Introduction
Breast cancer is the most common female cancer and
one of the leading causes of cancer-related deaths worldwide with a
relatively high incidence rate (1,2).
Surgery, chemotherapy and radiotherapy are the traditional
therapeutic methods for the treatment of breast cancer, and
radiotherapy is an important adjuvant therapy for breast cancer
patients (3).
However, resistance to radiotherapy often results in
treatment failure, particularly for loco-regional recurrence.
Tumor-suppressor genes that regulate the cell cycle and induce cell
apoptosis can reverse the radiation resistance of cancer cells
(4,5).
B-cell translocation gene 1 (BTG1) is a
tumor-suppressor gene, and belongs to the antiproliferative gene
family comprising pheochromacytoma cell-3, tetradecanoyl phorbol
acetate-inducible sequence 21, BTG3, transducer of ERBB2, 1 (TOB1)
and TOB2 (6,7). Proteins encoded by members of this
gene family induce growth arrest or apoptosis in a variety of cell
systems (8). Overexpression of BTG1
has been found to inhibit proliferation during normal erythroid
differentiation (9) and induce cell
cycle arrest or apoptosis in several cell types, including NIH3T3
murine fibroblasts (10), myoblasts
(11) and microglia (12). BTG1 is also essentially expressed in
many types of tumor cells and inhibits the proliferation of a
variety of cancer cells. Recent studies have demonstrated that BTG1
protein levels are significantly reduced in breast cancer and are
associated with the pathogenesis and progression of breast
carcinomas (13). Overexpression of
BTG1 inhibited breast cancer cell invasion and metastasis both
in vitro and in vivo (14). Downregulation of BTG1 by miR-454-3p
enhanced cellular radiosensitivity in renal carcinoma cells due to
the role of BTG1 in cell cycle progression (15). Importantly, we reported that in
breast cancer cells, BTG1 inhibits cell growth through induction of
cell cycle arrest and apoptosis (16). We, therefore, hypothesized that BTG1
plays an important role in the radiosensitivity of breast cancer
cells. Thus, the present study was undertaken to evaluate the
effect of BTG1 along with X-ray irradiation on breast cancer cell
(MDA-MB-231 and MCF-7) colony formation, cell cycle distribution
and apoptosis in vitro. Furthermore, we examined whether
these changes are also observed in vivo using a nude mouse
model.
Materials and methods
Cell culture
Human breast cancer MCF-7 and MDA-MB-231 cell lines
were obtained from the Shanghai Cell Bank (Shanghai, China). The
stable cell lines MDA-MB-231/BTG1, MDA-MB-231/Neo, MCF-7/BTG1 and
MCF-7/Neo which overexpressed BTG1 and control cell lines were
preserved in our laboratory (16),
and were maintained in Dulbecco's modified Eagle's medium (DMEM)
(Gibco-BRL, USA) supplemented with 10% fetal bovine serum (FBS) and
were cultured in an incubator at 37°C with 5% CO2.
Irradiation
The cells were irradiated with different doses of 0,
0.5, 1, 3, 6 and 9 Gy rays using a Primus High-Energy Siemens
irradiator at a dose rate of 200 cGy/min at room temperature.
Colony formation assay
For the colony formation assay, transfected
overexpression and control cells were plated in 60-mm culture
dishes at a cell density of 2×103 for 24 h. Cells were
exposed to 0, 0.5, 1, 3, 6 and 9 Gy doses of irradiation and then
incubated at 37°C for 2 weeks post-irradiation. Cells were fixed
with methanol and stained with Giemsa solution. Colonies containing
>50 cells were counted. Colony forming efficiency = number of
colonies formed/number of cells plated. A multi-target click model
[S = 1 − (1 − e−D/D0)N] was applied to
delineate the survival curve; D0, lethal dose. Then, Dq
(quasi-threshold dose) = D0 × LN (N) was calculated.
Flow cytometric analysis of cell cycle
distribution and apoptosis
For the cell cycle assay, transfected overexpression
and control cells were plated into 6-well plates at a cell density
of 5×105 and allowed to attach overnight. Cells were
exposed to 0, 1, 3, 6 and 9 Gy doses of radiation, incubated at
37°C for 24 h post-irradiation and then fixed with 70% ice-cold
ethanol at 4°C overnight. Fixed cells were washed with PBS and
stained with propidium iodide (100 µg/ml) for 30 min in the
dark before analysis. The cell cycle profiles were assayed using
the FACSCan ESP flow cytometer at 488 nm, and data were analyzed
using MultiCycle software (BD Biosciences, USA). For analysis of
apoptosis, cells were exposed to a 3 Gy dose of irradiation and
were then incubated at 37°C for 24 h post-irradiation and processed
as described in the Annexin V-FITC apoptosis detection kit (BD
Biosciences) and analyzed on a FC500 flow cytometer.
Western blot analysis
Protein concentration was determined with a protein
assay. Equal amounts of protein were separated using 10% SDS-PAGE
gel electrophoresis, transferred onto nitrocellulose membranes and
blocked with 5% skimmed milk. Following blocking, the membranes
were incubated with antibodies against β-actin, cyclin B1, p-p53,
AKT, p-AKT, Bcl-2 and Bax and were then incubated with
HRP-conjugated anti-mouse or anti-rabbit IgG antibodies (both from
Santa Cruz Biotechnology, Santa Cruz, CA, USA). Protein bands were
visualized with ECL solution.
DCFH-DA fluorescence probe analysis of
reactive oxygen species (ROS)
Transfected overexpression and control cells were
plated into 6-well plates at a cell density of 1×105 and
allowed to attach overnight. Cells were exposed to a 3 Gy dose of
irradiation and were then incubated at 37°C for 0.5, 1, 2, 4 and 24
h post-irradiation. Cells were harvested, resuspended in 100
µl PBS and 50 µl
chloromethyl-2′,7′-dichlorofluorescein diacetate (50 µM
DCFH-DA) was added for 15 min at 37°C in the dark. Then the cells
were washed with PBS and treated with 1 mg/l ROS for up to 15 min
at 37°C in the dark. The intensity of fluorescence was assayed
using the FC500 flow cytometer at 488 nm.
Chromosomal aberration analysis
Exponentially growing transfected overexpression and
control cells were exposed to 0, 1, 3, 6 and 9 Gy doses of
irradiation and were then treated with 400 ng/ml colchicine at 37°C
for 8 h post-irradiation. Cells were harvested, administered
hypotonic treatment of 0.075 M KCl, were fixed onto a slide glass
with methanol:acetic acid (3:1) for two times and stained with 10%
Giemsa. Chromosome aberrations including dicentric (dic) and
centromere ring (r) were counted by microscopic examination.
