Introduction
Pancreatic cancer (PC) is one of the most common
malignancies (1). More than 230,000
cases are diagnosed worldwide annually, mainly in developed
countries and with a slight male predominance (2). As a result, the prognosis of
pancreatic cancer is extremely poor: the 5-year survival rate is
6%, with only 20% of patients reaching two years of survival
(3). A number of risk factors such
as smoking, diabetes and chronic pancreatitis have been identified
in case-control and cohort studies, whereas at present results
remain inconclusive, mainly due to the role of candidate genes
(4).
The TP53 protein is a key tumor suppressor (5) that responds to various cell stresses,
such as DNA damage, hypoxia, metabolic stress and oncogene
activation (6). Previous findings
have shown that the tumor suppressor TP53 is involved in the cancer
development of various cancer types including breast, colon and
pancreatic cancer, while it is frequently mutated in pancreatic
cancer, and these mutations result in the absence or dysfunction of
the p53 protein (7). TP53 also
interacts with numerous cell proteins in the control of programmed
cell death, including tumor protein p53-induced nuclear protein 1
(TP53INP1) transcription, which regulates several phenotypes of
cancer cells involved in pancreatic cancer. TP53INP1 is a key
stress-response protein that is highly expressed during
pancreatitis (8). Previous findings
demonstrated that TP53INP1 deficiency accelerates the progression
of pancreatic cancer (9–11). However, the downregulation of
TP53INP1 expression has yet to be adequately investigated for
pancreatic cancer susceptibility.
MicroRNAs (miRNAs) are small non-coding RNAs that
regulate target genes post-transcriptionally through complementary
binding to their target mRNAs to induce translational silencing or
degradation (12). miRNAs have
emerged as oncogenes or tumor suppressors in networks that
establish regulatory circuits (13), which are involved in many key cell
processes, such as cell growth, proliferation and death. The
miR-17-92 cluster first attracted attention following a series of
observations that linked it with cancer pathogenesis. It was also
shown to be overexpressed in many types of cancer, including lung,
colon and breast cancer and neuroblastoma, and pancreatic cancer
(14). miR-17-92, reduced by p53 at
the transcriptional level, has an important function in cell
apoptosis for the regulation of cancer development (15). miR-19a and miR-19b (miR-19a/b) are
located in the miR-17-92 cluster because they were recently
identified to be the most important miRNAs in this cluster
(16). Thus, we aimed to examine
the relationships between miR-19a/b and cancer development and the
potential mechanisms. Using gain and loss-of-function assays, we
found that miR-a/b function was downregulated by TP53 expression.
Furthermore, the tumor suppressor TP53INP1 was demonstrated to be a
direct target of miR-19a/b. Our findings provide mechanistic
insight into the function of miR-19a/b and provide evidence
regarding the molecular mechanism involved in the development of
pancreatic cancer, which may be useful in the identification of new
therapeutic targets for pancreatic cancer.
Materials and methods
Clinical human pancreatic cancer
specimens and cell lines
Sixteen paired human pancreatic cancer and adjacent
pancreatic tissue were confirmed and examined to detect miR-19a/b,
TP53 and TP53INP1 expression levels. RNA was isolated from the
tissue samples according to the manufacturer's instructions.
The human PANC-1 and PC-3 pancreatic cancer cell
lines were obtained from the American Type Culture Collection
(ATCC; Manassas, VA, USA). The two cell lines were cultured in
RPMI-1640 (Sigma, St. Louis, MO, USA), supplemented with 10% (v/v)
fetal bovine serum, 1% PS (100 U/ml penicillin and 100 µg/ml
streptomycin). The cell lines were maintained in a humidified
atmosphere at 37°C with 5% CO2. Transient transfection
was performed using the Lipofectamine™ 2000 transfection reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA) according to the
manufacturer's instructions.
Antibodies, reagents and DNA
constructs
Mouse monoclonal antibody against Flag-tag and
rabbit polyclonal antibodies against TP53 and TP53INP1 were
purchased from Sigma. ASO-miR-19a/b and miR-19a/b were obtained
from Applied Biosystems (Carlsbad, CA, USA).
pcMV6/TP53 was constructed by using standard
techniques. pcMV6 was digested with XhoI and KpnI.
Two strands were annealed to clone a fragment of the TP53 protein
with XhoI and KpnI sites. This construct was inserted
into the pcMV6 vector. The two strands were then annealed to clone
a fragment of the TP53INP1 3′UTR containing the target site of
miR-19a/b with BamHI and XhoI sites. This construct
was inserted into a BamHI-XhoI digested EGFP
luciferase reporter vector. To generate a mutant containing a
mutation in the miR-19a/b target site, two strands were annealed
and inserted into a BamHI-XhoI digested
TP53INP1-3′UTR-mut reporter vector.
In silico analysis was performed using
TargetScan software (http://www.targetscan.org/) to determine whether the
3′UTR of human TP53INP1 contained conserved putative target site
for miR-19a/b miRNAs.
