Introduction
Ovarian cancer is a genetic disease, and the main
cause of death of women with a malignant tumor of the genital
system, showing a high morbidity rate in developing countries. It
is still the fifth major cause of death in women with cancer, in
spite of rapid progress in diagnosis and treatment of ovarian
cancer (1). Of all ovarian
carcinoma cases, epithelial ovarian cancer (EOC) accounts for 90%
of morbidity (2). Moreover, EOC can
spread to peritoneal cavity via peritoneal fluid, contributing to
the inefficiency of surgery and chemotherapy treatment. Most
patients die of recurrence. Previous findings confirmed that the
dysfunctional molecular mechanism targeted EOC, for instance, Kras,
Brca1/2, Tp53, Rb and PTEN (3-5). A
better understanding of the mechanisms involved in EOC and more
effective therapeutic approaches are urgently needed.
Long non-coding RNA (lncRNA) does not translate into
proteins and is longer than 200 nucleotides (6). Increasingly lncRNAs are emerging as
important regulators of tumor initiation and progression (7). The tempting potential in most of the
lncRNAs have stimulated keen interests, particularly the cancer
complexity. More recently, CCAT1-L has been shown to play a role in
MYC transcriptional regulation and to promote a long-range
chromatin looping (8-10). MALAT1 has been reported to promote
cancer metastasis (11) or resist
rapid RNA decay (12-14). The findings indicate that lncRNAs
may act as important regulators in tumorigenesis.
The present study describes the lncRNA growth
arrest-specific transcript 5 (GAS5), which is alternatively spliced
and is transcribed from locus 1q25.1 (15). GAS5 regulates a valuable biological
function since it is a multiple-snoRNA-host gene (16). Functionally, GAS5 competes with the
glucocorticoid response elements in the genome for binding to these
receptors and promotes cells apoptosis (17), which were originally identified in
leukemic and NIh3T3 cells (18).
Intriguingly, the inhibition of mammalian target of rapamycin
(mTOR) pathway depends on GAS5 (19), which negatively regulates miR-21
possibly through the RNA-induced silencing complex (20). Additionally, it was shown that GAS5
is down-regulated in some cancer cell lines and tissues (21-24).
For example, GAS5 inhibits malignant pleural mesothelioma (MPM)
cell growth by inhibiting hedgehog and PI3K/mTOR signal pathway in
MPM (23). GAS5 as a tumor
suppressor in non-small cell lung cancer (NSCLC) mediated by the
p53-independent and p53-dependent pathways (25). GAS5 is able to inhibit E2F1 and
cyclin D1, and thus leads to decreased gastric cancer cell
proliferation (24). However, the
role of GAS5 in ovarian cancer has not been previously
reported.
The fundamental mechanisms of GAS5 on tumorigenesis
remain largely unknown, although GAS5 has been indicated to take
part in suppression on malignant tumors. The potential mechanisms
can be related to the fact that GAS5 regulates nonsense-mediated
RNA decay pathway (19,26) or downregulates c-Myc (27). These findings provide strong
evidence that GAS5 plays an important role in guiding the cell fate
toward apoptosis. It is well known that apoptosis is a regulated
cell death process and plays a significant role in most of the
physiological processes. Apoptosis can be caused by the extrinsic
or intrinsic pathway; the former occurs through triggering the
transmembrane death receptors, while, the latter is initiated from
intracellular developmental cues or cell stress (28,29).
In the intrinsic apoptotic pathway, mitochondria is considered the
core of organelles (30). The
mitochondrial function can be mediated by Bcl-2 family proteins
(31). The potential role of
mitochondria-dependent apoptotic pathway in the apoptotic effect of
GAS5 has not been explored.
The present study demonstrated a significant
decrease in the expression of GAS5 in EOC tissues, which was
associated with clinicopathological parameters. Moreover, the
overexpression of GAS5 obviously promoted the apoptosis of ovarian
cancer cell lines. Together, these results reveal evidence for
apoptosis regulation between lncRNA GAS5 and ovarian cancer,
indicating a potential target of diagnosis and gene therapy in the
disease.
Materials and methods
Cell lines and tissue samples
The human ovarian cancer HO8910 (Bioleaf Biotech
Co., Ltd., Shanghai, China) and A2780 cells (Chuanbo Biotechnology
Co., Ltd., Nanjing, China) were grown in RPMI-1640 and Dulbecco's
modified Eagle's medium (DMEM) medium (both from HyClone, Beijing,
China), respectively, supplemented with 10% fetal bovine serum
(FBS) (Sijiqing, Zhejiang, China) in a humidified incubator (37°C,
5% CO2).
Specimens of EOC tissue (n=60, without radiation or
chemotherapy), normal ovarian epithelial tissues (n=13) and benign
ovarian epithelial lesions (n=10) were collected at the Second
Affiliated hospital of Harbin Medical University (China) between
March 2012 and April 2014 (median age 55 years, range 37-79). The
samples of normal ovarian tissue were collected from
hysterosalpingo-oophorectomy following uterine myoma, endometriosis
or adenomyosis. Written informed consent was obtained from all
participants. The study was approved by the Human Ethnics Committee
of the Second Affiliated Hospital of Harbin Medical University (no.
2015-yan-167).
Real-time polymerase chain reaction
Total RNA was extracted from tissues and cell lines
using TRIzol reagent (Invitrogen, CA, USA), and RNA purity was
identified by optical density (OD) 260/OD 280 nm. The reverse
transcription reactions were performed using oligo(dT) primers and
a reverse transcriptase kit (Bioneer, Shanghai, China). CDNAs were
synthesized from total RNA using the specific primers. The
20-μl reactions were incubated on a PCR system for 1 min at
56°C, 60 min at 50°C, 5 min at 95°C and then held at 4°C. Real-time
polymerase chain reaction (PCR) was performed with the ABI 7300
real-time PCR system (Applied Biosystems, CA, USA). The assay was
carried out in optical tubes at 95°C for 5 min, followed by 40
cycles of 95°C for 30 sec and 55°C for 30 sec. For all human
ovarian tissues and cell lines, real-time PCR was performed using
TaqMan MGB primers and probe specially for human GAS5 (designed by
GenePharma, Shanghai, China; forward primer, 3′-CTT
CTGGGCTCAAGTGATCCT-5′ and reverse primer,
3′-TTGTGCCATGAGACTCCATCAG-5′; probe, CCTCCCAGTG GTCTTT) and
eukaryotic 18S rRNA (GenePharma; forward primer,
5′-TTTGACTCAACACGGGAAACC-3′ and reverse primer,
5′-CACGGAATCGAGAAAGAGCTATC-3′; probe, CCGGACACGGACAGGATTGACAGAT)
served as the endogenous control. All of the real-time PCRs were
performed in triplicate. The threshold cycle (CT) data and
baselines were determined using auto-settings. The relative
quantification of GAS5 expression was calculated using the
2−ΔΔCt method relative to 18s rRNA.
Transfection
To overexpress GAS5, plasmid pCDNA-GAS5 was
constructed by Shanghai GenePharma Co., Ltd. of China containing
the whole genome sequence of GAS5 (NCBI Reference Sequence:
NR_002578.2).
