Introduction
Epidemiological studies have indicated that
consumption in the diet of fish oil, rich in n-3 polyunsaturated
fatty acids (n-3 PUFAs) has been associated with a reduced risk of
colorectal cancer via modulation of the inflammatory status of
cellular membranes (1–3). Experimental studies have identified a
role for n-3 PUFAs in colon carcinogenesis showing a growth
inhibitory effect on the progression of malignancy (4). Dietary n-3 PUFAs have been
demonstrated to reduce tumor load in mice with an APC defect
(5,6). Additionally, clinical evidence showed
that these fatty acids have specific effects on disease prevention,
reducing rectal polyp number and size in patients with familial
adenomatous polyposis (7).
Previously, in HepG2 hepatoma cells, we showed that
the exposure of these cells to the eicosapentaenoic acid (EPA) and
the arachidonic acid (ARA), belonging to the family of n-3 PUFAs
and n-6 PUFAs, respectively, led to cell growth arrest and the
promotion of apoptosis (8). The
inhibition of cell growth exerted by these PUFAs was due to a
strong inhibition of fatty acid synthase (FAS) and
3-hydroxy-3-methyl-glutaryl CoA reductase (HMGCoAR) gene
expression.
We previously performed an in vivo study
demonstrating that Mediterranean diet components, such as olive
oil, n-3 and n-6 PUFAs, when given to mice that spontaneously
develop intestinal polyps (ApcMin/+ mice), are able to
reduce polyp number and volume by decreasing proliferation and
increasing pro-apoptotic activity (9). These biological effects were
associated with the inhibition of FAS and HMGCoAR gene expression
and activity as well as an increased estrogen receptor β/estrogen
receptor α (ERβ/ERα) ratio.
Previous findings have demonstrated that ERβ is
abundantly expressed in normal colon but exhibits a progressively
decreased expression in human adenomatous sporadic polyps and in
ApcMin/+ mice (10–15).
By contrast, ERα is a well-known mediator of cell proliferation
activity (16), which acts by
enhancing the transcription of factors associated with cell
proliferation and shows an increased expression in colon cancer as
compared to normal surrounding tissue (16). In particular, ERα protein expression
has been demonstrated to play a role in the regulation of the
hedgehog (Hh) signaling pathway which has been demonstrated to be
activated in many types of cancer, including colorectal cancer
(17–19). Activation of the Hh pathway induces
an overexpression of Hh signaling pathway-associated genes, sonic
hedgehog (Shh) protein and the glioma-associated oncogene homolog-1
(GLI-1) (19,20).
Another important feature of malignant
transformation is loss of the cholesterol feedback inhibition
mechanism that regulates cholesterol synthesis (21,22).
The main cholesterol feedback defect in malignant cells has been
located at the HMGCoAR step. Cancer cells are characterized by a
reduced expression of LDL receptor (LDL-R) and as a consequence of
the inability to internalize the exogenous cholesterol, tend to
increase the endogenous synthesis, thereby increasing the activity
of HMGCoAR. Alterations of lipid metabolism, and high levels of FAS
and HMGCoAR expression and activity, are usual characteristics of
tumor cells and are essential in the onset and progression of tumor
(23).
The aim of the present study was to investigate
whether a diet enriched with n-3 PUFAs, known already to have an
anti-neoplastic efficacy, would reverse the development of
intestinal polyps in ApcMin/+ mice and to examine the
possible molecular mechanisms involved. The expression levels of
cell proliferation- and apoptosis-related proteins, as well as the
protein expression of LDL-R and the levels of FAS activity were
analyzed in mouse intestinal tissue.
Materials and methods
Animals and experimental study
design
Five-week-old C57BL/6J male mice with a heterozygote
mutation for the Apc gene (ApcMin/+) were obtained from
Charles River Laboratories Italia (Calco, Italy). The mice were
maintained under temperature-, air- and light-controlled conditions
and received food and water ad libitum, although they did
not receive any surgical or hormonal manipulation. All animals
received care in compliance with the 'Guide for the Care and Use of
Laboratory Animals'. The procedures regarding animal use were
communicated to the Italian Ministry of Health and approved.
The ApcMin/+ mice were randomly divided
into 3 groups of 5 animals each and fed as follows: control (ST1)
group, received a purified AIN-93M standard diet (12.5% protein,
12% soybean oil, 3% cellulose fiber) for 5 weeks; control (ST2)
group, received the same purified AIN-93M standard diet for 10
weeks; OM-3R group, received a purified AIN-93M standard diet for 5
weeks and a purified AIN-93M standard diet in which soybean oil was
replaced with salmon oil, rich in n-3 PUFAs (12.5% protein, 12%
salmon oil, 3% cellulose fiber) for an additional 5 weeks. Diet
fatty acid composition has been previously described (9), with the diets being isocaloric and
supplied as pellets (Mucedola Srl, Settimo Milanese, Italy). Body
weight and food intake of the mice were measured every 3 days.
Following establishment of the time period for the
dietary treatment, the animals were sacrificed by cervical
dislocation and the entire intestinal tract was immediately removed
and washed with cold phosphate-buffered saline (PBS). The small
intestine and colon were cut along the mesenteric insertion, placed
on a paper strip at 0–4°C and analyzed through a stereomicroscope
at ×3 magnification in order to calculate the number and the volume
of polyps. The small intestine was further divided into proximal,
medial and distal segments. A portion of the intestinal segments of
all the animals was immediately stored at −80°C, for western blot
analysis and enzymatic activity analyses.
