Introduction
The p53 tumor suppressor is a central 'cellular
gatekeeper', also referred to as 'the guardian of the genome',
which is critically implicated in monitoring diverse cellular
processes including cell cycle arrest, DNA repair, senescence,
apoptosis, differentiation, cell metabolism and autophagy under
various cellular stresses (1). p53
as a transcription factor can bind to the promoter regions of
hundreds of target genes and regulate their transcription to elicit
its functions (2). The
well-described p53 target genes include P21 involved in cell cycle
arrest, Bax, Puma and Noxa involved in apoptosis, Nanog involved in
differentiation, and PAI-1 involved in senescence (2). The p53 gene is commonly mutated in 50%
of all human cancers (3). Somatic
p53 missense mutations discrupt the p53 binding to DNA response
elements leading to the repression of target gene transcription.
Loss of wild-type p53 function is crucial for human cancer
progression. In addition to the loss-of-function or
dominant-negative effect, many mutant p53 proteins acquire
tumor-promoting functions via multiple mechanisms (4,5).
Moreover, many cancers expressing mutant p53 increases resistance
to chemotherapy and radiotherapy (6). Even in human cancers that bear
wild-type p53, the p53 upstream or downsteam regulatory pathways as
well as post-translational modifications are disrupted, suggesting
that essentially all cancers have alterations in the p53 pathway
(7). Based upon powerful
tumor-suppressive effects of p53, restoration of wild-type p53
function is thus an attractive therapeutic approach for the
treatment of human cancers. The strategies to restore wild-type p53
function consist of wild-type p53 gene therapy, activation of
endogenous wild-type p53 using SIRT1/2 inhibitor tenovins, nuclear
export inhibitor Crm1 and MDM2 inhibitors, reactivation of mutant
p53 using peptides or compounds, and activating other p53 family
member p73 (8). Among them,
adenovirus-based p53 cancer gene therapy such as Advexin and
Gendicine has been tested in clinical trials.
The inhibitor of growth 4 (ING4) is a novel tumor
suppressor belonging to type II tumor suppressor family including
ING1-ING5, the five known members (9). ING4 has been found to be commonly
deleted, mutated or downregulated in a large variety of cancers,
which contributes to tumorigenesis, cancer development and
prognosis (9). Accumulating
evidence has shown that ING4 can induce tumor growth suppression
via induction of cell cycle alteration, apoptosis and toxic
autophagy in a p53-dependent or -independent fashion in a broad
spectrum of cancer cells (10–15).
Notably, ING4 enhances the sensitivity of chemotherapy,
intracavitary radiotherapy or external beam radiotherapy in
heptocarcinoma (11,16), pancreatic cancer (17) and lung cancer (18). ING4 also suppresses oncogenes such
as myelocytomatosis viral related oncogene, neuroblastoma derived
(MYCN)- and myelocytomatosis viral oncogene homolog (MYC)-induced
loss of contact inhibition (19).
Furthermore, ING4 represses cancer spreading, migration and
invasion by interacting with liprin α1 at lamellipodia (20) and downregulating matrix
metalloproteinase (MMP)-2 and MMP-9 (13,21).
Additionally, ING4 downregulates the expression of IL-6 and IL-8
pro-angiogenic factors and consequently suppresses tumor
angiogenesis via repressing transcription activity of nuclear
factor-κB (NF-κB) (22,23) and hypoxia inducible factor-1α
(HIF-1α) (24,25) in a chromatin-remolding manner.
Interestingly, ING4 can act as a novel E3 ubiquitin ligase that
directly induces NF-κB p65 degradation and consequently terminates
NF-κB activation (26). More
recently, ING4 has been shown to interact with AUF1 and reduce
pro-oncogene c-myc translational expression (27). These findings revealed that ING4 as
a promising candidate tumor suppressor negatively regulating cancer
growth via targeting multiple pathways.
Breast cancer is the most frequently diagnosed
malignancy among women and the first leading cause of
cancer-related death in female worldwide, accounting for 23% of the
total new cases and 14% of the cancer deaths (28). Conventional therapies for breast
cancer such as surgery, chemotherapy, radiation therapy, hormone
therapy and targeted therapy have demonstrated a considerable
clinical success over the past few years. However, de novo
and acquired resistance is one of the major obstacles in treatment
of breast cancer, warranting the search for new therapeutic
alternatives for breast cancer. Gene therapy is considered as a
promising therapeutic modality for human cancers (29). Combined gene therapy may be an
effective practice in cancer gene therapy, which can achieve
greater therapeutic benefit by targeting multiple pathways
(29,30). Previous studies showed that tumor
suppressor p53 acetylation is indispensable for p53 activation
(31). Previous studies also showed
that tumor suppressor ING4 can physically interact with p300 and
p53, thereby enhancing p53 acetylation and its transcriptional
activity (10). In the present
study, we thus investigated whether adenovirus-mediated p53 and
ING4 combined gene therapy would produce enhanced antitumor effects
in MDA-MB-231 (mutant p53) human breast cancer in vitro and
in vivo in an athymic nude mouse model, and also elucidated
its molecular mechanisms.
Materials and methods
Vectors, cell lines, reagents and
mice
The polyA+hEF1a-eIF4g promoter expression
cassette-modified adenoviral transfer plasmid
pAdTrack-CMV/polyA+hEF1a-eIF4g with three independent promoters
including two cytomegalovirus (CMV) promoters driving the
interesting gene (1st CMV) and green fluorescent protein (GFP)
maker gene (2nd CMV) expression, respectively; and one inserted
hEF1a-eIF4g hybrid promoter driving the other interesting gene
expression were constructed in our laboratory (32). The pAdTrack-CMV/ING4 carrying
humanized ING4 coding sequence was also constructed in our
laboratory (13). The human
wild-type p53 open reading frame (ORF) clone was purchased form
Sino Biological Inc. (Beijing, China). The integrin-binding motif
Arg-Gly-Asp (RGD)-modified pAdEasy-1 (Ad5E1/E3-deleted) adenoviral
backbone plasmid (33) was kindly
provided by Professor Jim Xiang (Saskatoon Cancer Agency,
Saskatoon, SK, Canada). The BJ5183 bacteria and QBI-293A human
embryonic kidney cell line for adenovirus packaging and
amplification were kindly provided by Professor Jiang Zhong
(Department of Microbiology, College of Life Science, Fudan
University, Shanghai, China). The MDA-MB-231 (mutant p53, R280K)
(4) human breast cancer cell line
was purchased from the Cell Bank, Type Culture Collection of
Chinese Academy of Sciences (Shanghai, China). The Dulbecco's
modified Eagle's medium (DMEM) and fetal bovine serum (FBS) were
purchased from Hyclone (Logan, UT, USA). The MiniBEST Universal RNA
extraction kit was purchased from Takara (Dalian, Liaoning, China).
The reverse transcriptase polymerase MuMLV and OligodT18 were
purchased from Thermo Fisher Scientific (Shanghai, China). The
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
and mammalian cell lysis kit were purchased from Sigma (Shanghai,
China). The Annexin V-PE/7-AAD apoptosis detection kit was
purchased from BD Biosciences (Shanghai, China). The antibodies
specific for ING4, p53, Ac-p53 (K382), P21, Bax, PUMA, Noxa, Bcl-2,
caspase-9, caspase-3, poly(ADP-ribose) polymerase (PARP), β-actin
and CD31 were purchased from Santa Cruz Biotechnology (Shanghai,
China) and Cell Signaling Technology (Danvers, MA, USA). The
SuperEnhanced chemiluminescence detection kit was purchased from
Applygen Technology Inc. (Beijing, China). The terminal
deoxynucleotidyl transferase-mediated dUTP nick end labeling
(TUNEL) apoptosis detection kit was purchased from Beyotime
Biotechnology (Beijing, China). The UltraSensitive™ SP kit was
purchased from Maixin (Fuzhou, Fujian, China). The primers were
synthesized from Sangon Biotechnology Inc. (Shanghai, China). The
4-week-old female athymic BALB/c nude mice were purchased from
Shanghai Experimental Animal Center (Shanghai, China) and
maintained in the animal facility at Soochow University (Suzhou,
China) according to the Animal Research Committee guidelines of
Soochow University.
