Introduction
Colon cancer, one of the most common malignancies,
leads to major cancer morbidity and mortality. Although the current
diagnoses and treatment for colon cancer have greatly improved in
the last decades, the prognosis is still poor. The challenges for
colon cancer clinical treatment are the serious side-effects of the
current chemotherapy drugs and the metastasis of colon cancer
cells. Thus, there is a great need to develop new clinical
treatment regimens for colon cancer therapy.
Some natural products and their essential derived
bioactive components, including semi-synthetic and synthetic
analogs, have been used as anticancer agents for a few decades
(1–4), such as taxol, vincristine,
camptothecin, vinblastine, teniposide and etoposide (5). Hence, natural products play a major
role in cancer chemotherapy. Evodiamine (Evo), a quinolone alkaloid
from the traditional herb medicine Evodia rutaecarpa
(6), may be used to treat many
diseases, such as obesity, inflammation and cardiovascular
diseases, due to its versatile pharmacological functions (7). Increasing evidence supports that Evo
processes anticancer activity in various cancer types, such as
lung, colon and breast cancer (8–10).
This effect of Evo may be associated with downregulating ERK
(8), activating JNK (9) or degrading estrogen receptor (11). However, the exact molecular
mechanism underlay this effect of Evo in cancer remains
unclear.
Hypoxia-inducible factors (HIFs) are special
transcriptional factors that respond to the decrease of oxygen in
the cellular environment (12).
HIF-1 is composed of HIF-1α and HIF-1β subunits, and HIF-1α level
determines the transcriptional activity of HIF-1 since it has an
extremely short half-life (12).
HIF-1α forms a dimer with HIF-1β to regulate the expression of
downstream target genes associated with metabolism, proliferation,
apoptosis, inflammation, immunity, survival and angiogenesis
(13). Consequently, HIF-1α is very
important, not only for homeostasis, but also for blood vessel
formation in embryos and tumorigenesis (13,14).
HIF-1α has already been reported as a critical target to block
angiogenesis for cancer treatment (14), Evo can decrease the expression of
HIF-1α in cancer cells (15), but
it remains unknown whether Evo-induced downregulation of HIF-1α
could mediate this function in colon cancer cells, and how Evo
decreases the expression of HIF-1α.
Herein, we investigated the antiproliferation effect
of Evo in colon cancer cells, and dissected the possible mechanism
underlying this function.
Materials and methods
Chemicals and drug preparations
Evo was purchased from Hao-Xuan Bio-Tech Co., Ltd.
(Xi'an, China). LoVo cell line was purchased from the American Type
Culture Collection (ATCC). Evo was dissolved in dimethylsulfoxide
(DMSO) for in vitro test or prepared with 0.4%
carboxymethylcellulose sodium (CMC-Na) as suspension for in
vivo experiments. All antibodies were purchased from Santa Cruz
Biotechnology. Cells were maintained in the Dulbecco's modified
Eagle's medium (DMEM) with 10% fetal bovine serum (FBS), 100 U/ml
of penicillin and 100 µg/ml of streptomycin at 37°C in 5%
CO2.
Crystal violet viability assay
Crystal violet staining was conducted as reported
(16). Briefly, LoVo cells were
seeded into a 24-well plate and treated with different
concentrations of Evo. Cells were washed carefully with cold (4°C)
phosphate-buffered saline (PBS) and stained with 0.5% crystal
violet formalin solution at room temperature to visualize the cell
viability at the scheduled time points. For quantification, crystal
violet was extracted with 1 ml 20% acetic acid at room temperature
for 20 min with gentle shaking. Absorbance at 570 nm was measured.
Each assay was carried out in triplicate.
Clone formation assay
The clone formation assay was conducted as reported
(16). Briefly, sub-confluent LoVo
cells were treated with Evo at the indicated concentrations for 24
h. The cells were reseed at 12-well plate without Evo treating, and
maintained in culture for 14 days. The initial cell number was 100
or 200/well. Colonies were subjected to crystal violet staining.
Each assay condition was carried out in duplicate.
Construction of recombinant
adenovirus
The recombinant adenoviruses expressing human HIF-1α
(AdHIF-1α), RFP (AdRFP) and small interference RNA fragments
targeting HIF-1α (AdsiHIF-1α) were generated using the AdEasy
technology, as described (17,18).
Flow cytometry analysis for
apoptosis
Sub-confluent LoVo cells were seeded in 6-well
plates and treated with different concentrations of Evo for 48 h.
Then, cells were harvested and washed with cold PBS (4°C), followed
by incubating with Annexin V-EGFP and propidium iodide (PI)
according to kit instructions (KeyGen Biotech, Nanjing, China). The
stained cells were analyzed by fluorescence-activated cell sorting
(FACS). Each assay was carried out in triplicate.
Annexin V-EGFP staining for apoptosis
assay
Sub-confluent LoVo cells were seeded into 24-well
plates and treated with different concentrations of Evo for 24 h.
Cells were washed with PBS twice and incubated with 500 µl
of binding buffer and 2 µl of Annexin V-EGFP fusion protein
each well for 5 min, followed by washing with PBS twice. Images
were captured under a fluorescence microscope. Each assay was
carried out in triplicate.
Western blot assay
Sub-confluent LoVo cells were seeded into a 6-well
plate and were treated with different concentrations of Evo or
DMSO. At the scheduled time points, cells were washed with cold PBS
and lysed in 300 µl lysis buffer. The lysates were boiled
for 10 min, and then subjected to SDS-PAGE electrophoresis and
transfered to polyvinylidene fluoride membranes. The membranes were
immunoblotted with corresponding primary antibodies, and followed
by incubating with horseradish peroxidase (HRP)-conjugated
secondary antibodies. The target proteins were visualized with the
SuperSignal West Pico substrate (Pierce, Rockford, IL, USA). Each
assay was carried out in triplicate.
Reverse transcription and polymerase
chain reaction analysis (RT-PCR)
Sub-confluent LoVo cells were seeded in T25 flasks
and treated with different concentrations of Evo or DMSO. At the
scheduled time point, total RNAs were extracted with TRIzol
reagents (Invitrogen, Carlsbad, CA, USA) and used to generate cDNA
templates by RT reaction. Then, the cDNAs were used as templates
for PCR to detect the expression level of the genes of interest.
The primers used were available upon request. Each assay was
carried out in triplicate.
Xenograft tumor model of human colon
cancer
All animal experiments were approved by the
Institutional Animal Care and Use Committee (IACUC) of Chongqing
Medical University. Athymic nude mice (female, 4–6 weeks old,
5/group) were purchased from the Animal Center of Chongqing Medical
University (Chongqing, China). LoVo cells were collected and
resuspended in cold PBS to a final density of 2×107
cells/ml. Cells in 50 µl of cold PBS were injected into the
flanks of athymic mice. At 3 days after injection, animals were
treated with different doses of Evo (5, 10 and 20 mg/kg) or the
same volume of solvent by intragastric administration once a day.
The tumor size was measured once a week and the tumor volume (V,
mm3) was calculated as the following equation: V = π/6x
(Rmax × 6Rmin)2 (R, is the tumor
diameter). Five weeks after injection, the animals were sacrificed
and the tumor samples were retrieved for histological
evaluation.
