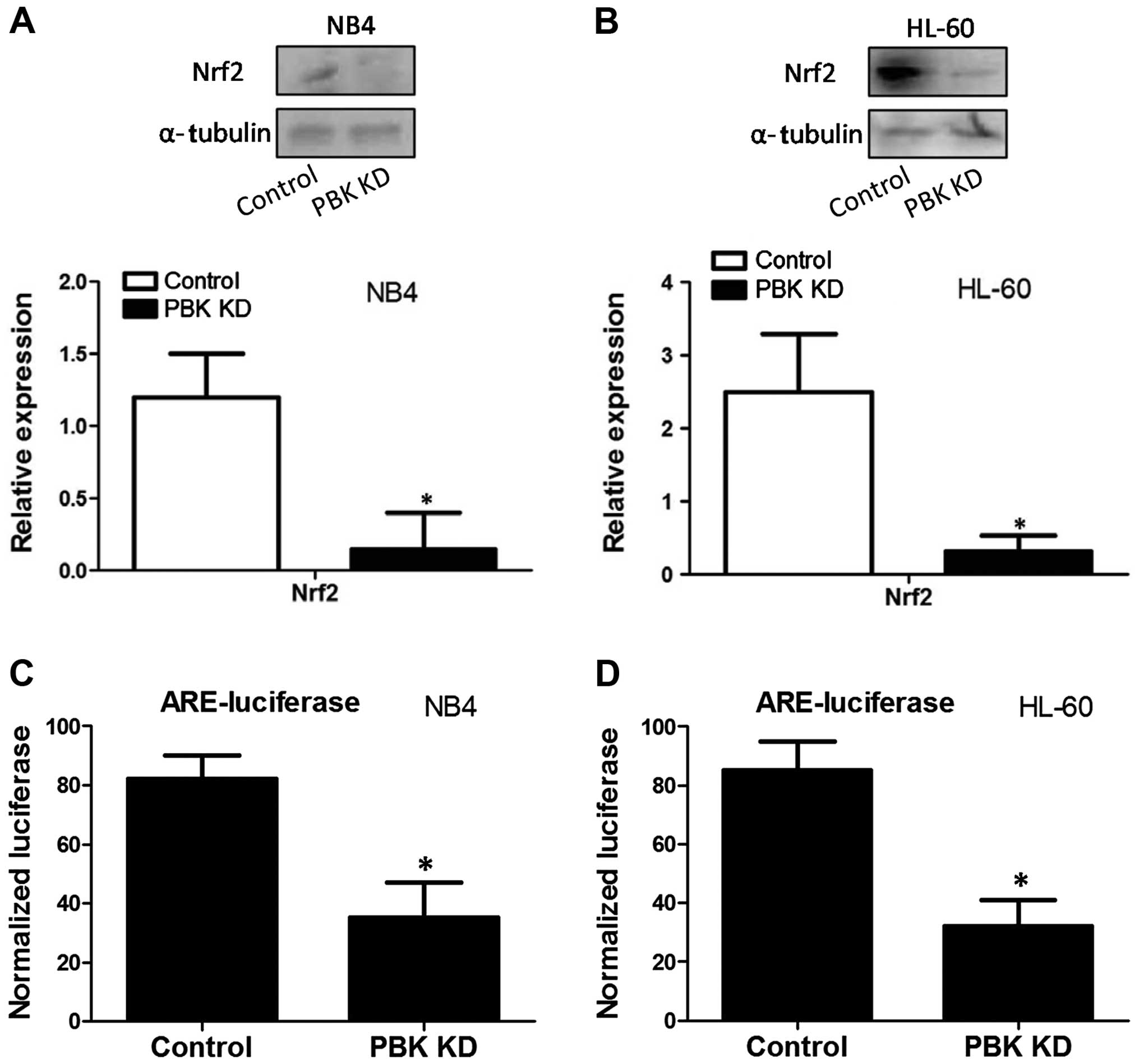
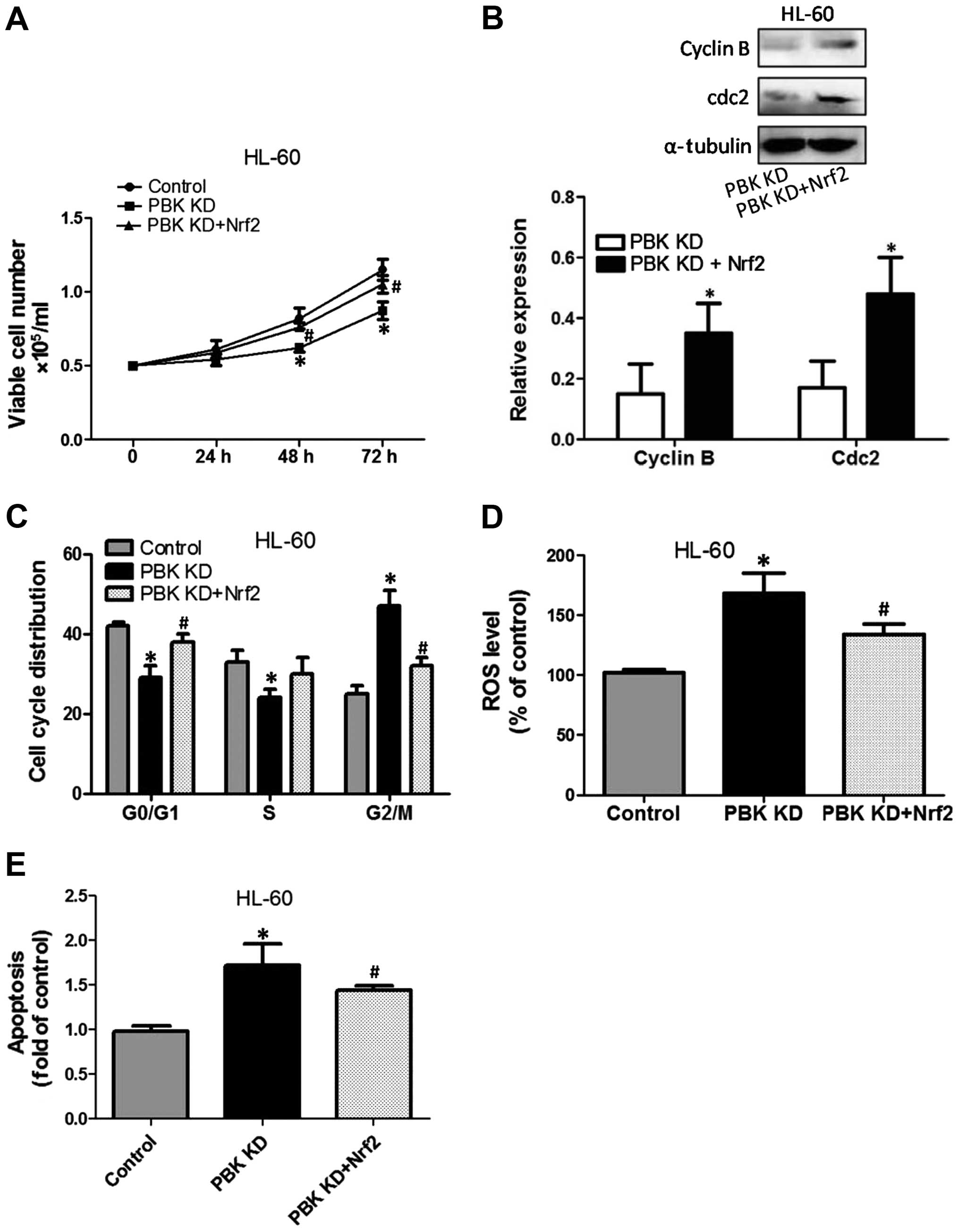
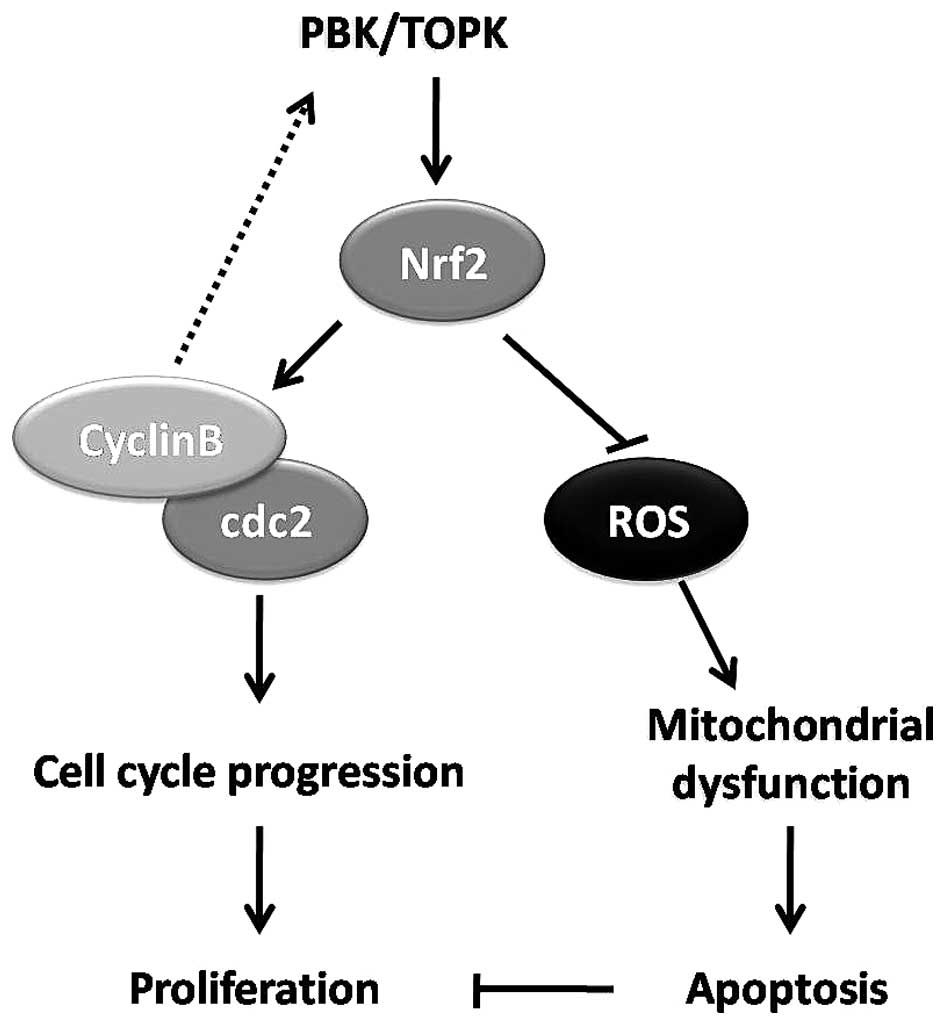
|
1
|
Rampal R and Mascarenhas J: Pathogenesis
and management of acute myeloid leukemia that has evolved from a
myeloproliferative neoplasm. Curr Opin Hematol. 21:65–71. 2014.
View Article : Google Scholar
|
|
2
|
Nasr R, Lallemand-Breitenbach V, Zhu J,
Guillemin MC and de Thé H: Therapy-induced PML/RARA proteolysis and
acute promyelocytic leukemia cure. Clin Cancer Res. 15:6321–6326.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Coombs CC, Tavakkoli M and Tallman MS:
Acute promyelocytic leukemia: Where did we start, where are we now,
and the future. Blood Cancer J. 5:e3042015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gaudet S, Branton D and Lue RA:
Characterization of PDZ-binding kinase, a mitotic kinase. Proc Natl