Construction of an adenovirus
co-expressing ING4 and p53 tumor suppressor genes
We previously generated two kinds of recombinant
adenovirus co-expressing ING4 and interleukin (IL)-24 using
polyA+hEF1a-eIF4g promoter expression cassette and internal
ribosome entry site (IRES) element, respectively (32). We demonstrated that inserted
hEF1a-eIF4g promoter more efficiently directed the translational
expression of downstream gene IL-24 than IRES element (32). In the study, we thus constructed an
adenovirus co-expressing ING4 and p53 double tumor suppressor genes
using polyA+hEF1a-eIF4g promoter expression cassette modality
(Fig. 1A). Briefly, the ING4 and
p53 coding sequence (CDS) fragments were amplified by PCR using
pAdTrack-CMV/ING4 plasmid (13) or
p53 ORF clone plasmid as a template and primers specific for human
ING4 (ING4 forward, 5′-tag aga tct acc atg gct gct ggg atg tat ttg
g-3′ and reverse, 5′-acc gtc gac cct att tct tct tcc gtt ctt g-3′)
or p53 (p53 forward, 5′-gca ctc gag acc atg gag gag ccg cag tca gat
c-3′ and reverse, 5′-gct tct aga tca gtc tga gtc agg ccc ttc-3′) as
primers, and then subcloned into pAdTrack-CMV/polyA+hEF1a-eIF4g
modified transfer plasmid (32) at
BglII, SalI; XhoI, XbaI sites,
respectively. To develop a more efficient gene delivery system, we
used Arg-Gly-Asp (RGD)-modified pAdEasy-1 (Ad5E1/E3-deleted) that
contains an integrin-binding motif RGD sequence in the HI loop of
adenoviral fiber as an adenoviral backbone plasmid (33). After its homologous recombination
with RGD-modified pAdEasy-1 in BJ5183 bacteria, the
replication-incompetent and RGD-modified adenovirus AdVING4/p53
expressing GFP marker gene was subsequently produced and abundantly
amplified in QBI-293A cells as described previously (34), and then purified by cesium chloride
(CsCl) density-gradient ultracentrifugation. The adenoviruses such
as AdVING4, AdVp53, AdV used for controls were also similarly
prepared.
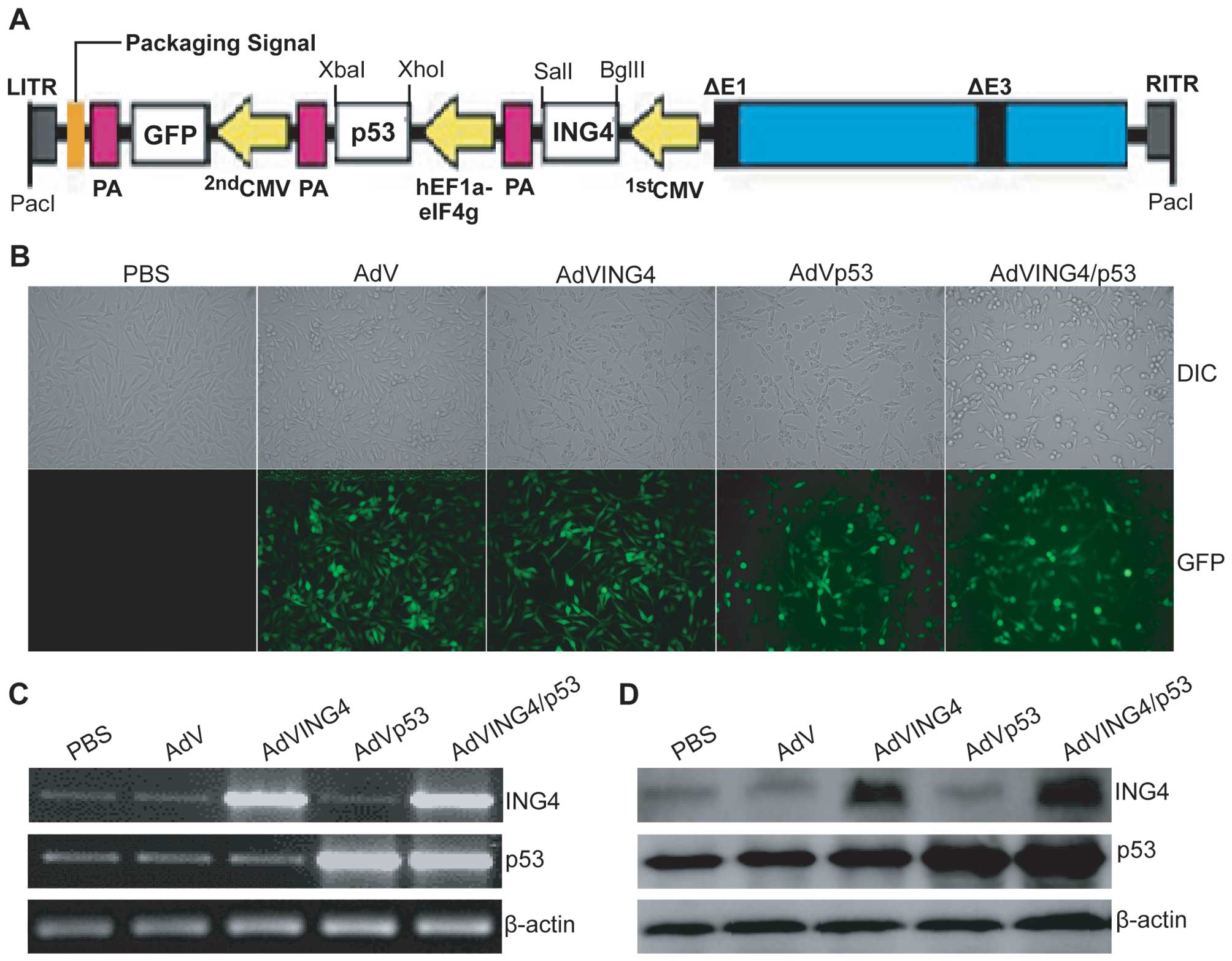 | Figure 1Adenovirus-mediated ING4 and p53
co-expression in breast cancer. (A) The construction strategy of an
adenovirus co-expressing ING4 and p53 (AdVING4/p53). The ING4 and
p53 CDS fragments were subcloned into
pAdTrack-CMV/polyA+hEF1a-eIF4g modified adenoviral transfer plasmid
at BglII, SalI; XhoI, XbaI sites, where
ING4 transcription is under control of CMV (1st CMV) promoter,
while p53 transcription is under control of hEF1a-eIF4g promoter.
The AdVING4/p53 adenovirus was then generated by its homologous
recombination with a RGD-modified pAdEasy-1 adenoviral backbone
plasmid in BJ5183 bacteria followed by packaging and amplification
in QBI-293A cells. CMV, cytomegalovirus promoter; hEF1a-eIF4g,
hybrid promoter coupled 5′-untranslated region (UTR) of the
translation initiation factor eIF4g to elongation factor 1α (EF1α)
promoter; GFP, green fluorescent protein; PA, SV40 polyadenylation
signal; LITR, left-hand inverted terminal repeat; RITR, right-hand
inverted terminal repeat. (B) Representative microphotos of
differential interference contrast (DIC) and GFP. The MDA-MB-231
human breast cancer cells were infected with AdVING4/p53, AdVING4,
AdVp53 or AdV at a MOI of 50 for 24 h, and then observed under DIC
and GFP fluorescence images by fluorescence microscopy. (C) RT-PCR
analysis of adenovirus-mediated ING4 and p53 transcriptional
expression. Total cellular RNAs were extracted from AdVING4/p53-,
AdVING4-, AdVp53- or AdV-infected and uninfected MDA-MB-231 human
breast cancer cells. The first-strand cDNAs were synthesized from
RNAs using reverse transcriptase; PCRs were conducted using primer
sets specific for human ING4, p53 and housekeep gene β-actin (an
internal control), respectively. (D) Western blot analysis of
adenovirus-mediated ING4 and p53 translational expression. Total
cellular lysates derived from AdVING4/p53-, AdVING4-, AdVp53- or
AdV-infected and uninfected MDA-MB-231 tumor cells were
immunoblotted with anti-ING4, p53 and β-actin antibody,
respectively. Data shown are representative of three independent
experiments. |
Fluorescence microscopic analysis
The titer of AdVING4/p53, AdVING4, AdVp53 and AdV
adenoviruses was determined using gene transfer unit (GTU) method
by calculating the number of GFP-expressing QBI-293A cells within
18 h after adenoviral infection under fluorescence microscopy.
Accordingly, the ratio of infectious adenovirus (GTU) to target
cells is called multiplicity of infection (MOI). To select an
optimal MOI for a maximal adenoviral infection and human ING4/p53
transgene expression in MDA-MB-231 tumor cells, the MDA-MB-231
human breast cancer cells (1×104 cells) were treated
with AdVING4/p53, AdVING4, AdVp53, AdV or PBS without adenovirus at
various MOIs (0, 10, 25, 50, 100 or 200). Twenty-four hours after
infection, the GFP expression and adenoviral infection efficiency
was observed by fluorescence microscopy.