Luciferase reporter assay
The pancreatic PANC-1 cancer cell lines were
co-transfected with ASO-miR-19a/b or miR-19a/b and the wild-type or
mutant 3′UTR of TP53INP1 compared with the control class in 48-well
plates. At 48 h after transfection, the Luciferase EGFP intensity
was measured with an F-4500 fluorescence spectrophotometer
(Hitachi, Chestertown, MD, USA), and EGFP luciferase activity was
normalized to that of RFP.
Reverse transcription-qPCR analysis
Total RNA was extracted by using High Pure RNA
Isolation kit (Roche) according to the protocol described by the
manufacturer. Reverse transcription-qPCR (RT-qPCR) was carried out
using the M-MLV reverse transcriptase (Invitrogen Life
Technologies). For the gene and miRNA analysis, PCR was performed
at a temperature of 58°C for 30 cycles in reaction mixture of 25
µl individually of each sample in an iQ5 real-time PCR
system (Bio-Rad Laboratories, Hercules, CA, USA). GAPDH served as
the control gene, and the human U6 RNA as a control small RNA.
Western blot analysis
Cell lysates were prepared using lysis buffer
containing 10 mM Tris-HCl (pH 7.4), 1% SDS, 1 mM
Na3VO4, 10 mM NaF and protease inhibitor
cocktail (Roche, Mannheim, Germany). Protein expression was
analyzed by western blot analysis. The separated proteins by
SDS-PAGE (8–15% gel) were transferred to PVDF membranes followed by
immunostaining with the primary antibodies at 4°C overnight.
Horseradish peroxidase-conjugated goat anti-mouse secondary
antibody was then applied at room temperature (25°C) for 2 h, and
the specific protein bands were detected using ECL (Amersham,
Piscataway, NJ, USA). After detection of the protein bands, the
blot was re-probed with anti-GAPDH antibody to confirm equal
loading of the samples. The following antibodies used were TP53 and
TP53INP1 at a dilution of 1:3,000 and 1:5,000, respectively.
Cell viability and TUNEL assay
The cell viability and proliferation of PANC-1 and
PC-3 cell lines with Si-TP53 or miR-19a/b mimics were determined by
