Introduction
Natural compounds exhibit a wide range of anticancer
effects, including cell cycle arrest, apoptosis, anti-angiogenesis,
and anticancer invasion and migration. Natural phytochemicals
containing phenolic compounds have been widely documented to have
the capability to prevent cancer metastasis (1). The Korean prostrate spurge
Euphorbia supina is a weed that belongs to the Euphorbiaceae
family. The plant has been used in folk medicine in Korea against a
variety of conditions such as bronchitis, jaundice, hemorrhage and
gastrointestinal diseases including gastritis, peptic ulcer,
diarrhea and hemorrhoid (2,3). It has been reported that this plant
contains a number of biologically important organic substances
(4–6). Among these, polyphenols have attracted
a great deal of interest due to their beneficial effects on human
health. Epidemiological studies have shown that polyphenols reduce
the risk of chronic diseases (7,8), and
have anti-oxidant, anti-aging, and anti-microbial properties
(9). According to Song et al
(10), nine polyphenols have been
isolated and identified from Korean E. supine, and quercetin
and kaempferol derivatives account for 84.8% of the total
polyphenols. Therefore, the inhibitory effects of E. supine
on cancer growth and metastasis could be expected; however, few
studies have been performed to demonstrate this effect.
Breast cancer is the most common cancer diagnosed in
Western European and North American women. Asian populations are
generally at the lowest risk, but the incidence has been steadily
increasing. Particularly, in Korea, the incidence of breast cancer
has increased by more than four times from 1996 to 2010, showing
the highest growth of breast cancer in the OECD countries (11). Most breast cancer patients virtually
die of metastasis. Cancer metastasis is the spread of cancer cells
from the primary neoplasm to distant sites, where secondary tumors
are formed. Its process involves several steps: the entrance of
cancer cells from the primary tumor into the vasculature, migration
to distant organs, adhesion to endothelial cells lining the blood
vessels, extravasation from the blood vessels, and the final
proliferation of secondary tumors (12). Thus, to enhance the survival of
cancer patients as well as the quality of life, the blockade of the
metastatic cascade with natural compounds has gained research
interest. In the present study, we investigated the effects of
polyphenol mixtures of Korean Euphorbia supina on the
invasion and metastasis of highly metastatic breast cancer
MDA-MB-231 cells.
Materials and methods
Preparation of polyphenol mixtures of
Euphorbia supine (E. supine)
Polyphenols from E. supine (PES) were
extracted and purified by Professor S.C. Shin as reported in Song
et al (10). Briefly, the
lyophilized E. supine tissue (10 g) was ground into powder
and extracted in ethyl acetate (300 ml) at 80°C for 20 h, and
eluted using a mixture of methanol:dichloromethane (1:5, 25 ml).
The isolated poly-phenol mixtures were identified by HPLC-MS/MS
according to a previous method (13). The nine polyphenols in the Korean
E. supina were as follows: gallic acid, protocatechuic acid,
nodakenin, quercetin-3-O-hexoside,
quercetin-3-O-pentoside, kaempferol 3-O-hexoside,
kaempferol 3-O-pentoside, quercetin and kaempferol.
Quercetin and kaempferol derivatives formed 84.8% of the total
polyphenols (10).
Materials
Anti-VCAM-1, anti-ICAM-1, anti-Snail,
anti-N-cadherin, anti-β-catenin, anti-E-cadherin, anti-VE-cadherin
and anti-LOX antibodies were purchased from Santa Cruz
Biotechnology (Santa Cruz, CA, USA). Anti-phospho-VE-cadherin
(phospho-Y658) antibody was purchased from Abcam (Cambridge, MA,
USA). Matrigel™ basement membrane matrix was supplied by BD
Biosciences (San Diego, CA, USA). Enhanced chemiluminescence (ECL)
western blotting detection reagent was obtained from Amersham
(Buckinghamshire, UK). All other chemicals, including β-actin, were
purchased from Sigma-Aldrich (St. Louis, MO, USA).
Cell culture
Human breast cancer cell line, MDA-MB-231, was
obtained from the Korean Cell Line Bank (Seoul, Korea) and grown in
RPMI-1640 supplemented with 10% fetal bovine serum (FBS), 2 mM
L-glutamine, 25 mM
N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid,
25 mM NaHCO3, 100 IU/ml penicillin and 10 µg/ml
streptomycin. Human umbilical vein endothelial cell line (EA.hy 926
cell) was obtained from the American Type Culture Collection (ATCC)
and grown in medium 199 supplemented with 20% FBS, 2 mM
L-glutamine, 5 U/ml heparin, 100 IU/ml penicillin, 10 µg/ml
streptomycin and 50 µg/ml EC growth supplements. Cells were
cultured in 100-mm dishes at 37°C in a humidified atmosphere of 95%
air and 5% cO2.
Cell viability assay
Cells were seeded at 104 cells/well in
24-well plates. Cells were treated with PES at the indicated doses
for 24 h. After treatments, 50 µl of 5 mg/ml MTT solution
was added to each well and incubated for 4 h. The supernatants were
aspirated, and the formazan crystals were dissolved with 200
µl of 4 N HCl-isopropanol in each well. The optical density
of the colored product was measured at 570 nm, as suggested by the
manufacturer, using an Infinite 200 microplate reader (Tecan
Austria GmbH, Grödig, Austria).
Western blot analysis
Western blot analysis was performed as described
previously (14), with minor
modifications. Briefly, cells were lysed using PRO-PREP protein
extraction solution (iNtRON Biotechnology, Seoul, Korea), and
proteins in conditioned media (CM) were concentrated 20-fold with
Pierce concentrator 7 ml/9K, MWCO devices (Thermo Pierce, Rockford,
IL, USA). The protein concentration was determined by the Bradford
method. Aliquots of 50 µg of protein were subjected to
7.5–12.5% sodium dodecyl sulfate-polyacryl-amide gel
electrophoresis (SDS-PAGE) and transferred onto
Hybond-P+ polyvinylidene difluoride membranes (Amersham
Biosciences UK Ltd.). The membranes were incubated with the
indicated primary antibodies. The bound antibodies were detected
with horseradish peroxidase-conjugated secondary antibodies and an
ECL western blotting detection reagent (Bionote, Gyeonggi-do,
Korea). β-actin was used as a loading control.
