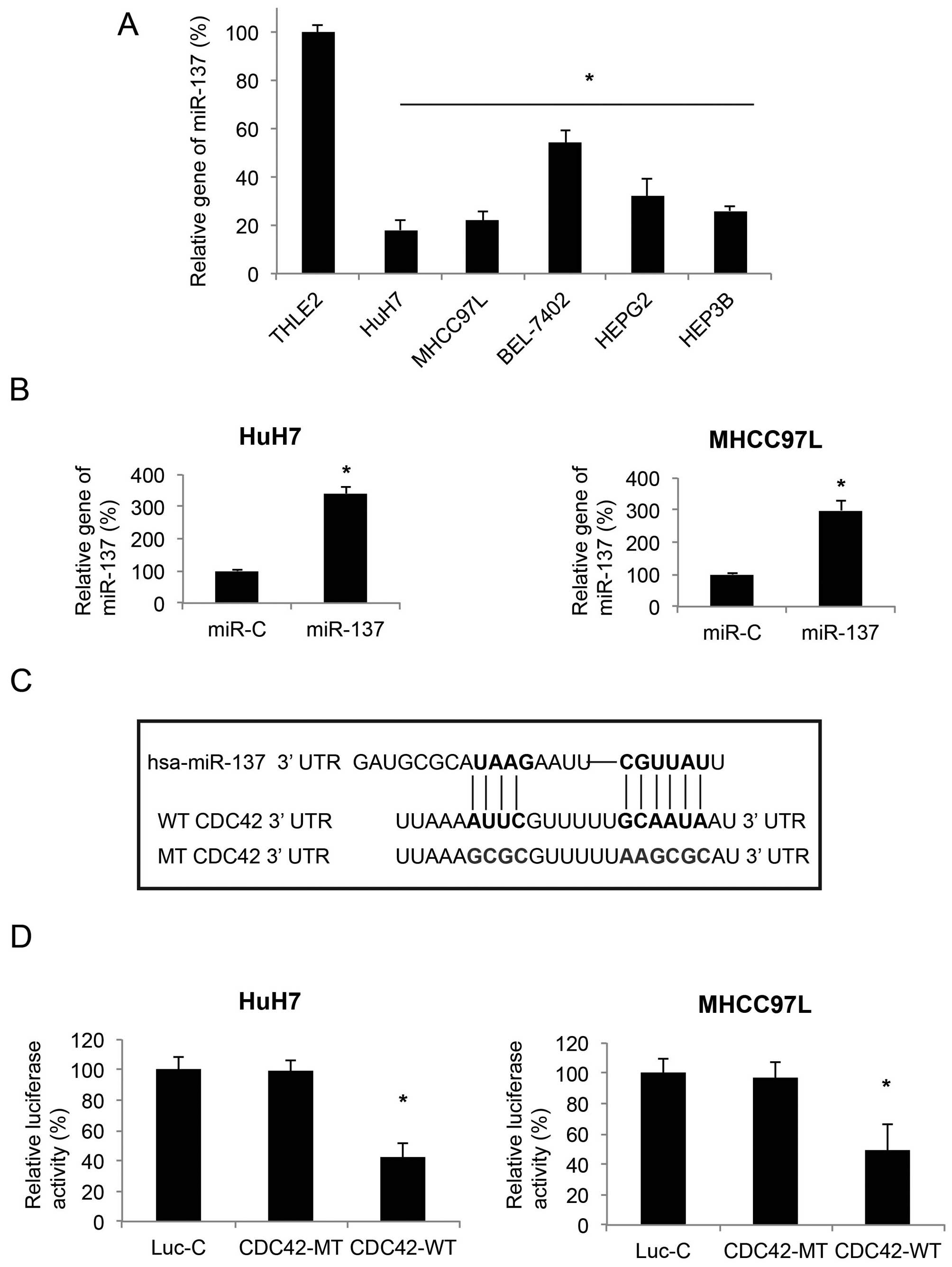
downregulated in HCC cell lines (13). In the present study, we firstly
verified this result by qRT-PCR. We found that the gene expression
levels of miR-137 were lower in all 7 probed HCC cell lines, when
compared with the expression level of miR-137 in THLE2, a normal
human hepatocyte cell line (P<0.05, Fig. 1A). We then used a lentiviral vector
to ectopically upregulate miR-137 in two HCC cell lines, HuH7 and
MHCC97L. The efficiency of lentivirus-mediated miR-137 upregulation
was confirmed by qRT-PCR (P<0.05, Fig. 1B).
In order to find the downstream molecular target of
miR-137 in HCC, we explored several online miRNA target softwares,
such as TargetScan (www.targetscan.org), microRNA (www.microRNA.org) and miTarget (www.cbit.snu.ac.kr/~miTarget) and found that CDC42 was
a possible hit (Fig. 1C). We then
carried out a dual-luciferase reporter assay and confirmed that in
HuH7 and MHCC97L cells, CDC42 was the downstream target of miR-137
(P<0.05, Fig. 1D).
