Introduction
Prostate cancer (PCa) is the second leading cause of
male tumor-related deaths in developed countries (1). The main treatment used in patients
with metastatic prostate cancer (mPCa) is androgen deprivation
therapy which has demonstrated an improved overall survival after
prostate-specific antigen detection (2). However, these patients often enter in
an androgen-independent stage associated with high mortality
(3). There are several treatment
options for these patients, but none includes surgical management
of the primary tumor outside of a clinical trial setting (3,4).
Surgeons are becoming more prone to surgical treatment in high risk
and locally advanced disease as a part of a multimodality approach
to the treatment of PCa, including the possibility to treat the
primary tumor in M1 patients (4).
There has arisen a question of whether removing the primary tumor
in patients with metastatic disease is relevant, and during the
last decade it has been addressed in several reports that analyze
information available in retrospective studies (5,6).
Currently, cytoreductive surgery (CS) is used rarely in mPCa
patients but only to relieve the patient from local side-effects
derived from tumor growth (7) and
not in the context of PCa treatment (3). The effect of a reduction in tumor mass
in a patient presenting with metastasis may be used as an
additional therapeutic measure to prolong patient survival, but
there are no current pre-clinical models that confirm or refuse
this hypothesis (4). From the
biological point of view, the primary tumor has an important role
in initiating and maintaining the metastatic process. The tumor has
the propensity for constantly delivering cells into circulation
(8,9) while it also can prepare the distant
pre-metastatic niche for successful implantation of disseminated
cells (10,11). These processes can occur early in
the disease, an observation that has clinical (12) and biological support (13). Treatment of the primary tumor may
not only have a positive effect on the localized consequences of
tumor growth but also in the distant tumors that grow as metastasis
(14). Considering this clinical
and biological background, we proposed that cytoreduction of the
primary tumor reduces or slows the progression of metastatic
disease in a mouse model of mPCa. To address this question we
previously generated a murine model of CaP that is surgically
resectable without significant side-effects (15). This model consistently generates
metastasis in a time-dependent fashion and enables us to perform CS
and study the behavior of tumors after treatment.
Materials and methods
Cells
PC3 cells were used for orthotopic injection. This
cell line is derived from a bone metastasis of a human PCa patient
and has a high tumorigenic and metastatic behavior when injected
intravenously and orthotopically. These cells are
androgen-independent and do not express PSA. In order to visualize
the cells once injected in the mouse prostate, they were transduced
with a gene that contains the sequence for firefly luciferase
(GenTarget Inc.®) (PC3-LUC cells) using viral particles
pGreenFire1-LUC-CMV-EF1-Puro
(cat. TR011VA-P; System Biosciences, Mountain View, CA, USA)
diluted in Dulbecco's modified Eagle's medium (DMEM)/F12 10% FBS
with Polybrene (cat. H9268; Sigma-Aldrich) at a concentration of 5
µg/ml. Cells were left overnight for infection, and
puromycin was added (1 µg/ml) to select cells that were
stably transduced. Integration was verified using qPCR and
fluorescence.
Animals and orthotopic injection
NOD-SCIDγ mice (NOD. Cg-Prkdcscid
Il2rgtm1Wjl/SzJ; Jackson Laboratory®,
Sacramento, CA, USA) were obtained from our High Safety Animal
Facility (Faculty of Medicine, University of Chile) and maintained
in a laminar flow room under specific pathogen-free conditions. All
food, water and litter were sterilized prior to use. Temperature
(20–21°C) and humidity (50–60%) were controlled. Daily light cycles
were 12-h light and 12-h dark. Cages were changed fully once or
twice a week. Animals were manipulated under sterile conditions.
Orthotopic injection was performed using the anterior lobe of the
mouse prostate as previously described (primer paper). All
experiments with animals were approved by the Bioethics Committee
of the Faculty of Medicine, University of Chile (protocol CBA#0487
FMUCH).
Bioluminescence
Primary and metastatic tumor growth was followed
using the IVIS Lumina II® (Caliper Life Sciences,
Hopkinton, MD, USA) system. Animals were anesthetized with a
combination of ketamine and xylazine and injected intraperitoneally
with 150 mg/kg of D-luciferin (potassium salt). Sixteen minutes
after injection of D-luciferin, images were captured with the IVIS
system. Images were taken in 5–7 day intervals until the end of the
experiment.
Study design
Two groups of mice were used with 5 mice in each
group. The first group (control) consisted of animals injected
orthotopically in the prostate and then followed by luminescence
imaging at regular intervals until completion of the experiments.
These animals received no further manipulation. The second group
(treatment) was injected orthotopically at time 0 and then
subjected to CS of the primary tumor at day 30 in which metastatic
growth had already occurred. These animals were followed by
luminescence in the same manner as the first group until the end of
the experiment.
CS
At day 30 post-injection of PC3-LUC cells, in the
treatment group, animals were anesthetized and a midline incision
was made on the skin and muscle, 1 cm caudal to the umbilicus. The
prostate tumor and its associated seminal vesicle was identified,
externalized and isolated. Using 6/0 absorbable suture, a ligature
was made at the base of the anterior lobe, above the deferent duct.
The prostate lobe was then cut over the ligature and removed with
attention not to rupture the seminal vesicle. The same procedure
was repeated for the left anterior prostatic lobe, along with its
associated seminal vesicle. After checking for hemorrhages, the
abdominal and skin incisions were closed in two planes. The tissue
obtained from the CS was submerged in 10% neutral buffered
formaldehyde. A luminescence image was taken after the procedure to
ensure that no more than 5% of the tumor luminescence remained
detectable. This percentage was considered a successful
treatment.