The plasmid carrying pCDNA-GAS5 (1
μg/μl) was transfected into ovarian cancer cell lines
using Lipofectamine 2000 (Invitrogen); Lipofectamine
2000:pCDNA-GAS5=1:1.4.
Cell proliferation and viability
Cell proliferation and viability of HO8910 and A2780
were evaluated using
3-[4,5-dimeth-ylthiazol-2-yl]-2,5-diphenyl-tetrazolium bromide
(MTT) (Amresco LLC, Oh, USA) assay. Briefly, after 6 h transfection
with pCDNA-GAS5, ~5×103 cells/well were seeded into a
96-well plate at 37°C. Each well was repeated six times. After
further incubation at different times (24, 48 and 72 h), 20
μl MTT (0.5 mg/ml) was added to each well and further
incubated for 4 h. Then the medium was removed and
dimethylsulfoxide was added to dissolve the MTT formazan crystals.
The cell viability and proliferation were determined by OD450
value. The experiments were performed three times.
Colony formation assay
Approximately 800 pCDNA-GAS5- or empty
vector-transfected HO8910 and A2780 cells/wells were placed onto a
six-well plate and maintained in a medium containing 10% FBS,
replacing the medium every three days. After 14 days, the colonies
were fixed with methanol and stained with 0.5% crystal violet
(Amresco, Shanghai, China). The visible colonies were manually
counted. The experiments were performed three times.
Transwell assay
Transwell assays were performed using a Costar
chamber (Corning Costar Corp., Cambridge, MA, USA). The bottom
chambers were filled with a culture medium containing 10% FBS.
Different samples of tansfected HO8910 or A2780 cells
(5×104) were suspended into a culture medium without
FBS, and then the cells were seeded into the upper chambers. The
Transwell chamber containing an 8-μm pore size polycarbonate
membrane filter was coated either with (for invasion) or without
(for migration) Matrigel. After 48 h of culture at 37°C, the upper
layer of cells were removed before visualization, and the cells on
the lower surface were fixed and stained with 0.5% crystal violet.
The cells were counted by Image-Pro Plus 6.0. (Media Cybernetics,
Rockville, MD, USA) in five random fields and photographed. The
experiments were performed three times.
Terminal deoxynucleotidyl transferase
dUTP nick-end labeling (TUNEL)
The HO8910 and A2780 cells were transfected with
pCDNA-GAS5 or empty vector, and cultured into six-well plates for
48 h. They were then fixed with 4% para-formaldehyde solution, the
apoptotic cells were labeled using the In Situ Cell Death Detection
kit (Beyotime, Shanghai, China), the fluorescence was detected
using a Nikon Eclipse TE2000-S fluorescence microscope (Nikon,
Tokyo, Japan) and counted by Image-Pro Plus 6.0 (Media Cybernetics)
in three different experiments, the red fluorescence marked cells
were apoptotic cells.
Cell apoptotic analysis
HO8910 and A2780 cells (1–2×105) were
treated with a pcDNA-GAS5 or an empty vector; then placed into
six-well plates. After 24-h incubation, the cells were trypsinized
and then fixed in 70% ethanol for 3 h at 4°C; 3 h later, the cells
were incubated with propidium iodide (PI) (×20) and RNase A (×50)
for 30 min in the dark. Cells were collected and analyzed for
apoptosis using a flow cytometer (BD Biosciences, Franklin Lakes,
NJ, USA) after PI staining. The results were analyzed by BD Accuri
C6 software. The experiments were repeated at least three
times.
Mitochondrial membrane potential
assay
The JC-1 probe (Beyotime) was performed to measure
mitochondrial depolarization in ovarian cancer cells. Briefly,
cells (0.5–2×105/ml) were cultured in six-well plates.
After treatment for 48 h, they were incubated with an equal volume
of a JC-1 staining solution (5 μg/ml) at 37°C for 40 min and
rinsed three times with PBS. Mitochondrial membrane potentials were
monitored by determining the relative amounts of dual emissions
from mitochondrial JC-1 monomers or aggregates using a fluorescent
microscope at 488-nm excitation. Mitochondrial depolarization was
assessed by the change in the green/red fluorescence intensity
ratio. The cells were counted using Image-Pro Plus 6.0 in five
random fields.
Western blot analysis
The pCDNA-GAS5- or empty vector-transfected HO8910
and A2780 cells were lysed using a lysis buffer (Beyotime) that
contained the phenylmethanesulfonyl fluoride. The protein
concentrations were determined by bicinchoninic acid protein assay.
The samples (50 μg) were electrophoresed on a 10% sodium
dodecylsulfate-polyacryl-amide gel electrophoresis and transferred
onto nitrocellulose membranes (BioTrance, MI, USA). The membranes
were blocked with 0.1% I-Block (Applied Biosystems) in TBS-T (0.1%
Tween-20) at room temperature for 1 h, then incubated with specific
antibodies at 4°C overnight. The membranes were washed with TBS-T
(0.1% Tween-20) and incubated for 1 h at room temperature with
horseradish peroxidase-conjugated secondary antibodies (Bio-Rad
Laboratories). Following washing, the specific bands were detected
using the enhanced chemiluminescence (Beyotime) chromogenic
substrate. The protein expression was analyzed using densitometry
(Quantity One software; Bio-Rad, hercules, CA, USA). β-actin
(ZSGB-BIO, Beijing, China) was used as a control. Additionally,
anti-caspase 3 and anti-BAX antibodies were purchased from Santa
Cruz Biotechnology (Santa Cruz, CA, USA). Anti-caspase 9 and
anti-BAK antibodies were purchased from Beyotime Institute of
Biotechnology (Shanghai, China).
Statistical analysis
All statistical analysis was performed with SPSS
17.0 (SPSS, Inc., Chicago, IL, USA). Data are presented as means ±
standard error of mean from at least three independent experiments.
Statistical analysis was performed using Chi-square and Student's
t-tests, or one-way analysis of variance followed by a post hoc
test, where appropriate. Differences were considered to indicate a
statistically significant result at P<0.05.
