Introduction
Ovarian cancer (OC) is the most lethal gynecologic
malignancy and the fifth most common cause of cancer-related death
in women (1). Surgical and
chemotherapeutic management are the standard of care for this
disease (2). However, most patients
relapse after primary treatment and succumb to disease progression.
In addition, although changes to both chemotherapy schedules and
routes of administration have improved patient survival to a
degree, it appears that a therapeutic ceiling with chemotherapy has
been reached (3,4). OC still lags behind a number of other
solid malignancies in terms of the sluggish incremental extension
in overall survival during the last 20 years. This has led to a
number of clinical trials in OC investigating the efficacy of
molecular targeted therapies which have undoubtedly revolutionized
the therapeutic landscape in oncology in recent years (5,6).
Angiogenesis plays crucial roles in the development
and progression of OC (7).
Anti-angiogenic therapies mainly include agents targeting the
vascular endothelial growth factor (VEGF) and agents targeting the
VEGF receptors in tumor-associated endothelial cells. Anti-VEGF
therapy seems to be a relevant strategy in OC (8). Bevacizumab (BV), a VEGF monoclonal
antibody, is the first anti-angiogenic therapy proven to slow
metastatic disease progression in patients with cancer. Addition of
BV (given as concurrent or maintenance) to conventional
chemotherapy has been shown to improve progression-free survival
(PFS) in relapsed, platinum-resistant OC (9). Unfortunately, the efficacy of BV is
limited and the clinical benefit is short-lived. Some patients do
not respond to BV and most patients with initial response will
rapidly develop resistant disease (5,10,11).
Currently, the molecular mechanisms underlying OC resistance to BV
are less clear. Comprehensive understanding of OC resistance to
anti-VEGF therapy help to identify early indicators of resistance
and exploit better anti-angiogenic therapies.
TCEB2 encodes the protein elongin B, which is a
subunit of the transcription factor B (SIII) complex and is also
reported to function as an adapter protein in the proteasomal
degradation of target proteins via different E3 ubiquitin ligase
complexes (12,13). In the present study, we identified
that TCEB2 is involved in the development of acquired resistance to
BV in OC cells via the mechanisms of suppression of VEGF-A
expression by promoting HIF-1α degradation and induction of
interleukin-8 (IL-8) expression. These findings thus may provide an
alternative therapeutic strategy of combining anti-VEGF and
anti-IL-8 regents for drug resistant OC cells.
Materials and methods
Cell culture
Human ovarian cancer cells SKOV3 and HO8910 were
maintained in RPMI-1640 (Gibco, San Diego, CA, USA) medium
supplemented with 10% fetal bovine serum (FBS), human umbilical
vein endothelial cells (HUVEC) was maintained in endothelial cell
medium containing 5% FBS and 1% endothelial cell growth supplement
(ScienceCell). All cells were cultured in a humidified incubator at
37°C with 5% CO2.
Reagents
MG132 and cycloheximide (CHX) were purchased from
Sigma-Aldrich (St. Louis, MO, USA). Human VEGF monoclonal antibody
bevacizumab (BV) was from Roche (Indianapolis, IN, USA), and human
IL-8 neutralizing antibody (IL-8 Ab) was from R&D Systems
(Minneapolis, MN, USA). Monoclonal anti-HIF1α was purchased from BD
Biosciences (San Jose, CA, USA). Monoclonal anti-V5-tag and
anti-β-actin were purchased from Santa Cruz Biotechnology (Santa
Cruz, CA, USA).
Reverse transcriptional (RT) real-time
PCR
RT real-time PCR was performed as described
(14). Total RNA was extracted and
1 µg RNA was reverse transcribed with a cDNA Synthesis kit
(Invitrogen, Carlsbad, CA, USA). Real-time PCR analysis was set up
with SYBR Green qPCR SuperMix kit (Invitrogen) and carried out in
the iCycler thermal cycler (Bio-Rad, Hercules, CA, USA). The
relative level of mRNA expression of each gene was determined by
normalizing with β-actin. The primers used are: β-actin,
5′-TACCACAGGCATTGTGATGG-3′ (forward) and 5′-TTTGATGTCACGCACGATTT-3′
(reverse); human TCEB2, 5′-GAGGCCCATTTCCCCCAATA-3′ (forward) and
5′-ACAGGACAGCACAGGAACTG-3′ (reverse); and human VEGF-A,
5′-TACCTCCACCATGCCAAGTG-3′ (forward) and
5′-ATGATTCTGCCCTCCTCCTTC-3′ (reverse).
Western blot analysis
Cells were harvested and lysed using ice-cold lysis
buffer [150 mM NaCl, 1% Triton X-100, 0.5% sodium deoxycholate,
0.1% SDS, 50 mM Tris (pH 8.0), protease inhibitor cocktail (Roche)]
for 45 min. Lysates were then centrifuged at 14,000 rpm for 10 min
at 4°C to collect the supernate. Equivalent amount of proteins were
separated by 12% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis and transferred to nitrocellulose membranes.
Membranes were block with 5% skim milk containing 0.1% Tween-20 for
1 h at room temperature. Appropriate primary antibodies were added
into the membranes overnight at 4°C. Membranes were then washed and
incubated with horseradish peroxidase conjugated secondary
antibodies for 1 h at room temperature. Signals were then detected
by chemiluminescence (Pierce, Rockford, IL, USA).
Transfection
TCEB2-overexpressing plasmid (pLenti6-V5/TCEB2) and
empty vector were purchased from DNASU Plasmid Repository (Tempe,
AZ, USA). For transfection, 2×105 SKOV3 cells were
seeded in 6-well plate with 60% confluence prior to the
transfection, 2.5 µg pLenti6-V5/TCEB2 or empty vector were
transfected into cells by Xfect™ transfection reagent (Clontech,
Palo Alto, CA, USA) according to the manufacturer's instructions.
