Introduction
Pancreatic cancer ranks the fourth most common cause
of cancer-related deaths in the USA (1). In 2014, there were 46,420 estimated
new pancreatic cancer cases and 39,590 estimated deaths in the USA
(1). Pancreatic cancer accounts for
3.5% of estimated new cancer deaths worldwide, and its 5-year
overall survival rate is below 6% (2,3).
Multiple genetic and epigenetic alterations have been identified in
pancreatic cancer (4–7), but the exact molecular mechanisms are
poorly defined for this aggressive malignancy. Endometrial
carcinoma (EC) is the most common gynecologic malignancy in the
USA. It is estimated that in 2014, there were 52,630 new
endometrial cancer cases and 8,590 EC-caused deaths in the USA,
which accounts for 6% of incidence and 3% of deaths for women's
cancers (1). Although the
etiologies including the effects of genetic, epigenetic and
hormonal factors for the development of EC have been well
documented, many cellular events and pathways contributing to EC
progression remain to be elucidated.
The HE4 gene was first cloned in 1991 from the human
epididymis by Kirchhoff et al (8). Structural analysis indicated that HE4
is a whey-acidic-protein (WAP) family member containing two
WAP-type four-disulphide core (WFDC) domains (9,10). HE4
gene is located to chromosome 20q12-13.1, a region harboring 14
homologous genes (9), including
secretory leukocyte proteinase inhibitor (SLPI; or
anti-leukoproteinase 1) and elafin, the two better characterized
WAP domain proteins. HE4 expression has been detected in some
normal tissues and a variety of malignant tissues. Low levels of
HE4 expression were observed in the epithelium of female genital
track (endocervical and endometrial glands, and fallopian tube),
breast, prostate gland, distal convoluted tubules of the kidney,
bronchial epithelium, salivary glands, anterior pituitary and
lacrimal gland (11,12). While normal pancreas was generally
negative in immunostaining using HE4 antibodies (13,14),
recent studies indicated that HE4 expression is present in many
pancreatic carcinomas (14,15). High levels of HE4 expression are
found in ovarian serous carcinomas, and moderate levels in
adenocarcinomas of the lung and pancreatic carcinomas, and
endometrial cancers (11,14–16).
Since HE4 is secreted to the circulation (13), the increased serum HE4 levels have
been extensively evaluated as a biomarker for the diagnosis of
ovarian and endometrial cancers (17–19).
Various studies have shown that the serum HE4 is detectable and
increased compared to healthy controls in pancreatic adenocarcinoma
patients (15,19,20).
WAP domain family proteins have been found to carry
out multiple functions including protease inhibitory, antimicrobial
and anti-inflammatory activities (21,22).
Recently, the protease inhibitor activity of HE4 was confirmed by
experiments showing that HE4 inhibits serine proteases, aspartyl
and cysteine proteases, and matrix metalloproteinases (23–25).
Though HE4 is upregulated in many malignancies, there is a limited
knowledge on the function of HE4 in cancer development. In
endometrial cancers, higher HE4 levels positively correlate with
more aggressive phenotype, larger tumor size and myometrial
invasion (26,27), and negatively correlate with the
overall survival, disease-free survival and progression-free
survival (12,26,28,29).
Using endometrial cancer cell lines and mouse ectopic subcutaneous
cancer models, this and other groups have shown that overexpression
of HE4 led to increased cell proliferation and faster cancer
progression. In ovarian cancer and EC, HE4 was found to promote
cancer cell growth in vitro (30–32).
HE4-induced anti-apoptosis and drug resistance in ovarian cancer
cell lines have also been reported (33,34).
It is noteworthy that previous data in endometrial cancer cells
were obtained from overexpression or knockdown experiments, whereas
the function of secreted, circulatory HE4 is unclear. Moreover, HE4
activities in pancreatic cancer cells have not been reported.
In the present study, we investigated whether the
recombinant, extracellular HE4 protein purified from human cells
could affect the growth of pancreatic cancer and EC cells. These
experiments explore the potential role(s) of extracellular HE4 for
pancreatic and endometrial cancer progression through
paracrine/endocrine actions.
Materials and methods
Cell culture and treatment
Human endometrial cancer cell line KLE was obtained
from the American Type Culture Collection (ATCC). The pancreatic
cancer cell line Suit-2 was a gift from Dr Kaustubh Datta,
University of Nebraska). Cells were grown in Dulbecco's modified
Eagle's medium (DMEM)/F12 medium (HyClone Laboratories, Inc.,
Logan, UT, USA) supplemented with 10% fetal bovine serum and 1%
penicillin/streptomycin (100X; Mediatech, Inc., Manassas, VA, USA).
Cell culture was maintained at 37°C and a 5% CO2
atmosphere. Recombinant HE4 protein was expressed and isolated from
human HEK 293 cells (cat. no. 12609-H08H; Sino Biological Inc.,
Beijing, China) and applied for cell treatment as described in each
specific experiment.
MTS cell viability assay
Suit-2 and KLE cells were seeded into 96-well plates
(1×104 cells/well in 100 µl medium). Cells were
treated with 10 nM of recombinant HE4 protein for 1 to 5 days.
Cells without HE4 treatment were used as controls at each time
point. At 1 h before each time point, 20 µl CellTiter 96
AQueous One Solution Reagent (cat. no. G3581; Promega, Madison, WI,
USA) was added, and the absorbance at 490 nM was measured on a
96-well plate reader (Modulus Microplate Multimode Reader; Turner
BioSystems, Inc., USA). The assay was performed in triplicate and
the background absorbance in wells without cells was used as blank
control.
Cell counting
Suit-2 and KLE cells were seeded into 12-well plates
(8×104 cells/well). Cells were treated with recombinant
HE4 protein (10 nM, final concentration) for 1 to 6 days and every
24 h cell numbers were counted. At each time point, cells were
detached and dispersed by digestion with 1X trypsin-EDTA. After
mixing with 0.4% trypan blue solution, cells were counted with a
haemocytometer under a microscope. The assay was performed in
triplicates and data were presented as mean ± SD.