Acad Sci USA. 97:5167–5172. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Abe Y, Matsumoto S, Kito K and Ueda N:
Cloning and expression of a novel MAPKK-like protein kinase,
lymphokine-activated killer T-cell-originated protein kinase,
specifically expressed in the testis and activated lymphoid cells.
J Biol Chem. 275:21525–21531. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao S, Dai J, Zhao W, Xia F, Zhou Z, Wang
W, Gu S, Ying K, Xie Y and Mao Y: PDZ-binding kinase participates
in spermatogenesis. Int J Biochem Cell Biol. 33:631–636. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nandi A, Tidwell M, Karp J and Rapoport
AP: Protein expression of PDZ-binding kinase is up-regulated in
hematologic malignancies and strongly down-regulated during
terminal differentiation of HL-60 leukemic cells. Blood Cells Mol
Dis. 32:240–245. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Côté S, Simard C and Lemieux R: Regulation
of growth-related genes by interleukin-6 in murine myeloma cells.
Cytokine. 20:113–120. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Simons-Evelyn M, Bailey-Dell K, Toretsky
JA, Ross DD, Fenton R, Kalvakolanu D and Rapoport AP: PBK/TOPK is a
novel mitotic kinase which is upregulated in Burkitt's lymphoma and
other highly proliferative malignant cells. Blood Cells Mol Dis.
27:825–829. 2001. View Article : Google Scholar
|
|
10
|
Matsumoto S, Abe Y, Fujibuchi T, Takeuchi
T, Kito K, Ueda N, Shigemoto K and Gyo K: Characterization of a
MAPKK-like protein kinase TOPK. Biochem Biophys Res Commun.
325:997–1004. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ayllón V and O'connor R: PBK/TOPK promotes
tumour cell proliferation through p38 MAPK activity and regulation
of the DNA damage response. Oncogene. 26:3451–3461. 2007.