Adhesion assay
ECs and MDA-MB-231 cells were treated with PES for
24 h and subsequently stimulated with TNF-α for 6 h. Thereafter,
MDA-MB-231 cells (7.5×105 cells/ml) were added to the
Ecs. After 30 min at 37°C, cell suspensions were withdrawn, and the
ECs were gently washed with PBS three times. The cells were then
counted under a light microscope, and images were taken using an
Olympus microscope (CKX41) equipped with a camera (DS-U3;
Nikon).
Matrigel invasion assay
The Matrigel invasion assays were performed using EC
coated-Matrigel. ECs were pretreated with PES for 24 h and then
washed with PBS three times. After ECs were stimulated with TNF-α
for 6 h, MDA-MB-231 cells were added to EC-Matrigel-coated wells
and incubated for 24 h. The non-invasive cells that remained on the
upper side of the insert were removed. The cells on the lower part
of the insert membranes were stained with
4′,6-diamino-2-phe-nylindole (DAPI) and counted under a light
microscope.
Gelatin zymography
Media were prepared from MDA-MB-231 cells and
concentrated 20-fold using protein concentrators (9K MWCO).
Proteins in the media were precipitated with 80% cold acetone.
Precipitated proteins were mixed with sample buffer (0.03%
bromophenol blue, 0.4 M Tris-HCl pH 7.4, 20% glycerol, 5% SDS) and
separated on 8% SDS-polyacrylamide gels containing gelatin (1
mg/ml). Thereafter, the gels were washed with renaturing buffer
(2.5% Triton X-100) for 1 h and subsequently incubated for 24 h at
37°C in developing buffer (50 mM Tris, 20 mM NaCl, 5 mM
CaCl2, 0.02% Brij35, pH 7.5). Gels were stained with
0.05% Coomassie Brilliant Blue R-250 and destained with 50%
methanol and 10% acetic acid. Within the blue background, clear
zones indicated MMP proteolytic activity.
Statistical analysis
Scanning densitometry was performed using Image
Master® VDS (Pharmacia Biotech Inc., San Francisco, CA,
USA). The treatment groups were compared using one-way analysis of
variance and the post hoc test by Scheffe. All data are expressed
as the mean ± standard error of the mean (SEM). P<0.05 was
considered to indicate a statistically significant result.
Results
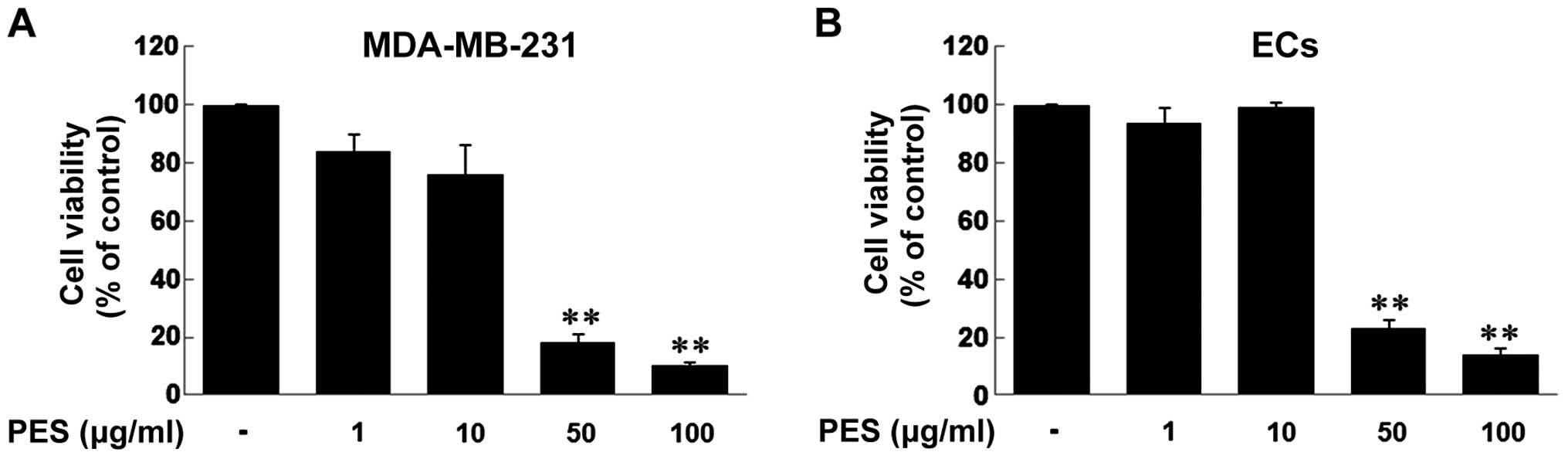
Effect of PES on the cell viability of
MDA-MB-231 breast cancer cells and ECs
First, we examined the cell viability of MDA-MB-231
breast cancer cells and ECs in response to PES treatment in a
dose-dependent manner (1, 10, 50 and 100 µg/ml). When
MDA-MB-231 cells and ECs were treated with the indicated doses of
PES for 24 h, the results revealed that the doses of 1 and 10
µg/ml of PES did not decrease the cell viability of both
MDA-MB-231 cells and ECs, but the doses of 50 and 100 µg/ml
significantly decreased the cell viability of both types of cells
(Fig. 1).