Further assay was performed 48 h after the transfection.
Co-culture and cell proliferation
assay
For SKOV3 cell proliferation assay, cells were
re-suspended in medium and cultured in 96-well plates at a
concentration of 2,000 cells/well. After the indicated treatment,
cell viability was assessed using a
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay (Roche). For co-culture system, SKOV3 cells were plated in
Transwell permeable support (0.4 µm pore size; Fisher
Scientific) and HUVEC cells were plated in 12-well plates on the
first day. Next day, Transwell inserts were placed on 12-well
plates and co-culture medium (M199; Life Technologies) containing
either vehicle, BV (1 mg/ml) or IL-8 Ab (0.5 µg/ml). After
the treatment, HUVEC cells were fixed with 4% paraformaldehyde for
10 min, stained with 0.5% crystal violet and washed twice, 0.5 ml
30% acetic acid was then added into the plates to dissolve the
crystal violet, and 0.2 ml aliquot of the solution was added into a
96-well plate to read the absorbance values (optical density, OD)
at 560 mm.
HRE-luciferase assay
Cells seeded in 24-well plates were transfected with
1 µg hypoxia response element (HRE) reporter gene luciferase
constructs (HRE-Luc) and 2 ng Renilla luciferase construct
as internal control. Forty-eight hours after transfection, HRE-Luc
activity was measured using dual luciferase assay kit according the
manufacturer's protocol (Promega, Madison, WI, USA).
Animal experiments
All experimental procedures were approved by the
Institutional Animal Care and Use Committee. SKOV3 cells
(3×106) were subcutaneously injected into 4-6 weeks old
athymic nude mice. Tumor volume was measured weekly after the
injection, when tumor volume reached 50 mm3, 10 mg/kg of
BV or vehicle (PBS) was injected intra-peritoneally twice weekly
for a total of 6 weeks. At the end of treatment, animals were
sacrificed and fresh tumor tissues were collected for further
studies.
Bioinformatic and statistical
analysis
RNA-sequence-based mRNA expression data for TCEB2
and VEGF-A of ovarian cancer samples from The Cancer Genome Atlas
(TCGA) were retrieved through the CGDS server of the cBioportal
hosted by the Memorial Sloan-Kettering Cancer Center. Pearson's
correlation analysis was performed to analyze the association
between TCEB2 and VEGF-A. All the in vitro and in
vivo data are presented as the mean ± SEM from at least three
independent experiments and the differences between two groups were
compared by the Student's t-test. All statistical analyses were
performed using SPSS 16.0 software.
Results
Heterogeneity of OC cells in response to
BV
BV has been shown to improve patient survival in OC,
however, the efficacy is limited and the clinical benefit is
short-lived, since the initial response rate of OV to BV is <40%
and most patients with initial response will eventually develop
resistant disease (5,10,11).
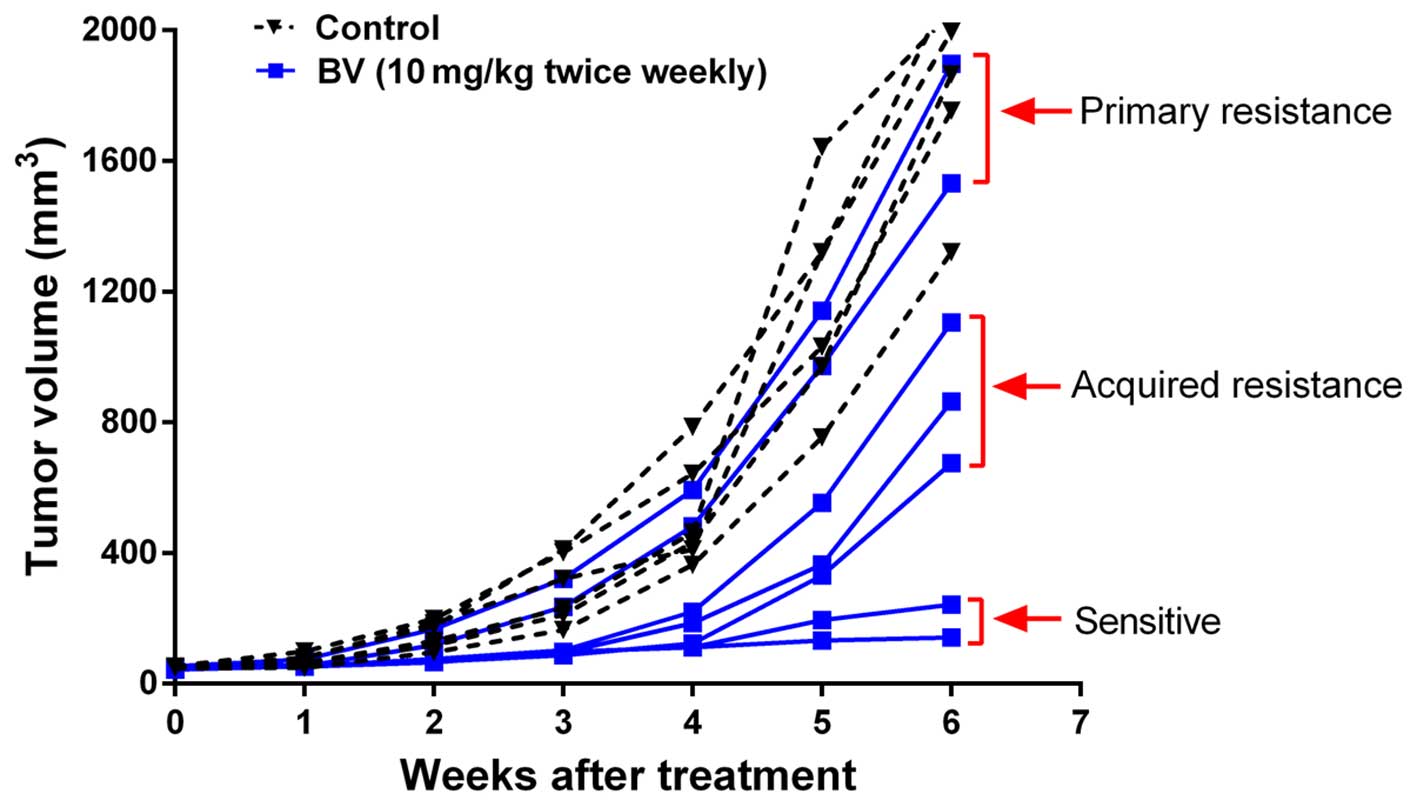
Here, we established OC xenograft models and treated tumors with BV
to determine 'sensitivity' or 'resistance'. The phenotypic
sensitivity of each individual tumor to treatment was defined by a
long-term trend toward tumor stasis (tumor volume increase of
<25%). In contrast, tumors that increased >25% of initial
volume and showed a long-term trend toward continued growth were
considered as resistance.