3-(4,5-dimethylthia-zolyl-2-yl)-2-5 diphenyl tetrazolium bromide
(MTT; Sigma) assay. The cells plated in 96-well plates at
5×103/well were performed according to the
manufacturer's instructions. The cell viability was normalized to
that of cells cultured in the culture medium with Si-NC. Three
independent experiments (three replicates in each) were
performed.
A TdT-mediated dUTP-biotin nick end-labeling (TUNEL)
assay was performed in PANC-1 cells by using Roche®
TUNEL kit as per the manufacturer's instructions. TUNEL-positive
nuclei were calculated at a magnification of ×20 under an Olympus
IX73 microscope (Tokyo, Japan). The assays were performed three
times.
Statistical analysis
The data are presented as the mean ± standard
deviation (SD). Statistical significances were calculated using the
Student's t-test. P≤0.05 was considered to indicate a statistically
significant result.
Results
TP53 regulates miR-19a/b and TP53INP1
expression in pancreatic cancer cells
TP53 is a tumor-suppressor gene and has a
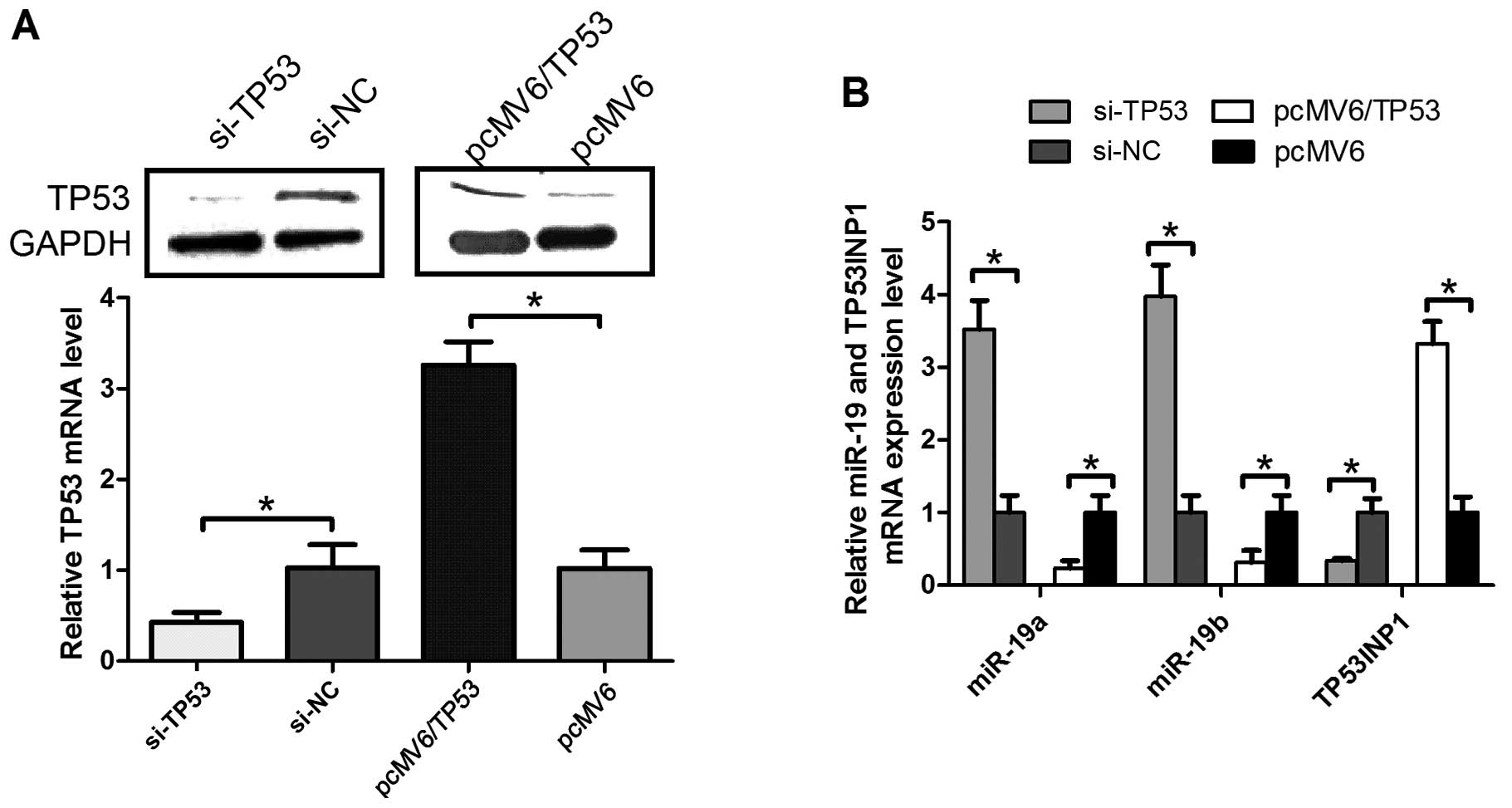
fundamental role in pancreatic cancer cell apoptosis (17). We constructed the pcMV6/TP53 vector
or obtained SiTP53 and infected them into the pancreatic cancer
cell lines. The results verified that the expression of TP53
protein (upper panel) or mRNA (lower panel) was lower in the cell
lines transfected with Si-TP53, compared with Si-NC (Fig. 1A). In addition, TP53 expression was
higher in the cell lines transfected with pcMV6/TP53, compared to
pcMV6 (Fig. 1A).
To determine whether TP53 proteins are involved in
the regulation of miR-19 cluster expression in pancreatic
carcinoma, RT-qPCR was employed to measure the miR-19a/b and
TP53INP1 level in PANC-1 cell lines, transfected with SiTP53 or
pcMV6/TP53 compared with Si-NC or pcMV6, respectively. Notably, the
expression of TP53 in the pancreatic cancer cell lines did not only
conversely correlate with the miR-19a/b level, but also correlated