Histology and immunohistochemistry
Tissues were fixed by immersion in neutral buffered
formalin for 24 h and then they were trimmed and placed in
histologic cassettes for dehydration, inclusion in paraffin and
staining with hematoxylin and eosin (H&E). Using the same
tissues, an indirect immunoperoxidase method was performed as
follows. Antigen retrieval was achieved by exposing the samples to
90°c for 30 min in citrate buffer (10 mM, pH 6.0). Then the samples
were incubated with the primary antibody [anti-human monoclonal
anti-mitochondria (ab3298; Abcam, Cambridge, MA, USA), mouse
monoclonal anti-CD-24 (CBL561; Chemicon, Temecula, CA, USA), rabbit
monoclonal anti-CD-44 (ab51037; Abcam), mouse monoclonal
anti-CD-133 (17A6.1; Millipore, Billerica, MA, USA) and mouse
monoclonal anti-KI-67 (clone MIB-1; DakoCytomation, Glostrup,
Denmark)] at 4°C overnight. The samples were treated with a
streptavidin-biotin detection method (Histostain®-Plus
Bulk kit, Zymed®, LAB-SA detection system and DAB-Plus
Substrate kit; all from Invitrogen, Camarillo, CA, USA) followed by
hematoxylin counterstaining. Images were obtained with a
Leica® microscope, model DM3000. For semi-quantititive
analysis of immunolabeling, a hybrid score (H-score) was calculated
for each marker. This score resulted from determining the
percentage of positive cells (from 0 to 100%) and the intensity of
staining (from 0–3 with 0, 1, 2, 3 grades corresponding to no
staining, weak, medium and high intensity, respectively). These two
numbers were then multiplied to obtain a score that ranged from 0
to 300. A proliferative index (PI) was calculated counting the
number of KI-67 positive cells in three images obtained at ×400 for
each of three samples per group. The number of total and positive
cells was obtained using the ImageJ software (ver. 1.48).
Statistics
Data were compiled and analyzed using Prism 6.0
software. Results were considered significantly different if
p<0.05, according to the specific statistical analysis used, as
described in each figure legend.
Results
Primary tumor growth
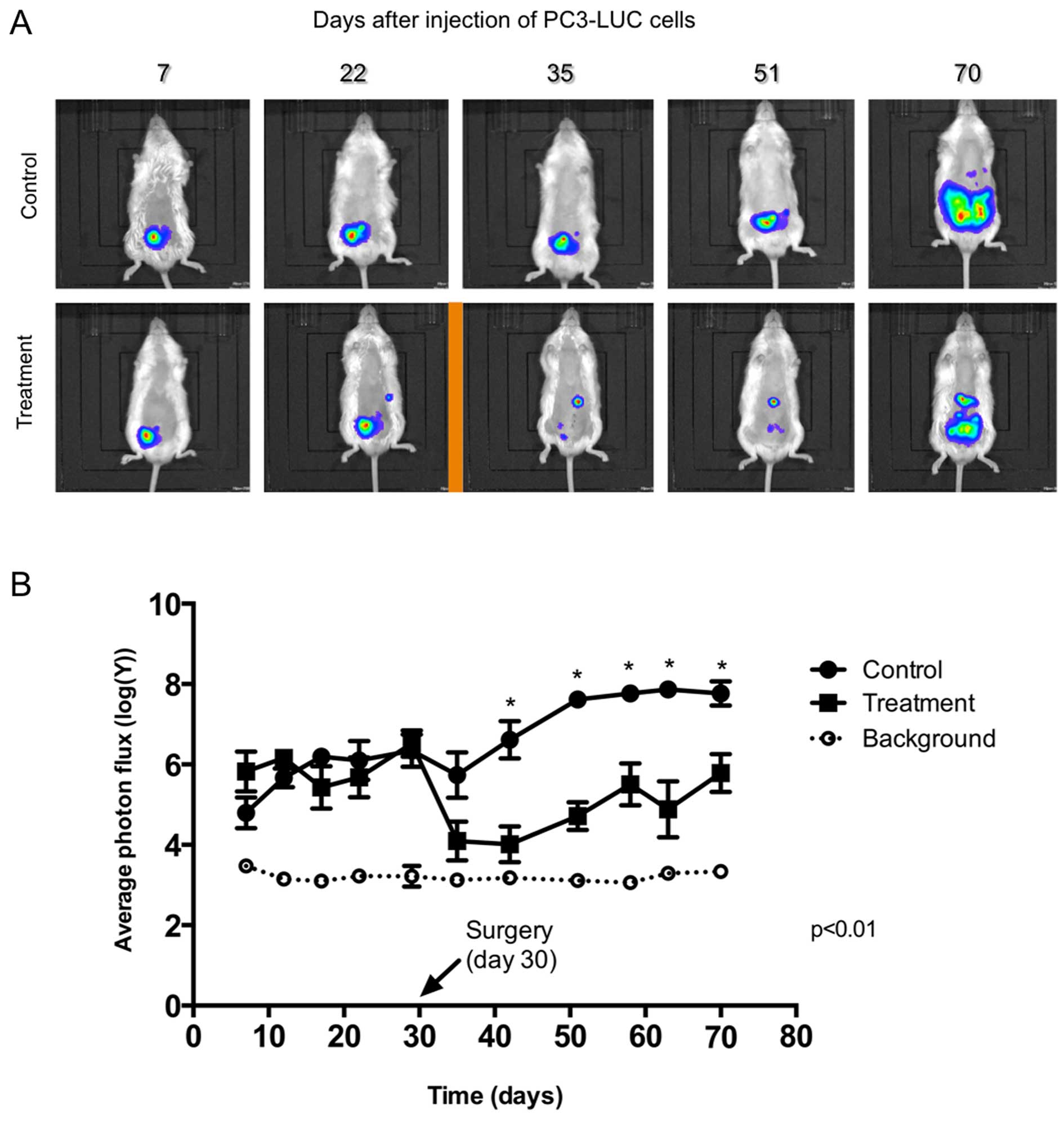
After injection of PC3-Luc cells to both the control
and treatment groups, we followed tumor growth by bioluminescence
(Fig. 1a, Table I). The IVIS system has a level of
background signal that is shown with a green line. This is the
minimum signal that can be measured when an animal injected with
cells but not with luciferin is photographed. CS was performed in
the treatment group at day 30 (arrow). In all cases, the
luminescence signal of the remnant tumors was <5% of the
original signal. On days 42, 51, 58, 63 and 70, luminescence of the
primary tumors in the treatment group was lower (p<0.01) than
that of the control group demonstrating the effect of surgery on
the tumor mass. The tumor mass was significantly reduced by the
procedure but it was not eliminated, simulating the case of an
advanced tumor which is not amenable to complete resection. At the
end of the experiment (day 70) a rise in the level of luminescence
was observed in the treatment group. This was still significantly
lower than the level in the tumors in the control group and lower
than the level in the tumors before surgery. In addition, in the
treatment group, tumor size was less homogenous as demonstrated by
their dispersion (SD, standard deviation; Table I).