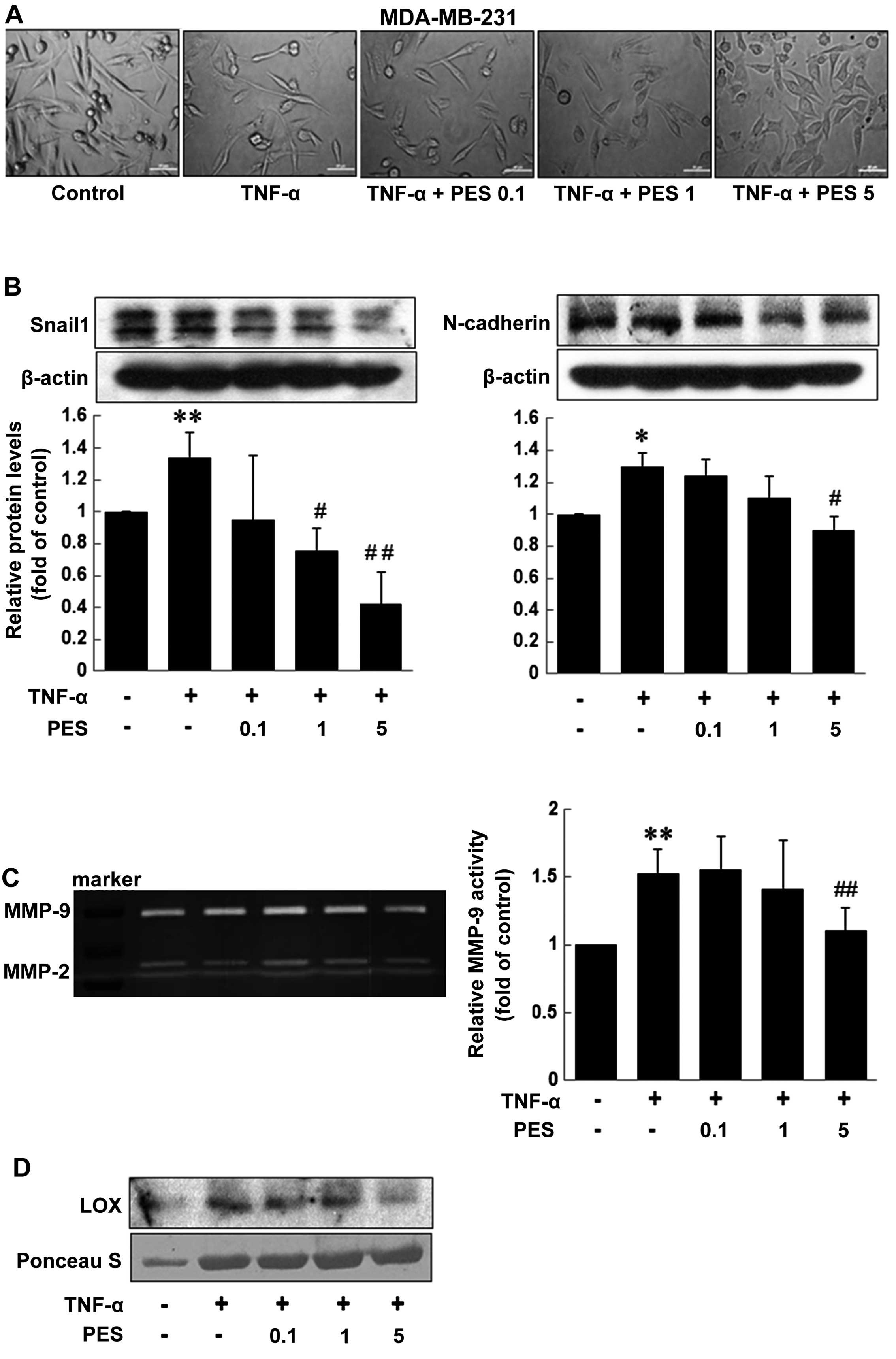
PES downregulates the levels of
mesenchymal markers and inhibits MMPs and lysyl oxidase (LOX)
secretion in TNF-α-treated MDA-MB-231 cells
Next, we observed changes in MDA-MB-231 cell
morphology after PES treatment. Fig.
2A showed that PES induced morphologic changes in the
MDA-MB-231 cells from a mesenchymal form to an epithelial form in a
dose-dependent manner. Furthermore, PES significantly reduced the
levels of mesenchymal markers Snail and N-cadherin at the dose of 1
or 5 µg/ml, but not β-catenin and E-cadherin levels (data
not shown). These results suggest that PES suppresses EMT by
downregulating the mesenchymal markers, Snail1 and N-cadherin
(Fig. 2B). Proteolytic digestion of
the extracellular matrix (ECM) by secreted MMPs is one of the major
steps for cancer invasion (reviewed in refs. 15 and 16), and MMP-9 expression is involved in
tumor-cell invasion and metastasis (17). In addition, LOX is overexpressed in
breast cancer patients and is known to be a novel mechanism for the
promotion of metastasis (18).
Thus, we examined the effect of PES on the activity of secreted
MMP-9 and the level of LOX in MDA-MB-231 cells in the presence of
TNf-α or not. PES suppressed the gelatinolytic activity of secreted
MMP-9 augmented by TNf-α in the MDA-MB-231 cells; the suppressive
effect was significant at 5 µg/ml of PES (Fig. 2C). Fig.