BrdU incorporation assay
Suit-2 and KLE cells were seeded on two-chamber
slides (1×105 cells/well) and cultured under standard
conditions. Following treatment with recombinant HE4 protein (10
nM, final concentration) for 48 h, 5-bromo-2′-deoxyuridine (BrdU)
(cat. no. 000103; Invitrogen, Rockville, MD, USA) was added into
the cultures to label the S-phase cells for 2 h. Following BrdU
labeling, cells were washed with cold phosphate-buffered saline
(PBS), fixed in 70% alcohol at 4°C for 30 min and permeabilized in
0.3% Triton X-100 for 30 min. BrdU immunocytochemical staining was
performed using the BrdU staining kit (cat. no. 933943; Invitrogen)
following the manufacturer's instructions. Images were captured by
Nikon DS-Fi2 camera (Nikon Instruments, Inc., Melville, NY, USA).
The BrdU-positive cells were counted and BrdU incorporation rates
were calculated. The assay was repeated three times and final
results were presented as mean ± SD.
RNA extraction and real-time PCR
Cultured cells were washed twice with cold PBS.
Total RNA was extracted with RNeasy plus mini kit (cat. no. 74136;
Qiagen, Valencia, CA, USA). RNA concentration was measured with the
use of NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific,
Wilmington, DE, USA). One microgram of total RNA was used for cDNA
synthesis with High Capacity RNA-to-cDNA kit (cat. no. 4387406;
Applied Biosystems, Foster, CA, USA) in 20 µl reactions.
cDNA was diluted to 100 µl with nuclease-free water.
Real-time PCR was conducted in 12 µl reactions containing 2
µl of diluted cDNA, 6 µl of real-time PCR reaction
mixture (cat. no. 75600; VeriQuest SYBR-Green real-time PCR master
mix with ROX; Affymetrix, Cleveland, OH, USA), 1 µl of 10
µM forward primer, 1 µl of 10 µM backward
primer and 2 µl of nuclease-free water. Real-time PCR
primers were designed using Primer3 software (Table I). Reactions were carried out under
the following conditions: initial denaturation at 95°C for 10 min,
followed by 40 cycles of denaturation at 95°C for 15 sec, annealing
and extension at 60°C for 1 min, using the ABI 7900HT fast
Real-Time PCR system (Applied Biosystems). PCR products were
resolved in 1.8% TAE agarose/ethidium bromide gels and the single
band pattern with predicted size of PCR product indicated specific
amplification. GAPDH was used as the internal reference gene.
Results were standardized by those from GAPDH. Relative changes in
mRNA expression were calculated using the 2−ΔΔCt method
(35) and presented as mean ±
SD.
 | Table IThe real-time PCR primers. |
Table I
The real-time PCR primers.
| NCBI accession
no. | Gene | Direction | Primer
sequence | Product size
(bp) |
|---|
| NM_002592.2 | PCNA | Forward |
5′-TGTCCAAAATACTAAAATGCGCCG-3′ | 82 |
| | Reverse |
5′-ACTAGCGCCAAGGTATCCGC-3′ | |
| NM_000389.4 | p21 | Forward |
5′-GCAGACCAGCATGACAGATTTC-3′ | 127 |
| | Reverse |
5′-ATGTAGAGCGGGCCTTTGAG-3′ | |
| NM_002046.5 | GAPDH | Forward |
5′-ACCATCTTCCAGGAGCGAGA-3′ | 71 |
| | Reverse |
5′-GACTCCACGACGTACTCAGC-3′ | |
Western blot analysis
Cultured cells were washed twice with cold PBS (pH
7.4) and lysed on ice in cold lysis buffer [150 mM NaCl, 50 mM
Tris-HCl (pH 7.4), 0.1% SDS, 0.5% sodium deoxycholate, 1% Nonidet
P-40] supplemented with 1% Halt protease inhibitor coktail (100X;
Thermo Scientific, Rockford, IL, USA), 1 mM of phenylmethylsulfonyl
fluoride (PMSF), 5 mM of sodium fluoride (NaF) and 1 mM of sodium
vanadate (Na3VO4). Cell suspensions were kept
on ice for 30 min before centrifugation at 18,400 × g at 4°C for 30
min. The supernatants were collected and stored at −80°C until use.
Forty micrograms of proteins was mixed with SDS-loading buffer,
boiled for 5 min, separated in 12 or 15% SDS-polyacrylamide gels,
and transferred onto PVDF membranes. Non-specific reaction was
blocked for 2 h at room temperature in TBS containing 5% non-fat
milk and 0.1% Tween-20. Primary antibodies (cat. no. SAB4502103,
rabbit polyclonal anti-PCNA antibody, 1:1,000; Sigma-Aldrich, St.
Louis, MO, USA; cat. no. sc-6246, mouse monoclonal anti-p21
antibody, 1:200; Santa Cruz Biotechnology, Santa Cruz, CA, USA)
diluted in blocking solution were applied and incubation was
carried out at room temperature for 2 h. Secondary antibodies (cat.
no. sc-2301, goat anti-rabbit IgG-HRP; cat. no. sc-2302, goat
anti-mouse IgG-HRP; both from Santa Cruz Biotechnology) were
1:6,000 diluted and incubation were performed at room temperature
for 40 min. The blots were developed with ECL system (Thermo
Scientific) and exposed to X-ray film. Membranes were stripped and
β-actin was detected with anti-β-actin mouse monoclonal antibody
(cat. no. A5316, 1:6,000; Sigma-Aldrich). Results of β-actin
detection served as protein loading controls.
Statistical analysis
Averages and standard deviations were calculated for
each experimental group. Student's t-tests were performed using
SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA) and P≤0.05 was
considered the level for statistical significance.