In vivo studies
The nude mouse models of breast cancer were
constructed using MDA-MB-231, MDA-MB-231/Neo and MDA-MB-231/BTG1
cells as previously described (16). Each group consisted of 12 nude mice,
and 6 nude mice were randomly selected to accept a 3 Gy dose of
irradiation. Every four days, the tumor diameter was measured, and
the volume was calculated according to the formula: V = 0.4 x
largest diameter x smallest diameter. Four weeks after injection of
the cells, the mice were sacrificed and the tumors were separated.
Tissue sections were deparaffinized, rehydrated and rinsed, prior
to hematoxylin and eosin (H&E) staining and examination for
metastatic nodules and cell pathology. The animal treatment
protocol used in the present study was approved by the
Institutional Animal Care and Use Committee.
Immunohistochemistry
Tumor tissue sections were treated with 0.03%
hydrogen peroxide for 5 min to block endogenous peroxidase activity
and were then incubated with CD31, vascular endothelial growth
factor (VEGF) and Bcl-2 antibodies (Santa Cruz Biotechnology)
diluted at 1:100 for 60 min at room temperature. Following washing
in PBS, the sections were incubated with labeled HRP-conjugated
anti-mouse antibody, for 30 min at room temperature, prior to
washing twice in PBS and incubating with diaminobenzene for 10 min.
Following washing, the sections were counterstained with
hematoxylin, washed and dipped briefly in a water bath containing
drops of ammonia, prior to dehydration and mounting in Diatex. The
stained sections were analyzed and scored using a Nikon microscope
(Nikon Corporation, Japan).
TUNEL staining assay
Terminal deoxynucleotidyl transferase-mediated
dUTP-biotin nick-end labeling (TUNEL) assay was performed using
recombinant terminal transferase (TdT) and biotin-16-dUTP (Sigma,
USA). Tumor tissue sections were processed following the
manufacturer's protocol, and then the stained sections were
reviewed and scored using a Nikon microscope.
Statistical analysis
Statistical analyses were carried out using SPSS
17.0. Each experiment was repeated 3 times. The results shown are
the means ± SD. A p-value of <0.05 was considered to indicate a
statistically significant difference.
Results
Effects of BTG1 on the radiosensitivity
of breast cancer cells
Each group of cells was irradiated at different
doses (0, 0.5, 1, 3, 6 and 9 Gy), respectively. After two weeks of
incubation, clonogenic survival of each group was assessed. The
cell survival curves were characterized according to the
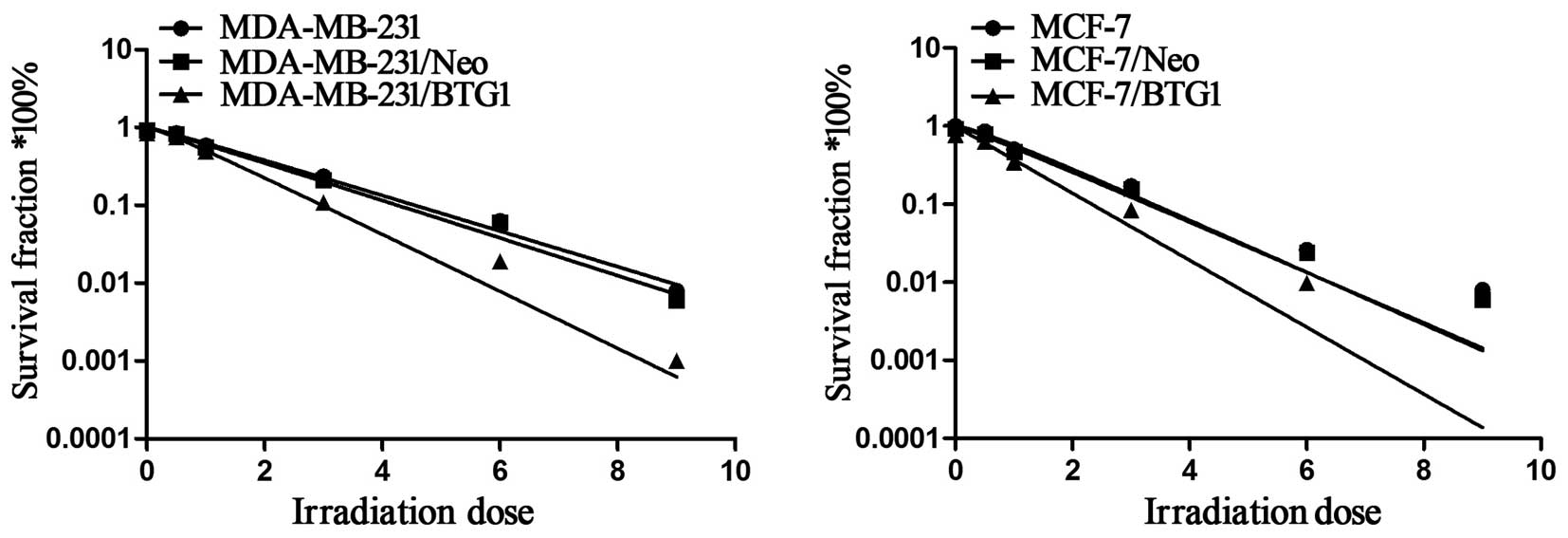
multi-target click model. As shown in Fig. 1, the cell survival curve of BTG1
overexpression cells shifted to the left both in the p53-mutant
cells (MDA-MB-231) and in the p53 wild-type cells (MCF-7),
indicating that cell radiosensitivity was increased. Table I shows the values of several
radiobiological parameters by the multi-target model. The D0 values
for the MDA-MB-231 and MDA-MB-231/Neo cells were 1.76 and 1.69 Gy,
yet the D0 value for the MDA-MB-231/BTG1 cells was reduced to 1.29
Gy. Thus, the radiosensitization ratios were 1.36 and 1.31, which
indicated that the radiosensitivity of the MDA-MB-231/BTG1 cells
was increased by 1.36 and 1.31. The same increasing trend was also
observed in the MCF-7 cells. The D0 value for both MCF-7 and
MCF-7/Neo cells was 1.79 Gy, yet the D0 value for the MCF-7/BTG1
cells was 1.32 Gy. Thus, the radiosensitization ratio was 1.36,
which indicated that the radiosensitivity of MCF-7/BTG1 cells was
increased by 1.36. These data indicate that overexpression of BTG1
enhanced the radiosensitivity of both the p53-mutant breast cancer
MDA-MB-231 cells and the p53 wild-type breast cancer MCF-7 cells
in vitro.