2D showed that induction in LOX secretion by TNf-α was also
prominently reduced at 5 µg/ml of PES.
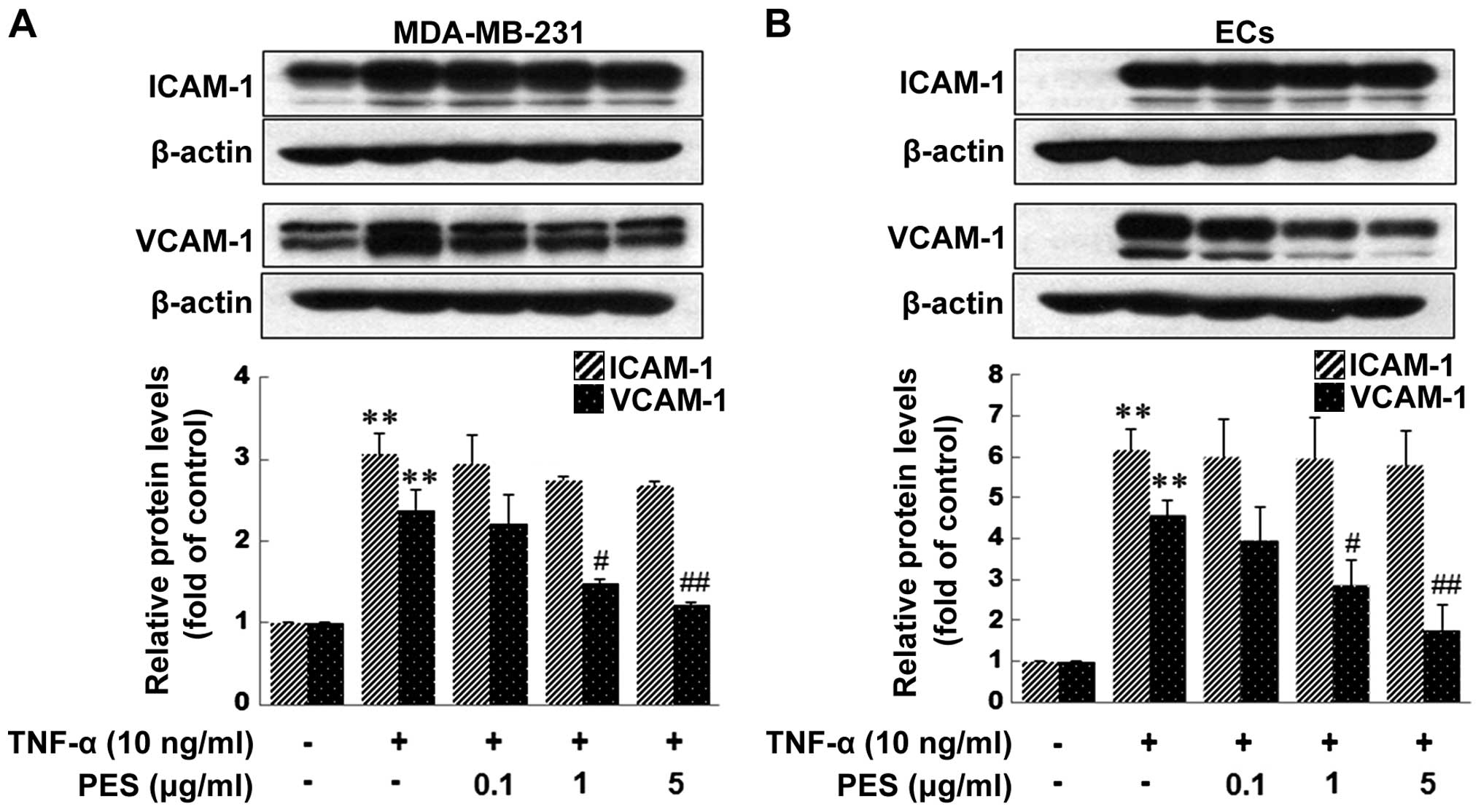
PES reduces the expression of VCAM-1
induced by TNF-α in MDA-MB-231 cells and ECs, and inhibits the
adhesion of MDA-MB-231 cells to TNF-α-stimulated ECs
The adhesion of circulating tumor cells to the
microvascular endothelium of distant organs is an important step in
blood-born metastasis. ICAM-1 and VCAM-1 have been shown to be
involved in cell-cell and cell-ECM interactions and are
mechanistically important for the extravasation of both monocytes
and cancer cells (19–21) during inflammation and metastasis,
respectively. Therefore, we examined whether PES inhibits VCAM-1
and ICAM-1 expression induced by TNF-α in ECs as well as in
MDA-MB-231 cells. Pretreatment of PES significantly inhibited
TNF-α-induced VCAM-1 expression, but not ICAM-1 expression in both
the MDA-MB-231 cells and ECs (Fig.
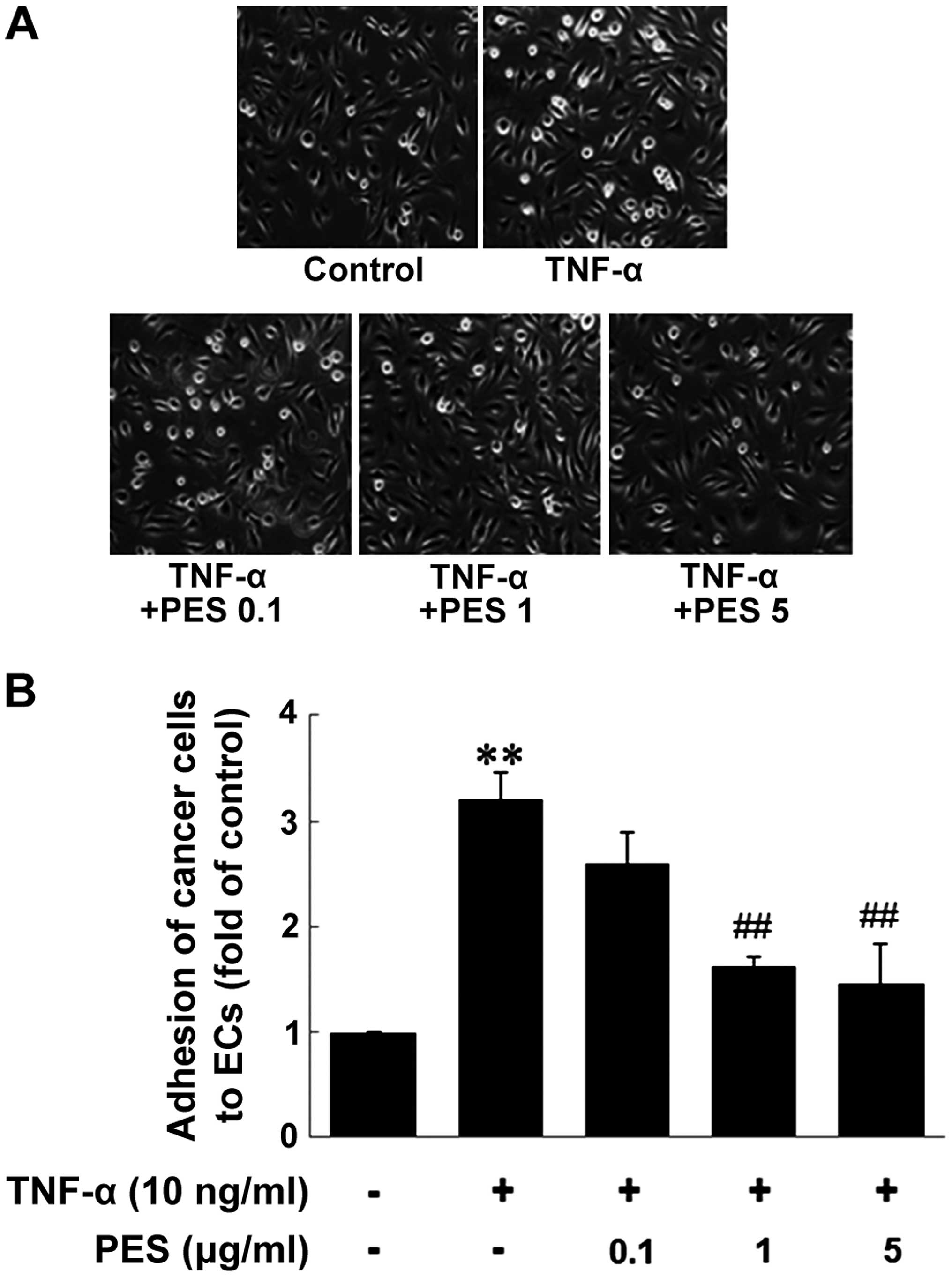
3). Then, we investigated the effect of PES on the adhesion of
MDA-MB-231 cells to ECs. The adhesion of the MDA-MB-231 cells to
ECs was markedly increased by TNF-α treatment (10 ng/ml, 6 h),
compared to unactivated ECs. In contrast, the treatment of PES
(0.1–5 µg/ml) to ECs for 1 h before TNF-α stimulation
resulted in a significant reduction in the adhesion of the
MDA-MB-231 cells to ECs (Fig.