Results
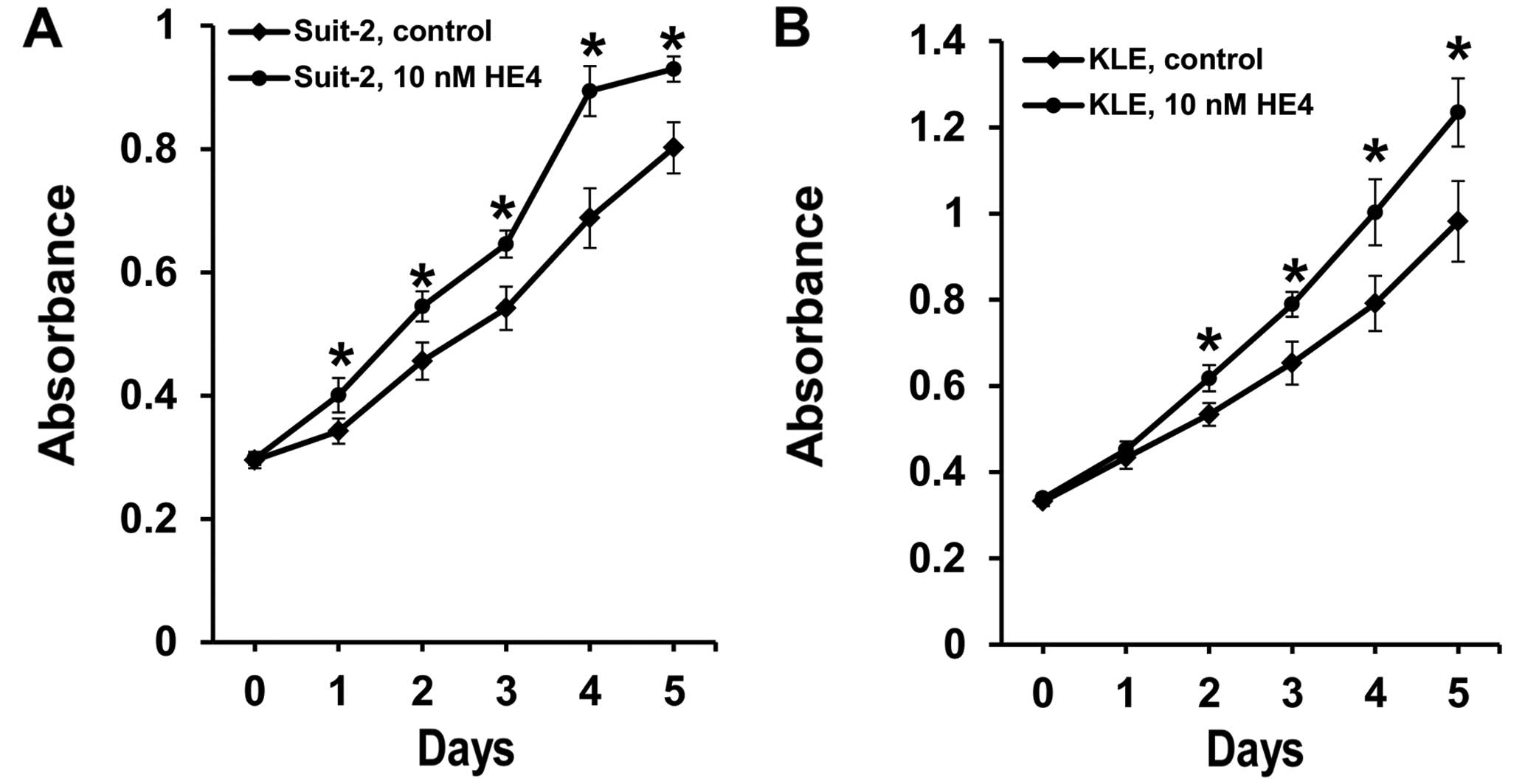
Recombinant HE4 protein promotes
pancreatic and endo-metrial cancer cell viability
Native HE4 is known to be a glycosylated and
secretory protein. In order to explore the function of
extracellular HE4, we used the glycosylated form of HE4 protein
produced in human HEK 293 cells (34) to treat pancreatic and endometrial
cancer cells. It was found that following the treatment with
relatively low concentration of recombinant HE4 protein (10 nM),
both pancreatic cancer and endometrial cancer cell lines displayed
a significantly increased cell viability when compared to the
control group without HE4 treatment (Fig. 1). This stimulation appeared to be
rapid, being detectable after one (Suit-2 cells) or two days (KLE
cells) of treatment, and sustainable throughout the time points
thereafter. Thus, extracellular HE4 has a robust effect on the
viability of both pancreatic and endometrial cancer cell lines.