Western blotting
Protein expression levels of ERα, ERβ, Shh, GLI-1,
STAT3 and p-STAT3Ser as well as PIAS-3, caspase-8, Bax, Bcl-2,
LDL-R and β-actin protein expression were evaluated in distal
tissue specimens by western blot analysis. Briefly, 50 µg
aliquots of total protein were separated in 4–12% pre-cast
polyacrylamide gels (Invitrogen, Life Technologies, Carlsbad, CA,
USA) and transferred onto a PVDF membrane with Transblot Turbo
(both from Bio-Rad Laboratories, Milan, Italy). The primary
antibodies anti-p-STAT3Ser, anti-STAT3 and anti-β-actin (Cell
Signaling Technology, Beverly, MA, USA); anti-ERα, anti-ERβ,
anti-Bax, anti-Bcl-2, anti-caspase-8, anti-Shh, anti-GLI-1 and
anti-PIAS-3 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA);
and anti-LDL-R (Abcam, Cambridge, MA, USA) were diluted at 1:500 in
blocking buffer. After overnight incubation, the membranes were
subsequently incubated with a horseradish peroxidase-conjugated
secondary antibody (Bio-Rad Laboratories). The proteins were
detected by chemiluminescence (ECL; Thermo Scientific, Rockford,
IL, USA) and the densitometric analysis of each protein-related
signal was obtained using the Molecular Imager Chemidoc™ (Bio-Rad
Laboratories) and normalized against β-actin expression.
FAS activity assay
FAS activity was determined on frozen distal
intestinal samples, as previously described (24), and expressed as picomoles of
incorporated 2-14C-malonyl-CoA/min/mg of total
proteins.
Statistical analysis
Data are presented as means ± SE. The significance
of the differences among experimental groups was evaluated by
one-way analysis of variance (ANOVA) and Tukey's multiple
comparison test. Differences were considered significant at a
P<0.05.
Results
Total number and volume of intestinal
polyps
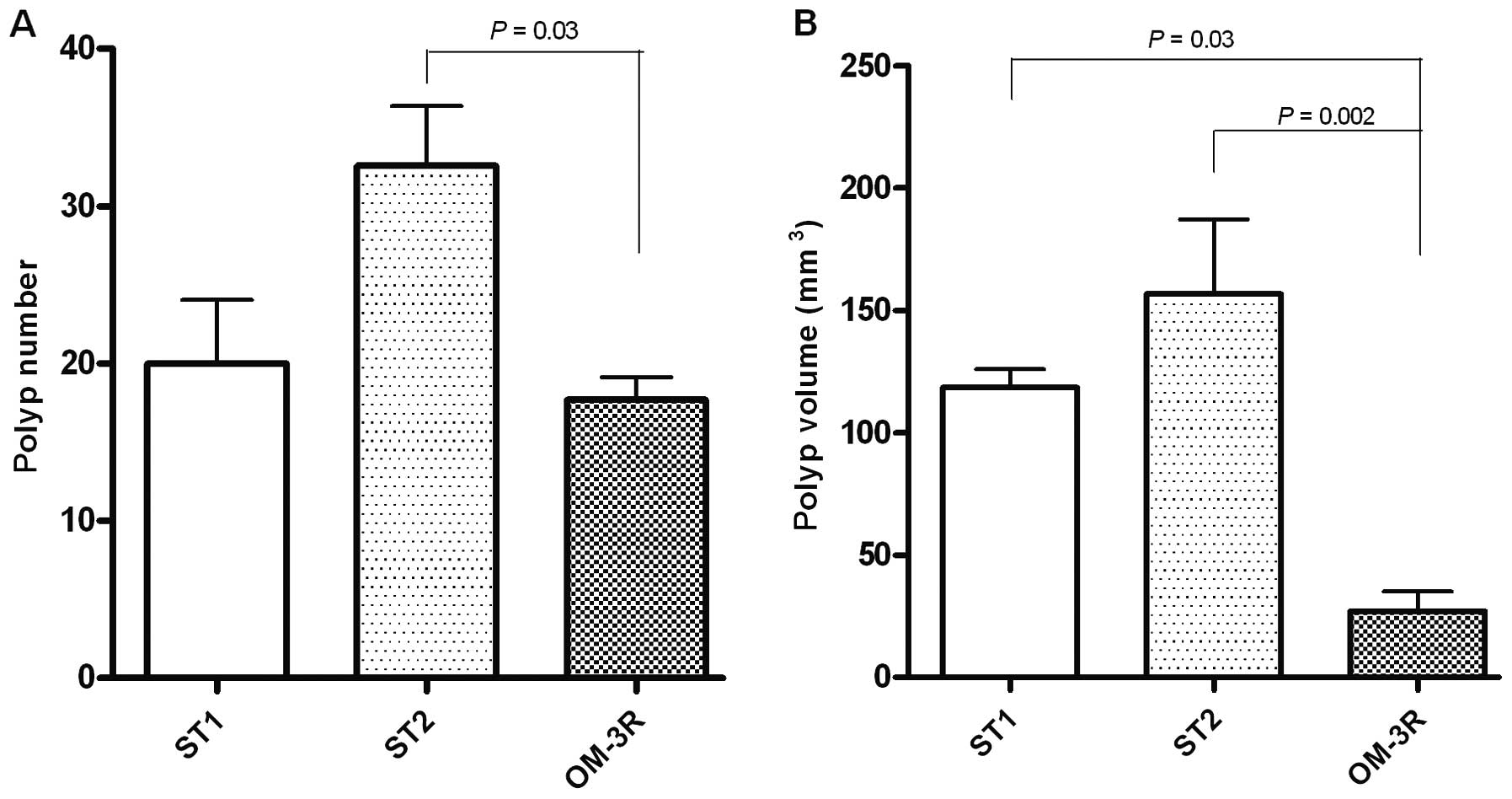
The number and volume of polyps evaluated along the
entire intestinal tract in three groups of mice were determined
(Fig. 1A and B). Compared to the
mice treated with standard diet for 5 weeks (ST1 group), the number
of polyps in the OM-3R group was decreased although the reduction
was not statistically significant. However, a statistically
significant difference in polyp number was present between the ST2
group (mice treated with standard diet for 10 weeks) and the OM-3R
group (P=0.03, ANOVA and Tukey's multiple comparison test)
(Fig. 1A).