 | Table IPhoton flux of the primary tumors. |
Table I
Photon flux of the primary tumors.
| Day | p-value | Control
| Treatment
|
|---|
| Mean [log(photon
flux)] | SD (±) | Mean [log(photon
flux)] | SD (±) |
|---|
| 7 | 0.140 | 4.798 | 0.851 | 5.821 | 1.108 |
| 12 | 0.118 | 5.667 | 0.522 | 6.161 | 0.355 |
| 17 | 0.217 | 6.193 | 0.494 | 5.429 | 1.173 |
| 22 | 0.563 | 6.099 | 1.083 | 5.680 | 1.109 |
| 29 | 0.743 | 6.346 | 0.899 | 6.522 | 0.729 |
| 35 | 0.058 | 5.736 | 1.265 | 4.095 | 1.078 |
| 42 | 0.004a | 6.612 | 1.036 | 4.012 | 1.001 |
| 51 | 0.00003a | 7.619 | 0.169 | 4.715 | 0.766 |
| 58 | 0.003a | 7.766 | 0.320 | 5.503 | 1.169 |
| 63 | 0.003a | 7.866 | 0.408 | 4.884 | 1.560 |
| 70 | 0.007a | 7.768 | 0.675 | 5.785 | 1.050 |
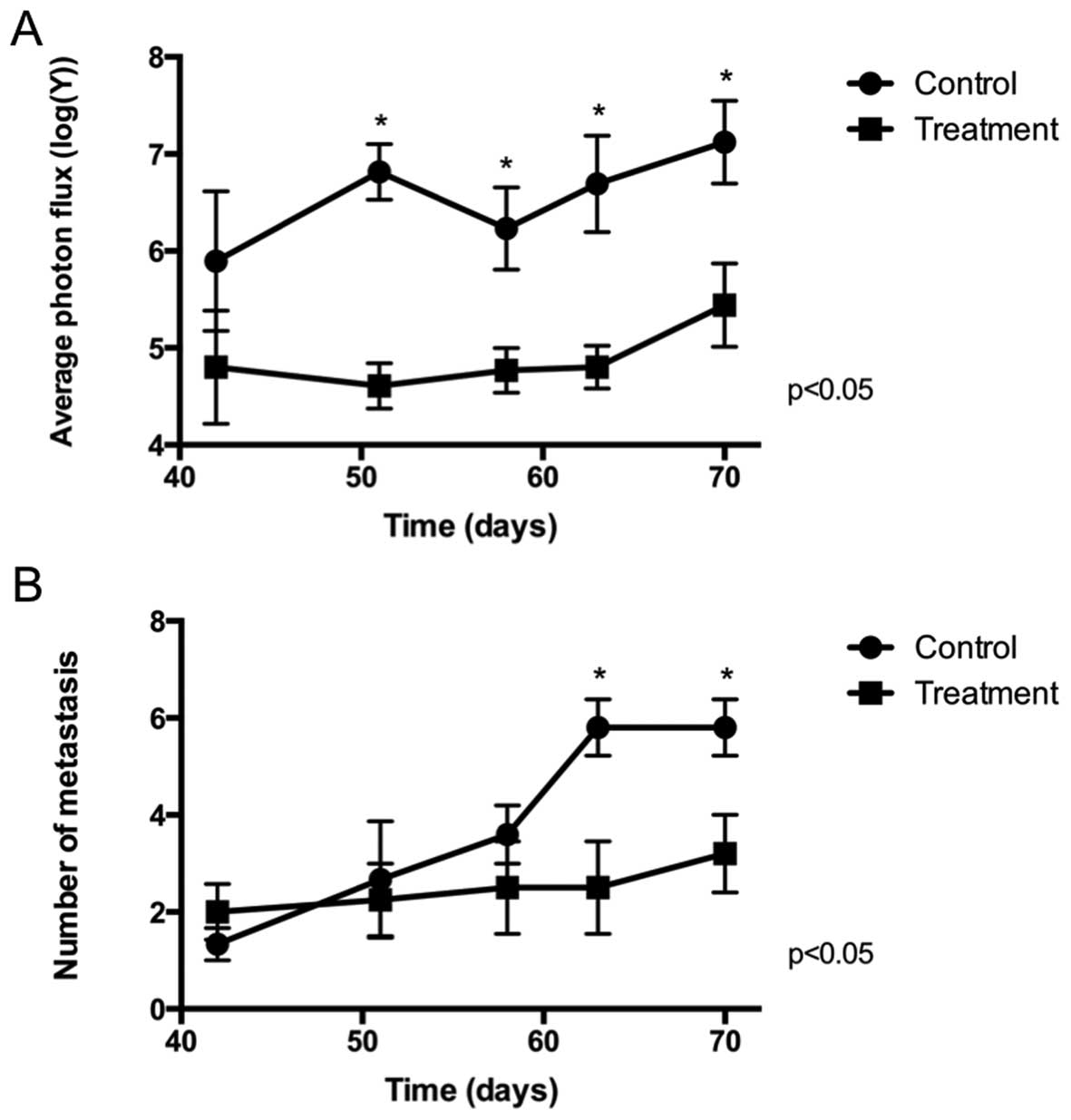
Metastasis
Fig. 2 and Tables II and III show the general results for the
metastases measured (Fig. 2A,
Table II) and counted (Fig. 2B, Table III) by bioluminescence from day 42
after injection of the PC3-LUC cells until the end of the
experiment at day 70. The size of the metastases (Fig. 2A), estimated by bioluminescence, was
lower in the treatment group from day 51 until the end of the
experiment. This size increased at the end of the experiment but
failed to reach that of the control group. The effect on size was
constant from day 42 until the end of the experiment showing that
the main bulk of the primary tumor is needed to allow for
metastasis to grow. The percentage of animals (Fig. 2B) that presented metastasis was
similar in both cases, confirmed by contingency tables that showed
no difference (p>0.05) in the frequency of animals affected.