The results showed that tumors treated with vehicle
control (control) showed continued growth. Of the 7 tumors treated
with BV, only 2 showed sensitivity, 2 were primary resistance to BV
and 3 exhibited initial response to the treatment during the first
3 weeks of treatment but rapidly acquired resistant phenotype after
that (Fig. 1). These results
indicate that heterogeneity exists in the response of OC to BV.
TCEB2 is associated with the development
of resistance to BV in OC cells
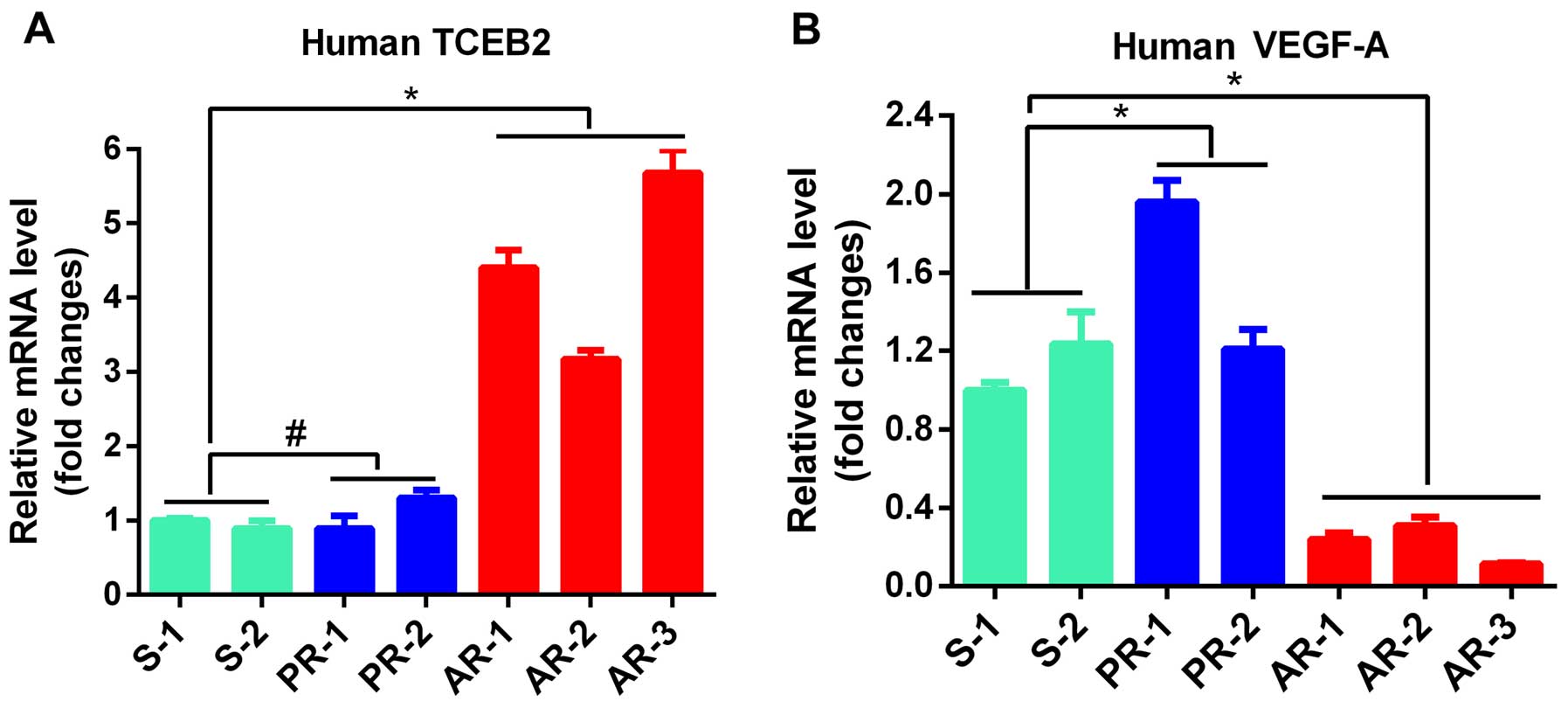
We analyzed the differential expression of genes
related to tumor angiogenesis in the sensitive (S), primary
resistant (PR) and acquired resistant (AR) tumors to identify
critical genes involved in the development of BV-resistance, and
found that human TCEB2 gene was significantly increased in all the
AR tumors (P<0.05), but no difference was observed in TCEB2
level between PR and S tumors (P>0.05, Fig. 2A), indicating that TCEB2 is
associated with the development of AR in OC cells. In addition, the
expression of human VEGF-A in tumors was determined, and the data
indicated that VEGF-A was significantly decreased in all AR tumors
compared to S tumors (P<0.05, Fig.