 | Table IRelative biological parameters of the
different cell groups after calculation using the multi-target
single-hit model. |
Table I
Relative biological parameters of the
different cell groups after calculation using the multi-target
single-hit model.
| Cell group | Fitting curve
equation | D0 | Dq | N |
|---|
| MDA-MB-231 | SF = 1 − (1 −
e−0.5683D)1.41 | 1.76 | 1.61 | 1.41 |
| MDA-MB-231/Neo | SF = 1 − (1 −
e−0.5933D)1.34 | 1.69 | 0.49 | 1.34 |
|
MDA-MB-231/BTG1 | SF = 1 − (1 −
e−0.7739D)1.33 | 1.29 | 0.37 | 1.33 |
| MCF-7 | SF = 1 − (1 −
e−0.5694D)1.74 | 1.79 | 0.99 | 1.74 |
| MCF-7/Neo | SF = 1 − (1 −
e−0.56790D)1.61 | 1.79 | 0.86 | 1.61 |
| MCF-7/BTG1 | SF = 1 − (1 −
e−0.7554D)1.19 | 1.32 | 0.22 | 1.19 |
Effects of BTG1 along with irradiation on
the cell cycle distribution of MDA-MB-231 and MCF-7 cells
The cells were irradiated at different doses of 0,
1, 3, 6 and 9 Gy and were then incubated at 37°C for 24 h
post-irradiation. Flow cytometry was used to analysis the cell
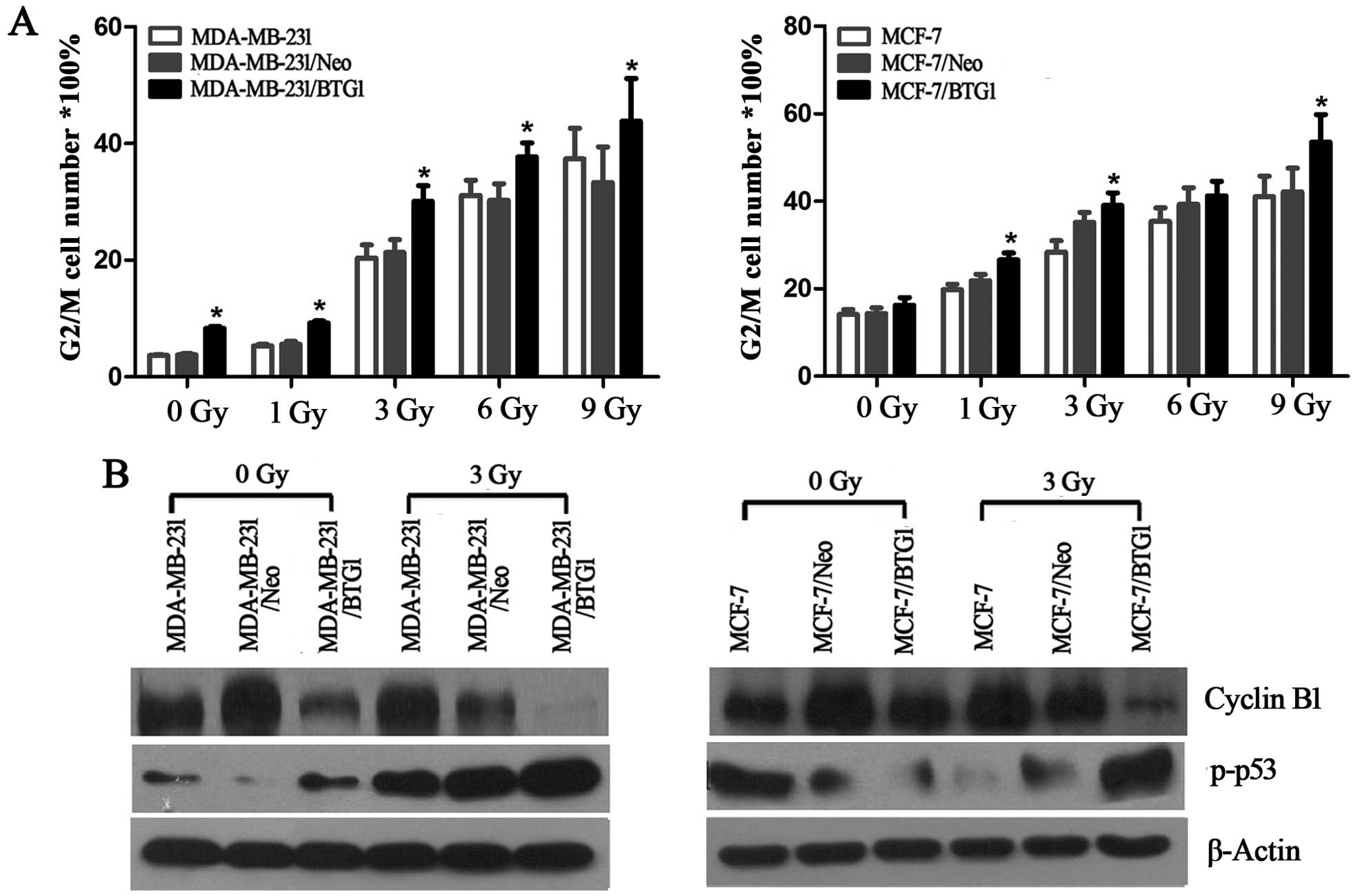
cycle distribution. As shown in Fig.
2A, with an increase in radiation dose, all groups of cells
underwent G2 phase arrest and the apoptosis rate was also increased
(p<0.05). In addition, the BTG1 overexpression group cells
(MDA-MB-231/BTG1 and MCF-7/BTG1) had significantly increased G2/M
phase arrest (p<0.05) when compared with the control group
cells.
Next, we evaluated the effects of BTG1 on the
expression of cell cycle proteins cyclin B1 and p-p53 in the
MDA-MB-231 and MCF-7 cells using western blot analysis. As shown in
Fig. 2B, overexpression of BTG1
along with irradiation treatment led to a significant inhibition of
cyclin B1 and promotion of p-p53. In summary, these results
revealed that after breast cancer cells underwent DNA damage caused
by irradiation, an increase in the level of BTG1 expression
inhibited cyclin B1 expression and promoted p-p53 expression, which
in turn affected the cell cycle distribution of the breast cancer
cells (MDA-MB-231 and MCF-7) and induced G2/M phase arrest.