This responds to the fact that surgery was performed in animals
that already had metastatic dissemination and therefore, surgery
did not revert the process but only delayed it. Although the number
of metastases was similar on days 42, 51 and 58, animals that
received surgery (treatment group) had a lower number of metastases
at the end of the experiment (days 63 and 70). This suggests that
surgery of the primary tumor prevents the growth of new metastases.
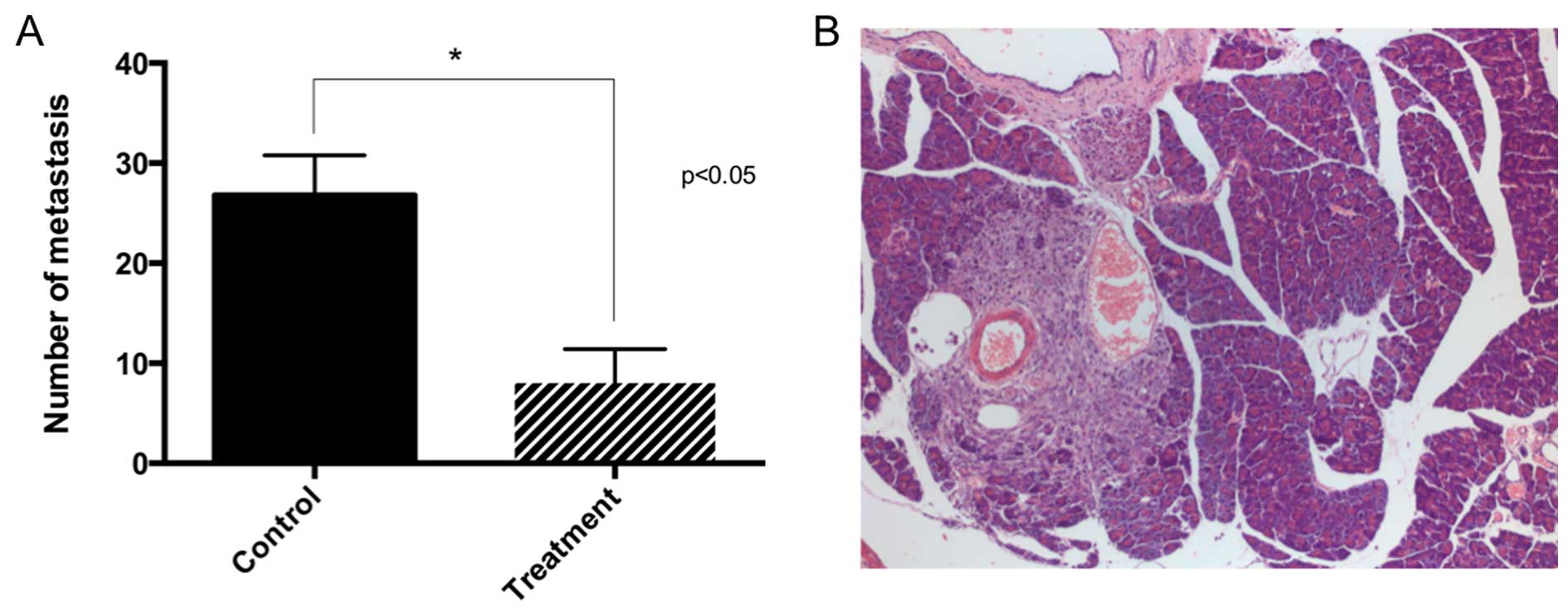
This result was also observed when counting metastases by
microscopy (Fig. 3).
 | Table IIPhoton flux of metastatic tumors. |
Table II
Photon flux of metastatic tumors.
| Day | p-value | Control
| Treatment
|
|---|
| Mean [log(photon
flux)] | SD (±) | N | Mean [log(photon
flux)] | SD (±) | N |
|---|
| 42 | 0.135 | 5.894 | 1.252 | 3 | 4.803 | 1.015 | 3 |
| 51 | 0.002a | 6.816 | 0.501 | 3 | 4.608 | 0.469 | 4 |
| 58 | 0.018a | 6.232 | 0.946 | 5 | 4.770 | 0.462 | 4 |
| 63 | 0.003a | 6.692 | 1.109 | 5 | 4.801 | 0.439 | 4 |
| 70 | 0.005a | 7.120 | 0.960 | 5 | 5.441 | 0.966 | 5 |
 | Table IIINumber of metastatic tumors. |
Table III
Number of metastatic tumors.
| Day | p-value | Control
| Treatment
|
|---|
| Mean | SD (±) | N (%) | Mean | SD (±) | N (%) |
|---|
| 42 | 0.600 | 1.33 | 0.58 | 3 (60) | 2.00 | 1.00 | 3 (60) |
| 51 | 0.726 | 2.67 | 2.08 | 3 (60) | 2.25 | 1.50 | 4 (80) |
| 58 | 0.296 | 3.60 | 1.34 | 5 (100) | 2.50 | 1.91 | 4 (80) |
| 63 | 0.003a | 5.80 | 1.30 | 5 (100) | 2.50 | 1.91 | 4 (80) |
| 70 | 0.012a | 5.80 | 1.30 | 5 (100) | 3.20 | 1.79 | 5 (100) |
Necropsy and histology
The most frequent site affected by metastasis was
the pancreas. It was followed by the hilum of the liver, spleen and
stomach, the hilum of the kidney, mesentery, liver (parenchyma) and
kidney (parenchyma). All mesenteric tumors were associated with a
mesenteric vessel. None of the animals had tumors in the abdominal
wall muscles or diaphragm, nor on the antimesenteric surface of the
intestine, all of which were considered signs of carcinomatosis.