2A), suggesting VEGF-A-independent manner of growth in AR
tumors.
TCEB2 negatively regulates VEGF-A in the
development of resistance to BV
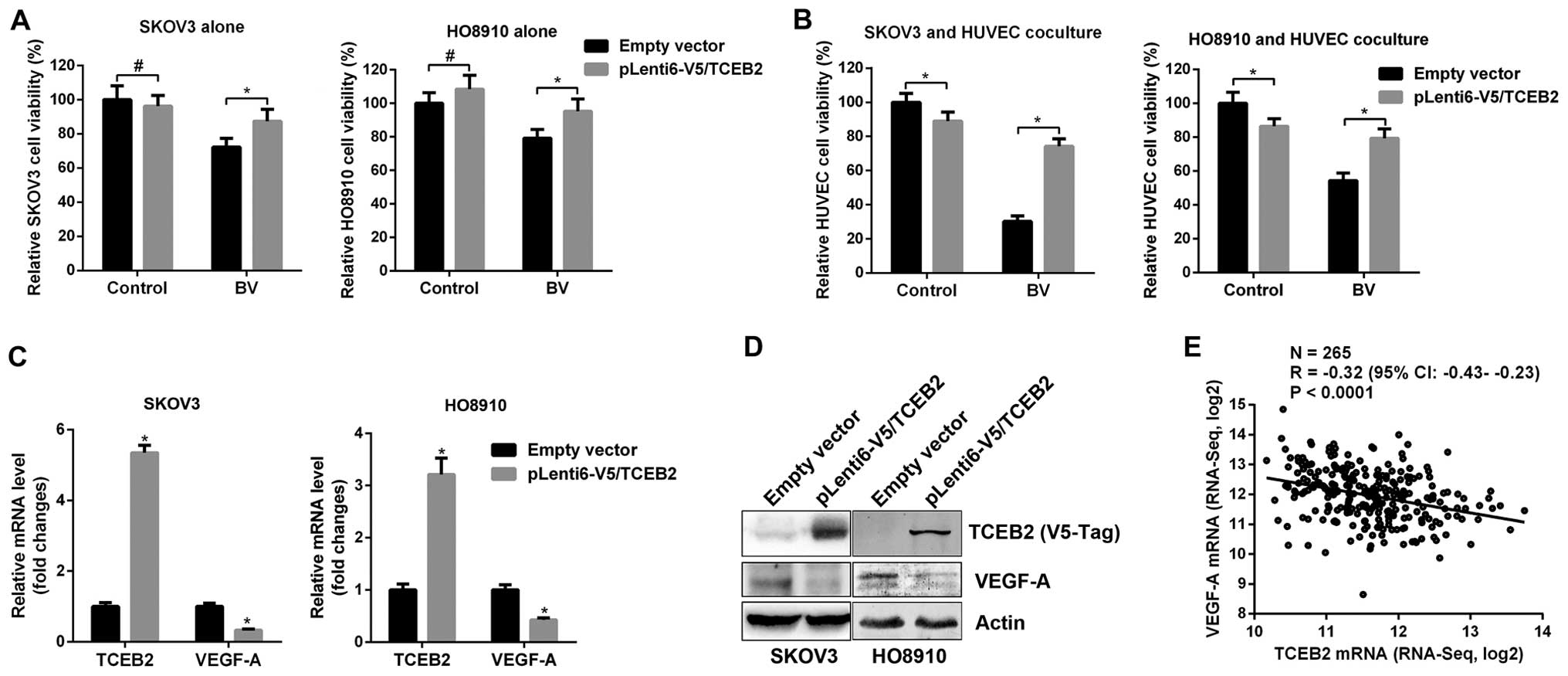
To further confirm the role of TCEB2 in the
development of BV-resistance, empty vector and TCEB2-overexpressing
(pLenti6-V5/TCEB2) SKOV3 and HO8910 cells were established. Cells
were then treated with BV and cell viability was examined.
TCEB2-overexpressing cells were relatively resistant to BV compared
to empty vector cells (Fig. 3A).
Since tumor-associated vascular endothelium are known to be crucial
for tumor growth and involved in resistance to anti-angiogenic
therapies (15), co-culture models
were used to mimic the interaction between tumor cells and vascular
endothelium. Empty vector and TCEB2-overexpressing cancer cells
co-cultured with HUVEC cells were then treated with BV, notably,
HUVEC co-cultured with empty vector cells showed sensitive to BV
but the HUVEC cells co-cultured with TCEB2-overexpressing cells
were much more resistant to BV (Fig.
3B). These data clearly demonstrated that TCEB2 plays an
essential role in the development of resistance to BV.
Additionally, since increased TCEB2 and decreased VEGF-A were
observed in AR tumors (Fig. 2), we
hypothesized that TCEB2 negatively regulated VEGF-A. Indeed,
TCEB2-overexpressing cells showed decreased VEGF-A mRNA and protein
expression (Fig. 3C and D).
Furthermore, by analysis of TCEB2 and VEGF-A expression in human OC
tissues from The Cancer Genome Atlas (TCGA), we found an inverse
correlation between these two genes in OC tissues (Fig. 3E). Taken together, these data
suggest that in the development of AR, TCEB2 may promote OC cells
to escape from BV treatment by VEGF-A suppression.