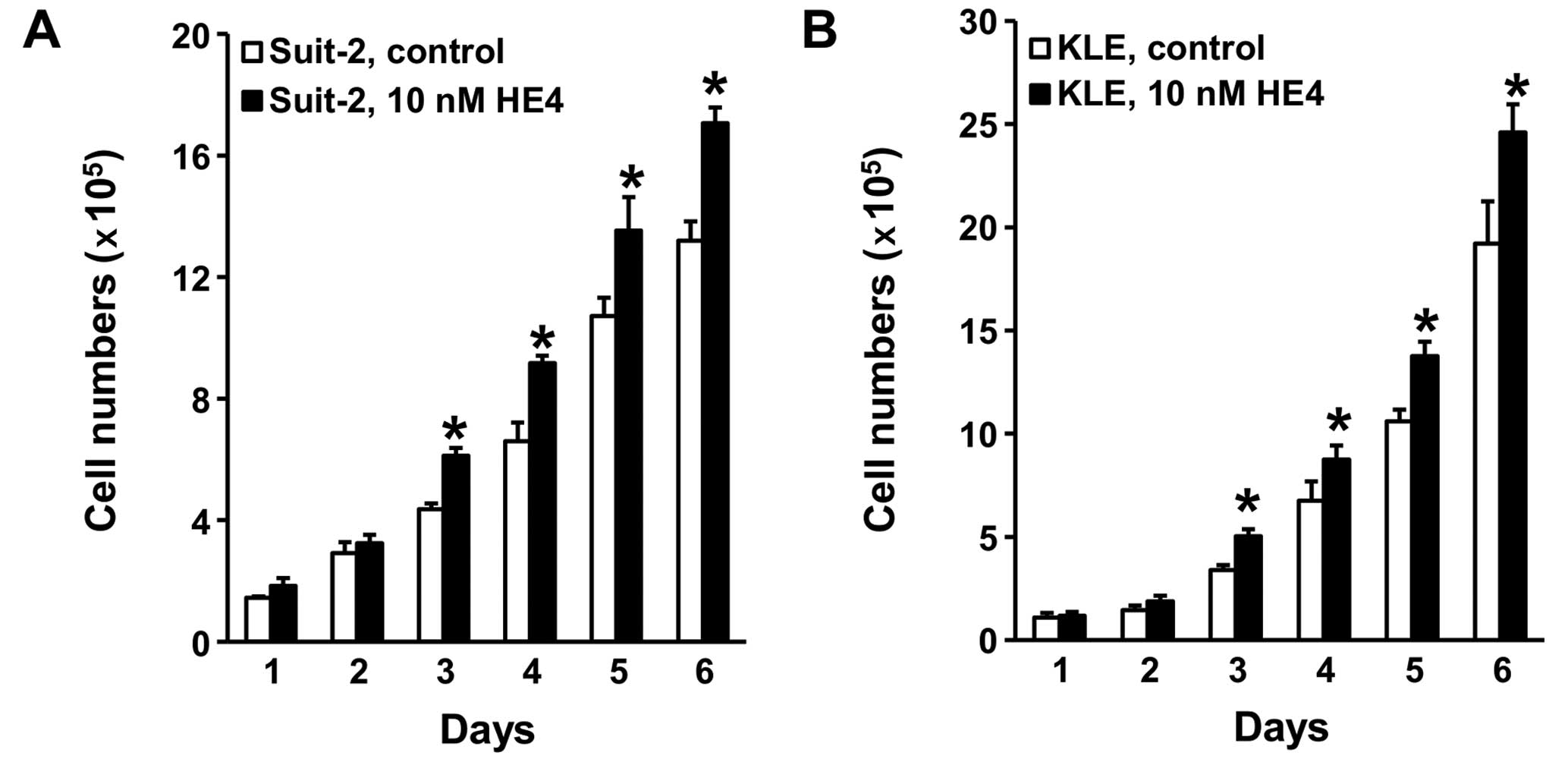
Recombinant HE4 promotes pancreatic
cancer and endometrial cancer cell growth
Since MTS experiment measures total cell vitality
rather than assessing cell growth, we performed cell counting assay
to determine the effect of extracellular HE4 on cell proliferation.
Following treatment with 10 nM of recombinant HE4 for three days,
pancreatic cancer Suit-2 cells grew significantly more rapidly than
the control group without HE4 treatment (Fig. 2A). The same activity of recombinant
HE4 protein was observed in endometrial cancer KLE cells (Fig. 2B). Consistent with the stimulatory
effect on cell viability, HE4-mediated promotion of cell growth
lasted throughout the time points observed.
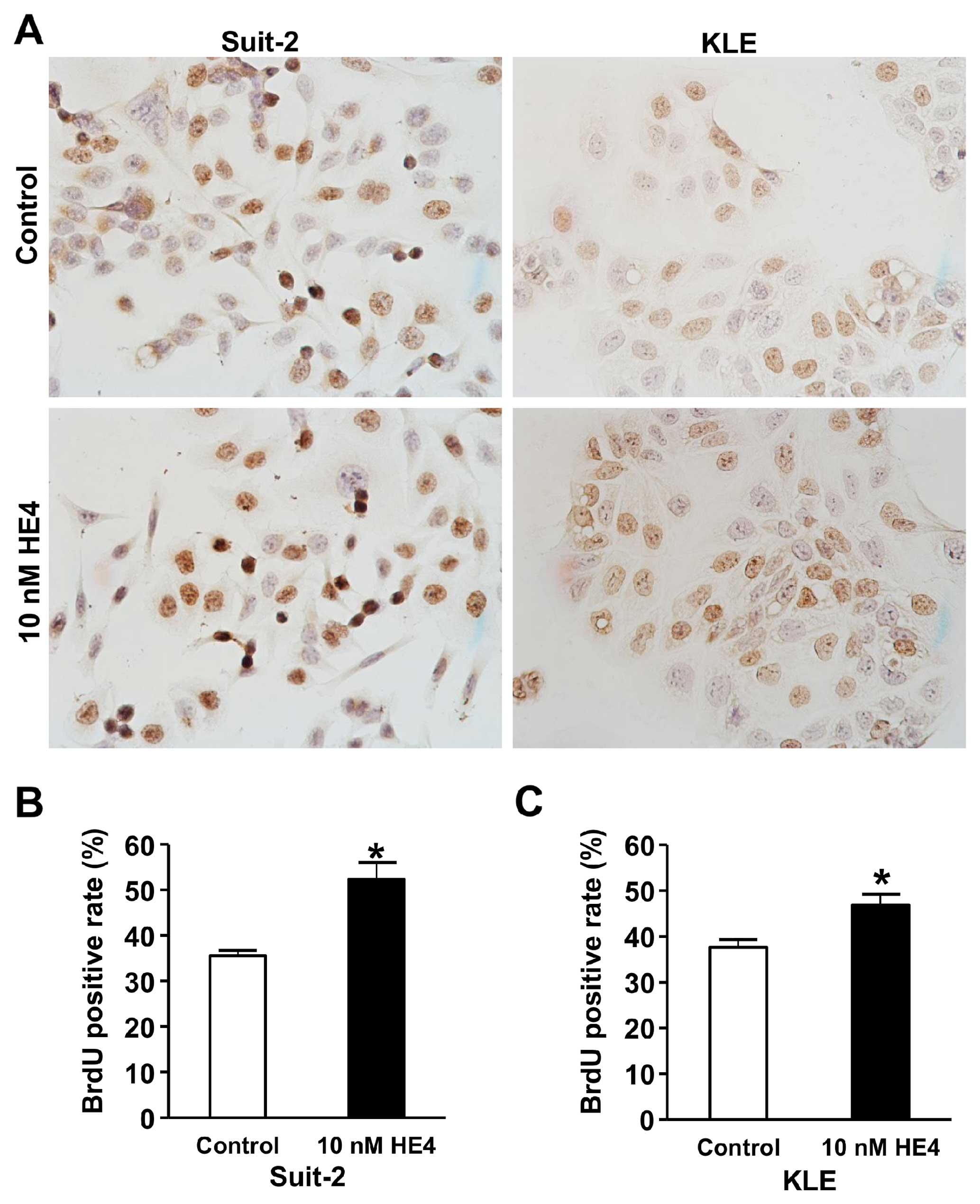
HE4 treatment increases DNA synthesis in
pancreatic and endometrial cancer cells
To investigate whether the recombinant HE4 protein
affects DNA synthesis, we compared the BrdU incorporation rates
between cells with or without HE4 treatment. As shown in Fig. 3, treatment with HE4 protein for 48 h
increased BrdU incorporation-positive Suit-2 cells from 35.6 to
52.3% (P<0.01) (Fig. 3B). For
endometrial cancer KLE cells, BrdU incorporation-positive cells
were increased from 37.6 to 46.9% (P<0.01) following the