Hence, all observed metastatic tumors were the result of
circulatory dissemination. No apparent difference was noted in the
histology of metastasis or the primary tumor when comparing both
groups. This morphology was described in a previous study (15). Smaller tumors had a solid growth
with scant amounts of stroma. Larger tumors had more stroma and
some had the formation of pseudoacinar structures. Treatment had no
effect on the microscopic structure of the tumors.
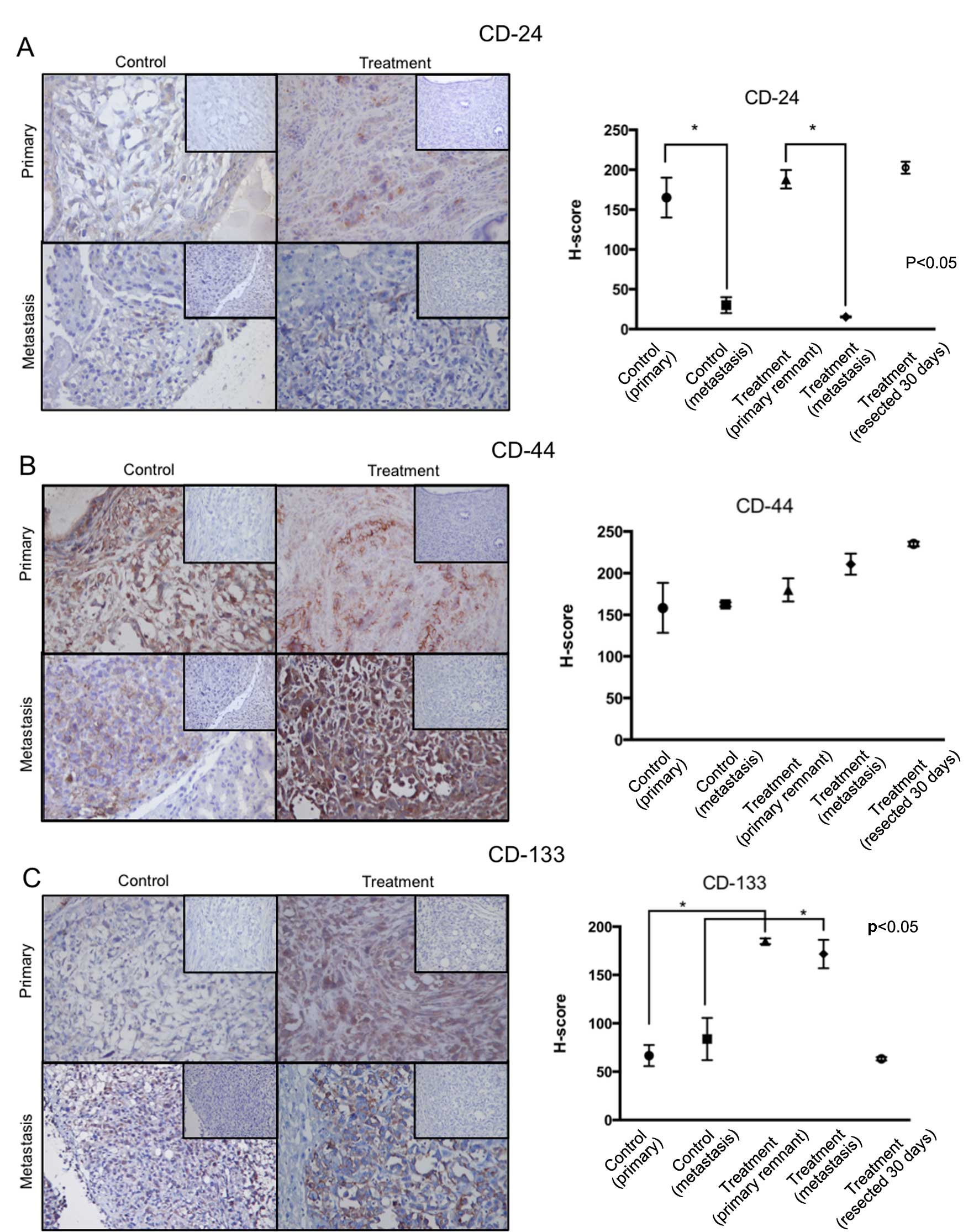
CD-24, CD-44, CD-133 immunoreactivity and
PI
Fig. 4 contains a
summary of these results. A hybrid score (H-score) was used as a
combined parameter of staining intensity and percentage of stained
cells. In this analysis, tumors resected from animals at day 30
(treatment group) were included and represent the marker status of
the primary tumors before surgery. CD-24 immunolabeling was high on
all primary tumors, including the tumors resected on day 30 in the
treatment group. In contrast, in both groups, CD-24 H-score was
lower in the metastatic tumors. There was no difference between
H-score in metastasis from the control and treatment group. CD-44
immunolabeling was high in all tumors and no differences were
observed between them. CD-133 immunolabeling was higher in all
tumors of the treatment group compared to the tumors in the control
group. Interestingly, CD-133 H-score in the resected tumors
(treatment group) was similar to the one observed in tumors in the
control group.