Results
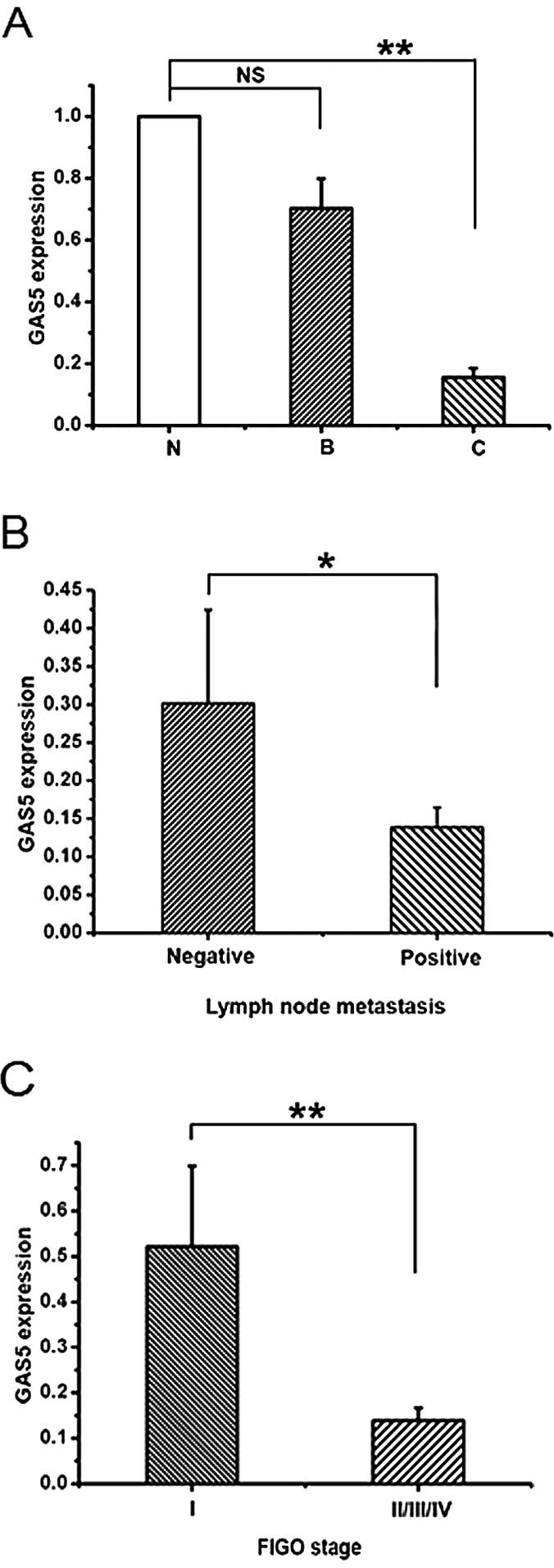
GAS5 is downregulated in EOC tissues
To investigate whether lncRNA GAS5 controls EOC,
real-time PCR was used to examine the GAS5 expression in normal
ovarian epithelial tissues, benign ovarian epithelial lesions and
EOCs. The GAS5 expression of controls, both normal ovarian
epithelial tissues (N) and benign epithelial lesions (B), showed no
statistical significance (Fig. 1A).
While, there appears to be a significant reduction of GAS5
expression in EOCs (C) compared with normal ovarian epithelial
tissues (6.44-fold) (Fig. 1A).
Then, the clinicopathological parameters, such as age,
differentiation, location, lymph node metastasis and FIGO stage,
were analyzed to assess the expression of GAS5 and clinical
significance of EOC. As shown in Fig.
1B and C and Table I, samples
with lymph node metastasis and advanced FIGO stage had low
expression of GAS5. These data indicate that the decreased
expression of GAS5 is related to EOC.
 | Table ICorrelation between GAS5 expression
and clinico-pathological parameters of EOC. |
Table I
Correlation between GAS5 expression
and clinico-pathological parameters of EOC.
| Clinicopathological
parameters | No. of cases | Relative GAS5
expression
| P-valuea |
|---|
| Low | High |
|---|
| Age (years) | | | | 0.381 |
| ≤50 | 22 | 13 | 9 | |
| >50 | 38 | 18 | 20 | |
|
Differentiation | | | | 0.944 |
| Well,
moderate | 26 | 14 | 12 | |
| Poor | 34 | 18 | 16 | |
| Location | | | | 0.965 |
| Unilateral | 25 | 13 | 12 | |
| Bilateral | 35 | 18 | 17 | |
| Lymph node | | | | 0.025b |
| metastasis | | | | |
| Positive | 40 | 28 | 12 | |
| Negative | 20 | 8 | 12 | |
| FIGO stage | | | | 0.035b |
| I | 15 | 5 | 10 | |
| II/III/IV | 45 | 29 | 16 | |
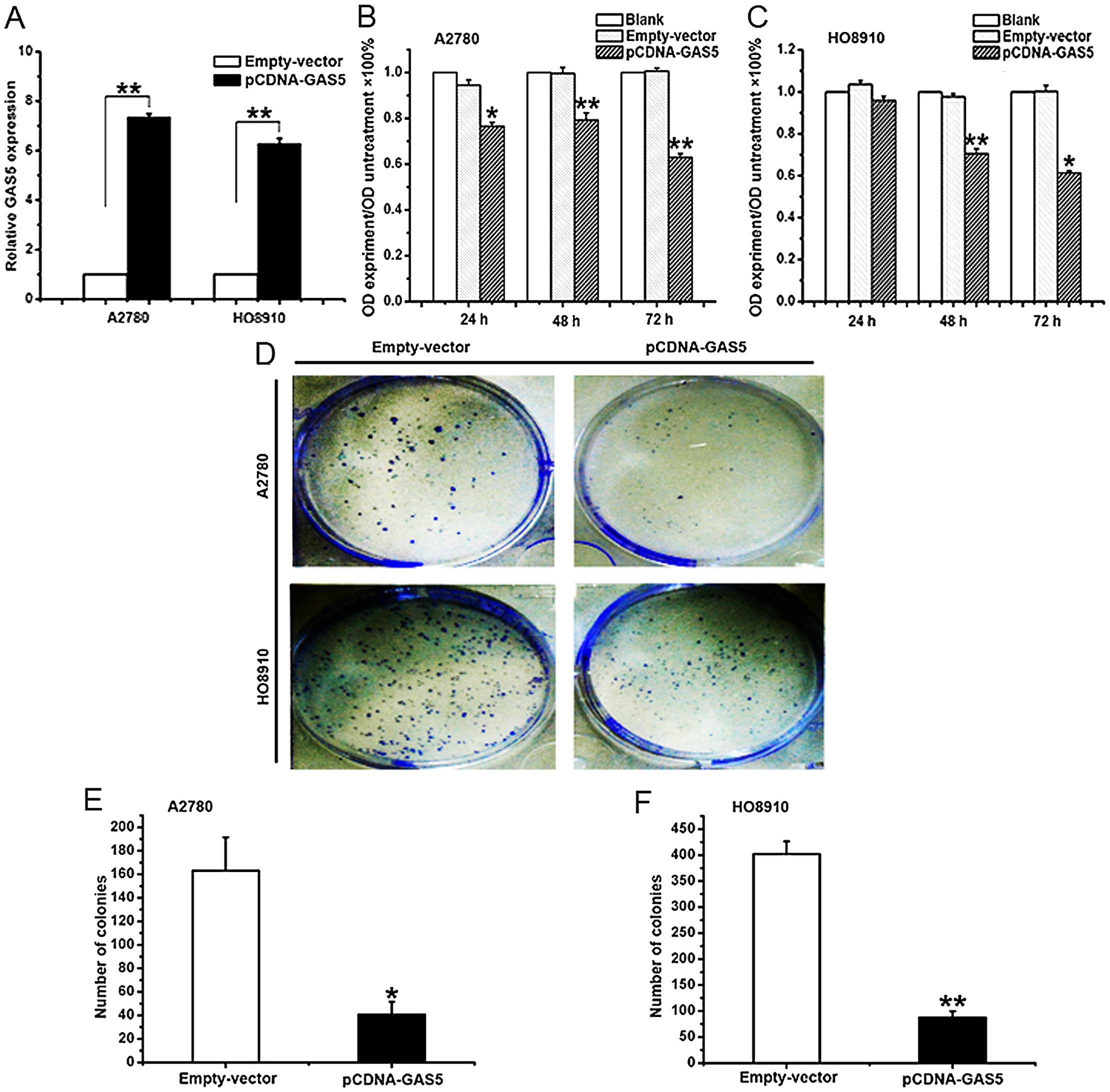
The effect of GAS5 on proliferation in
ovarian cancer cell lines
With the aim of manipulating the GAS5 expression in
ovarian cancer cells, the pCDNA-GAS5 or an empty vector was
transfected into A2780 and HO8910 cells. After 24 h of
transfection, the level of GAS5 was well upregulated in A2780
(74-fold) and HO8910 (63-fold) cells, respectively (Fig. 2A). To ascertain the role of GAS5 in
the proliferation of EOC, the overexpression of A2780 and HO8910
cells of GAS5 were analyzed. MTT assay was used to assess the
biological role of GAS5 in proliferation. Compared with the cells
transfected with empty vector, the ones transfected with pCDNA-GAS5
demonstrated significantly decreased viability (Fig. 2B and C). Besides, it was found that
the overexpression of GAS5 greatly weakened the ability of colony
forming using the colony formation assay (Fig. 2D–F). The results demonstrated that
GAS5 inhibited the proliferation of ovarian cancer cells.