4).
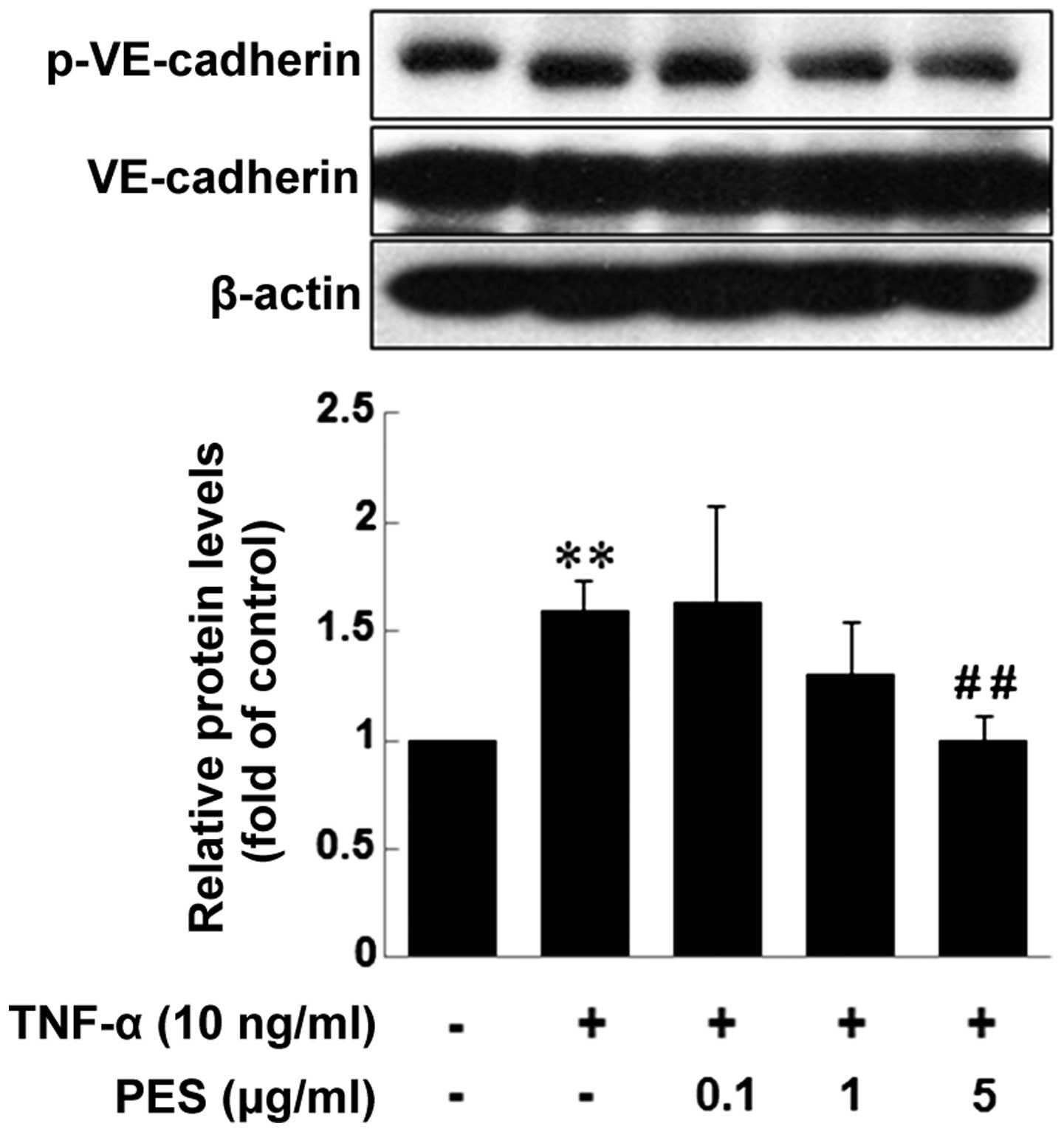
PES inhibits the phosphorylation of
VE-cadherin mediated by TNF-α in ECs
Tyrosine phosphorylation of VE-cadherin is known to
be associated with weak junctions and impaired barrier function.
Therefore, we investigated the effect of PES on the phosphorylation
of VE-cadherin at tyrosine residue 658 (Y658) by western blotting.
PES treatment 1 h prior to TNF-α decreased TNF-α-induced
phospho-VE-cadherin from 1 µg/ml of PES, showing a
significant inhibition at 5 µg/ml (Fig. 5).