Luciferase reporter assay
Sub-confluent LoVo cells were seeded into T25 flask
and transfected with 2 µg HIF-1 responsive element
luciferase reporter plasmid (pBGluc-HIF-1α) per flask using
Lipofectamine (19), and replacing
the medium 4 h later with fresh complete medium. After incubating
for 12 h, cells were reseeded into a 24-well plate and treated with
different concentrations of Evo or DMSO. Twenty-four hours after
treatment, cells were lysed and subjected to luciferase assays
using luciferase assay kit (E1500; Promega). Each assay was carried
out in triplicate.
Histological evaluation and
immunohistochemical staining
Retrieved tumor masses were fixed in 10% formalin
and embedded in paraffin. Serial sections of the embedded specimens
were stained with hematoxylin and eosin. For immunohistochemical
staining, slides were deparaffinized and then rehydrated in a
graduated fashion. The deparaffinized slides were subjected to
antigen retrieval and probed with primary antibody, or isotype IgG
as control, followed by incubation with biotinylated secondary
antibody and streptavidin-conjugated HRP. The target proteins were
visualized by DAB staining and imaged under a microscope (20).
Statistical analysis
All quantitative experiments were performed in
triplicate. Data are expressed as mean ± SD. Statistical
significances between vehicle treatments vs. drug treatment were
determined by the Student's t-test. A p-value of <0.05 was
considered to indicate a statistically significant result.
Results
Evo shows antiproliferation effect on
LoVo cells
We initiated the investigation by analyzing whether
Evo could inhibit the proliferation in LoVo cells. Crystal violet
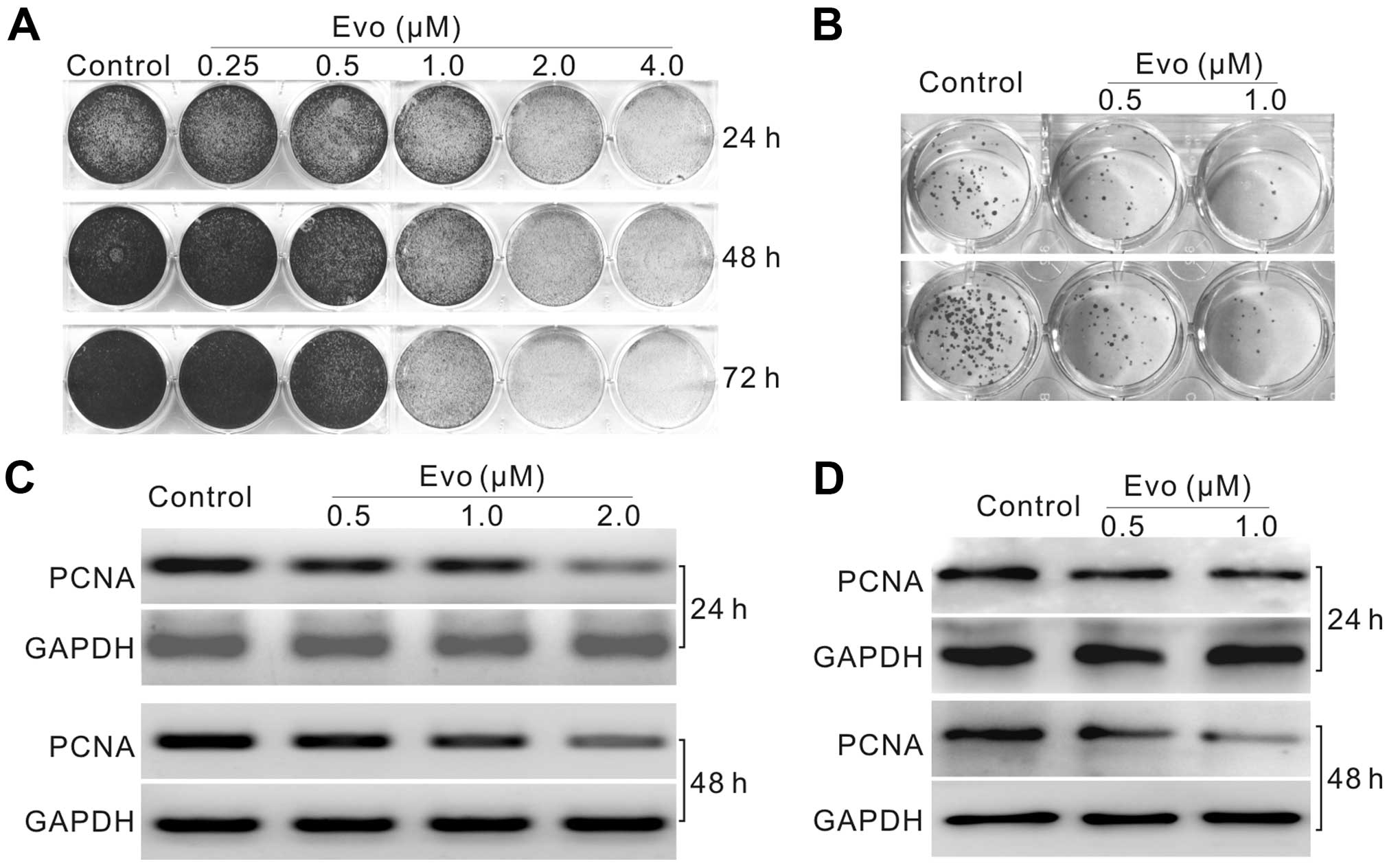
staining results showed that Evo inhibits the proliferation of LoVo
cells concentration- and time-dependently (Fig. 1A and B). PCR and western blot
results showed that Evo suppresses significantly the expression of
proliferating cell nuclear antigen (PCNA) (Fig. 1C and D). These results suggested
that Evo can inhibit the proliferation of LoVo cells.