TCEB2 promotes HIF-1α degradation
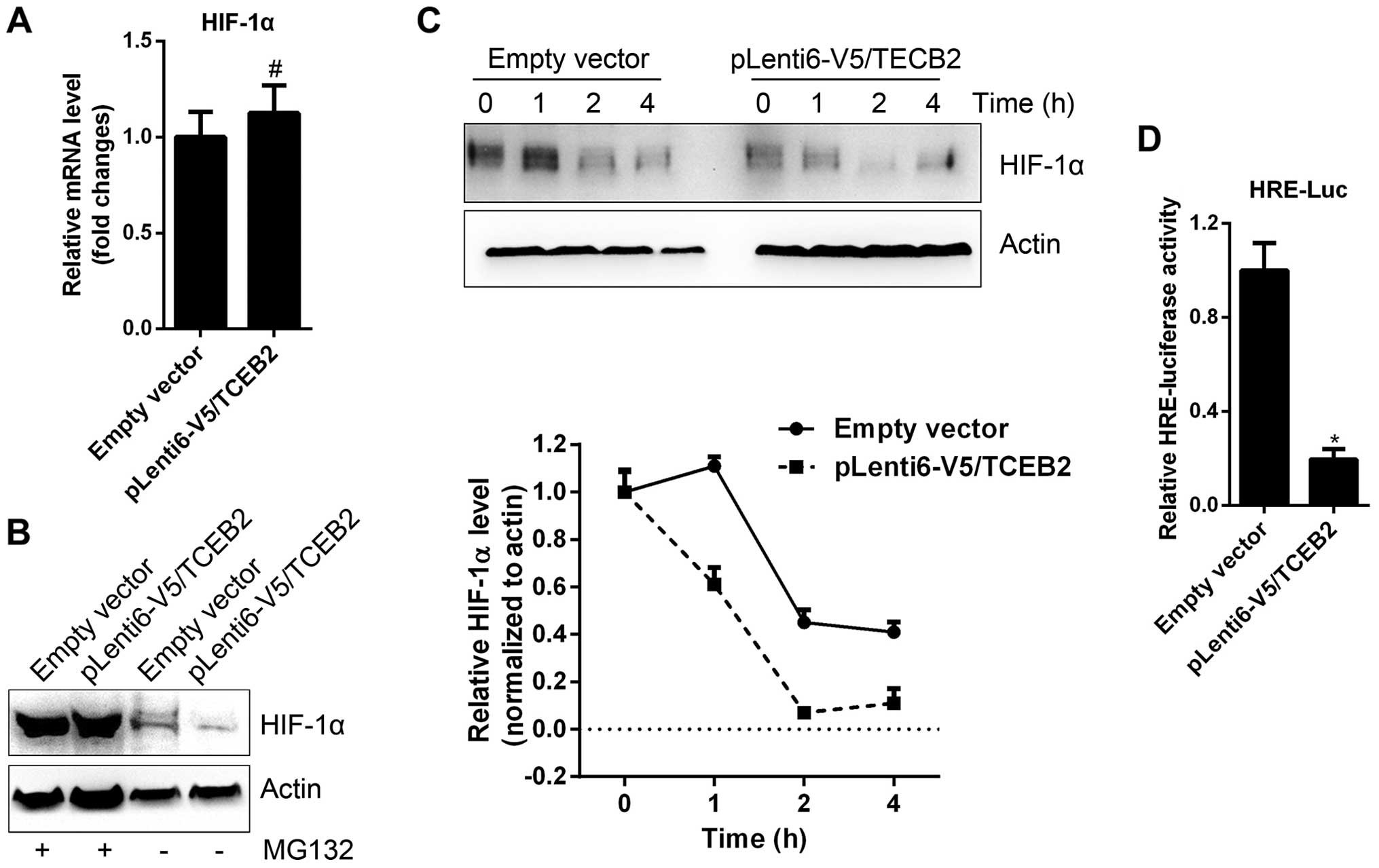
HIF-1α is a well-known transcription factor directly
controlling VEGF-A expression in most tumor cells (16). We examined the expression of HIF-1α
in empty vector and TCEB2-overexpressing SKOV3 cells, and found
HIF-1α mRNA level was unchanged (Fig.
4A). However, TCEB2-overexpressing cells exhibited decreased
HIF-1α protein, and the treatment of a proteasome inhibitor, MG132,
abolished the difference in HIF-1α level between empty vector and
TCEB2-overexpressing cells (Fig.
4B). In addition, after the treatment of a protein biosynthesis
inhibitor, cycloheximide (CHX), the half-life of HIF-1α protein in
TCEB2-overexpressing cells was much shorter than empty vector cells
(Fig. 4C). These data indicates
that TCEB2 promotes HIF-1α protein degradation in OC cells. Along
with the reduction of HIF-1α protein in TCEB2-overexpressing cells,
the transcriptional activity (HRE-luciferase) of HIF-1α was also
decreased (Fig. 4D).