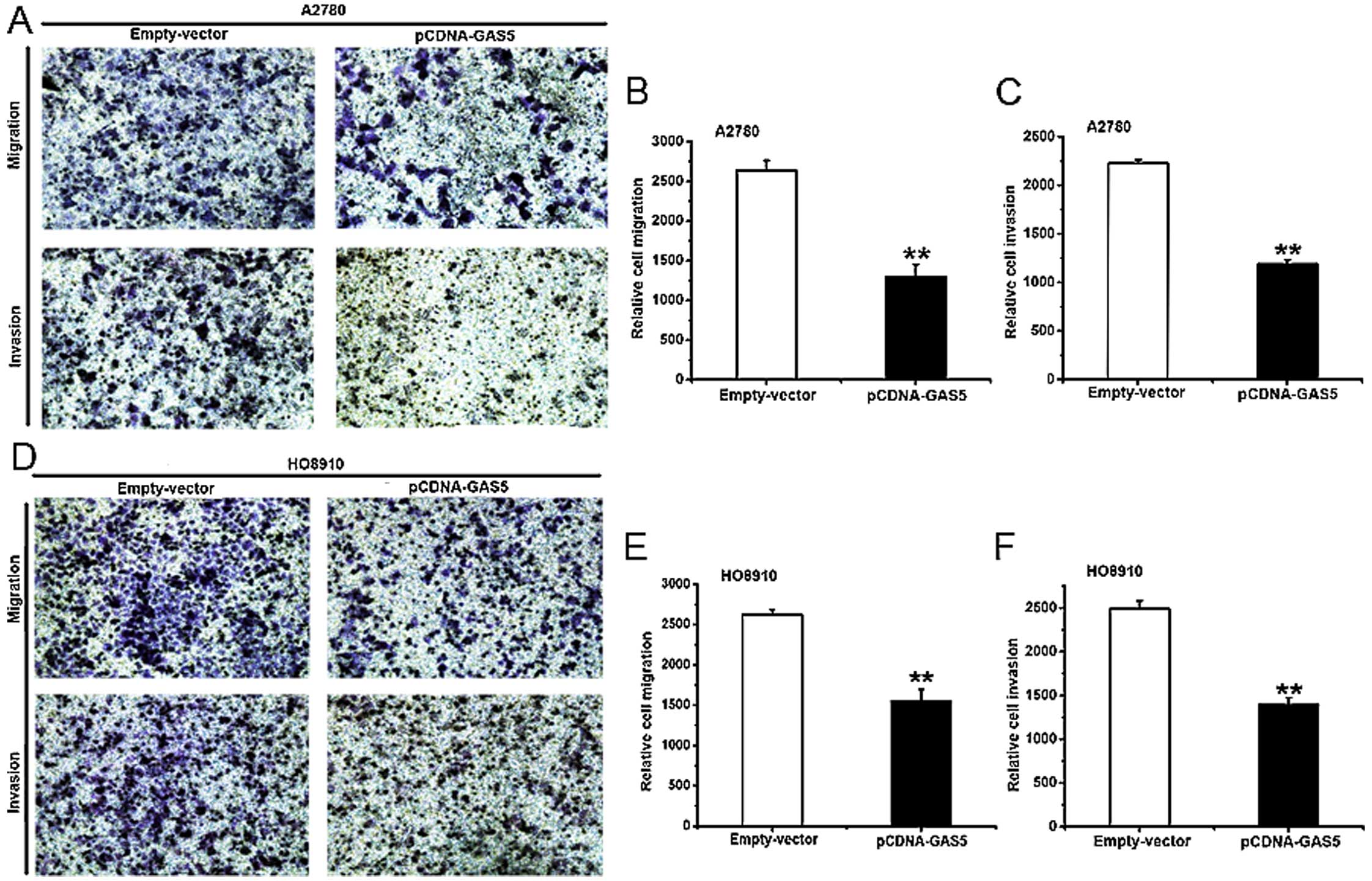
The effect of GAS5 on migration and
invasion in ovarian cancer cell lines
Most ovarian cancer patients die of tumor
metastasis, and there some research reports concerning the
relationship between lncRNAs and neoplastic metastasis (32,33).
To study the effect of GAS5 in vitro on migration and
invasion of ovarian cancer cell lines, a Costar chamber without
(for migration) or with (for invasion) Matrigel was used. The
treatment of A2780 and HO8910 cells is the same as described above.
Compared with the ones treated with empty vector, the ability of
migration and invasion was weakened in GAS5-overexpressed A2780
(Fig. 3A–C) and HO8910 (Fig. 3D and E) cells. Conclusively, GAS5
can inhibit migration and invasion in ovarian cancer cells.