Evo induces apoptosis in LoVo cells
We next analyzed whether Evo could induce LoVo cells
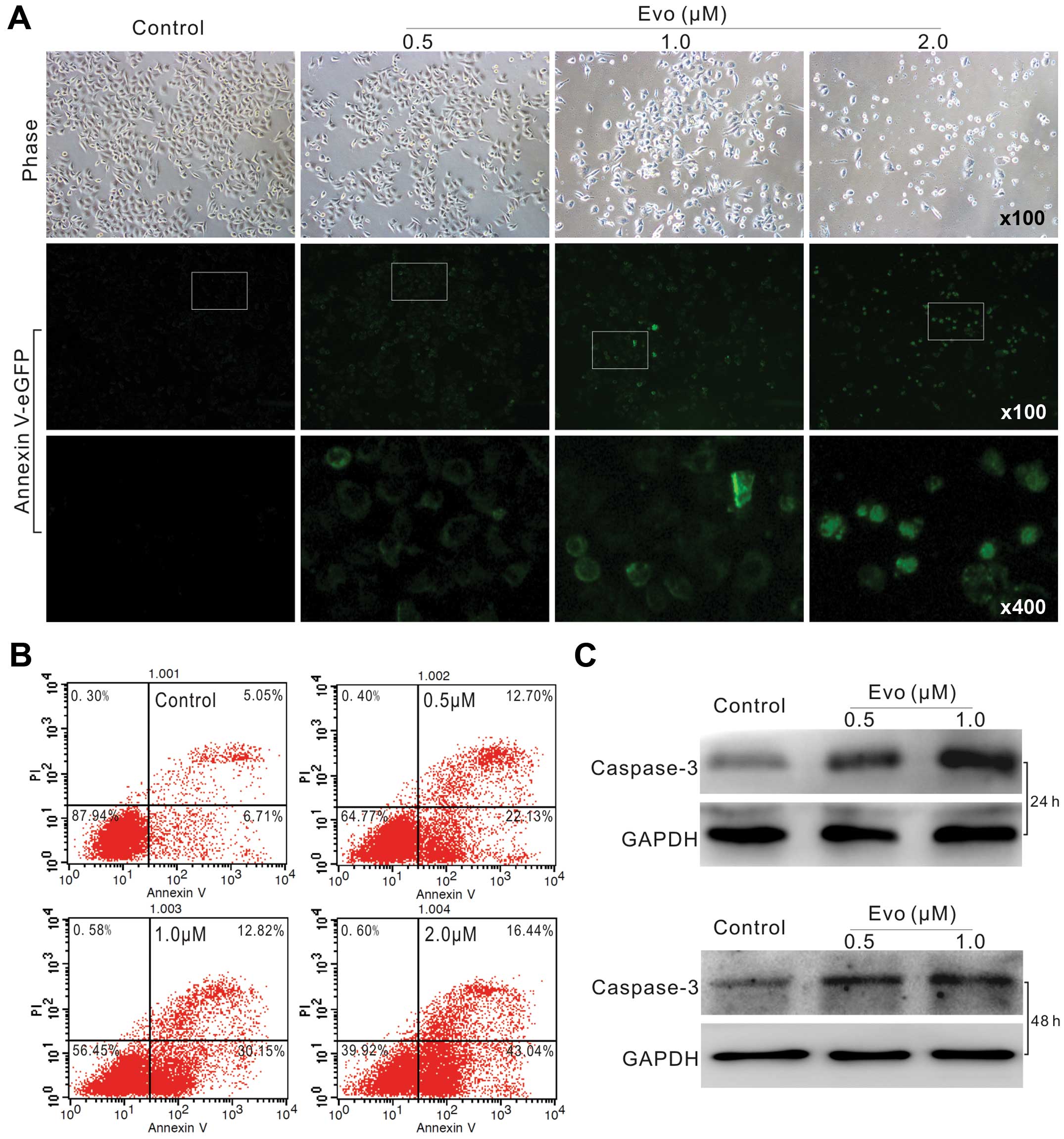
to undergo apoptosis. Annexin V-EGFP staining results showed that
Evo can effectively induce apoptosis even at concentration of 0.5
µM (Fig. 2A). FACS assay
results showed that the percentage of apoptotic cells increased
concentration-dependently (Fig.
2B). Caspase-3 as an executor of apoptosis, and often used as a
definite marker for apoptosis detection. Western blot results
showed that Evo increases the level of caspase-3 (Fig. 2C). Thus, these data indicated that
Evo can induce apoptosis in LoVo cells.
Evo inhibits tumor growth in a xenograft
tumor model of human colon cancer
Next, we injected LoVo cells subcutaneously into the
flanks of athymic nude mice, and then treated the mice with
different doses of Evo (5, 10 and 20 mg/kg) or solvent by
intragastric administration to assess the anticancer activity of
Evo. The results showed that Evo suppresses the tumor growth in a
dose-dependent manner, compared with the solvent control group
(Fig. 3A and B). Hematoxylin and
eosin (H&E) staining result showed that more necrotic cells
were found in Evo treated groups than that of control group
(Fig. 3C). These results showed
that Evo can inhibit the proliferation of colon cancer cells.