The volume of polyps detected along the entire
intestinal tract of each mouse was calculated by considering polyps
as hemispheres (1/2×3/4 π r3). Analysis of the polyp
volume revealed a statistically significant reduction in the OM-3R
group as compared to animals fed with the standard diet for 5 weeks
(ST1) and for 10 weeks (ST2) (P=0.03 and P=0.002, respectively,
ANOVA and Tukey's multiple comparison test) (Fig. 1B). These results clearly indicated
that n-3 PUFAs reversed the intestinal polyp formation in
ApcMin/+ mice.
Protein expression of estrogen
receptors
To examine the underlying molecular mechanisms of
polyp reversion, we studied the two cell signals of proliferation
and of apoptosis.
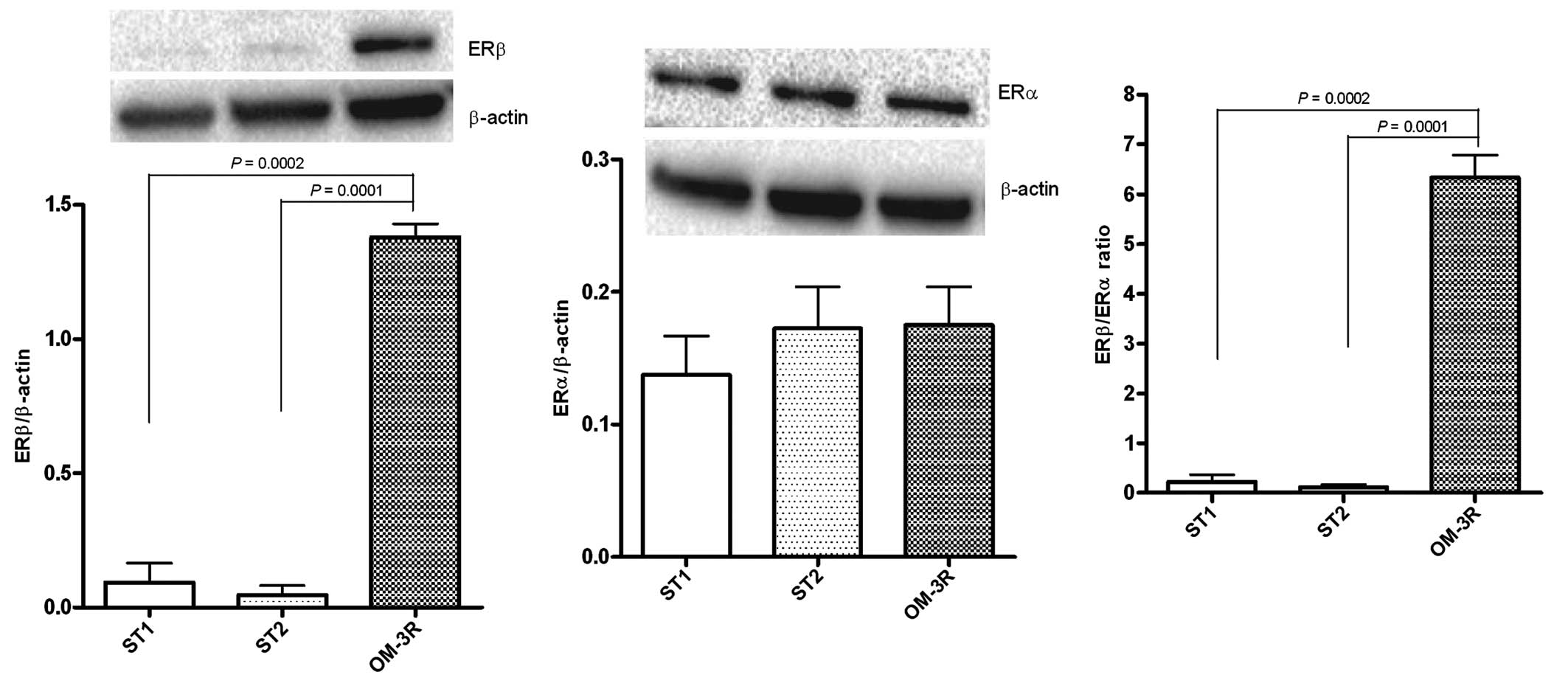
The role of ERβ in counteracting tumor progression
was confirmed. Fig. 2 shows a
significant increase of ERβ protein expression in the OM-3R group
versus the ST1 and ST2 groups (P=0.0002 and P=0.0001, respectively,
ANOVA and Tukey's multiple comparison test), whereas the ERα
protein levels were not altered. These results led to a significant
induction of ERβ/ERα ratio, evident in the OM-3R group as compared
to the ST1 and ST2 groups (P=0.0002 and P=0.0001,
respectively).