treatment with recombinant HE4 protein (Fig. 3C). These results demonstrated that
extracellular HE4 protein can stimulate DNA synthesis in both
pancreatic and endometrial cancer cell lines.
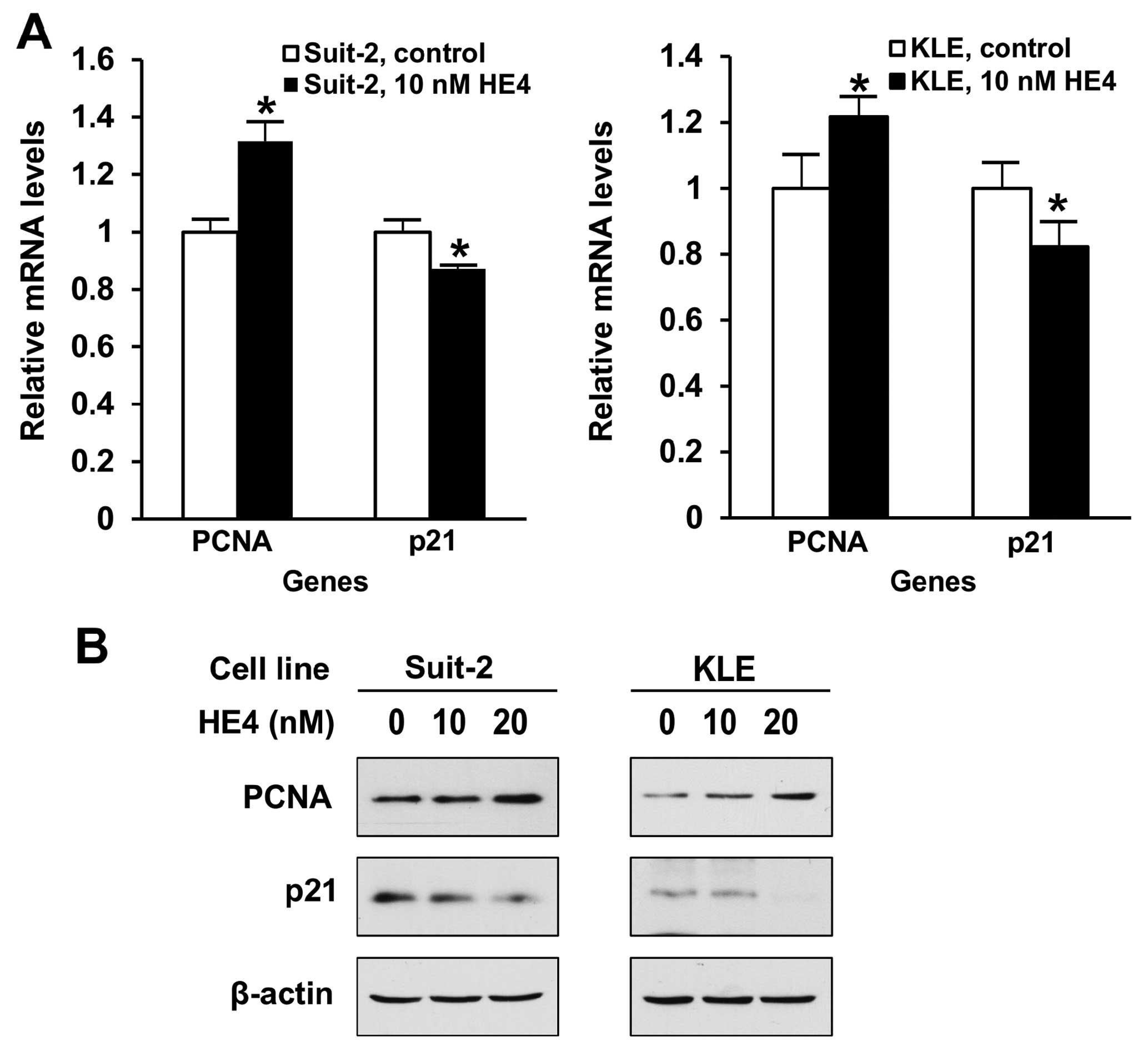
HE4 regulates PCNA and p21 gene
expression in pancreatic and endometrial cancer cells
PCNA is a key molecule for DNA synthesis and its
expression levels are considered an important marker for cell
division. p21 is a critical cell cycle regulator and its
deregulation is required for G1-S transition. We examined how the
recombinant HE4 protein treatment may affect the expression of cell
cycle marker/regulator. As shown in Fig. 4A, results of real-time PCR show that
treatment with 10 nM recombinant HE4 protein for 24 h upregulated
PCNA, but downregulated cell cycle inhibitor p21, mRNA levels, in
both Suit-2 and KLE cell lines. On the protein level, results of
western blotting indicated that following 48 h of treatment with 10
nM HE4, PCNA and p21 expression underwent changes in the same
directions as observed on mRNA levels (Fig. 4B). Treatment with 20 nM of HE4 led
to stronger effects in both cell lines. These results indicated
that recombinant HE4 protein may promote pancreatic and endometrial
cancer cell proliferation via regulating the expression of cell
cycle regulators.