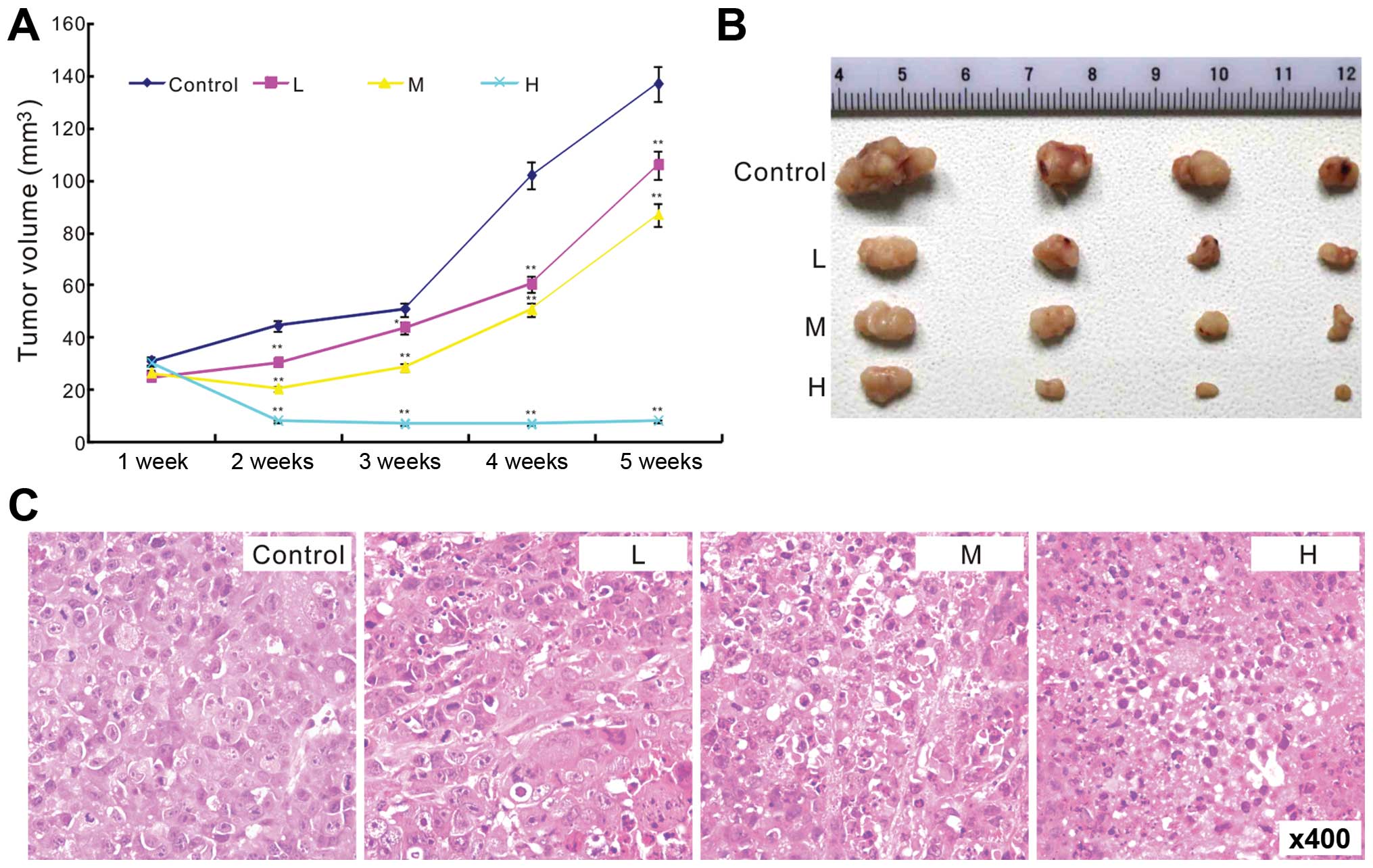 | Figure 3Effect of Evo on tumor growth in
xenograft tumor model of human colon cancer. (A) The effect of Evo
on colon cancer tumor growth (*p<0.05 and
**p<0.01, compared with control group). (B)
Representative tumor masses show the effect of Evo on colon cancer
tumor growth (L, low-dose, 5 mg/kg; M, middle-dose, 10 mg/kg; H,
high-dose, 20 mg/kg). (C) H&E staining results show the
antiproliferation effect of Evo in colon cancer (L, low-dose, 5
mg/kg; M, middle-dose, 10 mg/kg; H, high-dose, 20 mg/kg). |
Evo downregulates the expression of
HIF-1α in LoVo cells
We next sought to investigate the possible mechanism
underlying the antiproliferation effect of Evo in human colon
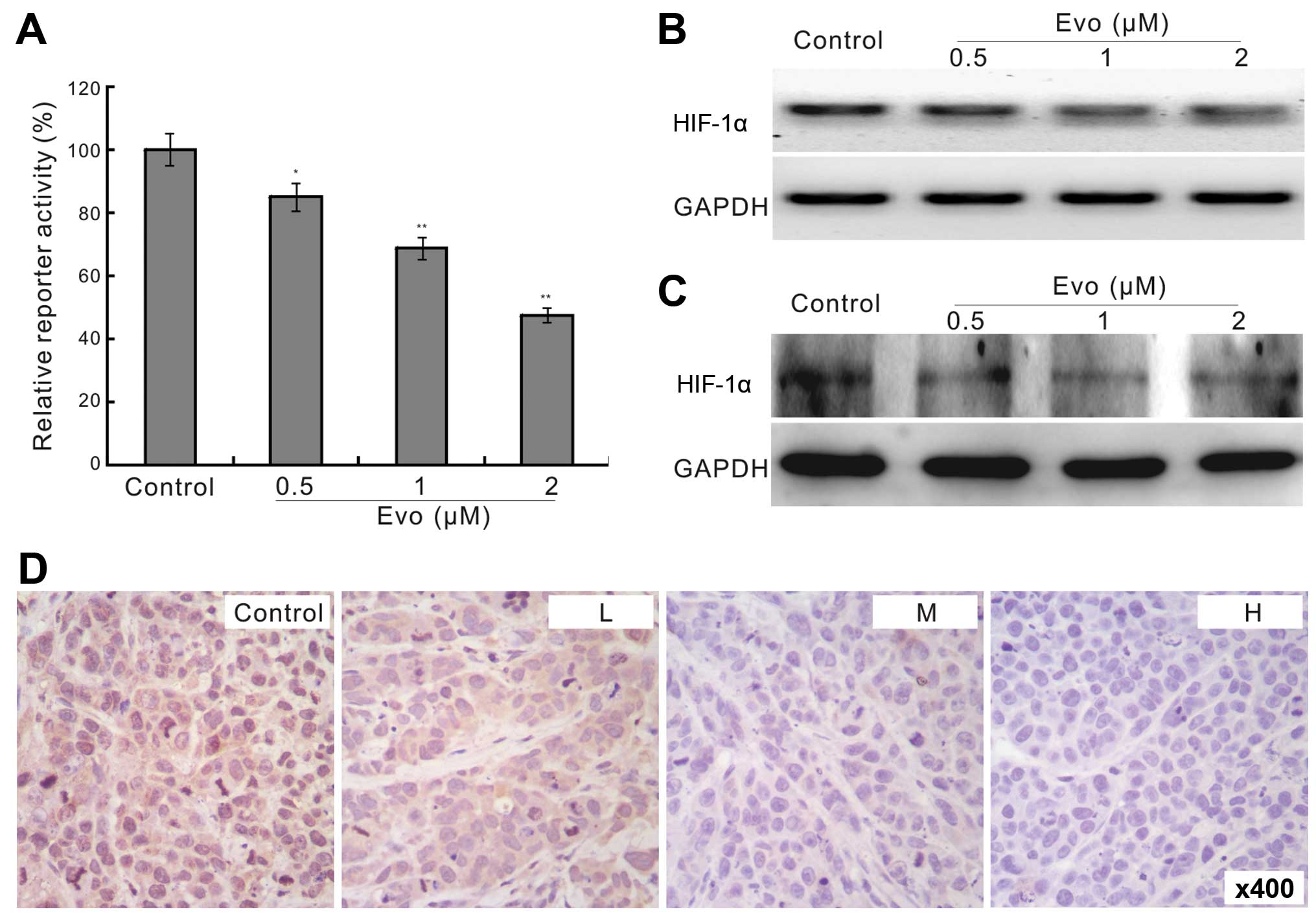
cancer cells. With HIF-1α responsive element reporter assay, we
found that Evo can significantly decrease the transcriptional
activity of the reporter (Fig. 4A).
This suggested that HIF-1α may be involved in the antiproliferation
effect of Evo in colon cancer cells. Using PCR and western blot
assay, we found that Evo inhibited the expression of HIF-1α in LoVo
cells (Fig. 4B and C), which was
confirmed by immunohistochemical staining for the tumor tissues
retrieved from the in vivo experiment (Fig. 4D). These results implied that HIF-1α
may be associated with the antiproliferation effect of Evo in LoVo
cells.