Effects of BTG1 along with irradiation on
the apoptosis of MDA-MB-231 and MCF-7 cells
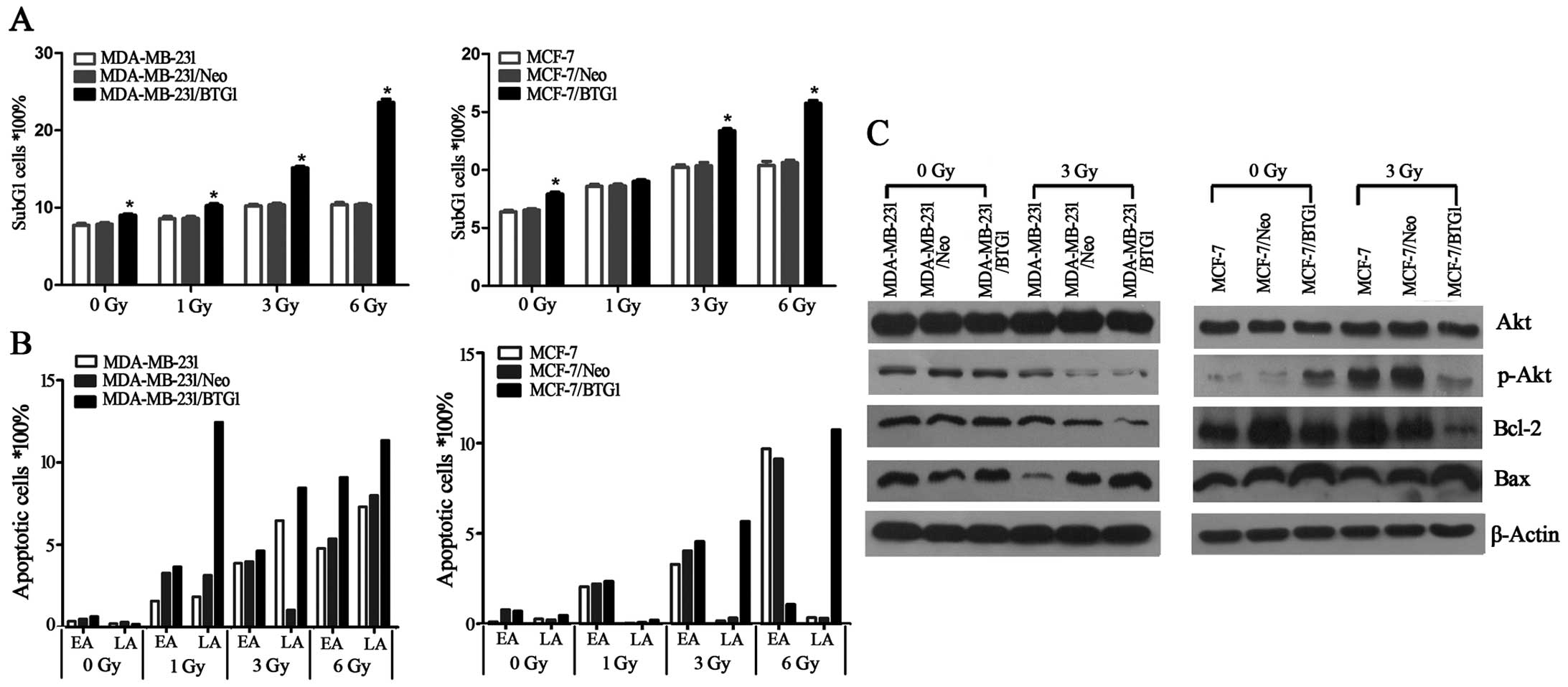
Annexin V staining was used to detect cell
apoptosis. As determined by the Annexin V assay, in the MCF-7/BTG1
and MDA-MB-231/BTG1 cells the percentage of apoptotic cells and the
sub-G1 peaks were higher than that in the control cells (Fig. 3A and B), after cells were exposed to
irradiation. Next, we detected the expression of anti-apoptotic
factor Bcl-2 and pro-apoptotic factors Bax and PI3K/Akt using
western blot analysis. As shown in Fig.
3C, overexpression of BTG1 along with irradiation treatment
suppressed the PI3K/Akt signaling pathway, downregulated the
expression of the anti-apoptotic protein Bcl-2 and upregulated the
expression of the pro-apoptotic protein Bax in the MCF-7 and
MDA-MB-231 cells. These results indicated that the promotive effect
on cell apoptosis by BTG1 along with irradiation is most likely
mediated by Bcl-2 and Bax which is regulated by the PI3K/Akt
signaling pathway in breast cancer cells.
Effects of BTG1 along with irradiation on
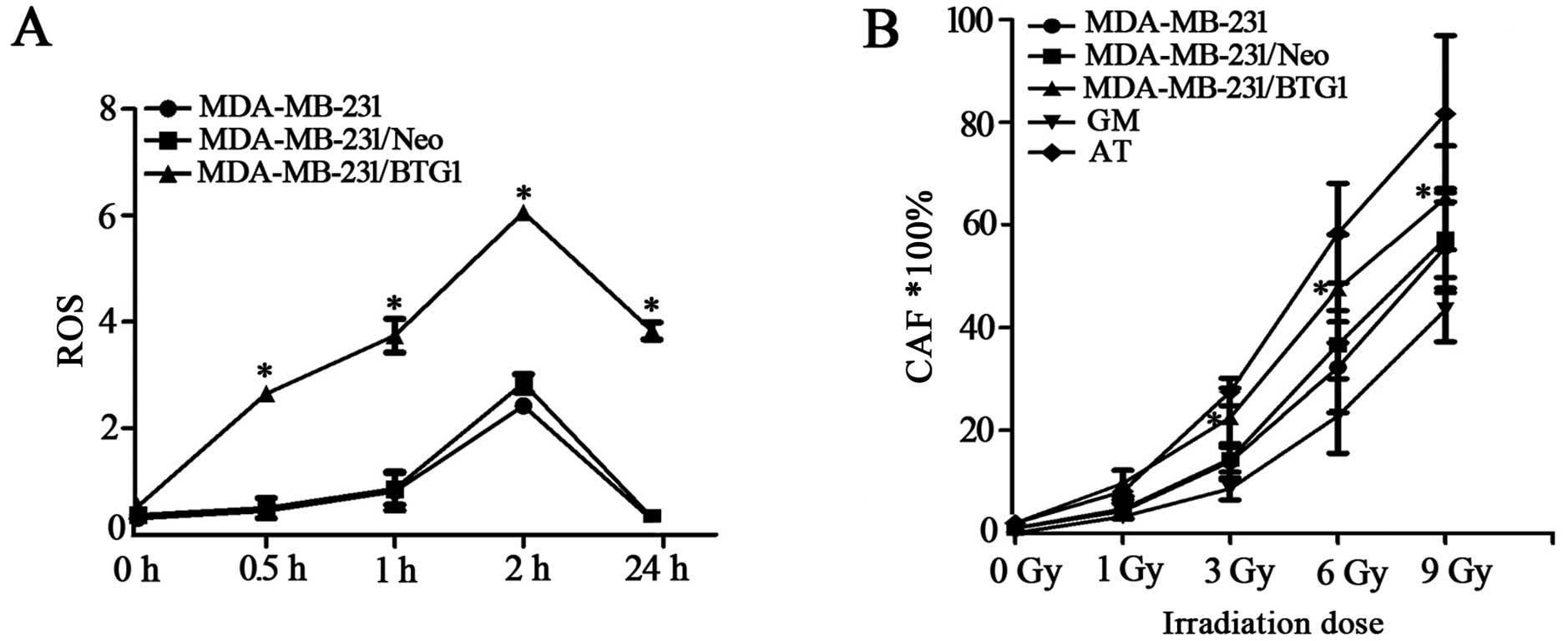
the intracellular ROS of MDA-MB-231 cells
Intracellular ROS accumulation can activate several
pathways important for the induction of apoptosis; thus, we
examined the involvement of ROS in BTG1 and irradiation-induced
cell death. As shown in Fig. 4A,
ROS levels in the BTG1 overexpression group cells were markedly
higher than the levels in the control group cells after irradiation
of 3 Gy treatment for 0.5–24 h. This result indicates that
upregulation of BTG1 promotes the formation of ROS in breast cancer
cells, thereby enhancing cell radiosensitivity.