MicroRNA-137 is inversely correlated with
CDC42 in both HCC tumors and cell lines
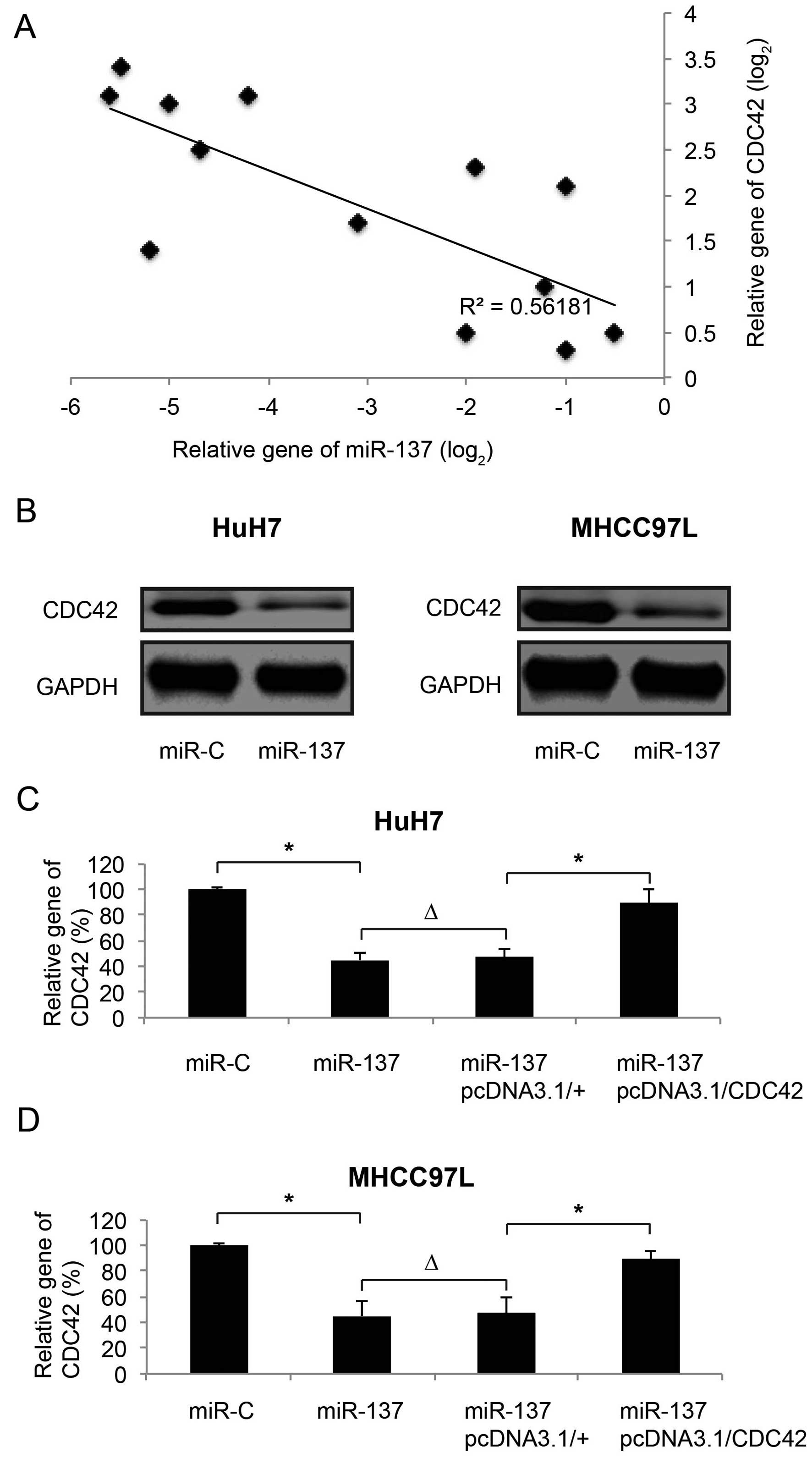
We then evaluated the correlation between miR-137
and CDC42 in both HCC tumors and cell lines. Firstly, 13 clinically
obtained HCC tumor samples underwent gene expression analysis by
qRT-PCR. We found that, through a linear regression method, the
gene expression level of miR-137 was inversely correlated with the
mRNA expression level of CDC42 in the human HCC tumors (Fig. 2A). We also examined the protein
expression of CDC42 in HCC cell lines. We found that, while miR-137
was upregulated by lentiviral transfection, CDC42 protein levels
were substantially downregulated in the HuH7 and MHCC97L cells
(Fig. 2B). The downregulation of
CDC42 by miR-137 upregulation in HCC cells was further confirmed by
qRT-PCR (Fig. 2C and D; miR-C vs.
miR-137, P<0.05).
After CDC42 was downregulated by miR-137
upregulation, we re-introduced CDC42, through the transection of an
overexpression vector pcDNA3.1/CDC42, back into HuH7 and MHCC97L
cells. In the control experiment, the HCC cells were transfected
with an empty vector pcDNA3.1/+. We found that, while pcDNA3.1+ had
no effect on CDC42 gene expression (Fig. 2C and D; miR-137 vs.
miR-137+pcDNA3.1/+, P>0.05), pcDNA3.1/CDC42 significantly
upregulated CDC42 mRNA in the HuH7 and MHCC97L cells (Fig. 2C and D; miR-137+pcDNA3.1/+ vs.
miR-137+ pcDNA3.1/CDC42, P<0.05).