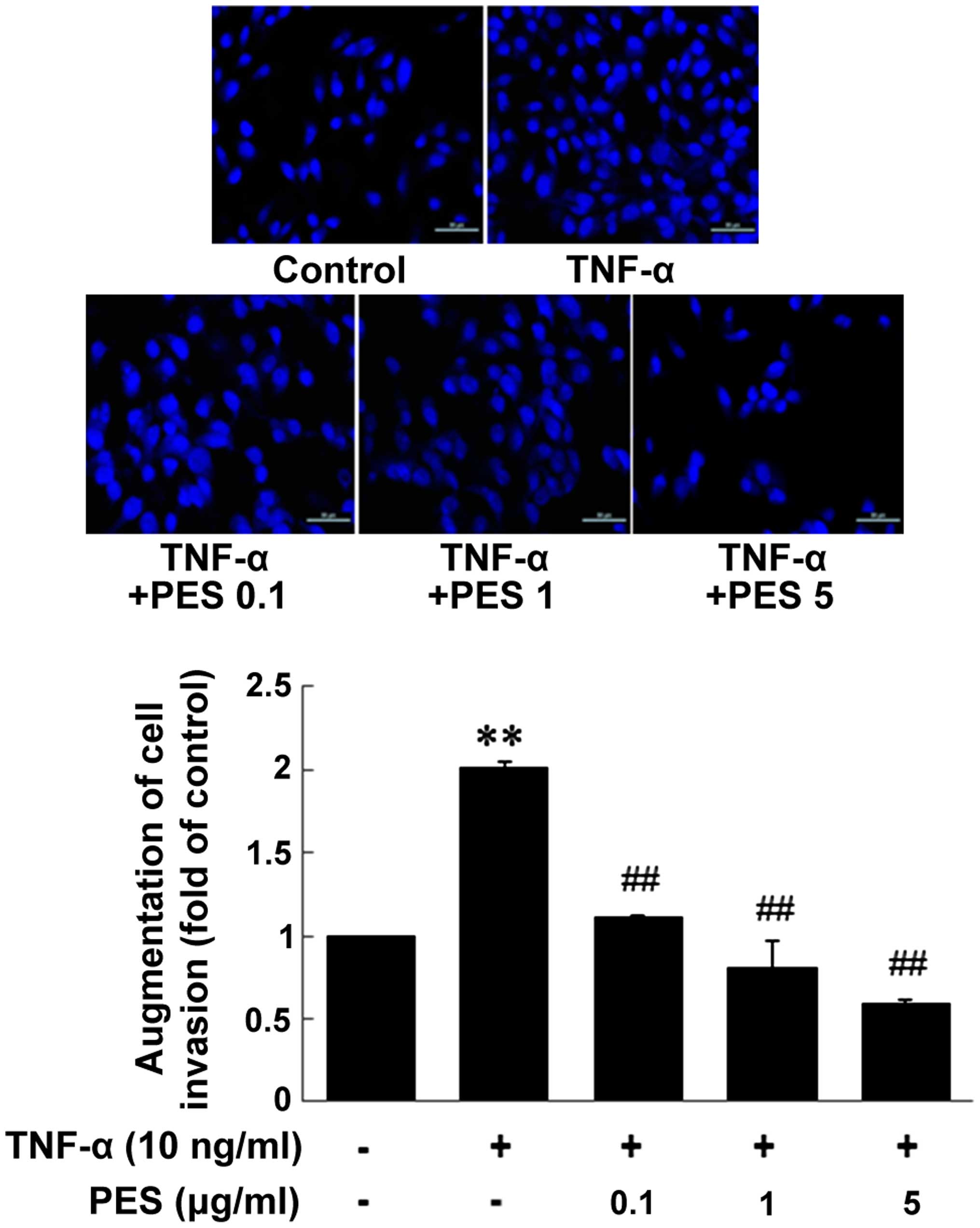
PES downregulates MDA-MB-231 cell
invasion induced by TNF-α
Finally, we examined the effect of PES on MDA-MB-231
cell invasion through ECs. ECs were pretreated with PES for 1 h and
stimulated with TNF-α for an additional 6 h. Then, MDA-MB-231 cells
were added to the EC-Matrigel-coated wells and incubated for 24 h.
As shown in Fig. 6, PES
dose-dependently inhibited MDA-MB-231 cell invasion through
TNF-α-stimulated ECs.
Discussion
Great advances in medical science have been
achieved. Yet, the population of individuals diagnosed with cancer
is steadily increasing, and cancer patients virtually die due to
metastasis. Therefore, it is important to develop a strategy to
inhibit cancer metastasis with minimal toxicity to normal cells to
enhance the quality of life of patients. In this regard, much
research has focused on natural compounds with anticancer effects.
Particularly, natural phytochemicals containing phenolic compounds
are known to prevent cancer metastasis (1). The Korean prostrate spurge
Euphorbia supina is reported to contain a number of
biologically significant organic substances such as polyphenols and
has been used as a folk medicine in Korea against a variety of
inflammatory conditions. Therefore, we aimed to ascertain whether
the polyphenols in Euphorbia supina (PES) have a suppresive
effect on the invasion and metastasis of breast cancer cells.
Cancer metastasis involves several steps in which
cellular responses between cancer cells and normal cells are
coordinately involved. These include the entrance of cancer cells
from the primary tumor into the vasculature, migration to distant
organs, adhesion to endothelial cells lining the blood vessels,
extravasation from the blood vessels and proliferation of secondary
tumors. EMT is a process that converts an epithelial cell to a
mesenchymal cell by promoting the loss of cell-cell adhesion,
leading to the release of cells from the surrounding tissue, and
finally enables cells to acquire the migratory capability to
invade. Therefore, EMT can be regarded as an initial process of
metastasis, and EMT occurring during tumor progression is
considered to be the major mechanism that is responsible for the
invasion and metastasis of cancer cells (22–24).
Within the tumor microenvironment, tumors release several factors
which can promote cancer metastasis; MMPs are involved in the
proteolytic digestion of the ECM as well as angiogenesis, which are
major steps in cancer invasion. In addition, adhesion molecules
such as ICAM-1 and VCAM-1 are involved in cell-cell and cell-ECM
interactions and are mechanistically important for the
extravasation of cancer cells during metastasis (20,21).
In particular, VCAM-1 is expressed preferentially or highly on
breast cancer endothelium compared to normal endothelium (25,26).
Another important factor is endothelial cell membrane permeability
regulated by transmembrane endothelial adherens junctions (AJs). In
endothelial cells, AJs are largely composed of VE-cadherin. The
phosphorylation, cleavage and internalization of VE-cadherin are
thought to affect endothelial permeability (27). Thus, in the present study, we
determined the effect of PES on the invasion and metastasis of
highly metastatic breast cancer MDA-MB-231 cells.
As expected, PES significantly suppressed EMT by
downregulating the mesenchymal markers, Snail1 and N-cadherin. In
addition, PES significantly inhibited MMP-9 activity induced by
TNF-α at 5 µg/ml. Moreover, the release of LOX, an enzyme
that crosslinks EcM proteins such as collagen and promotes breast
cancer metastasis, was induced by TNF-α, which was inhibited by PES
treatment (mainly at 5 µg/ml). Then, we determined the
effect of PES on the expression of adhesion molecules and the
phosphorylation of VE-cadherin. The results showed that PES
effectively reduced TNF-α-induced VCAM-1 but not ICAM expression in
both the MDA-MB-231 cells and ECs, resulting in the decreased
adhesion of MDA-MB-231 cells to ECs. Furthermore, PES suppressed
LOX secretion by TNf-α, suggesting that PES efficiently inhibited
the invasion of MDA-MB-231 cells. Finally, when we assessed whether
PES inhibits the invasion of MDA-MB-231 cells through ECs, the
results showed that PES effectively inhibited MDA-MB-231 cell
invasion through ECs at a very low concentration (0.1 µg/ml)
where it showed no cytotoxicity on cancer cells and ECs.