Reverse transcription (RT)-PCR
analysis
The adenovirus-mediated human ING4/p53
transcriptional expression was analyzed by reverse transcription
(RT)-PCR. The MDA-MB-231 human breast cancer cells
(2×106 cells) were treated with AdVING4/p53 (50 MOI),
AdVING4 (50 MOI), AdVp53 (50 MOI), AdV (50 MOI) or PBS without
adenovirus for 24 h. Total cellular RNAs were purified from
AdVING4/p53-, AdVING4-, AdVp53-, AdV-infected MDA-MB-231 tumor
cells or uninfected control cells and the first-strand cDNAs were
synthesized from RNAs using reverse transcriptase MuMLV. The PCR
reactions were then carried out using cDNA as a template and
primers specific for human ING4 and p53 described above, or
housekeep gene β-actin (β-actin forward, 5′-tgc gtg aca tta agg aga
ag-3′ and reverse, 5′-ctg cat cct gtc ggc aat g-3′) as primers. The
reaction products were analyzed on 1% agarose gel electrophoresis
with ethidium bromide staining.
Cell viability assay
The effect of AdVING4/p53 on the MDA-MB-231 human
breast cancer cell growth in vitro was assessed by MTT
assay. Briefly, the MDA-MB-231 tumor cells were dispensed into
96-well plates at 1×104 cells/well/200 µl culture
medium, i.e. DMEM supplemented with 10% FBS. After 24 h of
incubation, the MDA-MB-231 tumor cells were infected with
AdVING4/p53, AdVING4, AdVp53 or AdV at the optimal MOI of 50 in
culture medium and treated for the indicated time periods. The
medium containing PBS without adenovirus was used as a cell control
(PBS control). Before infection (day 0) and at days 1, 2, 3 and 4
after infection, the viability of MDA-MB-231 tumor cells was
analyzed using MTT kit according to the manufacturer's
protocols.
Flow cytometric analysis
The effect of AdVING4/p53 on MDA-MB-231 human breast
cancer cell apoptosis in vitro was evaluated by flow
cytometric analysis with Annexin V-PE (early apoptotic marker) and
7-AAD (late apoptotic marker) double staining according to the
company protocols. Briefly, the MDA-MB-231 tumor cells
(1×106 cells) were treated with AdVING4/p53 (50 MOI),
AdVING4 (50 MOI), AdVp53 (50 MOI), AdV (50 MOI) or PBS without
adenovirus in culture medium for 24 h. The uninfected and
AdVING4/p53-, AdVING4-, AdVp53- or AdV-infected MDA-MB-231 tumor
cells were harvested and washed with cold PBS. The above tumor
cells (1×105 cells) were then incubated in the presence
of 5 µl Annexin V-PE and 7-AAD in 100 µl of 1X
Annexin V binding buffer at room temperature. After 15 min
incubation, 400 µl of 1X binding buffer was added and the
apoptotic cells were analyzed by flow cytometry.
Western blot analysis
The MDA-MB-231 human breast cancer cells
(1×107 cells) were treated with AdVING4/p53 (50 MOI),
AdVING4 (50 MOI), AdVp53 (50 MOI), AdV (50 MOI) or PBS without
adenovirus. After 24 h of treatment, the AdVING4/p53-, AdVING4-,
AdVp53-, AdV-infected MDA-MB-231 tumor cells or uninfected control
cells were collected, washed with cold PBS and lysed in lysis
buffer (1×107 cells/1 ml lysis buffer) for preparation
of total cellular lysates using mammalian cell lysis kit following
the kit instructions. The protein concentration was determined by
BCA protein assay using a spectrophotometer. The total cellular
lysates (100 µg/lane) were then subjected to 12% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and
western blot analysis using a panel of primary antibodies specific
for human ING4, p53, Ac-p53 (K382), P21, Bax, PUMA, Noxa, Bcl-2,
caspase-9, caspase-3, PARP and β-actin following by corresponding
horseradish peroxidase (HRP)-conjugated secondary antibody,
respectively. After washing the membrane was developed using
SuperEnhanced chemiluminescence detection kit. The bands were
visualized after exposure of the membrane to Kodak X-ray film.
Animal study
The female athymic BALB/c nude mice were
subcutaneously (s.c.) implanted with 2×106 MDA-MB-231
human breast cancer cells and monitored daily for tumor growth.
Tumor volume was measured with a caliper and calculated by the
following formula: tumor size = ab2/2, where
a is the larger of the two dimensions and b is the
smaller. When the tumors grew to a mean tumor volume of around 100
mm3, MDA-MB-231 human breast cancer s.c. xenografted
tumor-bearing mice were subjected to gene therapy (5 mice each
group) by multi-point intratumoral (i.t.) injection of AdVING4/p53
(1×108 GTU), AdVING4 (1×108 GTU), AdVp53
(1×108 GTU), AdV (1×108 GTU) or PBS every
other day for a total of 5 times. Tumor progression was monitored
and tumor volume was measured. The tumor-bearing mice were
sacrificed 2 weeks after treatment. The xenografted tumors were
then removed, weighted, fixed by 10% neutral formalin and embedded
in paraffin for hematoxylin and eosin (H&E) staining and
immunohistochemistry analysis.
Immunohistochemistry analysis
The expression of p53, Ac-p53 (K382), P21, Bax,
PUMA, Noxa, Bcl-2, cleaved caspase-3 and CD31 in MDA-MB-231 human
breast cancer s.c. xenografted tumors was examined by
immunohistochemistry analysis using UltraSensitive™ SP kit. The
integral optical density (IOD) of immunohistochemical staining was
quantitatively assessed by Image-Pro Plus 6.0 software (Media
Cybernetics, Bethesda, MD, USA). Microvessel density (MVD) was
determined by CD31 immunostaining. Any endothelial cell cluster
immunoreactive for CD31 clearly separated from adjacent
microvessels was considered as a single countable vessel. To detect
the apoptotic cells in vivo in MDA-MB-231 xenografted
tumors, tumor sections were further analyzed for apoptosis using
TUNEL apoptosis detection kit following manufacturer's
instructions. The IOD, MVD and TUNEL-positive cells was counted at
5 randomly selected high-power (×200 magnification) fields of each
section by microscopy.
Statistical analysis and evaluation of
combinatorial interaction
All data are presented as the mean ± standard
deviation (SD) and statistically processed by one-way and two-way
repeated measures analysis of variance (ANOVA) and multiple
comparisons using SPSS 10.0 software (SPSS, Chicago, IL, USA). A
value of P<0.05 was considered statistically significant. The
interactive effect of ING4 and p53 was evaluated by Q-value
calculated by the formula (35),
Q=F(A+B)/FA+(1-FA) FB, where F(A+B) represents the fraction
affected by treatment with AdVING4/p53 compared to the untreated
control group, FA represents the fraction affected by AdVING4, and
FB represents the fraction affected by AdVp53. A value of Q>1.15
indicates a synergistic effect, Q<0.85 indicates an antagonistic
effect, and Q between 0.85 and 1.15 indicates an additive
effect.
Results
Adenovirus-mediated ING4 and p53 double
gene co-expression
To select an optimal MOI for a maximal
adenovirus-mediated ING4 and/or p53 transgene expression but a
minimal adenovirus itself-induced cytotoxic effect, the MDA-MB-231
human breast cancer cells were infected with marker gene
GFP-expressing AdVING4/p53, AdVING4, AdVp53 or AdV at different
MOIs for 24 h, then the GFP expression was examined by fluorescence
microscopy. More than 90% of GFP expression was found in
AdVING4/p53-, AdVING4-, AdVp53- or AdV-infected MDA-MB-231 tumor
cells at a MOI of 50 (Fig. 1B) or
above (data not shown), whereas GFP expression was not found in
uninfected MDA-MB-231 control tumor cells. Furthermore, there was
almost no adenovirus-elicited cytotoxicity in 50 MOI blank
adenovirus AdV-infected MDA-MB-231 tumor cells compared to control
MDA-MB-231 tumor cells (Fig. 1B).
RT-PCR (Fig. 1C) and western blot
analysis (Fig. 1D) further
demonstrated that adenovirus-mediated ING4 and/or p53 tumor
suppressor gene was abundantly expressed at both the
transcriptional and translational levels in 50 MOI AdVING4/p53-,
AdVING4-, AdVp53-infected MDA-MB-231 tumor cells but not in
AdV-infected and uninfected control cells. In addition, the
endogenous mutant p53 was also obviously detected in AdV- or
AdVING4-infected and uninfected control cells (Fig. 1C and D). These data indicated that
50 MOI can be employed as an optimal infection dose for
adenovirus-mediated ING4 and p53 gene co-transfer in MDA-MB-231
tumor cells.