IL-8 mediates the resistance of OV cells
to BV
In order to clarify the mechanisms of TCEB2-induced
resistance to BV, we investigated the expression of IL-8, which has
been reported to mediate VEGF-A-independent angiogenesis in many
cancer (17–19). Interestingly, IL-8 was significantly
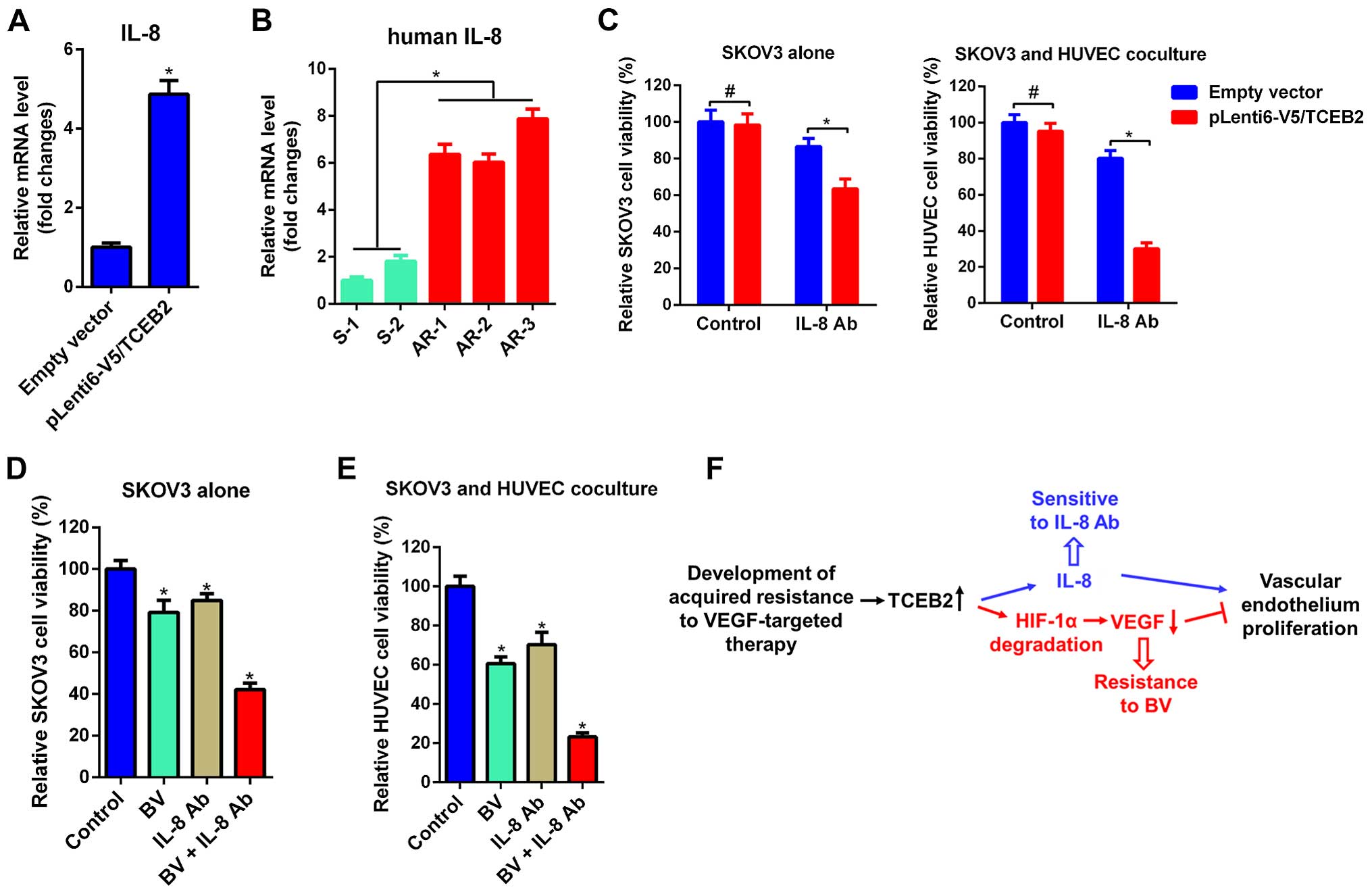
increased in TCEB2-overexpressing cells (Fig. 5A) and AR tumors exhibited
significantly elevated IL-8 compared to S tumors (Fig. 5B). Although TCEB2-overexpressing
cells and the HUVEC cells co-cultured with TCEB2-overexpressing
cells were shown to be resistant to BV (Fig. 3A and B), both of them were sensitive
to IL-8 neutralizing antibody (IL-8 Ab, Fig. 5C). Furthermore, we investigated the
potential implication of a novel strategy which combined BV and
IL-8 Ab in OC cells, and found that simultaneous treatment of BV
and IL-8 Ab exhibited enhanced effect of growth inhibition on
co-cultured HUVEC and SKOV3 cells (Fig.
5D and E). Collectively, these data indicate that TCEB2
promotes OC cells resistance to BV via upregulation of IL-8
(Fig. 5F).
Discussion
Clinically, BV has been approved as a single agent
for glioblastoma (20), and also
approved in combination with standard chemotherapy or immunotherapy
for the treatment of metastatic colorectal cancer (21), non-small cell lung cancer (22), renal cell carcinoma (23) and advanced ovarian cancer (24,25).
In OC, the clinical efficacy of BV has been extensively evaluated
in a number of trials. BV as single agent and in combination with
chemotherapy has shown to improve PFS to a certain degree (24,26),
but results obtained from these trails seem to be mixed. Even
though BV was administered at the same schedule, in the study of
Burger et al (24), 21.0%
clinical responses [including complete responses (CR) and partial
responses (PR)] and 40.3% improved FPS for at least 6 months were
observed, however, the study of Cannistra et al (9) only demonstrated a 15.9% PR rate, also
an 11% risk for gastrointestinal perforation, resulting in early
termination of the experiment because of safety concerns. Our
results from animal models suggest a low response rate of OC cells
to BV (2/6 sensitivity, 2/6 primary resistance and 3/6 with initial
response but rapidly developing resistance). Indeed, low response
to BV have been shown in other solid tumors. For example,
anti-angiogenic therapies are the first-line treatment for
metastatic renal cell carcinoma, however, the response rate of
patient to BV is only 31% (27).
The low response rate, rapid development of resistance and high
drug toxicity observed in some cases make it necessary to either
understand the mechanisms of resistance, or to exploit better
therapeutic strategies.