The most abundant flavonoids in the diet, flavonols,
exhibit numerous biological and pharmacological effects including
anticancer-related properties (28), and quercetin and kaempferol
derivatives are the major components of total polyphenols isolated
and identified from Korean E. supine. Quercetin
(3,5,7,3′,4′-pentahydroxyflavone) is an active component of
flavonoids that abundantly exists in many fruits and vegetables,
and dietary food sources. Quercetin exhibits various beneficial
biological activities, such as anti-oxidant, anti-inflammatory,
anti-atherosclerotic, and anti-tumorigenic activities (29–31).
Quercetin was found to inhibit hL-60 leukemia cell proliferation in
association with the inhibition of cytosolic protein kinase C and
membrane tyrosine protein kinase in vitro (32). Furthermore, quercetin exerted
anti-proliferative effects against glioma and breast cancer cells
(33–35). It has been suggested that quercetin
may be a potential anti-invasive compound in breast cancer cells
(36,37). In addition, kaempferol and its
derivatives also exhibit a wide range of pharmacological activities
including anti-oxidant, anti-inflammatory, anticancer,
anti-microbial, cardioprotective, neuroprotective, and
anti-diabetic activities (38).
Kaempferol induced apoptosis in HL60 leukemia cells which was
accompanied by significant DNA condensation and increased ATP
levels. It also altered the expression of caspase-3 and
apoptosis-inducing factor (39).
The regular consumption of foods containing kaempferol has been
positively correlated to a reduction in the risk for developing
several disorders including cancer. In breast cancer, similar to
the activity of quercetin, kaempferol exhibited inhibitory activity
on the invasiveness and MMP-3 levels in MDA-MB-231 cells (37). Based on our results and these
studies, PES containing quercetin and kaempferol may suppress the
process of metastasis of highly metastatic breast cancer cells by
regulating the adhesion of MDA-MB-231 cells to ECs by inhibiting
VCAM-1 expression in ECs, and by reducing EC permeability and
inhibiting EMT in MDA-MB-231 cells. Finally, PES may serve as a
therapeutic agent against cancer metastasis with minimal
cytotoxicity to normal cells.
Acknowledgments
The present study was supported by grants from the
National R&D Program for Cancer Control, the Ministry of Health
and Welfare, Republic of Korea (no. 0820050).
Abbreviations:
|
AJs
|
adherens junctions
|
|
CAM
|
cell adhesion molecule
|
|
CM
|
conditioned media
|
|
DAPI
|
4′,6-diamino-2-phenylindole
|
|
ECs
|
endothelial cells
|
|
ECM
|
extracellular matrix
|
|
ECL
|
enhanced chemiluminescence
|
|
EMT
|
epithelial-mesenchymal transition
|
|
FBS
|
fetal bovine serum
|
|
ICAM
|
intracellular adhesion molecule
|
|
LOX
|
lysyl oxidase
|
|
MTT
|
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide
|
|
PBS
|
phosphate-buffered saline
|
|
SDS
|
sodium dodecyl surfate
|
|
TBS
|
Tris-buffered saline
|
|
TNF
|
tumor necrosis factor
|
|
VCAM
|
vascular cell adhesion molecule
|
|
VE-cadherin
|
vascular endothelial cadgerin
|
References
|
1
|
Sliva D: Suppression of cancer
invasiveness by dietary compounds. Mini Rev Med Chem. 8:677–688.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tanaka R, Kurimoto M, Yoneda M and
Matsunaga S: 17β,21β-Epoxyhopan-3β-ol and β-alnincanol from
Euphorbia supina. Phytochemistry. 29:2253–2256. 1990. View Article : Google Scholar
|
|
3
|
An RB, Kwon JW, Kwon TO, Chung WT, Lee HS
and Kim YC: Chemical constituents from the whole plants of
Euphorbia supina Rafin. Korean J Pharmacognosy. 38:291–295.
2007.
|
|
4
|
Agata I, Hatano T, Nakaya Y, Sugaya T,
Nishibe S, Yoshida T and Okuda T: Tannins and related polyphenols
of euphorbiaceous plants. VIII. Eumaculin A and eusupinin A, and
accompanying polyphenols from Euphorbia maculata L. and E. supina
Rafin. Chem Pharm Bull (Tokyo). 39:881–883. 1991. View Article : Google Scholar
|
|
5
|
Lee SH, Tanaka T, Nonaka G and Nishioka I:
Tannins and related compounds. CV. Monomeric and dimeric
hydrolyzable tannins having a dehydrohexahydroxydiphenoyl group,
supinanin, euphorscopin, euphorhelin and jolkianin, from Euphorbia
species. Chem Pharm Bull (Tokyo). 39:630–638. 1991. View Article : Google Scholar
|
|
6
|
Fang Z, Zeng X, Zhang Y and Zhou G:
Chemical constituents of spotted leaf euphorbia (Euphorbia supina).
Zhongcaoyao. 24:230–233. 1993.
|
|
7
|
Erlund I: Review of the flavonoids
quercetin, hesperetin, and naringenin. Dietary sources,
bioactivities, bioavailability, and epidemiology. Nutr Res.
24:851–874. 2004. View Article : Google Scholar
|
|
8
|
Le Marchand L: Cancer preventive effects
of flavonoids - a review. Biomed Pharmacother. 56:296–301. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu YC, Leung SW, Yeung DK, Hu LH, Chen GH,
Che CM and Man RY: Structure-activity relationships of flavonoids
for vascular relaxation in porcine coronary artery. Phytochemistry.
68:1179–1188. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Song Y, Jeong SW, Lee WS, Park S, Kim YH,
Kim GS, Lee SJ, Jin JS, Kim CY, Lee JE, et al: Determination of
polyphenol components of Korean prostrate spurge (Euphorbia supina)
by using liquid chromatography-tandem mass spectrometry: Overall
contribution to antioxidant activity. J Anal Methods Chem.
2014:4186902014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Korean Breast Cancer Society: Korean
breast cancer data of 1996. J Korean Surg Soc. 55:621–635.
1998.
|
|
12
|
Lu X and Kang Y: Hypoxia and
hypoxia-inducible factors: Master regulators of metastasis. Clin
Cancer Res. 16:5928–5935. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim HG, Kim GS, Park S, Lee JH, Seo ON,
Lee SJ, Kim JH, Shim JH, Abd El-Aty AM, Jin JS, et al: Flavonoid
profiling in three citrus varieties native to the Republic of Korea
using liquid chromatography coupled with tandem mass spectrometry:
Contribution to overall antioxidant activity. Biomed Chromatogr.