with TP53INP1 mRNA (P<0.05). The association between TP53 and
miR-19a/b or TP53INP1 identified that TP53 regulates miR-19a/b and
TP53INP1 expression in pancreatic cancer cells.
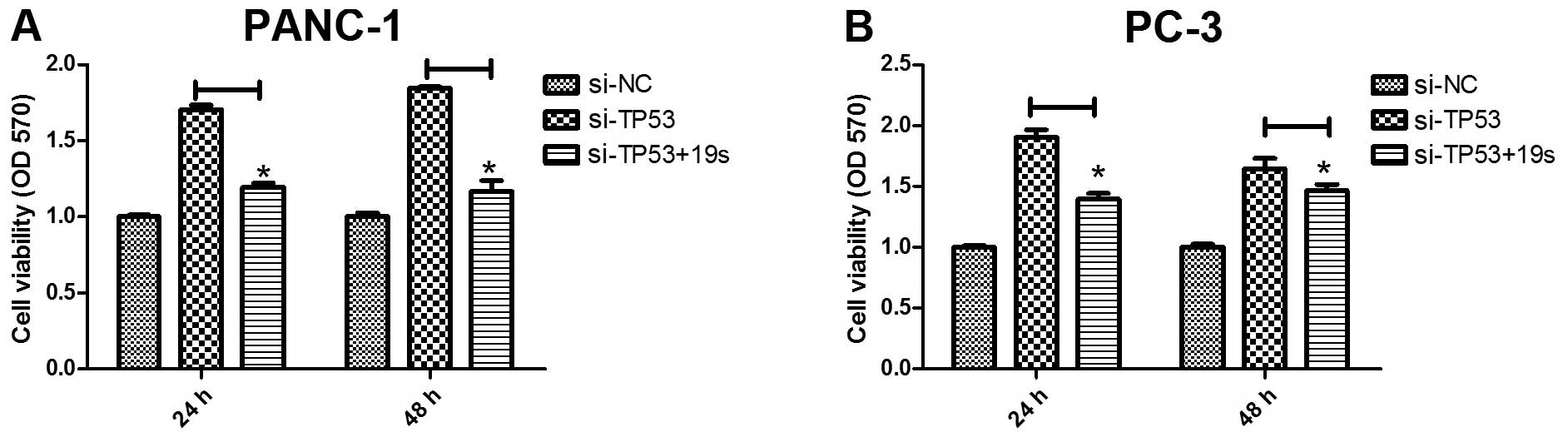
Effects of TP53 on cell proliferation in
pancreatic cancer are partially restored by miR-19a/b
miR-19a/b was previously regarded as an oncogene and
detected to regulate pancreatic development (16). Thus, we hypothesized that the
overexpression of miR-19a/b may partially restore TP53 function in
pancreatic cancer cell proliferation. To assess the cell viability
of miR-19a/b, we transfected Si-NC, Si-TP53, and miR-19s into the
PANC-1 and PC-3 pancreatic cancer cell lines and detected
absorbances at 570 nm using a microplate reader. As shown in
Fig. 2A, Si-TP53 transfection in
the PANC-1 pancreatic cancer cells induced cell proliferation,
compared to the Si-NC group (P<0.05). However, miR-19a/b
expression partially restored TP53 function in Si-TP53-transfected
cells compared to the Si-TP53 group (P<0.05) (Fig. 2A). Similar results were observed in
PC-3as pancreatic cancer cells (Fig.
2B). These results suggested that miR-19a/b partly restored the
role of TP53 protein in pancreatic cancer cell proliferation.
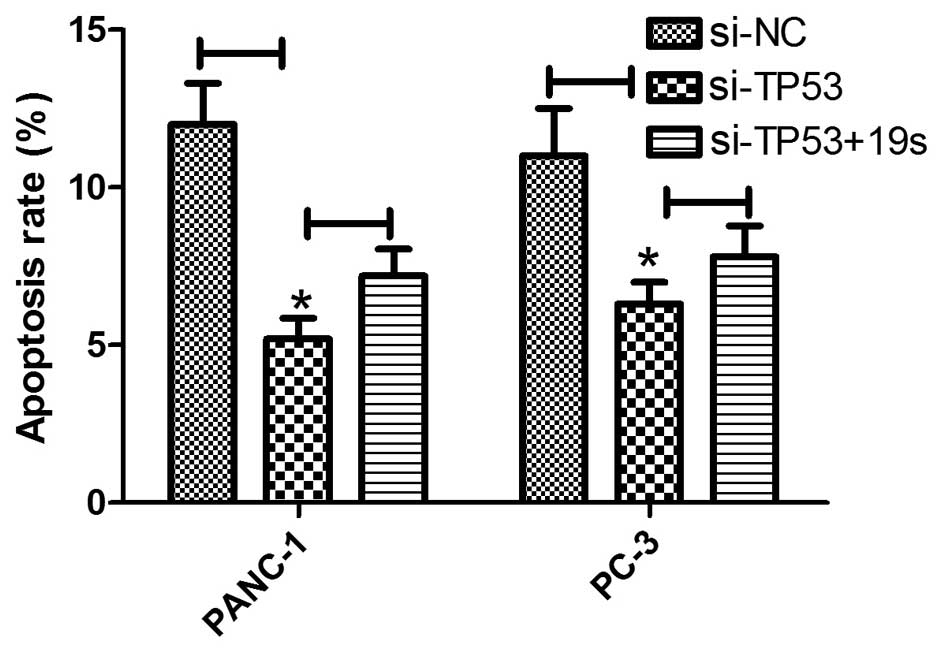
TP53-induced cell apoptosis is partly
restored by miR-19a/b
To analyze the miR-19a/b function in pancreatic
cancer cell development, Si-NC-, Si-TP53, and miR-19s were
transfected into PANC-1 and PC-3 cell lines and a TUNEL assay was
employed to detect the role of miR-19a/b in pancreatic cancer. The
number of TUNEL-positive cells in PANC-1 transfected with Si-TP53
were significantly elevated in the Si-NC-transfected groups.
However, miR-19a/b expression partially restored TP53 function in
Si-TP53-transfected cells compared to the Si-TP53 group (Fig. 3, left panel) (P<0.05). Similar
results were observed in pancreatic cancer cells PC-3 as indicated
in Fig. 3, right panel.