Discussion
Pancreatic ductal adenocarcinoma, accounting for
more than 90% of pancreatic tumors, is one of the most deadly
malignancies (4). Most patients are
diagnosed at advanced, unresectable stage at which currently
available treatment procedures have only limited effects (36). Poor understanding on the pathologic
mechanisms impede the development of new treatment modalities.
Applying the large-scale serial analysis of gene expression (SAGE)
technique, Missiaglia et al observed that HE4 mRNA levels
were upregulated in pancreatic cancers (37). Ryu et al reported that HE4
was not expressed in normal pancreas but highly expressed in
pancreatic cancer cells lines including Capan-1, Capan-2, PL45 and
AsPc1, as well as primary pancreatic adenocarcinomas (14). Immunostaining of tissue microarray
also indicated that HE4 protein was not detectable in normal
pancreas (11,13), but there was weak HE4 protein
staining in 3 cases, strong HE4 staining in 4 cases, and negative
HE4 staining in 1 case out of 8 cases of pancreaticobiliary
carcinoma (11). O'Neal et
al indicated that HE4 was not expressed in pancreatic
intraepithelial neoplasia (PanIN), but 46.8% of pancreatic
adenocarcinomas had HE4 expression (16). In addition, serum levels of HE4 were
also upregulated in pancreatic cancer (15,19,20).
These data indicated that HE4 could be a candidate diagnosis marker
for pancreatic cancers and HE4 may play an important role in
pancreatic cancer progression.
Endometrial cancer is the most common gynecologic
malignancy in developed countries. Unlike pancreatic cancer, most
endometrial cancers are diagnosed at early stages due to the
symptom of postmenopausal bleeding. However, 30% of EC patients are
asymptomatic, making the early detection a significant issue for
these cases (38). Multiple studies
have demonstrated that serum HE4 is a useful tumor biomarker of EC
(17,28). HE4 expression levels correlates
positively with aggressive phenotype, tumor size and myometrial
invasion of EC (11,18,26,27),
but inversely with overall EC patients survival (26,28,29).
We have reported that in EC cell culture and mouse xenograft model
forced overexpression of HE4 resulted in more aggressive phenotypes
(31).
In the present study, we demonstrated that the
recombinant, exogenous HE4 protein was able to promote cell
proliferation in pancreatic and endometrial cancer cell lines
(Figs. 1 and 2). Moreover, the addition of recombinant
HE4 protein to Suit-2 and KLE cell cultures increased DNA synthesis
(Fig. 3) and induced correspondent
changes in the expression of cell cycle regulators. This is the
first study on the bioactivity of extracellular HE4 in pancreatic
cancer cells. Previously, several studies have shown that
overexpression of HE4 affected cell proliferation in ovarian and
endometrial cancer cell lines (31,33,39,40).
While we could not exclude the bioactivity of intracellular HE4,
the new data indicated that extracellular HE4 secreted into culture
medium may contribute to the overall HE4 effects observed in
previous overexpression models.
In cancer patients, HE4 produced by cancer cells is
secreted into the extracellular plasma. This raises the possibility
that through affecting the surrounding cancer and non-cancer cells
HE4 may exerts a paracrine function. Similarly, circulatory HE4
protein released by primary tumor may play an endocrine role by
affecting the proliferation of remote cell/tissues, e.g., the
metastatic cancer. Thus, our new findings point to a new model of
cell-cell interaction mediated by extracellular HE4. The presence
and significance of this action in cancer progression need to be
examined by specifically designed in vivo experiments.
Our data raise a possibility that extracellular HE4
could affect cell proliferation through directly binding to its
receptor or receptor-like proteins on the cell membrane, delivering
its stimulatory signals into cytoplasm. For this potential
mechanism, HE4 protein remains in the outer space of cell membrane.
Although as a 25 kDa glycosylated peptide, HE4 protein could not
directly pass through cell membrane, we could not exclude the
possibility that through actions like pinocytosis, some HE4
molecules could enter cytoplasm and/or nuclei and thereby exerting
its function. In this initial investigation, we did not delineate
the detailed mechanism by which recombinant HE4 protein exerts its
bioactivity. Nevertheless, the present study provided evidence
showing that recombinant HE4 protein could regulate PCNA and p21
gene expression and modulate cell proliferation in pancreatic and
endometrial cancer cell lines. As shown in Fig. 4, recombinant HE4 protein increased
PCNA expression and decreased p21 expression on both mRNA and
protein levels in Suit-2 and KLE cells. PCNA plays important roles
in S-phase DNA synthesis and controls establishment of sister
chromatid cohesion during S-phase (41,42).
p21 binds to cyclin D1-CDK4, cyclin D2-CDK4, cyclin E-CDK2 and
cyclin A-CDK2, and inhibits their activities, resulting in an
increased phosphorylation of Rb (43,44).
In addition, p21 controls DNA replication by interaction with PCNA
(45,46). These findings indicated that HE4
function may be mediated by p21-CDK-Rb pathway.