HIF-1α affects the anticancer activity of
Evo in LoVo cells
We next scheduled to establish whether HIF-1α could
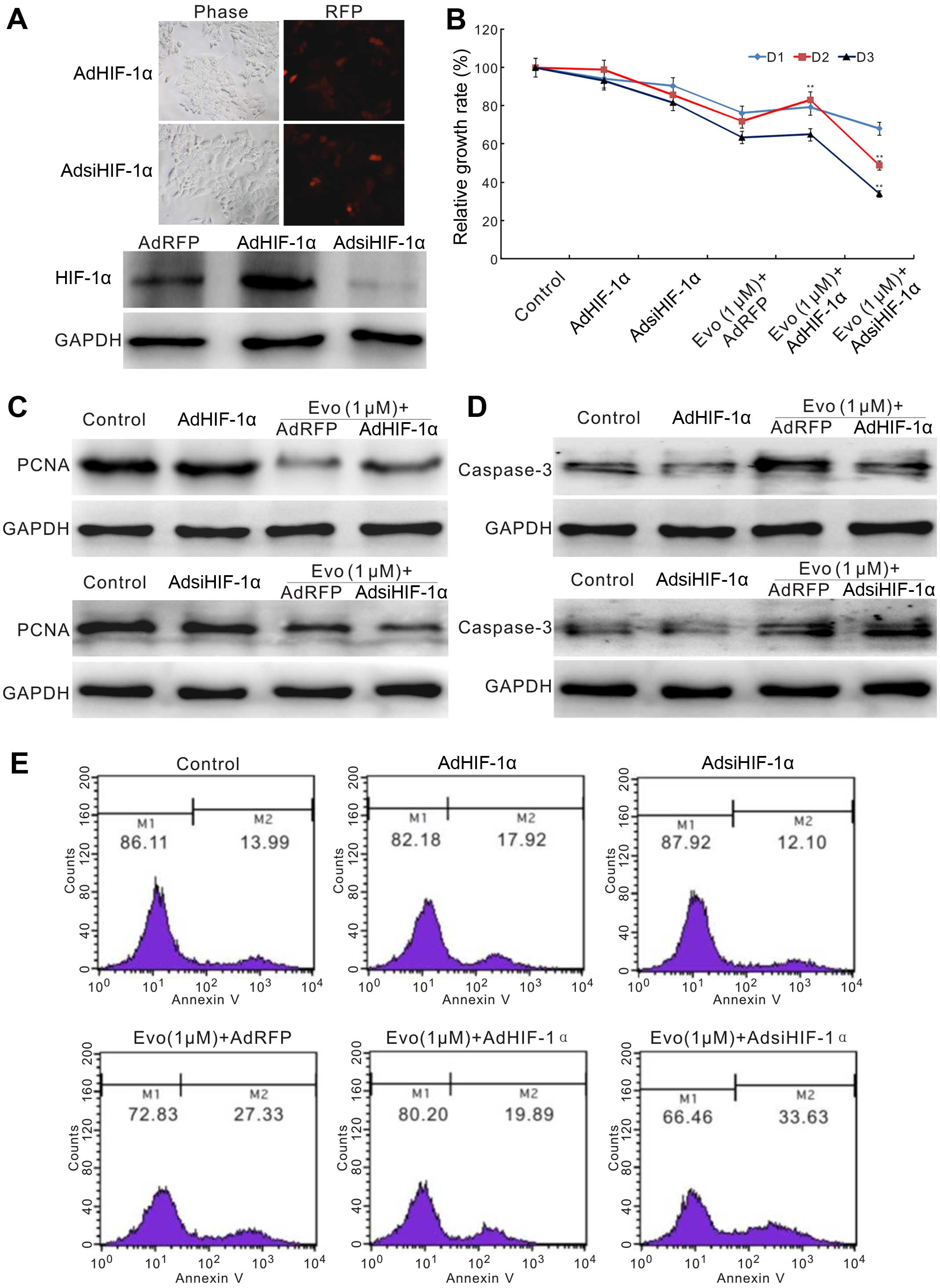
affect the anticancer activity of Evo in LoVo cells. We constructed
red fluorescent protein (RFP) tagged recombinant adenoviruses for
HIF-1α overexpression or siRNA fragments. Western blot analysis
showed that both recombinant adenoviruses were function well
(Fig. 5A). The crystal violet
staining results showed that exogenous expression of HIF-1α can
reverse Evo-induced proliferation inhibition partly, but HIF-1α
knockdown enhances prominently this effect (Fig. 5B). Exogenous expression of HIF-1α
can notably reverse the decrease of PCNA induced by Evo, while
HIF-1α knockdown can substantially promote the inhibitory effect of
Evo on PCNA (Fig. 5C). For
apoptosis, exogenous expression of HIF-1α reduces the level of
caspase-3 induced by Evo, while it is increased by HIF-1α knockdown
(Fig. 5D). Flow cytometric assay
results showed that the percentage of apoptotic cells induced by
Evo was decreased by exogenous expression of HIF-1α, but increased
by HIF-1α knockdown (Fig. 5E).
These results suggested that the anticancer activity of Evo is
associated with downregulation of HIF-1α.
Evo may decrease HIF-1α expression though
IGF-1/PI3K/Akt signaling in LoVo cells
Our results have demonstrated that the
antiproliferation effect of Evo in LoVo cells maybe partly mediated
by downregulating HIF-1α. However, the possible mechanism of this
process remains unknown. PI3K/Akt is important for cell survival
signaling, and HIF-1α is an important downstream target of this
signaling. Thus, the effect of Evo on HIF-1α may be associated with
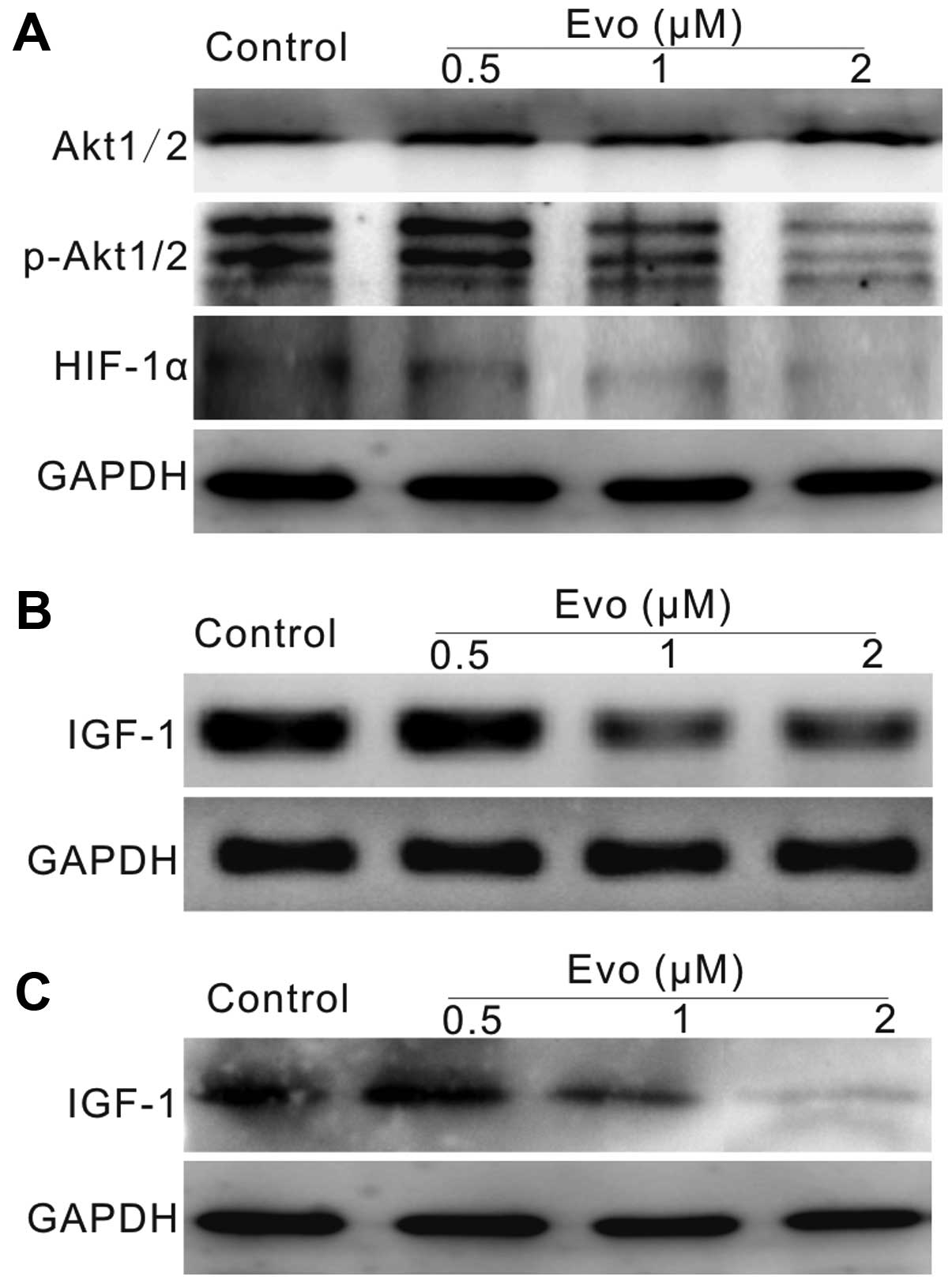
the inhibition of PI3K/Akt signaling. Using western blot analysis,
we found that Evo can decrease the phosphorylation of Akt1/2/3, as
well as HIF-1α (Fig. 6A). PI3K/Akt
signaling can be regulated by certain cytokines or growth factors.