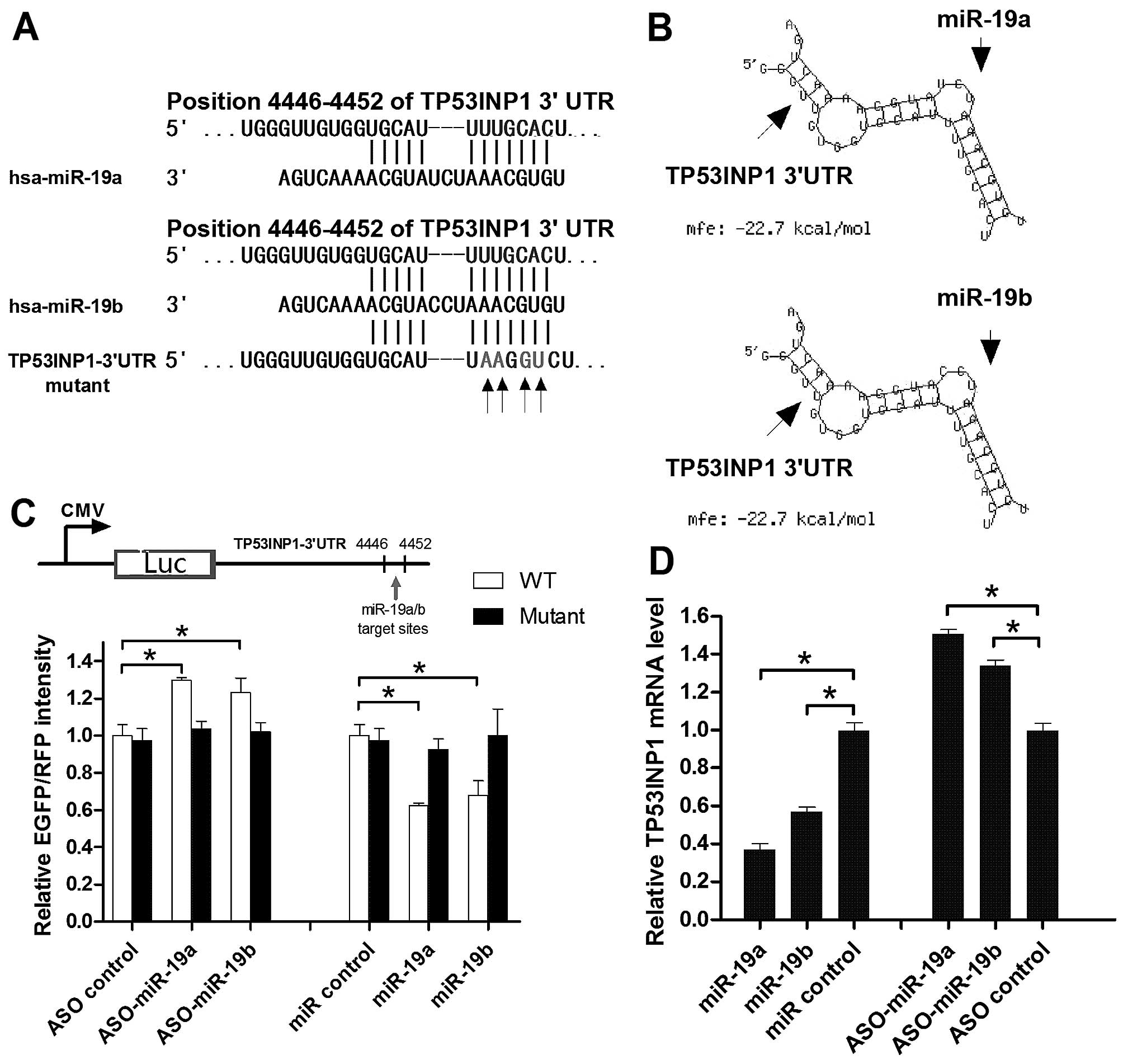
miR-19a/b directly targets TP53INP1
In silico analysis using TargetScan software
(http://www.targetscan.org/) showed that
the 3′UTR of human TP53INP1 contains a conserved putative target
site for miR-19a/b miRNAs (Fig.
4A). In the present study, miRNA identification and analysis
were developed through a primary tool, RNAhybrid (18), in order to identify the available
targets according to the secondary structure feature and minimum
free energy (MFE) between the miRNAs and target gene. To validate
these target sites, the 3′UTR of human TP53INP1 (Homo
sapiens, 4446–4452 bp) was amplified and inserted in both
orientations downstream of the EGFP luciferase gene in the
pcDNA3-control vector, generating sense (S) and antisense (AS)
constructs, collectively referred to as pcDNA3 (Fig. 4C, upper panel). One mutant clone
(mutant) was prepared in a similar manner. We also obtained the
ASO-miR-19a/b to inhibit the endogenous level in the pancreatic
cancer cell lines. The transfection of PANC-1 cells with
ASO-miR-19a or ASO-miR-19b significantly increased the expression
of pcDNA3/EGFP-TP53INP1 compared to the mutant, while with miR-19a
or miR-19b a decreased expression of pcDNA3/EGFP-TP53INP1 was
observed (Fig. 4C, lower panel).