Inhibition of HCC proliferation by
miR-137 is ameliorated by CDC42
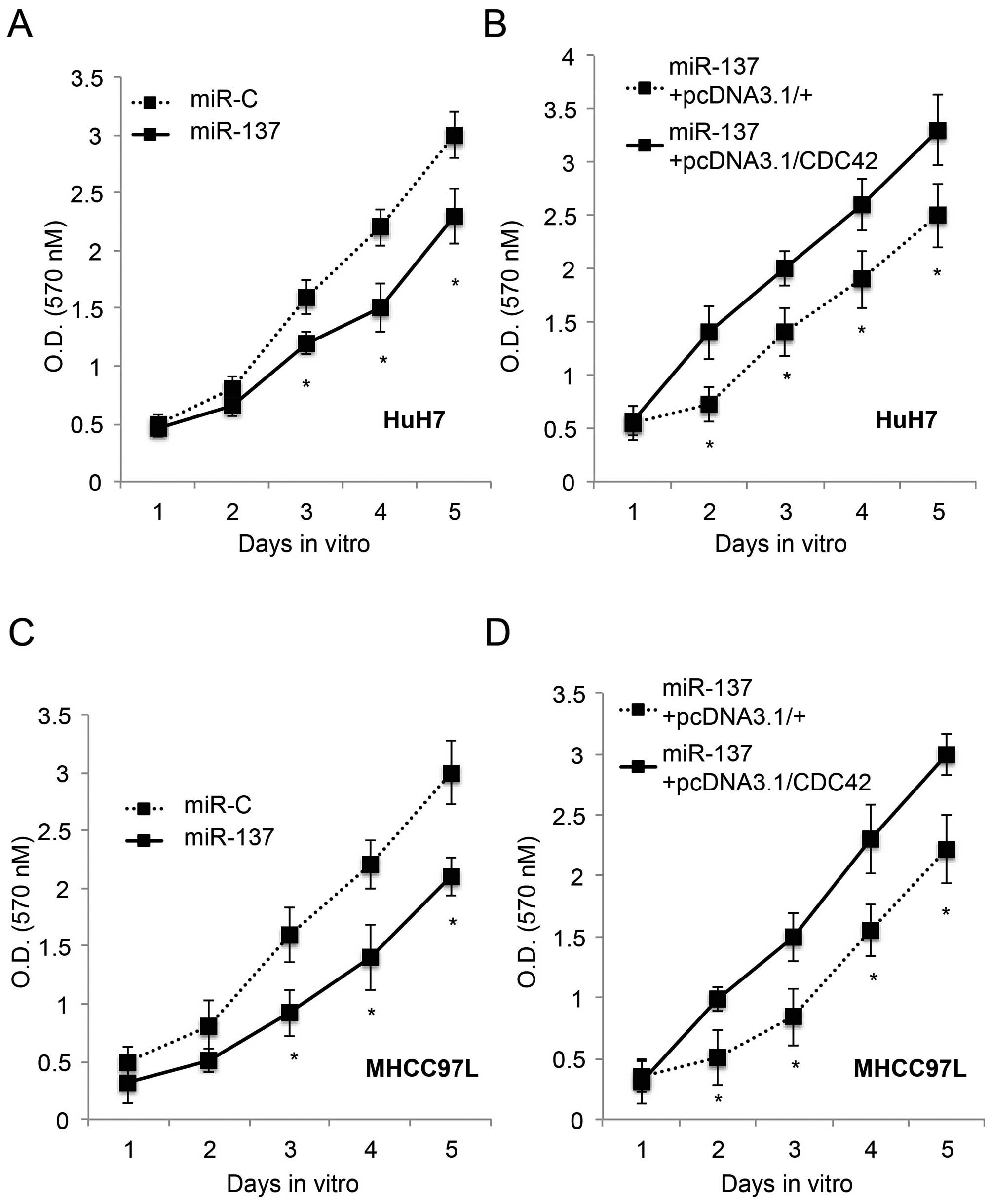
Since we discovered that CDC42 was inversely
regulated by miR-137 in HCC, we speculated that CDC42 may play a
functional role in HCC. To test this hypothesis, we firstly
upregulated miR-137 in the HuH7 and MHCC97L cells with an miR-137
lentivirus. Through a 5-day proliferation assay, we found that
miR-137 upregulation significantly inhabited cancer growth in both
the HuH7 and MHCC97L cells (P<0.05, Fig. 3A and C), in line with a previous
study (13). Secondly, 24 h after
lentiviral transfection to upregulate miR-137, CDC42 was
overexpressed in the HuH7 and MHCC97L cells for 24 h. Another 5-day
in vitro proliferation assay showed that re-introduction of
CDC42 restored the growth of HuH7 and MHCC97L cells (P<0.05,
Fig. 3B and D). Thus, our results
showed that overexpression of CDC42 ameliorated the inhibitory
effect of miR-137 on HCC proliferation.
Inhibition of HCC metastasis by miR-137
is ameliorated by CDC42
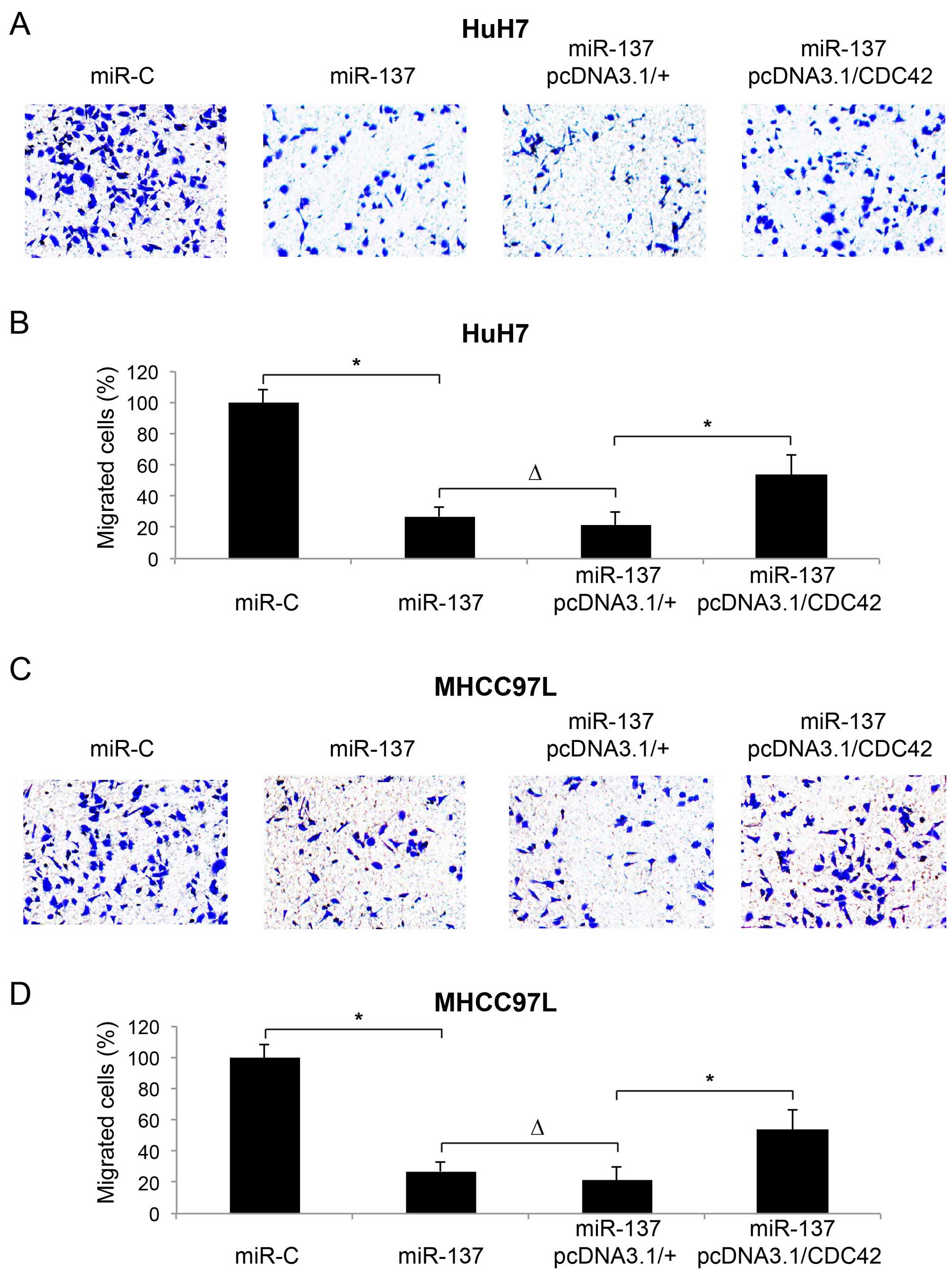
We speculated that CDC42 may play a functional role
in miR-137-mediated HCC metastasis. Firstly, we confirmed the
inhibitory effect of miR-137 on HCC metastasis (13). We transfected HuH7 and MHCC97L cells
with a lentivirus of miR-137 or miR-C, followed by a migration
assay to assess the metastasis in 24 h. The assay showed that in
both HCC cell lines, the metastatic capability of the cancer cells
was inhibited by miR-137 upregulation. Immunostaining showed that
significantly less migrated HCC cells were noted in the lower
chambers (Fig. 4A and C, miR-C vs.