In summary, using the recombinant HE4 protein
produced by human cells, we observed that extracellular HE4 is able
to promote the proliferation of pancreatic and endometrial cancer
cells. By this action, HE4 secreted from cancer cells may carry out
paracrine and endocrine functions in cancer patients. The novel HE4
action may have important implications for the progression and
metastasis of pancreatic and endometrial cancers. Moreover,
extracellular HE4 protein is able to modulate the expression of
cell cycle regulators p21 and PCNA. The exact mechanism by which
extracellular HE4 may exert its function in cell cycle regulation
remains to be elucidated.
Acknowledgments
We thank Dr Kaustubh Datta (University of Nebraska)
for providing the pancreatic cancer Suit-2 cell line. The present
study was supported by the Memorial Health University Medical
Center/Curtis and Elizabeth Anderson Cancer Institute and Mercer
University (ACI/MUSM) Pancreatic Cancer Research Program (Li J.;
Jiang S.-W.; and Brower S.); the ACI Excellence through Discovery
Laboratory Research Fund (Li J.; Jiang S.-W.; Brower S.; and
Glasgow W.); the Distinguished Cancer Scholar Program of Georgia
Cancer Coalition (Jiang S.-W.); the National High Technology
Research and Development Program of China (863 Program,
2014AA020521 to Wang J.; NSFC 81472450 Li J.); the Research
Supplement from Mercer University School of Medicine (Jiang S.-W.);
and the Research Start-up fund from Mercer University School of
Medicine (Li J.).
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar
|
|
4
|
Bardeesy N and DePinho RA: Pancreatic
cancer biology and genetics. Nat Rev Cancer. 2:897–909. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Muniraj T, Jamidar PA and Aslanian HR:
Pancreatic cancer: A comprehensive review and update. Dis Mon.
59:368–402. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sakai T, Toguchida J, Ohtani N, Yandell
DW, Rapaport JM and Dryja TP: Allele-specific hypermethylation of
the retinoblastoma tumor-suppressor gene. Am J Hum Genet.
48:880–888. 1991.PubMed/NCBI
|
|
7
|
Esteller M: CpG island hypermethylation
and tumor suppressor genes: A booming present, a brighter future.
Oncogene. 21:5427–5440. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kirchhoff C, Habben I, Ivell R and Krull
N: A major human epididymis-specific cDNA encodes a protein with
sequence homology to extracellular proteinase inhibitors. Biol
Reprod. 45:350–357. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Clauss A, Lilja H and Lundwall A: A locus
on human chromosome 20 contains several genes expressing protease
inhibitor domains with homology to whey acidic protein. Biochem J.
368:233–242. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bingle CD: Towards defining the complement
of mammalian WFDC-domain-containing proteins. Biochem Soc Trans.
39:1393–1397. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Galgano MT, Hampton GM and Frierson HF Jr:
Comprehensive analysis of HE4 expression in normal and malignant
human tissues. Mod Pathol. 19:847–853. 2006.PubMed/NCBI
|
|
12
|
Jiang SW, Chen H, Dowdy S, Fu A, Attewell
J, Kalogera E, Drapkin R, Podratz K, Broaddus R and Li J: HE4
transcription- and splice variants-specific expression in
endometrial cancer and correlation with patient survival. Int J Mol
Sci. 14:22655–22677. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Drapkin R, von Horsten HH, Lin Y, Mok SC,
Crum CP, Welch WR and Hecht JL: Human epididymis protein 4 (HE4) is
a secreted glycoprotein that is overexpressed by serous and
endometrioid ovarian carcinomas. Cancer Res. 65:2162–2169. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ryu B, Jones J, Blades NJ, Parmigiani G,
Hollingsworth MA, Hruban RH and Kern SE: Relationships and
differentially expressed genes among pancreatic cancers examined by
large-scale serial analysis of gene expression. Cancer Res.
62:819–826. 2002.PubMed/NCBI
|
|
15
|
Huang T, Jiang SW, Qin L, Senkowski C,
Lyle C, Terry K, Brower S, Chen H, Glasgow W, Wei Y, et al:
Expression and diagnostic value of HE4 in pancreatic
adenocarcinoma. Int J Mol Sci. 16:2956–2970. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
O'Neal RL, Nam KT, LaFleur BJ, Barlow B,
Nozaki K, Lee HJ, Kim WH, Yang HK, Shi C, Maitra A, et al: Human
epididymis protein 4 is up-regulated in gastric and pancreatic
adenocarcinomas. Hum Pathol. 44:734–742. 2013. View Article : Google Scholar
|
|
17
|
Angioli R, Miranda A, Aloisi A, Montera R,
Capriglione S, De Cicco Nardone C, Terranova C and Plotti F: A
critical review on HE4 performance in endometrial cancer: Where are
we now? Tumour Biol. 35:881–887. 2014. View Article : Google Scholar
|
|
18
|
Moore RG, Miller CM, Brown AK, Robison K,
Steinhoff M and Lambert-Messerlian G: Utility of tumor marker HE4
to predict depth of myometrial invasion in endometrioid
adenocarcinoma of the uterus. Int J Gynecol Cancer. 21:1185–1190.
2011.PubMed/NCBI
|
|
19
|
Faca VM, Song KS, Wang H, Zhang Q,
Krasnoselsky AL, Newcomb LF, Plentz RR, Gurumurthy S, Redston MS,
Pitteri SJ, et al: A mouse to human search for plasma proteome
changes associated with pancreatic tumor development. PLoS Med.
5:e1232008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Brand RE, Nolen BM, Zeh HJ, Allen PJ,
Eloubeidi MA, Goldberg M, Elton E, Arnoletti JP, Christein JD,
Vickers SM, et al: Serum biomarker panels for the detection of
pancreatic cancer. Clin Cancer Res. 17:805–816. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bingle CD and Vyakarnam A: Novel innate
immune functions of the whey acidic protein family. Trends Immunol.