Furthermore, miR-19a and miR-19b targeted TP53INP1 mRNA (Fig. 4D).
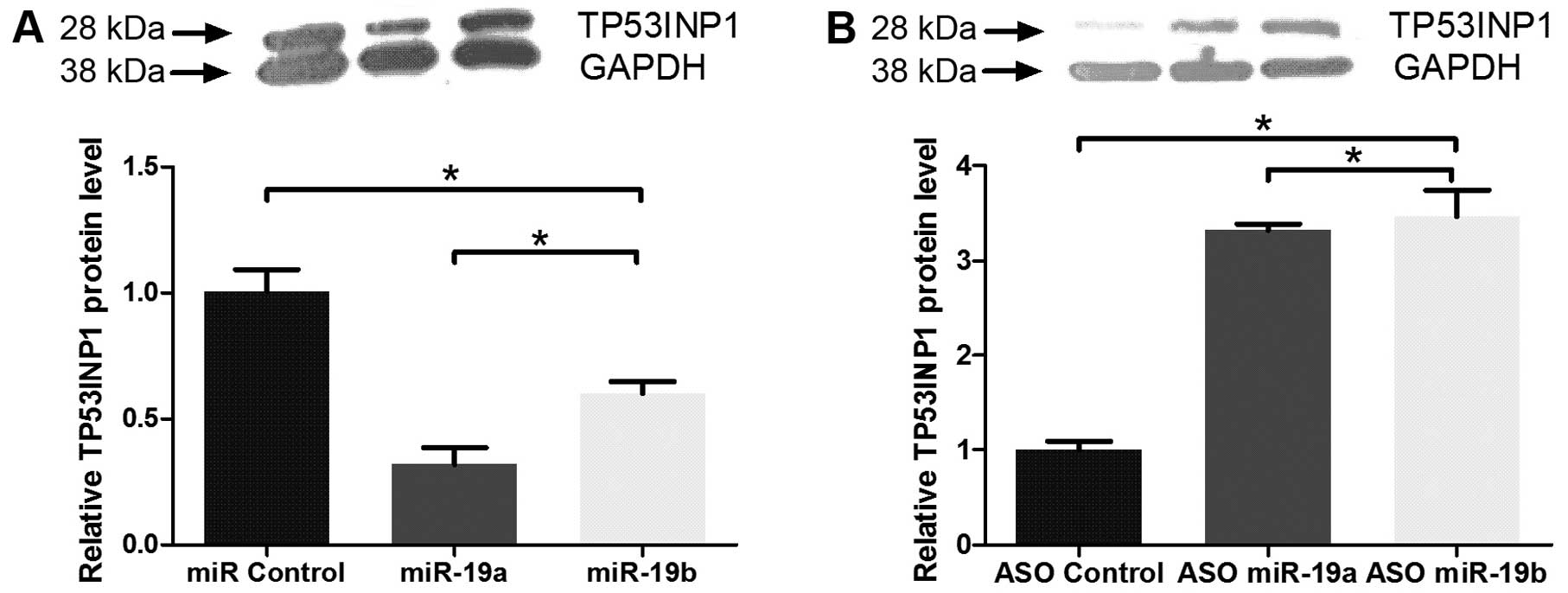
miR-19a/b represses TP53INP1 level in PC
cells
To determine the downregulation effects of miR-19a/b
at the TP53 protein level, we performed western blot assays using
an anti-TP53IP1 antibody. As expected, the transient transfection
of the PANC-1 pancreatic cancer cell with miR-19a or miR-19b
decreased TP53INP1 levels, compared to the miR control (Fig. 5A). However, cells transiently
transfected with ASO-miR-19a or ASO-miR-19b showed increased
TP53INP1 levels (Fig. 5B). These
results suggested that miR-19a/b miRNAs regulate MXD1 expression
in vivo at the post-transcriptional level.
Different expression of miR-19a/b in
pancreatic cancer tissues is conversely correlated with the TP53
and TP53INP1 protein
To examine the relationship between miR-19a/b and
TP53INP1, 16 paired human pancreatic cancer tissues and adjacent
pancreatic tissues were confirmed by pathological analysis and the
endogenous miR-19a/b and TP53INP1 level was measured using RT-qPCR
assay. As shown in Fig. 5A and B,
in human tissues, the expression levels of miR-19a and miR-19b were
conversely correlated with the TP53INP1 protein. To determine
whether miR-19a and miR-19b levels were regulated by TP53, we
performed RT-qPCR on 16 paired human pancreatic cancer tissues and
adjacent pancreatic tissues. miR-19a and miR-19b expression levels
were conversely correlated with the TP53 protein. These results
demonstrated that although miR-19a/b is capable of downregulating
TP53INP1 expression by binding its 3′UTR, the overexpression of
TP53 is also able to reduce miR-19a/b expression levels, indicating
a potential miRNA target gene regulatory loop between miR-19a/b
TP53, and TP53INP1.