The underlying mechanisms of tumor cell resistance
to anti-angiogenic agents are complicated. Firstly,
VEGF/VEGFR-independent signaling, such as, HGF/c-Met, FGF/FGFR,
Ang/Tie2 and immunocytokines (e.g., IL-8, IL-11) are also reported
to play important roles in angiogenesis, thus may escape from the
anti-VEGF and anti-VEGFR therapies (17,28).
Secondly, mutations in target genes during the treatment may lead
to off-target therapeutics (29).
Thirdly, insufficient drug concentration in the circulation and
incomplete blockage of angiogenic signaling (29). Fourthly, tumor microenvironmental
factors, such as inflammatory and stroma cells involved in
angiogenesis are not affected by the currently used agents
(15). Fifthly, tumor cell
metabolism reprogramming may help to adapt to the treatment and
survive in the poorly vascularized and low nutrition conditions
(30). The present study suggests
that when VEGF-A is suppressed during the treatment of BV, tumor
cells will shift to a complementary manner (IL-8 mediated
signaling) to maintain angiogenesis. Besides, decreased VEGF-A
expression in OC administered BV was also observed in the study of
Smerde et al (31).
TCEB2 can function as both a regulatory subunit in
the transcription factor B (SIII) complex and an adapter protein in
the proteasomal degradation of target proteins via different E3
ubiquitin ligase complexes, including the von Hippel-Lindau
ubiquitination complex (VHL) (12,13).
VHL specifically targets HIF-α for degradation in normoxic
condition (32). Our results
demonstrate that TCEB2 promotes HIF-1α degradation in OC cells, and
expectedly, suppresses VEGF-A which is a known direct target gene
of HIF-1α. TCEB2 also upregulates IL-8 which is a critical factor
mediating cells resistant to anti-VEGF agents, however, how TCEB2
regulates IL-8 is not defined in the present study and needs to be
further studied. In addition, the finding of TCEB2 associated with
OC cells resistant to BV also indicates that TCEB2 may serve as a
predictive biomarker of response for BV treatment, and thus may
help in stratifying patients before treatment. Although the
predictive implication of VEGF-A has not been studied in OC,
amplification of VEGF-A gene has been reported to predict
sensitivity to anti-angiogenic agent in hepatocellular carcinomas
(33). Furthermore, based on the
observation that IL-8 is upregulated in BV-resistant OC cells, this
study also provides an alternative strategy of simultaneously
targeting VEGF-A and IL-8 for OC.
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sonoda Y: Management of early ovarian
cancer. Oncology (Williston Park). 18:343–362. 2004.
|
|
3
|
Katsumata N, Yasuda M, Isonishi S,
Takahashi F, Michimae H, Kimura E, Aoki D, Jobo T, Kodama S,
Terauchi F, et al Japanese Gynecologic Oncology Group: Long-term
results of dose-dense paclitaxel and carboplatin versus
conventional paclitaxel and carboplatin for treatment of advanced
epithelial ovarian, fallopian tube, or primary peritoneal cancer
(JGOG 3016): A randomised, controlled, open-label trial. Lancet
Oncol. 14:1020–1026. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vaughan S, Coward JI, Bast RC Jr, Berchuck
A, Berek JS, Brenton JD, Coukos G, Crum CC, Drapkin R,
Etemadmoghadam D, et al: Rethinking ovarian cancer: Recommendations
for improving outcomes. Nat Rev Cancer. 11:719–725. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Coward JI, Middleton K and Murphy F: New
perspectives on targeted therapy in ovarian cancer. Int J Womens
Health. 7:189–203. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Teplinsky E and Muggia F: Targeting HER2
in ovarian and uterine cancers: Challenges and future directions.
Gynecol Oncol. 135:364–370. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bamberger ES and Perrett CW: Angiogenesis
in epithelian ovarian cancer. Mol Pathol. 55:348–359. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Longo R, Sarmiento R, Fanelli M,
Capaccetti B, Gattuso D and Gasparini G: Anti-angiogenic therapy:
Rationale, challenges and clinical studies. Angiogenesis.
5:237–256. 2002. View Article : Google Scholar
|
|
9
|
Cannistra SA, Matulonis UA, Penson RT,
Hambleton J, Dupont J, Mackey H, Douglas J, Burger RA, Armstrong D,
Wenham R, et al: Phase II study of bevacizumab in patients with
platinum-resistant ovarian cancer or peritoneal serous cancer. J
Clin Oncol. 25:5180–5186. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Garcia AA, Hirte H, Fleming G, Yang D,
Tsao-Wei DD, Roman L, Groshen S, Swenson S, Markland F, Gandara D,
et al: Phase II clinical trial of bevacizumab and low-dose
metronomic oral cyclophosphamide in recurrent ovarian cancer: A
trial of the California, Chicago, and Princess Margaret Hospital
phase II consortia. J Clin Oncol. 26:76–82. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Spannuth WA, Sood AK and Coleman RL:
Angiogenesis as a strategic target for ovarian cancer therapy. Nat
Clin Pract Oncol. 5:194–204. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Garrett KP, Aso T, Bradsher JN, Foundling
SI, Lane WS, Conaway RC and Conaway JW: Positive regulation of
general transcription factor SIII by a tailed ubiquitin homolog.