miR-137). Quantitative measurement showed that miR-137 upregulation
reduced the percentages of migrated cells to <40% in both the
HuH7 and MHCC97L cells (Fig. 4B and
D; miR-C vs. miR-137, P<0.05).
Secondly, 24 h after miR-137 transfection, we
carried out another transfection with either pcDNA3.1/CDC42 or
pcDNA3.1/+ in the HuH7 and MHCC97L cells, followed by a migration
assay to evaluate the effect of CDC42 overexpression on HCC
metastasis. Immunostaining showed that, after miR-137 upregulation,
re-introduction of CDC42 promoted more HCC cells to migrate into
the lower chambers (Fig. 4A and C;
miR-137 + pcDNA3.1/+ vs. miR-137 + pcDNA3.1/+CDC42). Furthermore,
quantification demonstrated that CDC42 restored the percentages of
migrated cells to ~60% of the original levels (Fig. 4B and D; miR-137 + pcDNA3.1/+ vs.
miR-137 + pcDNA3.1/+CDC42, P<0.05). It is also worth noting that
second transfection of the empty vector had no effect on HCC
metastasis (Fig. 4B and D; miR-137
vs. miR-137 + pcDNA3.1/+).
Therefore, the results of our migration assay
demonstrated that overexpression of CDC42 ameliorated the
inhibitory effect of miR-137 on HCC metastasis.
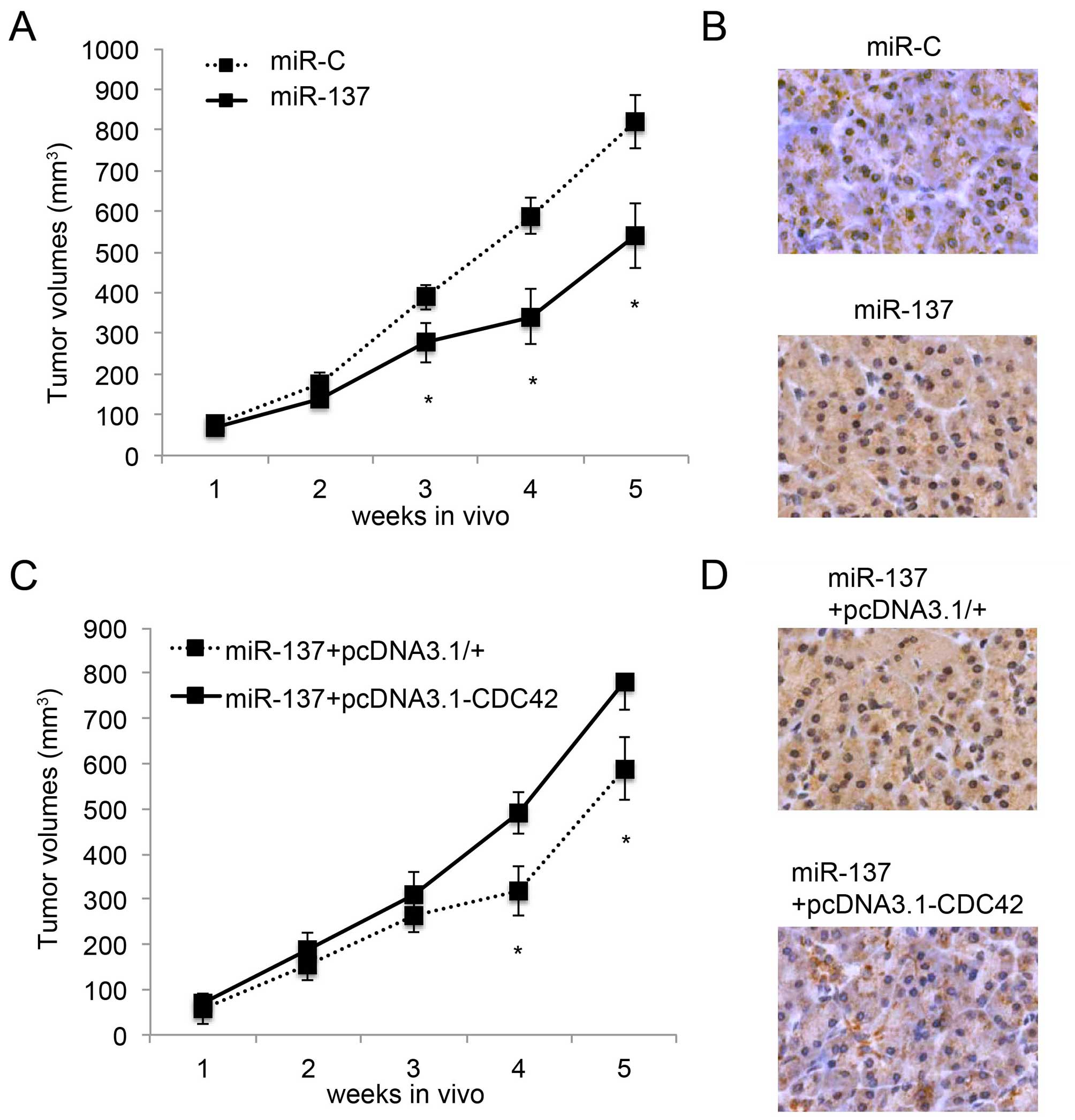
Inhibition of in vivo HCC tumor growth by
miR-137 is ameliorated by CDC42
We then examined the effect of the overexpression of
CDC42 on miR-137-mediated inhibition of in vivo HCC tumor
growth (13). Firstly, we
transfected HuH7 cells with the lentivirus of miR-137 or miR-C.