Discussion
Although miR-19a/b has been reported to have a broad
and significant roles in many types of cancer cells (16,19,20),
the exact function and the potential mechanisms in pancreatic
cancer remain to be investigated. The present study identified the
direct targets and the underlying functions of miR-19a/b by using
bioinformatics tools and gene manipulations using pancreatic cancer
cell. In summary, our novel findings are: i) overexpression of
miR-19a/b partly rescued TP53-induced cell apoptosis to promote
cell proliferation; ii) the luciferase reporter assays, RT-qPCR and
western blot analysis showed that miR-19a/b directly downregulated
TP53INP1 at the mRNA and protein levels; iii) and thus, the
miR-19a/b family exerts important functions in pancreatic cancer
development by regulating endogenous TP53INP3 protein and is
regulated by TP53 expression in vivo.
The role of the tumor-suppressor gene, TP53 has been
investigated in considerable depth in previous studies (21–23).
In the present study, we observed that miR-19a/b expression was
regulated by endogenous TP53 protein in most of the randomly
selected pancreatic cancer samples and the overexpression of
miR-19a and miR-19b partly rescued the TP53-mediated suppression of
the development of pancreatic cancer. Furthermore, MTT assays were
performed to examine the relationships between miR-19a/b miRNAs and
cell viability in pancreatic cancer using transient
miR-19a/b-expressing cells. Moreover, an anti-apoptotic function
was observed in the present study using TUNEL assays, which was
consistent with previous studies showing that miR-19a/b acts as an
oncomiR in pancreatic cancers.
TP53INP1 is a key tumor suppressor that modulates
p53 to induce tumor cell death (24–26).
We showed in a present study that miR-19a/b directly targeted
TP53INP1 3′UTR and regulated the TP53INP1 mRNA and protein level in
pancreatic cancer cells. Moreover, pancreatic cancer cells
transiently transfected with si-TP53 triggered miR-19s expression,
and that reduced endogenous TP53INP1 gene expression enhanced cell
viability to inhibit cell apoptosis. However, previous findings
have shown that TP53 directly binds to the TP53INP1 promoter region
and triggers TP53INP1 expression in various cancer types (27,28).
The patient tissues showed that miR-19a/b level in pancreatic
cancer tissues was conversely correlated with the expression of
TP53 and TP53INP1. The results of the present study suggested that
TP53INP1 regulated by miR-19s is one of the molecular mechanisms
driving p53 to induce cell apoptosis.
In conclusion, the results suggest that
overexpression of the miR-19a/b family partially restored cell
apoptosis induced by the endogenous TP53 protein. Therefore,
miR-19a/b improved cell proliferation through the repression of
novel target gene TP53INP1. The present findings indicate that the
positive regulatory network between TP53, miR-19a/b and TP53INP1
may be important in inhibiting the expression levels of oncogenic
miRNAs and may provide new insights into the mechanism involved in
the development of pancreatic cancer.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tempero MA, Arnoletti JP, Behrman SW,
Ben-Josef E, Benson AB III, Casper ES, Cohen SJ, Czito B, Ellenhorn
JD, Hawkins WG, et al National Comprehensive Cancer Networks:
Pancreatic Adenocarcinoma, version 2.2012: Featured updates to the
NCCN Guidelines. J Natl Compr Canc Netw. 10:703–713.
2012.PubMed/NCBI
|
|
3
|
Hidalgo M: Pancreatic cancer. N Engl J
Med. 362:1605–1617. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Burki TK: Whole-genome analysis of
pancreatic cancer. Lancet Oncol. 16:e1612015.
|
|
5
|
Levine AJ, Finlay CA and Hinds PW: P53 is
a tumor suppressor gene. Cell. 116:S67–S69, 61 p following S69.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Whibley C, Pharoah PD and Hollstein M: p53
polymorphisms: Cancer implications. Nat Rev Cancer. 9:95–107. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Petitjean A, Achatz MI, Borresen-Dale AL,
Hainaut P and Olivier M: TP53 mutations in human cancers:
Functional selection and impact on cancer prognosis and outcomes.
Oncogene. 26:2157–2165. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tomasini R, Samir AA, Vaccaro MI, Pebusque
MJ, Dagorn JC, Iovanna JL and Dusetti NJ: Molecular and functional
characterization of the stress-induced protein (SIP) gene and its
two transcripts generated by alternative splicing. SIP induced by
stress and promotes cell death. J Biol Chem. 276:44185–44192. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
N'guessan P, Pouyet L, Gosset G, Hamlaoui
S, Seillier M, Cano CE, Seux M, Stocker P, Culcasi M, Iovanna JL,
et al: Absence of tumor suppressor tumor protein 53-induced nuclear
protein 1 (TP53INP1) sensitizes mouse thymocytes and embryonic
fibroblasts to redox-driven apoptosis. Antioxid Redox Signal.