26:464–470. 2012. View
Article : Google Scholar
|
|
14
|
Joo YN, Jin H, Eun SY, Park SW, Chang KC
and Kim HJ: P2Y2R activation by nucleotides released from the
highly metastatic breast cancer cell MDA-MB-231 contributes to
pre-metastatic niche formation by mediating lysyl oxidase
secretion, collagen crosslinking, and monocyte recruitment.
Oncotarget. 5:9322–9334. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rucci N, Sanità P and Angelucci A: Roles
of metalloproteases in metastatic niche. Curr Mol Med. 11:609–622.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Deryugina EI and Quigley JP: Matrix
metalloproteinases and tumor metastasis. Cancer Metastasis Rev.
25:9–34. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Przybylowska K, Kluczna A, Zadrozny M,
Krawczyk T, Kulig A, Rykala J, Kolacinska A, Morawiec Z, Drzewoski
J and Blasiak J: Polymorphisms of the promoter regions of matrix
metalloproteinases genes MMP-1 and MMP-9 in breast cancer. Breast
Cancer Res Treat. 95:65–72. 2006. View Article : Google Scholar
|
|
18
|
Zoccoli A, Iuliani M, Pantano F,
Imperatori M, Intagliata S, Vincenzi B, Marchetti P, Papapietro N,
Denaro V, Tonini G, et al: Premetastatic niche: Ready for new
therapeutic interventions? Expert Opin Ther Targets. 16(Suppl 2):
S119–S129. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mousa SA: Cell adhesion molecules:
Potential therapeutic and diagnostic implications. Mol Biotechnol.
38:33–40. 2008. View Article : Google Scholar
|
|
20
|
Price JT and Thompson EW: Mechanisms of
tumour invasion and metastasis: Emerging targets for therapy.
Expert Opin Ther Targets. 6:217–233. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Balkwill F and Mantovani A: Inflammation
and cancer: Back to Virchow? Lancet. 357:539–545. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Christofori G: New signals from the
invasive front. Nature. 441:444–450. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mitra A, Mishra L and Li S: EMT, CTCs and
CSCs in tumor relapse and drug-resistance. Oncotarget.
6:10697–10711. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang J and Weinberg RA:
Epithelial-mesenchymal transition: At the crossroads of development
and tumor metastasis. Dev Cell. 14:818–829. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fox SB, Turner GD, Gatter KC and Harris
AL: The increased expression of adhesion molecules ICAM-3, E- and
P-selectins on breast cancer endothelium. J Pathol. 177:369–376.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nguyen M, Corless CL, Kräling BM, Tran C,
Atha T, Bischoff J and Barsky SH: Vascular expression of E-selectin
is increased in estrogen-receptor-negative breast cancer: A role
for tumor-cell-secreted interleukin-1 alpha. Am J Pathol.
150:1307–1314. 1997.PubMed/NCBI
|
|
27
|
Dejana E, Orsenigo F and Lampugnani MG:
The role of adherens junctions and VE-cadherin in the control of
vascular permeability. J Cell Sci. 121:2115–2122. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Middleton E Jr, Kandaswami C and
Theoharides TC: The effects of plant flavonoids on mammalian cells:
Implications for inflammation, heart disease, and cancer. Pharmacol
Rev. 52:673–751. 2000.PubMed/NCBI
|
|
29
|
Naderi GA, Asgary S, Sarraf-Zadegan N and
Shirvany H: Anti-oxidant effect of flavonoids on the susceptibility
of LDL oxidation. Mol Cell Biochem. 246:193–196. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mamani-Matsuda M, Kauss T, Al-Kharrat A,
Rambert J, Fawaz F, Thiolat D, Moynet D, Coves S, Malvy D and
Mossalayi MD: Therapeutic and preventive properties of quercetin in
experimental arthritis correlate with decreased macrophage
inflammatory mediators. Biochem Pharmacol. 72:1304–1310. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lotito SB and Frei B: Dietary flavonoids
attenuate tumor necrosis factor alpha-induced adhesion molecule
expression in human aortic endothelial cells. Structure-function
relationships and activity after first pass metabolism. J Biol
Chem. 281:37102–37110. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kang TB and Liang NC: Studies on the
inhibitory effects of quercetin on the growth of HL-60 leukemia
cells. Biochem Pharmacol. 54:1013–1018. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Braganhol E, Zamin LL, Canedo AD, Horn F,
Tamajusuku AS, Wink MR, Salbego C and Battastini AM:
Antiproliferative effect of quercetin in the human U138MG glioma
cell line. Anticancer Drugs. 17:663–671. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Indap MA, Radhika S, Motiwale L and Rao
KVK: Quercetin: Antitumor activity and pharmacological
manipulations for increased therapeutic gains. Indian J Pharm Sci.
68:465–469. 2006. View Article : Google Scholar
|
|
35
|
Choi EJ, Bae SM and Ahn WS:
Antiproliferative effects of quercetin through cell cycle arrest
and apoptosis in human breast cancer MDA-MB-453 cells. Arch Pharm
Res. 31:1281–1285. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lin CW, Hou WC, Shen SC, Juan SH, Ko CH,
Wang LM and Chen YC: Quercetin inhibition of tumor invasion via
suppressing PKc delta/ErK/AP-1-dependent matrix metalloproteinase-9
activation in breast carcinoma cells. Carcinogenesis. 29:1807–1815.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Phromnoi K, Yodkeeree S, Anuchapreeda S
and Limtrakul P: Inhibition of MMP-3 activity and invasion of the
MDA-MB-231 human invasive breast carcinoma cell line by
bioflavonoids. Acta Pharmacol Sin. 30:1169–1176. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Calderón-Montaño JM, Burgos-Morón E,
Pérez-Guerrero C and López-Lázaro M: A review on the dietary
flavonoid kaempferol. Mini Rev Med Chem. 11:298–344. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Leung HW, Lin CJ, Hour MJ, Yang WH, Wang
MY and Lee HZ: Kaempferol induces apoptosis in human lung non-small
carcinoma cells accompanied by an induction of antioxidant enzymes.
Food Chem Toxicol. 45:2005–2013. 2007. View Article : Google Scholar : PubMed/NCBI
|