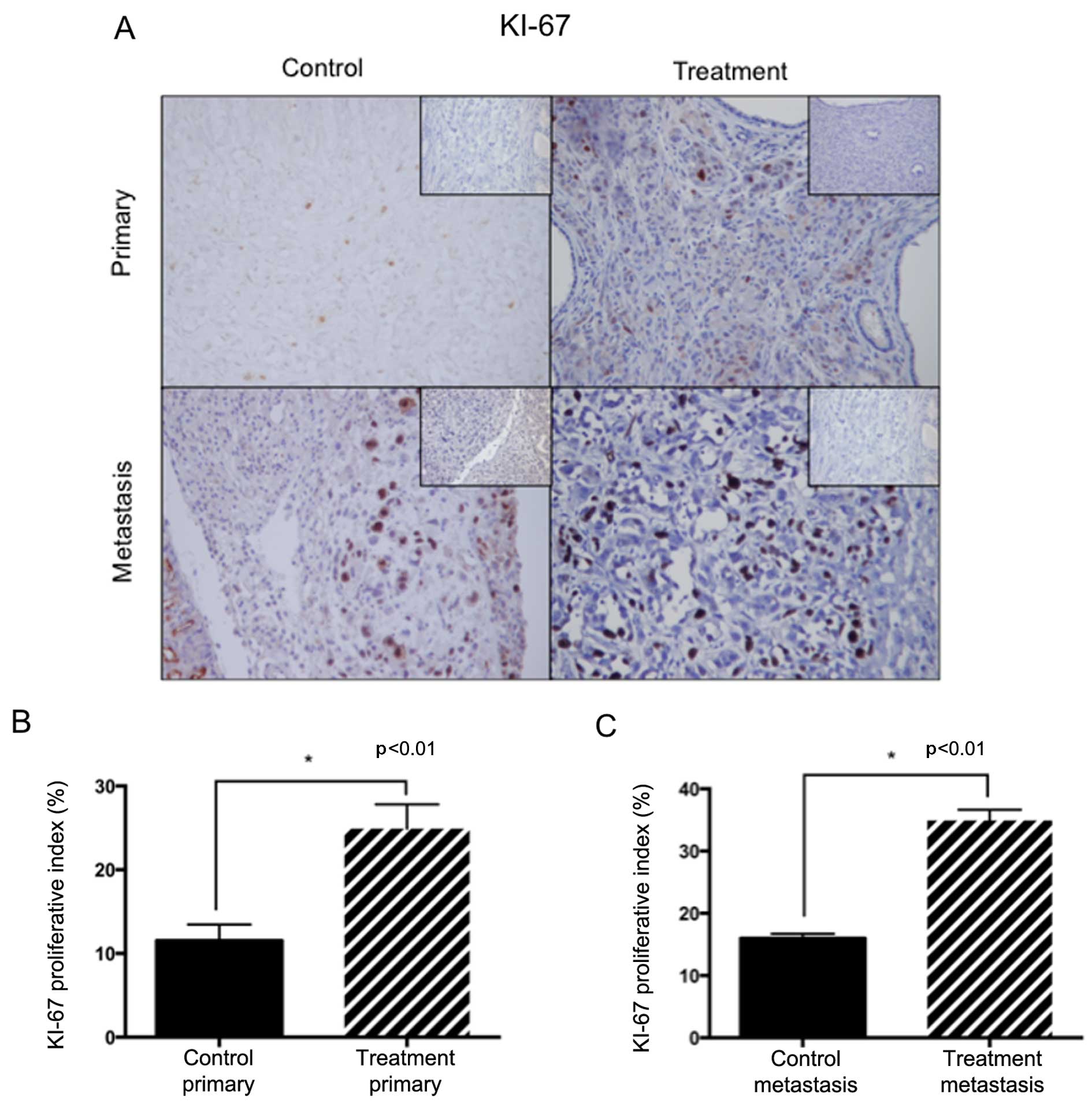
The PI (Fig. 5) was
calculated as a percentage of KI-67 positive cells in ×400 fields.
We compared the PI between primary and metastatic tumors in both
groups. When comparing PI in the primary tumors, a higher
percentage of KI-67-positive cells was found in the treatment
group. These tumors arose from the remaining cells after CS
suggesting that this is an effect of surgical treatment. Similarly,
PI was higher in metastatic tumors of the treatment group versus
metastases of the control group. These results suggest that
proliferation was activated in the metastatic tumors from mice that
received surgery. The effect of surgery on proliferation was,
therefore, observed in all tumors confirming a systemic effect of
treatment. When we compared PI between the primary tumors of the
control group (day 70) with those resected at day 30 from mice in
the treatment group (before any effect of surgery was possible) no
difference was found, further indicating that a higher
proliferative activity was a consequence of the surgical
procedure.
Discussion
Surgical removal of the primary tumor as a treatment
for metastatic cancer is an old idea that has been revisited
throughout the history of oncology (16). Several studies in the 70's and 80's
suggested that this treatment results in an increased number of
metastases (17,18). More recently, some evidence suggests
that treatment of the primary tumor may activate angiogenesis,
which in turn, may activate dormant metastatic cells (19). In the present study the observed
effect of CS was a reduction in size and number of metastatic foci
when compared with the non-treated mice. This effect suggests that
surgery not only slows down the development of already established
metastases but also reduces the number of new metastases, probably
arising from dormant sites. Escaping dormancy depends on several
factors, including angiogenesis, appropriate interaction with the
local microenvironment, response to immune surveillance, and cancer
stem cell presence (20,21). There is ample evidence showing a
promotive role of the primary tumor in preparing the
microenvironment or pre-metastatic niche and facilitating survival
and proliferation at distant sites (10,11).
This effect can be related to the mobilization of bone
marrow-derived hematopoietic progenitor cells (11), the upregulation of extracellular
matrix ligands and the expression of chemoattractants (22). All these factors may regulate the
angiogenic-dependent dormancy status (23). Our results are in accordance with
these observations. Another mechanism proposed for the primary
tumor to distantly regulate the pre-metastatic niche is the
delivery of microvesicles that can modify distant cells by
transferring several classes of molecules, such as microRNAs
(24). microRNAs have been proposed
as regulators of several steps involved in the metastatic cascade,
particularly regulating the occurrence of oligometastatic disease
(25). In this context, the removal
of the bulk mass of the primary tumor may alter delivery of these
molecules, resulting in a less aggressive presentation. This
hypothesis must be tested in an appropriate setting. Another
important mechanism regarding tumor development is the role of
cancer stem cells in initiating primary and metastatic tumors. To
address this issue we evaluated expression of CD-24, CD-44 and
CD-133 markers by immunolabeling. We found that the primary and
metastatic tumors in the treated group had low CD-24 and high CD-44
and CD-133 expression compared to levels in the tumors from the
non-treated animals. This combination is suggestive of a higher
presence of less differentiated cells with high expression of stem
cell markers. This denotes a possible switch in differentiation
markers. Potential roles for CSCs are initiation of tumors,
resistance to therapy and origination of metastasis (26,27).