Synergistic tumor suppression by
AdVING4/p53
To examine the combined effect of ING4 and p53
double tumor suppressors on breast cancer in vitro, we
performed AdVING4/p53-mediated gene co-transfer in MDA-MB-231 human
breast cancer cells and assessed the MDA-MB-231 tumor cell
viability by MTT assay. Compared with PBS and AdV control group,
adenovirus-mediated ING4 and/or p53 expression significantly
suppressed MDA-MB-231 tumor cell growth in vitro in a
time-dependent manner with a peak inhibition at day 4 after
infection (Fig. 2A, P<0.05).
Moreover, combination treatment by ING4 and p53 coexpression
resulted in a synergistic growth inhibition in MDA-MB-231 tumor
cells (P<0.05; Q=1.189, 1.158, 1.168 and 1.176 at days 1, 2, 3
and 4 after infection, respectively). To further determine whether
AdVING4/p53-mediated synergistic growth suppression observed in
vitro could be reproduced in vivo, we evaluated combined
effect of ING4 and p53 on MDA-MB-231 s.c. human breast cancer
xenografted tumor growth in athymic nude mice. As shown in Fig. 2B–D, AdVING4/p53 also synergistically
repressed MDA-MB-231 human breast cancer xenografted tumor growth
in vivo (P<0.05; Qvolume =0.898, 1.094, 1.005,
1.153, 1.163 and 1.183 at days 4, 6, 8, 10, 12 and 14 after
treatment, Qweight =1.197, respectively).
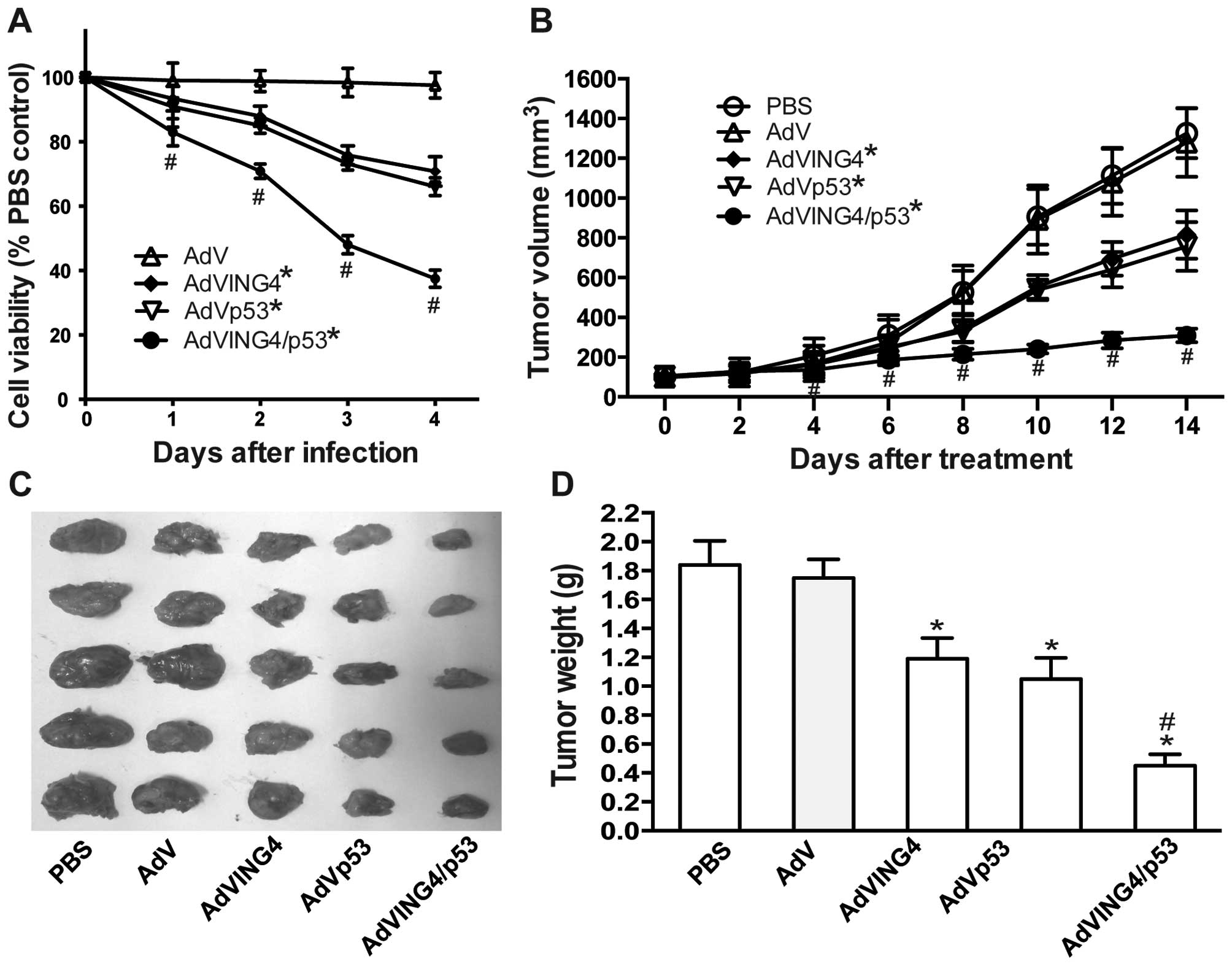 | Figure 2AdVING4/p53 induces synergistic tumor
suppression in breast cancer. (A) AdVING4/p53 synergistically
inhibits breast cancer growth in vitro. The MDA-MB-231 human
breast cancer cells were treated with AdVING4/p53 (50 MOI), AdVING4
(50 MOI), AdVp53 (50 MOI), AdV (50 MOI) or PBS without adenovirus
for the indicated time periods (0–4 days). The survival cells were
evaluated at days 0, 1, 2, 3 and 4 after treatment by MTT assay.
Tumor viability in vitro was calculated by comparison with
PBS-treated control group according to absorbance value (OD 570
nm). *P<0.05 compared with PBS and AdV group;
#P<0.05 compared with AdVING4 and AdVp53 group,
Q=1.189, 1.158, 1.168 and 1.176 at days 1, 2, 3 and 4 after
treatment, respectively, two-way repeated measures ANOVA and
multiple comparisons; n=4 replicates per condition. (B–D)
AdVING4/p53 synergistically inhibits breast cancer growth in
vivo. The athymic BALB/c nude mice bearing MDA-MB-231 s.c.
xenografted tumors were i.t. injected with AdVING4/p53
(1×108 GTU), AdVING4 (1×108 GTU), AdVp53
(1×108 GTU), AdV (1×108 GTU) or PBS every
other day for a total 5 times. The tumor volume (B) was measured
before and after treatment. The xenografted tumors were removed (C)
and tumor weight (D) was measured 2 weeks after treatment.
*P<0.05 compared with PBS and AdV group;
#P<0.05 compared with AdVING4 and AdVp53 group,
Qvolume=0.898, 1.094, 1.005, 1.153, 1.163 and 1.183 at days 4, 6,
8, 10, 12 and 14 after treatment, Qweight=1.197, two-way and
one-way repeated measures ANOVA and multiple comparisons; n=5
replicates per condition. Data shown are representative of three
independent experiments. |
Synergistic induction of apoptosis by
AdVING4/p53
To explore the cellular mechanism by which
AdVING4/p53 synergistically inhibits tumor cell growth, apoptosis
of MDA-MB-231 human breast cancer cells treated with AdVING4/p53,
AdVING4, AdVp53 or AdV for 24 h and untreated control cells were
analyzed using Annexin V-PE/7-AAD double staining by flow
cytometry. As shown in Fig. 3A and
B, treatment with AdVING4/p53, AdVING4 or AdVp53 remarkably
induced 35.2, 11.2 and 15.6% apoptosis including early and late
apoptotic cells in MDA-MB-231 tumor cells (P<0.05), whereas
there were only 0.7 and 1.5% spontaneously apoptotic MDA-MB-231
tumor cells occurring in those grown in the medium containing PBS
or AdV, respectively. Furthermore, AdVING4/p53 more efficiently
triggered MDA-MB-231 breast cancer cell apoptosis with a
synergistic effect (P<0.05; Q=1.447). To confirm
AdVING4/p53-induced apoptotic effect in vivo, we further
assessed the apoptosis in MDA-MB-231 human breast cancer s.c.
xenografted tumors by TUNEL assay (Fig.