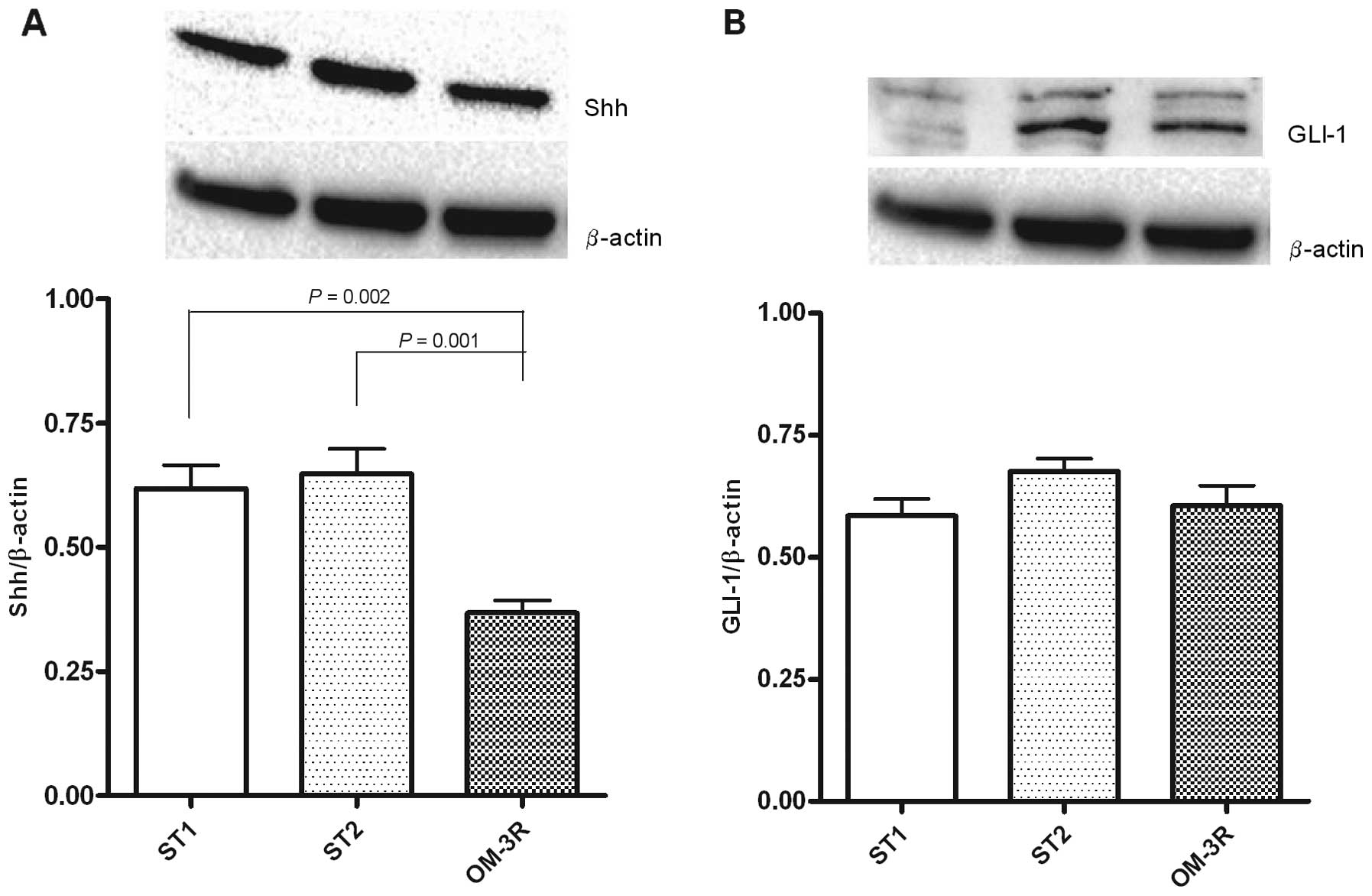
Protein expression of Shh and GLI-1
Since ERα, a mediator of cell proliferation,
regulates the Hh signaling pathway, we examined the protein
expression levels of Shh and GLI-1 in mouse intestinal tissue. The
results demonstrated that the two proteins were differentially
expressed following the n-3 PUFAs diet treatment: Shh protein
levels were significantly downregulated in the OM-3R group, whereas
the GLI-1 protein expression was unmodified when compared to the
ST1 and ST2 groups (Fig. 3).
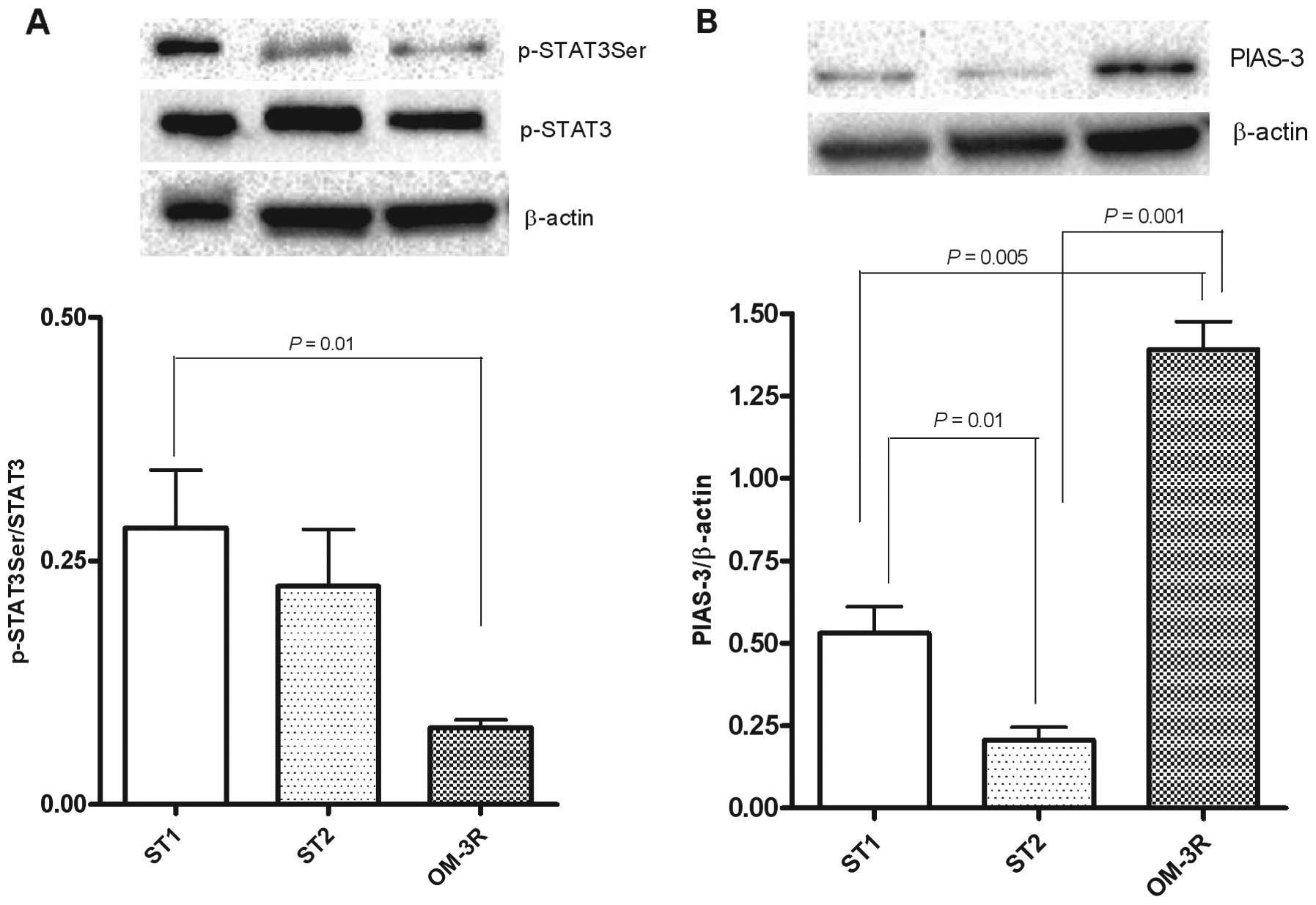
Protein expression of STAT3Ser/STAT3
ratio and PIAS-3
To better define the molecular connection between
n-3 PUFAs and cell proliferation, we evaluated the expression of
p-STAT3, known to play a role in cell growth by upregulating the