Twenty-four hours later, HuH7 cells were transplanted into
2-month-old null mice. The in vivo growth of tumors was
monitored for 5 weeks, followed by immunostaining of Ki67 at the
end of the in vivo assay. Both the in vivo assay
(P<0.05, Fig. 5A) and Ki67
immunostaining (Fig. 5B) confirmed
that miR-137 inhibited HCC tumor growth. Secondly, 24 h after
miR-137 transfection, HuH7 cells were further transfected with
either pcDNA3.1/CDC42 or pcDNA3.1/+, followed by the
transplantation assay. Both in vivo tumor growth assay
(P<0.05, Fig. 5C) and Ki67
immunostaining (Fig. 5D) showed
that overexpression of CDC42 ameliorated the inhibitory effect of
miR-137 on in vivo HCC tumor growth.
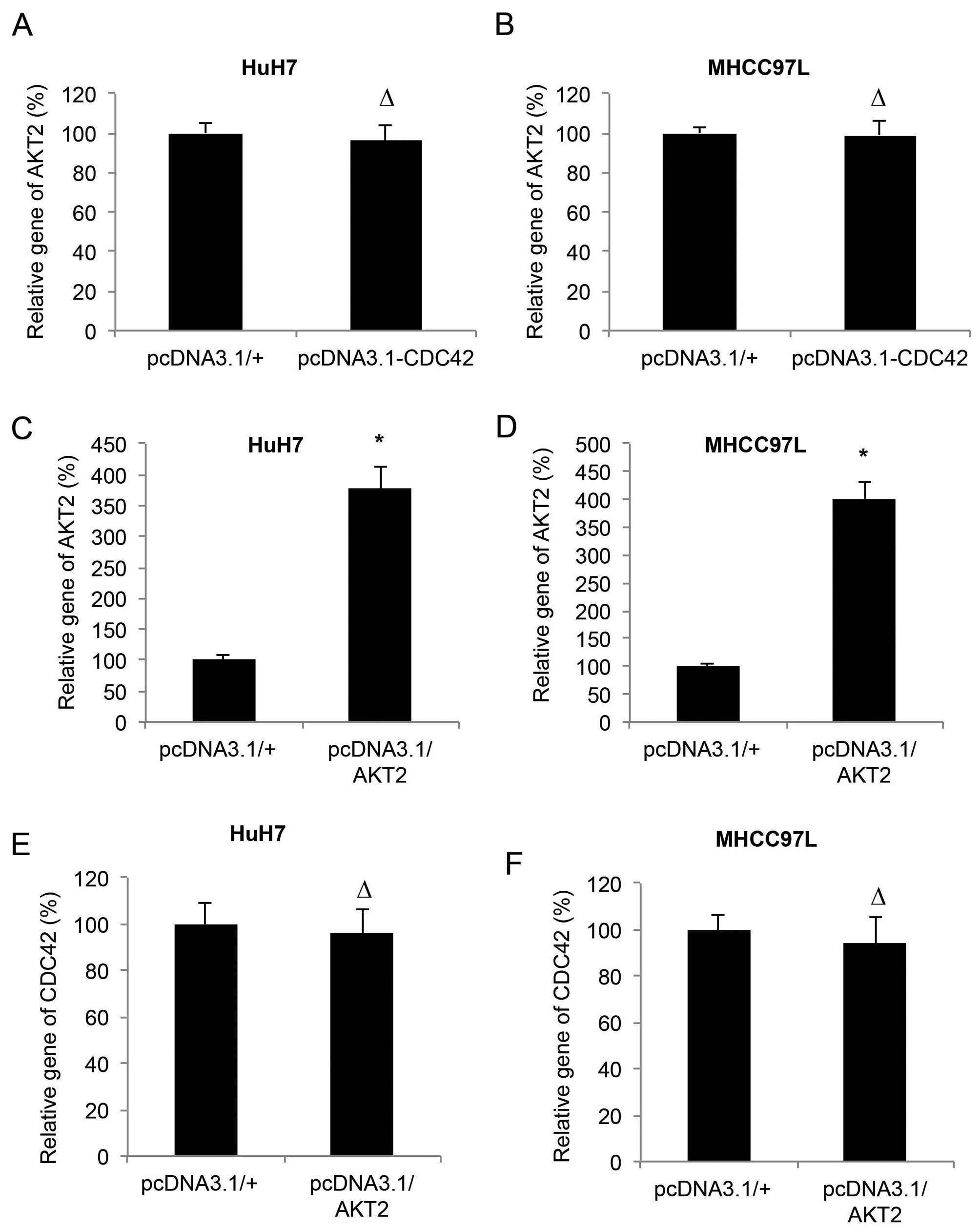
CDC42 and AKT2 are independently
expressed in HCC
A previous study demonstrated that miR-137 inhibited
HCC through AKT2 (13). Since we
demonstrated that CDC42 was also involved in miR-137-mediated HCC
inhibition, we aimed to ascertain whether CDC42 and AKT2 undergoes
crosstalk in HCC. We firstly examined the effect of the
overexpression of CDC42 on the mRNA expression level of AKT2 in the
HuH7 and MHCC97L cells. The results of qRT-PCR showed that CDC42
did not alter the expression level of AKT2 in the HCC cells
(P>0.05, Fig. 6A and B). We then
transfected the HuH7 and MHCC97L cells with another set of
overexpression vectors of pcDNA3.1/AKT2 and its control pcDNA3.1/+.
qRT-PCR showed that, in both HuH7 and MHCC97L cells, the expression
levels of AKT2 were significantly upregulated by pcDNA3.1/AKT2
(P<0.05, Fig. 6C and D), whereas
expression levels of CDC42 were not changed (P>0.05, Fig. 6E and F).