3C). Consistent with the results in vitro by flow
cytometric analysis of apoptosis, AdVING4/p53 also has a
synergistic efficacy for in vivo inducing MDA-MB-231 breast
tumor apoptosis in athymic nude mice (P<0.05; Q=1.360).
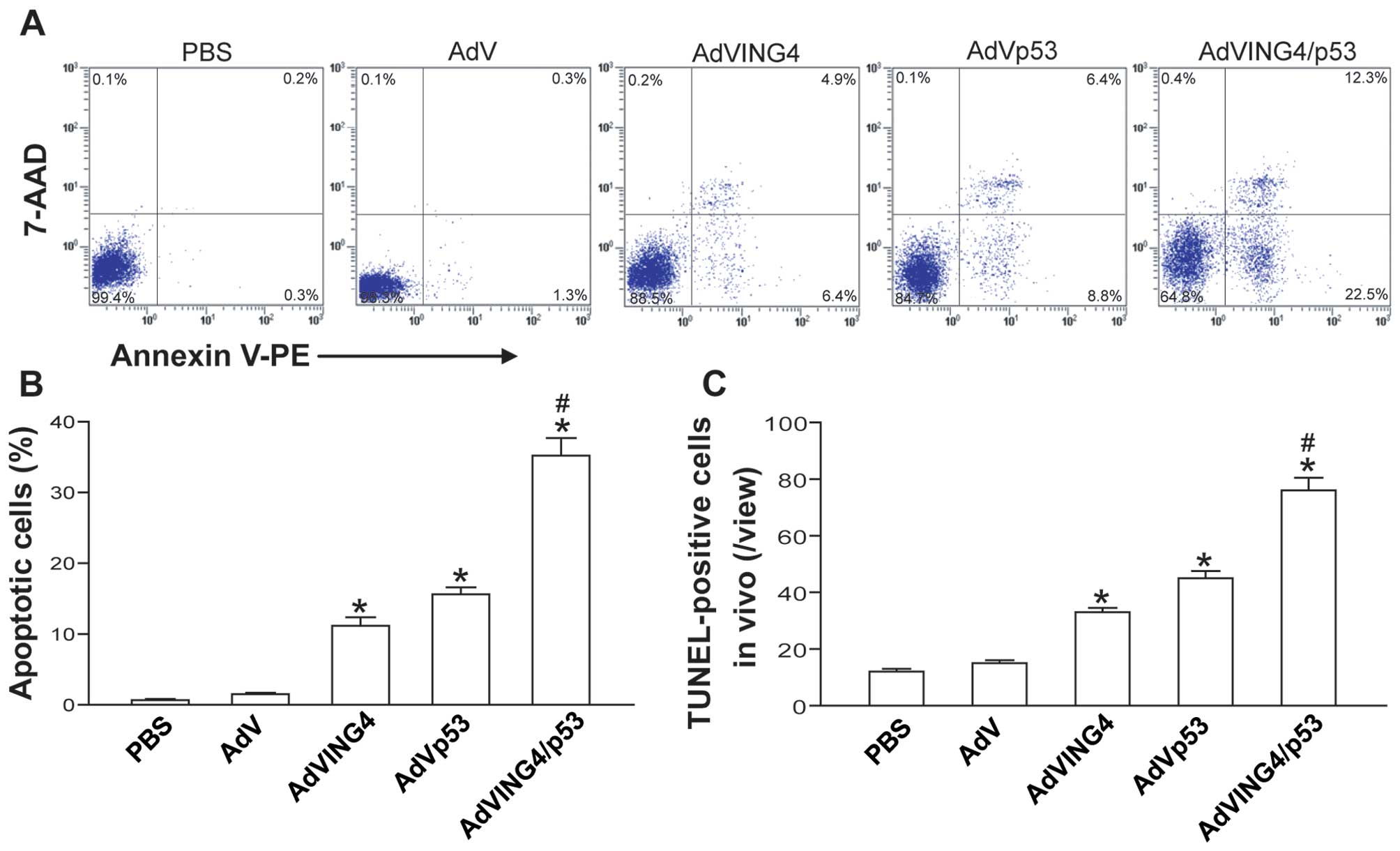 | Figure 3AdVING4/p53 induces synergistic tumor
apoptosis in breast cancer. (A and B) Flow cytometric analysis of
in vitro apoptosis. The MDA-MB-231 human breast cancer cells
were treated with AdVING4/p53 (50 MOI), AdVING4 (50 MOI), AdVp53
(50 MOI), AdV (50 MOI) or PBS without adenovirus. Twenty-four hours
after treatment, the tumor cells were harvested, stained with
Annexin V-PE and 7-AAD, and then analyzed by flow cytometry.
Representative flow cytometric images are shown (A). The Annexin
V-positive cells including Annexin V single-positive cells (early
apoptotic cells) and Annexin V/7-AAD double-positive cells (late
apoptotic cells) in the total cell population represent apoptotic
cells (B). *P<0.05 compared with PBS and AdV group;
#P<0.05 compared with AdVING4 and AdVp53 group,
Q=1.447, one-way repeated measures ANOVA and multiple comparisons;
n=3 replicates per condition. (C) TUNEL analysis of in vivo
apoptosis. The TUNEL-positive cells represent in vivo
apoptotic cells. *P<0.05 compared with PBS and AdV
group; #P<0.05 compared with AdVING4 and AdVp53
group, Q=1.360, one-way repeated measures ANOVA and multiple
comparisons; n=5 replicates per condition, n=5 sections per sample,
n=5 observations per section. Data shown are representative of
three independent experiments. |
Enhanced p53 acetylation and
transcriptional activity by ING4
To elucidate the molecular mechanism responsible for
AdVING4/p53 combined gene therapy-induced synergistic antitumor
effect, the expression of tumor suppressor p53/Ac-p53 (K382),
Cip/Kip family cyclin-dependent kinase inhibitor (CDI) P21 and
apoptosis-related proteins such as Bax, PUMA, Noxa, Bcl-2,
caspase-9, caspase-3 and PARP in MDA-MB-231 human breast cancer
cells after different treatment were analyzed by western blot
analysis. As shown in Fig. 4A, the
p53 and Ac-p53 (K382) was substantially increased in AdVp53 and
AdVING4/p53 group. Additionally, the P21, Bax, PUMA, Noxa, cleaved
caspase-9, cleaved caspase-3 and cleaved PARP in AdVING4, AdVp53
and AdVING4/p53 group was remarkably increased, whereas the Bcl-2
was significantly decreased. Moreover, AdVING4/p53 resulted in an
enhanced effect on upregulation of Ac-p53 (K382) and p53-downstream
genes such as P21, Bax, PUMA and Noxa, activation of caspase-9,
caspase-3 and cleavage of PARP as well as downregulation of Bcl-2.
The combined effect of AdVING4/p53 on in vivo expression of
p53, Ac-p53 (K382), P21, Bax, PUMA, Noxa, Bcl-2, cleaved caspase-3
in MDA-MB-231 human breast cancer s.c. xenografted tumors was
further confirmed by immunohistochemistry analysis (Fig. 4B).