expression of anti-apoptotic genes. The results clearly
demonstrated that p-STAT3Ser expression was significantly reduced
in mice fed on n-3 PUFAs as compared to mice receiving only the
standard diet for 5 weeks (P=0.01, ANOVA and Tukey's multiple
comparison test) (Fig. 4A).
Consequently, PIAS-3 levels, known to be an inhibitor of p-STAT3,
were significantly increased in the OM-3R mouse group as compared
to mice receiving only the standard diet, for 5 and 10 weeks
(P=0.005 and P=0.001, respectively) (Fig. 4b). Fig.
4b shows that PIAS protein expression was highly downregulated
after 10 weeks of treatment with the standard diet.
Expression level of apoptotic
proteins
The expression levels of caspase-8 (Fig. 5A), and Bax and Bcl-2 proteins
(Fig. 5B) were determined.
Apoptotic processes were not involved in the reversion process
induced by n-3 PUFAs, i.e., no change in the caspase-8, Bax and
Bcl-2 protein expression was observed in the OM-3R group as
compared to the ST1 and ST2 groups.
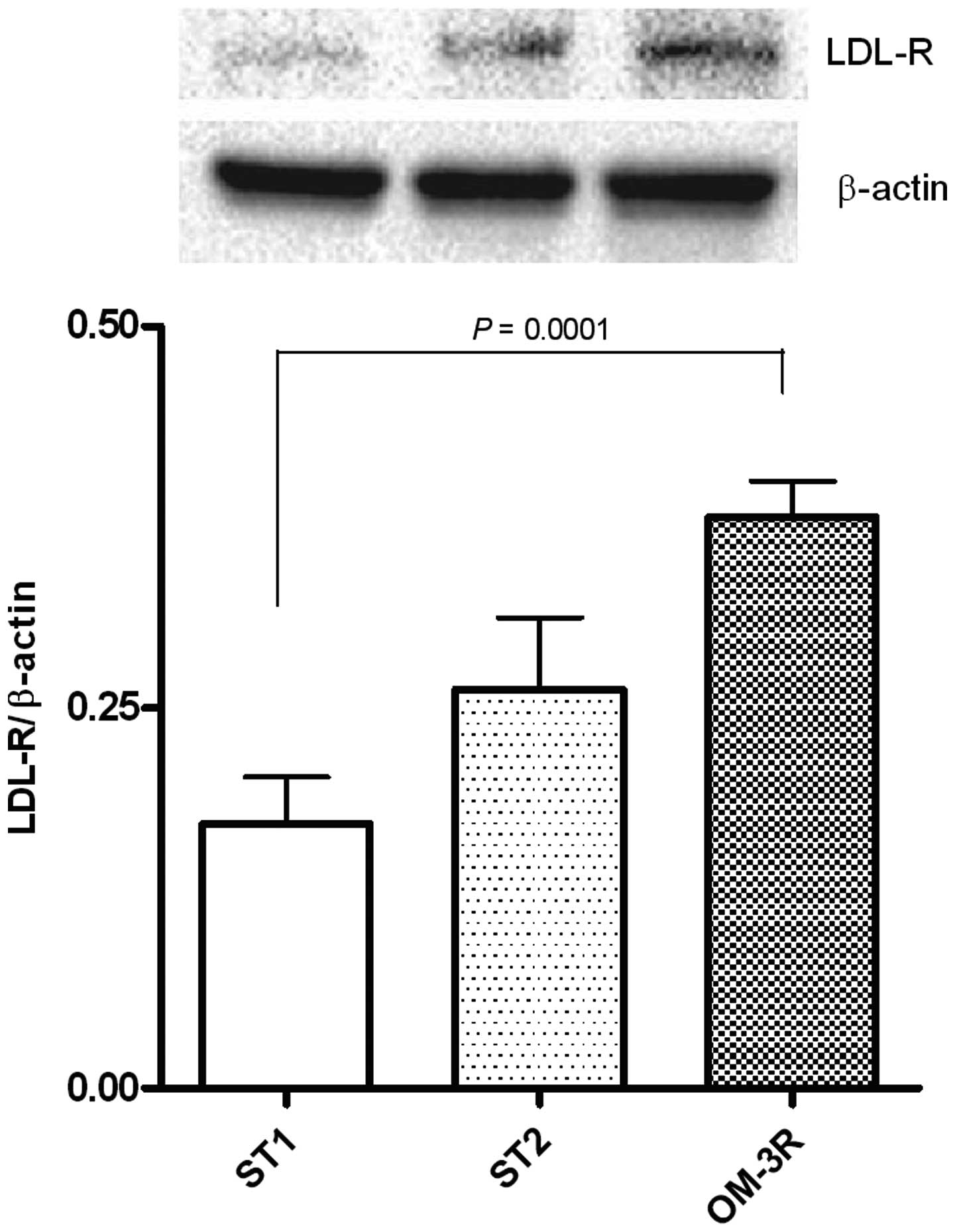
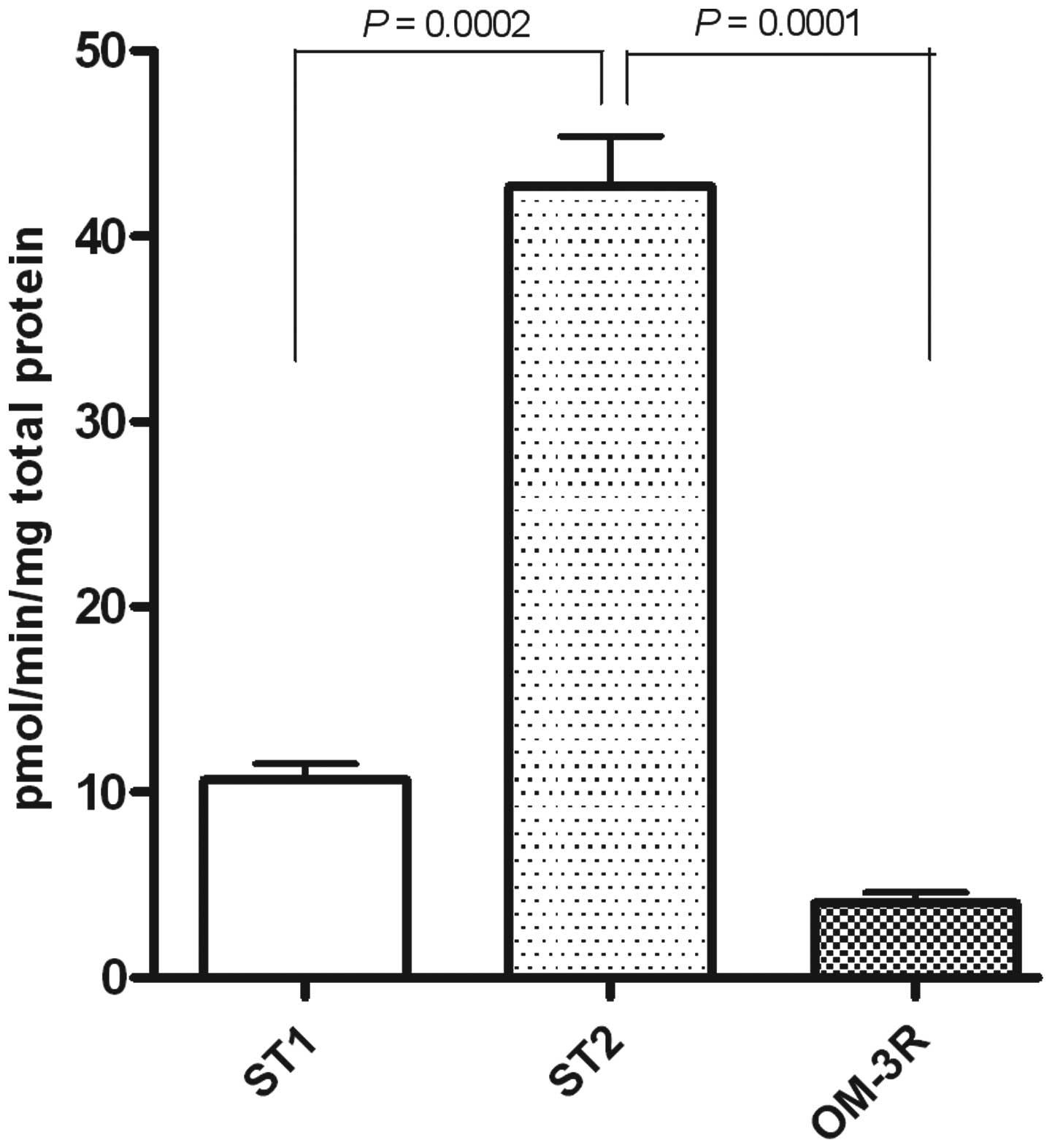
LDL-R protein expression and levels of
FAS activity
The dietary n-3 PUFAs treatment, in intestinal mice
tissue, caused a significant increase of LDL-R expression, able to
exert an inhibitory effect on tumor cell growth (Fig. 6) as well as a strong reduction of
FAs activity (Fig. 7). These
results confirmed that natural compounds such as n-3 PUFAs can
elicit their effects via the downregulation of lipogenic
enzymes.
Discussion
The present study shows for the first time the
ability of a diet enriched with n-3 PUFAs to invert the polyp
formation process in a ApcMin/+ mouse model.