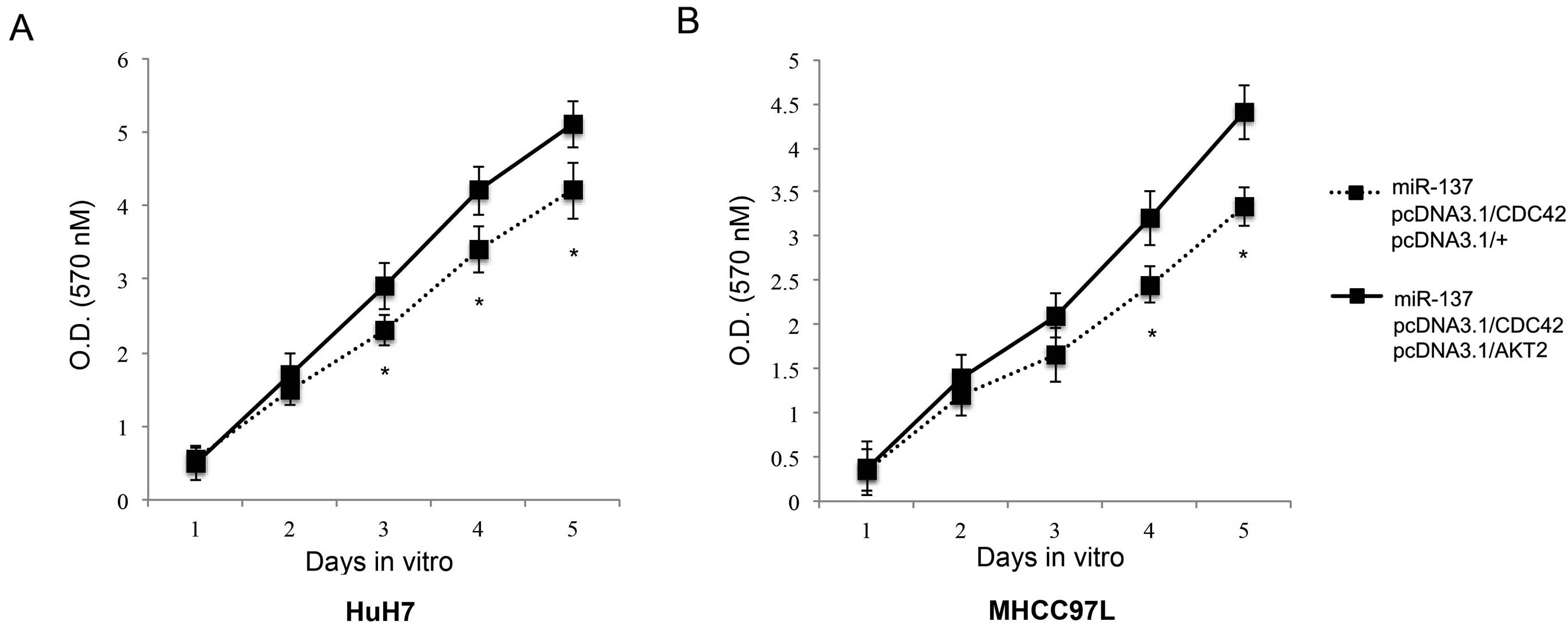
AKT2 contributes additively to CDC42 in
reversing the inhibitory effect of miR-137 on HCC proliferation and
metastasis
We then investigated the correlation between AKT2
and CDC42 in the regulation of miR-137-induced HCC inhibition.
Firstly, we studied the possible additive effect of AKT2 on HCC
proliferation. We transfected HuH7 and MHCC97L cells with the
miR-137 lentivirus. Twenty-four hours after that, we co-transfected
the cells with pcDNA3.1/CDC42 and pcDNA3.1/AKT2. The control cells
were co-transfected with pcDNA3.1/CDC42 and pcDNA3.1/+. The in
vitro proliferation assay demonstrated that AKT2
overexpression, in addition to CDC42 overexpression, further
rescued HCC proliferation from miR-137-induced inhibition
(P<0.05, Fig. 7). Secondly, we
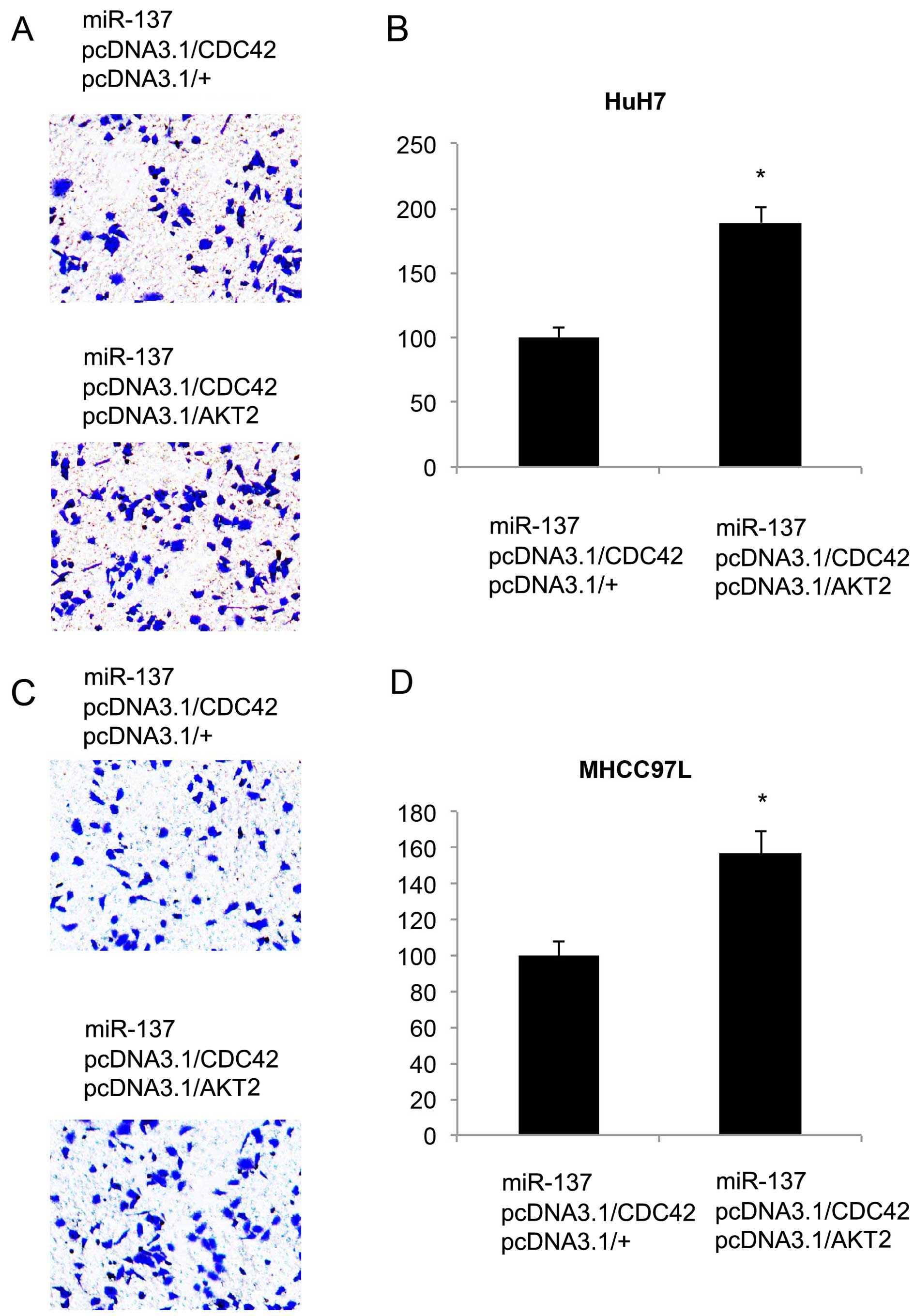
evaluated the additive effect of the overexpression of AKT2 on HCC
metastasis. Twenty-four hours after co-transfection of the
double-overexpressing vectors, an in vitro migration assay
was carried out. It showed that in both HCC cell lines, more cancer
cells migrated into the lower chambers (Fig. 8A and C). Quantitative measurement
also showed that AKT2 overexpression, in addition to CDC42
overexpression, further rescued HCC metastasis from miR-137-induced
inhibition (P<0.05, Fig. 8B and
D).
Discussion
It was recently reported that miR-137 is a new
member of the tumor-suppressing miRNAs found in human HCC (13). Liu et al found that miR-137
was lowly expressed in HCC tumors and cell lines, and was strongly
associated with survival in patients with HCC (13). In addition, forced miR-137
overexpression was able to exert inhibitory effects on HCC
proliferation and migration, both in vitro and in
vivo (13). In the present
study, we firstly confirmed that the gene expression levels of
miR-137 were low in 7 HCC lines, as compared to the expression
level of miR-137 in a normal human hepatocyte cell line (THLE2).
Importantly, one of the newly examined HCC cell lines in our study,
MHCC97L, was specifically derived from Chinese HCC patients with
low metastatic capability (24).
Thus, our result showing that miR-137 was downregulated in MHCC97L
cells, as in the other HCC cell lines, suggests that a low
expression pattern of miR-137 may be universal regardless of ethic
background.