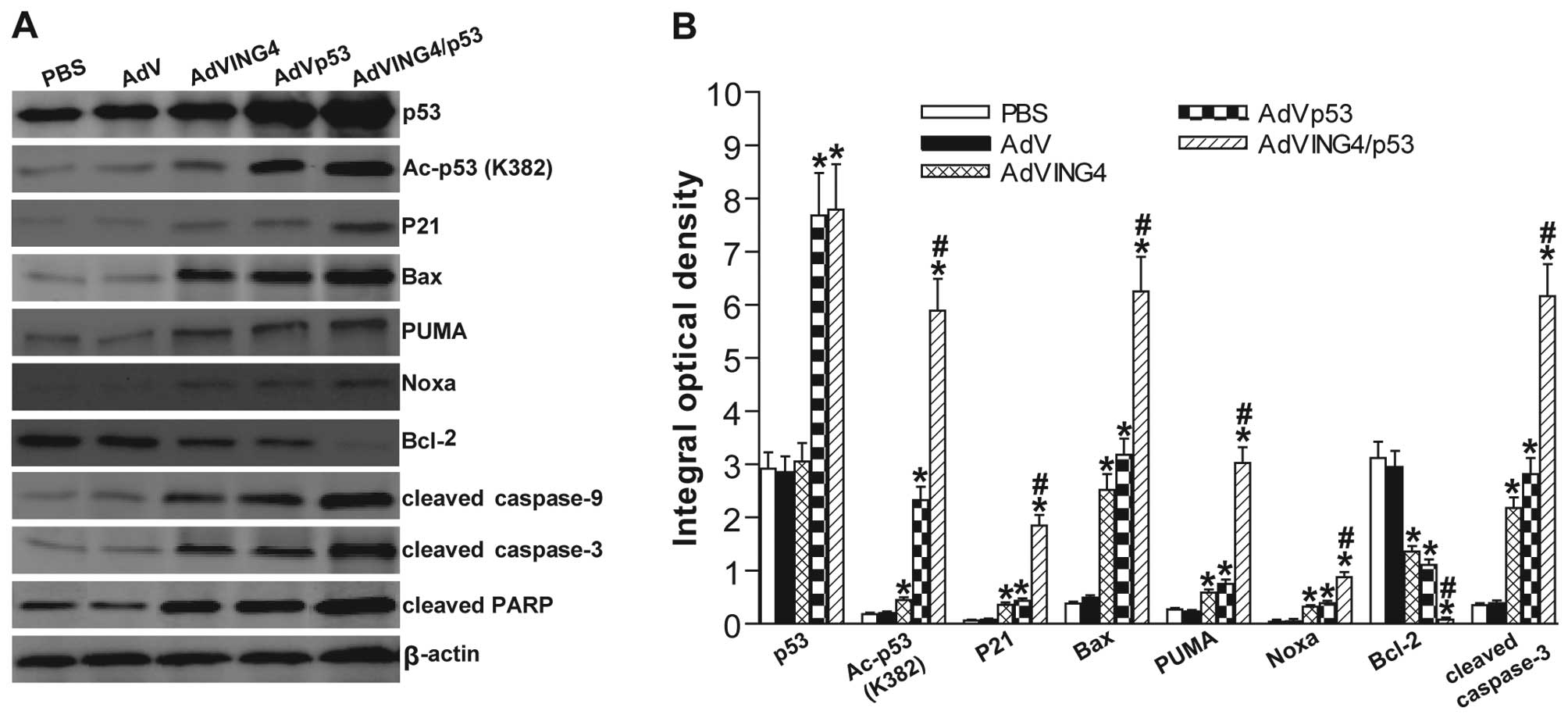 | Figure 4AdVING4/p53 enhances upregulation of
cyclin-dependent kinase inhibitor and activation of intrinsic
apoptosis via ING4-mediated enhancement of p53 acetylation and
transcriptional responses. (A) Western blot analysis of
cyclin-dependent kinase inhibitor and apoptosis-related proteins.
The total cellular lysates derived from AdVING4/p53-, AdVING4-,
AdVp53-, AdV- or PBS-treated MDA-MB-231 human breast cancer cells
were immunoblotted with a panel of antibodies specific for p53,
Ac-p53 (K382), P21, Bax, PUMA, Noxa, Bcl-2, caspase-9, caspase-3,
PARP and β-actin (an internal control), respectively. The
representative pictures of western blot analysis are shown. (B)
Immunohistochemistry analysis of cyclin-dependent kinase inhibitor
and apoptosis-related proteins in MDA-MB-231 s.c. xenografted
tumors. The immunostaining intensity of p53, Ac-p53 (K382), P21,
Bax, PUMA, Noxa, Bcl-2 and cleaved caspase-3 was quantified as
integral optical density (IOD) by Image-Pro Plus 6.0 software.
*P<0.05 compared with PBS and AdV group;
#P<0.05 compared with AdVING4 and AdVp53 group,
QAc-p53=2.365, QP21=2.676, QBax=1.203,
QPUMA=3.444, QNoxa=1.335, QBcl-2=1.153,
Qcleaved caspase-3=1.366, one-way repeated measures ANOVA and
multiple comparisons; n=5 replicates per condition, n=5 sections
per sample, n=5 observations per section. Data shown are
representative of three independent experiments. |
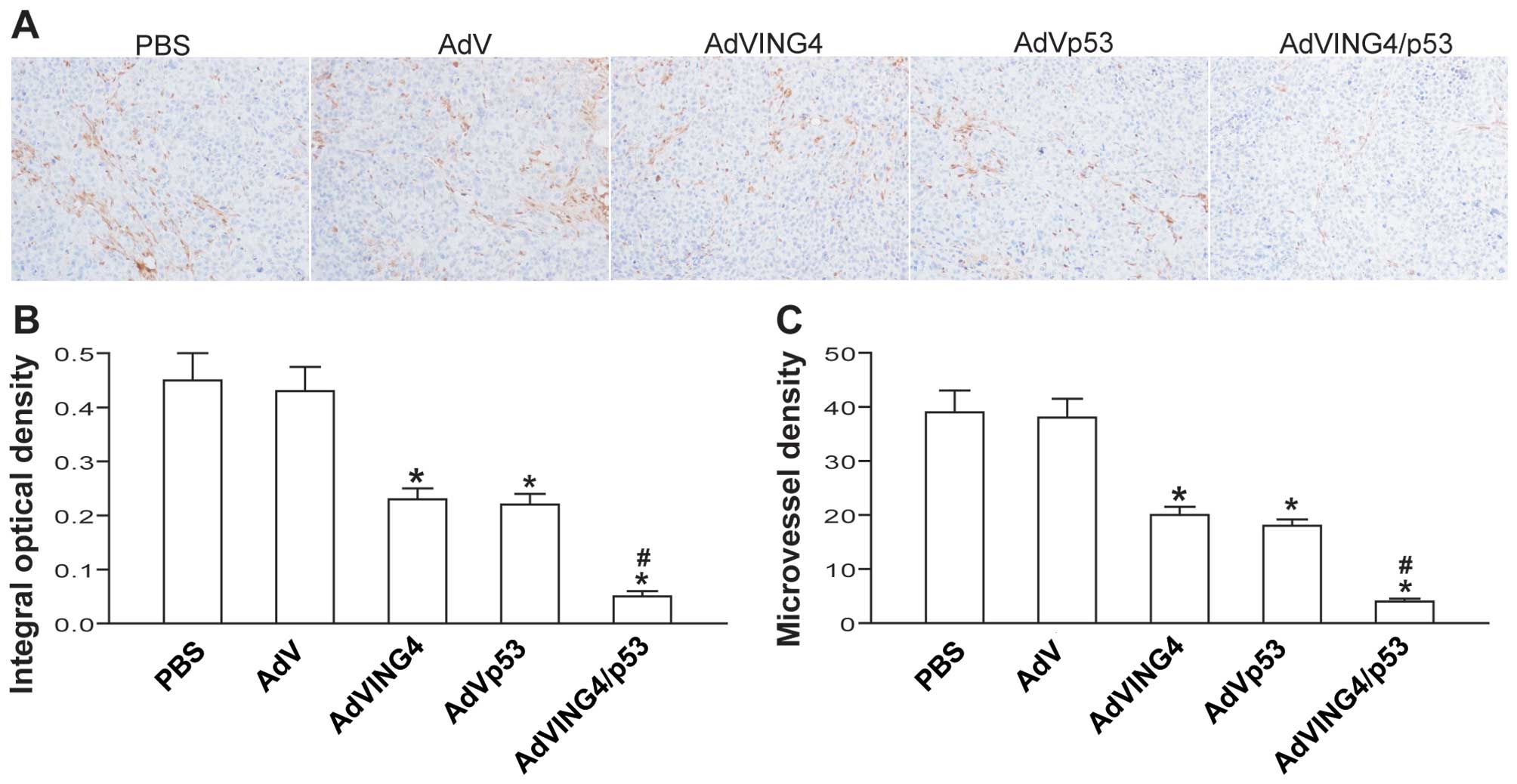
Synergistic inhibition of in vivo tumor
angiogenesis by AdVING4/p53
To investigate the combined effect of AdVING4/p53 on
tumor angiogenesis in vivo, the microvessel density (MVD) in
MDA-MB-231 human breast cancer s.c. xenografted tumors was detected
by CD31 immunohistochemistry analysis. The positive expression of
CD31 was mainly presented as brownish yellow or brownish granules
in tumor vascular endothelial cells of MDA-MB-231 xenografted
tumors (Fig. 5A). Compared with
PBS- and AdV-treated control group, the CD31 expression in
AdVING4-, AdVp53- and AdVING4/p53-treated group was weaker or less
(Fig. 5A and B, P<0.05). In
addition, the MVD counted in AdVING4, AdVp53 and AdVING4/p53 group
was significantly less than that in PBS and AdV group (Fig. 5C, P<0.05). Furthermore,
AdVING4/p53 combined gene therapy has a synergistic effect on
downregulation of CD31 and reduction of MVD in MDA-MB-231 breast
cancer xenografted tumors in athymic nude mice (P<0.05;
QCD31=1.185 and QMVD=1.176).