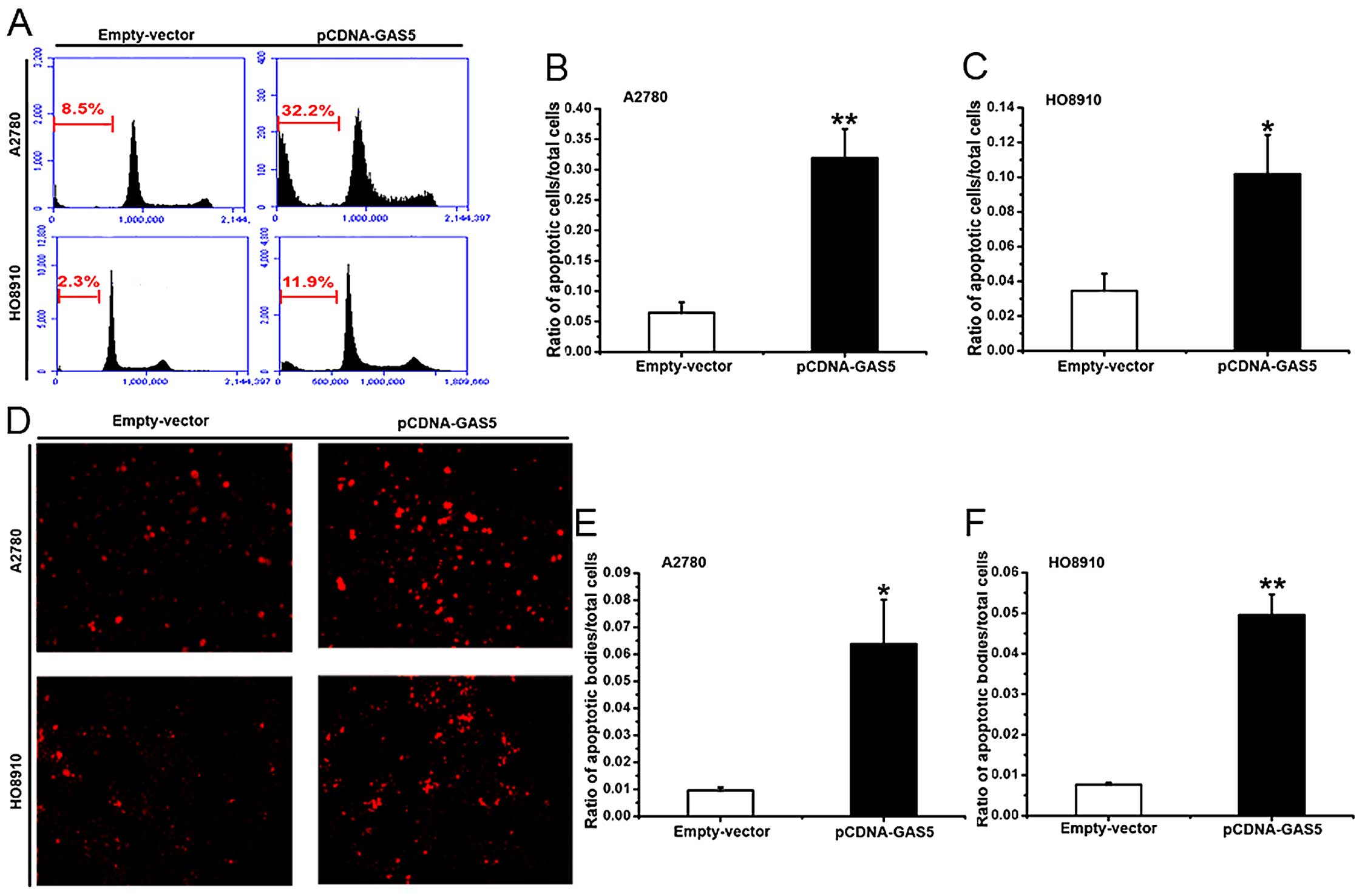
The effect of GAS5 on apoptosis in
ovarian cancer cell lines
Apoptosis-resistant phenotype is the major feature
of cancer cells (34). To study the
role of GAS5 in apoptosis, A2780 and HO8910 cells transfected with
pCDNA-GAS5 or empty vector were monitored using flow cytometry. As
shown in Fig. 4A–C, ectogenic GAS5
obviously promoted the apoptosis of the cells. Similarly, TUNEL
staining showed that the number of apoptotic cells was more in the
GAS5-overexpressed cells than in the controls (Fig. 4D–F). The above results suggest that
GAS5 displays a critical function in pro-apoptosis of ovarian
cancer cells.
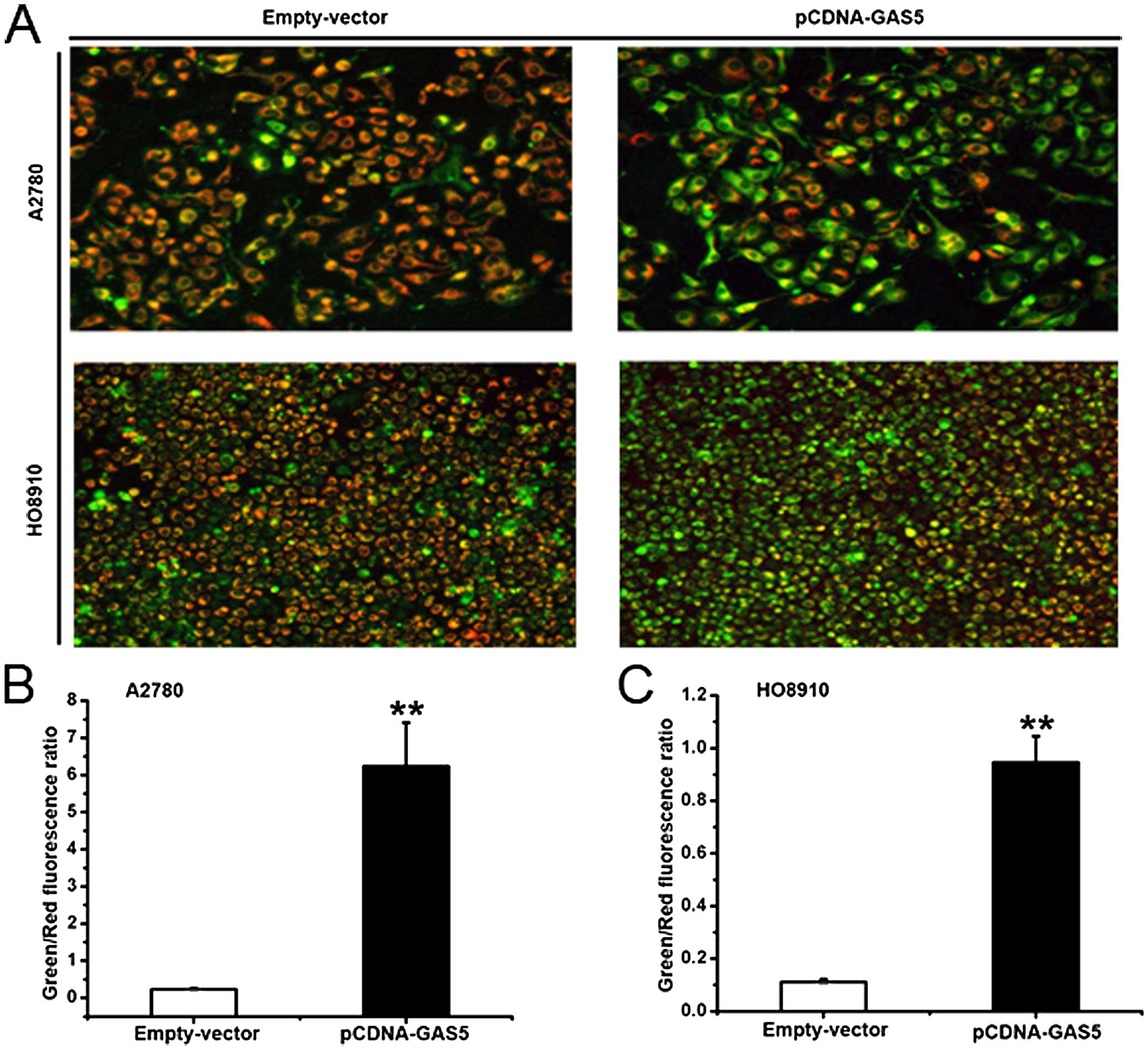
The effect of GAS5 on mitochondrial
depolarization in ovarian cancer cell lines
The statistics previously described demonstrated
that GAS5 played a substantial role in apoptosis. The disruption of
the mitochondrial membrane potential is an early event of
apoptosis. To understand the function of GAS5 on an early stage of
apoptosis, JC-1 probe staining was used to detect the effect. The
mitochondrial membrane potential of healthy mitochondria, which
were detected by JC-1, displayed red fluorescence. When the
mitochondrial membrane potential collapsed in apoptotic cells, the
JC-1 fluoresced green. As shown in Fig.