Moreover, in our study, we used a lentivirus to
ectopically upregulate miR-137 in the HuH7 and MHCC97L cells. We
found that miR-137 upregulation inhibited HCC proliferation and
metastasis in vitro and tumor growth in vivo
(Figs. 3Figure 4–5), further confirming the antitumor effect
of miR-137 in HCC as shown in a previous study (13). Moreover, we identified CDC42 as
another downstream target gene of miR-137 in HCC. Dual-luciferase
reporter and western blot assays showed that CDC42 was directly
regulated by miR-137 in HCC cells. Most importantly, while we used
an overexpression system to ectopically re-introduce CDC42 back
into HCC cells after miR-137 upregulation, we were able to
ameliorate or reverse the tumor-suppressive effects of miR-137 on
HCC in vitro proliferation, migration and in vivo
tumor growth. A previous study showed that CDC42 was lowly
expressed in liver tumors when compared to the expression in
non-tumor liver tissues (22),
suggesting that CDC42 may act as an antitumor (or tumor
suppressing) factor in HCC. Interestingly, another study showed
that CDC42 indeed acted as an oncogene in HCC as CDC42 knockdown
inhibited the migration of QGY-7703 cells (23). Although the results of our study
supported the idea of CDC42 as an oncogene, caution shall be taken
to draw such a conclusion as more complex signaling mechanisms may
be associated with CDC42 to determine whether it is an oncogene or
a tumor-suppressor in HCC.
Finally, we co-expressed CDC42 with AKT2, another
known miR-137 target gene in HCC (13), in the HuH7 and MHCC97L cells. We
found that AKT2 contributed additively to CDC42 to rescue the
inhibition of miR-137 on HCC proliferation and migration (Figs. 7 and 8), suggesting that CDC42 and AKT2 may
exert their oncogenic effects independently, although both are
regulated by miR-137. Future study may help to elucidate the
differential signaling pathways associated with CDC42 or AKT2 in
HCC regulation.
In summary, our study revealed that CDC42 is an
independent target gene of miR-137 in regulating HCC. These results
may help to identify possible biomarkers and elucidate the
underlying molecular mechanisms of human HCC.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Alvarez-Garcia I and Miska EA: MicroRNA
functions in animal development and human disease. Development.