Discussion
It is widely accepted that p53 is an important
molecular target in human cancers and restoring wild-type p53
function would provide an effective alternative for cancer therapy.
Post-translational modifications of p53 such as phosphorylation,
ubiquitination, methylation, sumoylation, neddylation and
acetylation are crucial for its activation and determining
p53-dependent cellular outcomes (1,36).
Among them, acetylation is indispensable for p53 activation
(31). It has also been reported
that ING4 as a novel candidate tumor suppressor can physically
interact with p53 and p300 in the nucleus and consequently enhance
p53 acetylation and its transcriptional activity via its bipartite
nuclear localization signal (NLS) domain (10,37).
In the study, we examined the combined effects of p53 and ING4
tumor suppressors on MDA-MB-231 breast cancer cells in vitro
and in vivo in an athymic nude mouse model by
adenovirus-mediated ING4 and p53 co-expression (AdVING4/p53). We
demonstrated that AdVING4/p53 induced in vitro synergistic
growth inhibition and apoptosis in p53-mutant MDA-MB-231 human
breast cancer cells. Moreover, AdVING4/p53 treatment also
synergistically suppressed in vivo breast cancer growth and
induced apoptosis in MDA-MB-231 s.c. xenografted tumors in athymic
nude mice.
To delineate the molecular mechanism underlying
AdVING4/p53-mediated synergistic antitumor effects, the expression
of cell cycle- and apoptosis-related molecules such as p53, Ac-p53
(K382), P21, Bax, PUMA, Noxa, Bcl-2, caspase-9, caspase-3 and PARP
in MDA-MB-231 breast cancer cells in vitro and in
vivo xenografted tumors after different treatment was analyzed
by western blot analysis and/or immunohistochemistry. The
cyclin-dependent kinase (CDK) inhibitor P21 and pro-apoptosis
proteins such as Bax, Puma and Noxa are the well-characterized p53
target genes involved in cell cycle arrest and apoptosis (2). P21 as an important member of CDK
inhibitor Cip/Kip family can prevent the activation of cyclin
E/A-CDK2 complex and induce cell cycle G1 phase arrest (38). Bcl-2 family is a key regulator of
apoptosis and a crucial determinant of cell fate (39). The ratio of pro- to anti-apoptotic
molecules such as Bax/Bcl-2 constitutes a rheostat that sets the
threshold of susceptibility to apoptosis for the intrinsic pathway
(39), including pore formation in
mitochondrial outer membrane, loss of mitochondrial integrity,
release of cytochrome c into the cytosol, activation of
caspase-9, caspase-3 and cleavage of PARP. In addition, PUMA and
Noxa BH3-only pro-apoptosis proteins can bind to Bcl-2
anti-apoptosis protein in the mitochondria and promote cytochrome
c release, leading to intrinsic apoptosis (39). In the present study, we found that
the levels of Ac-p53, P21, Bax, PUMA, Noxa, cleaved caspase-9,
cleaved caspase-3 and cleaved PARP was significantly upregulated in
AdVp53-treated MDA-MB-231 tumor cells, whereas the Bcl-2 was
downregulated. The increased Ac-p53 in AdVp53 group may be
accompanied by the adenovirus-mediated increased synthesis of p53.
In addition, AdVING4 single gene therapy also markedly increased
the P21, Bax, PUMA, Noxa, cleaved caspase-9, cleaved caspase-3 and
cleaved PARP while decreased the Bcl-2 in p53-mutant MDA-MB-231
tumor cells, which may be elicited by p53-independent mechanisms
such as other transcription factors involved and chromatin
remolding (12,40). Interestingly, AdVING4/p53 combined
therapy resulted in an enhanced effect on upregulation of Ac-p53
and p53-downstream genes such as P21, Bax, PUMA and Noxa,
downregulation of Bcl-2, activation of caspase-9, caspase-3 and
cleavage of PARP to a great extent via ING4-induced enhancement of
p53 acetylation and its transcriptional responses, which may be an
important mechanism accountable for AdVING4/p53-induced synergistic
growth suppression and apoptosis in MDA-MB-231 breast cancer
cells.
Tumor angiogenesis is a hallmark of cancer, which is
governed by two countervailing factors that either facilitate or
oppose angiogenesis (41). It has
been shown to be indispensable for progressive tumor growth and
metastasis, representing a potential therapeutic target in
anticancer therapy (41). A great
deal of data suggested that tumor angiogenesis inhibition and
vessel normalization is a promising and non-toxic anticancer
therapeutic strategy (42,43). It has been reported that p53 can
inhibit tumor angiogenesis by downregulating pro-angiogenic factor
VEGF expression via MDM2-mediated HIF-1α degradation (44) and miR-107-induced HIF-1β suppression
(45) as well as by upregulating
production of anti-angiogenic collagen fragments via promoting
collagen prolyl hydroxylase expression (46). It has also been shown that ING4 can
repress expression of pro-angiogenic factors such as IL-6 and IL-8
via inhibiting transcriptional activity of NF-κB and HIF-1α,
leading to the inhibition of tumor angiogenesis (22,25).
To investigate the combined effect of AdVING4/p53 on tumor
angiogenesis in vivo, the MVD in MDA-MB-231 human breast
cancer s.c. xenografted tumor tissues was evaluated by CD31
immunohistochemistry analysis. Our data showed that AdVING4/p53
synergistically downregulated CD31 expression and decreased MVD in
MDA-MB-231 breast cancer xenografted tumors, which may be another
important mechanism involved in AdVING4/p53-mediated in vivo
synergistic growth inhibition in human breast cancer xenografted
tumors in athymic nude mice.
In summary, adenovirus-mediated p53 and ING4
combined gene therapy induced synergistic growth inhibition and
apoptosis as well as enhanced effects on upregulation of acetylated
p53, P21, Bax, PUMA, Noxa, cleaved caspase-9, cleaved caspase-3 and
cleaved PARP, and downregulation of Bcl-2, CD31 and microvessel
density (MVD) in MDA-MB-231 breast cancer in vitro and/or
in vivo subcutaneous (s.c.) xenografted tumors. The
synergistic antitumor activity elicited by AdVING4/p53 was closely
associated with the enhanced activation of intrinsic apoptotic
pathway and synergistic inhibition of tumor angiogenesis, very
possibly via ING4-mediated enhancement of p53 acetylation and
activity. Thus, out results indicate that cancer gene therapy
combining two or more tumor suppressors such as p53 and ING4 may
constitute a novel and effective therapeutic modality for human
breast cancer and other cancers.
Acknowledgments
This research study was supported by grants from the
National Natural Science Foundation of China (NNSFC) (nos.
81372443, 81001016, 81272542 and 81572992) and the Science and
Technology Department of Jiangsu Province (nos. BL2014039 and
BY2015039).
References
|
1
|
Kruse JP and Gu W: Modes of p53
regulation. Cell. 137:609–622. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wei CL, Wu Q, Vega VB, Chiu KP, Ng P,
Zhang T, Shahab A, Yong HC, Fu Y, Weng Z, et al: A global map of
p53 transcription-factor binding sites in the human genome. Cell.
124:207–219. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kandoth C, McLellan MD, Vandin F, Ye K,
Niu B, Lu C, Xie M, Zhang Q, McMichael JF, Wyczalkowski MA, et al:
Mutational landscape and significance across 12 major cancer types.
Nature. 502:333–339. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Muller PA and Vousden KH: Mutant p53 in
cancer: New functions and therapeutic opportunities. Cancer Cell.
25:304–317. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Muller PA and Vousden KH: p53 mutations in
cancer. Nat Cell Biol. 15:2–8. 2013. View
Article : Google Scholar
|
|
6
|
Tchelebi L, Ashamalla H and Graves PR:
Mutant p53 and the response to chemotherapy and radiation. Subcell
Biochem. 85:133–159. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cheok CF, Verma CS, Baselga J and Lane DP:
Translating p53 into the clinic. Nat Rev Clin Oncol. 8:25–37. 2011.
View Article : Google Scholar
|
|
8
|
Lane DP, Cheok CF and Lain S: p53-based
cancer therapy. Cold Spring Harb Perspect Biol. 2:a0012222010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tallen G and Riabowol K: Keep-ING balance:
Tumor suppression by epigenetic regulation. FEBS Lett.