Effects of irradiation on chromosomal
aberrations in MDA-MB-231 cells
MDA-MB-231, MDA-MB-231/Neo, MDA-MB-231/BTG1, GM
(normal radiation sensitive) and AT (radiation sensitive) cells
were exposed to 0, 1, 3, 6 and 9 Gy doses of irradiation and
chromosomal aberrations were detected. As shown in Fig. 4B, the rates of chromosomal
aberrations in the MDA-MB-231/BTG1 cells were significantly higher
than those in the MDA-MB-231, MDA-MB-231/Neo and GM cells following
irradiation at doses of 3, 6 and 9 Gy (p<0.05), yet these rates
were lower than those in the AT cells. Those results revealed that
BTG1 increased the rate of chromosomal aberrations after breast
cancer MDA-MB-231 cells were exposed to moderate- or high-dose
irradiation.
Effects of BTG1 on the radiosensitivity
of breast cancer in vivo
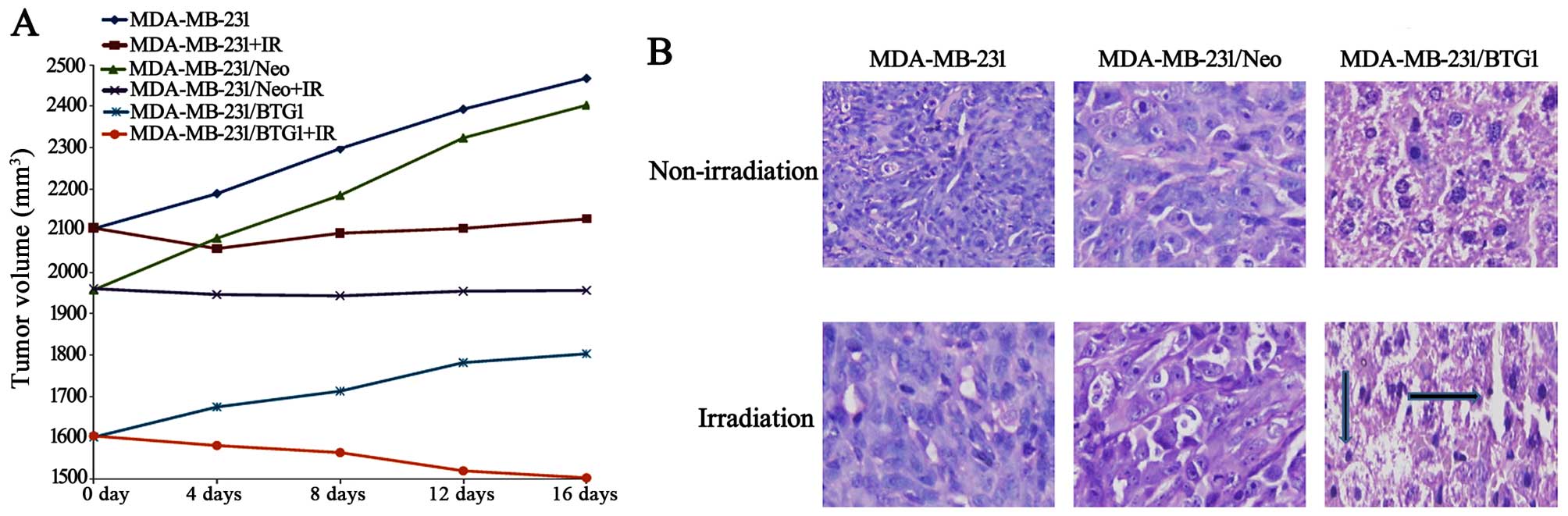
The three groups of MDA-MB-231 cells were
subcutaneously injected into nude mice. Each group consisted of 12
nude mice, and 6 nude mice were randomly selected to accept a 3 Gy
dose of radiation. After 16 days of growth, the tumor masses
obtained from the irradiated group xenografts were markedly smaller
than those from the non-irradiated group (Fig. 5A, p<0.05), and the BTG1
overexpression (MDA-MB-231/BTG1) and irradiation group xenografts
were smaller than the xenografts from the other groups (p<0.05).
H&E staining showed that tumor density in the MDA-MB-231/BTG1
cell tumors was decreased when compared to the control cell tumors
in both the irradiated and non-irradiated groups (Fig. 5B).