132:4653–4662. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sassen S, Miska EA and Caldas C: MicroRNA:
Implications for cancer. Virchows Arch. 452:1–10. 2008. View Article : Google Scholar
|
|
5
|
Takasaki S: Roles of microRNAs in cancers
and development. Methods Mol Biol. 1218:375–413. 2015. View Article : Google Scholar
|
|
6
|
Hung CH, Chiu YC, Chen CH and Hu TH:
MicroRNAs in hepatocellular carcinoma: Carcinogenesis, progression,
and therapeutic target. Biomed Res Int. 2014:4864072014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lim L, Balakrishnan A, Huskey N, Jones KD,
Jodari M, Ng R, Song G, Riordan J, Anderton B, Cheung ST, et al:
MicroRNA-494 within an oncogenic microRNA megacluster regulates
G1/S transition in liver tumorigenesis through suppression of
mutated in colorectal cancer. Hepatology. 59:202–215. 2014.
View Article : Google Scholar :
|
|
8
|
Ohta K, Hoshino H, Wang J, Ono S, Iida Y,
Hata K, Huang SK, Colquhoun S and Hoon DS: MicroRNA-93 activates
c-Met/PI3K/Akt pathway activity in hepatocellular carcinoma by
directly inhibiting PTEN and CDKN1A. Oncotarget. 6:3211–3224. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gao B, Gao K, Li L, Huang Z and Lin L:
miR-184 functions as an oncogenic regulator in hepatocellular
carcinoma (HCC). Biomed Pharmacother. 68:143–148. 2014. View Article : Google Scholar
|
|
10
|
Kim HS, Lee KS, Bae HJ, Eun JW, Shen Q,
Park SJ, Shin WC, Yang HD, Park M, Park WS, et al: MicroRNA-31
functions as a tumor suppressor by regulating cell cycle and
epithelial-mesenchymal transition regulatory proteins in liver
cancer. Oncotarget. 6:8089–8102. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bae HJ, Noh JH, Kim JK, Eun JW, Jung KH,
Kim MG, Chang YG, Shen Q, Kim SJ, Park WS, et al: MicroRNA-29c
functions as a tumor suppressor by direct targeting oncogenic SIRT1
in hepatocellular carcinoma. Oncogene. 33:2557–2567. 2014.
View Article : Google Scholar
|
|
12
|
Zhang JP, Zeng C, Xu L, Gong J, Fang JH
and Zhuang SM: MicroRNA-148a suppresses the epithelial-mesenchymal
transition and metastasis of hepatoma cells by targeting Met/Snail
signaling. Oncogene. 33:4069–4076. 2014. View Article : Google Scholar
|
|
13
|
Liu LL, Lu SX, Li M, Li LZ, Fu J, Hu W,
Yang YZ, Luo RZ, Zhang CZ and Yun JP: FoxD3-regulated microRNA-137
suppresses tumour growth and metastasis in human hepatocellular
carcinoma by targeting AKT2. Oncotarget. 5:5113–5124. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Luo C, Tetteh PW, Merz PR, Dickes E,
Abukiwan A, Hotz-Wagenblatt A, Holland-Cunz S, Sinnberg T, Schittek
B, Schadendorf D, et al: miR-137 inhibits the invasion of melanoma
cells through downregulation of multiple oncogenic target genes. J
Invest Dermatol. 133:768–775. 2013. View Article : Google Scholar
|
|
15
|
Wright C, Turner JA, Calhoun VD and
Perrone-Bizzozero N: Potential impact of miR-137 and its targets in
schizophrenia. Front Genet. 4:582013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen Q, Chen X, Zhang M, Fan Q, Luo S and
Cao X: miR-137 is frequently down-regulated in gastric cancer and
is a negative regulator of Cdc42. Dig Dis Sci. 56:2009–2016. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhu X, Li Y, Shen H, Li H, Long L, Hui L
and Xu W: miR-137 inhibits the proliferation of lung cancer cells
by targeting Cdc42 and Cdk6. FEBS Lett. 587:73–81. 2013. View Article : Google Scholar
|
|
18
|
Liu M, Lang N, Qiu M, Xu F, Li Q, Tang Q,
Chen J, Chen X, Zhang S, Liu Z, et al: miR-137 targets Cdc42
expression, induces cell cycle G1 arrest and inhibits invasion in
colorectal cancer cells. Int J Cancer. 128:1269–1279. 2011.
View Article : Google Scholar
|
|
19
|
Arias-Romero LE and Chernoff J: Targeting
Cdc42 in cancer. Expert Opin Ther Targets. 17:1263–1273. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Stengel K and Zheng Y: Cdc42 in oncogenic
transformation, invasion, and tumorigenesis. Cell Signal.
23:1415–1423. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
van Hengel J, D'Hooge P, Hooghe B, Wu X,
Libbrecht L, De Vos R, Quondamatteo F, Klempt M, Brakebusch C and
van Roy F: Continuous cell injury promotes hepatic tumori-genesis
in cdc42-deficient mouse liver. Gastroenterology. 134:781–792.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang Y, Takahashi S, Tasaka A, Yoshima T,
Ochi H and Chayama K: Involvement of microRNA-224 in cell
proliferation, migration, invasion, and anti-apoptosis in
hepatocellular carcinoma. J Gastroenterol Hepatol. 28:565–575.
2013. View Article : Google Scholar
|
|
23
|
Wang R, Zhao N, Li S, Fang JH, Chen MX,
Yang J, Jia WH, Yuan Y and Zhuang SM: MicroRNA-195 suppresses
angio-genesis and metastasis of hepatocellular carcinoma by
inhibiting the expression of VEGF, VAV2, and CDC42. Hepatology.
58:642–653. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li Y, Tang ZY, Ye SL, Liu YK, Chen J, Xue
Q, Chen J, Gao DM and Bao WH: Establishment of cell clones with
different metastatic potential from the metastatic hepatocellular
carcinoma cell line MHCC97. World J Gastroenterol. 7:630–636.
2001.
|