5A–C, A2780 and HO8910 cells transfected with pCDNA-GAS5 or
empty vector displayed a significant difference. Tumor
cell-overexpressed GAS5 showed increased ratios of green/red
fluorescence. Thus, these data provide evidence that the action of
GAS5 is required for the damage of mitochondrial potential.
The effect of GAS5 on apoptosis pathway
in ovarian cancer cell lines
The upregulation of GAS5 in cells was verified to
arrest growth (22). The results of
flow cytometry (Fig. 4A–C) and
TUNEL assay (Fig. 4D–F)
demonstrated that GAS5 plays an important role in apoptosis of
ovarian cancer cells. The early apoptosis stage was enhanced by
GAS5 assessed through JC-1 (Fig.
5A–C). It was, thus, further explored whether GAS5 promoted
apoptosis and the underlying pathway.
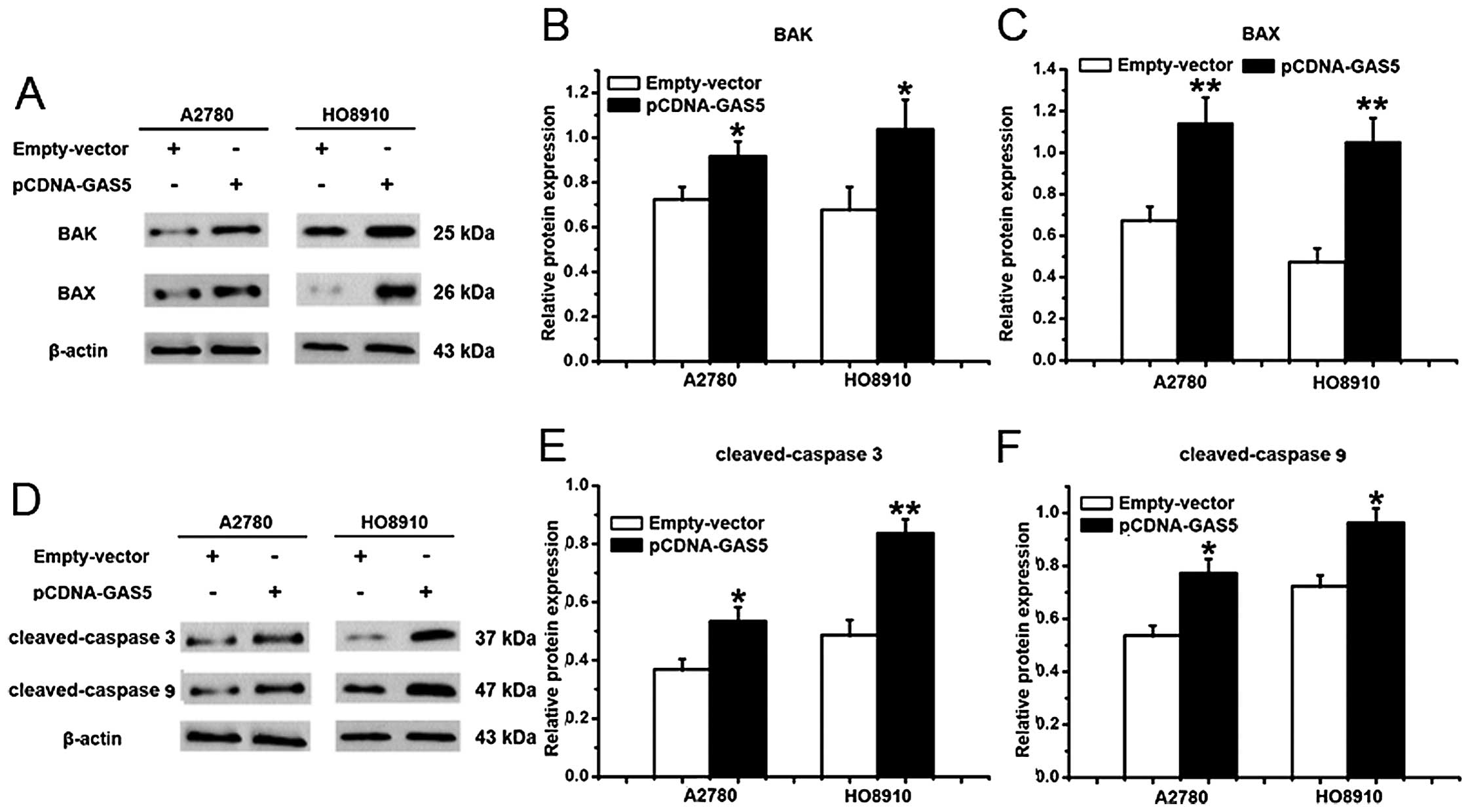
Pro-apoptotic BAX and BAK is a requisite gateway to
mitochondrial dysfunction and death (31), which were used to study the role in
GAS5-induced apoptosis of ovarian cancer cells. As shown in
Fig. 6A–C, there was an increase in
the expression of BAX and BAK in A2780 and HO8910 cells that were
overexpressed by the GAS5 gene. Taken together, these results
suggest that GAS5 promotes apoptosis potentially by the
mitochondrial-mediated apoptosis pathway.
Pro-apoptotic member, BAK, can lead to the release
of cytochrome c (35), which
can interact with caspase 9 and then activate caspase 3,
contributing to cell apoptosis (29,36).
Therefore, cleaved-caspase 3 and cleaved-caspase 9 were also
detected. After transfected with pCDNA-GAS5 for 24 h, the protein
expression of cleaved-caspase 3 and cleaved-caspase 9, which were
likely to be the critical molecules affected by GAS5, were both
significantly higher than the empty-vector-transfected HO8910 and
A2780 cells (Fig. 6D–F).
Discussion
Several important observations were demonstrated in
the present study. First, compared with epithelial tissues of
normal ovary and benign epithelial ovarian lesions, depressed
expression of GAS5 was detected in EOC. Moreover, GAS5 expression
appeared to be significantly correlated to lymph node metastasis
and FIGO stage of EOC. Second, in vitro, the ability of
proliferation, migration and invasion were weakened, while the
capability of apoptosis was strengthened by GAS5 overexpression.