588:2728–2742. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shiseki M, Nagashima M, Pedeux RM,
Kitahama-Shiseki M, Miura K, Okamura S, Onogi H, Higashimoto Y,
Appella E, Yokota J, et al: p29ING4 and p28ING5 bind to p53 and
p300, and enhance p53 activity. Cancer Res. 63:2373–2378.
2003.PubMed/NCBI
|
|
11
|
Zhang X, Xu LS, Wang ZQ, Wang KS, Li N,
Cheng ZH, Huang SZ, Wei DZ and Han ZG: ING4 induces G2/M cell cycle
arrest and enhances the chemosensitivity to DNA-damage agents in
HepG2 cells. FEBS Lett. 570:7–12. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Unoki M, Shen JC, Zheng ZM and Harris CC:
Novel splice variants of ING4 and their possible roles in the
regulation of cell growth and motility. J Biol Chem.
281:34677–34686. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xie Y, Zhang H, Sheng W, Xiang J, Ye Z and
Yang J: Adeno-virus-mediated ING4 expression suppresses lung
carcinoma cell growth via induction of cell cycle alteration and
apoptosis and inhibition of tumor invasion and angiogenesis. Cancer
Lett. 271:105–116. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cai L, Li X, Zheng S, Wang Y, Wang Y, Li
H, Yang J and Sun J: Inhibitor of growth 4 is involved in
melanomagenesis and induces growth suppression and apoptosis in
melanoma cell line M14. Melanoma Res. 19:1–7. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gong A, Ye S, Xiong E, Guo W, Zhang Y,
Peng W, Shao G, Jin J, Zhang Z, Yang J, et al: Autophagy
contributes to ING4-induced glioma cell death. Exp Cell Res.
319:1714–1723. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xie Y, Sheng W, Miao J, Xiang J and Yang
J: Enhanced antitumor activity by combining an adenovirus harboring
ING4 with cisplatin for hepatocarcinoma cells. Cancer Gene Ther.
18:176–188. 2011. View Article : Google Scholar :
|
|
17
|
Zhao Y, Su C, Zhai H, Tian Y, Sheng W,
Miao J and Yang J: Synergistic antitumor effect of
adenovirus-mediated hING4 gene therapy and (125)I radiation therapy
on pancreatic cancer. Cancer Lett. 316:211–218. 2012. View Article : Google Scholar
|
|
18
|
Ling C, Xie Y, Zhao D, Zhu Y, Xiang J and
Yang J: Enhanced radiosensitivity of non-small-cell lung cancer
(NSCLC) by adenovirus-mediated ING4 gene therapy. Cancer Gene Ther.
19:697–706. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim S, Chin K, Gray JW and Bishop JM: A
screen for genes that suppress loss of contact inhibition:
Identification of ING4 as a candidate tumor suppressor gene in
human cancer. Proc Natl Acad Sci USA. 101:16251–16256. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shen JC, Unoki M, Ythier D, Duperray A,
Varticovski L, Kumamoto K, Pedeux R and Harris CC: Inhibitor of
growth 4 suppresses cell spreading and cell migration by
interacting with a novel binding partner, liprin alpha1. Cancer
Res. 67:2552–2558. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li J, Martinka M and Li G: Role of ING4 in
human melanoma cell migration, invasion and patient survival.
Carcinogenesis. 29:1373–1379. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Garkavtsev I, Kozin SV, Chernova O, Xu L,
Winkler F, Brown E, Barnett GH and Jain RK: The candidate tumour
suppressor protein ING4 regulates brain tumour growth and
angiogenesis. Nature. 428:328–332. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nozell S, Laver T, Moseley D, Nowoslawski
L, De Vos M, Atkinson GP, Harrison K, Nabors LB and Benveniste EN:
The ING4 tumor suppressor attenuates NF-kappaB activity at the
promoters of target genes. Mol Cell Biol. 28:6632–6645. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ozer A, Wu LC and Bruick RK: The candidate
tumor suppressor ING4 represses activation of the hypoxia inducible
factor (HIF). Proc Natl Acad Sci USA. 102:7481–7486. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Colla S, Tagliaferri S, Morandi F, Lunghi
P, Donofrio G, Martorana D, Mancini C, Lazzaretti M, Mazzera L,
Ravanetti L, et al: The new tumor-suppressor gene inhibitor of
growth family member 4 (ING4) regulates the production of
proangiogenic molecules by myeloma cells and suppresses
hypoxia-inducible factor-1 alpha (HIF-1alpha) activity: Involvement
in myeloma-induced angiogenesis. Blood. 110:4464–4475. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hou Y, Zhang Z, Xu Q, Wang H, Xu Y and
Chen K: Inhibitor of growth 4 induces NFκB/p65 ubiquitin-dependent
degradation. Oncogene. 33:1997–2003. 2014. View Article : Google Scholar
|
|
27
|
Lu M, Pan C, Zhang L, Ding C, Chen F, Wang
Q, Wang K and Zhang X: ING4 inhibits the translation of
proto-oncogene MYC by interacting with AUF1. FEBS Lett.
587:1597–1604. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Brenner MK, Gottschalk S, Leen AM and Vera
JF: Is cancer gene therapy an empty suit? Lancet Oncol.
14:e447–e456. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wilson DR: Viral-mediated gene transfer
for cancer treatment. Curr Pharm Biotechnol. 3:151–164. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tang Y, Zhao W, Chen Y, Zhao Y and Gu W:
Acetylation is indispensable for p53 activation. Cell. 133:612–626.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xie Y, Lv H, Sheng W, Miao J, Xiang J and
Yang J: Synergistic tumor suppression by adenovirus-mediated
inhibitor of growth 4 and interleukin-24 gene cotransfer in
hepatocarcinoma cells. Cancer Biother Radiopharm. 26:681–695. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu Y, Ye T, Sun D, Maynard J and
Deisseroth A: Conditionally replication-competent adenoviral
vectors with enhanced infectivity for use in gene therapy of
melanoma. Hum Gene Ther. 15:637–647. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
He TC, Zhou S, da Costa LT, Yu J, Kinzler
KW and Vogelstein B: A simplified system for generating recombinant
adenoviruses. Proc Natl Acad Sci USA. 95:2509–2514. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang W, Qin SK, Chen BA and Chen HY:
Experimental study on antitumor effect of arsenic trioxide in
combination with cisplatin or doxorubicin on hepatocellular
carcinoma. World J Gastroenterol. 7:702–705. 2001.
|
|
36
|
Brooks CL and Gu W: Ubiquitination,
phosphorylation and acetylation: The molecular basis for p53
regulation. Curr Opin Cell Biol. 15:164–171. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang X, Wang KS, Wang ZQ, Xu LS, Wang QW,
Chen F, Wei DZ and Han ZG: Nuclear localization signal of ING4
plays a key role in its binding to p53. Biochem Biophys Res Commun.
331:1032–1038. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Massagué J: G1 cell-cycle control and
cancer. Nature. 432:298–306. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Danial NN and Korsmeyer SJ: Cell death:
Critical control points. Cell. 116:205–219. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hung T, Binda O, Champagne KS, Kuo AJ,
Johnson K, Chang HY, Simon MD, Kutateladze TG and Gozani O: ING4
mediates crosstalk between histone H3 K4 trimethylation and H3
acetylation to attenuate cellular transformation. Mol Cell.
33:248–256. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Welti J, Loges S, Dimmeler S and Carmeliet
P: Recent molecular discoveries in angiogenesis and antiangiogenic
therapies in cancer. J Clin Invest. 123:3190–3200. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shang B, Cao Z and Zhou Q: Progress in
tumor vascular normalization for anticancer therapy: Challenges and
perspectives. Front Med. 6:67–78. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ravi R, Mookerjee B, Bhujwalla ZM, Sutter
CH, Artemov D, Zeng Q, Dillehay LE, Madan A, Semenza GL and Bedi A:
Regulation of tumor angiogenesis by p53-induced degradation of
hypoxia-inducible factor 1alpha. Genes Dev. 14:34–44.
2000.PubMed/NCBI
|
|
45
|
Yamakuchi M, Lotterman CD, Bao C, Hruban
RH, Karim B, Mendell JT, Huso D and Lowenstein CJ: P53-induced
micro-RNA-107 inhibits HIF-1 and tumor angiogenesis. Proc Natl Acad
Sci USA. 107:6334–6339. 2010. View Article : Google Scholar
|
|
46
|
Teodoro JG, Parker AE, Zhu X and Green MR:
p53-mediated inhibition of angiogenesis through up-regulation of a
collagen prolyl hydroxylase. Science. 313:968–971. 2006. View Article : Google Scholar : PubMed/NCBI
|