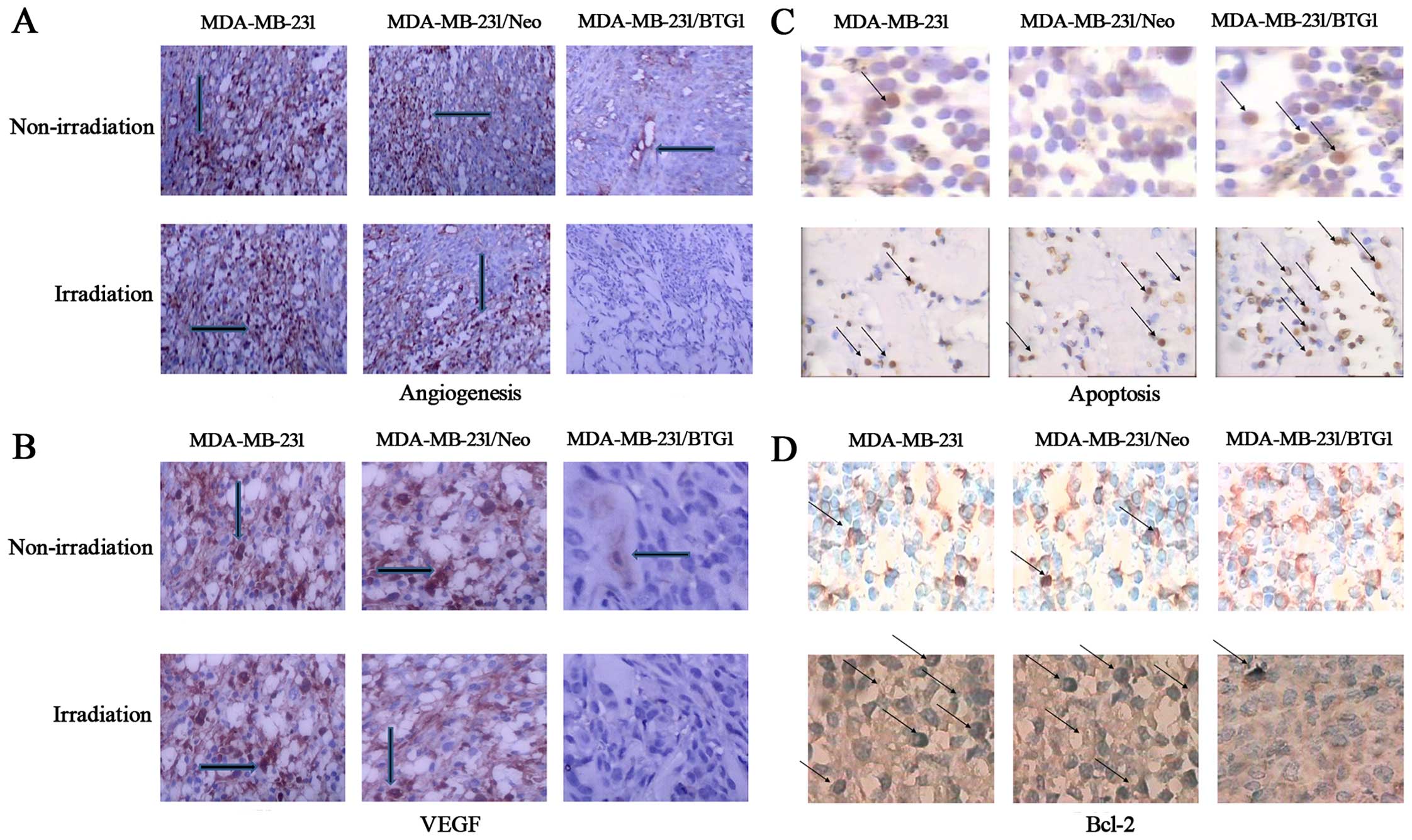
The effect of BTG1 overexpression on tumor
angiogenesis in vivo was then assessed using
immunohistochemistry. Anti-CD31 antibodies were utilized to detect
CD31 levels, which were used to reflect the level of vascular
endothelial proliferation. As shown in Fig. 6A, the number of tumor microvessels
in the MDA-MB-231 and MDA-MB-231/Neo tumor groups was 13 and 11,
respectively, and significantly more than the MDA-MB-231/BTG1 tumor
group (p<0.05). In contrast, the number of tumor microvessels in
the MDA-MB-231/BTG1 nude mice irradiated with 3 Gy X-ray was 2–3,
which was less than that in the other groups (p<0.05). In
addition, VEGF expression was lower or negative in the
MDA-MB-231/BTG1 tumor group whether irradiation or not (Fig. 6B). These results suggest that
overexpression of BTG1 along with irradiation may inhibit
angiogenesis through the downregulation of VEGF expression, thereby
inhibiting tumor metastasis.
Next, we assessed tumor necrosis and apoptosis by
TUNEL staining assay. As shown in Fig.
6C, various cells exhibited nuclear pyknosis and fragmented,
irregular, inconsistency in size and brownish yellow staining
indicating cell apoptosis. Thus, several visible sporadic apoptotic
cells were presented in the MDA-MB-231, MDA-MB-231/Neo tumor
tissue. In contrast, a high number of apoptotic cells was observed
in the MDA-MB-231/BTG1 tumor tissue. The immunochemical staining
showed that overexpression of BTG1 along with the irradiation
treatment downregulated the expression of the anti-apoptotic
protein Bcl-2 (Fig. 6D).
Clearly, these in vivo results strongly
confirm the effect observed in vitro indicating that BTG1
plays an important role in breast cancer cell growth and
radiosensitivity.
Discussion
Radiotherapy is arguably the most important
treatment for cancer, particularly for loco-regional tumors without
metastasis. Ionizing radiation is used to treat nearly all types of
solid tumors, yet to varying degrees of success (17,18).
The reasons for radiotherapy failure are multiple and varied. Cells
in different cell cycle phases have variant radiosensitivity. In
general, cells in the G0/G1 phase have a certain radiation
resistance and the highest sensitivity exists for cells in the
boundary of the G0/G1 and S phase. When cells enter the S phase,
the radiation resistance increases gradually and reaches the
highest at the late S phase, yet it is decreased in the G2/M phase
(19). In the present study, our
results showed that overexpression of BTG1 induced G2/M phase
arrest, which increased the ionizing radiosensitivity of breast
cancer cells.