IGF-1 is one of the most important activators of this signaling,
thus we detected whether Evo could affect the expression of IGF-1.
The PCR and western blot results showed that Evo can decrease the
expression of IGF-1 notably in LoVo cells (Fig. 6B and C). Taken together, these data
suggested that the effect of Evo on HIF-1α may be related with the
inhibition of IGF-1 in human colon cancer cells.
Discussion
Colon cancer is one of the major causes of cancer
morbidity and mortality. Although the diagnosis and treatment of
colon cancer have greatly improved in the last decades, the
prognosis is still poor for most patients. Thus, it is urgent to
develop new regimens for colon cancer treatment. In the present
study, we investigated the anticancer activity of evodiamine (Evo)
in human colon cancer cells. We found that Evo can effectively
inhibit the proliferation and promote apoptosis in LoVo cells.
Mechanistically, we found that the antiproliferation activity of
Evo in colon cancer may be mediated by downregulating HIF-1α
through inactivating IGF-1/PI3K/Akt signaling.
Although colon cancer is one of the most common
maligancies, it is still treatable and often curable if localized
to the bowel. The metastasis or recurrence is often the major
causes of death. At the late stage or recurrence of colon cancer,
chemotherapy or targeted therapy are the primary choices. Natural
products and their derived active components, including
semi-synthetic and synthetic analogs, have served as the major
source for anticancer agents (1–4).
Several plant-derived compounds have been used as anticancer drugs
for some years (5). Evo, a
quinolone alkaloid, was extracted from the traditional herbal
medicine Evodiae fructus (6). Evo shows pharmacological actions and
can be used to treat many diseases, such as obesity, inflammation
and cardiovascular diseases (7).
Expanding pool of evidence supports that Evo possesses anticancer
activities either in vitro or in vivo by inhibiting
proliferation, metastasis, and inducing apoptosis in various tumor
cell lines, such as lung, prostate, breast and colon cancer cells
(8–10). For colon cancer, the
antiproliferation effect of Evo has been well demonstrated
(9,21,22).
However, the exact molecular mechanism underlay this function
remains unknown, although JNK and caspase have been reported
associated with the anticancer activity of Evo in colon cancer
cells (9,21).
In this investigation, we analyzed the
antiproliferation effect of Evo in colon cancer cells, and then
tried to unveil the possible mechanism underlying this effect. Our
results from crystal violet and clone formation assay demonstrated
that Evo is a very potent proliferation inhibitor in LoVo cells
(Fig. 1A and B). Proliferating cell
nuclear antigen (PCNA) is an important factor for proliferation
(23), and can be used as a
potential target for cancer treatment (24). We found that Evo could decrease the
level of PCNA in a concentration dependent manner (Fig. 1C and D). As most of the current
anticancer agents are capable of inducing apoptosis, we analyzed
whether Evo also possesses this ability. Phosphatidylserine,
usually locates on the inner side of cell membrane, and can
specifically bind with Annexin V. At the early stage of apoptosis,
phosphatidylserine translocates to the outer side of cell membrane.
Thus, it is widely used for early apoptosis detection (25). Caspase-3 has been thought as the
executor for apoptosis (26). Our
results showed that Evo can increase the Annexin V-EGFP signal
greatly, as well as the protein level of caspase-3 (Fig. 2A–C). These data supported that Evo
can induce the colon cancer cells to undergo apoptosis. The in
vivo xenograft tumor assay results showed that Evo can inhibit
tumor growth (Fig. 3A–C). Taken
together, these data confirmed that Evo may be used as a potential
antiproliferation agent for colon cancer treatment alone or as an
adjuvant.
Hypoxia-inducible factors (HIFs), including HIF-1,
HIF-2 and HIF-3, are special transcriptional factors that respond
to the decrease of oxygen in cellular environment. For this reason,
HIFs are vital to development (27,28).
HIF-1 consists of HIF-1α and HIF-1β subunits, which form
heterodimers to regulate the downstream targets. HIF-1α and HIF-1β
are constitutively expressed, but HIF-1α has an extremely short
half-life, so HIF-1α level determines the transcriptional activity
of HIF-1 (12). HIF-1α is critical
for angiogenesis and new vascular formation, which is very
important for tumor growth. Therefore, HIF-1α and other
angiogenesis-related factors have been thought as potential targets
for cancer treatment, such as vascular endothelial growth factor
(VEGF) and epidermal growth factor receptor (EGFR) (14,29,30).
HIF-1α can regulate not only vascular formation, but also
proliferation, glucose metabolism, inflammation, survival and
apoptosis (13). In fact,
metabolism regulation is the principal function of HIF-1α (27,31).
Inhibition of HIF-1α has shown anticancer activity (14), and the level of HIF-1α is associated
with increasing metastasis and/or patient mortality (14,32,33).
With HIF-1 responsive element luciferase reporter, we found that
Evo can decrease the transcriptional activity of this reporter
(Fig. 4A), which suggested that Evo
may down-regulate the expression of HIF-1α. With further analysis,
we confirmed that Evo can apparently decrease the expression of
HIF-1α in LoVo cells (Fig. 4B and
C), this was consistent with the immunohistochemical staining
results for the tumor masses (Fig.
4D). Thus, it is definite that HIF-1α is a target of Evo in
colon cancer, but its role in the anticancer activity of Evo in
colon cancer remains unknown. We introduced recombinant
adenoviruses to mediate overexpression or knock down of HIF-1α. The
results showed that exogenous expression of HIF-1α have no apparent
effect on the proliferation of colon cancer cells, but it can
partly reverse the proliferation inhibitory effect of Evo;
knockdown of HIF-1α can enhance the antiproliferation effect of Evo
in colon cancer cells (Fig. 5B).