15:1639–1653. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cano CE, Gommeaux J, Pietri S, Culcasi M,
Garcia S, Seux M, Barelier S, Vasseur S, Spoto RP, Pébusque MJ, et
al: Tumor protein 53-induced nuclear protein 1 is a major mediator
of p53 antioxidant function. Cancer Res. 69:219–226. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gommeaux J, Cano C, Garcia S, Gironella M,
Pietri S, Culcasi M, Pébusque MJ, Malissen B, Dusetti N, Iovanna J,
et al: Colitis and colitis-associated cancer are exacerbated in
mice deficient for tumor protein 53-induced nuclear protein 1. Mol
Cell Biol. 27:2215–2228. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Croce CM: Causes and consequences of
microRNA dysregulation in cancer. Nat Rev Genet. 10:704–714. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Morimura R, Komatsu S, Ichikawa D,
Takeshita H, Tsujiura M, Nagata H, Konishi H, Shiozaki A, Ikoma H,
Okamoto K, et al: Novel diagnostic value of circulating miR-18a in
plasma of patients with pancreatic cancer. Br J Cancer.
105:1733–1740. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yan HL, Xue G, Mei Q, Wang YZ, Ding FX,
Liu MF, Lu MH, Tang Y, Yu HY and Sun SH: Repression of the
miR-17-92 cluster by p53 has an important function in
hypoxia-induced apoptosis. EMBO J. 28:2719–2732. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu Q, Yang Z, An Y, Hu H, Yin J, Zhang P,
Nie Y, Wu K, Shi Y and Fan D: MiR-19a/b modulate the metastasis of
gastric cancer cells by targeting the tumour suppressor MXD1. Cell
Death Dis. 5:e11442014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Naccarati A, Pardini B, Polakova V,
Smerhovsky Z, Vodickova L, Soucek P, Vrana D, Holcatova I, Ryska M
and Vodicka P: Genotype and haplotype analysis of TP53 gene and the
risk of pancreatic cancer: An association study in the Czech
Republic. Carcinogenesis. 31:666–670. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Krüger J and Rehmsmeier M: RNAhybrid:
microRNA target prediction easy, fast and flexible. Nucleic Acids
Res. 34(Web Server): W451–W454. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kurokawa K, Tanahashi T, Iima T, Yamamoto
Y, Akaike Y, Nishida K, Masuda K, Kuwano Y, Murakami Y, Fukushima
M, et al: Role of miR-19b and its target mRNAs in 5-fluorouracil
resistance in colon cancer cells. J Gastroenterol. 47:883–895.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang J, Xiao Z, Lai D, Sun J, He C, Chu
Z, Ye H, Chen S and Wang J: miR-21, miR-17 and miR-19a induced by
phosphatase of regenerating liver-3 promote the proliferation and
metastasis of colon cancer. Br J Cancer. 107:352–359. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Harris CC and Hollstein M: Clinical
implications of the p53 tumor-suppressor gene. N Engl J Med.
329:1318–1327. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Green DR and Kroemer G: Cytoplasmic
functions of the tumour suppressor p53. Nature. 458:1127–1130.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Venkatanarayan A, Raulji P, Norton W,
Chakravarti D, Coarfa C, Su X, Sandur SK, Ramirez MS, Lee J,
Kingsley CV, et al: IAPP-driven metabolic reprogramming induces
regression of p53-deficient tumours in vivo. Nature. 517:626–630.
2015. View Article : Google Scholar :
|
|
24
|
Seillier M, Peuget S, Gayet O, Gauthier C,
N'Guessan P, Monte M, Carrier A, Iovanna JL and Dusetti NJ:
TP53INP1, a tumor suppressor, interacts with LC3 and ATG8-family
proteins through the LC3-interacting region (LIR) and promotes
autophagy-dependent cell death. Cell Death Differ. 19:1525–1535.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Peuget S, Bonacci T, Soubeyran P, Iovanna
J and Dusetti NJ: Oxidative stress-induced p53 activity is enhanced
by a redox-sensitive TP53INP1 SUMOylation. Cell Death Differ.
21:1107–1118. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tomasini R, Seux M, Nowak J, Bontemps C,
Carrier A, Dagorn JC, Pébusque MJ, Iovanna JL and Dusetti NJ:
TP53INP1 is a novel p73 target gene that induces cell cycle arrest
and cell death by modulating p73 transcriptional activity.
Oncogene. 24:8093–8104. 2005.PubMed/NCBI
|
|
27
|
Okamura S, Arakawa H, Tanaka T, Nakanishi
H, Ng CC, Taya Y, Monden M and Nakamura Y: p53DINP1, a
p53-inducible gene, regulates p53-dependent apoptosis. Mol Cell.
8:85–94. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tomasini R, Samir AA, Pebusque MJ, Calvo
EL, Totaro S, Dagorn JC, Dusetti NJ and Iovanna JL: P53-dependent
expression of the stress-induced protein (SIP). Eur J Cell Biol.
81:294–301. 2002. View Article : Google Scholar : PubMed/NCBI
|