The regulation of the cell cycle is one of the main
influencing mechanisms of cellular radiosensitivity. Cell cycle
regulation after ionizing radiation occurs in the control points of
G1/S and G2/M phase, where DNA damage repair-related proteins are
activated preventing the unrepaired DNA into S phase (20). The p53 gene is an important effector
in the human cell cycle checkpoint, and can be phosphorylated by
DNA damage signal caused by irradiation. Then p53 starts p21 gene
transcription, promotes the expression of p21CIP1/WAF1,
and inhibits the activity of CDK2 to prevent cells from entering
the S phase (21,22). p53-mutant cells show G2 phase arrest
in DNA damage repair, and the length of G2 phase arrest is
proportional to the degree of DNA damage. G2 phase arrest leads to
an increase in cell death and enhanced cell radiosensitivity in
theory. Our results indicated that overexpression of BTG1 increased
p-p53 expression after cells were exposed to irradiation, which
enhanced breast cancer cell radiosensitivity. Cyclin B1 expression
is minimal at the initiation of the S phase and peaks at the G2-M
border; this peak in cyclin B1 activity is required for cells
entering mitosis (23). Thus,
overexpression of cyclin B1 leads to G2/M conversion, and even
leads to uncontrolled cell proliferation and malignant
transformation (24).
Phosphorylation of cyclin B1 inhibits Cdk1 activation through the
p21/Gadd45/Cdk1 pathway activated by p53 which result in G2/M phase
arrest (25). Cyclin B1
overexpression is also closely related to the pathological type of
tumor, tumor metastasis and prognosis, and causes tumor radiation
resistance (26). In the present
study, we found that overexpression of BTG1 decreased cyclin B1
expression after breast cancer cells were exposed to irradiation.
These data indicated that BTG1 participates in G2/M phase
checkpoint regulation through inhibition of cyclin B1 expression
and enhances breast cancer cell radiosensitivity.
Cell apoptosis is also one of the molecular
mechanisms that improves tumor cell radiosensitivity (27). The Bcl-2 family of proteins serves
as critical regulators of apoptosis that can be identified as
anti-apoptotic proteins. The anti-apoptotic Bcl-2/Bcl-xL and
pro-apoptotic Bax/Bak proteins can both negatively and positively
regulate events in apoptotic cell death through modulation of
cytochrome c release (28).
The PI3K/Akt pathway is an important intracellular signaling
pathway in the regulation of cell growth, survival, adhesion and
migration, particularly during cancer progression, metastasis and
radioresistance (29,30). In the present study, we demonstrated
that overexpression of BTG1 reduced p-Akt, Bcl-2 and increased Bax
expression after breast cancer cells were exposed to irradiation.
These results indicate that the PI3K/Akt pathway mediates BTG1
upregulation of breast cancer cell radiosensitivity.
Recent studies suggest that reactive oxygen species
(ROS) may play an important role during the induction of apoptosis
(31). Many stimuli such as tumor
necrosis factor-α, anticancer drugs and chemopreventive agents
stimulate cells to produce ROS (32–35).
Intracellular ROS can also be induced by irradiation and serve as
major mediators of radiation damage (36). When ROS generation exceeds the
cellular antioxidant defenses, cell damage ensues (37,38).
In the present study, we found that overexpression of BTG1 along
with irradiation treatment led to significant elevation in the
levels of intracellular ROS and cell apoptosis rates, which further
indicated that upregulation of BTG1 promoted the formation of ROS
in breast cancer cells, thereby enhancing cell
radio-sensitivity.
Importantly, the finding that BTG1 promoted ionizing
radiosensitivity of breast cancer cells in vitro was
confirmed in our animal model. After the nude mouse model was
treated with irradiation, the volume of established BTG1
overexpression xenografts was smaller than the volume in the vector
control group, and the immunochemical staining showed that the
levels of Bcl-2 and VEGF expression were lower than those in the
control group. Next, we found that the proportion of apoptotic
cells was increased and the tumor angiogenesis was reduced in the
experimental group xenografts, as detected by TUNEL and
immunohistochemical analysis.
In summary, the present study investigating the role
of BTG1 in breast cancer cell radiosensitivity demonstrated that
the protein can enhance cell radiosensitivity. The mechanism of
action may be related to cell cycle regulation, G2/M phase arrest,
ROS formation, promotion of cell apoptosis and inhibition of tumor
angiogenesis. These findings provide new insight into the role of
BTG1 in breast cancer radiosensitivity and may have important
implication for the development of radiation therapy for breast
cancer.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (nos. 81502758 and 81302383),
the Science and Technology Foundation of Suzhou (nos. SYS201215 and
SYS201417), and a Project Funded by the Priority Academic Program
Development of Jiangsu Higher Education Institutions (PAPD).
References
|
1
|
Printz C: American Cancer Society reports
progress in reducing cancer deaths: However, some groups still lag
behind this trend. Cancer. 117:4573–4574. 2011. View Article : Google Scholar
|
|
2
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
DeSantis C, Ma J, Bryan L and Jemal A:
Breast cancer statistics, 2013. CA Cancer J Clin. 64:52–62. 2014.
View Article : Google Scholar
|
|
4
|
Pekkola-Heino K, Servomaa K, Kiuru A and
Grenman R: Increased radiosensitivity is associated with p53
mutations in cell lines derived from oral cavity carcinoma. Acta
Otolaryngol. 116:341–344. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huerta S, Gao X, Dineen S, Kapur P, Saha D
and Meyer J: Role of p53, Bax, p21, and DNA-PKcs in radiation
sensitivity of HCT-116 cells and xenografts. Surgery. 154:143–151.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Berthet C, Guéhenneux F, Revol V, Samarut
C, Lukaszewicz A, Dehay C, Dumontet C, Magaud JP and Rouault JP:
Interaction of PRMT1 with BTG/TOB proteins in cell signalling:
Molecular analysis and functional aspects. Genes Cells. 7:29–39.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rouault JP, Rimokh R, Tessa C, Paranhos G,
Ffrench M, Duret L, Garoccio M, Germain D, Samarut J and Magaud JP:
BTG1, a member of a new family of antiproliferative genes. EMBO J.
11:1663–1670. 1992.PubMed/NCBI
|
|
8
|
Matsuda S, Rouault J, Magaud J and Berthet
C: In search of a function for the TIS21/PC3/BTG1/TOB family. FEBS
Lett. 497:67–72. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bakker WJ, Blázquez-Domingo M, Kolbus A,
Besooyen J, Steinlein P, Beug H, Coffer PJ, Löwenberg B, von
Lindern M and van Dijk TB: FoxO3a regulates erythroid
differentiation and induces BTG1, an activator of protein arginine
methyl transferase 1. J Cell Biol. 164:175–184. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Corjay MH, Kearney MA, Munzer DA, Diamond
SM and Stoltenborg JK: Antiproliferative gene BTG1 is highly
expressed in apoptotic cells in macrophage-rich areas of advanced
lesions in Watanabe heritable hyperlipidemic rabbit and human. Lab
Invest. 78:847–858. 1998.PubMed/NCBI
|
|
11
|
Rodier A, Marchal-Victorion S, Rochard P,
Casas F, Cassar-Malek I, Rouault JP, Magaud JP, Mason DY, Wrutniak
C and Cabello G: BTG1: A triiodothyronine target involved in the
myogenic influence of the hormone. Exp Cell Res. 249:337–348. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee H, Cha S, Lee MS, Cho GJ, Choi WS and
Suk K: Role of antiproliferative B cell translocation gene-1 as an
apoptotic sensitizer in activation-induced cell death of brain
microglia. J Immunol. 171:5802–5811. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sheng SH, Zhao CM and Sun GG: BTG1
expression correlates with the pathogenesis and progression of
breast carcinomas. Tumour Biol. 35:3317–3326. 2014. View Article : Google Scholar
|
|
14
|
Li W, Zou ST, Zhu R, Wan JM, Xu Y and Wu
HR: B-cell translocation 1 gene inhibits cellular
metastasis-associated behavior in breast cancer. Mol Med Rep.