29:444–453. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Scott A, Weldon S and Taggart CC: SLPI and
elafin: Multifunctional antiproteases of the WFDC family. Biochem
Soc Trans. 39:1437–1440. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chhikara N, Saraswat M, Tomar AK, Dey S,
Singh S and Yadav S: Human epididymis protein-4 (HE-4): A novel
cross-class protease inhibitor. PLoS One. 7:e476722012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
LeBleu VS, Teng Y, O'Connell JT, Charytan
D, Müller GA, Müller CA, Sugimoto H and Kalluri R: Identification
of human epididymis protein-4 as a fibroblast-derived mediator of
fibrosis. Nat Med. 19:227–231. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hua L, Liu Y, Zhen S, Wan D, Cao J and Gao
X: Expression and biochemical characterization of recombinant human
epididymis protein 4. Protein Expr Purif. 102:52–62. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bignotti E, Ragnoli M, Zanotti L, Calza S,
Falchetti M, Lonardi S, Bergamelli S, Bandiera E, Tassi RA, Romani
C, et al: Diagnostic and prognostic impact of serum HE4 detection
in endometrial carcinoma patients. Br J Cancer. 104:1418–1425.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kalogera E, Scholler N, Powless C, Weaver
A, Drapkin R, Li J, Jiang SW, Podratz K, Urban N and Dowdy SC:
Correlation of serum HE4 with tumor size and myometrial invasion in
endometrial cancer. Gynecol Oncol. 124:270–275. 2012. View Article : Google Scholar
|
|
28
|
Zanotti L, Bignotti E, Calza S, Bandiera
E, Ruggeri G, Galli C, Tognon G, Ragnoli M, Romani C, Tassi RA, et
al: Human epididymis protein 4 as a serum marker for diagnosis of
endometrial carcinoma and prediction of clinical outcome. Clin Chem
Lab Med. 50:2189–2198. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Angioli R, Plotti F, Capriglione S,
Montera R, Damiani P, Ricciardi R, Aloisi A, Luvero D, Cafà EV,
Dugo N, et al: The role of novel biomarker HE4 in endometrial
cancer: A case control prospective study. Tumour Biol. 34:571–576.
2013. View Article : Google Scholar
|
|
30
|
Lu R, Sun X, Xiao R, Zhou L and Gaoxand
Guo L: Human epididymis protein 4 (HE4) plays a key role in ovarian
cancer cell adhesion and motility. Biochem Biophys Res Commun.
419:274–280. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li J, Chen H, Mariani A, Chen D, Klatt E,
Podratz K, Drapkin R, Broaddus R, Dowdy S and Jiang SW: HE4 (WFDC2)
promotes tumor growth in endometrial cancer cell lines. Int J Mol
Sci. 14:6026–6043. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhu YF, Gao GL, Tang SB, Zhang ZD and
Huang QS: Effect of WFDC 2 silencing on the proliferation, motility
and invasion of human serous ovarian cancer cells in vitro. Asian
Pac J Trop Med. 6:265–272. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen Y, Mu X, Wang S, Zhao L, Wu Y, Li J
and Li M: WAP four-disulfide core domain protein 2 mediates the
proliferation of human ovarian cancer cells through the regulation
of growth-and apoptosis-associated genes. Oncol Rep. 29:288–296.
2013.
|
|
34
|
Wang H, Zhu L, Gao J, Hu Z and Lin B:
Promotive role of recombinant HE4 protein in proliferation and
carboplatin resistance in ovarian cancer cells. Oncol Rep.
33:403–412. 2015.
|
|
35
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ahrendt SA and Pitt HA: Surgical
management of pancreatic cancer. Oncology. 16:725–743.
2002.PubMed/NCBI
|
|
37
|
Missiaglia E, Blaveri E, Terris B, Wang
YH, Costello E, Neoptolemos JP, Crnogorac-Jurcevic T and Lemoine
NR: Analysis of gene expression in cancer cell lines identifies
candidate markers for pancreatic tumorigenesis and metastasis. Int
J Cancer. 112:100–112. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hamilton CA, Cheung MK, Osann K, Chen L,
Teng NN, Longacre TA, Powell MA, Hendrickson MR, Kapp DS and Chan
JK: Uterine papillary serous and clear cell carcinomas predict for
poorer survival compared to grade 3 endometrioid corpus cancers. Br
J Cancer. 94:642–646. 2006.PubMed/NCBI
|
|
39
|
Zou SL, Chang XH, Ye X, Cheng HY, Cheng
YX, Tang ZJ, Zhang ZJ, Gao L, Chen XH and Cui H: Effect of human
epididymis protein 4 gene silencing on the malignant phenotype in
ovarian cancer. Chin Med J. 124:3133–3140. 2011.PubMed/NCBI
|
|
40
|
Moore RG, Hill EK, Horan T, Yano N, Kim K,
MacLaughlan S, Lambert-Messerlian G, Tseng YD, Padbury JF, Miller
MC, et al: HE4 (WFDC2) gene overexpression promotes ovarian tumor
growth. Sci Rep. 4:35742014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kelman Z: PCNA: Structure, functions and
interactions. Oncogene. 14:629–640. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Moldovan GL, Pfander B and Jentsch S: PCNA
controls establishment of sister chromatid cohesion during S phase.
Mol Cell. 23:723–732. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Harper JW, Adami GR, Wei N, Keyomarsi K
and Elledge SJ: The p21 Cdk-interacting protein Cip1 is a potent
inhibitor of G1 cyclin-dependent kinases. Cell. 75:805–816. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Xiong Y, Hannon GJ, Zhang H, Casso D,
Kobayashi R and Beach D: p21 is a universal inhibitor of cyclin
kinases. Nature. 366:701–704. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Waga S, Hannon GJ, Beach D and Stillman B:
The p21 inhibitor of cyclin-dependent kinases controls DNA
replication by interaction with PCNA. Nature. 369:574–578. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chen J, Jackson PK, Kirschner MW and Dutta
A: Separate domains of p21 involved in the inhibition of Cdk kinase
and PCNA. Nature. 374:386–388. 1995. View Article : Google Scholar : PubMed/NCBI
|