Previous findings demonstrated in
ApcMin/+ mice that n-3 PUFAs significantly reduced the
number and volume of polyps, through a decrease of cell
proliferation and an increase of apoptosis in the adenomatous
tissue (9). By contrast, the
reverse process of polyp development, is likely due to the
activation of anti-proliferative mechanisms that exclude cell
apoptotic processes.
n-3 PUFAs were able to suppress intestinal polyps in
ApcMin/+ mice and significantly reverse polyp
development associated with the downregulation of cell
proliferation markers and with the induction of ERβ and LDL-R,
which are negative modulators of cell proliferation.
p-STAT3 has been shown to have pro-proliferative
effects and is responsible for the activation of metabolic pathways
involved in the regulation of cell proliferation (25–28).
Its reduction, following n-3 PUFAs treatment, is an index of the
shutdown of cell proliferation in mouse intestinal tissue.
Consequently, PIAS-3 levels were induced, since PIAS-3 is known to
control the extent and the duration of STAT3 activity in cells
preventing its oncogenic function (29).
Several chemopreventive agents, such as olive oil,
n-3 PUFAs, curcumin and silymarin (9,11,30)
have been demonstrated to suppress the spontaneous formation of
intestinal tumors in the ApcMin/+ mouse, confirming a
key role of diet in modulating colon cancer risk.
Different mechanisms have been suggested to explain
the protective effect of n-3 PUFAs on colon cancer development.
Authors have suggested that their antineoplastic effects would
involve the incorporation of n-3 PUFAs into cellular membranes
replacing the n-6 PUFAs with a consequent reduction of inflammation
(3,6).
Previously, we demonstrated that the
anti-proliferative effects of dietary n-3 PUFAs were associated
with an inhibition of FAS and HMGCoAR gene expression and activity
and to an increased ERβ/ERα ratio (9).
In the present study, we showed that induction of
the ERβ/ERα ratio is also involved in the reverse process of polyp
development associated with the upregulation of LDL-R
expression.
LDL-R has been found to play a role in cell growth
and tumorigenesis (31).
Previously, we showed that LDL-R was little expressed in colon
cancer and that the absence of LDL-R predicted shorter survival in
CRC patients (32). Thus, the
factors that upregulate LDL-R expression in normal and tumor cells,
consequently, are able to control cell proliferation and
transformation.
In addition, dietary n-3 PUFAs exerted a decrease of
p-STAT3Ser protein expression and FAs activity, confirming the
central role of n-3 PUFAs in the regulation of cell proliferation
metabolic pathways, as previously demonstrated.
In conclusion, n-3 PUFAs-induced metabolic changes
are able to counteract intestinal polyp formation in the mice and
to revert polyp growth. This noteworthy finding is important for a
translational study evaluating the therapeutic role of dietary
components on human health. In particular, the marked effect of
diets on polyp development in the absence of toxicity makes n-3
PUFAs an excellent candidate for the prevention and treatment of
subjects with gastrointestinal diseases.
References
|
1
|
Caygill CP, Charlett A and Hill MJ: Fat,
fish, fish oil and cancer. Br J cancer. 74:159–164. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kato I, Akhmedkhanov A, Koenig K, Toniolo
PG, Shore RE and Riboli E: Prospective study of diet and female
colorectal cancer: The New York University Women's Health Study.
Nutr Cancer. 28:276–281. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dupertuis YM, Meguid MM and Pichard C:
Colon cancer therapy: New perspectives of nutritional manipulations
using polyunsaturated fatty acids. Curr Opin Clin Nutr Metab Care.
10:427–432. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chapkin RS, Seo J, McMurray DN and Lupton
JR: Mechanisms by which docosahexaenoic acid and related fatty
acids reduce colon cancer risk and inflammatory disorders of the
intestine. Chem Phys Lipids. 153:14–23. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Petrik MB, McEntee MF, Chiu CH and Whelan
J: Antagonism of arachidonic acid is linked to the antitumorigenic
effect of dietary eicosapentaenoic acid in Apc(Min/+) mice. J Nutr.
130:1153–1158. 2000.PubMed/NCBI
|
|
6
|
Fini L, Piazzi G, Ceccarelli C, Daoud Y,
Belluzzi A, Munarini A, Graziani G, Fogliano V, Selgrad M, Garcia
M, et al: Highly purified eicosapentaenoic acid as free fatty acids
strongly suppresses polyps in Apc(Min/+) mice. Clin Cancer Res.
16:5703–5711. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
West NJ, Clark SK, Phillips RK, Hutchinson
JM, Leicester RJ, Belluzzi A and Hull MA: Eicosapentaenoic acid
reduces rectal polyp number and size in familial adenomatous
polyposis. Gut. 59:918–925. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Notarnicola M, Messa C, Refolo MG, Tutino
V, Miccolis A and Caruso MG: Polyunsaturated fatty acids reduce
fatty acid synthase and hydroxy-methyl-glutaryl CoA-reductase gene
expression and promote apoptosis in HepG2 cell line. Lipids Health
Dis. 10:102011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Barone M, Notarnicola M, Caruso MG, Scavo
MP, Viggiani MT, Tutino V, Polimeno L, Pesetti B, Di Leo A and
Francavilla A: Olive oil and omega-3 polyunsaturated fatty acids
suppress intestinal polyp growth by modulating the apoptotic
process in ApcMin/+ mice. Carcinogenesis. 35:1613–1619.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Di Leo A, Barone M, Maiorano E, Tanzi S,
Piscitelli D, Marangi S, Lofano K, Ierardi E, Principi M and
Francavilla A: ER-beta expression in large bowel adenomas:
Implications in colon carcinogenesis. Dig Liver Dis. 40:260–266.