Proc Natl Acad Sci USA. 92:7172–7176. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Stebbins CE, Kaelin WG Jr and Pavletich
NP: Structure of the VHL-ElonginC-ElonginB complex: Implications
for VHL tumor suppressor function. Science. 284:455–461. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou J, Wu K, Gao D, Zhu G, Wu D, Wang X,
Chen Y, Du Y, Song W, Ma Z, et al: Reciprocal regulation of
hypoxia-inducible factor 2α and GLI1 expression associated with the
radiore-sistance of renal cell carcinoma. Int J Radiat Oncol Biol
Phys. 90:942–951. 2014. View Article : Google Scholar
|
|
15
|
Cascone T, Herynk MH, Xu L, Du Z, Kadara
H, Nilsson MB, Oborn CJ, Park YY, Erez B, Jacoby JJ, et al:
Upregulated stromal EGFR and vascular remodeling in mouse xenograft
models of angiogenesis inhibitor-resistant human lung
adenocarcinoma. J Clin Invest. 121:1313–1328. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ke Q and Costa M: Hypoxia-inducible
factor-1 (HIF-1). Mol Pharmacol. 70:1469–1480. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Grepin R, Guyot M, Jacquin M, Durivault J,
Chamorey E, Sudaka A, Serdjebi C, Lacarelle B, Scoazec JY, Negrier
S, et al: Acceleration of clear cell renal cell carcinoma growth in
mice following bevacizumab/Avastin treatment: The role of CXCL
cytokines. Oncogene. 31:1683–1694. 2012. View Article : Google Scholar
|
|
18
|
Huang D, Ding Y, Zhou M, Rini BI, Petillo
D, Qian CN, Kahnoski R, Futreal PA, Furge KA and Teh BT:
Interleukin-8 mediates resistance to antiangiogenic agent sunitinib
in renal cell carcinoma. Cancer Res. 70:1063–1071. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Abraham RT: Chemokine to the rescue:
Interleukin-8 mediates resistance to PI3K-pathway-targeted therapy
in breast cancer. Cancer Cell. 22:703–705. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kreisl TN, Kim L, Moore K, Duic P, Royce
C, Stroud I, Garren N, Mackey M, Butman JA, Camphausen K, et al:
Phase II trial of single-agent bevacizumab followed by bevacizumab
plus irinotecan at tumor progression in recurrent glioblastoma. J
Clin Oncol. 27:740–745. 2009. View Article : Google Scholar :
|
|
21
|
Hurwitz H, Fehrenbacher L, Novotny W,
Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S,
Holmgren E, et al: Bevacizumab plus irinotecan, fluorouracil, and
leucovorin for metastatic colorectal cancer. N Engl J Med.
350:2335–2342. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sandler A, Gray R, Perry MC, Brahmer J,
Schiller JH, Dowlati A, Lilenbaum R and Johnson DH:
Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell
lung cancer. N Engl J Med. 355:2542–2550. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Escudier B, Bellmunt J, Négrier S, Bajetta
E, Melichar B, Bracarda S, Ravaud A, Golding S, Jethwa S and
Sneller V: Phase III trial of bevacizumab plus interferon alfa-2a
in patients with metastatic renal cell carcinoma (AVOREN): Final
analysis of overall survival. J Clin Oncol. 28:2144–2150. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Burger RA, Brady MF, Bookman MA, Fleming
GF, Monk BJ, Huang H, Mannel RS, Homesley HD, Fowler J, Greer BE,
et al Gynecologic Oncology Group: Incorporation of bevacizumab in
the primary treatment of ovarian cancer. N Engl J Med.
365:2473–2483. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Perren TJ, Swart AM, Pfisterer J,
Ledermann JA, Pujade-Lauraine E, Kristensen G, Carey MS, Beale P,
Cervantes A, Kurzeder C, et al ICON7 Investigators: A phase 3 trial
of bevacizumab in ovarian cancer. N Engl J Med. 365:2484–2496.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pujade-Lauraine E, Hilpert F, Weber B,
Reuss A, Poveda A, Kristensen G, Sorio R, Vergote I, Witteveen P,
Bamias A, et al: Bevacizumab combined with chemotherapy for
platinum-resistant recurrent ovarian cancer: The AURELIA open-label
randomized phase III trial. J Clin Oncol. 32:1302–1308. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Escudier B, Pluzanska A, Koralewski P,
Ravaud A, Bracarda S, Szczylik C, Chevreau C, Filipek M, Melichar
B, Bajetta E, et al AVOREN Trial investigators: Bevacizumab plus
interferon alfa-2a for treatment of metastatic renal cell
carcinoma: A randomised, double-blind phase III trial. Lancet.
370:2103–2111. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shojaei F, Lee JH, Simmons BH, Wong A,
Esparza CO, Plumlee PA, Feng J, Stewart AE, Hu-Lowe DD and
Christensen JG: HGF/c-Met acts as an alternative angiogenic pathway
in sunitinib-resistant tumors. Cancer Res. 70:10090–10100. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Figlin RA, Kaufmann I and Brechbiel J:
Targeting PI3K and mTORC2 in metastatic renal cell carcinoma: New
strategies for overcoming resistance to VEGFR and mTORC1
inhibitors. Int J Cancer. 133:788–796. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Metallo CM, Gameiro PA, Bell EL, Mattaini
KR, Yang J, Hiller K, Jewell CM, Johnson ZR, Irvine DJ, Guarente L,
et al: Reductive glutamine metabolism by IDH1 mediates lipogenesis
under hypoxia. Nature. 481:380–384. 2012.
|
|
31
|
Smerdel MP, Steffensen KD, Waldstrøm M,
Brandslund I and Jakobsen A: The predictive value of serum VEGF in
multi-resistant ovarian cancer patients treated with bevacizumab.
Gynecol Oncol. 118:167–171. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Haase VH: The VHL tumor suppressor: Master
regulator of HIF. Curr Pharm Des. 15:3895–3903. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Horwitz E, Stein I, Andreozzi M, Nemeth J,
Shoham A, Pappo O, Schweitzer N, Tornillo L, Kanarek N, Quagliata
L, et al: Human and mouse VEGFA-amplified hepatocellular carcinomas
are highly sensitive to sorafenib treatment. Cancer Discov.
4:730–743. 2014. View Article : Google Scholar : PubMed/NCBI
|