9:2374–2380. 2014.PubMed/NCBI
|
|
15
|
Wu X, Ding N, Hu W, He J, Xu S, Pei H, Hua
J, Zhou G and Wang J: Down-regulation of BTG1 by miR-454-3p
enhances cellular radiosensitivity in renal carcinoma cells. Radiat
Oncol. 9:1792014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhu R, Zou ST, Wan JM, Li W, Li XL and Zhu
W: BTG1 inhibits breast cancer cell growth through induction of
cell cycle arrest and apoptosis. Oncol Rep. 30:2137–2144.
2013.PubMed/NCBI
|
|
17
|
Peters LJ, Withers HR, Thames HD Jr and
Fletcher GH: Tumor radioresistance in clinical radiotherapy. Int J
Radiat Oncol Biol Phys. 8:101–108. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Deacon J, Peckham MJ and Steel GG: The
radioresponsiveness of human tumours and the initial slope of the
cell survival curve. Radiother Oncol. 2:317–323. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wyld L, Smith O, Lawry J, Reed MW and
Brown NJ: Cell cycle phase influences tumour cell sensitivity to
aminolaevulinic acid-induced photodynamic therapy in vitro. Br J
Cancer. 78:50–55. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Efeyan A and Serrano M: p53: Guardian of
the genome and policeman of the oncogenes. Cell Cycle. 6:1006–1010.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Duan X, Zhang H, Liu B, Li XD, Gao QX and
Wu ZH: Apoptosis of murine melanoma cells induced by heavy-ion
radiation combined with Tp53 gene transfer. Int J Radiat Biol.
84:211–217. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hill R, Bodzak E, Blough MD and Lee PW:
p53 binding to the p21 promoter is dependent on the nature of DNA
damage. Cell Cycle. 7:2535–2543. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hwang A, McKenna WG and Muschel RJ: Cell
cycle-dependent usage of transcriptional start sites. A novel
mechanism for regulation of cyclin B1. J Biol Chem.
273:31505–31509. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ozeki M, Tamae D, Hou DX, Wang T, Lebon T,
Spitz DR and Li JJ: Response of cyclin B1 to ionizing radiation:
Regulation by NF-kappaB and mitochondrial antioxidant enzyme MnSOD.
Anticancer Res. 24:2657–2663. 2004.PubMed/NCBI
|
|
25
|
Raj D, Liu T, Samadashwily G, Li F and
Grossman D: Survivin repression by p53, Rb and E2F2 in normal human
melanocytes. Carcinogenesis. 29:194–201. 2008. View Article : Google Scholar
|
|
26
|
Hassan KA, Ang KK, El-Naggar AK, Story MD,
Lee JI, Liu D, Hong WK and Mao L: Cyclin B1 overexpression and
resistance to radiotherapy in head and neck squamous cell
carcinoma. Cancer Res. 62:6414–6417. 2002.PubMed/NCBI
|
|
27
|
Lee CH, Jeon YT, Kim SH and Song YS:
NF-kappaB as a potential molecular target for cancer therapy.
Biofactors. 29:19–35. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cory S, Huang DC and Adams JM: The Bcl-2
family: Roles in cell survival and oncogenesis. Oncogene.
22:8590–8607. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chang L, Graham PH, Hao J, Ni J, Bucci J,
Cozzi PJ, Kearsley JH and Li Y: Acquisition of
epithelial-mesenchymal transition and cancer stem cell phenotypes
is associated with activation of the PI3K/Akt/mTOR pathway in
prostate cancer radioresistance. Cell Death Dis. 4:e8752013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chang L, Graham PH, Hao J, Bucci J, Cozzi
PJ, Kearsley JH and Li Y: Emerging roles of radioresistance in
prostate cancer metastasis and radiation therapy. Cancer Metastasis
Rev. 33:469–496. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schulz JB, Bremen D, Reed JC, Lommatzsch
J, Takayama S, Wüllner U, Löschmann PA, Klockgether T and Weller M:
Cooperative interception of neuronal apoptosis by BCL-2 and BAG-1
expression: Prevention of caspase activation and reduced production
of reactive oxygen species. J Neurochem. 69:2075–2086. 1997.
View Article : Google Scholar
|
|
32
|
Goossens V, De Vos K, Vercammen D,
Steemans M, Vancompernolle K, Fiers W, Vandenabeele P and Grooten
J: Redox regulation of TNF signaling. Biofactors. 10:145–156. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Simizu S, Takada M, Umezawa K and Imoto M:
Requirement of caspase-3(-like) protease-mediated hydrogen peroxide
production for apoptosis induced by various anticancer drugs. J
Biol Chem. 273:26900–26907. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chan WH, Yu JS and Yang SD: PAK2 is
cleaved and activated during hyperosmotic shock-induced apoptosis
via a caspase-dependent mechanism: Evidence for the involvement of
oxidative stress. J Cell Physiol. 178:397–408. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Vercammen D, Brouckaert G, Denecker G, Van
de Craen M, Declercq W, Fiers W and Vandenabeele P: Dual signaling
of the Fas receptor: Initiation of both apoptotic and necrotic cell
death pathways. J Exp Med. 188:919–930. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mikkelsen RB and Wardman P: Biological
chemistry of reactive oxygen and nitrogen and radiation-induced
signal transduction mechanisms. Oncogene. 22:5734–5754. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gamaley IA and Klyubin IV: Roles of
reactive oxygen species: Signaling and regulation of cellular
functions. Int Rev Cytol. 188:203–255. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cerutti PA: Prooxidant states and tumor
promotion. Science. 227:375–381. 1985. View Article : Google Scholar : PubMed/NCBI
|