View Article : Google Scholar
|
|
12
|
Kumagai A and Dunphy WG: Control of the
Cdc2/cyclin B complex in Xenopus egg extracts arrested at a G2/M
checkpoint with DNA synthesis inhibitors. Mol Biol Cell. 6:199–213.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Clarke PR, Klebe C, Wittinghofer A and
Karsenti E: Regulation of cdc2/cyclin b activation by ran, a
ras-related GTPase. J Cell Sci. 108:1217–1225. 1995.PubMed/NCBI
|
|
14
|
Mohammad RM, Muqbil I, Lowe L, Yedjou C,
Hsu HY, Lin LT, Siegelin MD, Fimognari C, Kumar NB, Dou QP, et al:
Broad targeting of resistance to apoptosis in cancer. Semin Cancer
Biol. Apr 28–2015.Epub ahead of print. View Article : Google Scholar
|
|
15
|
Flusberg DA and Sorger PK: Surviving
apoptosis: Life-death signaling in single cells. Trends Cell Biol.
Apr 25–2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sinha K, Das J, Pal PB and Sil PC:
Oxidative stress: The mitochondria-dependent and
mitochondria-independent pathways of apoptosis. Arch Toxicol.
87:1157–1180. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Balaban RS, Nemoto S and Finkel T:
Mitochondria, oxidants, and aging. Cell. 120:483–495. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Turrens JF: Mitochondrial formation of
reactive oxygen species. J Physiol. 552:335–344. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bonora M and Pinton P: The mitochondrial
permeability transition pore and cancer: Molecular mechanisms
involved in cell death. Front Oncol. 4:3022014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chistiakov DA, Sobenin IA, Revin VV,
Orekhov AN and Bobryshev YV: Mitochondrial aging and age-related
dysfunction of mitochondria. Biomed Res Int. 2014:2384632014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Correia-Melo C and Passos JF:
Mitochondria: Are they causal players in cellular senescence?
Biochim Biophys Acta. May 28–2015.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Singh KK: Mitochondria damage checkpoint,
aging, and cancer. Ann NY Acad Sci. 1067:182–190. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Penna C, Perrelli MG and Pagliaro P:
Mitochondrial pathways, permeability transition pore, and redox
signaling in cardioprotection: Therapeutic implications. Antioxid
Redox Signal. 18:556–599. 2013. View Article : Google Scholar
|
|
24
|
Osman MM, Lulic D, Glover L, Stahl CE, Lau
T, van Loveren H and Borlongan CV: Cyclosporine-A as a
neuroprotective agent against stroke: Its translation from
laboratory research to clinical application. Neuropeptides.
45:359–368. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rushworth GF and Megson IL: Existing and
potential therapeutic uses for N-acetylcysteine: The need for
conversion to intracellular glutathione for antioxidant benefits.
Pharmacol Ther. 141:150–159. 2014. View Article : Google Scholar
|
|
26
|
Kaspar JW, Niture SK and Jaiswal AK:
Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radic Biol
Med. 47:1304–1309. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hayes JD and McMahon M: NRF2 and KEAP1
mutations: Permanent activation of an adaptive response in cancer.
Trends Biochem Sci. 34:176–188. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang DD: Mechanistic studies of the
Nrf2-Keap1 signaling pathway. Drug Metab Rev. 38:769–789. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
DeNicola GM, Karreth FA, Humpton TJ,
Gopinathan A, Wei C, Frese K, Mangal D, Yu KH, Yeo CJ, Calhoun ES,
et al: Oncogene-induced Nrf2 transcription promotes ROS
detoxification and tumorigenesis. Nature. 475:106–109. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Niture SK and Jaiswal AK: Nrf2-induced
antiapoptotic Bcl-xL protein enhances cell survival and drug
resistance. Free Radic Biol Med. 57:119–131. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Burhans WC and Heintz NH: The cell cycle
is a redox cycle: Linking phase-specific targets to cell fate. Free
Radic Biol Med. 47:1282–1293. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rushworth SA, MacEwan DJ and O'Connell MA:
Lipo-polysaccharide-induced expression of NAD(P)H:quinone
oxidoreductase 1 and heme oxygenase-1 protects against excessive
inflammatory responses in human monocytes. J Immunol.
181:6730–6737. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rushworth SA, Bowles KM and MacEwan DJ:
High basal nuclear levels of Nrf2 in acute myeloid leukemia reduces
sensitivity to proteasome inhibitors. Cancer Res. 71:1999–2009.
2011. View Article : Google Scholar : PubMed/NCBI
|