Exogenous expression of HIF-1α can reverse the effect of Evo on
PCNA, while knockdown of HIF-1α can potentiate this effect
(Fig. 5C). Similar results were
found in Evo-induced apoptosis in LoVo cells (Fig. 5D and E). Taken together, all these
data suggested that the antiproliferation function of Evo in colon
cancer may be mediated by decreasing the expression of HIF-1α.
HIFs are special transcriptional factors that
respond to hypoxia, and are vital for development, and in cancer
therapy (31). Growth factors and
cytokines can also induce HIF-1α accumulation, such as vascular
endothelial growth factor A (VEGF-A), basic fibroblast growth
factor (bFGF), platelet-derived growth factor (PDGF) and tumor
necrosis factor α (TNF-α) (33,34).
Several signaling pathways, such as PI3K/Akt and NF-κB (35), are strongly involved in mediating
response of these factors to regulate the expression of HIF-1α.
PI3K/Akt signaling pathway is very important for differentiation,
proliferation and survival. Therefore, we hypothesized that the
Evo-induced decrease of HIF-1α may result from the inhibition of
PI3K/Akt signaling transduction. Western blot analysis supported
our hypothesis that Evo can decrease the phosphorylation level of
Akt1/2/3 concentration-dependently (Fig. 6A). PI3K/Akt signaling can be
regulated by various factor, such as insulin-like growth factor 1
(IGF-1) and PTEN (36,37). IGF-1 is one of the most potent
natural activators for PI3K/Akt signaling, while PTEN is the
negative regulator for this signaling (38,39).
Thus, we tested whether Evo can affect the expression of IGF-1, an
important component of IGFs signaling. IGF signaling is another
very complex system, comprising two ligands (IGF-1 and IGF-2), two
receptors (IGF-1R and IGF-2R), seven binding proteins (IGF binding
proteins, IGFBP-1 to IGFBP-7) with high affinity to IGFs, and
IGFBPs degrading enzymes. The IGF signaling is vital for regulating
the development and homeostasis, including differentiation,
proliferation and apoptosis (40–43).
Thus, the aberrant IGF signaling are implicated with the
development of cancer (44–47), including human colon cancer
(48). We found that Evo can
downregulate the expression of IGF-1 in a concentration-dependent
manner (Fig. 6B and C). Taken
together, these data demonstrated that Evo-induced downregulation
of HIF-1α may be the result of inhibition of the expression of
IGF-1.
In summary, our investigation elucidated that Evo
can efficiently inhibit the proliferation of colon cancer cells and
may be used alone or combination as a potential anticancer agent;
the antiproliferation effect of Evo in colon cancer cells may be
mediated by downregulating HIF-1α at least, followed by partly
decreasing the expression of IGF-1. Future investigations will
focus on how Evo affect the IGFs signaling in colon cancer
cells.
Acknowledgments
We thank Dr T.-C. He (University of Chicago Medical
Center, USA) for generously providing all the recombinant
adenoviruses and hypoxia responsive element reporter plasmid
(pBGluc-HIF-1). The present study was supported in part by research
grants from the Natural Science Foundation of China (NSFC 81372120
to B.-C.H.).
References
|
1
|
da Rocha AB, Lopes RM and Schwartsmann G:
Natural products in anticancer therapy. Curr Opin Pharmacol.
1:364–369. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mann J: Natural products in cancer
chemotherapy: Past, present and future. Nat Rev Cancer. 2:143–148.
2002. View
Article : Google Scholar
|
|
3
|
Koehn FE and Carter GT: The evolving role
of natural products in drug discovery. Nat Rev Drug Discov.
4:206–220. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Corson TW and Crews CM: Molecular
understanding and modern application of traditional medicines:
Triumphs and trials. Cell. 130:769–774. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang HL, Zhang XH and Chang TH: Effects of
tetrandrine on smooth muscle contraction induced by mediators in
pulmonary hypertension. Acta Pharmacol Sin. 23:1114–1120.
2002.PubMed/NCBI
|
|
6
|
Jiang J and Hu C: Evodiamine: A novel
anti-cancer alkaloid from Evodia rutaecarpa. Molecules.
14:1852–1859. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yu H, Jin H, Gong W, Wang Z and Liang H:
Pharmacological actions of multi-target-directed evodiamine.
Molecules. 18:1826–1843. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hong JY, Park SH, Min HY, Park HJ and Lee
SK: Anti-proliferative effects of evodiamine in human lung cancer
cells. J Cancer Prev. 19:7–13. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chien CC, Wu MS, Shen SC, Ko CH, Chen CH,
Yang LL and Chen YC: Activation of JNK contributes to
evodiamine-induced apoptosis and G2/M arrest in human colorectal
carcinoma cells: A structure-activity study of evodiamine. PLoS
One. 9:e997292014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Du J, Wang XF, Zhou QM, Zhang TL, Lu YY,
Zhang H and Su SB: Evodiamine induces apoptosis and inhibits
metastasis in MDA-MB-231 human breast cancer cells in vitro and in
vivo. Oncol Rep. 30:685–694. 2013.PubMed/NCBI
|
|
11
|
Wang KL, Hsia SM, Yeh JY, Cheng SC, Wang
PS and Wang SW: Anti-Proliferative Effects of evodiamine on human
breast cancer cells. PLoS One. 8:e672972013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pugh CW, O'Rourke JF, Nagao M, Gleadle JM
and Ratcliffe PJ: Activation of hypoxia-inducible factor-1;
definition of regulatory domains within the alpha subunit. J Biol
Chem. 272:11205–11214. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Palazon A, Goldrath AW, Nizet V and
Johnson RS: HIF transcription factors, inflammation, and immunity.
Immunity. 41:518–528. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Semenza GL: Defining the role of
hypoxia-inducible factor 1 in cancer biology and therapeutics.
Oncogene. 29:625–634. 2010. View Article : Google Scholar :
|
|
15
|
Sun HL, Liu YN, Huang YT, Pan SL, Huang
DY, Guh JH, Lee FY, Kuo SC and Teng CM: YC-1 inhibits HIF-1
expression in prostate cancer cells: Contribution of Akt/NF-kappaB
signaling to HIF-1alpha accumulation during hypoxia. Oncogene.
26:3941–3951. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
He BC, Gao JL, Zhang BQ, Luo Q, Shi Q, Kim
SH, Huang E, Gao Y, Yang K, Wagner ER, et al: Tetrandrine inhibits
Wnt/β-catenin signaling and suppresses tumor growth of human
colorectal cancer. Mol Pharmacol. 79:211–219. 2011. View Article : Google Scholar :
|
|
17
|
He TC, Zhou S, da Costa LT, Yu J, Kinzler
KW and Vogelstein B: A simplified system for generating recombinant
adenoviruses. Proc Natl Acad Sci USA. 95:2509–2514. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Luo J, Deng ZL, Luo X, Tang N, Song WX,
Chen J, Sharff KA, Luu HH, Haydon RC, Kinzler KW, et al: A protocol
for rapid generation of recombinant adenoviruses using the AdEasy
system. Nat Protoc. 2:1236–1247. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
El Naggar A, Clarkson P, Zhang F, Mathers
J, Tognon C and Sorensen PH: Expression and stability of hypoxia
inducible factor 1α in osteosarcoma. Pediatr Blood Cancer.