The presence of CSC markers suggests that after surgical trauma
more of these cells are able to induce the growth of the remaining
tumor. This may be happening in metastasis as well, suggesting that
after surgery, CSCs may have a role in repopulating the tumors
(either metastatic or primary) rather than generating new
metastatic foci.
The role of immunity has been proposed to explain a
potential negative effect of surgery. Some authors have suggested
that surgery-related stress results in suppression of cellular
mediated immunity, allowing metastatic development (28,29).
These authors propose that measures must be taken to avoid a
perioperative immune depressive state, which may lead to
uncontrolled metastatic development. However, they highlight that
this effect probably occurs mainly in early stages of tumor
development, before the immune system is co-opted by the tumor to
develop tolerance (28). In this
study we used NOD-SCID-γ mice, which have severely dysfunctional
innate and adaptive immunity (30)
and hence have no immune barrier for tumor development. This is
confirmed by the rapid development of primary and metastatic tumors
in non-treated animals. However, in this experiment the effect of
surgery on metastatic development was readily observable,
suggesting that maximum metastatic growth in these mice depended
not only on the lack of immune response but also on the presence of
a fully developed primary tumor. Therefore, we conclude that the
effect of surgery is, at least in part, independent of the immune
status.
We chose to perform CS in contrast to complete
removal of the tumor as this is more realistic in the context of a
PCa T3 tumor and it is similar to other cancers, notably ovarian
cancer (31). Our results show an
important reduction in the luminescence signal in primary tumors as
a consequence of treatment. After this reduction, a slow relapse
occurred. The relapsed tumors in the treatment group did not reach
the size of the primary tumors of the non-treated animals during
the time of the experiment. Furthermore, they did not reach the
size of the tumors before surgery. Although we saw a higher PI in
the relapsed tumors, this was not sufficient to overcome the
overall effect of surgery. A similar tendency was observed in the
metastatic tumors, where a higher PI was observed in metastases
from the treated animals. Apparently this is a late effect from
surgery and may translate to future tumor growth. The significant
reduction in primary and metastatic tumor size and numbers and the
delay of their development, suggest that this treatment may
translate to a survival benefit for mPCa patients, supporting the
observations made in clinical trials (32).
CS is an accepted treatment for metastatic kidney
(33), breast (34) and ovarian (31) cancer. This treatment depends on
anatomical presentation and feasibility of surgery. This is clear
in ovarian carcinoma in which patients with suboptimal tumor
debulking derive no benefit from the surgical procedure and are
only exposed to its complications (31). Furthermore, in PCa patients with
locally advanced disease (T3 and/or N1), treatment of the primary
tumor only achieves significant results when they are at high risk
to die but not in those with a slow progression of the disease
(35). If CS is to be used in mPCa,
pre-clinical and clinical evidence is necessary. There are several
reports of retrospective studies that suggest this treatment may
result in a survival benefit (6,32).
Recently, a large retrospective clinical study analyzed data from
PCa patients in stage IV (M1) that had definitive treatment of the
prostate tumor (5). The conclusion
of this study suggests that there is a survival benefit for this
treatment option. However, an editorial comment about this article
points out that this treatment modality is not supported by any
published guidelines and that more evidence is necessary to
recommend the treatment of the primary tumor in mPCa patients
outside of a clinical trial (4);
moreover, concludes that well-thought-out clinical trials need
proper patient selection (4). In
agreement with this, here we showed in a pre-clinical model, that
CS of the primary tumor alone can reduce metastatic burden and may
translate to a survival benefit for cancer patients. Current
modalities for treatment of mPCa patients include mainly androgen
blockage and, when resistance develops, variable application of
chemotherapy and radiotherapy (3).
Surgery of the primary tumor may be a useful addition to these
sequential approaches with the main goal of prolonging survival.
Here we show evidence that may promote the design of prospective
studies that address this subject.
Acknowledgments
The present study was supported by grants FONDECYT
1140417 (EAC) and 1151214 (HRC). F.F.C. and R.V. are granted with
PhD CONICYT fellowships nos. 211006631 and 21100651, respectively.
We thank Ms. Graciela Caroca and Mr. Miguel Sepulveda for their
excellent technical assistance and Mr. Dagoberto Donoso for his
expert animal handling.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tangen CM, Hussain MH, Higano CS,
Eisenberger MA, Small EJ, Wilding G, Donnelly BJ, Schelhammer PF,
Crawford ED, Vogelzang NJ, et al: Improved overall survival trends
of men with newly diagnosed M1 prostate cancer: A SWOG phase III
trial experience (S8494, S8894 and S9346). J Urol. 188:1164–1169.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Valenca LB, Sweeney CJ and Pomerantz MM:
Sequencing current therapies in the treatment of metastatic
prostate cancer. Cancer Treat Rev. 41:332–340. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chapin BF, Mcguire SE and Aparicio A: Is
treatment of the primary tumor in metastatic prostate cancer
justified? Eur Urol. 65:1067–1068. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Culp SH, Schellhammer PF and Williams MB:
Might men diagnosed with metastatic prostate cancer benefit from
definitive treatment of the primary tumor? A SEER-based study. Eur
Urol. 65:1058–1066. 2014. View Article : Google Scholar
|
|
6
|
Swanson G, Thompson I, Basler J and
Crawford ED: Metastatic prostate cancer - Does treatment of the
primary tumor matter? J Urol. 176:1292–1298. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Qin XJ, Ma CG, Ye DW, Yao XD, Zhang SL,
Dai B, Zhang HL, Shen YJ, Zhu Y, Zhu YP, et al: Tumor cytoreduction
results in better response to androgen ablation - a preliminary
report of palliative transurethral resection of the prostate in
metastatic hormone sensitive prostate cancer. Urol Oncol.