2008. View Article : Google Scholar
|
|
11
|
Barone M, Tanzi S, Lofano K, Scavo MP,
Pricci M, Demarinis L, Papagni S, Guido R, Maiorano E, Ingravallo
G, et al: Dietary-induced ERbeta upregulation counteracts
intestinal neoplasia development in intact male ApcMin/+
mice. Carcinogenesis. 31:269–274. 2010. View Article : Google Scholar
|
|
12
|
Barone M, Scavo MP, Papagni S, Piscitelli
D, Guido R, Di Lena M, Comelli MC and Di Leo A: ERβ expression in
normal, adenomatous and carcinomatous tissues of patients with
familial adenomatous polyposis. Scand J Gastroenterol.
45:1320–1328. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Konstantinopoulos PA, Kominea A, Vandoros
G, Sykiotis GP, Andricopoulos P, Varakis I, Sotiropoulou-Bonikou G
and Papavassiliou AG: Oestrogen receptor beta (ERbeta) is
abundantly expressed in normal colonic mucosa, but declines in
colon adenocarcinoma paralleling the tumour's dedifferentiation.
Eur J cancer. 39:1251–1258. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rudolph A, Toth C, Hoffmeister M, Roth W,
Herpel E, Jansen L, Marx A, Brenner H and Chang-Claude J:
Expression of oestrogen receptor β and prognosis of colorectal
cancer. Br J Cancer. 107:831–839. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Weihua Z, Andersson S, Cheng G, Simpson
ER, Warner M and Gustafsson JA: Update on estrogen signaling. FEBS
Lett. 546:17–24. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Thomas C and Gustafsson JÅ: The different
roles of ER subtypes in cancer biology and therapy. Nat Rev Cancer.
11:597–608. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kameda C, Nakamura M, Tanaka H, Yamasaki
A, Kubo M, Tanaka M, Onishi H and Katano M: Oestrogen receptor-α
contributes to the regulation of the hedgehog signalling pathway in
ERalpha-positive gastric cancer. Br J Cancer. 102:738–747. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang H, Li YY, Wu YY and Nie YQ:
Expression and clinical significance of hedgehog signaling pathway
related components in colorectal cancer. Asian Pac J cancer Prev.
13:2319–2324. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bian YH, Huang SH, Yang L, Ma XL, Xie JW
and Zhang HW: Sonic hedgehog-Gli1 pathway in colorectal
adenocarcinomas. World J Gastroenterol. 13:1659–1665. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gulino A, Ferretti E and De Smaele E:
Hedgehog signalling in colon cancer and stem cells. EMBO Mol Med.
1:300–302. 2009. View Article : Google Scholar
|
|
21
|
Notarnicola M, Messa C, Pricci M, Guerra
V, Altomare DF, Montemurro S and Caruso MG: Up-regulation of
3-hydroxy-3-methyl glutaryl coenzyme A reductase activity in
left-sided human colon cancer. Anticancer Res. 24:3837–3842.
2004.
|
|
22
|
Caruso MG and Notarnicola M: Biochemical
changes of mevalonate pathway in human colorectal cancer.
Anticancer Res. 25:3393–3397. 2005.PubMed/NCBI
|
|
23
|
Swinnen JV, Brusselmans K and Verhoeven G:
Increased lipo-genesis in cancer cells: New players, novel targets.
Curr Opin Clin Nutr Metab Care. 9:358–365. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Notarnicola M, Miccolis A, Tutino V,
Lorusso D and Caruso MG: Low levels of lipogenic enzymes in
peritumoral adipose tissue of colorectal cancer patients. Lipids.
47:59–63. 2012. View Article : Google Scholar
|
|
25
|
Goodman WA, Young AB, McCormick TS, Cooper
KD and Levine AD: Stat3 phosphorylation mediates resistance of
primary human T cells to regulatory T cell suppression. J Immunol.
186:3336–3345. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gao SP, Mark KG, Leslie K, Pao W, Motoi N,
Gerald WL, Travis WD, Bornmann W, Veach D, Clarkson B, et al:
Mutations in the EGFR kinase domain mediate STAT3 activation via
IL-6 production in human lung adenocarcinomas. J Clin Invest.
117:3846–3856. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Koukos G, Polytarchou C, Kaplan JL,
Morley-Fletcher A, Gras-Miralles B, Kokkotou E, Baril-Dore M,
Pothoulakis C, Winter HS and Iliopoulos D: MicroRNA-124 regulates
STAT3 expression and is down-regulated in colon tissues of
pediatric patients with ulcerative colitis. Gastroenterology.
145:842–52.e2. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Behera R, Kumar V, Lohite K, Karnik S and
Kundu GC: Activation of JAK2/STAT3 signaling by osteopontin
promotes tumor growth in human breast cancer cells. Carcinogenesis.
31:192–200. 2010. View Article : Google Scholar
|
|
29
|
Borghouts C, Tittmann H, Delis N,
Kirchenbauer M, Brill B and Groner B: The intracellular delivery of
a recombinant peptide derived from the acidic domain of PIAS3
inhibits STAT3 transactivation and induces tumor cell death. Mol
Cancer Res. 8:539–553. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pettan-Brewer C, Morton J, Mangalindan R
and Ladiges W: Curcumin suppresses intestinal polyps in APC Min
mice fed a high fat diet. Pathobiol Aging Age Relat Dis.
1:70132011.
|
|
31
|
Notarnicola M, Messa C, Refolo MG, Tutino
V, Miccolis A and Caruso MG: Synergic effect of eicosapentaenoic
acid and lovastatin on gene expression of HMGCoA reductase and LDL
receptor in cultured HepG2 cells. Lipids Health Dis. 30:1352010.
View Article : Google Scholar
|
|
32
|
Caruso MG, Notarnicola M, Santilo MR,
Cavallini A and Di Leo A: Enhanced 3-hydroxy-3-methyl-glutaryl
coenzyme a reductase activity in human colorectal cancer not
expressing low density lipoprotein receptor. Anticancer Res.
19:451–454. 1999.PubMed/NCBI
|