59:1215–1222. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
He BC, Gao JL, Luo X, Luo J, Shen J, Wang
L, Zhou Q, Wang YT, Luu HH, Haydon RC, et al: Ginsenoside Rg3
inhibits colorectal tumor growth through the down-regulation of
Wnt/ß-catenin signaling. Int J Oncol. 38:437–445. 2011. View Article : Google Scholar
|
|
21
|
Zhang C, Fan X, Xu X, Yang X, Wang X and
Liang HP: Evodiamine induces caspase-dependent apoptosis and S
phase arrest in human colon LoVo cells. Anticancer Drugs.
21:766–776. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ogasawara M, Matsubara T and Suzuki H:
Inhibitory effects of evodiamine on in vitro invasion and
experimental lung metastasis of murine colon cancer cells. Biol
Pharm Bull. 24:917–920. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Leonardi E, Girlando S, Serio G, Mauri FA,
Perrone G, Scampini S, Dalla Palma P and Barbareschi M: PCNA and
Ki67 expression in breast carcinoma: Correlations with clinical and
biological variables. J Clin Pathol. 45:416–419. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang SC: PCNA: A silent housekeeper or a
potential therapeutic target? Trends Pharmacol Sci. 35:178–186.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vermes I, Haanen C, Steffens-Nakken H and
Reutelingsperger C: A novel assay for apoptosis. Flow cytometric
detection of phosphatidylserine expression on early apoptotic cells
using fluorescein labelled Annexin V. J Immunol Methods. 184:39–51.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Walters J, Pop C, Scott FL, Drag M, Swartz
P, Mattos C, Salvesen GS and Clark AC: A constitutively active and
uninhibitable caspase-3 zymogen efficiently induces apoptosis.
Biochem J. 424:335–345. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Formenti F, Constantin-Teodosiu D,
Emmanuel Y, Cheeseman J, Dorrington KL, Edwards LM, Humphreys SM,
Lappin TR, McMullin MF, McNamara CJ, et al: Regulation of human
metabolism by hypoxia-inducible factor. Proc Natl Acad Sci USA.
107:12722–12727. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Benizri E, Ginouvès A and Berra E: The
magic of the hypoxia-signaling cascade. Cell Mol Life Sci.
65:1133–1149. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Macarulla T, Sauri T and Tabernero J:
Evaluation of aflibercept in the treatment of metastatic colorectal
cancer. Expert Opin Biol Ther. 14:1493–1505. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gasparini G, Buttitta F, D'Andrea MR,
Tumolo S, Buonadonna A, Pavese I, Cordio S, De Tursi M, Mosconi S,
Stumbo L, et al: Optimizing single agent panitumumab therapy in
pre-treated advanced colorectal cancer. Neoplasia. 16:751–756.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Semenza GL: Hypoxia-inducible factors in
physiology and medicine. Cell. 148:399–408. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vaupel P, Mayer A and Höckel M: Tumor
hypoxia and malignant progression. Methods Enzymol. 381:335–354.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hoffmann AC, Mori R, Vallbohmer D,
Brabender J, Klein E, Drebber U, Baldus SE, Cooc J, Azuma M,
Metzger R, et al: High expression of HIF1a is a predictor of
clinical outcome in patients with pancreatic ductal adenocarcinomas
and correlated to PDGFA, VEGF, and bFGF. Neoplasia. 10:674–679.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wu Y, Lucia K, Lange M, Kuhlen D, Stalla
GK and Renner U: Hypoxia inducible factor-1 is involved in growth
factor, glucocorticoid and hypoxia mediated regulation of vascular
endothelial growth factor-A in human meningiomas. J Neurooncol.
119:263–273. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
van Uden P, Kenneth NS and Rocha S:
Regulation of hypoxia-inducible factor-1alpha by NF-kappaB. Biochem
J. 412:477–484. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ma X and Bai Y: IGF-1 activates the
P13K/AKT signaling pathway via upregulation of secretory clusterin.
Mol Med Rep. 6:1433–1437. 2012.PubMed/NCBI
|
|
37
|
Song MS, Salmena L and Pandolfi PP: The
functions and regulation of the PTEN tumour suppressor. Nat Rev Mol
Cell Biol. 13:283–296. 2012.PubMed/NCBI
|
|
38
|
Zhu C, Qi X, Chen Y, Sun B, Dai Y and Gu
Y: PI3K/Akt and MAPK/ERK1/2 signaling pathways are involved in
IGF-1-induced VEGF-C upregulation in breast cancer. J Cancer Res
Clin Oncol. 137:1587–1594. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Blanco-Aparicio C, Renner O, Leal JF and
Carnero A: PTEN, more than the AKT pathway. Carcinogenesis.
28:1379–1386. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gluckman P, Klempt N, Guan J, Mallard C,
Sirimanne E, Dragunow M, Klempt M, Singh K, Williams C and Nikolics
K: A role for IGF-1 in the rescue of CNS neurons following
hypoxic-ischemic injury. Biochem Biophys Res Commun. 182:593–599.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Valentinis B and Baserga R: IGF-I receptor
signalling in transformation and differentiation. Mol Pathol.
54:133–137. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fu P, Thompson JA, Leeding KS and Bach LA:
Insulin-like growth factors induce apoptosis as well as
proliferation in LIM 1215 colon cancer cells. J Cell Biochem.
100:58–68. 2007. View Article : Google Scholar
|
|
43
|
LeRoith D, Werner H, Beitner-Johnson D and
Roberts CT Jr: Molecular and cellular aspects of the insulin-like
growth factor I receptor. Endocr Rev. 16:143–163. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
LeRoith D and Roberts CT Jr: The
insulin-like growth factor system and cancer. Cancer Lett.
195:127–137. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Weroha SJ and Haluska P: The insulin-like
growth factor system in cancer. Endocrinol Metab Clin North Am.
41:335–350. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang Z, Wang Z, Liang Z, Liu J, Shi W, Bai
P, Lin X, Magaye R and Zhao J: Expression and clinical significance
of IGF-1, IGFBP-3, and IGFBP-7 in serum and lung cancer tissues
from patients with non-small cell lung cancer. Onco Targets Ther.
6:1437–1444. 2013.PubMed/NCBI
|
|
47
|
Chen D, Siddiq A, Emdad L, Rajasekaran D,
Gredler R, Shen XN, Santhekadur PK, Srivastava J, Robertson CL,
Dmitriev I, et al: Insulin-like growth factor-binding protein-7
(IGFBP7): A promising gene therapeutic for hepatocellular carcinoma
(HCC). Mol Ther. 21:758–766. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Vanamala J, Reddivari L, Radhakrishnan S
and Tarver C: Resveratrol suppresses IGF-1 induced human colon
cancer cell proliferation and elevates apoptosis via suppression of
IGF-1R/Wnt and activation of p53 signaling pathways. BMC Cancer.
10:2382010. View Article : Google Scholar : PubMed/NCBI
|