Finally, evidence exists that GAS5 promoted apoptosis of ovarian
cancer cells via the mitochondrial-dependent apoptosis pathway. The
present study provides a novel finding that ovarian cancer is
modulated by GAS5.
GAS5 was found downregulated in EOC tissues compared
to the normal ovarian epithelium, and lower GAS5 correlated with
more transferred lymph nodes and advanced FIGO stage. Consistently,
earlier studies showed that GAS5 was downregulated in NSCLC
compared with the adjacent normal lung tissues; importantly, lower
GAS5 expression correlated with larger tumor size and advanced
clinical stage (25). Additionally,
findings showed that GAS5 expression was markedly depressed in
gastric cancer tissues, and the lower GAS5 mainly appeared in the
larger tumor size; an advanced pathologic stage. Moreover, patients
with lower GAS5 expression had poorer disease-free survival and
overall survival (24). More
recently, studies showed that the GAS5 expression was decreased in
cervical cancer tissues compared to the adjacent normal tissues,
and depressed GAS5 expression was correlated with the advanced FIGO
stage, deeper invasion and more lymph node metastasis. Patients
with lower GAS5 expression had poorer overall survival (37). Taken together, GAS5 may serve as a
tumor suppressor in human tumors.
Cell apoptosis is a complex process that is involved
in a variety of regulatory mechanisms and is closely related to
tumorigenesis. It has been indicated that GAS5 stimulates apoptosis
through significantly different intron or exon composition of these
GAS5 transcripts (22). Moreover,
the study confirmed that GAS5 leads to the dysfunctional
mitochondrial membrane potential. The current study found that
GAS5-induced mitochondrial-mediated pro-apoptotic proteins, BAD and
BAK (released more from the ovarian cancer cells) were
overexpressed by GAS5, indicating that the mitochondrion-modulated
apoptosis pathway is required for the pro-apoptotic effect of GAS5
in human ovarian cancer cells.
Mitochondria have been illustrated as the guardian
of cell death, and they play a key role in regulating pathways of
cell apoptosis (31,38). Besides, the Bcl-2 family proteins
and caspases execute a crucial role in the mitochondria-mediated
apoptosis pathways. Studies indicate that it is necessary to
activate BAX or BAK to initiate mitochondrial dysfunction and cell
apoptosis (31). The activation of
BAX or BAK has been proposed to result in the formation of a
voltage-dependent anion channel-containing pore (39) or permeabilization of mitochondrial
membranes (40) to initiate
cytochrome c release. Then cytochrome c binds the
C-terminal domain of the apoptotic protease-activating factor-1.
Later, Apaf1, pro-caspase 9, and released cytochrome c form
the apoptosome that drives the cleaved-caspase 3, one of the most
important apoptosis executioners, leading to the mitochondrial
dysfunction and cell apoptosis (40).
The present study confirms that GAS5 leads to the
dysfunctional mitochondrial membrane potentials in vitro. To
further verify the GAS5-induced mitochondria-dependent apoptosis,
the expression of BAK, BAX, cleaved-caspase 9 and cleaved-caspase 3
were evaluated. Consistent with previous studies, significant
expression of the proteins was observed after the GAS5
transfection. Taken together, these findings prove that lncRNA GAS5
acts as a tumor-suppressor in human EOC, and the regulating pathway
is mitochondrion adjusted. however, there are certain aspects that
still need to be resolved: i) more patients samples should be
collected to verify the credibility of GAS5 repression function in
EOC; ii) the interacting molecular mechanisms and signal pathways
on how GAS5 changes the mitochondrion-independent apoptosis should
be clarified; and iii) the mechanisms of decreased capabilities of
migration and invasion by GAS5 in ovarian cancer cells should be
explicated.
In conclusion, the findings of the present study
show that GAS5 overexpression can promote apoptosis, inhibit
proliferation, and reduce migration and invasion in ovarian cancer
cells. In addition, GAS5 expression is lower in EOC tissues than in
the normal ovarian epithelium, and the lower expression of GAS5 is
correlated to more lymph node metastasis and advanced FIGO stage of
EOC, indicating that GAS5 may play a role as a suppressor of
tumorigenesis for EOC patients. These findings have major
implications for devising a strategy for ovarian cancer
treatment.
Acknowledgments
The authors would like to acknowledge the generous
assistance of all the staff of the Key Laboratory of Harbin Medical
University (Daqing). The authors also acknowledge the female
patients who participated in the present study.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Naora H: The heterogeneity of epithelial
ovarian cancers: Reconciling old and new paradigms. Expert Rev Mol
Med. 9:1–12. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mullany LK, Fan HY, Liu Z, White LD,
Marshall A, Gunaratne P, Anderson ML, Creighton CJ, Xin L, Deavers
M, et al: Molecular and functional characteristics of ovarian
surface epithelial cells transformed by KrasG12D and loss of Pten
in a mouse model in vivo. Oncogene. 30:3522–3536. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Quinn BA, Brake T, Hua X, Baxter-Jones K,
Litwin S, Ellenson LH and Connolly DC: Induction of ovarian
leiomyo-sarcomas in mice by conditional inactivation of Brca1 and
p53. PLoS One. 4:e84042009. View Article : Google Scholar
|
|
5
|
Szabova L, Yin C, Bupp S, Guerin TM,
Schlomer JJ, Householder DB, Baran ML, Yi M, Song Y, Sun W, et al:
Perturbation of Rb, p53, and Brca1 or Brca2 cooperate in inducing
metastatic serous epithelial ovarian cancer. Cancer Res.
72:4141–4153. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Clark MB, Johnston RL, Inostroza-Ponta M,
Fox AH, Fortini E, Moscato P, Dinger ME and Mattick JS: Genome-wide
analysis of long noncoding RNA stability. Genome Res. 22:885–898.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gutschner T and Diederichs S: The
hallmarks of cancer: A long non-coding RNA point of view. RNA Biol.
9:703–719. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Alaiyan B, Ilyayev N, Stojadinovic A,
Izadjoo M, Roistacher M, Pavlov V, Tzivin V, Halle D, Pan H, Trink
B, et al: Differential expression of colon cancer associated
transcript1 (CCAT1) along the colonic adenoma-carcinoma sequence.
BMC Cancer. 13:1962013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ling H, Spizzo R, Atlasi Y, Nicoloso M,
Shimizu M, Redis RS, Nishida N, Gafa R, Song J, Guo Z, et al:
CCAT2, a novel noncoding RNA mapping to 8q24, underlies metastatic
progression and chromosomal instability in colon cancer. Genome
Res. 23:1446–1461. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xiang JF, Yin QF, Chen T, Zhang Y, Zhang
XO, Wu Z, Zhang S, Wang HB, Ge J, Lu X, et al: human colorectal
cancer-specific CCAT1-L lncRNA regulates long-range chromatin
interactions at the MYC locus. Cell Res. 24:513–531. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tee AE, Ling D, Nelson C, Atmadibrata B,
Dinger ME, Xu N, Mizukami T, Liu PY, Liu B, Cheung B, et al: The
histone demethylase JMJD1A induces cell migration and invasion by
up-regulating the expression of the long noncoding RNA MALAT1.