30:145–149. 2012. View Article : Google Scholar
|
|
8
|
Nguyen DX: Tracing the origins of
metastasis. J Pathol. 223:195–204. 2011. View Article : Google Scholar
|
|
9
|
Miyamoto DT, Sequist LV and Lee RJ:
Circulating tumour cells - monitoring treatment response in
prostate cancer. Nat Rev Clin Oncol. 11:401–412. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sceneay J, Smyth MJ and Möller A: The
pre-metastatic niche: Finding common ground. Cancer Metastasis Rev.
32:449–464. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kaplan RN, Riba RD, Zacharoulis S, Bramley
AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, et
al: VEGFR1-positive haematopoietic bone marrow progenitors initiate
the pre-metastatic niche. Nature. 438:820–827. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yu C, Shiozawa Y, Taichman RS, McCauley
LK, Pienta K and Keller E: Prostate cancer and parasitism of the
bone hematopoietic stem cell niche. Crit Rev Eukaryot Gene Expr.
22:131–148. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hüsemann Y, Geigl JB, Schubert F, Musiani
P, Meyer M, Burghart E, Forni G, Eils R, Fehm T, Riethmüller G, et
al: Systemic spread is an early step in breast cancer. Cancer Cell.
13:58–68. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Morgan SC and Parker CC: Local treatment
of metastatic cancer - killing the seed or disturbing the soil? Nat
Rev Clin Oncol. 8:504–506. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cifuentes FF, Valenzuela RH, Contreras HR
and Castellón EA: Development of an orthotopic model of human
metastatic prostate cancer in the NOD-SCIDγ mouse (Mus musculus)
anterior prostate. Oncol Lett. 10:2142–2148. 2015.
|
|
16
|
Demicheli R, Retsky MW, Hrushesky WJ, Baum
M and Gukas ID: The effects of surgery on tumor growth: A century
of investigations. Ann Oncol. 19:1821–1828. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Clamage DM, Sanford CS, Vander AJ and Mouw
DR: Effects of psychosocial stimuli on plasma renin activity in
rats. Am J Physiol. 231:1290–1294. 1976.PubMed/NCBI
|
|
18
|
Fisher B, Gunduz N, Coyle J, Rudock C and
Saffer E: Presence of a growth-stimulating factor in serum
following primary tumor removal in mice. Cancer Res. 49:1996–2001.
1989.PubMed/NCBI
|
|
19
|
Camphausen K, Moses MA, Beecken WD, Khan
MK, Folkman J and O'Reilly MS: Radiation therapy to a primary tumor
accelerates metastatic growth in mice. Cancer Res. 61:2207–2211.
2001.PubMed/NCBI
|
|
20
|
Aguirre-Ghiso JA: Models, mechanisms and
clinical evidence for cancer dormancy. Nat Rev Cancer. 7:834–846.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Giancotti FG: Mechanisms governing
metastatic dormancy and reactivation. Cell. 155:750–764. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Peinado H, Lavotshkin S and Lyden D: The
secreted factors responsible for pre-metastatic niche formation:
Old sayings and new thoughts. Semin Cancer Biol. 21:139–146. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ghajar CM, Peinado H, Mori H, Matei IR,
Evason KJ, Brazier H, Almeida D, Koller A, Hajjar KA, Stainier DY,
et al: The perivascular niche regulates breast tumour dormancy. Nat
Cell Biol. 15:807–817. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Antonyak MA and Cerione RA: Microvesicles
as mediators of intercellular communication in cancer. Methods Mol
Biol. 1165:147–173. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Uppal A, Ferguson MK, Posner MC, Hellman
S, Khodarev NN and Weichselbaum RR: Towards a molecular basis of
oligometastatic disease: Potential role of micro-RNAs. Clin Exp
Metastasis. 31:735–748. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tu SM and Lin SH: Prostate cancer stem
cells. Clin Genitourin Cancer. 10:69–76. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shackleton M, Quintana E, Fearon ER and
Morrison SJ: Heterogeneity in cancer: Cancer stem cells versus
clonal evolution. Cell. 138:822–829. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ben-Eliyahu S: The promotion of tumor
metastasis by surgery and stress: Immunological basis and
implications for psychoneuroimmunology. Brain Behav Immun. 17(Suppl
1): S27–S36. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Neeman E and Ben-Eliyahu S: Surgery and
stress promote cancer metastasis: New outlooks on perioperative
mediating mechanisms and immune involvement. Brain Behav Immun.
30(Suppl): S32–S40. 2013. View Article : Google Scholar
|
|
30
|
Shultz LD, Goodwin N, Ishikawa F, Hosur V,
Lyons BL and Greiner DL: Human cancer growth and therapy in
immunodeficient mouse models. Cold Spring Harb Protoc.
2014:694–708. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Narasimhulu DM, Khoury-Collado F and Chi
DS: Radical surgery in ovarian cancer. Curr Oncol Rep. 17:162015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Faiena I, Singer EA, Pumill C and Kim IY:
Cytoreductive prostatectomy: Evidence in support of a new surgical
paradigm (Review). Int J Oncol. 45:2193–2198. 2014.PubMed/NCBI
|
|
33
|
Krabbe LM, Haddad AQ, Westerman ME and
Margulis V: Surgical management of metastatic renal cell carcinoma
in the era of targeted therapies. World J Urol. 32:615–622. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Neuman HB, Morrogh M, Gonen M, Van Zee KJ,
Morrow M and King TA: Stage IV breast cancer in the era of targeted
therapy: Does surgery of the primary tumor matter? Cancer.
116:1226–1233. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Verhagen PC, Schröder FH, Collette L and
Bangma CH: Does local treatment of the prostate in advanced and/or
lymph node metastatic disease improve efficacy of
androgen-deprivation therapy? A systematic review. Eur Urol.
58:261–269. 2010. View Article : Google Scholar : PubMed/NCBI
|