Oncotarget. 5:1793–1804. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gutschner T, Hämmerle M and Diederichs S:
MALAT1 - a paradigm for long noncoding RNA function in cancer. J
Mol Med. 91:791–801. 2013. View Article : Google Scholar
|
|
13
|
Gutschner T, Hammerle M, Eissmann M, Hsu
J, Kim Y, Hung G, Revenko A, Arun G, Stentrup M, Gross M, et al:
The noncoding RNA MALAT1 is a critical regulator of the metastasis
phenotype of lung cancer cells. Cancer Res. 73:1180–1189. 2013.
View Article : Google Scholar :
|
|
14
|
Brown JA, Bulkley D, Wang J, Valenstein
ML, Yario TA, Steitz TA and Steitz JA: Structural insights into the
stabilization of MALAT1 noncoding RNA by a bipartite triple helix.
Nat Struct Mol Biol. 21:633–640. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Raho G, Barone V, Rossi D, Philipson L and
Sorrentino V: The gas 5 gene shows four alternative splicing
patterns without coding for a protein. Gene. 256:13–17. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Smith CM and Steitz JA: Classification of
gas5 as a multi-small-nucleolar-RNA (snoRNA) host gene and a member
of the 5′-terminal oligopyrimidine gene family reveals common
features of snoRNA host genes. Mol Cell Biol. 18:6897–6909. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kino T, Hurt DE, Ichijo T, Nader N and
Chrousos GP: Noncoding RNA gas5 is a growth arrest- and
starvation-associated repressor of the glucocorticoid receptor. Sci
Signal. 3:ra82010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Coccia EM, Cicala C, Charlesworth A,
Ciccarelli C, Rossi GB, Philipson L and Sorrentino V: Regulation
and expression of a growth arrest-specific gene (gas5) during
growth, differentiation, and development. Mol Cell Biol.
12:3514–3521. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fingar DC and Blenis J: Target of
rapamycin (TOR): An integrator of nutrient and growth factor
signals and coordinator of cell growth and cell cycle progression.
Oncogene. 23:3151–3171. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang Z, Zhu Z, Watabe K, Zhang X, Bai C,
Xu M, Wu F and Mo YY: Negative regulation of lncRNA GAS5 by miR-21.
Cell Death Differ. 20:1558–1568. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pickard MR, Mourtada-Maarabouni M and
Williams GT: Long non-coding RNA GAS5 regulates apoptosis in
prostate cancer cell lines. Biochim Biophys Acta. 1832:1613–1623.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mourtada-Maarabouni M, Pickard MR, Hedge
VL, Farzaneh F and Williams GT: GAS5, a non-protein-coding RNA,
controls apoptosis and is downregulated in breast cancer. Oncogene.
28:195–208. 2009. View Article : Google Scholar
|
|
23
|
Renganathan A, Kresoja-Rakic J, Echeverry
N, Ziltener G, Vrugt B, Opitz I, Stahel RA and Felley-Bosco E: GAS5
long non-coding RNA in malignant pleural mesothelioma. Mol Cancer.
13:1192014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sun M, Jin FY, Xia R, Kong R, Li J, Xu T,
Liu Y, Zhang E, Liu X and De W: Decreased expression of long
noncoding RNA GAS5 indicates a poor prognosis and promotes cell
proliferation in gastric cancer. BMC Cancer. 14:3192014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xuefei Shi MS, Liu H, Yao Y, Kong R, Chen
F and Song Y: A critical role for the long non-coding RNA GAS5 in
proliferation and apoptosis in non-small-cell lung cancer. Mol
Carcinog. 54(Suppl 1): E1–E12. 2015. View
Article : Google Scholar
|
|
26
|
Hay N and Sonenberg N: Upstream and
downstream of mTOR. Genes Dev. 18:1926–1945. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang FJ and Chan WH: Effects of
ochratoxin a on mouse oocyte maturation and fertilization, and
apoptosis during fetal development. Environ Toxicol. Dec
15–2014.Epub ahead of print. View Article : Google Scholar
|
|
28
|
Danial NN and Korsmeyer SJ: Cell death:
Critical control points. Cell. 116:205–219. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hengartner MO: The biochemistry of
apoptosis. Nature. 407:770–776. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Crow MTMK, Nam YJ and Kitsis RN: The
mitochondrial death pathway and cardiac myocyte apoptosis. Circ
Res. 95:957–970. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wei MC, Zong WX, Cheng EH, Lindsten T,
Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB and
Korsmeyer SJ: Proapoptotic BAX and BAK: A requisite gateway to
mitochondrial dysfunction and death. Science. 292:727–730. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Guttman M and Rinn JL: Modular regulatory
principles of large non-coding RNAs. Nature. 482:339–346. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gupta RA, Shah N, Wang KC, Kim J, Horlings
HM, Wong DJ, Tsai MC, Hung T, Argani P, Rinn JL, et al: Long
non-coding RNA HOTAIR reprograms chromatin state to promote cancer
metastasis. Nature. 464:1071–1076. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Meier P, Finch A and Evan G: Apoptosis in
development. Nature. 407:796–801. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Vyssokikh MY, Zorova L, Zorov D, Heimlich
G, Jürgensmeier JM and Brdiczka D: Bax releases cytochrome c
preferentially from a complex between porin and adenine nucleotide
translocator. Hexokinase activity suppresses this effect. Mol Biol
Rep. 29:93–96. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Slee EA, Harte MT, Kluck RM, Wolf BB,
Casiano CA, Newmeyer DD, Wang HG, Reed JC, Nicholson DW, Alnemri
ES, et al: Ordering the cytochrome c-initiated caspase cascade:
hierarchical activation of caspases-2, -3, -6, -7, -8, and -10 in a
caspase-9-dependent manner. J Cell Biol. 144:281–292. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cao S, Liu W, Li F, Zhao W and Qin C:
Decreased expression of lncRNA GAS5 predicts a poor prognosis in
cervical cancer. Int J Clin Exp Pathol. 7:6776–6783.
2014.PubMed/NCBI
|
|
38
|
Green DR and Kroemer G: The
pathophysiology of mitochondrial cell death. Science. 305:626–629.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shimizu S, Narita M and Tsujimoto Y: Bcl-2
family proteins regulate the release of apoptogenic cytochrome c by
the mitochondrial channel VDAC. Nature. 399:483–487. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kluck RM, Esposti MD, Perkins G, Renken C,
Kuwana T, Bossy-Wetzel E, Goldberg M, Allen T, Barber MJ, Green DR,
et al: Pro-apoptotic proteins, Bid and Bax, cause a limited
permeabilization of the mitochondrial outer membrane that is
enhanced by cytosol. J Cell Biol. 147:809–822. 1999. View Article : Google Scholar : PubMed/NCBI
|